Abstract
Staphylococcal enterotoxin-like K (SEl-K) is a potent mitogen that elicits T-cell proliferation and cytokine production at very low concentrations. However, unlike the classical enterotoxins SEB and toxic shock syndrome toxin 1 (TSST-1), the gene for SEl-K is commonly present in more than half of all Staphylococcus aureus clinical isolates and is present in almost all USA300 community-acquired methicillin-resistant S. aureus (CA-MRSA) isolates. Sequencing of the sel-k gene in over 20 clinical isolates and comparative analysis with all 14 published sel-k sequences indicate that there are at least 6 variants of the sel-k gene, including one that is conserved among all examined USA300 strains. Additionally, we have developed a highly sensitive enzyme-linked immunosorbent assay (ELISA) that specifically detects and measures SEl-K protein in culture supernatants and biological fluids. Quantification of in vitro SEl-K secretion by various S. aureus isolates using this novel capture ELISA revealed detectable amounts of SEl-K secretion by all isolates, with the highest secretion levels being exhibited by MRSA strains that coexpress SEB. In vivo secretion was measured in a murine thigh abscess model, where similar levels of SEl-K accumulation were noted regardless of whether the infecting strain exhibited high or low secretion of SEl-K in vitro. We conclude that SEl-K is commonly expressed in the setting of staphylococcal infection, in significant amounts. SEl-K should be further explored as a target for passive immunotherapy against complicated S. aureus infection.
INTRODUCTION
Staphylococcus aureus can express a large and diverse repertoire of virulence factors, including many different surface proteins, enzymes, and secreted toxins (1). Among the most potent of these virulence factors are the members of a family of secreted, heat-stable proteins known as enterotoxins. More than 20 staphylococcal enterotoxins (SEs) have been discovered that demonstrate superantigenic (SAg) properties against T cells (2, 3). Superantigens can activate 20% to 50% of the T cell population by bypassing the traditional pathway of major histocompatibility complex (MHC)-dependent presentation of antigens to T cell receptors (TCRs). Instead, superantigens bind simultaneously to MHC class II (MHC-II) and TCRs, outside their antigen-binding groove, resulting in an excessive inflammatory response that can lead to toxic shock, multiorgan failure, and death. Superantigens are also associated with other immune-mediated diseases, including Kawasaki disease, atopic dermatitis, and chronic rhinosinusitis (4).
The classical superantigens toxic shock syndrome toxin 1 (TSST-1), SEA, and SEB have been extensively studied due to their causative roles in toxic shock syndrome and food poisoning (5). However, advancements in sequencing methodologies have enabled the discovery of many more SEs whose roles in pathogenesis remain unknown (6). One of these toxins, SEl-K, has been shown to exhibit superantigenic properties, including Vβ-specific T cell activation, pyrogenicity, emesis, and lethality in primates (7–9). Epidemiological studies have demonstrated the SEl-K-encoding genes to be among the most common SE genes in S. aureus clinical isolates (10–14). Additionally, SEl-K is the only SE gene to our knowledge that is significantly associated with community-acquired methicillin-resistant S. aureus (CA-MRSA) strains of several clonal lineages (10, 15, 16). Interestingly, the neighboring SEl-Q enterotoxin is not significantly associated with MRSA (10). However, studies examining the role of SEl-K in staphylococcal pathogenesis have been hampered by a lack of sensitive methods to measure expression of this toxin in vivo. Here we report the development of a sensitive and specific immunoassay for the measurement of SEl-K in biological fluids and demonstrate, for the first time, expression and accumulation of SEl-K in the setting of staphylococcal infection. To our knowledge, this is the first systematic investigation of SEl-K secretion and gene variation in clinical S. aureus strains. The implications of our results are discussed.
MATERIALS AND METHODS
Toxins.
Purified toxins SEA, SEB, and TSST-1 were purchased from Toxin Technology (Sarasota, FL) in accordance with CDC biosafety regulations.
Purification of SEl-K.
The full-length sel-k gene from USA300 clinical isolate W-83b, encoding residues 1 to 219, was subcloned into H-MBT-T vector (17) using primers sel-k_BamH1_F (3′-GGGGGATCCCAAGGTGATATAGGAATTGATAAT-5′) and sel-k_Sal1_R (3′-GGGGTCGACTTATATCGTTTCTTTATAAGAAATATCGAC-5′). H-MBP-T SEl-K plasmid was then transformed into XL10 Escherichia coli (Stratagene) and purified by standard methods for sequencing. After sequence verification, the H-MBP-T SEl-K plasmid was transformed into BL21 E. coli (Stratagene) for protein expression. Cells were grown for 5 h at 37°C in LB-ampicillin (LB-amp) media after induction with 0.5 mM IPTG (isopropyl-β-d-thiogalactopyranoside) at an optical density at 600 nm (OD600) of 0.6. Cells were harvested, resuspended in 20 mM Tris (pH 7.5) with protease inhibitor tablets (Roche), and then lysed by sonication. The sonicated suspension was centrifuged at 16,000 rpm for 30 min, and the supernatant was syringe filtered with a 0.2-μm-pore-size filter to eliminate cellular debris. Filtered supernatant was flowed through a 5-ml column of Talon affinity resin (Clontech), and the MBP:SEl-K fusion protein was eluted by 200 mM imidazole. The eluted fusion protein was digested with thrombin overnight at 4°C to cleave the H-maltose binding protein (MBP) tag, and the excess imidazole was removed by dialysis into 20 mM Tris (pH 7.5). The MBP fusion tag and other impurities were removed by a series of subsequent washes through Talon affinity resin and then amylase resin columns. The fractions which contained SEl-K were pooled and passed through a size exclusion column preequilibrated with buffer (20 mM Tris [pH 7.5] with 10 mM dithiothreitol [DTT]) to remove high-molecular-weight soluble aggregates. The protein was dialyzed into phosphate-buffered saline (PBS) to remove excess Tris and DTT and was found to be >99% pure by SDS-PAGE. SEl-K stocks were prepared at 1 mg/ml in 10% glycerol, and aliquots were stored at −20°C.
MAbs.
