Abstract
Smear microscopy has suboptimal sensitivity, and there is a need to improve its performance since it is commonly used to diagnose tuberculosis (TB). We prospectively evaluated the diagnostic accuracy of the small membrane filtration (SMF) method, an approach that uses a vacuum manifold and is designed to concentrate bacilli onto a filter that can be examined microscopically. We enrolled hospitalized adults suspected to have pulmonary TB in Kampala, Uganda. We obtained a clinical history and three spontaneously expectorated sputum specimens for smear microscopy (direct, concentrated, and SMF), MGIT (mycobacterial growth indicator tube) 960 and Lowenstein-Jensen (LJ) cultures, and Xpert MTB/RIF testing. We performed per-specimen (primary) and per-patient analyses. From October 2012 to June 2013, we enrolled 212 patients (579 sputum specimens). The participants were mostly female (63.2%), and 81.6% were HIV infected; their median CD4 cell count was 47 cells/μl. Overall, 19.0%, 20.4%, 27.1%, 25.2%, and 25.9% of specimens tested positive by direct smear, concentrated smear, MGIT culture, LJ culture, and Xpert test, respectively. In the per-specimen analysis, the sensitivity of the SMF method (48.5%; 95% confidence interval [CI], 37.4 to 59.6) was lower than those of direct smear (60.9%; 51.4 to 70.5 [P = 0.0001]) and concentrated smear (63.3%; 53.6 to 73.1 [P < 0.0001]). Subgroup analyses showed that SMF performed poorly in specimens having a low volume or low bacterial load. The SMF method performed poorly compared to standard smear techniques and was sensitive to sample preparation techniques. The optimal laboratory SMF protocol may require striking a fine balance between sample dilution and filtration failure rate.
INTRODUCTION
Tuberculosis (TB) remains a major global health problem (1). Despite the recent development of new diagnostic modalities (2), in much of the world, the diagnosis of pulmonary TB continues to rely on microscopic examination of stained sputum smears. Acid-fast bacillus (AFB) smear microscopy is inexpensive and rapid, has high positive predictive value in areas of high TB prevalence, and identifies the most infectious subset of TB patients (3). However, despite its widespread use, the sensitivity of routine smear microscopy ranges from 20% to 75% and its performance is highly dependent on the number of bacilli in the specimen (4, 5). The sensitivity of smear microscopy is notably poor in populations with a high burden of TB and under conditions that lead to a low mycobacterial burden in clinical specimens, such as in children, patients with HIV/AIDS-associated TB, early TB disease, or pleural or meningeal TB (2, 4). Overall, the suboptimal sensitivity of smear microscopy results in delays in diagnosis with consequent disease progression, poor outcomes, and uninterrupted transmission of M. tuberculosis (6).
Concentration of bacilli is a strategy to increase the sensitivity of sputum AFB smear microscopy. Concentration through centrifugation or gravity sedimentation results in modest (5 to 10%) gains in sensitivity (3), but centrifugation carries a risk of aerosol generation and requires equipment, while gravity sedimentation increases the specimen testing time. The small membrane filtration (SMF) method uses a vacuum manifold to concentrate bacilli present in a clinical sample onto a small membrane that can subsequently be stained and examined microscopically for AFB. By increasing the concentration of bacilli in the microscopic field, the SMF method may increase the sensitivity of smear microscopy. The SMF method reduces biohazard potential through the use of bleach (sodium hypochlorite), which kills M. tuberculosis bacilli (7), and a sealed processing system that limits sample manipulation, and thus, the method might be amenable for use in the existing global network of facilities that already use smear microscopy. Two prior studies using earlier, more rudimentary (full manual operation) versions of the vacuum manifold showed promising results for both HIV-uninfected (8) and HIV-infected (9) patients, but the processing failure rates were high, since 5 to 20% of samples failed to fully filter through the membrane.
