Abstract
In October 2001, the first disseminated biological warfare attack was perpetrated on American soil. Initially, a few clinical microbiology laboratories were testing specimens from acutely ill patients and also being asked to test nasal swabs from the potentially exposed. Soon after, a significant number of clinical microbiology and public health laboratories received similar requests to test the worried well or evaluate potentially contaminated mail or environmental materials, sometimes from their own break rooms. The role of the clinical and public health microbiology laboratory in response to a select agent event or act of bioterrorism is reviewed.
INTRODUCTION
In October 2001, on the heels of the terrorist attacks on New York City and the Pentagon, the first geographically disseminated biological warfare attack on American soil was launched and unwittingly disseminated by the U.S. Postal Service. Many local and state public health laboratories were immediately overwhelmed with requests for testing. A few clinical microbiology laboratories were testing clinical specimens from acutely ill patients and also being asked to test nasal swabs from the potentially exposed for both treatment prophylaxis and support of the concurrent epidemiological and criminal investigations. Soon after, a significant number of clinical microbiology laboratories across the country received similar requests to test the worried well or evaluate potentially contaminated mail or environmental materials, sometimes from their own break rooms. Many laboratory directors were informally consulted for interpretation of environmental cultures for epidemiological surveillance and return-to-work decisions. In addition, many were called upon by law enforcement for diagnostic and interpretive services in the ensuing criminal investigations. In very short order, microbiologists across the country were being called upon to advise and provide laboratory services in a scenario heretofore popularized in novels and movies. In a conundrum that only a microbiologist could appreciate, the tragedy of this event provided many in our profession a once-in-a-lifetime opportunity to respond, lead, innovate, and communicate science to the public.
BRIEF CHRONOLOGY
As the 20th century drew to a close, the published literature on biological warfare and associated clinical presentation was historically expansive (1, 2). More-recent literature had increased understanding of specific microorganisms, soon to be described as select agents (3, 4). Gaps in the technical literature existed due to concerns over proprietary techniques and national security interests in the international community. But a biological attack on U.S. soil had not yet been perpetrated, and requests for resources to prepare for such a scenario were thought to be overstated. As the 1990s drew to a close, federal funding for emergency preparedness was not a popular alternative to competing domestic budget priorities for the “peace dividend” from the U.S. military drawdown in Europe. Federal laboratories in the Department of Defense (DOD) and the Department of Health and Human Services (HHS) and, to some small extent, at the U.S. Department of Agriculture (USDA) and the Department of Justice (DOJ) were surviving through mission-specific direct-line budget appropriations and research grants. In 1996, limited public health laboratory funding resulted from the Metropolitan Medical Response System (MMRS), which was part of a series of initiatives to prepare for the possibility of a domestic terrorist attack with a weapon of mass destruction (WMD). In 1999, a nascent public health Laboratory Response Network (LRN) received its first federal funding (5). The events of 2001 brought public visibility to the national infrastructure of emergency preparedness and led to legislative focus and budget review. Ultimately, funding was directed to state and federal laboratories for assay development, reagents and equipment, personnel, and new or renovated facilities to increase levels of biosafety containment.
LOOKING BACK
A tale of two cities.
On 3 October 2001, the Florida Department of Health Bureau of Public Health Laboratories (BPHL) in Jacksonville received a specimen from a commercial laboratory in southern Florida that had isolated an organism from a patient with meningitis and could not rule out Bacillus anthracis (6) (see Table 1.). Traditional phenotypic techniques were required to confirm B. anthracis because molecular techniques, although recently validated and available at federal laboratories, had not been implemented at the reference laboratory level for identification at that time. The BPHL confirmed the isolate to be B. anthracis on 4 October 2001. The next day, the Federal Bureau of Investigation (FBI) and Centers for Disease Control and Prevention (CDC), with assistance from the BPHL, collected environmental samples from places that the index patient had visited in the 60 days prior to the onset of symptoms. Of 56 samples, two grew B. anthracis; neither sample was from a potentially natural source (7). It was a pivotal moment in the investigation because it pointed toward an intentional release of a biological terrorism agent.
TABLE 1.
A week in the life of the Laboratory Response Network (Florida)
| Date in 2001 | Eventa | Location |
|---|---|---|
| Monday 1 October | 73-year-old man hospitalized with a respiratory infection | A Miami-Dade County hospital |
| Tuesday 2 October | 63-year-old man (index patient) hospitalized with symptoms of meningitis | A Palm Beach County hospital |
| Cerebrospinal fluid and blood collected for microbiological analysis from index patient | A Palm Beach County hospital | |
| Index patient microbiology results: from CSF, WBC, 4,750/mm3, RBC, 1,375/mm3, glucose, 57 mg/dl, protein, 666 mg/dl; from blood culture, Gram-positive bacillus, nonhemolytic on 5% sheep blood agar, nonmotile; unable to rule out Bacillus anthracis | A commercial laboratory in Fort Lauderdale | |
| Wednesday 3 October | Index patient isolate forwarded to reference laboratory | Florida Bureau of Public Health Laboratories—Jacksonville |
| Thursday 4 October | Index patient isolate confirmed to be Bacillus anthracis and reported to CDC | Florida Bureau of Public Health Laboratories—Jacksonville |
| Index patient isolate flown to CDC | Centers for Disease Control and Prevention, Atlanta, GA | |
| Friday 5 October | Index patient dies of inhalational anthrax | A Palm Beach County hospital |
| 56 samples (44 nonworkplace samples and 12 workplace samples) obtained for culture collected by Federal Bureau of Investigation (FBI) and CDC Epidemic Intelligence Service (EIS) | Environmental sampling from workplace and locations visited by index patient in the 60 days prior to onset of symptoms | |
| Sunday 7 October | Of the 56 samples collected 5 October 2001, B. anthracis was isolated from two, the index patient's workplace computer keyboard and the index patient's mailbox in the company mailroom, confirming suspicion that this represents an intentional release of an agent of biological terrorism | Florida Bureau of Public Health Laboratories—Lantana/Miami |
| A nasal swab sample collected 5 October 2001 from a patient hospitalized since 1 October, the 2nd case patient, who was a coworker of the index patient at American Media Inc. (AMI), was tested; microbiology results confirmed that the isolate was B. anthracis | Florida Bureau of Public Health Laboratories—Miami |
WBC, white blood cells; RBC, red blood cells.