Monoclonal antibodies (MAbs) to SEl-K were generated from SEl-K-immunized BALB/c mice in the Hybridoma Facility of Albert Einstein College of Medicine as described here. All mice were immunized with full-length SEl-K (purchased from Toxin Technologies, Sarasota, FL) in complete Freund's adjuvant (CFA). The mouse with the highest Ab titer for SEl-K was selected for spleen harvest and hybridoma generation. Hybridoma supernatants were screened for reactivity to SEl-K by an enzyme-linked immunosorbent assay (ELISA), with positive reactivity being defined as absorbance 3-fold higher than the background value. Six MAbs (4G3, 4H3, 5G2, 6G1, 9H2, and 10C12) were selected and used in this study. Specificity of MAbs for SEl-K was determined by Western blotting and ELISA according to standard methods. MAbs were harvested from concentrated hybridoma supernatants, and their concentrations were determined by ELISA using commercial antibody standards (18).
Staphylococcus aureus strains.
S. aureus isolates from bacteremic patients were obtained from three hospitals in the Bronx, NY, and were previously genotyped by PCR for 19 SE genes and classified by pulsed-field gel electrophoresis (PFGE), multilocus sequence typing (MLST), spa typing, and staphylococcal cassette chromosome mec (SCCmec) typing (10). See Table 1 for a more detailed description of each strain. Isolates were stored in −80°C, grown on blood agar plates, and confirmed as S. aureus by a standard laboratory protocol.
TABLE 1.
Strain lista
| Strainb | mecA | SCC | ACME | MLST | Toxinsc | SEl-K (ng/ml)d |
|---|---|---|---|---|---|---|
| MSSA | ||||||
| B-88 | − | MSSA | − | CC 8 | sed,sel-j, sel-k, sel-p, sel-r | 2 |
| B-3 | − | MSSA | − | CC 8 | sec, sed, sel-j, sel-k, sel-l, sel-p, sel-r, sel-u, tsst-1 | 2 |
| B-63 | − | MSSA | − | CC 8 | sed, sel-j, sel-k, sel-r | 2 |
| W-133 | − | MSSA | − | CC 8 | pvl, sed, sel-j, sel-k, sel-r | 70 |
| W-84 | − | MSSA | − | CC 8 | pvl, sed, sel-j, sel-k, sel-p, sel-r | 96 |
| B-41b | − | MSSA | − | CC 8 | sej, sel-k, sel-p, sel-r | 2 |
| W-136 | − | MSSA | − | CC 8 | seb, sel-j, sel-k, sel-q, sel-r | 582 |
| W-67 | − | MSSA | − | CC 8 | sea, seb, sel-k, sel-p, sel-q, sel-r, sel-u | 993 |
| W-183 | − | MSSA | − | CC 8 | seb, sel-i, sel-k, sel-p, sel-r | 2 |
| W-162 | − | MSSA | − | CC 59 | seb, sed, sel-k, sel-p, sel-r | 327 |
| W-100a | − | MSSA | − | CC 59 | seb, sel-k | 7 |
| W-116a | − | MSSA | − | CC 20 | seb, sel-g, sel-k, sel-m, sel-n, sel-p, sel-r | 2 |
| MRSA | ||||||
| W-112b | + | IV | + | CC 8 | sel-k, sel-r, sel-u | 90 |
| B-46a | + | IV | − | CC 8 | sed, sel-j, sel-k, sel-m, sel-p, sel-r, sel-u | 2 |
| W-110a | + | IV | − | CC 8 | sel-k, sel-r, sel-u | 7 |
| B-74b | + | IV | − | CC 8 | sel-k, sel-p, sel-r, sel-u | 41 |
| W-75a | + | IV | − | CC 8 | sed, sel-j, sel-k, sel-p, sel-r, sel-u | 69 |
| W-165 | + | II | − | CC 5 | sed, sel-g, sel-i, sel-j, sel-k, sel-m | 1 |
| B-45 | + | IV | − | CC 8 | seb, sed, sel-j, sel-k, sel-m, sel-p, sel-r, sel-u | 350 |
| B-2 | + | IV | − | CC 8 | seb, sec, sed, sej, sel-k, sel-l, sel-r, sel-u, tsst-1 | 641 |
| B-4 | + | IV | − | CC 8 | seb, sec, sed, sel-k, sel-p, sel-r, sel-u | 745 |
| B-47a | + | IV | − | CC 8 | seb, sel-k, sel-p, sel-r, sel-u | 647 |
| B-86 | + | IV | − | CC 8 | seb, sel-k, sel-p, sel-r | 385 |
| B-62b | + | IV | − | CC 8 | sea, seb, sel-k, sel-r, sel-u | 704 |
| W-132 | + | IV | − | ND | seb, sel-k, sel-r | 688 |
| B-1 | + | IV | − | CC5 | sec, sed, sel-i, sel-j, sel-l, sel-m, sel-n, sel-r, sel-u, tsst-1 | 0 |
| USA300 | ||||||
| W-144 | + | IV | + | CC 8 | pvl, sel-j, sel-k, sel-r | 82 |
| W-129 | + | IV | + | CC 8 | pvl, sel-k, sel-q, sel-r | 67 |
| W-98a | + | IV | + | CC 8 | pvl, sel-k, sel-q | 69 |
| W-130 | + | IV | + | CC 8 | pvl, sel-k, sel-r | 52 |
| W-118 | + | IV | + | CC 8 | pvl, sed, sel-k, sel-p, sel-r | 90 |
| W-123 | + | IV | + | CC 8 | pvl, sel-k, sel-p, sel-r | 81 |
| W-85b | + | IV | + | CC 8 | pvl, sel-k, sel-p, sel-r | 44 |
ACME, arginine catabolic mobile element; CC, clonal complex; ND, not determined; pvl, Panton-Valentine leukocidin (PVL). USA300 data were defined by pulsed-field gel electrophoresis and determination of CC 8, SCCmec IV, and positivity for both PVL and the ACME.
W, wound isolate; B, blood isolate.
Toxin profiles were previously determined by PCR amplification with primer sets for 19 different enterotoxins.
