Abstract
Results from 3,263 QuantiFERON-TB Gold in-tube (QFT-GIT) assays were analyzed to determine the impact of age on test performance. The proportion of indeterminate results was significantly higher in pediatric and elderly (9.1% and 7.4%, respectively) than in adult (2.6%; chi-square test, P < 0.0001) patients. A detailed analysis of indeterminate QFT-GIT assay results is presented.
TEXT
Interferon gamma release assays (IGRA) are widely used for the diagnosis of tuberculosis (TB) infection in resource-rich countries (1). Currently, two IGRA are commercially available, the QuantiFERON-TB (QFT) Gold assay (Cellestis/Qiagen, Carnegie, Australia) and the T-SPOT.TB assay (Oxford Immunotec, Abingdon, United Kingdom). Both assays rely on the detection of interferon gamma produced by sensitized T cells in response to stimulation with the comparatively Mycobacterium tuberculosis-specific antigens early-secretory antigenic target 6 (ESAT-6) and the 10-kDa culture filtrate protein (CFP-10), which are absent from all Mycobacterium bovis BCG vaccine strains and most nontuberculous mycobacteria (2, 3). The QFT Gold In-Tube (QFT-GIT) assay incorporates TB7.7 as an additional antigen.
QFT-GIT results are classified as either determinate (i.e., positive or negative) or indeterminate. In brief, the latter occur when interferon gamma production in response to stimulation with phytohemagglutinin (PHA) in the positive-control sample is insufficient or when the interferon gamma background concentration in the unstimulated (nil) control sample is high (4). Previous reports have shown that indeterminate QFT-GIT results are more common in patients with immunodeficiency and those receiving immunosuppressive therapy (5–7). In addition, delayed sample incubation has been shown to increase the likelihood of indeterminate results (8).
Indeterminate results pose a considerable dilemma for clinical management, as they generally convey no information regarding the TB infection status of the patient. Previous publications, including our own, have shown that indeterminate QFT-GIT results occur in a considerable proportion of children evaluated for TB infection (5, 9, 10). However, few publications have specifically focused on indeterminate results in adults, and even fewer have included a comparison of the performance of QFT-GIT assays between pediatric and adult patients (11, 12).
This study aimed to compare the performance of the QFT-GIT assay in children with that in adults, to determine the impact of age on test performance, and to describe associations between immunocompromise and indeterminate assay results.
Data from the QFT-GIT assay performed at a regional public health laboratory in the southeast of England over a 3-year period (January 2011 to December 2013) were analyzed. The laboratory provides microbiology services for a large tertiary-care referral hospital (University Hospital Southampton [UHS]), several regional hospitals, and general practitioner (GP) surgeries. UHS is a regional referral center for the south of England, which overall has a comparatively low incidence of TB (<5/100,000 population); however, certain areas within this region, such as inner-city Southampton, have higher rates of TB incidence (up to 25/100,000 population; for further details, see http://www.hpa.org.uk/webc/HPAwebFile/HPAweb_C/1317139689583). The age distribution of TB cases reflects global trends, with comparatively few cases in childhood and a peak in young adulthood. The laboratory is fully accredited as a diagnostic laboratory and has extensive experience with the QFT-GIT assay, having processed these assays since 2007. All QFT-GIT assays were performed and interpreted according to the manufacturer's instructions. Following incubation, supernatants were harvested manually and enzyme-linked immunosorbent assays (ELISAs) were performed with an Opsys MR microplate reader (Dynex Technologies, Worthing, United Kingdom). The manufacturer's proprietary software package (Cellestis/Qiagen; version 2.62) was used for data analysis and calculation of the test results. The study was approved by UHS.
For statistical analyses, three age groups were created: children and adolescents (<18 years of age), adults (18 to 64 years of age), and elderly (≥65 years of age). The data were analyzed with STATA (version 12; StataCorp, College Station, TX, USA) and Prism (version 5.0; GraphPad, La Jolla, CA, USA). Mann-Whitney U and chi-square tests were used for the analysis of continuous and categorical data, respectively.
