Abstract
We describe an immunodeficient adult with Ogilvie's syndrome preceding a disseminated papulovesicular skin rash in whom varicella-zoster virus infection was demonstrated by PCR assay in cutaneous and colonic biopsy specimens. In view of the significant morbidity and mortality that this condition carries, early and accurate molecular diagnosis and timely treatment are strongly recommended.
CASE REPORT
A 62-year-old white man was admitted in June 2013 because of a 6-day history of severe and worsening abdominal pain and vomiting. He had been diagnosed with stage IIIA, diffuse large B-cell lymphoma in 2004, when complete remission was achieved following immunochemotherapy (rituximab, cyclophosphamide, adriamycin, vincristine, and prednisone [R-CHOP]). In 2008, he received immunochemotherapy as a salvage treatment for relapse (rituximab, etoposide, cytarabine, cisplatinum, and methylprednisolone [R-ESHAP]) and an autologous hematopoietic stem-cell transplant, reaching complete remission once more. Afterwards, the patient was given consolidation chemotherapy until October 2012, bendamustine being the last agent prescribed. A positron emission tomography scan performed in May 2013 displayed normal findings. His medical history also included diabetes mellitus and an episode of herpes zoster on the right T 12 dermatome 15 months before admission. He denied opiate, phenothiazines, or calcium channel blocker administration, chronic constipation, or recent trauma or surgical intervention. He was not on immunosuppressive therapy.
On admission, the patient was afebrile and hemodynamically stable. Physical examination revealed profound abdominal distention and decreased bowel sounds but no signs of peritoneal irritation. Blood tests were significant for leucopenia and lymphopenia (2.3 × 109/liter leukocytes, 71.4% neutrophils, 16.8% lymphocytes), mild anemia (hemoglobin, 119 g/liter), and an accelerated erythrocyte sedimentation rate (45 mm/h). His total T-cell count and CD4+ T-cell count were 0.317 × 109/liter and 0.012 × 109/liter, respectively. An abdominal computed tomography (CT) scan showed marked colonic dilatation and a possible narrowing at the rectosigmoid junction; pneumoperitoneum or ascites were absent (Fig. 1). An ensuing colonoscopy ruled out strictures and was remarkable only for a solitary sigmoidal ulcerative lesion (Fig. 2) which was subjected to a biopsy procedure (Fig. 3). Simultaneous control biopsy specimens taken from an endoscopically normal right colon revealed normal findings. An upper gastrointestinal endoscopy displayed no abnormalities. At that time, emergency, internal medicine, and surgery physicians were unable to ascertain the origin of the large bowel distension. Moreover, the patient's complaints required intravenous tramadol, paracetamol, and metamizole treatment. On the third hospital day, he developed a cutaneous, diffuse exanthematous papulovesicular rash most consistent with disseminated herpes zoster (Fig. 4)—one of these lesions was subjected to a biopsy procedure on the same day. As abdominal pain had subsided after the rash appeared and there were no signs of extraintestinal visceral involvement, he was started on oral valacyclovir instead of intravenous acyclovir on the fourth hospital day. A skin biopsy specimen showed an ulcerative lesion with epidermal necrosis and evidence of viral cytopathic effect (Fig. 5). The PCR assay performed in the skin specimen was positive for varicella-zoster virus (VZV) DNA but showed lack of amplification for herpes simplex virus 1 (HSV-1) and HSV-2, herpesvirus (HV) 6, 7, and 8, cytomegalovirus (CMV), Epstein-Barr virus (EBV), and enterovirus. These results were received on the seventh hospital day. Afterward, a PCR analysis of a colonic ulcer biopsy specimen was requested, and the result turned out to be positive for VZV DNA and negative for HSV-1 and HSV-2, HV 6, 7, and 8, CMV, EBV, and enterovirus. The PCR method used to detect VZV and enterovirus was that of a Clart Entherpex (Genomica SAU, Madrid, Spain) kit. This assay for human herpesvirus and enterovirus genotyping is based on viral-genome-specific fragment amplification via multiplex real-time PCR and subsequent detection via hybridization with microorganism-specific binding probe arrays. This PCR method shows specificity higher than 97%, because it uses both a sequence corresponding to a highly preserved region within the viral genome and binding probes specific to each human herpesvirus and enterovirus type. The analytical sensitivity of the assay for VZV is 10 copies. Immunohistochemical staining in biopsy specimens for VZV was not done because such a diagnostic method is not available in our reference university hospital. Moreover, the presence of VZV DNA was not assessed retrospectively in the blood or stool of our patient prior to the appearance of the rash. The results of serologic testing for HIV were negative. Additionally, there was no evidence of circulating lymphoma cells. Abdominal pain eventually resolved when crusting appeared, and he was discharged as asymptomatic 19 days after admission. At a follow-up visit 9 months after discharge, the patient remained symptom free.
