Abstract
Chagas disease is one of the main public health issues in Latin America. Increasingly during the past few decades, Trypanosoma cruzi infection has been detected in North America, Europe, and the Western Pacific, mainly as a result of population movement. The limited availability of rapid serological diagnostic tests hinders rapid diagnosis and early treatment in areas of endemicity and nonendemicity. In collaboration with 11 national reference laboratories (NRLs) from different geographical areas, we evaluated the performances of commercialized serological rapid diagnostic tests (RDT) for T. cruzi infection. Eleven commercialized T. cruzi infection RDTs were evaluated on a total of 474 samples extensively tested with at least three different techniques for Chagas disease, maintained at controlled low temperatures, and stored in the serum banks of the 11 NRLs. We measured the sensitivity, specificity, and concordance of each RDT and provided an additional questionnaire to evaluate its ease of use. The selected RDTs in this study were performed under controlled laboratory conditions. Out of the 11 RDTs, we found 8 of them to be useful, with the cassette format favored over the strip. We did not observe significant differences in RDT performances in the different regions. Overall, the performance results were lower than those disclosed by the manufacturers. The results of this evaluation validate the possibility of using RDTs to diagnose Chagas disease, thereby decreasing the time to treatment at a primary health care facility for patients who are willing to be treated. Further studies should be conducted in the laboratory and in the field to confirm these data, expressly to evaluate reproducibility in resource-limited settings, or using whole blood in clinical settings in areas of endemicity and nonendemicity.
INTRODUCTION
Chagas disease is one of the main public health problems in Latin America. It is estimated that between 8 and 11 million people (see http://www.cdc.gov/chagas) have the disease and another 100 million are at risk of acquiring the disease (1). In recent decades, mainly due to population movements, this public health challenge has spread and is no longer limited to Latin America (2, 3); cases can also be found in the United States (4), Canada, Europe, and the Western Pacific region, mainly in Japan and Australia (5–10).
Diagnosis during the chronic phase is performed by detecting circulating Trypanosoma cruzi-specific IgG antibodies. Many serodiagnostic tests, based on different principles and immunological targets, such as enzyme-linked immunosorbent assay (ELISA) (11, 12), indirect immunofluorescence assay (IFA) (13, 14), indirect hemagglutination assay (IHA) (15), or chemiluminescent assays (ChLIA) (16), are available for laboratory diagnosis of Chagas disease.
Such laboratory tests require qualified staff, specific equipment, and infrastructure, which is either unaffordable or unavailable in most regions where the disease is endemic. Therefore, the lack of access to diagnosis is one of the main obstacles to initiating treatment for Chagas disease (17).
Currently, several rapid diagnostic tests (RDTs) to detect T. cruzi infection using whole blood, serum, or plasma samples are commercially available. Few of these tests have been evaluated independently (not by the manufacturers). RDTs may be either qualitative or semiquantitative and are characterized by the delivery of quick results without the need for electrical equipment. In general, these tests rely on different test principles: immunochromatography, particle agglutination, immunofiltration, or immunodot.
Since 2007, in various forums, scientists and governmental and nongovernmental organizations have expressed the urgent need for new and simplified diagnostic tools, ideally through RDTs, to increase the diagnosis of T. cruzi infection/Chagas disease and decrease the underdiagnosis in remote areas where the diagnosis is not accessible by conventional techniques. These RDTs must be inexpensive and not require skilled laboratory staff, external equipment, or refrigeration (18–21).
These tests also must be highly sensitive, specific, and easy to use (22). The need for highly sensitive and specific RDTs was reiterated in 2010 when the 63rd World Health Assembly outlined, through its resolution WHA63.20, the need “to promote and encourage operational research on control of Chagas disease in order to establish systems of early detection” and “to integrate, at the primary health care level, diagnosis and treatment of Chagas disease in patients in both acute and chronic phases of the disease,” in countries that are endemic and nonendemic for the disease (23).
In recent years, several RDTs have been developed. However, to date, no independent performance evaluations have been conducted in different geographical areas. Therefore, in mid-2010, the WHO and Médecins Sans Frontières (MSF) Technical Group III “Diagnostic Chagas Disease” decided to coordinate a multicenter study to evaluate the performances of all available commercialized RDTs using existing serum banks in national reference laboratories (NRLs) in different geographical areas.
MATERIALS AND METHODS
Selection of RDTs.
For inclusion in the study, a commercialized RDT had to meet some specific criteria. The test had to: be defined as a rapid test, be intended for routine diagnosis of T. cruzi infection (not restricted to research use) by the manufacturer, generate results on the same day, not require a reader or other sophisticated instrumentation, be available for immediate purchase, and be manufactured with recognized quality certificates, such as CE mark, FDA-cleared, or manufactured with ISO accreditation (24).
A systematic review was conducted, and a total of 15 RDTs were found to be available from 14 different manufacturers. After contacting the respective manufacturers, 1 test was excluded from the list due to it being research use only, and 3 RDTs were no longer produced by the manufacturers, resulting in a final list of 11 RDTs used in this study (Table 1).
TABLE 1.