Seven hundred miles to the north, on 15 October 2001, a letter was opened in the U.S. Senate Hart Building office of Senator Tom Daschle (see Table 2.) A white powdery substance disseminated rapidly through the 2-story office complex. After a rapid test was positive for B. anthracis, the heating, ventilation, and air conditioning (HVAC) system was shut down and a hot-zone investigation was initiated to determine the extent of human exposure and environmental contamination. Swabs were collected from the anterior nares of office personnel and first responders and forwarded to the clinical microbiology laboratories at the National Naval Medical Center (NNMC) in Bethesda, MD. All personnel on the 6th floor office that were present when the letter was opened, and 5 of 5 first responders to the 6th floor office, tested presumptively positive for B. anthracis by conventional culture. The letter and presumptively positive cultures were confirmed at the United States Army Medical Research Institute for Infectious Diseases (USAMRIID; Ft. Detrick, MD) by culture, PCR, and gamma phage analysis.
TABLE 2.
A week in the life of the Laboratory Response Network (National Capital region, Washington, DC)
| Date or week in 2001 | Eventa | Location |
|---|---|---|
| Monday 15 October | Letter opened in Senator Tom Daschle's office | U.S. Senate Hart Building, Washington, DC |
| National Capitol Police first responders deploy and report rapid test positive for anthrax | ||
| Office of the Attending Physician for Congress notified; initial material sent for culture | ||
| Tuesday 16 October | Establish hot zone; 6,000 nasal and environmental swabs submitted for culture over the next 72 h | U.S. Senate Hart Building, Washington, DC |
| Positive results of initial cultures characteristic of B. anthracis | National Navy Medical Center (NNMC), Bethesda, MD | |
| B. anthracis confirmed by culture, PCR, and gamma phage testing | USAMRIID, Ft. Detrick, MD | |
| Wednesday 17 October | Senate leadership is informed of 31 presumptively positive cultures | U.S. Senate Hart Building, Washington, DC |
| Laboratory consortium informally constituted in National Capital region (NCR) | NNMC (Bethesda, MD), WRAMC (DC), NIH (Bethesda, MD), AFIP (DC) | |
| Thursday 18 October | Resource and management planning: staffing, supply, containment (biosafety and biosecurity), information management, law enforcement | Walter Reed Army Medical Center (WRAMC), Washington, DC |
| Friday 19 October | 1,900 nasal swab cultures received and processed in clinical microbiology BSL-3 laboratories | WRAMC, Washington, DC |
| Week of 15–22 October | Level A LRN clinical microbiology laboratory granted level B confirmatory status by CDC | WRAMC, Washington, DC |
| Week of 15–22 October | CDC deploys EIS team to investigate Brentwood Post Office; eventually sets up environmental test processing in BSL-3 clinical laboratory | WRAMC, Washington, DC |
| Week of 15–22 October | CDC begins daily teleconferences to inform and prepare HHS daily press conference | CDC, FBI, WRAMC, NNMC, NIH, USAMRIID, AFIP, NVSL,a others |
NVSL, National Veterinary Services Laboratories.
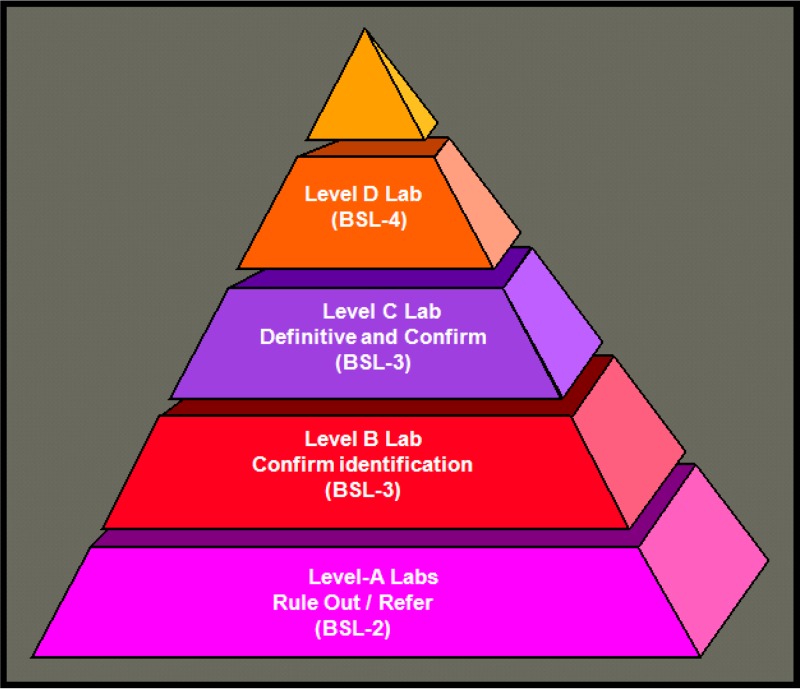
The index case was identified in Florida on 4 October 2001, and the sentinel event for the nascent Laboratory Response Network (LRN) worked according to the organizational design (see Fig. 1). The clinical laboratory (level A sentinel) that received the initial specimen could not rule out B. anthracis and referred the isolate to the state public health laboratory (level B or C confirmatory), which upon confirmation forwarded the isolate to the federal public health laboratory (level D definitive) for definitive characterization. In Florida, an additional asymptomatic patient was identified (1/1,075 nasal swab specimens tested positive) through clinical sampling of the index patient's colleagues and contacts at the workplace intervention clinic supporting American Media Inc. in Boca Raton, FL. Environmental sampling of the workplace by the FBI, CDC, and BPHL resulted in 20/136 samples positive for B. anthracis by culture, including samples from the company mailroom and mail van. Information was released to the media on 10 October that there was suspicion regarding the U.S. Postal Service, meaning that anyone who received mail was a potential victim. In Washington, DC, the first patients were postal workers (8). Without a local LRN-certified public health laboratory, the proximity of U.S. Department of Defense (DOD) sentinel, confirmatory, and definitive identification laboratories allowed flexibility in the laboratory support of the clinical response and criminal investigation. For clinical support, the existing tertiary care clinical laboratories in the National Capital region (NCR) spread the workload among themselves. For environmental testing, both the Armed Forces Institute of Pathology (AFIP, Washington, DC) and USAMRIID were the primary laboratories in support of the investigations. Two unique hybrid activities emerged at the Walter Reed Army Medical Center (WRAMC) in Washington, DC. The non-public-health level A clinical microbiology laboratory was granted level B confirmatory status by the CDC and in turn provided laboratory space to CDC Epidemiological Investigative Service (EIS) laboratory personnel to support the Brentwood Post Office environmental surveillance. In both Florida and Washington, DC, a number of unusual but similar activities were occurring regarding which clinical microbiologists were uniquely prepared to consult or participate. These activities included consultation on first-responder test kit use and interpretation, operation of therapeutic intervention clinics utilizing the Strategic National Stockpile, evidence collection and processing, and providing information for local and federal press releases. These events highlighted the essential role of the microbiology laboratory in supporting a public health and criminal investigation related to a bioterrorism (BT) event, including the laboratory diagnosis of clinical cases and identification of exposed persons and environmental contamination. When considered together, these capabilities were able to focus evidence for a potential source, exposure, and mode of transmission.
