Abstract
Nineteen natural cases of etiologically undetermined encephalitides in free-ranging cetaceans were studied retrospectively. Histological examination of the brains revealed variable degrees of nonsuppurative encephalitis or meningoencephalitis, characterized predominantly by perivascular lymphohistiocytic infiltrates. A PCR assay was used on brain and other available tissues to detect the presence of morbillivirus, herpesvirus, West Nile virus, Toxoplasma gondii, and Brucella spp. In addition, immunohistochemical (IHC) staining was performed on selected tissues to determine the presence of morbilliviral antigens. Six animals (5 striped dolphins and 1 common dolphin) showed IHC and/or molecular evidence of morbilliviral antigens and/or genomes, mainly in brain tissue. Conventional nested PCR detected herpesviral DNA in brain tissue samples from two striped dolphins. There was no evidence of West Nile virus, T. gondii, or Brucella spp. in any of the brain tissue samples examined. The information presented here increases the number of confirmed morbillivirus-positive cases within the Canarian archipelago from two previously reported cases to eight. Furthermore, a new nested-PCR method for the detection of morbillivirus is described here. Regarding herpesvirus, the phylogenetic analysis performed in the current study provides valuable information about a possible pathogenic branch of cetacean alphaherpesviruses that might be responsible for some fatal cases worldwide.
INTRODUCTION
The Canary Islands have one of the most diverse populations of cetaceans in the Northeast Atlantic, with 28 different species. Natural causes of death have so far accounted for the majority of the cases in the cetaceans stranded along the coasts of the Canary Islands, including infectious and noninfectious diseases, neonatal pathology, and intra- and interspecific interactions (1). Several cases of nonsuppurative (NS) meningoencephalitis have been described based on histopathological examinations of cetaceans of different species, but the striped dolphin (Stenella coeruleoalba) has been the most commonly affected species.
Dolphin morbillivirus (DMV) (2, 3) (genus Morbillivirus, subfamily Paramyxovirinae, family Paramyxoviridae [see http://www.ictvdb.rothamsted.ac.uk/Ictv/index.htm]) is one of the three strains of cetacean morbillivirus (CeMV), which also includes porpoise morbillivirus (PMV) (2, 4) and pilot whale morbillivirus (PWMV) (5). Dolphin morbillivirus is the main agent described in cases of NS meningoencephalitis in striped dolphins during the last two epizootics in the Mediterranean Sea (3, 6–10). Other causative agents of NS meningoencephalitis in marine mammals include herpesviruses (HV), Toxoplasma gondii, Brucella spp., and West Nile virus.
Herpesvirus (family Herpesviridae, which includes three subfamilies, Alphaherpesvirinae, Betaherpesvirinae, and Gammaherpesvirinae) infections in humans and animals result in a wide variety of responses, ranging from unapparent infections to fatal disseminated disease (11). Members of the Alphaherpesvirinae subfamily, which includes herpes simplex and varicella viruses, have been implicated in two previous cases of herpesviral encephalitis in cetaceans (12, 13).
T. gondii, an apicomplexan protozoan parasite, infects a range of hosts worldwide, including several marine mammal species, in which it may cause abortion, lethal systemic disease (14), and nonsuppurative (NS) encephalitis (15–18).
Since the first description of marine mammal brucellae (19, 20), several cases have been described in different marine mammal species worldwide (21), such as placentitis, abortion (22), and NS encephalitis (23, 24).
West Nile virus, a single-stranded RNA virus of the genus Flavivirus, has been associated with a spectrum of clinical conditions from asymptomatic infections to sudden death in humans and animals. In marine mammals, a polioencephalomyelitis case in a harbor seal (Phoca vitulina) (25), and an NS encephalitis case in a killer whale (Orcinus orca) (26) have been reported.
The present report involves a retrospective study performed to determine the infectious causative agents of NS meningoencephalitis in archived tissues of stranded cetaceans from the Canary Islands from 1996 to 2011.
MATERIALS AND METHODS
Samples.
One hundred sixty-eight carcasses of cetaceans stranded along the coasts of the Canary Islands were examined and necropsied according to standard procedures (27). During necropsy, the tissue samples of all the major organs and lesions were collected and preserved in neutral buffered 10% formalin solution. The fixed tissue samples were trimmed, routinely processed, embedded in paraffin, sectioned at 5 μm, and stained with hematoxylin and eosin (HE) for examination by light microscopy.
In order to determine the possible causative agent(s) of NS meningoencephalitis, a retrospective study was conducted using archived tissues from 19 specimens: 15 striped dolphins, 2 common dolphins (Delphinus delphis), and 2 rough-toothed dolphins (Steno bredanensis). The animals were of both sexes and ranged in age from neonatal to adult, according to biological and morphometric parameters (28). The selection criteria for these cases were a carcass that was necropsied within 24 h postmortem and a histologic diagnosis of NS meningoencephalitis. The animal data and histologic findings are summarized in Table S1 in the supplemental material.
Samples from the skin, blubber, skeletal muscle, lung, liver, lymph nodes (prescapular, lung-associated, and/or mesenteric), kidney, brain, and spleen were collected during necropsy and stored frozen at −80°C until processed for PCR. All the fresh frozen tissues available from each animal were tested for CeMV and HV (Table 1), while only brain tissue was tested by PCR for the presence of T. gondii, Brucella spp., and West Nile virus.
TABLE 1.