List of commercial rapid diagnostic tests for the serological detection of T. cruzi (Chagas disease) used in this study
| Test no. | Name | Manufacturer (country) | Formata | Storage temp (°C) | Sample typeb | Sample vol (μl) | No. or vol of buffer drops added | Reading time (min) | Additional material required? (i.e., pipette) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | OnSite Chagas Ab Rapid test | CTK Biotech (United States) | IC/cassette | 2–30 | WB | 40–50 | 1 | 15 | Yes |
| S/P | 20 | 2 | |||||||
| 2 | WL Check Chagas | Wiener Lab (Argentina) | IC/cassette | 2–30 | WB-S/P | 40 | 3 | 25–35 | Yes |
| 3 | Chagas Instantest | Silanes (Mexico) | IC/cassette | 2–30 | S/P | 10 | 3–4 | 15–25 | Yes |
| 4 | Trypanosoma Detect Rapid Test | InBios, Inc. (United States) | IC/strip | 2–28 | WB | 20 | 3–4 | 10–15 | Yes |
| S | 10 | ||||||||
| 5 | Chagas Quick Test | Cypress Diagnostic (Belgium) | IC/strip | 2–28 | WB | 20 | 3–4 | 10–15 | Yes |
| S | 10 | ||||||||
| 6 | Chagas Stat-Pak assay | Chembio (United States) | IC/cassette | 8–30 | WB | 10 | 6 | 15 | No |
| S/P | 5 | ||||||||
| 7 | Immu-Sure Chagas (T. Cruzi) | Millennium Biotech (United States) | IC/cassette | 8–30 | WB | 10 | 6 | 5–25 | Yes |
| S/P | 5 | ||||||||
| 8 | SD Chagas Ab Rapid | Standard Diagnostic (Korea) | IC/cassette | 1–30 | WB-S/P | 100 | None | 15 | Yes |
| 9 | Simple Chagas WB | Operon (Spain) | IC/cassette | 8–25 | WB | 25 | 100 μl | 10–15 | No |
| S/P | 1:15 dilution or alternativec | Yes | |||||||
| 10 | Serodia Chagas | Fujirebio, Inc. (Japan) | Agglutination card | 2–8 | S/P | 25 μl (prediluted) | Multiple reagent/bufferd | 120 | Yes |
| 11 | ImmunoComb II Chagas Ab | Orgenics (Israel) | IE | 2–8 | S/P | 10 | Multiple reagent/bufferd | 60 | Yes |
IC, immunochromatographic assay; IE, immunoenzymatic assay.
WB, whole blood; S/P, serum or plasma.
The manufacturer said to use a 1:15 dilution, but an example in the instructions said “Add 10 μl buffer to 140 μl of sample.” One recommendation was used in half of the tests, and the other recommendation was used in the other half of the tests.
These tests required multiple dilutions with buffer.
All the RDTs were bought by MSF-Logistique, based in Bordeaux (France). For each NRL, MSF-Logistique prepared two parcels containing the RDTs and a temperature-monitoring device. All packages were sent to their destination using a commercial shipping service and received at the NRLs from November 2011 up to June 2012.
Identification of the national reference laboratories.
The NRLs that participated in the study were located in countries of endemicity and nonendemicity and were representative of all geographical areas from where Chagas disease is reported. NRLs from countries of endemicity (vectorial transmission) in Central and South America included laboratories in Argentina, Brazil, Colombia, Costa Rica, and Mexico. The participating NRLs from countries of nonendemicity (nonvectorial transmission) included laboratories in North America (United States), Europe (France and Spain), and the Asia-Pacific region (Japan).
Each NRL also met the following selection criteria: the laboratory had to be recognized within the country as the national reference for Chagas disease diagnosis, have a serum bank available (with positive- and negative-confirmed Chagas samples), conduct regular internal and/or external quality controls, not present any conflict of interest at the time of the study, and follow standard good laboratory practices and ethical procedures, according to the national requirements. The study was conducted in a total of 10 NRLs from 9 different countries, in –areas of nonendemicity (in Japan, Central Blood Institute, Blood Service HQ, the Japanese Red Cross Society; in France, Service de Parasitologie-Mycologie, Groupe Hospitalier Pitié-Salpêtrière; in Spain, Servicio de Parasitología, the Instituto de Salud Carlos III; United States, Division of Parasitic Diseases and Malaria, Centers for Disease Control and Prevention, Transmissible Diseases Department, American Red Cross Holland Laboratory) and endemicity (in Argentina, Departamento de Diagnóstico, Instituto Nacional de Parasitología Dr. Mario Fatala Chabén; in Brazil, Laboratório de Pesquisa da Doença de Chagas, Hospital das Clínicas, Universidade Federal de Goiás; in Colombia, Grupo de Parasitología, Subdirección Red Nacional de Laboratorios, Instituto Nacional de Salud; in Costa Rica, Centro Nacional de Referencia en Parasitología, INCIENSA; and in Mexico, Departamento de Parasitología, Instituto de Diagnóstico y Referencia Epidemiológicos).
An additional laboratory, the Programa Nacional Controle de Qualidade (PNCQ) in Brazil, provided a lyophilized standard control panel for Chagas disease to be used in the 10 NRLs. The PNCQ is an accredited laboratory that specializes in quality control programs for clinical laboratories, blood bank services, and research units in Latin America and Europe.
Sample selection.
The study samples were selected from the existing serum bank at each NRL. All samples were stored in the serum bank at each NRL, were thawed with no additional preservatives, and were previously tested for T. cruzi infection using two conventional tests (ELISA/IHA/IFA), as recommended by the WHO.
The samples were selected using a randomized numbers method. The average of the samples processed were 25 samples confirmed positive for T. cruzi infection and 25 samples that were proven negative. The selected positive samples were categorized according to reactivity as low reactivity (optical density greater than the cutoff between 10% and 30%) and medium reactivity (optical density greater than the cutoff between 30% and 60%) using the data available in the corresponding NRL.
Quality control.
Each NRL received, in addition to the RDTs, a panel of 4 lyophilized quality control (QC) samples prepared by the PNCQ laboratory in Brazil, with unknown results. This panel contained instructions on how to reconstitute, as well as the blinded vials. The QC samples were reconstituted and processed according to the PNCQ instructions.
RDT testing.
The RDTs were used according to each manufacturer's instructions. The same tests were evaluated for all 11 RDTs in each NRL.
Ease-of-use assessment.
