Abstract
A 42-plex clustered regularly interspaced short palindromic repeat (CRISPR)-based typing technique (spoligotyping) was recently developed at the French National Reference Center for Legionella. It allows the subtyping of the Legionella pneumophila sequence type 1/Paris pulsotype. In this report, we present the transfer of the membrane-based spoligotyping technique to a microbead-based multiplexed format. This microbead-based high-throughput assay uses devices such as Luminex 200 or the recently launched Magpix system (Luminex Corp., Austin, TX). We designated this new technique LP-SPOL (for L. pneumophila spoligotyping). We used two sets of samples previously subtyped by the membrane-based spoligotyping method to set up and validate the transfer on the two microbead-based systems. The first set of isolates (n = 56) represented the whole diversity of the CRISPR patterns known to date. These isolates were used for transfer setup (determination of spacer cutoffs for both devices). The second set of isolates (n = 245) was used to validate the transfer to the two microbead-based systems. The results obtained by the Luminex 200 system were 100% concordant with those obtained by the Magpix system for the 2 sets of isolates. In total, 10 discrepant results were observed when comparing the membrane-based method to the microbead-based method. These discrepancies were further resolved by repeating either the membrane-based or the microbead-based assay. This new assay is expected to play an emerging role for surveillance of L. pneumophila, starting with one of the most frequent genotypes, the sequence type 1/Paris pulsotype. However, the generalization of this typing method to all L. pneumophila strains is not feasible, since not all L. pneumophila strains contain CRISPRs.
INTRODUCTION
Legionella pneumophila is a Gram-negative facultative intracellular pathogenic bacterium, identified as the infectious agent of Legionnaires' disease (LD) in 1977 (1). Several species of the Legionella genus are responsible for LD; however, L. pneumophila is responsible for the majority of cases of LD, with >90% of all identified clinical cases within serogroup 1, which accounts for ∼85% of all cases (2, 3). The organism is quite ubiquitous in aqueous environments, whether natural or artificial (4).
L. pneumophila may replicate in phagocytic protozoa and in human macrophages. Its pathogenicity is related to pulmonary infections, mainly consisting of an acute pneumonia that might be fatal in healthy or immunodeficient patients. As an example, the case fatality rate was 13% in France from 1998 until 2008 (5). Transmission mechanisms are related to environmental factors, such as transmission through contaminated water droplets and airborne transmission.
The European reference method to study the molecular epidemiology of L. pneumophila is sequence-based typing (SBT), and clusters are referred to as sequence types (STs) (6). Restriction enzyme analysis using pulsed-field gel electrophoresis (PFGE), although laborious, remains a highly discriminating method and defines clusters referred to as pulsotypes. Other technical choices, such as monoclonal antibody typing and, more recently, multilocus variable-number tandem-repeat analysis (MLVA), have been developed (7). However, such methods have limitations in molecular epidemiological investigations due to their lack of power of discrimination, and they are also tedious and slow to perform. A restriction fragment length polymorphism-insertion sequence analysis method (RFLP-IS) has also been developed and is expected to increase the capacity of molecular investigations of legionellosis outbreaks (8).
In previous work, we showed that in certain cases, some strains that are indistinguishable (either by SBT or by PFGE), particularly within the L. pneumophila ST1/Paris pulsotype, could be efficiently classified further using the genetic diversity of their clustered regularly interspaced short palindromic repeats (CRISPRs), and we developed a membrane-based method, spoligotyping (9). The name of this technique is based on that of the original spoligotyping method, which was first used for Mycobacterium tuberculosis complex (MTC) (10). Indeed, spoligotyping can be used to design a generic method, since polymorphic CRISPR regions are described at an increased pace and for more and more pathogens (11–13). Spoligotyping gained great acceptance internationally as a first-line method to genotype MTC for molecular epidemiological studies and allowed an understanding of its global phylogeographical structure (14).
The possibility to run spoligotyping on devices other than membranes, such as multiplex microbead-based flow cytometers or fluorescence imager devices, provides a number of advantages. These microbead-based systems allow high-throughput and a better standardization of assays. The multiplexing level is up to 100 for the Luminex 200 system and up to 50 for the Magpix system. These systems are well suited for use in routine analyses and for surveillance and control of infectious disease. Hence, the purpose of this study was to transfer the previously developed membrane-based technique to the microbead-based format, as was done for M. tuberculosis and, more recently, for Salmonella enterica serovar Typhimurium (12, 15, 16).
MATERIALS AND METHODS
Spoligotyping oligonucleotides and microspheres.