This study prospectively evaluated the diagnostic accuracy of the SMF method using a new, multitest vacuum manifold that allowed semiautomation and a new laboratory protocol intended to reduce the filtration failure rate by dilution of the sputum sample. The study focused mainly on HIV-infected adults with suspected pulmonary TB—a population predicted to include a large proportion of individuals with relatively low bacillary burdens in their sputum and in whom the concentration effect of the SMF method would be most useful.
MATERIALS AND METHODS
Participants.
We prospectively enrolled hospitalized adults at Mulago National Referral Hospital in Kampala, Uganda. Uganda is on the WHO list of high-burden countries, with an estimated annual TB incidence rate in 2012 of 179 cases per 100,000 and with an HIV prevalence of approximately 50% among TB patients (1). Nonstudy clinicians referred potentially eligible, interested individuals to study personnel. All consecutive individuals were eligible to participate provided they were (i) ≥18 years old, (ii) suspected of active pulmonary TB based on having had a cough for ≥2 weeks and one or more of fever, night sweats, or unexplained weight loss, and (iii) willing and able to comply with the study protocol, including HIV testing. Individuals were excluded if they had received ≥2 days of antituberculous treatment within the previous 60 days. This study was approved by institutional review boards of Johns Hopkins Medicine, Boston University Medical Center, the Mulago Hospital Research and Ethics Committee, and the Uganda National Council for Science and Technology, and all participants provided written informed consent.
Clinical data.
We used a questionnaire to collect demographic and clinical information. Individuals with no documented HIV results were offered HIV testing; a CD4+ lymphocyte cell count was measured in HIV-infected patients. For each participant, two sputum samples were collected on the day of enrollment (spot specimens), and a third sputum sample was collected, usually the day after enrollment but within 4 days (morning specimen). A posteroanterior chest radiograph was performed on all nonpregnant study participants.
SMF manifold.
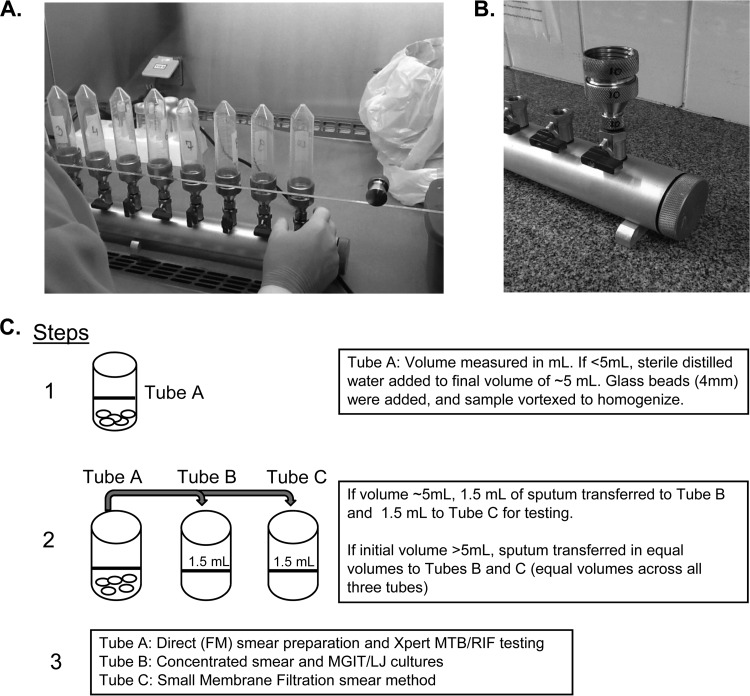
The SMF manifold (version 5.0) device consists of a cylindrical manifold connected to a single vacuum and uses separate control valves at each station for independent operation (Fig. 1A and B). The manifold is made of anodized aluminum, which is durable and lightweight, has large port openings for easy cleaning and sanitizing, and has 1/4-in. stainless steel drain plugs, chrome-plated brass valves, Viton O rings, and 1/4-in. nylon adapters. The prototype manifold shown contains 10 workstations, each fitted with a stainless steel screw-on port that holds an SMF filter apparatus. Each SMF filter apparatus is comprised of four parts, as follows: (i) a reservoir with a membrane filter, (ii) a first inlet-outlet port in communication with a second reservoir, (iii) a second reservoir containing a prefilter, positioned adjacent to the second surface, and (iv) a third reservoir designed to work with standard 50-ml centrifuge tubes, which eliminates sample transfer and cross contamination risk. This filter apparatus allows efficient staining of bacilli after they have been collected and immobilized on the membrane, which minimizes membrane loss or disruption. When