At the end of the study, the working group was asked to complete a questionnaire to assess their experiences with the respective tests. The questionnaire was adapted from a conventional format used in several similar studies led by the WHO/Foundation for Innovative New Diagnostics [FIND]/MSF/Epicentre from 2001 (25, 26). Each questionnaire item was scored according to the instructions in the tool, with a maximum score of 52. An average score was calculated for each parameter from the scores submitted by the NRLs.
Statistical methods.
The data were analyzed in aggregate rather than by each participating laboratory. Once completed, the results of the analyses were shared and discussed with all participating NRLs. The validity of each RDT was determined by its sensitivity and specificity (27), which measured the capacity of the test correctly to identify healthy (true-negative) or infected (true-positive) individuals. The definitions of positive or negative for each NRL were used to define true-positive and true-negative samples, as well as for calculations of sensitivity and specificity. The degree of agreement with the NRL expected results and RDT results was assessed by the kappa test (28, 29).
RESULTS
Assay performance.
A total of 474 samples (424 serum and 50 plasma samples) previously tested for T. cruzi infection following two conventional tests (ELISA/IHA/IFA), as recommended by the WHO, were evaluated from areas of endemicity and nonendemicity. Among them, 46% were women, 42% were men, and 12% had no information. In relation to the country of origin of the patients, 42% of the samples were from Bolivia, followed by 18% from Brazil, 13% from Mexico, 10% from Colombia, 7% from Argentina, 9.8% from Costa Rica, and 0.2% from Nicaragua.
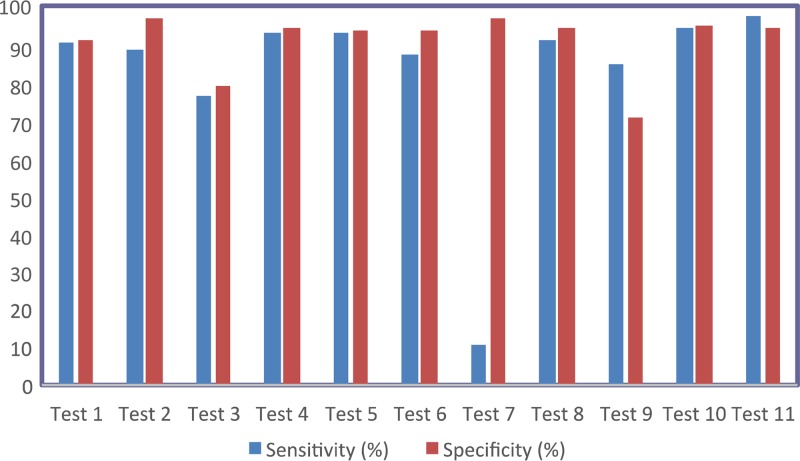
Based on the total number of samples testing positive or negative as defined by the NRLs, the sensitivities and specificities of the rapid diagnostic tests were calculated (Table 2 and Fig. 1). Kappa-type statistics were also calculated to compare the agreement between the actual and expected results. The tests were stratified into groups based on the relative strength of agreement associated with the kappa statistics (Table 3). Sensitivity and specificity were very high with tests 10 and 11, followed by tests 8, 4, 5, and 2.
TABLE 2.
Sensitivities and specificities of tests in serum
| Test no. | Sensitivity (%) | Specificity (%) | Kappa value |
|---|---|---|---|
| 1 | 90.1 | 91 | 0.81 |
| 2 | 88.7 | 97 | 0.85 |
| 3 | 76.6 | 79 | 0.56 |
| 4 | 92.9 | 94 | 0.87 |
| 5 | 92.9 | 93.2 | 0.86 |
| 6 | 87.2 | 93.2 | 0.80 |
| 7 | 10.6 | 97 | 0.07 |
| 8 | 90.7 | 94 | 0.85 |
| 9 | 84.9 | 70.7 | 0.56 |
| 10 | 94.2 | 94.7 | 0.89 |
| 11 | 97.2 | 94 | 0.91 |
FIG 1.
Sensitivity (blue) and specificity (red) by test for each of the 11 commercialized T. cruzi detection tests.
TABLE 3.
RDT classification by kappa value
| Kappa value | Category | RDTs |
|---|---|---|
| 0.00–0.20 | Poor | 7 |
| 0.21–0.40 | Slight | None |
| 0.41–0.60 | Moderate | 3, 9 |
| 0.61–0.80 | Substantial | 6 |
| 0.81–1.00 | Almost perfect | 1, 2, 4, 5, 8, 10, 11 |
We tested 50 plasma samples; the results showed lower values of sensitivity and specificity than the results found in the serum samples. However, additional studies will be necessary to confirm these results.
Quality control results and validation of performance results.
The panel of four lyophilized serum samples contained one sample that was nonreactive for Chagas disease, two samples reactive for Chagas disease, and one sample that was either positive or negative (randomly chosen by PCNQ). Only the coordinator of the study knew the results of the panel in each national reference laboratory, and this was used to monitor the quality of the results and confirm the performance data of the RDTs.
The agreement of expected results obtained with the QC panel from PNCQ varied from 100% for the Trypanosoma Detect Rapid test, Chagas Stat-Pak, Chagas Quick test, SD-Bioline Chagas Ab Rapid test, and Serodia-Chagas tests, to ≤25% for the Chagas-Instantest, Immu-Sure Chagas, and Simple Chagas WB tests. These results are consistent with the level of agreement seen with the NRL sera (Table 4).
TABLE 4.