The protocol used for the spoligotyping on a membrane has been published previously (9). For the microbead-based spoligotyping, the DR-F primer was labeled at the 5′ end with biotin (instead of digoxigenin [DIG] as for the membrane-based method). All probes were synthesized with a 5′ amino-C12 linker (instead of a C6 linker as for the membrane-based method) to increase the gyration radius (Eurofins MWG Synthesis, Ebersberg, Germany). Microspheres were MagPlex (paramagnetic coated microspheres) when using the MagPix system and MicroPlex (polystyrene microspheres) when using the Luminex 200 system and were purchased from Luminex BV (Oosterhout, The Netherlands). Coupling of the oligonucleotides to the microspheres was performed according to standard procedures, and the PCR was done as previously described (16). Briefly, the total reaction volume was 25 μl per sample: 8 μl of sterilized water, 5 μl of 5× betaine, 2.5 μl of 10× Q buffer [0.2 M Tris-HCl (pH 8.75), 0.1 M KCl, 0.1 M (NH4)2SO4, 20 mM MgSO4, 15 mM MgCl2, 1% Triton], both primers (10 μM; 2.5 μl each), deoxynucleoside triphosphate (dNTP) mix (2 mM; 2.5 μl), and 1 U of Taq polymerase. Alternatively, another protocol with commercial buffer was also used, in which Q buffer was replaced with 5 μl of 5× Promega Flexi buffer, adding 1.5 μl of 25 mM MgCl2 and 0.2 μl (1 U) of GoTaq from Promega (Mannheim, Germany). The PCR program was as follows: 20 cycles of 95°C for 5 min, 95°C for 20 s, 57°C for 30 s, and 72°C for 40 s. The hybridization took place in a thermocycler at 52°C for 20 min after 10 min of denaturation at 95°C. These steps were performed in a 50-μl volume with 2 μl of PCR product, 15 μl of Tris-EDTA (TE), and 33 μl of bead working solution in 1.5× TMAC (tetramethylammonium chloride) containing around 2,000 beads/analyte on the Luminex 200 or 1,200 beads/analyte on the Magpix. After 5 min of incubation at 52°C in the Luminex analyzer in the presence of 25 μl of 5-ng/μl streptavidin-phycoerythrin (SA-PE; Interchim, Montluçon, France), fluorescence reading was performed by following the Luminex instruction manual and with the use of the Exponent 3 software (Luminex BV, Oosterhout, The Netherlands).
Multiplex analyzers.
We used two Luminex high-throughput systems, the flow cytometry-based Luminex 200 system (two lasers) and the fluorescent imager MagPix (Luminex Corp., Austin, TX), with a charge-coupled-device (CCD) camera and light-emitting diodes (LEDs). The oligonucleotide-precoupled MicroPlex and MagPlex beads that were used throughout the study (research use only) are available from our public genotyping Beads4Med services platform within our institute (UMR8621, Institute of Genetics and Microbiology, Orsay, France; http://www.igmors.u-psud.fr).
Clinical and environmental isolates and DNA extraction.
The DNA samples studied in this work were provided by the French Legionella National Reference Center (NRC) in Lyon. Two sets, totaling 301 DNA samples (set 1 [n = 56] for method development and set 2 [n = 245] as a validation set), were extracted using the QIAamp DNA extraction kit (Qiagen, Les Ulis, France) from L. pneumophila clinical and environmental isolates. The 56 isolates of the 1st set were selected to represent the complete diversity of CRISPR patterns known to date. The 245 isolates of the 2nd set were selected based on the presence of the CRISPR loci. All isolates of the 2 sets have been spoligotyped independently and in a blind fashion by the membrane-based spoligotyping method by the NRC (9). Their membrane spoligotype results were made available once experiments were run in a blind fashion on the Luminex 200 and Magpix systems. The sequence-based typing (SBT) and pulse-field gel electrophoresis (PFGE) genotypes of all isolates had also previously been determined by the NRC. Out of the 301 isolates, 264 isolates had an ST1/Paris pulsotype, 20 isolates had an ST1/non-Paris pulsotype, 11 isolates had a non-ST1/Paris pulsotype, and 6 isolates had a non-ST1/non-Paris pulsotype.
Principle, interpretation of values, statistics, and cutoffs.