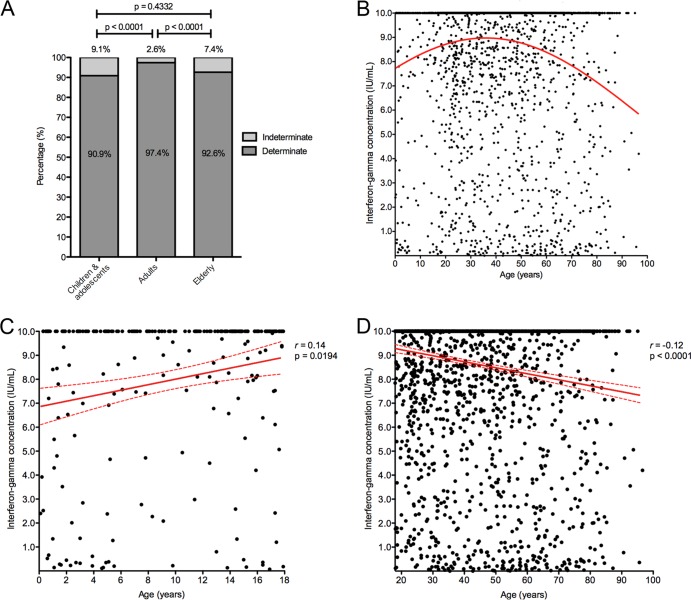
During the period investigated, a total of 3,263 QFT-GIT assays were performed on 263 children and adolescents, 2,622 adults, and 378 elderly patients. Overall, 562 (17.2%) had a positive, 2,582 (79.1%) a negative, and 119 (3.6%) an indeterminate result. The proportion of indeterminate results was significantly higher in the child and adolescent group and the elderly group than in the adult group (Fig. 1A).
FIG 1.
(A) Proportions of determinate and indeterminate QuantiFERON-TB Gold In-Tube assay results in children and adolescents (<18 years of age), adults (18 to 64 years of age), and elderly patients (≥65 years of age) (n = 3,263). Mann Whitney U test P values are shown above the bars. (B) Interferon gamma concentrations in the positive-control samples according to age (n = 3,263). The nonlinear regression line is indicated in red. (C) Correlation between age and interferon gamma concentrations in the positive-control samples in the group of children and adolescents (n = 263). The dotted lines indicate the 95% confidence interval. (D) Correlation between age and interferon gamma concentrations in the positive-control samples for adult and elderly patients (n = 3,000). The dotted lines indicate the 95% confidence interval.
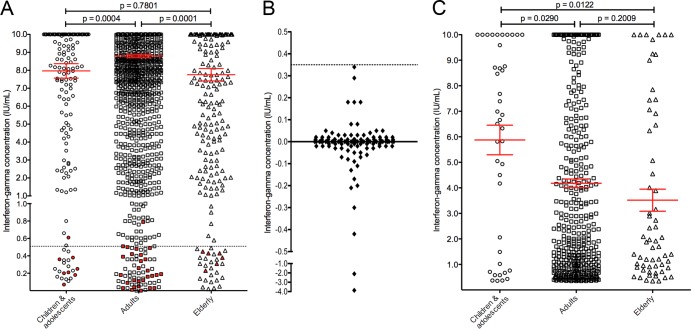
The majority of indeterminate QFT-GIT results were due to failed positive controls (n = 114; 95.8%); few were due to high interferon gamma concentrations in the nil control sample (n = 5; 4.2%). Figure 1B shows the correlation between age and interferon gamma concentration in the positive-control sample (data were censored at 10 IU/ml according to the manufacturer's instructions). In children and adolescents, there was a significant positive correlation between age and interferon gamma concentrations (Fig. 1C) (Spearman's correlation coefficient [r] = 0.1444; 95% confidence interval [CI], 0.0200 to 0.2644; P = 0.0194). In contrast, in adult and elderly patients, a significant inverse relationship was observed (Fig. 1D) (r = −0.1203; 95% CI, −0.1565 to −0.0839; P < 0.0001). Interferon gamma concentrations in the positive-control samples were significantly higher in the adult group than in the other two age groups (Fig. 2A).
FIG 2.
(A) Interferon gamma concentrations in the positive-control sample according to age group (n = 3,263). Mann Whitney U test P values are shown above the chart. The red lines indicate the mean and the standard error of the mean. Results from immunocompromised patients (i.e., those with known immunodeficiency or on immunosuppressive therapy) are highlighted as red circles, squares, and triangles. The dotted line indicates an interferon gamma concentration of 0.5 IU/ml. (B) Background-corrected interferon gamma responses in patients with indeterminate QFT-GIT results resulting from failed positive controls (n = 114). The dotted line indicates an interferon gamma concentration of 0.35 IU/ml. (C) Interferon gamma concentrations in the antigen-stimulated sample (uncorrected) in patients with a positive QuantiFERON-TB Gold In-Tube assay result according to age group (n = 562). Mann Whitney U test P values are shown above the chart. The red lines indicate the mean and the standard error of the mean.