Quality control results
| Country | Agreement (%) by test noa: |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |
| Costa Rica | 100 | 100 | 25 | 100 | 100 | 100 | 25 | 100 | 100 | 100 | 100 |
| Spain | 100 | 100 | 75 | 100 | 100 | 100 | 0 | 100 | 25 | 100 | 100 |
| France | 100 | 100 | 75 | 100 | 100 | 100 | 50 | 100 | 25 | 100 | 100 |
| Colombia | 100 | 100 | 75 | 100 | 100 | 100 | 25 | 100 | 75 | 100 | 100 |
| Mexico | 100 | 100 | 50 | 100 | 100 | 100 | 50 | 100 | 75 | 100 | 100 |
| Brazil | 100 | 100 | 50 | 100 | 100 | 100 | 25 | 100 | 25 | 100 | 100 |
| United States (ARCb) | 75 | 75 | 50 | 100 | 100 | 100 | 50 | 100 | 100 | 100 | 50 |
| Japan | 100 | 100 | 75 | 100 | 100 | 100 | 25 | 100 | 100 | 100 | 100 |
Interpretation: 100%, agreement with 4 samples; 75%, agreement with 3 samples; 50%, agreement with 2 samples; 25%, agreement with one sample.
ARC, American Red Cross.
Ease-of-use evaluation.
The technical staff responsible for performing testing for the study completed an ease-of-use questionnaire for each RDT in the study. The summary of the scores for each RDT is illustrated in Fig. 2. The Trypanosoma Detect Rapid test scored the highest, with 43.89 points on average, out of 52 possible points.
FIG 2.
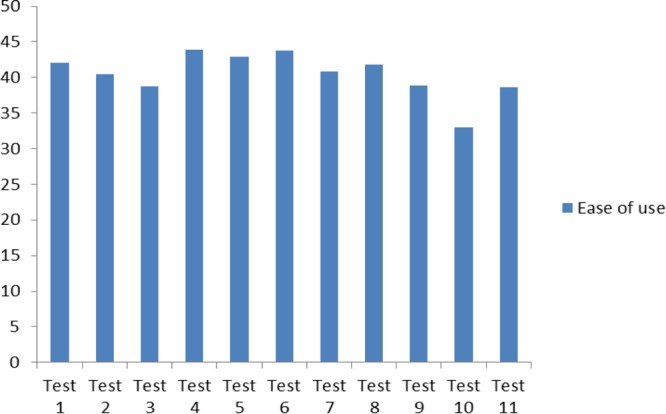
Ease-of-use score (of a possible total of 52 points) for each test.
A separate analysis of the individual criteria did not reveal any additional significant findings, but some specific observations can be shared. The evaluators agreed that the SD-Bioline Chagas Ab Rapid test was the simplest to use, as it is a single-step test. The most complex tests were determined to be the Serodia-Chagas and the ImmunoComb II Chagas Ab kit, both with procedures of ≥3 steps. The Chagas Stat-Pak test required the lowest volume (10 μl) and the SD-Bioline Chagas Ab Rapid test the most (100 μl). Nine of the 11 RDTs did not require cold chain for storage and included all necessary materials with the kit. The Serodia-Chagas test and the ImmunoComb II Chagas Ab kit required maintenance of a cold chain, and both required numerous additional materials not provided in the kit. Similarly, the time to results was <20 min for nine of the 11 RDTs; the Serodia-Chagas tes and the ImmunoComb II Chagas Ab kit required >1 h to complete.
Generally, the tests in cassette format received higher scores than the ones prepared as strips. The quality of the manufacturer's instructions was generally good for all RDTs, with the exception of the Simple Chagas WB test, which received the lowest score for the quality of the instructions in its kit, mainly due to confusion about the instructions for sample preparation prior to testing. After reviewing these findings, it was evident that the Serodia-Chagas and the ImmunoComb II Chagas Ab kit tests have characteristics that do not comply with the conventional definition of a rapid diagnostic test; these tests would be better considered semirapid.
Validity of results.
The validity of the results, according to the criteria defined by the manufacturer, is determined by the appearance of the control line and the sample line. In tests 3 and 7, this control line often did not appear, which resulted in a high level of invalid tests (Table 5). Another difficulty encountered in these tests was the appearance of faint lines, making the results difficult to read.
TABLE 5.
Invalid and repeat results of RDTs
| Test no. | Total no. of samples | No. of results that were: |
|||
|---|---|---|---|---|---|
| Positive | Negative | Invalid | Repeated | ||
| 1 | 474 | 225 | 249 | 0 | 0 |
| 2 | 474 | 213 | 261 | 0 | 0 |
| 3 | 474 | 221 | 222 | 28 | 50 |
| 4 | 474 | 221 | 253 | 0 | 0 |
| 5 | 474 | 216 | 258 | 0 | 0 |
| 6 | 474 | 209 | 263 | 0 | 2 |
| 7 | 474 | 44 | 419 | 61 | 50 |
| 8 | 474 | 233 | 240 | 1 | 0 |
| 9 | 424 | 238 | 184 | 0 | 2 |
| 10 | 474 | 230 | 241 | 0 | 3 |
| 11 | 474 | 241 | 233 | 90 | 107 |
Analysis of performance by region.
We compared the sensitivity and specificity values in 6 regions among all NRLs that processed serum samples to determine if there were differences in test performance according to geographical area, as well as to give some indication about the suitability for use of these tests in particular regions. For this particular analysis, we did not find a statistical difference between the values of sensitivity and specificity between the regions. However, in areas of nonendemicity (Europe and the United States), the NRLs in these areas found similar performances of the RDTs; the Serodia-Chagas test, Trypanosoma detect Rapid test, and Chagas Quick test gave the best results. The SD-Bioline Chagas Ab Rapid test had the best performance results of the NRLs in Asia.
In areas of endemicity, the performances of the tests varied but were generally >90%. The Serodia-Chagas test and ImmunoComb II Chagas Ab kit had higher performance results, followed by those of the SD-Bioline Chagas Ab Rapid test.
DISCUSSION
The RDTs provide the opportunity to give reliable and accurate results depending on performance (sensitivity and specificity results). In recent years, the use of rapid tests to diagnose infectious diseases has increased (e.g., for malaria and dengue) (30, 31). The RDTs for Chagas disease provide an opportunity to give rapid, reliable, and accurate results if the test used is sensitive and specific.