Tissues tested for cetacean morbillivirus and herpesvirus in the current study and positive results
| Case no. | Species | Tissues analyzed for CeMV and HVa | CeMV-positive sample type(s) | HV-positive sample type(s) |
|---|---|---|---|---|
| 1 | Stenella coeruleoalba | Skin, muscle, lung, liver, kidney, brain | Skin | |
| 2 | Steno bredanensis | Skin, muscle, lung, liver, kidney, brain | ||
| 3 | S. coeruleoalba | Skin, muscle, lung, liver, kidney, brain | ||
| 4 | S. coeruleoalba | Skin, muscle, lung, liver, kidney, spleen, brain | ||
| 5 | S. coeruleoalba | Skin, muscle, lung, kidney, brain | Lung, kidney, brain | |
| 6 | Delphinus delphis | Muscle, liver, kidney, brain | Brainb | |
| 7 | S. coeruleoalba | Skin, muscle, lung, liver, kidney, spleen, brain | Brain | Brain |
| 8 | S. coeruleoalba | Skin, muscle, lung, liver, kidney, spleen, mesenteric lymph node, brain | Brainb | |
| 9 | S. coeruleoalba | Skin, muscle, lung, liver, kidney, spleen, brain | ||
| 10 | S. coeruleoalba | Skin, muscle, lung, liver, kidney, spleen, brain | ||
| 11 | S. bredanensis | Skin, muscle, lung, liver, kidney, spleen, mesenteric lymph node, brain | ||
| 12 | S. coeruleoalba | Skin, muscle, lung, liver, spleen, mesenteric lymph node, brain | ||
| 13 | S. coeruleoalba | Skin, muscle, lung, liver, kidney, spleen, mesenteric lymph node, brain | Brain | |
| 14 | S. coeruleoalba | Skin, muscle, lung, liver, kidney, spleen, mesenteric lymph node, brain | ||
| 15 | S. coeruleoalba | Skin, muscle, lung, liver, kidney, spleen, mesenteric lymph node, brain | ||
| 16 | S. coeruleoalba | Skin, muscle, lung, liver, kidney, mesenteric lymph node, brain | Lung, brain | |
| 17 | S. coeruleoalba | Skin, muscle, lung, liver, kidney, spleen, mediastinal lymph node, brain | Skin, brain | |
| 18 | S. coeruleoalba | Skin, muscle, lung, liver, kidney, spleen, mesenteric lymph node, brain | ||
| 19 | D. delphis | Skin, muscle, lung, liver, kidney, spleen, mesenteric lymph node, brain |
CeMV, cetacean morbillivirus; HV, herpesvirus.
Only IHC-positive tissues.
Molecular study.
Molecular analyses were performed at the Athens Veterinary Diagnostic Laboratory (AVDL), College of Veterinary Medicine, University of Georgia, Athens, GA, USA. Approximately 0.5 g of fresh frozen tissue was mechanically macerated and subsequently centrifuged. The supernatants were collected, and RNA and DNA extractions were carried out from a 500-μl and 250-μl sample, respectively, using the RNeasy and the DNeasy tissue kits, respectively, according to the manufacturer's instructions (Qiagen, Inc., Valencia, CA). When no frozen tissue samples were available, nucleic acid extractions were made from available formalin-fixed paraffin-embedded tissue (FFPE) samples. Briefly, thick (10-μm) tissue sections were cut from the paraffin-embedded blocks, deparaffinized, and rehydrated. In order to disrupt the aldehyde bonds or cross-linking of the released DNA, different set temperatures of incubation within a digestion buffer for proteinase K were applied for 30 min.
In the current study, primers were designed based on the available sequence data, except for the molecular detection of CeMV, in which degenerate primers were generated and designed based upon the alignment of selected sequences from PMV, PDV, DMV, and CDV. The following primers were used: forward primer MVP2202 (5′-KKC TCR TGG TWC CW R CAG GC-3′), reverse primer MVP2480R (5′-TCT CTY CTG TGC CCT TTT TAA TGG-3′), and internal primer MVP A (5′-AGA TGA GAG CTC TCT CGA GA-3′). A conventional seminested reverse transcription-PCR (RT-PCR), which amplifies 300 bp within a conserved region in the phosphoprotein (P) gene of CeMV, was used for this purpose. Two negative controls (for extraction and amplification) and an amplification-positive control (APC) of PMV cell culture supernatant fluid were included. Previously titrated CDV and PMV stock viruses were used to test the sensitivity of the primer sets in triplicate, using 10-fold dilutions of previously titrated CDV and PMV virus stocks, starting from 105.3 tissue culture infectious doses (TCID50) and 104.2 TCID50, respectively. Herpesvirus DNA was detected by conventional nested PCR using degenerate primers (29, 30) designed to amplify a region of the DNA polymerase gene. Equine herpesvirus type I was used as the APC. The expected size of the amplicon for the herpesvirus group ranged between 215 and 315 bp. For the detection of West Nile virus, a conventional nested RT-PCR was performed (31). Selected primers amplified a 248-bp size portion of the E region of the genome of West Nile virus (WNV) genotype NY99, which encodes the envelope protein (GenBank accession no. AF196835). A WNV cell culture supernatant was used as the APC. A real-time PCR targeting a 529-bp repeat element of T. gondii was performed (32). Frozen tissue confirmed positive for T. gondii was used as the APC. A real-time PCR for Brucella spp. was performed (33, 34). Primers amplified a 223-bp fragment of the bcsp31 gene (Brucella abortus). Pure culture of Brucella canis was used as the APC. Additionally, one-step real-time RT-PCR for the housekeeper gene glyceraldehyde 3-phosphate dehydrogenase (G3PDH) was used as an internal control in selected samples (35).
Sequencing.
The PCR products were electrophoresed (2% agarose gels stained with ethidium bromide). The amplicons were purified by using the Millipore Ultrafree-DA kit (Millipore, Billerica, MA, USA) and submitted to SeqWright, DNA Technology Services (Houston, TX), for sequencing. The amplicon identities were confirmed with BLAST (http://www.ncbi.nlm.nih.gov/BLAST). The nucleotide sequences obtained from CeMV-positive cases in the current study were aligned with previously published GenBank sequences of CeMV (GenBank accession no. AF333347, FJ842381, HQ829972, HQ829973, EU039963, JX195718, EF451565, AJ608288, AF200817, JN210891, and KC572861), porpoise morbillivirus (PMV), described in 1993 (2), and the other five species of morbillivirus (PPRV, RPV, MeV, CDV, and PDV) (GenBank accession no. X74443, X98291, K01711, AF014953, and D10371, respectively), and a phylogenetic tree was generated. A p-distance matrix based on nucleotide differences among the current results and those of the two previously published DMV sequences from the epizootics of 1990 and 2007 was calculated. The nucleotide sequences obtained from HV-positive cases in the current study were aligned with the previously published cetacean HV sequences (GenBank accession no. GQ888669, GQ888670, GQ888671, GQ888673, GQ888674, GQ888675, AY757301, DQ295063, AB510473, GQ429150, DQ295064, AY608707, AF196646, AY949832, GU068981, GU066291, HQ214675, and JN863234).
MEGA 5.0 (36) was used to construct maximum likelihood phylogenetic trees. A bootstrap resampling (1,000 replicates) was used to assess the reliability of the trees.
Immunohistochemical study.
Parallel immunohistochemical (IHC) investigations for morbillivirus were also carried out on FFPE lung, kidney, and brain tissue samples from the 19 animals of the current study but not for the other etiologic agents. IHC staining was not performed for HV because of the lack of commercial antibodies, and it was not performed for the other targeted etiologic agents because the histologic analyses did not yield any lesions suggestive of the presence of those agents; hence, no confirmatory testing was necessary.