FIG 1.
LRN infrastructure in 2001.
The role of the microbiology laboratory in emergency response.
Prior to 2001, federal efforts to address bioterrorism preparedness included nonlegislated and underresourced Presidential Directives and CDC contingency planning (9, 10, 11, 12). In 1999, in a remarkably coordinated and somewhat clairvoyant effort that seems to defy coincidence, a select few dedicated individuals serving the federal government in the U.S. Department of Health and Human Services (HHS) and Department of Justice (DOJ) had the foresight to launch an initiative to coordinate public health infrastructure and standardize laboratory processes and procedures for such events (13, 14). Soon after, the U.S. Army Medical Department met with the CDC to discuss a potential role of the Department in such efforts, given USAMRIID's mission and public health laboratory capability. Also during this time, the Association of Public Health Laboratories (APHL) published a white paper describing and suggesting the unique capability of public health laboratories in emergency response (15). They recommended that state public health laboratories (PHL) provide support in disaster preparedness planning, laboratory testing, and public health response. The culmination of these efforts became the Laboratory Response Network (LRN), a network of laboratories providing the necessary infrastructure for a tiered capability of response to an event. Networked laboratories would also benefit from a technical infrastructure, including centralized communication, standardized reagents and equipment and test protocols, reporting policies, and shipping and transportation guidelines. Since 1999, the PHLs as part of the LRN have become an essential part of national laboratory preparedness. The LRN is also responsible for participating in the development and implementation of related legislation concerning select agents, biosecurity, and biosurety (16).
Regulatory infrastructure related to clinical and public health laboratories.
Beyond the Biological Weapons Convention Treaty of 1972, there had been limited legislation to address biological agents (9, 10, 11, 12, 17). In 1996, as a result of the World Trade Center and Oklahoma City bombings and the consequent Antiterrorism and Effective Death Penalty Act of 1996, 42 Code of Federal Regulations (CFR) Part 72 addressed provisions in the Act concerning the use and transfer of select agents. Since 2001, a number of laboratory-focused regulations have been legislated. The Uniting and Strengthening America by Providing Appropriate Tools Required to Intercept and Obstruct Terrorism Act (USAPATRIOT Act) was signed in October 2001. This act included terms that laboratory personnel must comply with, including a list of “select agents,” defined as those pathogens and toxins that are a potential threat to public health and safety, and “personnel reliability,” the requirement that all laboratory workers having contact with select agents must have background checks. In June 2002, the Public Health Security and Bioterrorism Preparedness and Response Act directed enhanced control and regulation of dangerous biological agents and toxins. This Act enabled interagency coordination for HHS (CDC) and USDA to regulate biological agents and toxins. The specific language regarding these agents and toxins can be found in various Code of Federal Regulations rules (42 CFR Part 73, 7 CFR Part 331, 9 CFR Part 121) (18). The Select Agent Program (SAP) is covered under both of the acts mentioned and provides guidance for the possession, use, and transfer of biological select agents and toxins which have the potential to pose a severe threat to human, animal, or plant health or to animal or plant products. Laboratory facilities that possess, use, or transport select agents must meet stringent documentation requirements for the SAP. The CFRs and the SAP are regularly updated, and in 2013, two updates that had a direct impact on microbiology laboratories were included: (i) Coccidioides immitis was taken off the Select Agent list and (ii) tier 1 agents were designated and requirements imposed for laboratories possessing, shipping, or receiving such agents to enhance biosecurity barriers and biosurety procedures to include biosafety, occupational health and suitability, incident response contingency planning, and training of personnel.
So what does all this mean to public health and clinical laboratories? For the LRN reference laboratory (e.g., the state or local PHL), laboratory personnel must obtain clearance through fingerprinting and background checks to work with select agents. This process is often referred to as biosurety. Laboratories are required to adopt biosafety procedures, defined as the discipline addressing the safe handling and containment of infectious microorganisms and hazardous biological materials to prevent unintentional exposure (19). To comply, laboratories are required to practice appropriate aseptic and containment practices and ensure that the equipment and the facility infrastructure are in place to protect laboratory personnel, the environment, and the public from exposure to infectious organisms or materials that are handled and stored in the laboratory. Laboratories must also address biosecurity, defined as the discipline addressing the security of microbiological agents and toxins and the threats posed to human and animal health, the environment, and the economy by deliberate misuse or release (19). To comply, laboratories are required to have the appropriate practices, equipment, and infrastructure to protect biological pathogens and toxins from theft, loss, or misuse; e.g., refrigerators and incubators must have locks and personal identification numbers (PINs) and two-person access. Additional responsibilities for such laboratories include protocols for the collection of information; appropriate retention of documentation and evidence for law enforcement; tracked documentation of the transfer, acquisition, possession, and destruction of agents; and implementation of checklisted items for federal inspection to ensure compliance with legislation.