The principle of interpretation of the LP-SPOL (L. pneumophila spoligotyping) raw values obtained on Luminex 200 or on Magpix is the same as the one used previously for other microbead-based methods (17). The positive and negative cutoffs used to interpret whether a spacer is positive or negative were computed using the raw results output file. The isolates of set 1 (n = 56) were used in the first step to determine statistically the cutoffs of all spacers. A script previously developed in the R software was applied for statistical calculation (http://www.r-project.org) using a modified receiver operating characteristic (ROC) method. Using these spoligotypes (the set 1, n = 56) as reference results, a pair of cutoffs (positive cutoff and negative cutoff) was calculated for each spacer. All cutoffs were determined for the LP-SPOL method experiment on both the Luminex 200 and Magpix systems (Table 1; see also Table S1 in the supplemental material). Once the cutoffs were determined, they were applied to automatically interpret the raw values of each spacer for each new sample by comparison. The positive values of one spacer (presence of that spacer) are values above the positive cutoff, and negative values are those below the negative cutoff. Raw values between the two cutoffs (positive cutoff and negative cutoff) are intermediate and have to be interpreted carefully by an expert, or a sample can be repeated with an increased amount of the PCR product in the analysis. To evaluate the quality of the probes, a signal/noise ratio was calculated by the same script for each probe (Table 1). Signal is considered the mean of raw fluorescence values of isolates in which the spacer is present, and noise is considered the mean of raw fluorescence values of strains, which are devoid of the spacer. Spoligotyping results obtained by the previous membrane-based method for the first 56 isolates were compared to those obtained by the microbead-based method on the same isolates to validate spacer presence or absence and calculation of cutoffs. The second set was run in a blind fashion in light of these cutoffs.
TABLE 1.
Signal/noise ratio of the 42 probes in the Luminex 200 and Magpix systemsa
| Probe | Luminex 200 |
Magpix |
||||
|---|---|---|---|---|---|---|
| Mean of pos RFU | Mean of neg RFU | Ratio | Mean of pos RFU | Mean of neg RFU | Ratio | |
| Sp1 | 2,082 | 47 | 44 | 1,313 | 70 | 19 |
| Sp2 | 1,797 | 76 | 24 | 1,489 | 102 | 15 |
| Sp3 | 1,927 | 97 | 20 | 1,723 | 113 | 15 |
| Sp4 | 2,374 | 244 | 10 | 1,841 | 204 | 9 |
| Sp5 | 1,984 | 42 | 47 | 1,511 | 72 | 21 |
| Sp6 | 2,364 | 57 | 41 | 1,830 | 141 | 13 |
| Sp7 | 2,130 | 63 | 34 | 1,689 | 90 | 19 |
| Sp8 | 2,330 | 76 | 31 | 1,815 | 84 | 22 |
| Sp9 | 2,114 | 80 | 27 | 1,730 | 112 | 16 |
| Sp10 | 1,911 | 73 | 26 | 1,537 | 85 | 18 |
| Sp11 | 1,835 | 153 | 12 | 1,434 | 101 | 14 |
| Sp12 | 1,602 | 116 | 14 | 1,398 | 92 | 15 |
| Sp13 | 2,350 | 60 | 39 | 1,748 | 86 | 20 |
| Sp14 | 2,198 | 97 | 23 | 1,647 | 102 | 16 |
| Sp15 | 2,467 | 81 | 31 | 1,916 | 75 | 26 |
| Sp16 | 1,591 | 111 | 14 | 1,263 | 89 | 14 |
| Sp17 | 1,814 | 58 | 31 | 1,475 | 60 | 25 |
| Sp18 | 1,968 | 67 | 29 | 1,617 | 95 | 17 |
| Sp19 | 1,477 | 63 | 23 | 1,108 | 76 | 15 |
| Sp20 | 2,055 | 62 | 33 | 1,689 | 96 | 18 |
| Sp21 | 1,966 | 58 | 34 | 1,601 | 83 | 19 |
| Sp22 | 2,216 | 64 | 34 | 1,910 | 219 | 9 |
| Sp23 | 933 | 208 | 4 | 919 | 262 | 4 |
| Sp24 | 1,861 | 101 | 18 | 1,687 | 274 | 6 |
| Sp25 | 2,547 | 125 | 20 | 2,232 | 631 | 4 |
| Sp26 | 2,233 | 93 | 24 | 1,662 | 123 | 13 |
| Sp27 | 2,082 | 58 | 36 | 1,728 | 101 | 17 |
| Sp28 | 1,623 | 75 | 22 | 1,570 | 96 | 16 |
| Sp29 | 2,097 | 176 | 12 | 1,725 | 130 | 13 |
| Sp30 | 2,033 | 189 | 11 | 1,543 | 201 | 8 |
| Sp31 | 2,336 | 80 | 29 | 1,876 | 124 | 15 |
| Sp32 | 2,101 | 89 | 24 | 1,426 | 118 | 12 |
| Sp33 | 2,523 | 77 | 33 | 1,872 | 133 | 14 |
| Sp34 | 2,522 | 88 | 29 | 1,834 | 144 | 13 |
| Sp35 | 2,532 | 89 | 28 | 2,179 | 672 | 3 |
| Sp36 | 988 | 86 | 11 | 838 | 100 | 8 |
| Sp37 | 2,039 | 62 | 33 | 1,634 | 116 | 14 |
| Sp38 | 2,152 | 96 | 23 | 1,636 | 111 | 15 |
| Sp39 | 1,429 | 87 | 16 | 1,065 | 95 | 11 |
| Sp40 | 2,231 | 141 | 16 | 1,742 | 301 | 6 |
| Sp41 | 1,825 | 69 | 26 | 1,456 | 88 | 17 |
| Sp42 | 1,160 | 101 | 12 | 851 | 118 | 7 |
Fifty-six well-characterized isolates were used. Pos, positive; neg, negative; RFU, relative fluorescence units.