The primary morbilliviral antibody (a mouse monoclonal IgG2B [kappa light chain] against the nucleoprotein [NP] antigen of CDV [VMRD, Pullman, WA, USA]) was used as previously described (37). Positive tissue controls consisted of FFPE canine brain samples with CDV inclusion bodies. As a negative control, the primary antibody was eliminated and substituted with normal mouse serum.
Nucleotide sequence accession numbers.
The new morbillivirus sequences identified in the present study were deposited in GenBank under accession no. KJ139452 (Sc/2007/ENoAt), KJ139453 (Sc/2009/ENoAt), KJ139451 (Sc/2002/ENoAt), and KJ139454 (Sc/2011/ENoAt). The new herpesvirus sequences identified in the present study were deposited in GenBank under accession no. KJ156332 (Sc/2011/ENoAt Skin), KJ156331 (Sc/2011/ENoAt Brain), and KJ156330 (Sc/2007/ENoAt Brain).
RESULTS
The histopathological study determined the presence of NS meningoencephalitis in 16% of the stranded cetaceans in the Canarian archipelago during the study period, characterized by multifocal mild to moderately severe perivascular lymphohistiocytic inflammation, scattered foci of microgliosis, neuronophagia, and mild lymphocytic perivascular cuffs within the cerebral cortex and/or meninges.
The causative agent was determined through IHC and/or molecular methods in 7 out of 19 (36.8%) cases with NS meningoencephalitis. The results, shown in Table 1, included a CeMV and HV coinfection in the brain of case no. 7.
Morbillivirus.
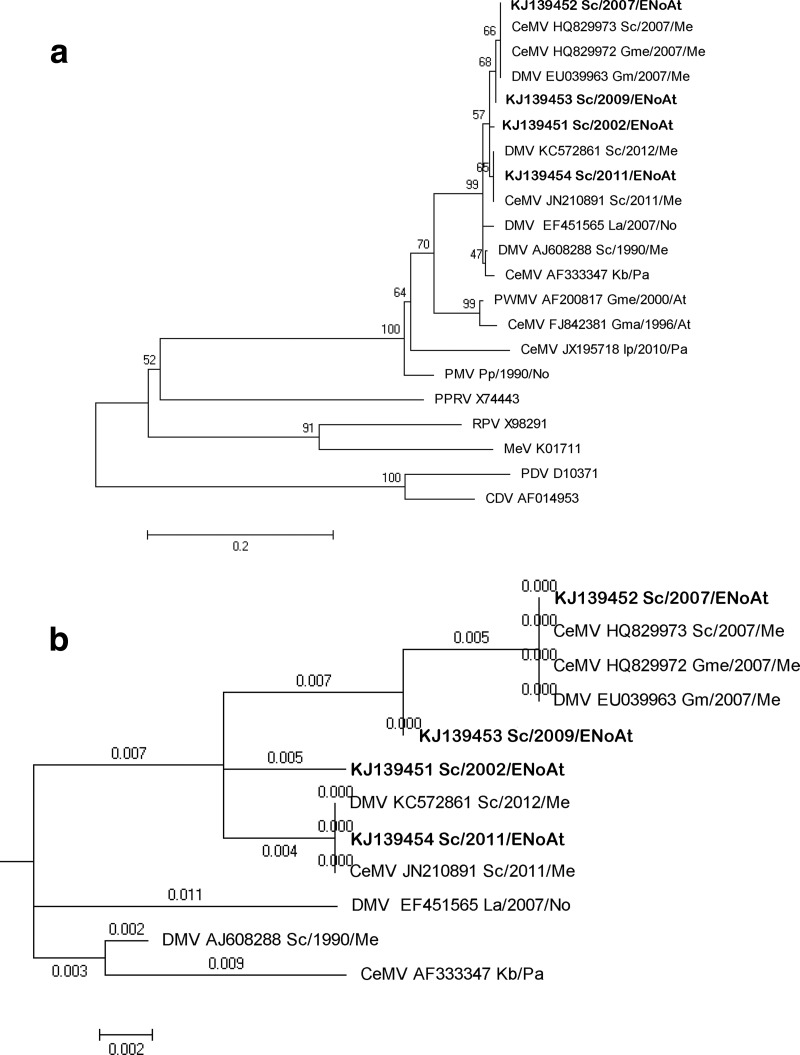
Morbillivirus was the main pathogen involved in 6 out of 19 (31.5%) cetaceans in the current study. The sequencing results confirmed the presence of CeMV (99% identity to DMV strain) in the brain samples of 4 (all S. coeruleoalba specimens) of the 19 animals tested. The constructed phylogenetic tree shows three different clusters within the DMV isolates (Fig. 1a), with all the sequences obtained in this study belonging to the same cluster, supported by a 57% bootstrap value. The sequence obtained from a 2007 case (case no. 7) forms a subclade with all the published sequences obtained during the 2007 Mediterranean epizootic, and the sequence corresponding to 2011 (case no. 16) is in the same subclade as those from 2011 and 2012 obtained from striped dolphins in the Mediterranean Sea. All the Mediterranean and northeastern Atlantic sequences are much closer except that from the first Mediterranean epizootic, which is located in a separate branch of bifurcation a distance of 0.003 nucleotide substitutions per site from the common root of the DMV cluster (Fig. 1b). Regarding the sensitivity of the new tested PCR, the limit of detection (LOD) for the CDV nested conventional PCR was 10−5 for serial dilutions of RNA extraction, starting with a 105.3 TCID50, while for PMV nested conventional PCR, the LOD was 10−6, starting with a virus titer of 104.2 TCID50.
FIG 1.
(a) Phylogram of morbillivirus phosphoprotein gene sequences. MEGA 5.0 was used to construct the maximum likelihood phylogenetic trees. A Tamura-Nei substitution model and a bootstrap resampling (1,000 replicates) were used to assess the reliability of the trees. Bootstrapping values are indicated as percentages next to the bifurcations. The new isolates from this study are shown in bold type. The scale bar shows the number of nucleotide substitutions per site. The name of the each sequence includes the virus name (DMV, dolphin morbillivirus; CeMV, cetacean morbillivirus; PMV, porpoise morbillivirus; PWMV, pilot whale morbillivirus; PPRV, peste-des-petits-ruminants virus; RPV; rinderpest virus; CDV, canine distemper virus; PDV, phocine distemper virus; MeV, measles virus), GenBank accession number, cetacean species (Gma, Globicephala macrorhynchus; Gme, Globicephala melas; Kb, Kogia breviceps; La, Lagenorhynchus albirostris; Pp, Phocoena phocoena; Sc, S. coeruleoalba; Tt, Tursiops truncatus), and the year and the geographic area of the stranding (At, Atlantic Ocean; ENoAt, Northeast Atlantic Ocean; Me, Mediterranean Sea; No, North Sea; Pa, Pacific Ocean). (b) DMV subtree branch length. Each branch length reflects the number of nucleotide substitutions per site.