For the LRN sentinel laboratory (e.g., the clinical microbiology laboratory), the main focus is implementing laboratory guidelines published by American Society for Microbiology (ASM) for the ruling out and appropriate referral of suspected bioterrorism organisms or select agents. The select agent protocols can be found at http://www.asm.org/index.php/guidelines/sentinel-guidelines and are referenced in Table 3. Additionally, clinical laboratories must securely store agents (and residual specimens) while awaiting confirmation results, document transfer, and document onsite agent destruction when appropriate. Offsite destruction via a biomedical waste service is prohibited. Sentinel laboratories also need to document personnel that work with suspect agents and whether any potential laboratory exposure could have occurred. Laboratories need to be aware of the implications for their laboratory and the federally mandated consequences if regulations are not carefully followed. One example is the case of Thomas Butler, a Texas Tech University professor, who was arrested and prosecuted in 2003 by the Justice Department for illegal transportation of plague samples (and other charges). In 2003 he had reported 30 vials of plague missing from his laboratory to safety officers at Texas Tech University, and this triggered a bioterrorism response plan and his eventual arrest. This case was criticized by some for the aggression with which a prominent scientist was prosecuted and represents, unfortunately, a cautionary tale for working with select agents (20).
TABLE 3.
Checklist items for sentinel laboratoriesa
| Issue or event | Action(s) | Resource(s) | Source(s) |
|---|---|---|---|
| Be prepared for an event | Have an institutional emergency response plan | Local institution (ASPR HPP) | http://www.phe.gov/PREPAREDNESS/PLANNING/HPP/Pages/default.aspx |
| Have a specific bioterrorism response plan | Local institution | ||
| Train staff on packaging and shipping | PHLs, APHL, ASM, CDC | DOT (http://www.phmsa.dot.gov/staticfiles/PHMSA/DownloadableFiles/Files/Transporting_Infectious_Substances_brochure.pdf), IATA (http://www.iata.org/training/courses/Pages/tcgp43.aspx), USDA (http://www.aphis.usda.gov/programs/ag_selectagent/) | |
| Train staff on ruling out select agents/BT organisms | PHL and/or DOT/IATA | ||
| Train staff on select agents | PHL | ||
| Train staff on communications, e.g., who to call | PHL | ||
| Perform proficiency testing/take part in proficiency exercise | PHL or other entity to participate: CAP LPX, PHL | CAP survey catalogue 2014 | |
| Maintain supply of materials for testing to rule out BT organisms | |||
| Know what to do if you suspect that you have a BT organism/select agent | Follow procedures for ruling out BT | ASM sentinel laboratory guidelines | ASM (http://www.asm.org/index.php/guidelines/sentinel-guidelines) |
| Initiate and maintain communication with departmental/hospital leadership and infection control | Institutional policy | ||
| Contact BT personnel at LRN reference laboratory | LRN reference laboratory (your local or state PHL) | Your local or state PHL | |
| Ship isolate to reference laboratory (public health laboratory) | LRN reference laboratory (your local or state PHL) | Your local or state PHL | |
| Document courrier transfer, e.g., institutional or commercial courrier tracking no. | Institutional courier, FedEx, UPS, etc. | ||
| Secure all potential select agent(s) and residual samples | Code of Federal Regulations: 42 CFR Part 73 | Select agents (http://www.selectagents.gov/Regulations.html) | |
| Document personnel (biosecurity) with access to potential select agent(s) | Code of Federal Regulations: 42 CFR Part 73 | Select agents (http://www.selectagents.gov/Regulations.html) | |
| Document personnel (biosafety) who have worked with suspect select agent and those present in laboratory if exposure occurred | Biosafety risk assessment | BMBL, 5th ed. (American Biological Safety Association; http://www.cdc.gov/biosafety/publications/bmbl5/) | |
| Know what to do if you have a confirmed select agent | Follow directions from PHL: destroy or transfer all isolates/specimens | LRN reference laboratory (your local or state PHL) | 42 CFR Part 73 |
| Document identification of select agent(s) (APHIS/CDC Form 4) | 42 CFR Part 73, LRN reference laboratory (your local or state PHL) | Select agents (http://www.selectagents.gov/Forms.html) | |
| Document disposition of select agent using APHIS/CDC Form 4 for destruction or APHIS/CDC Form 2 for transfer to a select agent-registered facility; confirm that select agents must not be transferred without prior authorization from CDC or USDA | 42 CFR Part 73, LRN reference laboratory (your local or state PHL) | Select agents (http://www.selectagents.gov/Forms.html) | |
| Document laboratory exposures; work with PHL or state or local health department for postexposure prophylaxis | 42 CFR Part 73, LRN reference laboratory (your local or state PHL) | Select agents (http://www.selectagents.gov/Forms.html) | |
| Keep abreast of updates | Periodically review ASM website for updates on sentinel laboratory guidelines, APHL training on sentinel guideline updates | ASM, APHL, LRN reference laboratory (your local or state PHL) | ASM guidelines (http://www.asm.org/index.php/guidelines/sentinel-guidelines), APHL (http://www.aphl.org/aphlprograms/preparedness-and-response/Pages/default.aspx) |
BMBL, Biosafety in Microbiological and Biomedical Laboratories; DOT, Department of Transportation; IATA, International Air Transport Association; LPX, Laboratory Preparedness Exercise; UPS, United Parcel Service.
Public health and clinical laboratory infrastructure—the Laboratory Response Network and funding post-2001.
The mission of the Laboratory Response Network is to develop, maintain, and strengthen an integrated domestic and international network of laboratories to respond quickly to biological and chemical threats and other high-priority public health emergencies through training, rapid testing, timely notification, and secure communication of laboratory results (16).