This study represents the first multicenter study evaluation of all commercially available RDTs for Chagas disease. In this study, conducted in NRLs in areas of endemicity and nonendemicity, the sensitivity and specificity results were lower than those reported in similar previous studies or those reported by the respective manufacturers.
Some specific technical problems were noted with the various tests. The Chagas-Instantest was difficult to interpret due to the presence of a faint pink line on a reddish background. Out of the 474 tests performed, 28 were invalid (absence of control line), which represented >5%. More than 50% of the positive samples or positive controls tested with the Immu-Sure Chagas test were negative.
The manufacturer's instructions for preparing the sample prior to testing in the Simple Chagas WB (Operon) test were not clear. The manufacturer recommends diluting the serum sample 1:15, but the volumes described in the instructions did not correspond to the correct dilution. The instructions stated, “add 10 μl of buffer to 140 μl of sample.” During the study, 50% of the NRLs processed this test using a correct dilution process, and the second half followed the manufacturer's recommended dilution. There was a significant difference between the 2 methods in terms of sensitivity and specificity. Sensitivity increased in the group that used the quantity of blood and buffer mentioned by manufacturer, but specificity was decreased. Assay sensitivity was decreased in the group that made a true 1:15 dilution, but specificity increased. Operon needs to revise the total procedure and modify the instructions accordingly.
The shipping and delivery times for the RDTs were variable due to customs clearance procedures; the average number of days of delivery for North America and Europe was 2 days. The average time to receipt in the Central American, Andean region, and Brazilian NRLs was 30 days. In Argentina, the RDTs were held in customs for >90 days, mainly due to changes in importation procedures. Despite the 90-day delay in Argentina, the tests remained stored according to the manufacturer's instructions, and no break in the cold chain occurred during this period.
In this study, the sensitivity and specificity of the Chagas Stat-Pak test using serum samples were 87% and 95%, respectively, which is less than those reported in 6 independent studies conducted between 2003 and 2010 (26, 32–37). The performance data for the Trypanosoma Detect Rapid test (93.5% sensitivity and 95.2% specificity) are close to the data reported in 2010 by Reithinger et al. (38) and in other similar studies (39). The SD-Bioline Chagas Ab Rapid test and the OnSite Chagas Ab Rapid test-cassette results in serum were low compared to those reported in 2009 (40).
Different studies evaluating the performance of the Simple Chagas WB test (Operon) showed results that were contradictory to the results found in this study. The sensitivity of the Simple Chagas WB test ranged from 50% to 100%, as reported by Flores-Chávez et al. in 2012 (44; also see 41–43). This range may be due to different interpretations of the manufacturer's instructions, as mentioned above, or a different batch of reactive that was used in this evaluation.
The generally lower levels of sensitivity and specificity seen in this study compared to those in previous reports or manufacturer's claims may be due to the nature of the samples used in the current evaluation. The reactivities of most of the samples were classified as medium or low, and most were banked serum samples that were an average of 2 years old. Additionally, each NRL likely used different assays to determine positivity, which impacts the classification of samples as medium or low, leading to further variability. Another possible explanation for the differences in the test performances observed here may be related to antigenic variability in different regions in which Chagas disease is transmitted. These possible antigenic differences may result in the production of some antibodies that may not be detected equally by all of the RDTs evaluated here.
Moreover, we observed an important difference in performance according to the type of sample used. Plasma samples gave much lower values in this study than those reported by the manufacturers or than those with serum samples. However, only 50 plasma samples were evaluated in this study, so these data need to be confirmed using a larger sample size.
Although the ImmunoComb II Chagas Ab kit and the Serodia-Chagas test demonstrated excellent sensitivities (>95% using serum samples), these two tests, despite being defined as rapid by the manufacturers, were more complicated than immunochromatographic tests. The ImmunoComb II Chagas Ab kit was more similar to an ELISA, and the Serodia-Chagas test was an agglutination test. Also, additional criteria, such as reading time or the length of procedure and ease of use, indicate that the two tests do not meet the conventional definition of RDTs, and their implementation requires laboratory resources. For all those reasons, these two tests should therefore be classified as semirapid diagnostic tests. A rapid test for Chagas disease should include all tests for which results can be delivered to the patient within 1 h after sample collection, and the semirapid test the ones in which result can be 2 to 3 h after sample collection.
Excluding the semirapid tests mentioned above, the 6 RDTs produced in cassette or strip formats had the best performances and the highest scores in the ease-of-use questionnaire. The six are the Trypanosoma Detect Rapid test, SD-Bioline Chagas Ab Rapid test, the OnSite Chagas Ab Rapid test-cassette, the WL Check Chagas test, the Chagas Quick test, and the Chagas Stat-Pak test. These results are similar to those in the study by Roddy and contributors in 2008 (26). These tests can be recommended for screening and surveillance in areas of endemicity and nonendemicity, but the results should be confirmed in a reference laboratory.
The choice of tests to be used will depend on the context and resources available. Semirapid tests need a minimum degree of infrastructure and may be considered for use in areas of endemicity and nonendemicity where laboratory facilities are readily available. Rapid tests can be used anywhere (i.e., in all regions) and are especially recommended in primary health care sectors in which laboratory facilities are scarce or nonexistent, as well as for epidemiological surveillance programs or studies.
The use of quality control (QC) samples was very important in this study because the results further identified tests with variable performance in the different laboratories around the world that obtained similar results with the QC samples. We recommend this kind of QC measure to improve the comparability between labs.