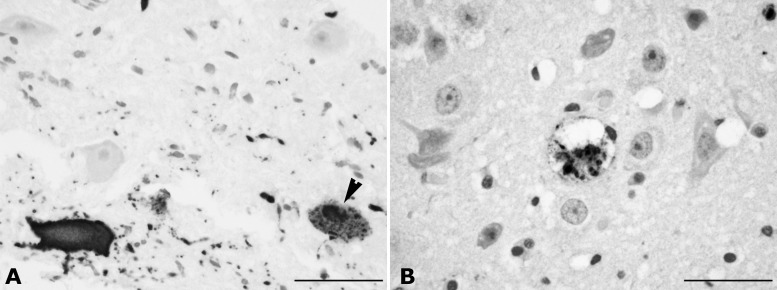
Positive immunoreactions in 6 out of 19 brain samples examined appeared as diffuse or finely granular brown staining mainly within the nuclei and cytoplasm of neurons, axonal processes, and glial cells (Fig. 2A). Morbillivirus antigen was found predominantly within lesions, although some chronic lesions were devoid of viral antigen. In case no. 16, endothelial and meningeal cells and choroid plexus epithelia were also immune positive against CDV antibodies. In case no. 7, a moderately severe spongiform neurodegeneration of both immune-positive and unstained cells within the white matter was observed (Fig. 2B). Two dolphins were positive by IHC but negative by RT-PCR for CeMV and for G3PDH RNA internal control due to the lack of fresh tissue samples from the affected organs. Despite the PCR-positive result obtained in the lung samples from cases no. 5 and 16, positive IHC staining was limited to scattered mononuclear cells within the interstitium and the lumina of the vessels. In the single kidney that was IHC positive for morbillivirus antigen and also positive by PCR for CeMV (case no. 5), immunopositive renal tubular cells were observed.
FIG 2.
Brain. (A) Cerebral cortex sample, striped dolphin, case no. 8, morbillivirus immunohistochemistry. Morbilliviral antigen is expressed in the cytoplasm and nuclei (arrowhead) of neurons and axonal processes. Immunoperoxidase technique with a monoclonal antibody to the nucleoprotein of canine distemper virus as primary antibody. Hematoxylin counterstain. Scale bar, 50 μm. (B) Cerebral cortex sample, case no. 7, morbillivirus immunohistochemistry. Spongiform neurodegeneration of an immune-positive cell within the white matter. Hematoxylin counterstain. Scale bar, 50 μm.
Herpesvirus.
Herpesviral DNA was detected in 3 (all striped dolphins) out of 19 animals (15.8%). Positive samples included those from the skin (cases no. 1 and 17) and the brain (cases no. 7 and 17). Brain coinfection for CeMV and HV was observed in one animal (case no. 7) (Table 1). Despite the molecular evidence of HV within the brain sample of case no. 7, no characteristic histopathological lesions generally attributed to HV infection were found. However, in addition to severe diffuse NS meningoencephalitis, other brain lesions observed in case no. 17 were more consistent with HV pathogenicity for such a host and included multifocal granulomatous encephalomalacia, especially located in the thalamus, pons, olivary nucleus, and medulla oblongata, as well as congestion and hemorrhages within the meninges, cerebellum, choroid plexus, and central area of the medulla. Focal neuronophagic nodules were associated with widespread necrotic neurons, and severe spongiosis within the white matter was observed, especially in the caudate nucleus. Large solid intranuclear amphophilic inclusion bodies in neurons and glial cells, often occupying the whole nucleus, were observed in the caudal colliculus, red nucleus, and surrounding the mesencephalic aqueduct, associated with a granulomatous focus of encephalitis (Fig. 3). There was also mild diffuse microgliosis in the cerebral cortex. Grossly, hyperpigmented skin areas of oval morphology along the flanks and caudal peduncle were observed, which microscopically corresponded with pigmented and vacuolated keratinocytes within the germinal stratum; we also found abnormal nuclear morphology, including chromatin margination in association with basophilic intranuclear inclusions. Similar lesions were observed in the skin of case no. 1, in which there was NS perivascular dermatitis.
FIG 3.
Brain sample, striped dolphin, case no. 17. Large solid intranuclear amphophilic inclusion bodies in neurons and glial cells, often occupying the whole nucleus, were observed to be associated with a granulomatous focus of encephalitis herpesvirus infection. Inset: higher magnification of the intranuclear inclusion bodies (arrowheads). HE stain. Scale bar, 50 μm.
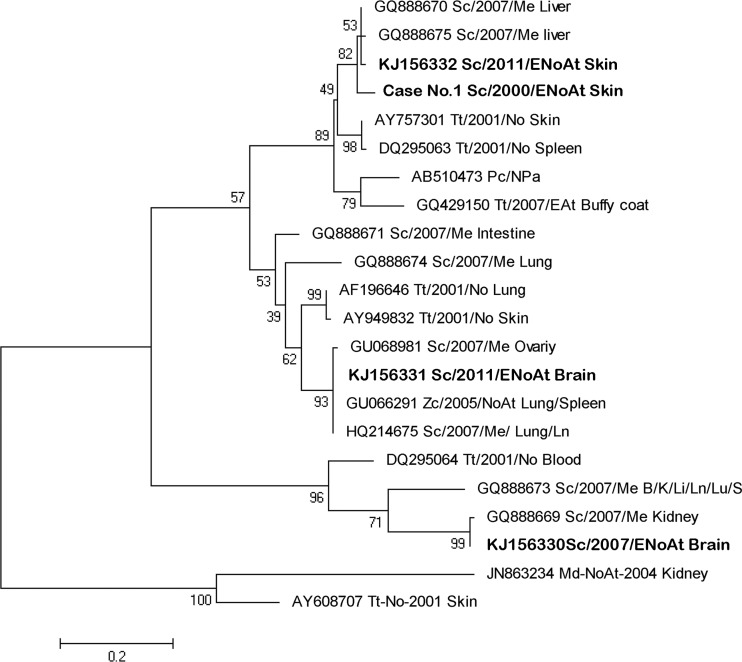
Four new sequences of alphaherpesvirus (α-HV) were obtained from the current study, with two of them originating from the same animal (case no. 17). Three different groups were observed within the phylogenetic tree (Fig. 4); the two first groups correspond to α1-HV, while the last one is formed by α2-HV sequences.