FIG 1.
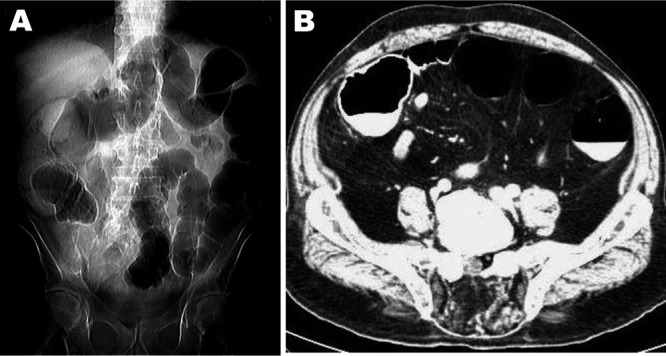
(A) CT scan of the abdomen revealing diffuse dilatation of the large bowel (initial plain view). (B) Contrast-enhanced abdominal CT scan showing striking dilatation of colonic loops (axial view).
FIG 2.

Colonoscopy displaying an isolated sigmoidal ulceration (arrow).
FIG 3.
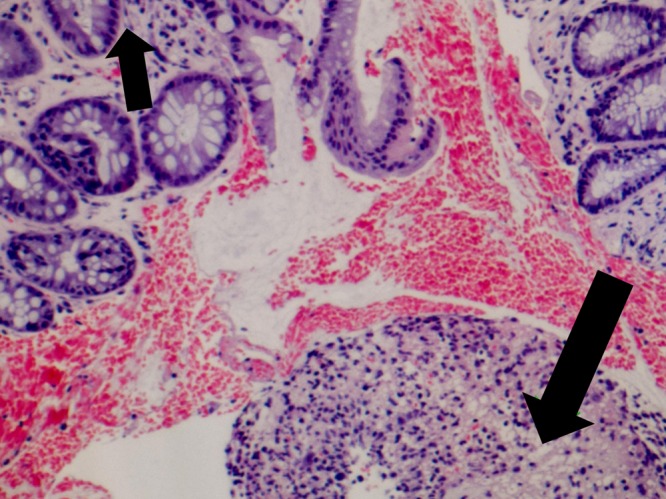
Colonic ulcer biopsy specimen revealing mucosal regeneration (small arrow) and a predominantly neutrophilic inflammatory infiltrate (large arrow) (hematoxylin-eosin staining; original magnification, ×40).
FIG 4.
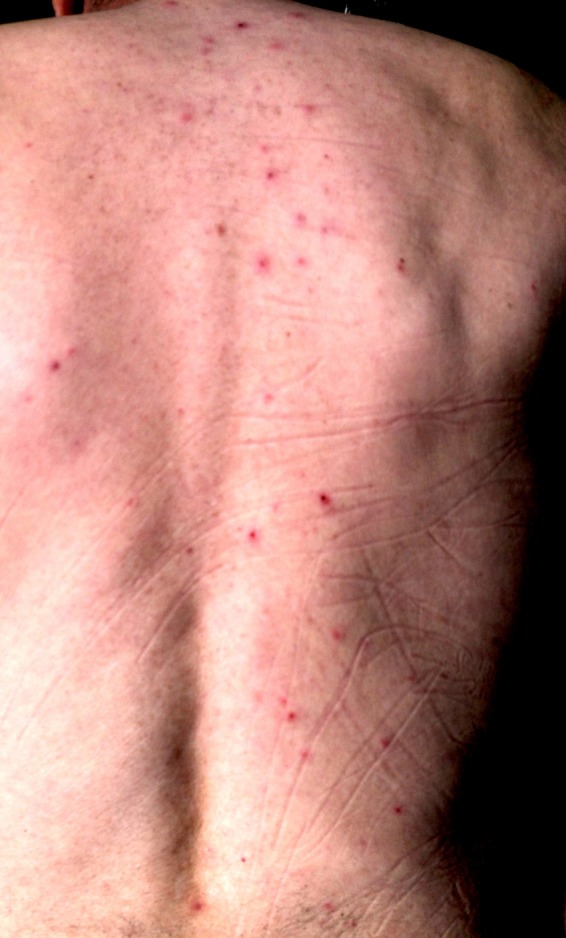
Generalized papulovesicular lesions with partial crusting can be appreciated on the patient's back (disseminated herpes zoster).