In the present study, we evaluated the performances of 11 commercialized RDTs. Out of 11 RDTs, 8 were considered valuable for use. The performance results are lower than those disclosed by the manufacturers. This study sought to test the selected RDTs under controlled laboratory conditions. To confirm the present data, especially the reproducibility in limited-resource settings, further studies should be conducted in various laboratory and field or clinic settings in areas of endemicity and nonendemicity using whole-blood samples. The results of this evaluation demonstrate the value of using RDTs to help diagnose Chagas disease and thereby improve access to treatment as soon as possible, starting at the primary health care level. At present, diagnosis is made using conventional tests in reference laboratories and the results might be ready in ≥1 month in most of the laboratories.
ACKNOWLEDGMENTS
We thank all the institutions/laboratories that participated in this study, and we especially thank MSF-Logistique (Bordeaux) for the purchase, packing, and transport of RDTs to the sites. We deeply appreciate all the laboratory personnel in the technical group who processed and tested the samples in the respective centers: Maria Flores-Chavez, Instituto Salud Carlos III (ISCIII), Spain; Erick Campos Fuentes, Centro Nacional de Referencia en Parasitología (CNRP), INCIENSA, Costa Rica; Lyda Muñoz Galindo, Instituto Nacional de Salud (INS), Colombia; Chieko Matsumoto, Japanese Red Cross, Japan; Clothilde Quiblier and Josiane Zanoni, Hopital Pitié-Salpêtrière, France; Suelene B. do Nascimento Tavares and Rozangela Amaral Oliveira, Hospital das Clínicas, Universidade Federal de Goiás, Brazil; Sergio Pastén Sánchez, Departamento de Parasitología, Instituto de Diagnóstico y Referencia Epidemiológicos, Mexico; Megan L. Nguyen, American Red Cross Holland Laboratory, USA; Rebecca L. Townsend, American Red Cross Scientific Support Office, USA; Karina Dopacio and Flavia Maldonado, Departamento de Diagnóstico, Instituto Nacional de Parasitología Dr. Mario Fatala Chabén, Argentina; and Frank Steurer, Centers for Disease Control and Prevention (CDC), USA. We also thank Pablo Garita Rivas, Rieko Sobata, Yusuke Sayama, Sachio Miura, François-Xavier Lescure, Liliane da Rocha Siriano, Ana María De Rissio, Charles Todd, Beatriz Carmona Camps, and Juli Gilabert.
Claudia L. Sánchez-Camargo is supported by a grant from Universidad Antonio Nariño (Colombia).
Footnotes
Published ahead of print 7 May 2014
REFERENCES
- 1.Organización Panamerica de la Salud. 2006. Estimación cuantitativa de la enfermedad de Chagas en las Américas. Document OPS/HDM/CD/425-06. Organización Panamerica de la Salud, Montevideo, Uruguay: (In Spanish.) http://www.bvsops.org.uy/pdf/chagas19.pdf [Google Scholar]
- 2.Schmunis GA. 2007. Epidemiology of Chagas disease in non-endemic countries: the role of international migration. Mem. Inst. Oswaldo Cruz 102(Suppl 1):75–85. 10.1590/S0074-02762007005000093 [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. 2007. Blood donor screening for Chagas disease—United States, 2006–2007. MMWR Morb. Mortal. Wkly. Rep. 56:141–143 [PubMed] [Google Scholar]
- 4.Bern C, Montgomery SP. 2009. An estimate of burden of Chagas disease in the Unites States. Clin. Infect. Dis. 49:e52–e54. 10.1086/605091 [DOI] [PubMed] [Google Scholar]
- 5.Schmunis G, Yadon ZE. 2010. Chagas disease: a Latin American health problem becoming a world health problem. Acta Trop. 115:14–21. 10.1016/j.actatropica.2009.11.003 [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. 2010. Control and prevention of Chagas disease in Europe: report of a WHO informal consultation (jointly organized by WHO headquarters and the WHO Regional Office for Europe) Geneva, Switzerland, 17 to 18 December 2009. WHO/HTM/NTD/IDM/2010.1. http://www.fac.org.ar/1/comites/chagas/Chagas_WHO_Technical%20Report_16_06_10.pdf
- 7.Gascon J, Bern C, Pinazo MJ. Chagas disease in Spain, the United States and other non-endemic countries. Acta Trop. 115:22–27. 10.1016/j.actatropica.2009.07.019 [DOI] [PubMed] [Google Scholar]
- 8.Arandes AS, Gutierrez JM, Navarro MV, Castells Doménech C, Portús Vinyeta M, Gascón Brustenga J. 2009. Prevalence of Chagas disease in the Latin American immigrant population in a primary health centre in Barcelona (Spain). Acta Trop. 112:228–230. 10.1016/j.actatropica.2009.07.014 [DOI] [PubMed] [Google Scholar]
- 9.Muñoz J, Gómez i Prat J, Gállego M, Gimeno F, Treviño B, López-Chejade P, Ribera O, Molina L, Sanz S, Pinazo MJ, Riera C, Posada EJ, Sanz G, Portús M, Gascon J. 2009. Clinical profile of Trypanosoma cruzi infection in a non-endemic setting: immigration and Chagas disease in Barcelona (Spain). Acta Trop. 111:51–55. 10.1016/j.actatropica.2009.02.005 [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization. 2013. Sustaining the drive to overcome the global impact of neglected tropical diseases: second WHO report on neglected tropical diseases. WHO/HTM/NTD/2013.1. World Health Organization, Geneva, Switzerland [Google Scholar]