samples are ready for processing, vacuum is applied to draw a sample through the metal holders containing a nylon net prefilter (pore size, 30 μm; Millipore) and a smaller Isopore membrane filter (pore size, 0.8 μm; Millipore). The version of the manifold prototype (version 5.0) evaluated in this study had several design and technical improvements over the manifold used (version 4.0) in a recent study in Brazil (9), including (i) a modification of the neck holder in order to fit the screw caps of 50-ml polypropylene tubes (to minimize cross-contamination), (ii) a modification to the tube holder to allow membrane staining, (iii) replacement of O rings, and (iv) fine adjustment of the rotation key to turn or adjust the holder's set screws.
FIG 1.
(A) Small membrane filtration (SMF) manifold (version 5.0) evaluated. The SMF manifold device consists of a cylindrical manifold connected to a single vacuum and uses separate control valves at each station for independent operation. (B) The prototype manifold shown contains 10 workstations, each fitted with a stainless steel screw-on port that holds an SMF filter apparatus. (C) Sputum processing and aliquoting protocol.
Laboratory assessments.
All laboratory testing was performed on site in Kampala, Uganda, in the Mycobacteriology (biosafety level 3 [BSL-3]) laboratory of the Department of Medical Microbiology, College of Health Sciences, Makerere University, with internationally acceptable quality standards. Sputum testing was initiated directly after collection and delivery to the laboratory. Upon arrival, sputum samples were visually examined for volume and viscosity; sterile distilled water was added to specimens of <5 ml to obtain a total sample volume of ∼5 ml. Samples were then homogenized using sterile glass beads (4 mm; Fisher Scientific, Hampton, NH, USA) and split into three aliquots (in tubes labeled A, B, and C) of approximately equal volume (Fig. 1C). The tube A aliquot (original specimen container) underwent processing for direct smear review using standardized WHO/IUATLD fluorescence microscopy (FM) methods and was examined for acid-fast bacilli (AFB) and graded (10). An additional portion of the tube A specimen was tested on the Xpert MTB/RIF (Cepheid, Sunnyvale, CA, USA) platform according to the manufacturer's instructions (May 2012, 2:1 [vol/vol] sample reagent/sputum volume). The specimen in tube B was digested and decontaminated using N-acetyl-l-cysteine (NALC)–NaOH (11) and resuspended with approximately 2 ml of phosphate-buffered saline (PBS); a 0.05-ml portion of the sputum sediment was smeared and reviewed using standardized WHO/IUATLD FM methods and smear grading scheme (10). An additional 0.5 ml of the sediment was cultured using the Bactec MGIT (mycobacterial growth indicator tube) 960 system (Becton and Dickinson, Sparks, MD, USA), and 0.2 ml was inoculated onto Lowenstein-Jensen (LJ) medium (prepared in-house at the National Tuberculosis Reference Laboratory). Cultures were incubated using either the automated MGIT 960 system for up to 6 weeks or at 37°C on LJ medium for up to 8 weeks. Cultures positive for growth were assessed for AFB using Ziehl-Neelsen (ZN) staining and light microscopy, and mycobacterial growth was identified to the species level as M. tuberculosis complex versus not M. tuberculosis complex using an anti-MPB64 antibody assay (SD MPT64TB Ag kit; SD Bioline, South Korea).