FIG 5.
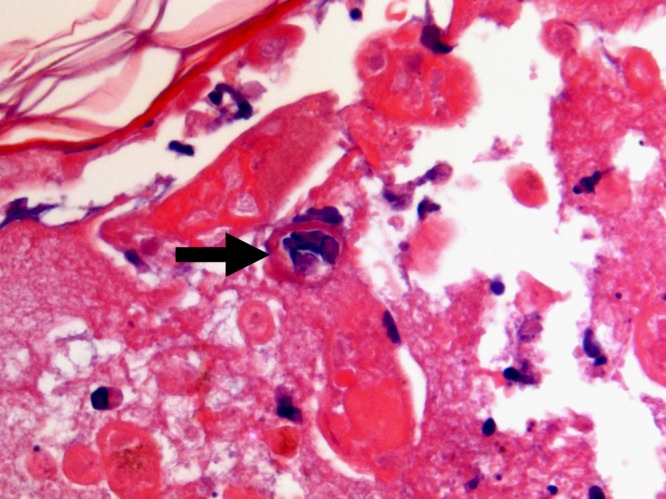
Cutaneous biopsy specimen demonstrating the presence of some multinucleated epithelial cells with chromatin margination and intranuclear inclusions inside the necrotic tissue (arrow) (hematoxylin-eosin staining; original magnification, ×100).
Primary infection with VZV results in varicella, during which the virus causes diffuse rash and viremia and establishes lifelong latency in neurons of dorsal-root, cranial-nerve, and enteric nervous system (ENS) ganglia (2–5). Specifically, VZV carried by peripheral T-lymphocytes and retrograde axonal transport from dorsal-root ganglion neurons infected through their epidermal projections are regarded as the two potential ways that allow VZV to reach the ENS (4). Thus, whatever induces VZV reactivation in dorsal-root ganglia is likely to induce the same effect in the ENS neurons (4). Herpes zoster develops when VZV reactivates from latency (in dorsal-root and cranial-nerve neurons) in some 30% of individuals, and its incidence is increasing (2, 4). Major risk factors for herpes zoster are older age and impaired T-cell immunity, although the risk is also higher for women, white people, and individuals with a family history of this condition (2, 4). Immunosuppressed patients are prone to have particular manifestations, including generalized skin disease and involvement of multiple organs (e.g., brain, lung, liver, pancreas, and gastrointestinal tract) (2, 6). As chemotherapeutic agents used in the treatment of diffuse large B-cell lymphoma have the potential to cause persistent lymphopenia, it is likely that the cellular immunodeficiency observed in our patient could have been induced by one or some of these drugs, especially bendamustine (7, 8).
The present case illustrates that acute colonic pseudo-obstruction (Ogilvie's syndrome) can be produced by VZV infection and that it usually occurs in immunocompromised persons (4, 9–17). VZV-induced Ogilvie's syndrome carries significant rates of morbidity and mortality, and its diagnosis requires a high index of suspicion because visceral symptomatology may antecede the appearance of the characteristic skin eruption (11, 14). However, the coexistence of herpes zoster and Ogilvie's syndrome is uncommon and has received scant attention in the medical literature. The largest case series published on this association involved 29 patients from 21 studies (1950 to 2008) (11). That review included 22 men and 7 women aged 32 to 87 years, relevant comorbidities being present in 45% of cases (malignancies in 8 [28%] patients). Most (83%) of the individuals were managed conservatively, and two (7%) patients died. Interestingly, colonic pseudo-obstruction started between 1 day and several weeks before the occurrence of skin rash in 14 (48%) patients. In 8 patients, cutaneous lesions preceded intestinal symptoms by 1 day to 1 month; in 7 patients, skin manifestations were diagnosed simultaneously with pseudo-obstruction.