- 11.Voller A, Draper C, Bidwell DE, Bartlett A. 1975. Microplate enzyme-linked immunosorbent assay for Chagas' disease. Lancet. i:426–428 [DOI] [PubMed] [Google Scholar]
- 12.Ferreira AW, Belem ZR, Lemos EA, Reed SG, Campos-Neto A. 2001. Enzyme-linked immunosorbent assay for serological diagnosis of Chagas' disease employing a Trypanosoma cruzi recombinant antigen that consists of four different peptides. J. Clin. Microbiol. 39:4390–4395. 10.1128/JCM.39.12.4390-4395.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fife EH, Jr, Muschel LH. 1959. Fluorescent antibody technique for serodiagnosis of Trypanosoma cruzi infection. Proc. Soc. Exp. Biol. Med. 101:540–543 [DOI] [PubMed] [Google Scholar]
- 14.Camargo ME. 1966. Fluorescent antibody test for the serodiagnosis of American trypanosomiasis. Technical modification employing preserved culture forms of Trypanosoma cruzi in a slide test. Rev. Inst. Med. Trop. São Paulo;. 8:227–234 [PubMed] [Google Scholar]
- 15.Knierim F, Sandoval J, Muñoz E. 1973. Indirect hemagglutination test in chronic Chagas disease. Bol. Chil. Parasitol. 28:54–57 (In Spanish.) [PubMed] [Google Scholar]
- 16.Chang CD, Cheng KY, Jiang LX, Salbilla VA, Haller AS, Yem AW, Bryant JD, Kirchhoff LV, Leiby DA, Schochetman G, Shah DO. 2006. Evaluation of a prototype Trypanosoma cruzi antibody assay with recombinant antigens on a fully automated chemiluminescence analyzer for blood donor screening. Transfusion. 46:1737–1744. 10.1111/j.1537-2995.2006.00965.x [DOI] [PubMed] [Google Scholar]
- 17.Gomes YM, Lorena VM, Luquetti AO. 2009- Diagnosis of Chagas disease: what has been achieved? What remains to be done with regard to diagnosis and follow up studies? Mem. Inst. Oswaldo Cruz 104(Suppl 1): 115–121. 10.1590/S0074-02762009000900017 [DOI] [PubMed] [Google Scholar]
- 18.Senior K. 2007. Chagas disease: moving towards global elimination. Lancet Infect. Dis. 7:572. 10.1016/S1473-3099(07)70194-9 [DOI] [PubMed] [Google Scholar]
- 19.WHO—World Health Organization. 2007. New global effort to eliminate Chagas disease. World Health Organization, Geneva, Switzerland: http://www.who.int/mediacentre/news/releases/2007/pr36/en/index.html [Google Scholar]
- 20.Villa L, Morote S, Bernal O, Bulla D, Albajar-Vinas P. 2007. Access to diagnosis and treatment of Chagas disease/infection in endemic and non-endemic countries in the XXI century. Mem. Inst. Oswaldo Cruz 102(Suppl 1):87–94. 10.1590/S0074-02762007005000081 [DOI] [PubMed] [Google Scholar]
- 21.Basile L, Jansà JM, Carlier Y, Salamanca DD, Angheben A, Bartoloni A, Seixas J, Van Gool T, Cañavate C, Flores-Chávez M, Jackson Y, Chiodini PL, Albajar-Viñas P, Working Group on Chagas Disease 2011. Chagas disease in European countries: the challenge of a surveillance system. Euro Surveill. 16:pii=19968 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19968 [PubMed] [Google Scholar]
- 22.Médecins Sans Frontières, Campaign for Access to Essential Medicines. 2008. International meeting: new diagnostic tests are urgently needed to treat patients with Chagas disease. Rev. Soc. Bras. Med. Trop. 41:315–319. 10.1590/S0037-86822008000300020 [DOI] [PubMed] [Google Scholar]
- 23.World Health Organization. 2010. WHA 63.20. Chagas disease: control and elimination. World Health Organization, Geneva, Switzerland: http://www.who.int/neglected_diseases/mediacentre/WHA_63.20_Eng.pdf [Google Scholar]
- 24.Peeling RW, Holmes KK, Mabey D, Ronald A. 2006. Rapid tests for sexually transmitted infections (STIs): the way forward. Sex. Transm. Infect. 82(Suppl 5):v1–v6. 10.1136/sti.2006.024265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guthmann JP, Ruiz A, Priotto G, Kiguli J, Bonte L, Legros D. 2002. Validity, reliability and ease of use in the field of five rapid tests for the diagnosis of Plasmodium falciparum malaria in Uganda. Trans. R Soc. Trop. Med. Hyg. 96:254–257. 10.1016/S0035-9203(02)90091-X [DOI] [PubMed] [Google Scholar]
- 26.Roddy P, Goiri J, Flevaud L, Palma PP, Morote S, Lima N, Villa L, Torrico F, Albajar-Viñas P. 2008. Field evaluation of a rapid immunochromatographic assay for detection of Trypanosoma cruzi infection by use of whole blood. J. Clin. Microbiol. 46:2022–2027. 10.1128/JCM.02303-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Altman D, Bland JM. 1994. Diagnostic tests. 1: sensitivity and specificity. BMJ 308:1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martín M, Horna O, Nedel F, Navarro A. 2010. Fundamentos de estadística en ciencias de la salud. Servicio de Publicaciones Universidad Autónoma de Barcelona, Barcelona, Spain [Google Scholar]
- 29.Landis JR, Koch GG. 1977. The measurement of observer agreement for categorical data. Biometrics 33:159–174. 10.2307/2529310 [DOI] [PubMed] [Google Scholar]