RESULTS
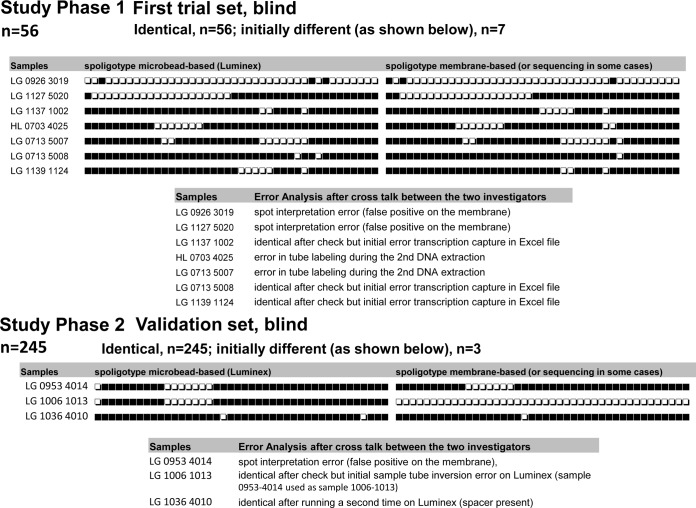
We developed the technique on one set of samples covering the known CRISPR diversity in L. pneumophila and validated it using a second independent set. When comparing the quantitative outputs of Luminex (median fluorescence intensities [MFIs]) with the results from membranes of the first 56 isolates, we observed a very strong positive correlation between MFI by the two systems (Luminex and Magpix) and the presence of spacers, except for 13 specific data points for 7 isolates. Indeed, for all spacers but those for 13 of the 56 tested isolates (2,339/2,352 spoligotype data points), high MFI values corresponded to spacers detected as positive (presence of spacer) with the membrane-based method; conversely, low MFI values corresponded to spacers detected as negative (absence of spacer) with the membrane-based method. The 13 discrepant points correspond to low MFI values for 5 spacers positive by the membrane-based method and to high MFI values for 8 spacers negative by the membrane-based method (see Tables S2 and S3 in the supplemental material for MFI values). These results strongly suggested artifacts for the corresponding isolates, and these 7 samples were checked first by rereading the membranes and then, if necessary, by repeating the membrane-based spoligotyping (Fig. 1). These verifications led to the corrections of the results obtained by the membrane-based method for all these 13 discrepant points (Fig. 2). The initial discrepancies were due to some errors in the membrane technique, whether due to misinterpretation of hybridization signals (2 isolates), error in transcription of source results into Excel (3 isolates), or a labeling error of the sample (2 isolates) (Fig. 2).
FIG 1.