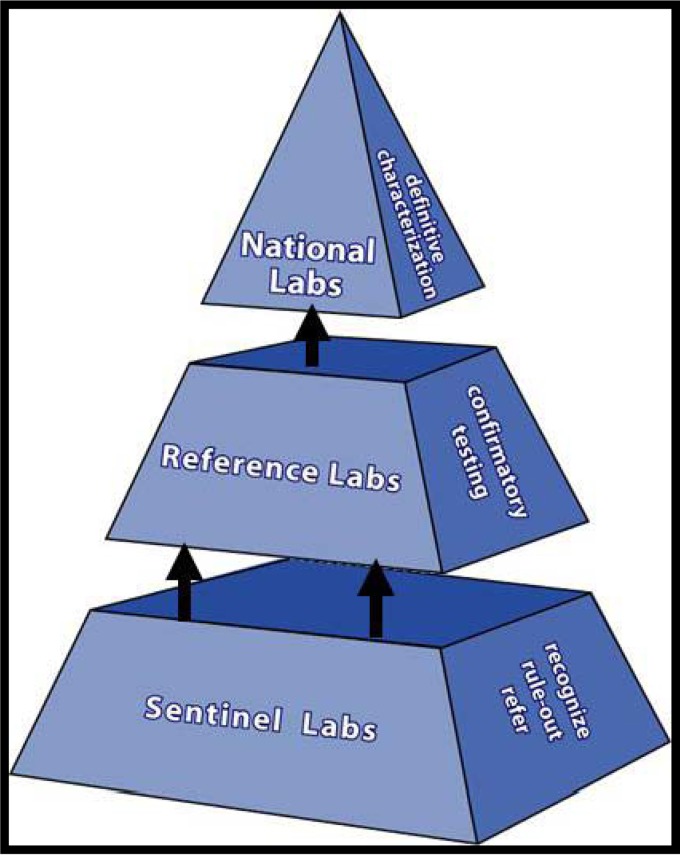
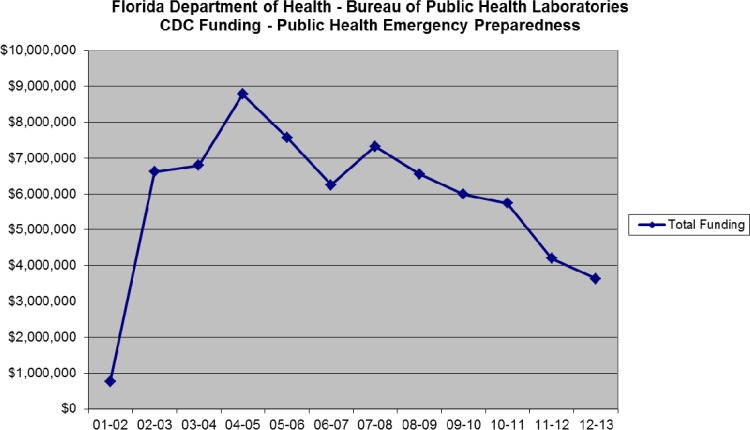
The current three-tiered structure of the LRN can be seen in Fig. 2. There are many partners that make up the network: CDC/HHS and FBI/DOJ are considered the founding partners (1999), with DOD and APHL partnering soon after (2000). There are additional partners, including but not limited to the USDA, Environmental Protection Agency (EPA), ASM, Food and Drug Administration (FDA/HHS), Department of Energy (DOE), Department of Homeland Security (DHS), American Association of Veterinary Laboratory Diagnosticians (AAVLD), and College of American Pathologists (CAP). Funding for the LRN at the reference laboratory level rose considerably after 2001 but has decreased in recent years. Figure 3 shows Public Health Emergency Preparedness (PHEP) funding for the Florida BPHL since 2000. Due to this funding, the capacity for PHLs to perform testing at the reference level as part of the LRN has greatly expanded. In 2001, there were 84 reference laboratories; there are now more than 150. In 2001, less than half of the states in the United States had PHLs with biosafety level 3 (BSL-3) capabilities; now, all 50 states have this capability. Funding for sentinel laboratories has also been supported. For example, the Hospital Preparedness Program (HPP), administered by the Office of the Assistant Secretary for Preparedness and Response (ASPR), provides leadership and funding to improve surge capacity and enhance community and hospital preparedness to respond to bioterrorism and other public health emergencies (21, 22).
FIG 2.
LRN infrastructure in 2014.
FIG 3.
Public Health Emergency Preparedness (PHEP) HHS/CDC cooperative agreement funding for the Florida BPHL since 2000.
Given that the LRN had been established only 2 years prior, it was surprisingly successful during the anthrax attacks of 2001 in executing the infrastructure for ensuring that sentinel laboratories were able to recognize and refer a potential bioterrorism agent, forward a sample(s) to a confirmatory laboratory, and in turn proceed up the LRN pyramid for definitive characterization at national laboratories. Levels of communication and coordination between public health personnel, first responders, law enforcement officers, and workers in local hospitals/laboratories differed across the country and were often dependent on the workload associated with the worried well. There were many lessons learned and, in some cases, written into legislation which ultimately resulted in improved infrastructure and increased technical capability. As with any centralized solution, there was also a proportionate increase in administrative bureaucracy associated with implementing, upgrading, and documenting biosurety, biosafety, and biosecurity in the laboratory.
LOOKING FORWARD
Surge capacity.