Tube C was processed for SMF testing. The SMF method was performed by trained laboratory personnel according to a written manual of procedures. Briefly, aliquot C was transferred to a 50-ml conical tube, a mixture of NALC-sodium citrate was added (equal to one-half volume of the original sample), and the sample was vortexed. One milliliter of this sample was then mixed with 2 ml 5% sodium hypochlorite (JIK brand; Reckitt Benckiser, Kampala, Uganda) and 3 ml Triton X-100-ethanol (final concentration, 95% ethanol–1% Triton X-100) (Triton X-100 was from Sigma-Aldrich, USA), for a final filterable volume of ∼6 ml. The conical tube was then attached to the SMF manifold, and the sample was filtered. Once the sample had been completely filtered, the 0.8-μm Isopore membrane filter containing trapped material was attached to a standard glass microscopy slide (Fisher Scientific), heat fixed at 80°C for 10 min, and stained and graded using standardized WHO/IUATLD FM methods (10). All methods were quality controlled, and the personnel interpreting the SMF smears were blinded to the results of other diagnostic tests.
Statistical methods.
We report the results according to the Standards for Reporting of Diagnostic Accuracy (STARD) guidelines (12, 13). Bivariate comparisons were made using Fisher's exact test for dichotomous variables and the Wilcoxon rank sum test for continuous variables. Sensitivity and specificity were calculated at both a per-specimen and per-participant level using culture and Xpert MTB/RIF results as separate reference standards. Smears were considered positive if they were graded scanty, 1+, 2+, or 3+. A specimen was considered culture positive if the MGIT and/or LJ culture was positive for M. tuberculosis, culture negative if both the MGIT and LJ culture were negative for M. tuberculosis, and contaminated if both the MGIT and LJ cultures were contaminated. A participant was considered culture positive if any culture was positive for M. tuberculosis, culture negative if all cultures were negative for M. tuberculosis, and contaminated if all cultures were contaminated. Cultures positive only for nontuberculous mycobacteria were considered negative for M. tuberculosis. A ratio estimator was used to adjust confidence intervals for clustering in the per-specimen analysis. All participants were included irrespective of the number of specimens provided. Specimens were excluded from the SMF diagnostic accuracy analysis if all cultures were contaminated. Comparisons between smear methods were made using McNemar's test. Analyses were performed using SAS version 9.3. All P values are two sided, with statistical significance defined as a P value of <0.05.
RESULTS
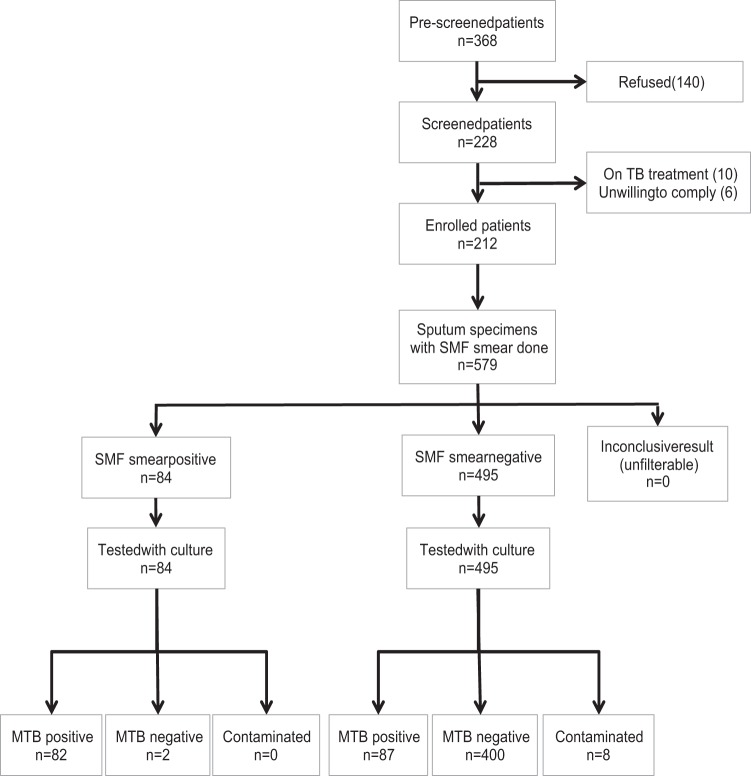
From October 2012 to June 2013, we screened 368 potentially eligible patients for study participation (Fig. 2). Of the 228 patients with possible TB who consented, we excluded 16 (7%) patients (10 were on TB treatment and 6 were unable or unwilling to comply). This analysis includes the 212 patients with possible TB with ≥1 sputum test (per-patient analysis) and the 579 corresponding sputum specimens (per-specimen analysis).