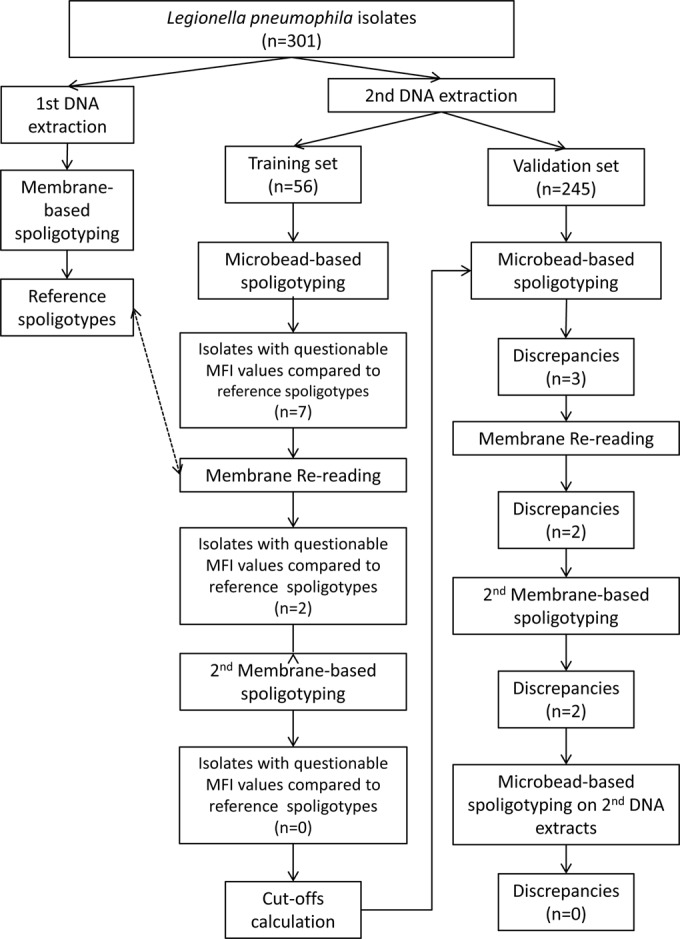
Flowchart of the analytical process.
FIG 2.
Description of solution of discrepant data points.
The signal/noise ratios, computed as the ratio between the mean of all positive values and the mean of all negative values for each spacer independently, were high for both systems (see Table S1 in the supplemental material). The negative and positive cutoff values were computed independently for Luminex and Magpix (see Table S1 in the supplemental material). These cutoffs were used to interpret the second set of isolates (validation set). For this set of isolates, 3 discrepant spoligotypes between microbead-based assays and membrane-based assays were also observed. One was resolved by repeating the membrane-based assay; the two others were resolved by repeating the microbead-based assay (Fig. 2). On the validation set, we thus finally obtained 100% concordance for all the unknown isolates (n = 245), whatever the method (membrane- or microbead-based assays developed either on the MagPix or on the Luminex 200) (see Table S4 in the supplemental material).
The signal/noise ratio was, on average, higher on the microfluidic laser-based system, Luminex 200, than on the fluorescence imaging magnetic system, Magpix (see Tables S2 and S3 in the supplemental material) (Student paired t test difference = 8.8; df = 41; P < 0.001). More data points fell in the gray zone between the maximum of the negatives and the minimum of the positives with the second technique (75, compared to 27 for Luminex 200, out of 12,768 data points). A visual inspection taking into account the quantitative values obtained for the same sample on other spacers could still allow an interpretation of most values. Altogether, the spoligotyping method allowed the identification of 43 different spoligotypes for the 264 isolates that were of the ST1/Paris pulsotype, 5 different spoligotypes for the 20 isolates that were of the ST1/non-Paris pulsotype, 5 for the 11 isolates that were of the non-ST1/Paris pulsotype, and 3 for the 6 isolates that were of the non-ST1/non-Paris pulsotype. Four spoligotypes were shared by 2 or more of these 4 groups, leading to a total of 51 different spoligotypes for the 301 isolates. STs and pulsotypes of non-ST1 and/or non-Paris pulsotype strains were very diverse; spoligotyping allowed a better differentiation of isolates in these groups only once for 6 isolates with the same ST and pulsotype (ST540/Paris pulsotype), which could be subtyped into 2 spoligotypes (see Table S4 in the supplemental material). None of the 26 non-Paris pulsotype clusters was more discernible by spoligotyping in this study (data not shown).
DISCUSSION
We successfully transferred the membrane-based spoligotyping method of Legionella pneumophila to two high-throughput microbead systems (9). The concordance between the microbead-based system and the membrane-based method was virtually complete after careful reexamination of a few discrepant points.
The discrepancies initially observed between the two techniques were due to (i) error in tube labeling during the 2nd DNA extraction, (ii) sample tube inversion, (iii) interpretation errors regarding intensities of spots of the assays obtained by the membrane-based technique, and (iv) error during the transcription of results from the membrane to a digital format. The first two points are technical mistakes that are independent of the methods used. The last two points were linked to the use of the membrane-based method. Similar observations concerning the last two points were made in the study of Abadia et al. (18) that compared membrane-based M. tuberculosis spoligotyping results to those from microbead-based systems. Concerning these points, as seen in this study, their study highlights the quality improvement of results by the microbead-based method. This improvement is due first to the better standardization of result interpretation based on quantitative measurements and the use of predefined cutoffs compared to the membrane-based method, for which the interpretation is subjective and operator dependent. It is due also to the absence of manual transcription of results to a digital format, a step during which transcription errors could occur with the membrane-based method.