SEl-K secretion was measured by capture ELISA, using MAbs 4G3 and 10C12, after 16 h of growth in BHI at 37°C with shaking at 220 RPM.
Sequence analysis of variant sel-k alleles.
Genomic DNA was isolated from 23 previously described clinical isolates of S. aureus (6 USA300 strains, 12 non-USA300 MRSA strains, and 5 methicillin-sensitive S. aureus [MSSA] strains) and used as the template for amplification of the sel-k gene by PCR with the primers sel-k_seq_F (5′-CGACATCCAAATGGAATTTCTCAGACTCTACAG-3′) and sel-k_seq_R (5′-GCAGAGAATTTTCATTTGGATGTAGAGATTTCATATGAG-3′). An additional 18 sel-k nucleotide sequences were obtained from NCBI GenBank. Predicted translated amino acid sequences were determined using the ExPASy Translation Tool, and amino acid sequence alignments were made using the ClustalW2 program.
SDS-PAGE and Western blotting.
Purified toxins SEA, SEB, SEl-K, and TSST-1 at 100 ng each were dissolved in 30 μl of sample buffer with β-mercaptoethanol (βME) and boiled for 6 min. After centrifugation for 10 s, the toxins were resolved on a 10% SDS-polyacrylamide gel, and the fractionated proteins were transferred from the gel onto a polyvinylidene difluoride (PVDF) membrane (Millipore) in a semidry transblot apparatus. The membrane was blocked with 5% milk–PBS–Tween for 2 h. The blots were washed and incubated with a 1:1,000 dilution of MAbs at a concentration of 2 mg/ml for 1 h. Later, the blots were washed with PBS-Tween and further incubated with horseradish-peroxidase (HRP)-conjugated goat anti-mouse IgG (1:10,000). After washing, development was performed by a chemiluminescence method according to the instructions of the manufacturer (Fisher Scientific).
Mouse experiments.
The thigh infection model was described elsewhere (19). Briefly, female BALB/c mice 6 to 8 weeks of age were anesthetized, and a small incision was made into the lateral aspect of the quadriceps muscle. The thigh wound was then inoculated with 105 CFU (in 5 μl of PBS) of either MRSA strain W-132 or USA300 strain W-85b, and a suture was embedded to provide a foreign-body substrate for bacterial attachment. On day 5, mice were euthanized, and abscesses were excised. The abscesses were homogenized in 1 ml of PBS, analyzed by ELISA for SEl-K quantification, and plated for CFU determination.
Ethics statement.
Animal experiments were performed with the approval of the Albert Einstein College of Medicine Animal Institute Committee in accordance with their rules and regulations.
Statistical analysis.
Standard curves, concentrations, and trend lines were generated and calculated using Excel software (Microsoft). Statistical analysis was performed with Student's t tests (two-tailed, unpaired) and analysis of variance (ANOVA) with a posttest using Prism 6 software.
RESULTS
Clinical isolates of S. aureus encode variant SEl-K toxins.
The gene encoding SEl-K was sequenced in 25 strains of S. aureus (4 MSSA strains and 21 MRSA strains, 9 of which were USA300 clones) from our published collection of clinical isolates (10). Deduced translated amino acid sequences were aligned with all 14 sel-k gene sequences currently published on NIH databases. Comparative analysis of all 39 sequences revealed the existence of 6 SEl-K toxin alleles (Fig. 1). Moreover, the predicted amino acid sequences of SEl-K in all examined USA300 strains, including the two published USA300 sequences, were found to be 100% identical and contained 5 amino acid mutations, including 3 in the signal peptide and 2 in the coding region, that were unique to the USA300 clone.
FIG 1.
Alignment of 6 variant SEl-K alleles. The COL strain sequence of SEl-K was used as the standard to which all other sequences are compared. The number to the left of each strain name represents the number of strains that have been found to have that corresponding sequence. The number to the right of each strain name indicates amino acid positions. Highlighted residues represent residues that differ from the COL strain sequence. The arrow indicates the cleavage site between the signal peptide and the mature, secreted SEl-K peptide.
Clinical isolates of S. aureus secrete SEl-K in vitro.
A panel of six stable hybridoma cell lines, each secreting a distinct anti-SEl-K MAb (four IgG1-specific and two IgG2b-specific cell lines), was generated by fusion of myeloma cells with splenic B cells from SEl-K-immunized mice. The hybridoma clones producing MAbs with the highest affinity to SEl-K were identified by an ELISA screen and subcloned to ensure that each clone produced MAbs of a single isotype. A Western blot analysis in which the individual MAbs were probed against a panel of purified enterotoxins (under denaturing conditions) revealed binding by all six MAbs to epitopes on SEl-K (Fig. 2A). None of the MAbs recognized epitopes on SEB or TSST-1. However, MAbs 4G3 and 4H3 did exhibit cross-reactivity with an epitope of SEA. Three clinical isolates of S. aureus, W-85b, W-132, and B-1 (Table 1), were grown overnight in brain heart infusion (BHI), and the resulting supernatants were probed with the 6 anti-SEl-K MAbs individually. USA300 strain W-85b and MRSA strain W-132, both verified by PCR to carry the sel-k gene, produced supernatants that bound the anti-SEl-K MAbs (Fig. 2B). USA300 strain W-85b also exhibited less overnight secretion of SEl-K than MRSA strain W-132, as measured by all 6 MAbs. No cross-reaction was observed with the overnight supernatant from MRSA strain B-1, which contains 10 different enterotoxin genes (sec, sed, sel-i, sel-j, sel-l, sel-m, sel-n, sel-r, sel-u, and tsst-1) but not sel-k.
FIG 2.