FIG 2.
Study profile. SMF, small membrane filtration method; MTB, Mycobacterium tuberculosis; NTM, nontuberculous mycobacteria.
Study population.
The study participants were mostly female (63.2%) and had a median age of 32 years (interquartile range [IQR], 27 to 41) (Table 1). The majority (81.6%) were HIV infected, with a median CD4 count of 47 cells/μl (IQR, 11 to 222). The distribution of clinical symptoms among study participants was as follows: cough (100%), fatigue (97.6%), poor appetite (97.2%), fever (92.5%), weight loss (92.0%), and night sweats (72.2%). Of the 187 (88%) patients with chest X-ray results, 69.5% were abnormal, and the most common radiographic findings were infiltrates (97.7%), adenopathy (64.6%), and pleural disease/effusion (27.7%); 9.6% had lung cavitations.
TABLE 1.
Demographic and clinical description of study population
| Parametera | Median no.b [interquartile range] or no. (%) |
|---|---|
| Patients | 212 |
| Age (yr) | 32 [27–41] |
| Female | 134 (63.2) |
| History of TB | 24 (11.3) |
| HIV status | |
| HIV infected | 173 (81.6) |
| CD4 cell count (cells/ml) | 47 [11–222] |
| ART status | 134 |
| Currently on ART | 80 (59.7) |
| Prior ART | 7 (5.2) |
| Never on ART | 47 (35.1) |
| Result of chest radiograph | 187 |
| Normal | 57 (30.5) |
| Abnormal | 130 (69.5) |
| Infiltrates | 127 (67.9) |
| Adenopathy | 84 (44.9) |
| Pleural disease/effusion | 36 (19.3) |
| Cavitation | 18 (9.6) |
| Miliary infiltrate | 10 (5.3) |
| Otherc | 6 (3.2) |
| Sputum specimens | 579 |
| Collection time | |
| Spot | 407 (70.3) |
| Early morning | 172 (29.7) |
| Vol (ml) | 3 [2–4] |
| Visual description | |
| Blood stained | 30 (5.2) |
| Watery | 255 (44.0) |
| Viscous | 302 (52.2) |
| Very viscous | 22 (3.8) |
| AFB smear microscopy (direct/concentrated sputum) | |
| Negative | 469 (81.0)/461 (79.6) |
| Scanty | 25 (4.3)/30 (5.2) |
| 1+ | 35 (6.0)/27 (4.7) |
| 2+ | 17 (2.9)/19 (3.3) |
| 3+ | 33 (5.7)/42 (7.2) |
| Sputum culture | |
| MGIT 960 positive | 157 (27.1) |
| LJ medium positive | 146 (25.2) |
| <50 colonies | 34 (23.3) |
| 1+ (50–100 colonies) | 37 (25.3) |
| 2+ (100–200 colonies) | 29 (19.9) |
| 3+ (200–500 colonies) | 36 (24.7) |
| 4+ (>500 colonies) | 10 (6.9) |
| Xpert MTB/RIF positive | 150 (25.9) |
ART, antiretroviral therapy; AFB, acid-fast bacillus; MGIT, mycobacterial growth indicator tube; LJ medium, Lowenstein-Jensen medium.
Number of patients or specimens.