As VZV reactivation is lethal to the neurons in which it develops, involvement of the ENS in this process might be associated with different gastrointestinal tract disorders, such as idiopathic gastroparesis, chronic intestinal idiopathic pseudo-obstruction, and acute colonic pseudo-obstruction (4). Nevertheless, the possible pathogenesis of Ogilvie′s syndrome combined with VZV infection remains not fully understood. In this regard, various hypotheses have been proposed to explain the origin of colonic dilatation. The first is parietal and visceral peritoneal inflammation linked to vesicular eruption (14). The second is viral involvement of the extrinsic autonomic nervous system, either through infection of anterior horn motor neurons or by involvement of the celiac plexus ganglion (14). The third refers to direct VZV injury of both the ENS (submucosal and myenteric plexuses) and the colonic muscularis propria (4, 14). Another hypothesis suggests hemorrhagic infarction of the abdominal sympathetic ganglia as the main mechanism of the colonic pseudo-obstruction (16). An additional theory proposes that VZV injury of the thoracolumbar or sacral lateral columns may interrupt sacral parasympathetic nerves and subsequently decrease intestinal contractility (18). Finally, a recent study put forward the hypothesis that thoracic herpes zoster may prevent afferent C-fibers from facilitating gastrointestinal motility (by tachykinins), with ensuing acute intestinal pseudo-obstruction (10).
The limited number of published reports on the coexistence of Ogilvie's syndrome and VZV infection and the absence of laboratory diagnosis in most of the cases prevent a definitive assessment of the diagnostic methods potentially available in this setting. Thus, it is plausible in such a context to apply the same general considerations that are valid when diagnosing herpes zoster. In one report on laboratory diagnosis of VZV performed in vesicle specimens from 100 patients, PCR assay was compared with other diagnostic methods (19). The sensitivity and specificity of PCR for detecting VZV DNA were 95% and 100%, respectively, whereas the sensitivity and specificity for immunofluorescence testing for VZV antigen were 82% and 76%. The detection rate of VZV by cell cultures was only 20%, although the specificity was 100%. Moreover, in 48% of patients there was a serological response to specific IgM and IgA antibodies within 4 days after the onset of rash. The authors concluded that PCR should be regarded as the method of choice for rapid diagnosis of zoster. In contrast to PCR, conventional methods for diagnosing VZV infection are time-consuming and/or have reduced sensitivity and specificity and are of limited value to the physician. Other direct techniques for identifying VZV (electron microscopy or cytomorphological tests) need extensive experience and usually fail to differentiate HSV from VZV. Moreover, a recent review on herpes zoster has added no new data about the diagnostic accuracy of PCR and immunofluorescence assays for the diagnosis of VZV infection (2). This is, to our knowledge, the first time that VZV DNA has been demonstrated by specific PCR testing in cutaneous and colonic biopsy specimens from a patient diagnosed with Ogilvie's syndrome.
The presence of unexplained acute abdominal pain and acute colonic pseudo-obstruction in an immunocompromised patient should prompt clinicians to consider early diagnosis and suitable treatment (in particular, the use of intravenous acyclovir) of VZV-induced Ogilvie's syndrome (2, 14). In this regard, PCR detection of VZV DNA in blood, bowel lesions, and maybe feces prior to the development of the skin rash can represent an invaluable diagnostic tool (20).
Footnotes
Published ahead of print 7 May 2014
REFERENCES
- 1. Reference deleted.