Until now, and based on whole-genome sequencing of L. pneumophila isolates, CRISPR sequences have distinguished 3 classes of L. pneumophila isolates: (i) those with no CRISPR, such as Philadelphia, Lorraine, HL 0604 1035, Corby, LPE509, and ATCC 43290; (ii) those with a type IF CRISPR (or linked to IF), as described for the Lens and Alcoy strains (19); and (iii) those with a type II CRISPR, as described in studies on the Paris strains previously sequenced and on the 130b strain (9, 20). The type II CRISPR was detected in almost 100% of the ST1 and/or Paris pulsotype isolates and in less than 10% of other L. pneumophila isolates (9). Among the latter isolates, some harbored different sequences of spacers (124 new spacers could be found in 5 isolates), much more than the 42 used in the currently developed technique (9). Only L. pneumophila serogroup 1 isolates of the ST1/Paris pulsotype showed a strong likelihood of CRISPR presence in their genomes. The L. pneumophila spoligotyping technique had worked for all isolates of the L. pneumophila serogroup 1 sequence type 1/Paris pulsotype (9). Considering the discrimination into 43 different spoligotypes for the 264 undistinguishable ST1/Paris pulsotype isolates, the L. pneumophila spoligotyping technique can be recommended as a complementary method for discriminating among these isolates. Spoligotyping allows more discrimination of isolates harboring identical STs and PFGE patterns than does the combination of SBT and PFGE methods. Thus, spoligotyping methods could be developed for the subtyping of other worldwide clinically predominant L. pneumophila sequence types harboring one CRISPR locus, such as the ST62, which is now emerging in Germany (21, 22).
The microbead-based system offers the possibility of multiplexing up to 50 analytes in the Magpix, up to 100 in the Luminex 200, and up to 500 in the FlexMap 3D. As the number of L. pneumophila genome sequences will increase and the spacer catalog is likely to concomitantly expand, more knowledge obtained through the use of data-mining softwares such as Sipina (http://eric.univ-lyon2.fr/∼ricco/sipina.html) and/or Weka (http://www.cs.waikato.ac.nz/ml/weka/) to select the most informative spacers will become feasible and necessary, similar to what has been done for the M. tuberculosis model (23). New spacers can be added to the previous set of 42 spacers in a new spoligotyping format that would extend LP-SPOL applicability to all ST1 isolates, all Paris pulsotype isolates, or eventually to other worldwide clinically predominant L. pneumophila sequence types harboring one CRISPR locus.
In conclusion, the transfer of L. pneumophila spoligotyping techniques to the microbead-based system is an important step that allows (i) a quality improvement in obtained results, (ii) a throughput that is 5× higher (200 isolates in two working weeks with membranes, versus two working days on microbeads), and (iii) a possible implementation to new international public health laboratories and an internationally connected database for surveillance.
The high-throughput and computerized interpretation of results will also promote cost-effective genotyping of a larger number of interesting isolates during epidemiological investigations of L. pneumophila outbreaks or of a larger number of environmental samples to prevent new outbreaks. We are currently working on an international evaluation of the microbead-based LP spoligotyping method, beginning with isolates from Italy.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to M. F. Topin and J. van Gils from Luminex BV (Oosterhout, The Netherlands) for technical support.
This work was supported by a Ph.D. grant given by Fondation Mérieux and the CNRS to Michel K. Gomgnimbou.
Footnotes
Published ahead of print 23 April 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JCM.00219-14.