Pericardial effusion (n = 5) and bone disease (n = 1).
Sputum characteristics and standard laboratory results.
Of the 212 patients enrolled, 164 (77%) provided three sputum specimens, 39 (18%) provided two specimens, and 9 (4%) provided a single specimen, totaling 579 sputum specimens available for analysis (Table 1). Among the sputum specimens, 255/579 (44.0%) were watery and 302/579 (52.2%) were viscous. There was a wide variation in the volume of sputum specimens (median, 3 ml; IQR, 2 to 4 ml; range, 0.2 to 30 ml).
In the per-specimen analysis shown in Table 1, 110/579 (19.0%) specimens were positive by direct smear microscopy and 118/579 (20.4%) were positive by concentrated smear microscopy; 157/579 (27.1%), 146/579 (25.2%), and 150/579 (25.9%) specimens were positive for M. tuberculosis by MGIT culture, LJ culture, and Xpert test, respectively. In the per-patient analysis, 54/212 (25.5%) participants were positive by direct smear and 59/212 (27.8%) were positive by concentrated smear; 70/212 (33.0%), 72/212 (34.0%), and 70/212 (33.0%) of participants were positive for M. tuberculosis by MGIT culture, LJ culture, and Xpert test, respectively.
Sensitivity of SMF method (per-specimen analysis).
All specimens were successfully filtered using the SMF method (i.e., no filtration failures). The median filtration time was 2 min 47 s (IQR, 1 min 37 s to 4 min 59 s). The sensitivities of the three smear methods (direct, concentrated, and SMF) compared to those of culture (any positive culture result) and Xpert test as reference standards in a per-specimen analysis are shown in Table 2. Using culture as the reference standard, the sensitivity of the SMF method (82/169, 48.5%) was significantly lower than those of both the direct (103/169, 60.9%, P = 0.0001) and the concentrated (107/169, 63.3%, P < 0.0001) smear methods. We observed similar results using Xpert as the reference standard (Table 2).
TABLE 2.
Per-specimen smear results by different reference methods
| Reference standard test and smear method | No. of specimens (n = 579) with indicated result in reference standard test that were positive by indicated smear method |
Sensitivity [no. positive,b % (95% CIa)] | P valuec | Specificity [no. negative, % (95% CI)]d | P value | PPVe ([no. positive/total no. (%)] | NPVf ([no. negative/total no. (%)] | ||
|---|---|---|---|---|---|---|---|---|---|
|
M. tuberculosis |
Contaminated (culture) or invalid/error (Xpert) | ||||||||
| Not detected | Detected | ||||||||
| Cultureg | 402 | 169 | 8 | ||||||
| Direct | 7 | 103 | 0 | 103, 60.9 (51.4–70.5) | 0.0001 | 395, 98.3 (97.0–99.5) | 0.219 | 103/110 (93.6) | 395/461 (85.7) |
| Concentrated | 11 | 107 | 0 | 107, 63.3 (53.6–73.1) | <0.0001 | 391, 97.3 (95.7–98.9) | 0.004 | 107/118 (90.7) | 391/453 (86.3) |
| SMF | 2 | 82 | 0 | 82, 48.5 (37.4–59.6) | 400, 99.5 (98.8–100) | 82/84 (97.6) | 400/487 (82.1) | ||
| Xpert test | 429 | 150 | 0 | ||||||
| Direct | 6 | 104 | 0 | 104, 69.3 (60.4–78.2) | 0.0001 | 423, 98.6 (97.2–100) | 0.219 | 104/110 (94.5) | 423/469 (90.2) |
| Concentrated | 10 | 108 | 0 | 108, 72.0 (63.0–81.0) | <0.0001 | 419, 97.7 (96.0–99.3) | 0.008 | 108/118 (91.5) | 419/461 (90.9) |
| SMF | 2 | 82 | 0 | 82, 54.7 (43.4–65.9) | 427, 99.5 (98.9–100) | 82/84 (97.6) | 427/495 (86.3) | ||
Ratio estimators were used to adjust confidence intervals for specimen clustering resulting from multiple specimens per participant.