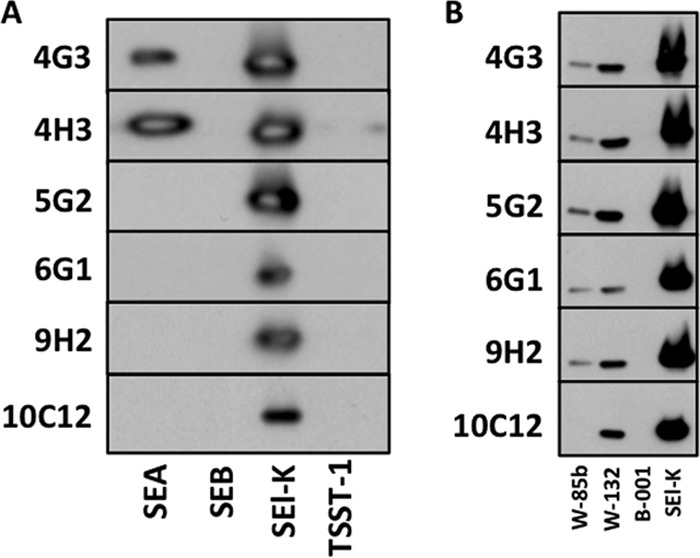
Detection of SEl-K secretion in vitro. (A) All six MAbs were individually probed by Western blotting against purified enterotoxins SEA, SEB, SEl-K, and TSST-1 at 100 ng each. (B) A panel of supernatants from overnight cultures of 3 S. aureus clinical isolates (W-85b [a USA300 strain that is PCR positive for sel-k, sel-p, and sel-r], W-132 [an MRSA strain that is PCR positive for sel-k, seb, and sel-r], and B-1 [an MRSA strain that is PCR positive for 10 different SEs but not sel-k]) were probed by Western blotting using each of the 6 anti-SEl-K MAbs. Identical overnight growth curves were observed for all strains. Purified SEl-K (100 ng) served as a positive control in the right-most lane.
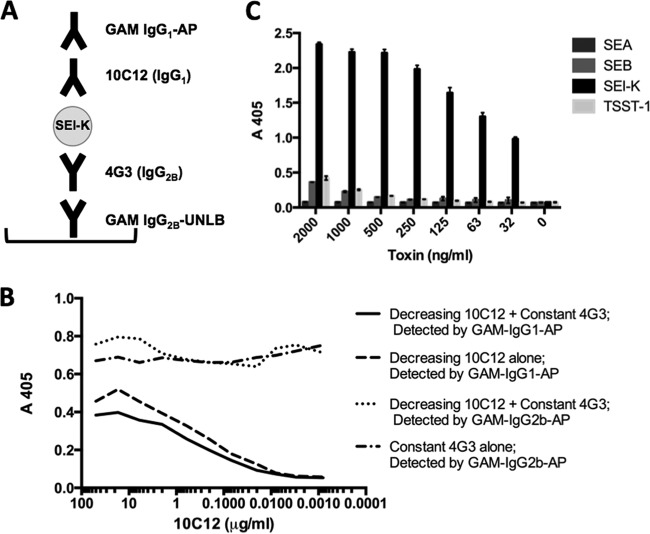
Development of ELISA to measure SEl-K secretion.
We evaluated our anti-SEl-K MAbs in combinations of two, and in different sandwich ELISA configurations, to identify the combination(s) that produced the most sensitive ELISA for the measurement of SEl-K. The combinations of MAbs 10C12 with 4G3, and 10C12 with 4H3, regardless of which MAb in the combination was used for capture or detection of SEl-K, produced ELISAs with sensitivities of down to 500 pg/ml of SEl-K (Table 2). The other combinations did not yield standard curves good enough to allow fitting of trend lines for quantification of SEl-K either because they bound to toxin only at high concentrations of SEl-K or because they exhibited competitive binding for the epitope(s) on SEl-K (data not shown). The ELISA configuration used in all subsequent SEl-K quantification experiments, with 4G3 as the capture Ab and 10C12 as the detection Ab, is outlined in Fig. 3A. An ELISA-based assay in which 10C12 and 4G3 were added simultaneously to SEl-K revealed no competition, from which we conclude that they bind different epitopes on SEl-K (Fig. 3B). To evaluate the specificity of our capture ELISA for SEl-K, we assayed equivalent amounts of other staphylococcal enterotoxins, including SEA, SEB, and TSST-1. Our data demonstrate that the ELISA is specific for SEl-K (Fig. 3C). As expected, the ELISA was not sensitive to SEA, even at high concentrations, and became marginally sensitive to SEB or TSST-1 only at concentrations greater than 1 μg/ml.
TABLE 2.
Sensitivities of different capture ELISAs tested
| Capture MAb (isotype) | Detection MAb | Sensitivity (ng/ml)a |
|---|---|---|
| 5G2 (IgG1) | 4G3 | 333 |
| 4H3 | ND | |
| 6G1 (IgG1) | 4G3 | ND |
| 4H3 | ND | |
| 9H2 (IgG1) | 4G3 | ND |
| 4H3 | ND | |
| 10C12 (IgG1) | 4G3 | 0.5 |
| 4H3 | 0.5 | |
| 4G3 (IgG2b) | 10C12 | 0.5 |
| 5G2 | 37 | |
| 6G1 | ND | |
| 9H2 | ND | |
| 4H3 (IgG2b) | 10C12 | 0.5 |
| 5G2 | ND | |
| 6G1 | ND | |
| 9H2 | ND |
Sensitivity is defined by the lowest concentration of SEl-K from which the starting concentration (1 μg/ml) could be calculated by using the linear-fit standard curve generated in each ELISA. Antibody combinations without a sensitivity value listed did not successfully bind SEl-K in this assay; hence, calculations were not done (ND).
FIG 3.
Development of SEl-K capture ELISA. (A) Configuration of sandwich ELISA for quantification of SEl-K. GAM, goat anti-mouse; AP, alkaline phosphatase; UNLB, unlabeled. GAM antibodies were purchased from SouthernBiotech (Birmingham, AL). (B) Competition assay. A 96-well ELISA plate was coated with 25 ng of SEl-K per well, blocked with 1% bovine serum albumin (BSA), and then treated with MAbs 10C12 (IgG1) and 4G3 (IgG2b) simultaneously. The experiments were performed in duplicate. (C) Specificity assay. A panel of purified enterotoxins, at concentrations equal to that of SEl-K, was assayed by capture ELISA with MAbs 4G3 and 10C12. The experiment was conducted in triplicate, and the error bars reflect standard deviations.
Measurement of SEl-K secretion in culture supernatants.