- 30.WHO. 2011. Malaria rapid diagnostic test performance. Results of WHO product testing of malaria RDTs: round 3 (2010–2011). World Health Organization, Geneva, Switzerland: http://www.who.int/tdr/publications/documents/rdt3.pdf?ua=1 [Google Scholar]
- 31.Peeling RW, Artsob H, Pelegrino JL, Buchy P, Cardoso MJ, Devi S, Enria DA, Jeremy F, Gubler DJ, Guzman MC, Halstead SB, Hunsperger E, Kliks S, Margolis HS, Nathanson CM, Nguyen VC, Rizzo N, Vázquez S, Yoksan S. 2010. Evaluation of diagnostic tests: dengue. Nat. Rev. Microbiol. 8(12 Suppl):S30–S38. 10.1038/nrmicro2459 [DOI] [PubMed] [Google Scholar]
- 32.Luquetti AO, Ponce C, Ponce E, Esfandiari J, Schijman A, Revollo S, Añez N, Zingales B, Ramgel-Aldao R, Gonzalez A, Levin M, Umezawa ES, Franco da Silveira J. 2003. Chagas' disease diagnosis: a multicentric evaluation of Chagas Stat-Pak, a rapid immunochromatographic assay with recombinant proteins of Trypanosoma cruzi. Diagn. Microbiol. Infect. Dis. 46:265–271. 10.1016/S0732-8893(03)00051-8 [DOI] [PubMed] [Google Scholar]
- 33.Ponce C, Ponce E, Vinelli E, Montoya R, de Aguilar V, González A, Zingales B, Rangel-Aldao R, Levin MJ, Esfandiari J, Umezawa ES, Luquetti AO, da Silveira JF. 2005. Validation of a rapid and reliable test for diagnosis of Chagas' disease by detection of Trypanosoma cruzi-specific antibodies in blood of donors and patients in Central America. J. Clin. Microbiol. 43:5065–5068. 10.1128/JCM.43.10.5065-5068.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sosa-Estani S, Gamboa-León MR, del Cid-Lemus J, Althahe F, Alger J, Almendares O, Cafferata ML, Chippaux JP, Dumonteil E, Gibbons L, Padilla-Raygoza N, Schneider D, Belizán JM, Buekens P, Working Group 2008. Short report: use of a rapid test on umbilical cord blood to screen for Trypanosoma cruzi infection in pregnant women in Argentina, Bolivia, Honduras, and México. Am. J. Trop. Med. Hyg. 79:755–759 [PubMed] [Google Scholar]
- 35.Chippaux JP, Santalla JA, Postigo JR, Romero M, Salas Clavijo NA, Schneider D, Brutus L. 2009. Sensitivity and specificity of Chagas Stat-Pak test in Bolivia. Trop. Med. Int. Health 14:732–735. 10.1111/j.1365-3156.2009.02288.x [DOI] [PubMed] [Google Scholar]
- 36.Verani JR, Seitz A, Gilman RH, LaFuente C, Galdos-Cardenas G, Kawai V, de LaFuente E, Ferrufino L, Bowman NM, Pinedo-Cancino V, Levy MZ, Steurer F, Todd CW, Kirchhoff LV, Cabrera L, Verastegui M, Bern C. 2009. Geographic variation in the sensitivity of recombinant antigen-based rapid tests for chronic Trypanosoma cruzi infection. Am. J. Trop. Med. Hyg. 80:410–415 [PubMed] [Google Scholar]
- 37.Chapuis F, Mauris A, Holst M, Albajar-Viñas P, Jannin J, Luquetti AO, Jackson Y. 2010. Validation of a rapid immunochromatographic assay for diagnosis of Trypanosoma cruzi infection among Latin-American migrants in Geneva, Switzerland. J. Clin. Microbiol. 48:2948–2952. 10.1128/JCM.00774-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reithinger R, Grijalva MJ, Chiriboga RF, de Noya BA, Torres JR, Pavia-Ruz N, Manrique-Saide P, Cardinal MV, Gürtler RE. 2010. 2010. Rapid detection of Trypanosoma cruzi in human serum by use of an immunochromatographic dipstick test. J. Clin. Microbiol. 48:3003. 10.1128/JCM.02474-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lorca M, Contreras MdC, Salinas P, Guerra A, Raychaudhuri S. 2008. Evaluación de una prueba rápida para el diagnóstico de la infección por Trypanosoma cruzi en suero. Parasitol. latinoam. 63:29–33. 10.4067/S0717-77122008000100005 [DOI] [Google Scholar]
- 40.Ji MJ, Noh JS, Cho BK, Cho YS, Kim SJ, Yoon BS. 2009. Evaluation of SD Bioline Chagas Ab Rapid kit. Korean J. Lab. Med. 29:48–52 (In Korean.) 10.3343/kjlm.2009.29.1.48 [DOI] [PubMed] [Google Scholar]
- 41.Flores-Chávez M, Cruz I, Rodríguez M, Nieto J, Franco E, Gárate T, Cañavate C. 2010. Comparación de técnicas serológicas convencionales y no convencionales para el diagnóstico de la enfermedad de Chagas importada en España. Enferm. Infecc. Microbiol. Clin. 28:284–293. 10.1016/j.eimc.2009.07.005 [DOI] [PubMed] [Google Scholar]
- 42.López-Chejade P, Roca C, Posada E, Pinazo MJ, Gascon J, Portús M. 2010. Utilidad de un test inmunocromatográfico para el cribado de la enfermedad de Chagas en asistencia primaria. Enferm. Infecc. Microbiol. Clin. 28:169–171. 10.1016/j.eimc.2009.04.007 [DOI] [PubMed] [Google Scholar]
- 43.Ramos JM, Milla A, Sánchez V, Vergés M, Toro C, Gutiérrez F. 2011. Cribado por la infección de Trypanosoma cruzi en la comunidad entre inmigrantes de Paraguay y Bolivia residentes en Elche, España. Enferm. Emerg. 13(Suppl 1):61–63 [Google Scholar]
- 44.Flores-Chavez M, Cruz I, Nieto J, Gárate T, Navarro M, Pérez-Ayala A, López-Vélez R, Cañavate C. 2012. Sensitivity and specificity of an operon immunochromatographic test in serum and whole-blood samples for the diagnosis of Trypanosoma cruzi infection in Spain, an area of nonendemicity. Clin. Vaccine Immunol. 19:1353–1359. 10.1128/CVI.00227-12 [DOI] [PMC free article] [PubMed] [Google Scholar]