For 22 children and adolescents with indeterminate results, clinical data were available (no clinical data were available for 2 patients, as the samples were sent from another hospital or GP surgery); of those, 8 (36.4%) had a known immunodeficiency or were receiving immunosuppressive therapy (n = 1 or n = 7, respectively). Immunosuppressive treatment in this group comprised corticosteroids (n = 3), methotrexate (n = 4), 6-mercaptopurine (n = 3), and infliximab (n = 1); 3 patients were receiving two or more immunosuppressive drugs. For 46 adults with indeterminate results, clinical data were available (no clinical data were available for 21); of those 46, 28 (60.9%) had a known immunodeficiency or were receiving immunosuppressive therapy (n = 2 or n = 26, respectively). Immunosuppressive treatment in this group comprised corticosteroids (n = 20), methotrexate (n = 6), azathioprine (n = 2), cyclosporine (n = 1), adalimumab (n = 1), and tocilizumab (n = 1); 4 patients were receiving two or more immunosuppressive drugs. For 22 elderly patients with indeterminate results, clinical data were available (no clinical data were available for 6); of those 22, 6 (27.3%) were receiving immunosuppressant drugs, comprising corticosteroids (n = 5) and methotrexate (n = 1). In immunocompromised patients with indeterminate results, interferon gamma concentrations in the positive-control sample rarely exceeded 0.5 IU/ml (3 of 42 patients) (Fig. 2A).
Figure 2B shows the background-corrected interferon gamma responses (i.e., interferon gamma concentration in the antigen-stimulated sample minus the interferon gamma concentration in the nil control sample) among patients with indeterminate QFT-GIT results. Only in five (4.4%) of those individuals were interferon gamma responses that exceeded 0.1 IU/ml observed (universally, <0.35 IU/ml). No clinical information was available for four of these patients (samples sent from another hospital or GP surgery); the remaining patient (with a background-corrected interferon gamma response of 0.18 IU/ml), who had a QFT-GIT assay performed as part of a routine workup for inflammatory bowel disease, had no known risk factors for TB infection.
Figure 2C shows the interferon gamma concentrations in the antigen-stimulated samples among patients with a positive QFT-GIT result. The concentrations in children and adolescents were significantly higher than in adult and elderly patients.
Our data highlight the suboptimal performance of the QFT-GIT assays at both ends of the age spectrum and provide several novel insights. First, indeterminate QFT-GIT results are significantly more common in children and adolescents and in the elderly than in young and middle-aged adults. Other reports, including our own, have previously documented that indeterminate QFT-GIT results are comparatively common in children, with rates ranging from 5% to 35% reported by different pediatric studies (5, 10). In contrast, the published data regarding indeterminate QFT-GIT results in the elderly are very limited.
The clear majority of indeterminate results occurred due to insufficient interferon gamma production in response to stimulation with PHA, which is consistent with data from other studies (10–12). Our results show that there is a significant relationship between age and PHA-induced interferon gamma responses, with an increasing trend in the first few years of life and a decreasing trend during adult life, likely reflecting immune maturation and subsequent immunosenescence. Unlike with the adult group, where the majority of patients with indeterminate results were immunodeficient or were receiving immunosuppressive therapy, only a minority of children and adolescents and elderly patients with indeterminate results were immunocompromised, further supporting the notion that age is a key determinant for the magnitude of PHA-induced interferon gamma responses. The use of age-specific cutoffs for positive controls or, alternatively, the incorporation of another control stimulant that produces similar responses across all age groups may help to reduce the unacceptably high rate of indeterminate assay results in young and elderly patients.
Interestingly, our data also show that antigen-induced interferon gamma responses are significantly higher in children than in adults, suggesting that young age does not negatively impact the ability of T cells to generate M. tuberculosis-specific interferon gamma responses. This observation is in accordance with data from a previous study that evaluated an earlier version of the QFT assay, which reported that both ESAT-6 and CFP-10 responses were on average higher in children than in adults (13). In conjunction, these data indicate that in contrast to PHA-induced responses, age-specific adjustments are likely not required for the interpretation of responses induced by mycobacterial antigens.
ACKNOWLEDGMENTS
We thank David Browning at Public Health England South-East Regional Microbiology Services, for his kind help with data retrieval.
No authors have commercial or other associations that might pose a conflict of interest.
M.T. is supported by a Clinical Lectureship provided by the United Kingdom National Institute for Health Research and a research grant provided by the United Kingdom Technology Strategy Board. M.T., P.E., and S.N.F. are supported by the Southampton NIHR Respiratory Biomedical Research Unit. S.N.F., H.D.G., and P.S. are supported by the Southampton NIHR Wellcome Trust Clinical Research Facility.
(Parts of the data were presented at the 32nd Annual Meeting of the European Society for Paediatric Infectious Diseases on 6 to 10 May 2014 in Dublin, Ireland.)