It is unlikely that only one clinical microbiology laboratory will be involved in the next laboratory response to a bioterrorism event. Unlike culturing a select agent in an area of endemicity, such as Yersinia pestis in the southwestern United States, a bioterrorism event will likely include patients presenting to multiple health care facilities. Laboratories in support will include clinical microbiology laboratories (sentinel) in direct support of the health care facility, clinical and environmental reference laboratories, and state and local public health (reference) and federal (national) laboratories. Additional laboratories such as those of the EPA, HHS regional BSL-3 facilities, and the National Veterinary Services Laboratories in Ames, IA, and Plum Island, NY, may be called upon for their experience in the 2001 events or their select agent expertise. Sentinel and surge capacity laboratories may find themselves being asked to test a range of environmental and clinical samples from an amazing variety of sources, from the psychologically wounded well to the office dairy creamer spill and other environmental samples obtained to define the extent of exposure and contamination. Since 2001, a great deal of preparation has gone into training first responders and the development of national standards for testing environmental samples, and sentinel laboratories should not receive environmental samples where possible. In addition, reference laboratories should now be receiving only samples from credible threats as determined by the FBI or suitably qualified local law enforcement (23, 24). The human and logistical resource drain is likely to be severe and was so intense during 2001 to 2002 that one director of an institute declared that it could never again support a select agent epidemiological or criminal investigation without suffering corporate mission failure (personal communication). Laboratory directors who coordinate and plan with local sentinel laboratories, supporting reference laboratories, and even national laboratories will ensure a more rapid, efficient, and coordinated laboratory response effort for the next event.
Processing bioterrorism event-related samples.
Biosafety containment, supply, and work flow issues will dominate the early laboratory response to a disseminated select agent event. Since the laboratory personnel may not initially know the identity of the agent or if it is related to a single-agent event, the initial containment should be maintained at no less than BSL-3 until the etiology is established. Once BSL-4 agents are ruled out, continued processing and workup of BSL-3 agents may be performed under BSL-2 conditions using BSL-3 precautions with appropriate personal protective equipment (PPE). If the laboratory is asked to support environmental monitoring, a biosafety risk assessment should be performed, as different agents present different challenges. For example, in 2001 it was common for the clinical laboratory to receive requests for environmental testing. While B. anthracis is a BSL-2 organism, its ability to be delivered as spores and contaminate the laboratory or facility must be assessed for the risk to the health care facility and patients.
Supply lines will be challenged. For example, sheep blood agar plates were not commercially available for about 2 weeks in the National Capital region in the fall of 2001. Ongoing assessment of media and reagent needs will be required for sentinel laboratories, as will flexibility of phenotypic protocols with respect to evolving supply constraints. Despite the best contingency planning, reference laboratories may face rapid exhaustion of initial stocks of LRN reagents, with delays in resupply. Laboratory staff will be challenged to develop efficient work flows for mass processing, setup, and reading and reporting of culture results. Ongoing planning and exercise of surge capacity contingencies should help to mitigate some of the strain. Clinical and therapeutic expectations, along with external public pressures to report results as soon as possible, will likely require laboratories to modify 2nd- and 3rd-shift staffing to 1st-shift models. And it is very likely that specimens will be received with non-Laboratory Information System (LIS)-integrated or even nonelectronic hand-written specimen logs and labeling that cannot be interfaced to the LIS. Additional LIS data entry time and quality assurance reviews of reports must be considered in contingency planning. A checklist for sentinel laboratories is provided in Table 3.
Criminal investigations.
Pre-event interaction between LRN reference laboratories and local FBI Weapons of Mass Destruction (WMD) offices is essential. Threat assessments by the FBI should precede accepting any environmental sample. The FBI is rarely involved in processing of clinical specimens but in the event of a threat would coordinate with referral and sentinel laboratories. The FBI will accept the clinical laboratory's laboratory information management system as auditable documentation. When suspected select agents are shipped, the tracking number from the courier becomes part of the chain of custody. If sentinel laboratories are in the practice of using additional documentation for other potential legal cases, e.g., child abuse sexually transmitted disease (STD) cases, then this same documentation may be used for select agent specimens.
Laboratory Response Network.
In addition to its response to the anthrax events of 2001, the LRN has demonstrated successes in responding to many public health emergencies: monkeypox from prairie dogs in 2003; severe acute respiratory syndrome; deployment of highly pathogenic influenza A virus (H5N1) and Middle East respiratory syndrome (MERS) coronavirus assays; naturally occurring infections with BT organisms; and countless incidents threatening the use of a weapon of mass destruction, including several cases where ricin toxin was detected. However, challenges remain for the LRN and its centralized approach to planning for future biological terrorism events. Despite considerable funding since 2001, the ability of laboratories to build surge capacity is limited at all levels of the LRN. Even with molecular testing, there are only so many samples a laboratory can test in a single day. A good example of this was the influenza A virus H1N1 outbreak in 2009 which resulted in public health laboratories being stretched beyond capacity to keep up with the demand for influenza testing. Although this surge testing was directed by the Influenza Division at CDC and not through the LRN, the LRN/PHEP was able to provide surge capacity through public health laboratory support of increased testing demands.
Perhaps the greatest challenge facing the LRN is that of sentinel (clinical) microbiology laboratories responding to rapidly evolving technology in a health care environment that is simultaneously responding to legislation-driven initiatives to increase cost efficiencies. Clinical laboratory managers are increasingly asked to produce positive returns on investment for new technology and validate those investments by demonstrating improvements in patient outcomes. The result is that most clinical laboratories are rightly focused on improving technology for patient care while attempting to maintain sentinel laboratory compliance. Clinical microbiology laboratories are increasingly performing culture-independent molecular testing that does not isolate an organism or use methods such as mass spectrometry and sequencing to identify organisms with reliance on FDA-approved or laboratory-validated databases for identification. These changes in methods might mean that a select agent or emerging pathogen would be misidentified or that there would be no isolate with which to conduct confirmatory and epidemiological analysis, monitor emerging antibiotic resistance, or support microbial forensics.
Validation of centralized protocols, procurement of standardized reagents and platforms, and proficiency testing is a never-ending task for CDC-certified reference and national laboratories and for any other laboratories that want to be involved in public health result reporting. Non-LRN laboratories with advanced technologies and similar platforms are often willing to provide surge capacity with their own validated assays but are excluded unless certified for LRN response and using assays and platforms validated by the CDC. The capacity and capability of these laboratories are juxtaposed against a lack of standardization of protocols, reagents, and instrumentation which could confuse public health interpretation or compromise a criminal investigation in support of an event. It should be pointed out that LRN reference laboratories have only a limited number of assays that are FDA approved. Before each new assay is released by the CDC to the reference laboratories, an extensive multicenter validation study is performed and approved by both the CDC and FBI before distribution. Reference laboratories must then verify performance characteristics before implementation, and local Institutional Review Boards (IRBs) have the final say for clinical laboratories that seek to provide confirmatory and definitive laboratory testing using CDC assays that are not FDA approved.