FIG 4.
Neighbor-joining phylogram of 22 selected sequences from the polymerase gene of a cetacean alphaherpesvirus. MEGA 5.0 was used to construct the maximum likelihood phylogenetic trees. A Tamura-Nei substitution model and a bootstrap resampling (1,000 replicates) were used to assess the reliability of the tree. The new isolates from this study are shown in bold type. The scale bar shows the number of nucleotide substitutions per site. The name of each sequence includes the GenBank accession number (when available); cetacean species (Md; Mesoplodon densirostris; Me; Mesoplodon europaeus; Pc; Pseudorca crassidens; Sc, S. coeruleoalba Tt, T. truncatus; Zc; Ziphius cavirostris), the year and the geographic area of the stranding (At, Atlantic Ocean; EnoAt, Northeast Atlantic Ocean; EAt, East Atlantic Ocean; Me, Mediterranean Sea; No, North Sea; NoAt, North Atlantic Ocean; Npa; North Pacific Ocean; Pa, Pacific Ocean), and the tissues in which they were detected (B, brain; I, intestine; K, kidney; Li, liver; Ln, lymph node; Lu, lung; ovary; skin; S, spleen).
No evidence of West Nile virus, T. gondii, or Brucella infection was detected in any of the tested tissue samples.
DISCUSSION
The IHC and PCR results presented herein indicate that CeMV (DMV strain) was the most common etiological agent associated with NS meningoencephalitis in the retrospective study of stranded cetaceans in the Canary Islands. In addition to brain lesions, morbillivirus-infected cetaceans in our study also showed various levels of bronchointerstitial pneumonia, although lung samples were IHC and/or PCR positive to morbillivirus in only two cases. Therefore, the majority of the lung lesions observed might be related to other etiologies, such as parasitic infections, as has been proposed (38). The lack of typical morbilliviral lesions, such as syncytial cells or viral inclusions (39, 40), further supports our speculation that etiologies other than morbillivirus might have been responsible for the majority of the bronchointerstitial pneumonia cases.
A partial P genome sequence of DMV was obtained in 4 out of the 6 IHC-positive animals. The negative results in two dolphins, in which only FFPE material was available, suggested that RNA in the formalin-fixed samples may have been too fragmented to be amplified. Archived FFPE material is sometimes the unique source of nucleic acids in retrospective molecular studies, although the lack of standardization of formalin fixation or well-defined nucleic acid standard operating procedures can adversely affect molecular results (41).
The results of this study might help better understand the circulation of morbilliviruses within cetacean populations worldwide (8, 42–44). Compared to the relatedness of sequences in previous morbillivirus epizootics, the sequences obtained in the Canary Islands are very similar (almost identical) to those obtained from the Mediterranean Sea. The results of the current study and some cases of morbilliviruses reported on the Atlantic side of the Strait of Gibraltar at the end of 1990 (7) suggest that populations of striped dolphins in the Mediterranean are not as isolated from those in the Northeast Atlantic as previously thought based on morphological and genetic studies of the striped dolphin population within these areas (45, 46). Moreover, the results support the previously suggested idea that interanimal transmission might occur through the Strait of Gibraltar (8, 44).
In one common dolphin, positively stained cells were observed within the epithelial cells of the choroid plexus, with no expression of morbilliviral antigen anywhere else in the nervous system, suggesting that the virus reached the central nervous system (CNS) through the hematogenous route without spreading through the cerebrospinal fluid (47).
Based on the PCR results, an unspecified α-HV was the second most common cause (10.5%) of NS meningoencephalitis in the current study, affecting two striped dolphins, one of which (case no. 7) was coinfected with CeMV, without typical herpesviral lesions (48–50). In addition, two different α-HV sequences were obtained from the skin samples from two dolphins with minimal lesions. A high association of herpesvirus infection with skin lesions has been described, varying from minimal oval rough and depressed areas to severe necrotizing dermatitis (50–53).
During the recent morbillivirus epizootic in the Mediterranean Sea, eight different HV sequences were detected in subclinically infected striped dolphins coinfected with DMV (54). In the current study, the coinfection was observed in a juvenile male found stranded in the Canary Islands in April 2007, in the middle of the Mediterranean epizootic, and the novel sequence obtained showed a 99% homology to those obtained from the kidney sample of the same host species in the 2007 epizootic (54). Such a finding supports our speculation above regarding viral transmission between striped dolphins from the Mediterranean Sea and the North Atlantic Ocean through the Strait of Gibraltar, as has been reported (8, 44). However, although HV infections are often not associated with clinical signs, severe diseases can occur. Evidence of herpesviral pathogenicity has been described in cetaceans, with the main lesions being cellular necrosis and viral intranuclear inclusions (12, 48–50, 55).
The striped dolphin of the current study, with NS encephalitis attributed only to HV, showed features in common with previously reported cases of fatal cetacean HV infection, such as the presence of intranuclear inclusions in the cells of the respective affected tissues. However, the extensive damage of the lymphoid system described in some cases was not observed in the case described herein. The phylogenetic analysis placed the HV brain sample sequence of this case within a cluster of the alphaherpesvirus 1 subfamily, which also included the sequences obtained from severe lymphoid necrosis in a beaked whale (49) and from the lymph node and the ovary of two striped dolphins coinfected with DMV during the 2007 Mediterranean epizootic (50, 54). The current report describes a primary HV infection causing lesions in a striped dolphin without DMV coinfection and also reports on an underdescribed case of NS encephalitis of HV origin in a cetacean species.
In summary, the results presented herein suggest that cetaceans from the Northeast Atlantic waters are affected by morbillivirus, mainly or exclusively with a nervous system presentation. The information presented here increases the number of morbillivirus-positive cases within the Canarian Archipelago from two (56, 57) to eight cases. Regarding HV, the phylogenetic analysis performed in the current study provides valuable information about a possible pathogenic branch of alphaherpesviruses in cetaceans that might be responsible for some fatal cases worldwide.
Determining the causes of mortality in stranded cetaceans provides vital information about the health status of the marine environment. Marine mammals are probably one of the best sentinel organisms in aquatic and coastal environments because many species have long life spans and feed at a high trophic level (58). The manner in which infectious diseases can potentially impact these animals and, therefore, affect human populations is still unpredictable, but the detection of diseases that impact these species may increase human awareness of deteriorating ocean health issues. Indeed, virtually all threats to marine mammals are ultimately related to the population size, growth rate, and the consumption patterns and behaviors of humans (59).
Supplementary Material
ACKNOWLEDGMENTS
This work was partially supported by a project of the Canary Island government (ProID20100091), Spain.