- 2.Cohen JI. 2013. Herpes zoster. N. Engl. J. Med. 369:255–263. 10.1056/NEJMcp1302674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gershon AA, Chen J, Davis L, Krinsky C, Cowles R, Reichard R, Gershon M. 2012. Latency of varicella zoster virus in dorsal root, cranial, and enteric ganglia in vaccinated children. Trans. Am. Clin. Climatol. Assoc. 123:17–33 [PMC free article] [PubMed] [Google Scholar]
- 4.Chen JJ, Gershon AA, Li Z, Cowles RA, Gershon MD. 2011. Varicella zoster virus (VZV) infects and establishes latency in enteric neurons. J. Neurovirol. 17:578–589. 10.1007/s13365-011-0070-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gershon AA, Chen J, Gershon MD. 2008. A model of lytic, latent, and reactivating varicella-zoster virus infections in isolated enteric neurons. J. Infect. Dis. 197(Suppl 2):S61–S65. 10.1086/522149 [DOI] [PubMed] [Google Scholar]
- 6.Remmerswaal RG, de Vries AC, Ramsoekh D, van Buuren HR. 2012. Varicella zoster-associated gastric ulcers, hepatitis and pancreatitis in an immunocompromised patient. Endoscopy 44:E140. 10.1055/s-0030-1256934 [DOI] [PubMed] [Google Scholar]
- 7.Plosker GL, Figgitt DP. 2003. Rituximab. Drugs 63:803–843. 10.2165/00003495-200363080-00005 [DOI] [PubMed] [Google Scholar]
- 8.Layman RM, Ruppert AS, Lynn M, Mrozek E, Ramaswamy B, Lustberg MB, Wesolowski R, Ottman S, Carothers S, Bingman A, Reinbolt R, Kraut EH, Shapiro CL. 2013. Severe and prolonged lymphopenia observed in patients treated with bendamustine and erlotinib for metastatic triple negative breast cancer. Cancer Chemother. Pharmacol. 71:1183–1190. 10.1007/s00280-013-2112-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou SR, Liu CY. 2012. A case report of abdominal distension caused by herpes zoster. World J. Gastroenterol. 18:4627–4628. 10.3748/wjg.v18.i33.4627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hosoe N, Sakakibara R, Yoshida M, Wakabayashi T, Kikuchi H, Yamada T, Kishi M. 2011. Acute, severe constipation in a 58-year-old Japanese patient. Gut 60:1059, 1093. 10.1136/gut.2009.183392 [DOI] [PubMed] [Google Scholar]
- 11.Edelman DA, Antaki F, Basson MD, Salwen WA, Gruber SA, Losanoff JE. 2010. Ogilvie syndrome and herpes zoster: case report and review of the literature. J. Emerg. Med. 39:696–700. 10.1016/j.jemermed.2009.02.010 [DOI] [PubMed] [Google Scholar]
- 12.Precupanu CM, Girodet J, Mariani P, Zanni M, Mathiot C, Escande MC, Brault P, Decaudin D. 2009. Pseudo-bowel obstruction due to varicella zoster virus infection after autologous stem cell transplantation. Am. J. Hematol. 84:127–128. 10.1002/ajh.21309 [DOI] [PubMed] [Google Scholar]
- 13.Rodrigues G, Kannaiyan L, Gopasetty M, Rao S, Shenoy R. 2002. Colonic pseudo-obstruction due to herpes zoster. Indian J. Gastroenterol. 21:203–204 [PubMed] [Google Scholar]
- 14.Pui JC, Furth EE, Minda J, Montone KT. 2001. Demonstration of varicella-zoster virus infection in the muscularis propria and myenteric plexi of the colon in an HIV-positive patient with herpes zoster and small bowel pseudo-obstruction (Ogilvie's syndrome). Am. J. Gastroenterol. 96:1627–1630. 10.1111/j.1572-0241.2001.03808.x [DOI] [PubMed] [Google Scholar]
- 15.Herath P, Gunawardana SA. 1997. Acute colonic pseudo-obstruction associated with varicella zoster infection and acyclovir therapy. Ceylon Med. J. 42:36–37 [PubMed] [Google Scholar]
- 16.Nomdedéu JF, Nomdedéu J, Martino R, Bordes R, Portorreal R, Sureda A, Domingo-Albós A, Rutllant M, Soler J. 1995. Ogilvie's syndrome from disseminated varicella-zoster infection and infarcted celiac ganglia. J. Clin. Gastroenterol. 20:157–159. 10.1097/00004836-199503000-00020 [DOI] [PubMed] [Google Scholar]
- 17.Walsh TN, Lane D. 1982. Pseudo obstruction of the colon associated with varicella-zoster infection. Ir. J. Med. Sci. 151:318–319. 10.1007/BF02940213 [DOI] [PubMed] [Google Scholar]
- 18.Tribble DR, Church P, Frame JN. 1993. Gastrointestinal visceral motor complications of dermatomal herpes zoster: report of two cases and review. Clin. Infect. Dis. 17:431–436. 10.1093/clinids/17.3.431 [DOI] [PubMed] [Google Scholar]
- 19.Sauerbrei A, Eichhorn U, Schacke M, Wutzler P. 1999. Laboratory diagnosis of herpes zoster. J. Clin. Virol. 14:31–36. 10.1016/S1386-6532(99)00042-6 [DOI] [PubMed] [Google Scholar]
- 20.de Jong MD, Weel JF, van Oers MH, Boom R, Wertheim-van Dillen PM. 2001. Molecular diagnosis of visceral herpes zoster. Lancet 357:2101–2102. 10.1016/S0140-6736(00)05199-0 [DOI] [PubMed] [Google Scholar]