Next, we tested the utility of our capture ELISA in measuring in vitro secretion of SEl-K by S. aureus. Thirty clinical isolates that carry the sel-k gene were selected for this experiment and grouped according to their SE profile and clone type (6 strains per group). S. aureus strains were grown overnight (16 h) in BHI. SEl-K concentrations in supernatants were measured by capture ELISA. MRSA strains that carry both sel-k and seb secreted significantly more SEl-K (350 to 750 ng/ml) than strains that do not cocarry the seb genes (1 to 100 ng/ml) (Fig. 4). These differences in SEl-K secretion are not a product of growth characteristics that differed between strains, as the growth curves obtained for all strains were equal (data not shown).
FIG 4.
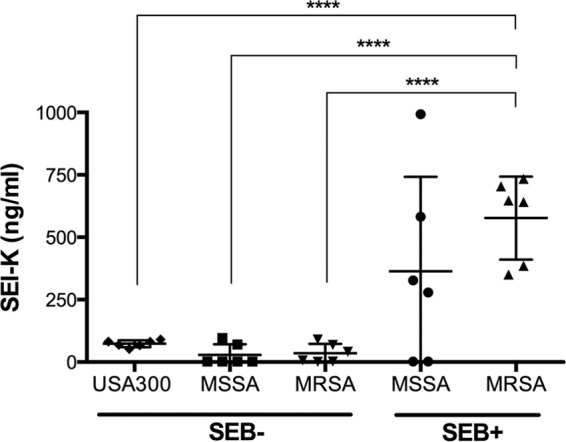
Measurement of in vitro SEl-K secretion. A total of 30 clinical isolates of S. aureus (6 different strains per group) were grown overnight in BHI, and capture ELISA, performed with MAbs 4G3 and 10C12, was used to measure SEl-K. USA300 strains: W-98a, W-118, W-123, W-129, W-130, and W-144. MSSA/B− strains: B-3, B-41b, B-63, W-84, B-88, and W-133. MRSA/B− strains: B-46a, B-74b, W-75a, W-110a, W-112b, and W-165. MSSA/B+ strains: W-67, W-100a, W-116a, W-136, W-162, and W-183. MRSA/B+ strains: B-2, B-4, B-45, B-47a, B-62b, and B-86. Each point represents the analysis of a single strain (performed in duplicate). The lower detection limit of the ELISA was 500 pg/ml. The horizontal bars represent the means ± standard deviations. Four asterisks (****) indicate a significant difference (P > 0.00001) between results determined for the SEl-K/SEB-copositive MRSA strains and strains that do not coexpress SEB. Statistically significant differences between the groups were determined by ANOVA with a posttest (P < 0.0001). See Table 1 for the exact measurements of overnight SEl-K secretions for each strain.
Measurement of in vivo SEl-K secretion in murine abscesses.
Next, we applied our capture ELISA to the detection of SEl-K in abscesses resulting from infection with S. aureus. Two groups of mice (5 mice per group) were infected with 105 CFU of either the in vitro high producer of SEl-K, MRSA strain W-132, or the in vitro low producer, USA300 strain W-85b, resulting in a localized abscess after 5 days (Fig. 5A). An accumulation of SEl-K of between 3 and 6 ng/ml was measured in all 10 abscesses regardless of the infecting S. aureus strain (Fig. 5B). The levels of CFU in the abscesses between these two groups were comparable (Fig. 5C). Likewise, in a single sample of surgically removed human abscess, our ELISA was able to detect 1 ng/ml of SEl-K. The MRSA strain cultured from this human abscess produced 100 ng/ml SEl-K when grown overnight in BHI (data not shown). In summary, these data confirm that our assay can quantify secretion of SEl-K in infected tissue and indicate that in vitro secretion may not correlate with in vivo secretion.
FIG 5.
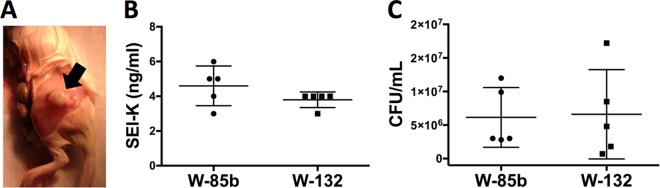
Detection of SEl-K in the murine thigh abscess model. (A) Representative abscess (arrow) of a mouse that was infected with 105 CFU of S. aureus clinical isolate W-85b or W132 in 5 μl of PBS. (B) Measurement of SEl-K concentrations in mouse thigh abscesses. Each point represents the analysis of one mouse abscess (performed in duplicate) by ELISA with MAbs 4G3 and 10C12. Horizontal lines represent means ± standard deviations. The lower detection limit of the ELISA was 500 pg/ml. (C) Levels of CFU in the abscesses were not significantly different between the two groups.
DISCUSSION
Several epidemiological studies have shown that sel-k is one of the most prevalent enterotoxin genes in clinical isolates of S. aureus (10, 12–16). Moreover, in several of these studies, SEl-K was significantly associated with CA-MRSA strains (10, 15, 16), which included USA300, USA400, and other clones. The USA300 clone in particular is now a major epidemic strain in several countries over 5 continents, and its clinical and epidemiological features, internationally, mirror those observed in the United States (20). The cause of hypervirulence and rapid transmission of USA300 strains relative to other staphylococcal strains is still been actively investigated (21). Importantly, we have generated anti-SEl-K MAbs that now provide a tool that facilitates the study of SEl-K expression in vivo and its strain-dependent variability. Such MAbs could potentially be further developed for adjunctive therapy in the treatment of S. aureus disease as described for SEB-specific MAbs (22).
Our analysis of predicted amino acid sequences from 39 S. aureus strains revealed the existence of at least 6 variants of SEl-K, including one that is conserved and unique to all examined USA300 strains. Naturally occurring variations in the coding region of TSST-1, SEB, and SEC have been shown to result in altered biological properties, including mitogenicity, immunogenicity, and lethality (23–25). Potential differences in biological properties between these 6 SEl-K variants must be explored, especially in regard to USA300 strains, which carry the gene encoding SEl-K in almost all cases and carry conserved unique mutations in their signal and coding regions.