Footnotes
Published ahead of print 14 May 2014
REFERENCES
- 1.Tebruegge M, Connell T, Curtis N. 2012. Tuberculosis in children. N. Engl. J. Med. 367:1568. 10.1056/NEJMc1210173#SA2 [DOI] [PubMed] [Google Scholar]
- 2.Tebruegge M, Connell T, Ritz N, Bryant PA, Curtis N. 2010. Discordance between TSTs and IFN-gamma release assays: the role of NTM and the relevance of mycobacterial sensitins. Eur. Respir. J. 36:214–215. 10.1183/09031936.00025510 [DOI] [PubMed] [Google Scholar]
- 3.Andersen P, Munk ME, Pollock JM, Doherty TM. 2000. Specific immune-based diagnosis of tuberculosis. Lancet 356:1099–1104. 10.1016/S0140-6736(00)02742-2 [DOI] [PubMed] [Google Scholar]
- 4.Cellestis/Qiagen. March 2013. QuantiFERON-TB Gold in-tube package insert (document no. US05990301L). http://www.quantiferon.com/irm/content/PI/QFT/2PK/US.pdf
- 5.Haustein T, Ridout DA, Hartley JC, Thaker U, Shingadia D, Klein NJ, Novelli V, Dixon GL. 2009. The likelihood of an indeterminate test result from a whole-blood interferon-gamma release assay for the diagnosis of Mycobacterium tuberculosis infection in children correlates with age and immune status. Pediatr. Infect. Dis. J. 28:669–673. 10.1097/INF.0b013e3181a16394 [DOI] [PubMed] [Google Scholar]
- 6.Kobashi Y, Mouri K, Obase Y, Fukuda M, Miyashita N, Oka M. 2007. Clinical evaluation of QuantiFERON TB-2G test for immunocompromised patients. Eur. Respir. J. 30:945–950. 10.1183/09031936.00040007 [DOI] [PubMed] [Google Scholar]
- 7.Richeldi L, Losi M, D'Amico R, Luppi M, Ferrari A, Mussini C, Codeluppi M, Cocchi S, Prati F, Paci V, Meacci M, Meccugni B, Rumpianesi F, Roversi P, Cerri S, Luppi F, Ferrara G, Latorre I, Gerunda GE, Torelli G, Esposito R, Fabbri LM. 2009. Performance of tests for latent tuberculosis in different groups of immunocompromised patients. Chest 136:198–204. 10.1378/chest.08-2575 [DOI] [PubMed] [Google Scholar]
- 8.Herrera V, Yeh E, Murphy K, Parsonnet J, Banaei N. 2010. Immediate incubation reduces indeterminate results for QuantiFERON-TB Gold in-tube assay. J. Clin. Microbiol. 48:2672–2676. 10.1128/JCM.00482-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bamford AR, Crook AM, Clark JE, Nademi Z, Dixon G, Paton JY, Riddell A, Drobniewski F, Riordan A, Anderson ST, Williams A, Walters S, Kampmann B. 2010. Comparison of interferon-gamma release assays and tuberculin skin test in predicting active tuberculosis (TB) in children in the UK: a paediatric TB network study. Arch. Dis. Child. 95:180–186. 10.1136/adc.2009.169805 [DOI] [PubMed] [Google Scholar]
- 10.Connell TG, Tebruegge M, Ritz N, Bryant PA, Leslie D, Curtis N. 2010. Indeterminate interferon-gamma release assay results in children. Pediatr. Infect. Dis. J. 29:285–286. 10.1097/INF.0b013e3181c4822f [DOI] [PubMed] [Google Scholar]
- 11.Rose MV, Kimaro G, Nissen TN, Kroidl I, Hoelscher M, Bygbjerg IC, Mfinanga SG, Ravn P. 2012. QuantiFERON™-TB Gold in-tube performance for diagnosing active tuberculosis in children and adults in a high burden setting. PLoS One 7:e37851. 10.1371/journal.pone.0037851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Banfield S, Pascoe E, Thambiran A, Siafarikas A, Burgner D. 2012. Factors associated with the performance of a blood-based interferon-gamma release assay in diagnosing tuberculosis. PLoS One 7:e38556. 10.1371/journal.pone.0038556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hesseling AC, Mandalakas AM, Kirchner HL, Chegou NN, Marais BJ, Stanley K, Zhu X, Black G, Beyers N, Walzl G. 2009. Highly discordant T cell responses in individuals with recent exposure to household tuberculosis. Thorax 64:840–846. 10.1136/thx.2007.085340 [DOI] [PubMed] [Google Scholar]