We thank Ingrid Fernández-Marrero, Sarah Bates, and Paula Bartlett for helpful assistance with laboratory skills at the Athens Lab of the University of Georgia.
We have no financial or personal relationships with other people or organizations that might have inappropriately influenced the present work.
Footnotes
Published ahead of print 23 April 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JCM.02906-13.
REFERENCES
- 1. Arbelo M, Los Monteros AE, Herráez P, Andrada M, Sierra E, Rodríguez F, Jepson PD, Fernández A. 2013. Pathology and causes of death of stranded cetaceans in the Canary Islands (1999–2005). Dis. Aquat. Organ. 103:87–99. 10.3354/dao02558 [DOI] [PubMed] [Google Scholar]
- 2. Barrett T, Visser IK, Mamaev L, Goatley L, van Bressem MF, Osterhaust AD. 1993. Dolphin and porpoise morbilliviruses are genetically distinct from phocine distemper virus. Virology 193:1010–1012. 10.1006/viro.1993.1217 [DOI] [PubMed] [Google Scholar]
- 3. Domingo M, Ferrer L, Pumarola M, Marco A, Plana J, Kennedy S, McAlisey M, Rima BK. 1990. Morbillivirus in dolphins. Nature 384:21. [DOI] [PubMed] [Google Scholar]
- 4. Kennedy S. 1998. Morbillivirus infections in aquatic mammals. J. Comp. Pathol. 119:201–225. 10.1016/S0021-9975(98)80045-5 [DOI] [PubMed] [Google Scholar]
- 5. Taubenberger JK, Tsai MM, Atkin TJ, Fanning TG, Krafft AE, Moeller RB, Kodsi SE, Mense MG, Lipscomb TP. 2000. Molecular genetic evidence of a novel morbillivirus in a long-finned pilot whale (Globicephalus melas). Emerg. Infect. Dis. 6:42–45. 10.3201/eid0601.000107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Domingo M, Visa J, Pumarola M, Marco AJ, Ferrer L, Rabanal R, Kennedy S. 1992. Pathologic and immunocytochemical studies of morbillivirus infection in striped dolphins (Stenella coeruleoalba). Vet. Pathol. 29:1–10. 10.1177/030098589202900101 [DOI] [PubMed] [Google Scholar]
- 7. Aguilar A, Raga JA. 1993. The striped dolphin epizootic in the Mediterranean Sea. Ambio 22:524–528 [Google Scholar]
- 8. Raga JA, Banyard A, Domingo M, Corteyn M, Van Bressem MF, Fernández M, Aznar FJ, Barrett T. 2008. Dolphin morbillivirus epizootic resurgence, Mediterranean Sea. Emerg. Infect. Dis. 14:471–473. 10.3201/eid1403.071230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Keck N, Kwiatek O, Dhermain F, Dupraz F, Boulet H, Danes C, Laprie C, Perrin A, Godenir J, Micout L, Libeau G. 2010. Resurgence of morbillivirus infection in Mediterranean dolphins off the French coast. Vet. Rec. 166:654–655. 10.1136/vr.b4837 [DOI] [PubMed] [Google Scholar]
- 10. Soto S, González R, Alegre F, González B, Medina P, Raga JA, Marco A, Domingo M. 2011. Epizootic of dolphin morbillivirus on the Catalonian Mediterranean coast in 2007. Vet. Rec. 169:22. 10.1136/vr.d4014 [DOI] [PubMed] [Google Scholar]
- 11. Cheville NF. 1994. Ultrastructural pathology: an introduction to interpretation. Iowa State University Press, Ames, IA [Google Scholar]
- 12. Kennedy S, Lindstedt IJ, McAliskey MM, McConnell SA, McCullough SJ. 1992. Herpesviral encephalitis in a harbor porpoise (Phocoena phocoena). J. Zoo Wildl. Med. 23:374–379 [Google Scholar]
- 13. Esperón F, Fernández A, Sánchez-Vizcaíno JM. 2008. Herpes simplex-like infection in a bottlenose dolphin stranded in the Canary Islands. Dis. Aquat. Organ. 81:73–76. 10.3354/dao01915 [DOI] [PubMed] [Google Scholar]
- 14. Dubey JP, Zarnke R, Thomas NJ, Wong SK, Van Bonn W, Briggs M, Davis JW, Ewing R, Mense M, Kwok OC, Romand S, Thulliez P. 2003. Toxoplasma gondii, Neospora caninum, Sarcocystis neurona, and Sarcocystis canis-like infections in marine mammals. Vet. Parasitol. 116:275–296. 10.1016/S0304-4017(03)00263-2 [DOI] [PubMed] [Google Scholar]
- 15. Resendes AR, Almería S, Dubey JP, Obón E, Juan-Sallés C, Degollada E, Alegre F, Cabezón O, Pont S, Domingo M. 2002. Disseminated toxoplasmosis in a Mediterranean pregnant Risso's dolphin (Grampus griseus) with transplacental fetal infection. J. Parasitol. 88:1029–1032. 10.1645/0022-3395(2002)088[1029:DTIAMP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 16. Di Guardo G, Di Cesare A, Otranto D, Casalone C, Iulini B, Mignone W, Tittarelli C, Meloni S, Castagna G, Forster F, Kennedy S, Traversa D. 2011. Genotyping of Toxoplasma gondii isolates in meningo-encephalitis affected striped dolphins (Stenella coeruleoalba) from Italy. Vet. Parasitol. 183:31–36. 10.1016/j.vetpar.2011.07.004 [DOI] [PubMed] [Google Scholar]
- 17. Di Guardo G, Proietto U, Di Francesco CE, Marsilio F, Zaccaroni A, Scaravelli D, Mignone W, Garibaldi F, Kennedy S, Forster F, Iulini B, Bozzetta E, Casalone C. 2010. Cerebral toxoplasmosis in striped dolphins (Stenella coeruleoalba) stranded along the Ligurian Sea coast of Italy. Vet. Pathol. 47:245–253. 10.1177/0300985809358036 [DOI] [PubMed] [Google Scholar]
- 18. Dubey JP, Mergl J, Gehring E, Sundar N, Velmurugan GV, Kwok OC, Grigg ME, Su C, Martineau D. 2009. Toxoplasmosis in captive dolphins (Tursiops truncatus) and walrus (Odobenus rosmarus). J. Parasitol. 95:82–85. 10.1645/GE-1764.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ross HM, Foster G, Reid RJ, Jahans KL, MacMillan AP. 1994. Brucella species infection in sea-mammals. Vet. Rec. 134:359. 10.1136/vr.134.14.359-b [DOI] [PubMed] [Google Scholar]