Additional challenges include biosurety, biosecurity, communications, and funding. During the next event, desperately needed surge capacity laboratories may have to rapidly establish biosurety for the workforce and increased biosecurity for their physical infrastructure. These requirements, especially for non-LRN laboratories, are considered onerous by some, leaving little flexibility for institution-specific human resource or facility management. Improving communications, from a system for alerting LRN partners about an emerging threat to secure electronic reporting of results with public health implications, is a constant challenge at all levels. Continued outreach between the LRN tiers and to sentinel laboratories is needed. There are still several cases of laboratory exposures to organisms such as Brucella species which highlight the need for increased education and training, particularly at the local, clinical microbiology laboratory level. Lastly, funding for emergency preparedness in the laboratory has decreased steadily in recent years and laboratories are frequently asked to do more with less. One particular area of fiscal concern is the increased cost of regulatory compliance, such as the additional facility requirements for tier 1 select agent-registered laboratories as discussed above.
CONCLUSION
Between 4 October and 20 November 2001, 22 individuals were diagnosed with B. anthracis infections; 11 cases were inhalational, and 5 of the 11 cases were fatal. Laboratories in direct support of the outbreak and an FBI investigation received over 30,000 specimens for testing in the fall of 2001 and early 2002. Across the country, state public health laboratories were quickly overwhelmed and then quickly recovered to support the analysis of specimens ranging from clinical samples to environmental samples such as carpet and countertops. Surge capacity was developed in real time, and laboratory support lingered due to the volume of environmental contamination resulting from a spore-forming bacillus. Without human-to-human transmission, potential pressures on a laboratory providing results for quarantine and containment decisions were not realized. But many laboratories gained insight into this type of national concern as the public awaited reassurance from supporting laboratories and public health officials that it was safe to return to work at the postal facilities and for the public to receive routine distribution of the mail. It is our hope that a reminder of the events of 2001 and the lessons learned, a review of the regulations, and a checklist of resources will be of practical assistance to clinical and public health microbiologists as we plan and prepare for the next event.
EPILOGUE: NO LABORATORY WHODOESIT WOULD BE COMPLETE WITHOUT A WHODUNIT
In February 2010, the FBI released their report on the investigation of the 2001 anthrax attacks (25). The report concluded that the late Dr. Bruce Ivins, a scientist and former employee at USAMRIID, acted alone in planning and executing the attacks. Because of his suicide, Dr. Ivins was never charged with the crime. In February 2011, a National Research Council Committee (NRCC) was asked to examine the scientific approaches used and conclusions reached by the FBI in their report (26). The NRCC report concluded that it was not possible to reach a definitive conclusion about the origins of the B. anthracis spores in letters mailed to New York City and Washington, DC, based solely on the available scientific evidence. The report concurred with the FBI's scientific conclusions about the genetic similarities of the outbreak isolates to flasks recovered from Dr. Ivin's laboratory. The report also pointed out that a specific flask (RMR-1029) was not the immediate source of spores in the letters, also aligning with the FBI's conclusion that one or more derivative growth steps were required to produce the spores in the attack letters. The report proposed other possible explanations for the similarities, such as independent, parallel evolution, which were not definitively explored during the investigation. The Committee also recommended that realistic expectations and limitations regarding the use of forensic science in such investigations need to be clearly communicated to the public.
ACKNOWLEDGMENTS
D.W.C. thanks Scott Riddell for his editorial assistance and significant laboratory contributions and recognizes Bill Nauschuetz, Ted Hadfield, Dave Rockabrand, Frank Witebsky, and Greg Martin for their key laboratory and clinical management decisions during the disease outbreak in Washington, DC.
Historical events from 2001 were documented from written and published accounts and from our personal experience. The accounts and opinions herein are solely ours and do not necessarily represent the official views of the Department of Defense or American Public Health Laboratory Society.
Biography

David W. Craft is the Medical Director of the Clinical Microbiology Laboratory at the Penn State Milton Hershey Medical Center and an Associate Professor of Pathology in the College of Medicine. He just concluded a 30-year career in the U.S. Army, during which he was Director of the Infectious Disease Laboratories at Walter Reed Army Medical Center in Washington, DC, during the anthrax mailings in October 2001. His laboratory served as the only CDC-certified confirmatory laboratory in the District of Columbia and supported the clinical hot-zone analysis of the Hart Senate Office Building. Dr. Craft has a B.S. and an M.S. in Microbiology from the University of Alabama, a Ph.D. in Microbiology from the University of Georgia College of Veterinary Medicine, and a postdoctoral fellowship in Medical and Public Health Microbiology from the University of North Carolina Hospitals. He is board certified and is a diplomate of the American Board of Medical Microbiology.