Three of these mutations are located in a presumed signal peptide as determined on the basis of results from sequence prediction program Signal IP v1.1 (7). It is conceivable that these signal peptide mutations contribute to the consistently low secretion of SEl-K exhibited by all USA300 strains in vitro. Future studies are needed to determine if these USA300-specific sequence alterations in the sel-k gene are relevant for expression levels. Also plausible, however, is that SEl-K expression is regulated by factors encoded in the same pathogenicity island in which SEB is cocarried. Several epidemiological studies have shown that SEl-K and SEB are often encoded on the same mobile genetic elements, namely, S. aureus pathogenicity island 1 (SaPI1), SaPI2, SaPI3, and SaPI5 (16, 26–28). Moreover, our in vitro data show that strains carrying both sel-k and seb expressed SEl-K at significantly higher levels than those that do not. Regulatory factors SaeRS and σ(B) have been shown to enhance and inhibit SEB transcription, respectively, but to have no effect on SEl-K. Thus, additional studies are needed to identify the regulatory factors of SEl-K (27).
Interestingly, despite a 10-fold difference between MRSA strain W-132 and USA300 strain W-85b in their levels of in vitro SEl-K secretion, infection of mice with either of these 2 strains resulted in equal accumulations of SEl-K in our murine thigh abscess model. This finding is consistent with studies on the regulation of SEs, which have also revealed that the in vivo expression of SEs may not correlate with in vitro observations (26, 29). One reason could be that the pathogen burden and/or the in vivo growth conditions potentially affect toxin production (30–32). Further experimentation is needed to identify the factors that influence SE expression in vivo.
The contribution of SEl-K to S. aureus pathogenesis is not well understood. However, the available data indicate that SEl-K is a potent stimulator of T cells, and now we demonstrate that the toxin is abundantly expressed in a large of portion of S. aureus strains. SEl-K accumulates in abscesses to similar degrees regardless of whether the infecting strain exhibits low or high secretion of SEl-K in vitro. This finding supports the notion that SEl-K, similarly to SEB, is not degraded or cleared from an abscess in vivo. Thus, our findings suggest that SEl-K may play a previously underappreciated role in S. aureus-mediated skin and soft-tissue infection. This finding is consistent with previous findings that documented SEl-K secretion in 14 of 36 S. aureus clinical isolates by Western blotting (7). However, the SEl-K secretion was not quantified and none of the strains were USA300 clones.
S. aureus is the second-most-common pathogen recovered in nosocomial bloodstream infections in the United States, and emergent USA300 strains in particular are reported to cause the majority of complicated skin and soft-tissue infections (33–36). Despite sensitivity to antibiotics, complicated S. aureus infections still have high mortality and treatment failure rates (37, 38). Several studies demonstrate that neutralization of SAgs with drugs or Abs improves outcomes in both intoxication and infection models (39–43). Specifically, a recent study from our laboratory performed with MAbs specific to SEB provides encouraging data with respect to the development of such Abs as adjunctive therapy for complicated S. aureus infections (44). Given that several anti-infective MAbs are now FDA licensed, more efforts should be focused on developing additional MAbs as therapeutics to neutralize SEs (45, 46). Because SEs are heat stable and resistant to enzymatic degradation, they are expected to persist over time in an abscess even if pathogen growth is suppressed by antibiotic treatment. Therefore, in the setting of a complicated skin and soft-tissue infection with deep-seated abscess formation, possibly also complicated by osteomyelitis, passive immunotherapy that targets SEl-K in combination with other commonly expressed toxins may enhance the therapeutic efficacy of current antibiotic therapies and improve outcomes. Our SEl-K-specific capture ELISA may prove helpful in examining such future clinical investigations.
ACKNOWLEDGMENTS
This work was supported by the NIH National Institute of Allergy and Infectious Diseases, grant U54-AI057158, and NIH MSTP training grant T3GM07288.
We thank Manxia Fan, Susan Buhl, and the staff at the Hybridoma Facility at the Albert Einstein College of Medicine for their technical help.
Footnotes
Published ahead of print 7 May 2014
REFERENCES
- 1.Lowy FD. 1998. Staphylococcus aureus infections. N. Engl. J. Med. 339:520–532. 10.1056/NEJM199808203390806 [DOI] [PubMed] [Google Scholar]
- 2.Spaulding AR, Salgado-Pabon W, Kohler PL, Horswill AR, Leung DY, Schlievert PM. 2013. Staphylococcal and streptococcal superantigen exotoxins. Clin. Microbiol. Rev. 26:422–447. 10.1128/CMR.00104-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pinchuk IV, Beswick EJ, Reyes VE. 2010. Staphylococcal enterotoxins. Toxins 2:2177–2197. 10.3390/toxins2082177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu SX, McCormick JK. 2012. Staphylococcal superantigens in colonization and disease. Front. Cell. Infect. Microbiol. 2:52. 10.3389/fcimb.2012.00052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dinges MM, Orwin PM, Schlievert PM. 2000. Exotoxins of Staphylococcus aureus. Clin. Microbiol. Rev. 13:16–34, table of contents. 10.1128/CMR.13.1.16-34.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lina G, Bohach GA, Nair SP, Hiramatsu K, Jouvin-Marche E, Mariuzza R, International Nomenclature Committee for Staphylococcal Superantigens 2004. Standard nomenclature for the superantigens expressed by Staphylococcus. J. Infect. Dis. 189:2334–2336. 10.1086/420852 [DOI] [PubMed] [Google Scholar]