- 20. Ewalt DR, Payeur JB, Martin BM, Cummins DR, Miller WG. 1994. Characteristics of a Brucella species from a bottlenose dolphin (Tursiops truncatus). J. Vet. Diagn. Invest. 6:448–452. 10.1177/104063879400600408 [DOI] [PubMed] [Google Scholar]
- 21. Nymo IH, Tryland M, Godfroid J. 2011. A review of Brucella infection in marine mammals, with special emphasis on Brucella pinnipedialis in the hooded seal (Cystophora cristata). Vet. Res. 42:1297–9716. 10.1186/1297-9716-42-93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Miller WG, Adams LG, Ficht TA, Cheville NF, Payeur JP, Harley DR, House C, Ridgway SH. 1999. Brucella-induced abortions and infection in bottlenose dolphins (Tursiops truncatus). J. Zoo Wildl. Med. 30:100–110 [PubMed] [Google Scholar]
- 23. González L, Patterson IA, Reid RJ, Foster G, Barberán M, Blasco JM, Kennedy S, Howie FE, Godfroid J, MacMillan AP, Schock A, Buxton D. 2002. Chronic meningoencephalitis associated with Brucella sp. infection in live-stranded striped dolphins (Stenella coeruleoalba). J. Comp. Pathol. 126:147–152. 10.1053/jcpa.2001.0535 [DOI] [PubMed] [Google Scholar]
- 24. Davison NJ, Cranwell MP, Perrett LL, Dawson CE, Deaville R, Stubberfield EJ, Jarvis DS, Jepson PD. 2009. Meningoencephalitis associated with Brucella species in a live-stranded striped dolphin (Stenella coeruleoalba) in south-west England. Vet. Rec. 165:86–89. 10.1136/vetrec.165.3.86 [DOI] [PubMed] [Google Scholar]
- 25. Del Piero F, Stremme DW, Habecker PL, Cantile C. 2006. West Nile flavivirus polioencephalomyelitis in a harbor seal (Phoca vitulina). Vet. Pathol. 43:58–61. 10.1354/vp.43-1-58 [DOI] [PubMed] [Google Scholar]
- 26. St Leger J, Wu G, Anderson M, Dalton L, Nilson E, Wang D. 2011. West Nile virus infection in killer whale, Texas, USA, 2007. Emerg. Infect. Dis. 17:1531–1533. 10.3201/eid1708.101979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kuiken T, García Hartmann M. 1993. Cetacean pathology: dissection techniques and tissue sampling. ECS Newsl. 17:1–39 [Google Scholar]
- 28. Geraci JR, Lounsbury VJ. 2005. Specimen and Data collection, p 182–184 In Geraci JR, Lounsbury VL. (ed), Marine mammals ashore: a field guide for strandings, 2nd ed. Texas A&M University Sea Grant College Program, Galveston, TX [Google Scholar]
- 29. VanDevanter DR, Warrener P, Bennett L, Schultz ER, Coulter S, Garber RL, Rose TM. 1996. Detection and analysis of diverse herpesviral species by consensus primer PCR. J. Clin. Microbiol. 34:1666–1671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ehlers B, Borchers K, Grund C, Frölich K, Ludwig H, Buhk HJ. 1999. Detection of new DNA polymerase genes of known and potentially novel herpesviruses by PCR with degenerate and deoxyinosine-substituted primers. Virus Genes 18:211–220. 10.1023/A:1008064118057 [DOI] [PubMed] [Google Scholar]
- 31. Johnson DJ, Ostlund EN, Pedersen DD, Schmitt BJ. 2001. Detection of North American West Nile virus in animal tissue by a reverse transcription-nested polymerase chain reaction assay. Emerg. Infect. Dis. 7:739–741. 10.3201/eid0704.017425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Edvinsson B, Lappalainen M, Evengård B, ESCMID Study Group for Toxoplasmosis 2006. Real-time PCR targeting a 529-bp repeat element for diagnosis of toxoplasmosis. Clin. Microbiol. Infect. 12:131–136. 10.1111/j.1469-0691.2005.01332.x [DOI] [PubMed] [Google Scholar]
- 33. Probert WS, Schrader KN, Khuong NY, Bystrom SL, Graves MH. 2004. Real-time multiplex PCR assay for detection of Brucella spp., B. abortus, and B. melitensis. J. Clin. Microbiol. 42:1290–1293. 10.1128/JCM.42.3.1290-1293.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Baily GG, Krahn JB, Drasar BS, Stoker NG. 1992. Detection of Brucella melitensis and Brucella abortus by DNA amplification. J. Trop. Med. Hyg. 95:271–275 [PubMed] [Google Scholar]
- 35. Peters IR, Helps CR, Batt RM, Day MJ, Hall EJ. 2003. Quantitative real-time RT-PCR measurement of mRNA encoding alpha-chain, pIgR and J-chain from canine duodenal mucosa. J. Immunol. Methods 275:213–222. 10.1016/S0022-1759(03)00056-5 [DOI] [PubMed] [Google Scholar]
- 36. Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28:2731–2739. 10.1093/molbev/msr121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Stone BM, Blyde DJ, Saliki JT, Blas-Machado U, Bingham J, Hyatt A, Wang J, Payne J, Crameri S. 2011. Fatal cetacean morbillivirus infection in an Australian offshore bottlenose dolphin (Tursiops truncatus). Aust. Vet. J. 89:452–457. 10.1111/j.1751-0813.2011.00849.x [DOI] [PubMed] [Google Scholar]
- 38. Bossart GD, Reif JS, Schaefer AM, Goldstein J, Fair PA, Saliki JT. 2010. Morbillivirus infection in free-ranging Atlantic bottlenose dolphins (Tursiops truncatus) from the Southeastern United States: seroepidemiologic and pathologic evidence of subclinical infection. Vet. Microbiol. 143:160–166. 10.1016/j.vetmic.2009.11.024 [DOI] [PubMed] [Google Scholar]
- 39. Duignan PJ, Geraci JR, Raga JA, Calzada N. 1992. Pathology of morbillivirus infection in striped dolphins (Stenella coeruleoalba) from Valencia and Murcia, Spain. Can. J. Vet. Res. 56:242–248 [PMC free article] [PubMed] [Google Scholar]
- 40. Domingo M, Vilafranca M, Visa J, Prats N, Trudgett A, Visser I. 1995. Evidence for chronic morbillivirus infection in the Mediterranean striped dolphin (Stenella coeruleoalba). Vet. Microbiol. 44:229–239. 10.1016/0378-1135(95)00016-4 [DOI] [PubMed] [Google Scholar]