REFERENCES
- 1. McDade JE, Shepard CC, Fraser DW, Tsai TR, Redus MA, Dowdle WR. 1977. Legionnaires' disease: isolation of a bacterium and demonstration of its role in other respiratory disease. N. Engl. J. Med. 297:1197–1203. 10.1056/NEJM197712012972202 [DOI] [PubMed] [Google Scholar]
- 2. Doleans A, Aurell H, Reyrolle M, Lina G, Freney J, Vandenesch F, Etienne J, Jarraud S. 2004. Clinical and environmental distributions of Legionella strains in France are different. J. Clin. Microbiol. 42:458–460. 10.1128/JCM.42.1.458-460.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yu VL, Plouffe JF, Pastoris MC, Stout JE, Schousboe M, Widmer A, Summersgill J, File T, Heath CM, Paterson DL, Chereshsky A. 2002. Distribution of Legionella species and serogroups isolated by culture in patients with sporadic community-acquired legionellosis: an international collaborative survey. J. Infect. Dis. 186:127–128. 10.1086/341087 [DOI] [PubMed] [Google Scholar]
- 4. Edagawa A, Kimura A, Doi H, Tanaka H, Tomioka K, Sakabe K, Nakajima C, Suzuki Y. 2008. Detection of culturable and nonculturable Legionella species from hot water systems of public buildings in Japan. J. Appl. Microbiol. 105:2104-2114. 10.1111/j.1365-2672.2008.03932.x [DOI] [PubMed] [Google Scholar]
- 5. Campese C, Bitar D, Jarraud S, Maine C, Forey F, Etienne J, Desenclos JC, Saura C, Che D. 2011. Progress in the surveillance and control of Legionella infection in France, 1998–2008. Int. J. Infect. Dis. 15:e30–e37. 10.1016/j.ijid.2010.09.007 [DOI] [PubMed] [Google Scholar]
- 6. Gaia V, Fry NK, Afshar B, Luck PC, Meugnier H, Etienne J, Peduzzi R, Harrison TG. 2005. Consensus sequence-based scheme for epidemiological typing of clinical and environmental isolates of Legionella pneumophila. J. Clin. Microbiol. 43:2047–2052. 10.1128/JCM.43.5.2047-2052.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nederbragt AJ, Balasingham A, Sirevag R, Utkilen H, Jakobsen KS, Anderson-Glenna MJ. 2008. Multiple-locus variable-number tandem repeat analysis of Legionella pneumophila using multi-colored capillary electrophoresis. J. Microbiol. Methods 73:111–117. 10.1016/j.mimet.2008.02.007 [DOI] [PubMed] [Google Scholar]
- 8. Vergnes M, Ginevra C, Kay E, Normand P, Thioulouse J, Jarraud S, Maurin M, Schneider D. 2011. Insertion sequences as highly resolutive genomic markers for sequence type 1 Legionella pneumophila Paris. J. Clin. Microbiol. 49:315–324. 10.1128/JCM.01261-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ginevra C, Jacotin N, Diancourt L, Guigon G, Arquilliere R, Meugnier H, Descours G, Vandenesch F, Etienne J, Lina G, Caro V, Jarraud S. 2012. Legionella pneumophila sequence type 1/Paris pulsotype subtyping by spoligotyping. J. Clin. Microbiol. 50:696–701. 10.1128/JCM.06180-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kamerbeek J, Schouls L, Kolk A, van Agterveld M, van Soolingen D, Kuijper S, Bunschoten A, Molhuizen H, Shaw R, Goyal M, van Embden J. 1997. Simultaneous detection and strain differentiation of Mycobacterium tuberculosis for diagnosis and epidemiology. J. Clin. Microbiol. 35:907–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mokrousov I, Limeschenko E, Vyazovaya A, Narvskaya O. 2007. Corynebacterium diphtheriae spoligotyping based on combined use of two CRISPR loci. Biotechnol. J. 2:901–906. 10.1002/biot.200700035 [DOI] [PubMed] [Google Scholar]
- 12. Fabre L, Zhang J, Guigon G, Le Hello S, Guibert V, Accou-Demartin M, de Romans S, Lim C, Roux C, Passet V, Diancourt L, Guibourdenche M, Issenhuth-Jeanjean S, Achtman M, Brisse S, Sola C, Weill FX. 2012. CRISPR typing and subtyping for improved laboratory surveillance of Salmonella infections. PLoS One 7:e36995. 10.1371/journal.pone.0036995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lopez-Sanchez MJ, Sauvage E, Da Cunha V, Clermont D, Ratsima Hariniaina E, Gonzalez-Zorn B, Poyart C, Rosinski-Chupin I, Glaser P. 2012. The highly dynamic CRISPR1 system of Streptococcus agalactiae controls the diversity of its mobilome. Mol. Microbiol. 85:1057–1071. 10.1111/j.1365-2958.2012.08172.x [DOI] [PubMed] [Google Scholar]