- 7.Orwin PM, Leung DY, Donahue HL, Novick RP, Schlievert PM. 2001. Biochemical and biological properties of Staphylococcal enterotoxin K. Infect. Immun. 69:360–366. 10.1128/IAI.69.1.360-366.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Omoe K, Hu DL, Ono HK, Shimizu S, Takahashi-Omoe H, Nakane A, Uchiyama T, Shinagawa K, Imanishi K. 2013. Emetic potentials of newly identified staphylococcal enterotoxin-like toxins. Infect. Immun. 81:3627–3631. 10.1128/IAI.00550-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Günther S, Varma AK, Moza B, Kasper KJ, Wyatt AW, Zhu P, Rahman AK, Li Y, Mariuzza RA, McCormick JK, Sundberg EJ. 2007. A novel loop domain in superantigens extends their T cell receptor recognition site. J. Mol. Biol. 371:210–221. 10.1016/j.jmb.2007.05.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Varshney AK, Mediavilla JR, Robiou N, Guh A, Wang X, Gialanella P, Levi MH, Kreiswirth BN, Fries BC. 2009. Diverse enterotoxin gene profiles among clonal complexes of Staphylococcus aureus isolates from the Bronx, New York. Appl. Environ. Microbiol. 75:6839–6849. 10.1128/AEM.00272-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xie Y, He Y, Gehring A, Hu Y, Li Q, Tu SI, Shi X. 2011. Genotypes and toxin gene profiles of Staphylococcus aureus clinical isolates from China. PLoS One 6:e28276. 10.1371/journal.pone.0028276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Djahmi N, Messad N, Nedjai S, Moussaoui A, Mazouz D, Richard JL, Sotto A, Lavigne JP. 2013. Molecular epidemiology of Staphylococcus aureus strains isolated from inpatients with infected diabetic foot ulcers in an Algerian University Hospital. Clin. Microbiol. Infect. 19:E398–E404. 10.1111/1469-0691.12199 [DOI] [PubMed] [Google Scholar]
- 13.Machuca MA, Sosa LM, Gonzalez CI. 2013. Molecular typing and virulence characteristic of methicillin-resistant Staphylococcus aureus isolates from pediatric patients in Bucaramanga, Colombia. PLoS One 8:e73434. 10.1371/journal.pone.0073434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Indrawattana N, Sungkhachat O, Sookrung N, Chongsa-nguan M, Tungtrongchitr A, Voravuthikunchai SP, Kong-ngoen T, Kurazono H, Chaicumpa W. 2013. Staphylococcus aureus clinical isolates: antibiotic susceptibility, molecular characteristics, and ability to form biofilm. BioMed Res. Int. 2013:314654. 10.1155/2013/314654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shukla SK, Karow ME, Brady JM, Stemper ME, Kislow J, Moore N, Wroblewski K, Chyou PH, Warshauer DM, Reed KD, Lynfield R, Schwan WR. 2010. Virulence genes and genotypic associations in nasal carriage, community-associated methicillin-susceptible and methicillin-resistant USA400 Staphylococcus aureus isolates. J. Clin. Microbiol. 48:3582–3592. 10.1128/JCM.00657-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu D, Li X, Yang Y, Zheng Y, Wang C, Deng L, Liu L, Li C, Shang Y, Zhao C, Yu S, Shen X. 2011. Superantigen gene profiles and presence of exfoliative toxin genes in community-acquired meticillin-resistant Staphylococcus aureus isolated from Chinese children. J. Med. Microbiol. 60:35–45. 10.1099/jmm.0.023465-0 [DOI] [PubMed] [Google Scholar]
- 17.Alexandrov A, Dutta K, Pascal SM. 2001. MBP fusion protein with a viral protease cleavage site: one-step cleavage/purification of insoluble proteins. Biotechniques 30:1198–1204 [DOI] [PubMed] [Google Scholar]
- 18.Cook E, Wang X, Robiou N, Fries BC. 2007. Measurement of staphylococcal enterotoxin B in serum and culture supernatant with a capture enzyme-linked immunosorbent assay. Clin. Vaccine Immunol. 14:1094–1101. 10.1128/CVI.00183-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuklin NA, Pancari GD, Tobery TW, Cope L, Jackson J, Gill C, Overbye K, Francis KP, Yu J, Montgomery D, Anderson AS, McClements W, Jansen KU. 2003. Real-time monitoring of bacterial infection in vivo: development of bioluminescent staphylococcal foreign-body and deep-thigh-wound mouse infection models. Antimicrob. Agents Chemother. 47:2740–2748. 10.1128/AAC.47.9.2740-2748.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nimmo GR. 2012. USA300 abroad: global spread of a virulent strain of community-associated methicillin-resistant Staphylococcus aureus. Clin. Microbiol. Infect. 18:725–734. 10.1111/j.1469-0691.2012.03822.x [DOI] [PubMed] [Google Scholar]
- 21.Thurlow LR, Joshi GS, Richardson AR. 2012. Virulence strategies of the dominant USA300 lineage of community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA). FEMS Immunol. Med. Microbiol. 65:5–22. 10.1111/j.1574-695X.2012.00937.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Varshney AK, Wang X, MacIntyre J, Zollner RS, Kelleher K, Kovalenko OV, Pechuan X, Byrne FR, Fries BC. 5 May 2014. Humanized staphylococcal enterotoxin B (SEB)-specific monoclonal antibodies protect from SEB intoxication and Staphylococcus aureus infections alone or as adjunctive therapy with vancomycin. J. Infect. Dis. 10.1093/infdis/jiu198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee PK, Kreiswirth BN, Deringer JR, Projan SJ, Eisner W, Smith BL, Carlson E, Novick RP, Schlievert PM. 1992. Nucleotide sequences and biologic properties of toxic shock syndrome toxin 1 from ovine- and bovine-associated Staphylococcus aureus. J. Infect. Dis. 165:1056–1063. 10.1093/infdis/165.6.1056 [DOI] [PubMed] [Google Scholar]