- 41. Hewitt SM, Lewis FA, Cao Y, Conrad RC, Cronin M, Danenberg KD, Goralski TJ, Langmore JP, Raja RG, Williams PM, Palma JF, Warrington JA. 2008. Tissue handling and specimen preparation in surgical pathology: issues concerning the recovery of nucleic acids from formalin-fixed, paraffin-embedded tissue. Arch. Pathol. Lab Med. 132:1929–1935 [DOI] [PubMed] [Google Scholar]
- 42. Duignan PJ, House C, Geraci JR, Early G, Copland HG, Walsh MT, Bossart GD, Cray C, Sadove S, Aubin DJST, Moore M. 1995. Morbillivirus infection in two species of pilot whales (Globicephala sp.) from the western Atlantic. Mar. Mammal Sci. 11:150–162. 10.1111/j.1748-7692.1995.tb00514.x [DOI] [Google Scholar]
- 43. Van Bressem M, Waerebeek KV, Jepson PD, Raga JA, Duignan PJ, Nielsen O, Di Beneditto AP, Siciliano S, Ramos R, Kant W, Peddemors V, Kinoshita R, Ross PS, López-Fernandez A, Evans K, Crespo E, Barrett T. 2001. An insight into the epidemiology of dolphin morbillivirus worldwide. Vet. Microbiol. 81:287–304. 10.1016/S0378-1135(01)00368-6 [DOI] [PubMed] [Google Scholar]
- 44. Van Bressem MF, Raga JA, Di Guardo G, Jepson PD, Duignan PJ, Siebert U, Barrett T, Santos MC, Moreno IB, Siciliano S, Aguilar A, Van Waerebeek K. 2009. Emerging infectious diseases in cetaceans worldwide and the possible role of environmental stressors. Dis. Aquat. Organ. 86:143–157. 10.3354/dao02101 [DOI] [PubMed] [Google Scholar]
- 45. Archer FII. 1996. Morphological and genetic variation of striped dolphins (Stenella coeruleoalba Meyen 1833). Ph.D. thesis University of California at San Diego, Scripps Institution of Oceanography, San Diego, CA [Google Scholar]
- 46. Gaspari S. 2004. Social and population structure of striped and Risso's dolphins in the Mediterranean Sea. Ph.D. dissertation School of Biological and Biomedical Sciences, University of Durham, Durham, United Kingdom [Google Scholar]
- 47. Rudd PA, Cattaneo R, von Messling V. 2006. Canine distemper virus uses both the anterograde and the hematogenous pathway for neuroinvasion. J. Virol. 80:9361–9370. 10.1128/JVI.01034-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Blanchard TW, Santiago NT, Lipscomb TP, Garber RL, McFee WE, Knowles S. 2001. Two novel alphaherpesviruses associated with fatal disseminated infections in Atlantic bottlenose dolphins. J. Wildl. Dis. 37:297–305. 10.7589/0090-3558-37.2.297 [DOI] [PubMed] [Google Scholar]
- 49. Arbelo M, Sierra E, Esperón F, Watanabe TT, Bellière EN, Espinosa de los Monteros A, Fernández A. 2010. Herpesvirus infection with severe lymphoid necrosis affecting a beaked whale stranded in the Canary Islands. Dis. Aquat. Organ. 89:261–264. 10.3354/dao02208 [DOI] [PubMed] [Google Scholar]
- 50. Soto S, González B, Willoughby K, Maley M, Olvera A, Kennedy S, Marco A, Domingo M. 2012. Systemic herpesvirus and morbillivirus coinfection in a striped dolphin (Stenella coeruleoalba). J. Comp. Pathol. 146:269–273. 10.1016/j.jcpa.2011.04.002 [DOI] [PubMed] [Google Scholar]
- 51. Smolarek Benson KA, Manire CA, Ewing RY, Saliki JT, Townsend FI, Ehlers B, Romero CH. 2006. Identification of novel alpha- and gammaherpesviruses from cutaneous and mucosal lesions of dolphins and whales. J. Virol. Methods 136:261–266. 10.1016/j.jviromet.2006.03.033 [DOI] [PubMed] [Google Scholar]
- 52. Barr B, Dunn JL, Daniel MD, Banford A. 1989. Herpes-like viral dermatitis in a beluga whale (Delphinapterus leucas). J. Wildl. Dis. 25:608–611. 10.7589/0090-3558-25.4.608 [DOI] [PubMed] [Google Scholar]
- 53. Martineau D, Lagacé A, Béland P, Higgins R, Armstrong D, Shugart LR. 1988. Pathology of stranded beluga whales (Delphinapterus leucas) from the St. Lawrence Estuary, Québec, Canada. J. Comp. Pathol. 98:287–311 [DOI] [PubMed] [Google Scholar]
- 54. Bellière EN, Esperón F, Arbelo M, Muñoz MJ, Fernández A, Sánchez-Vizcaíno JM. 2010. Presence of herpesvirus in striped dolphins stranded during the cetacean morbillivirus epizootic along the Mediterranean Spanish coast in 2007. Arch. Virol. 155:1307–1311. 10.1007/s00705-010-0697-x [DOI] [PubMed] [Google Scholar]
- 55. Arbelo M, Bellière EN, Sierra E, Sacchinni S, Esperón F, Andrada M, Rivero M, Diaz-Delgado J, Fernández A. 2012. Herpes virus infection associated with interstitial nephritis in a beaked whale (Mesoplodon densirostris). BMC Vet. Res. 8:243. 10.1186/1746-6148-8-243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bellière EN, Esperón F, Fernández A, Arbelo M, Muñoz MJ, Sánchez-Vizcaíno JM. 2011. Phylogenetic analysis of a new cetacean morbillivirus from a short-finned pilot whale stranded in the Canary Islands. Res. Vet. Sci. 90:324–328. 10.1016/j.rvsc.2010.05.038 [DOI] [PubMed] [Google Scholar]
- 57. Sierra E, Zucca D, Arbelo M, García-Álvarez N, Andrada M, Déniz S, Fernández A. 2014. Fatal systemic morbillivirus infection in bottlenose dolphin, Canary Islands, Spain. Emerg. Infect. Dis. 20:269–271. 10.3201/eid2002.131463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Reddy ML, Dierauf LA, Gulland FMD. 2001. Marine mammals as sentinels of ocean health. In Dierauf LA, Gulland FMD. (ed), CRC handbook of marine mammal medicine. CRC Press, Boca Raton, FL [Google Scholar]
- 59.Marine Mammal Commission. 2004. Annual report to Congress. The Marine Mammal Commission, Bethesda, MD [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.