Footnotes
Published ahead of print 19 March 2014
REFERENCES
- 1. Christopher GW, Cieslak TJ, Pavlin JA, Eitzen EM., Jr 1997. Biological warfare. A historical perspective. JAMA 278:412–417 [PubMed] [Google Scholar]
- 2. Franz DR, Jahrling PB, Friedlander AM, McClain DJ, Hoover DL, Bryne WR, Pavlin JA, Christopher GW, Eitzen EM., Jr 1997. Clinical recognition and management of patients exposed to biological warfare agents. JAMA 278:399–411. 10.1001/jama.1997.03550050061035 [DOI] [PubMed] [Google Scholar]
- 3. Klietmann WF, Ruoff KL. 2001. Bioterrorism: implications for the clinical microbiologist. Clin. Microbiol. Rev. 14:364–381. 10.1128/CMR.14.2.364-381.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Inglesby TV, Henderson DA, Bartlett JG, Ascher MS, Eitzen EM, Jr, Friedlander AM, Hauer J, McDade J, Osterholm MT, O'Toole T, Parker G, Perl TM, Russell PK, Tonat K. 1999. Anthrax as a biological weapon: medical and public health management. Working Group on Civilian Biodefense. JAMA 281:1735–1745 [DOI] [PubMed] [Google Scholar]
- 5. Koblentz GD. 2001. Overview of Federal programs to enhance state and local preparedness for terrorism with weapons of mass destruction. BCSIA 2001-5, ESDP-2001-03. John F. Kennedy School of Government, Harvard University, Cambridge, MA [Google Scholar]
- 6. Bush LM, Abrams BH, Beall A, Johnson CC. 2001. Index case of fatal inhalational anthrax due to bioterrorism in the United States. N. Engl. J. Med. 345:1607–1610. 10.1056/NEJMoa012948 [DOI] [PubMed] [Google Scholar]
- 7. Traeger MS, Wiersma ST, Rosenstein NE, Malecki JM, Shepard CW, Raghunathan PL, Pillai SP, Popovic T, Quinn CP, Meyer RF, Zaki SR, Kumar S, Bruce SM, Sejvar JJ, Dull PM, Tierney BC, Jones JD, Perkins BA, Florida Investigation Team 2002. First case of bioterrorism-related inhalational anthrax in the United States, Palm Beach County, Florida. Emerg. Infect. Dis. 8:1029–1034. 10.3201/eid0810.020354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Borio L, Frank D, Mani V, Chiriboga C, Pollanen M, Ripple M, Ali S, DiAngelo C, Lee J, Arden J, Titus J, Fowler D, O'Toole T, Masur H, Bartlett J, Inglesby T. 2001. Death due to bioterrorism-related inhalational anthrax: report of 2 patients. JAMA 286:2554–2559. 10.1001/jama.286.20.2554 [DOI] [PubMed] [Google Scholar]
- 9.Office of the President. 21 June 1995. Presidential Decision Directive 39 (PDD-39), U.S. policy on counterterrorism. http://www.fas.org/irp/offdocs/pdd39.htm
- 10.Office of the President. 22 May 1998. Presidential Decision Directive 62 (PDD-62), Protection against unconventional threats to the homeland and Americans overseas. https://www.fas.org/irp/offdocs/pdd-62.htm
- 11.Association for Professionals in Infection Control and Epidemiology Inc. and Centers for Disease Control and Prevention. 1999. Bioterrorism readiness plan: a template for healthcare facilities. ED Manag. 11(Suppl 1):1–16 [PubMed] [Google Scholar]
- 12.Centers for Disease Control. 1998. Preventing emerging infectious diseases: a strategy for the 21st century. Overview of the updated CDC plan. MMWR Recommend. Rep. 47(RR-15):1–14 [PubMed] [Google Scholar]
- 13.Centers for Disease Control. 2000. Biological and chemical terrorism: strategic plan for preparedness and response: recommendations of the CDC Strategic Planning Workgroup. MMWR Recommend. Rep. 49(RR-4):1–14 [PubMed] [Google Scholar]
- 14. Rotz LD, Khan AS, Lillibridge SR, Ostroff SM, Hughes JM. 2002. Public health assessment of potential biological terrorism agents. Emerg. Infect. Dis. 8:225–230. 10.3201/eid0802.010164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centers for Disease Control. 2002. Core functions and capabilities of state public health laboratories: a report of the Association of Public Health Laboratories. MMWR Recommend. Rep. 51(RR-14):1–8 [PubMed] [Google Scholar]
- 16.CDC. 1995. Laboratory Response Network. http://www.bt.cdc.gov/lrn/
- 17.The United Nations Office at Geneva. April 1972. Biological weapons convention treaty. http://www.unog.ch
- 18.U.S. Departments of Health and Human Services (HHS) and Agriculture (USDA). 2012. Select agent regulations. http://www.selectagents.gov/Regulations.html
- 19. Chosewood LC, Wilson DE. 2009. Biosafety in microbiological and biomedical laboratories, 5th ed. U.S. Department of Health and Human Services, Washington, DC [Google Scholar]
- 20. Murray BE, Anderson KE, Arnold K, Bartlett JG, Carpenter CC, Falkow S, Hartman JT, Lehman T, Reid TW, Ryburn FM, Jr, Sack RB, Struelens MJ, Young LS, Greenough WB., III 2005. Destroying the life and career of a valued physician-scientist who tried to protect us from plague: was it really necessary? Clin. Infect. Dis. 40:1644–1648. 10.1086/431348 [DOI] [PubMed] [Google Scholar]
- 21.Office of the Assistant Secretary for Preparedness Response. 2007. Medical surge capacity and capability: managing medical and public health responses to emergencies and disasters through a tiered response, 2nd ed. Office of the Assistant Secretary for Preparedness Response, Washington, DC [Google Scholar]
- 22.Office of the Assistant Secretary for Preparedness Response. 2009. Medical surge capacity and capability: the healthcare coalition in emergency response and recovery. Office of the Assistant Secretary for Preparedness Response, Washington, DC [Google Scholar]
- 23.American Society for Testing and Materials Committee E54. 2010. Standard guide for operational guidelines for the initial response to a suspected biothreat agent. ASTM International. Designation E2770-10. http://www.astm.org/DHS/E2770.pdf
- 24.Association of Public Health Laboratories. 2011. Model practice. algorithm and guidelines for responding to an incident involving a suspicious non-clinical sample. Version 1.0. http://www.aphl.org/AboutAPHL/publications/Documents/PHPR_2011June_Algorithm-and-Guidelines-for-Responding-to-an-Incident-Involving-a-Suspicious-Non-Clinical-Sample.pdf
- 25.U.S. Department of Justice. 2010. Amerithrax Investigative Summary February 19, 2010. http://www.justice.gov/archive/amerithrax/docs/amx-investigative-summary.pdf
- 26.National Research Council. 2011. Review of the scientific approaches used during the FBI's investigation of the 2001 anthrax letters. The National Academies Press, Washington, DC: [PubMed] [Google Scholar]