- 14. Brudey K, Driscoll J, Rigouts L, Prodinger WM, Gori A, Al-Hajoj SAM, Allix C, Aristimuno L, Arora J, Baumanis V, Binder L, Cafrune P, Cataldi A, Cheong S, Diel R, Ellermeier C, Evans JT, Fauville-Dufaux M, Ferdinand S, Garcia de Viedma D, Garzelli C, Gazzola L, Gomes HM, Gutierrez MC, Hawkey PM, van Helden PD, Kadival GV, Kreiswirth BN, Kremer K, Kubin M, Kulkarni SP, Liens B, Lillebaek T, Ly HM, Martin C, Martin C, Mokrousov I, Narvskaia O, Ngeow YF, Naumann L, Niemann S, Parwati I, Rahim MZ, Rasolofo-Razanamparany V, Rasolonavalona T, Rossetti ML, Rüsch-Gerdes S, Sajduda A, Samper S, Shemyakin IG, Singh UB, Somoskovi A, Skuce R, Van Soolingen D, Streicher EM, Suffys PN, Tortoli E, Tracevska T, Vincent V, Victor TC, Warren R, Yap SF, Zaman K, Portaels F, Rastogi N, Sola C. 2006. Mycobacterium tuberculosis complex genetic diversity: mining the fourth international spoligotyping database (SpolDB4) for classification, population genetics, and epidemiology. BMC Microbiol. 6:23. 10.1186/1471-2180-6-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cowan LS, Diem L, Brake MC, Crawford JT. 2004. Transfer of a Mycobacterium tuberculosis genotyping method, spoligotyping, from a reverse line-blot hybridization, membrane-based assay to the Luminex multianalyte profiling system. J. Clin. Microbiol. 42:474–477. 10.1128/JCM.42.1.474-477.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang J, Abadia E, Refregier G, Tafaj S, Boschiroli ML, Guillard B, Andremont A, Ruimy R, Sola C. 2010. Mycobacterium tuberculosis complex CRISPR genotyping: improving efficiency, throughput and discriminative power of ‘spoligotyping' with new spacers and a microbead-based hybridization assay. J. Med. Microbiol. 59:285–294. 10.1099/jmm.0.016949-0 [DOI] [PubMed] [Google Scholar]
- 17. Gomgnimbou MK, Abadia E, Zhang J, Refregier G, Panaiotov S, Bachiyska E, Sola C. 2012. “Spoligoriftyping,” a dual-priming-oligonucleotide-based direct-hybridization assay for tuberculosis control with a multianalyte microbead-based hybridization system. J. Clin. Microbiol. 50:3172–3179. 10.1128/JCM.00976-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Abadia E, Zhang J, Ritacco V, Kremer K, Ruimy R, Rigouts L, Gomes HM, Elias AR, Fauville-Dufaux M, Stoffels K, Rasolofo-Razanamparany V, Garcia de Viedma D, Herranz M, Al-Hajoj S, Rastogi N, Garzelli C, Tortoli E, Suffys PN, van Soolingen D, Refregier G, Sola C. 2011. The use of microbead-based spoligotyping for Mycobacterium tuberculosis complex to evaluate the quality of the conventional method: providing guidelines for quality assurance when working on membranes. BMC Infect. Dis. 11:110. 10.1186/1471-2334-11-110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. D'Auria G, Jimenez-Hernandez N, Peris-Bondia F, Moya A, Latorre A. 2010. Legionella pneumophila pangenome reveals strain-specific virulence factors. BMC Genomics 11:181. 10.1186/1471-2164-11-181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gunderson FF, Cianciotto NP. 2013. The CRISPR-associated gene cas2 of Legionella pneumophila is required for intracellular infection of amoebae. mBio 4(2):e00074-13. 10.1128/mBio.00074-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Harrison TG, Afshar B, Doshi N, Fry NK, Lee JV. 2009. Distribution of Legionella pneumophila serogroups, monoclonal antibody subgroups and DNA sequence types in recent clinical and environmental isolates from England and Wales (2000–2008). Eur. J. Clin. Microbiol. Infect. Dis. 28:781–791. 10.1007/s10096-009-0705-9 [DOI] [PubMed] [Google Scholar]
- 22. Lück C. 2013. Epidemiological typing methods for Legionella pneumophila, p 22 Abstr. 8th Int. Conf. Legionella 2013, Melbourne, Australia [Google Scholar]
- 23. Gomgnimbou MK, Hernandez-Neuta I, Panaiotov S, Bachiyska E, Palomino JC, Martin A, Del Portillo P, Refrégier G, Sola C. 2013. Tuberculosis-spoligo-rifampin-isoniazid typing: an all-in-one assay technique for surveillance and control of multidrug-resistant tuberculosis on Luminex devices. J. Clin. Microbiol. 51:3527–3534. 10.1128/JCM.01523-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.