- 24.Kohler PL, Greenwood SD, Nookala S, Kotb M, Kranz DM, Schlievert PM. 2012. Staphylococcus aureus isolates encode variant staphylococcal enterotoxin B proteins that are diverse in superantigenicity and lethality. PLoS One 7:e41157. 10.1371/journal.pone.0041157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turner TN, Smith CL, Bohach GA. 1992. Residues 20, 22, and 26 determine the subtype specificities of staphylococcal enterotoxins C1 and C2. Infect. Immun. 60:694–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Novick RP. 2003. Mobile genetic elements and bacterial toxinoses: the superantigen-encoding pathogenicity islands of Staphylococcus aureus. Plasmid 49:93–105. 10.1016/S0147-619X(02)00157-9 [DOI] [PubMed] [Google Scholar]
- 27.Kusch K, Hanke K, Holtfreter S, Schmudde M, Kohler C, Erck C, Wehland J, Hecker M, Ohlsen K, Broker B, Engelmann S. 2011. The influence of SaeRS and sigma(B) on the expression of superantigens in different Staphylococcus aureus isolates. Int. J. Med. Microbiol. 301:488–499. 10.1016/j.ijmm.2011.01.003 [DOI] [PubMed] [Google Scholar]
- 28.Diep BA, Carleton HA, Chang RF, Sensabaugh GF, Perdreau-Remington F. 2006. Roles of 34 virulence genes in the evolution of hospital- and community-associated strains of methicillin-resistant Staphylococcus aureus. J. Infect. Dis. 193:1495–1503. 10.1086/503777 [DOI] [PubMed] [Google Scholar]
- 29.Pragman AA, Schlievert PM. 2004. Virulence regulation in Staphylococcus aureus: the need for in vivo analysis of virulence factor regulation. FEMS Immunol. Med. Microbiol. 42:147–154. 10.1016/j.femsim.2004.05.005 [DOI] [PubMed] [Google Scholar]
- 30.Yarwood JM, McCormick JK, Schlievert PM. 2001. Identification of a novel two-component regulatory system that acts in global regulation of virulence factors of Staphylococcus aureus. J. Bacteriol. 183:1113–1123. 10.1128/JB.183.4.1113-1123.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yarwood JM, Schlievert PM. 2000. Oxygen and carbon dioxide regulation of toxic shock syndrome toxin 1 production by Staphylococcus aureus MN8. J. Clin. Microbiol. 38:1797–1803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yarwood JM, Schlievert PM. 2003. Quorum sensing in Staphylococcus infections. J. Clin. Invest. 112:1620–1625. 10.1172/JCI20442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moran GJ, Krishnadasan A, Gorwitz RJ, Fosheim GE, McDougal LK, Carey RB, Talan DA, Group EMINS 2006. Methicillin-resistant S. aureus infections among patients in the emergency department. N. Engl. J. Med. 355:666–674. 10.1056/NEJMoa055356 [DOI] [PubMed] [Google Scholar]
- 34.Laupland KB. 2013. Incidence of bloodstream infection: a review of population-based studies. Clin. Microbiol. Infect. 19:492–500. 10.1111/1469-0691.12144 [DOI] [PubMed] [Google Scholar]
- 35.Sowash MG, Uhlemann AC. 2014. Community-associated methicillin-resistant Staphylococcus aureus case studies. Methods Mol. Biol. 1085:25–69. 10.1007/978-1-62703-664-1_2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Uhlemann AC, Otto M, Lowy FD, Deleo FR. 3 May 2013. Evolution of community- and healthcare-associated methicillin-resistant Staphylococcus aureus. Infect. Genet. Evol. 10.1016/j.meegid.2013.04.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ippolito G, Leone S, Lauria FN, Nicastri E, Wenzel RP. 2010. Methicillin-resistant Staphylococcus aureus: the superbug. Int. J. Infect. Dis. 14(Suppl 4):S7–S11. 10.1016/j.ijid.2010.05.003 [DOI] [PubMed] [Google Scholar]
- 38.Low DE. 2013. Toxic shock syndrome: major advances in pathogenesis, but not treatment. Crit. Care Clin. 29:651–675. 10.1016/j.ccc.2013.03.012 [DOI] [PubMed] [Google Scholar]
- 39.Karauzum H, Chen G, Abaandou L, Mahmoudieh M, Boroun AR, Shulenin S, Devi VS, Stavale E, Warfield KL, Zeitlin L, Roy CJ, Sidhu SS, Aman MJ. 2012. Synthetic human monoclonal antibodies toward staphylococcal enterotoxin B (SEB) protective against toxic shock syndrome. J. Biol. Chem. 287:25203–25215. 10.1074/jbc.M112.364075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Larkin EA, Stiles BG, Ulrich RG. 2010. Inhibition of toxic shock by human monoclonal antibodies against staphylococcal enterotoxin B. PLoS One 5:e13253. 10.1371/journal.pone.0013253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Varshney AK, Wang X, Cook E, Dutta K, Scharff MD, Goger MJ, Fries BC. 2011. Generation, characterization, and epitope mapping of neutralizing and protective monoclonal antibodies against staphylococcal enterotoxin B-induced lethal shock. J. Biol. Chem. 286:9737–9747. 10.1074/jbc.M110.212407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tilahun ME, Kwan A, Natarajan K, Quinn M, Tilahun AY, Xie C, Margulies DH, Osborne BA, Goldsby RA, Rajagopalan G. 2011. Chimeric anti-staphylococcal enterotoxin B antibodies and lovastatin act synergistically to provide in vivo protection against lethal doses of SEB. PLoS One 6:e27203. 10.1371/journal.pone.0027203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tilahun ME, Rajagopalan G, Shah-Mahoney N, Lawlor RG, Tilahun AY, Xie C, Natarajan K, Margulies DH, Ratner DI, Osborne BA, Goldsby RA. 2010. Potent neutralization of staphylococcal enterotoxin B by synergistic action of chimeric antibodies. Infect. Immun. 78:2801–2811. 10.1128/IAI.01121-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Varshney AK, Wang X, Scharff MD, MacIntyre J, Zollner RS, Kovalenko OV, Martinez LR, Byrne FR, Fries BC. 2013. Staphylococcal enterotoxin B-specific monoclonal antibody 20B1 successfully treats diverse Staphylococcus aureus infections. J. Infect. Dis. 208:2058–2066. 10.1093/infdis/jit421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fox JL. 2013. Anthrax drug first antibacterial mAb to win approval. Nat. Biotechnol. 31:8. 10.1038/nbt0113-8 [DOI] [PubMed] [Google Scholar]
- 46.Storch GA. 1998. Humanized monoclonal antibody for prevention of respiratory syncytial virus infection. Pediatrics 102:648–651. 10.1542/peds.102.3.648 [DOI] [PubMed] [Google Scholar]