Sensitivity: for culture, n = 169, and for Xpert test, n = 150.
P values were calculated using McNemar's test. All comparisons are against SMF smear method.
Specificity: for culture, n = 402, and for Xpert test, n = 429.
PPV, positive predictive value.
NPV, negative predictive value.
Includes any culture-positive (MGIT or Lowenstein-Jensen medium) result for the same specimen.
Sensitivity of SMF method (per-patient analysis).
The sensitivities of the three smear methods in a per-patient analysis compared to those of culture and Xpert test as the reference standards are shown in Table 3. Using culture as the reference standard, the sensitivity of the SMF method (39/76, 51.3%) was significantly lower than those of the direct (51/76, 67.1% [P = 0.0004]) and concentrated (52/76, 68.4% [P = 0.0002]) smear methods. Again, the use of Xpert test as the reference standard did not significantly change these results (Table 3).
TABLE 3.
Per-patient smear results by different reference methods
| Reference standard and smear method | No. of patients (n = 212) with indicated result in reference standard test that were positive by indicated smear method |
Sensitivity [no. positive, % (95% CI)]a | P valueb | Specificity [no. negative, % (95% CI)]c | P value | PPVd ([no. positive/total no. (%)] | NPVe ([no. negative/total no. (%)] | ||
|---|---|---|---|---|---|---|---|---|---|
|
M. tuberculosis |
Contaminated (culture) or invalid/error (Xpert) | ||||||||
| Not detected | Detected | ||||||||
| Culturef | 134 | 76 | 2 | ||||||
| Direct | 3 | 51 | 0 | 51, 67.1 (55.4–77.5) | 0.0004 | 131, 97.8 (93.6–99.5) | 0.625 | 51/54 (94.4) | 131/156 (84.0) |
| Concentrated | 7 | 52 | 0 | 52, 68.4 (56.8–78.6) | 0.0002 | 127, 94.8 (89.5–97.9) | 0.031 | 52/59 (88.1) | 127/151 (84.1) |
| SMF | 1 | 39 | 0 | 39, 51.3 (39.6–63.0) | 133, 99.3 (95.9–100) | 39/40 (97.5) | 133/170 (78.2) | ||
| Xpert test | 142 | 70 | 0 | ||||||
| Direct | 4 | 50 | 0 | 50, 71.4 (59.4–81.6) | 0.0004 | 138, 97.2 (92.9–99.2) | 0.625 | 50/54 (92.6) | 138/158 (87.3) |
| Concentrated | 8 | 51 | 0 | 51, 72.9 (60.9–82.8) | 0.0002 | 134, 94.4 (89.2–97.5) | 0.031 | 51/59 (86.4) | 134/153 (87.6) |
| SMF | 2 | 38 | 0 | 38, 54.3 (41.9–66.3) | 140, 98.6 (95.0–99.8) | 38/40 (95.0) | 140/172 (81.4) | ||
Sensitivity: for culture, n = 76, and for Xpert test, n = 70.
P values were calculated using McNemar's test. All comparisons are against SMF smear method.
Specificity: for culture, n = 134, and for Xpert test, n = 142.
PPV, positive predictive value.
NPV, negative predictive value.
Includes any culture-positive (MGIT or Lowenstein-Jensen medium) result for the same patient.
Specificity of SMF method.
In the per-specimen analysis (Table 2), the specificity for all three AFB smear methods using culture or Xpert test as the reference standard was high (>97%); however, the specificity of the concentrated smear method was significantly lower than that of the SMF method (P = 0.004 for culture as the reference standard and P = 0.008 for Xpert as the reference standard). Similarly, in the per-patient analysis with culture as the reference standard (Table 3), the specificity of the concentrated smear method was lower (94.8%, P = 0.031) than that of the SMF method.
Subgroup analyses.
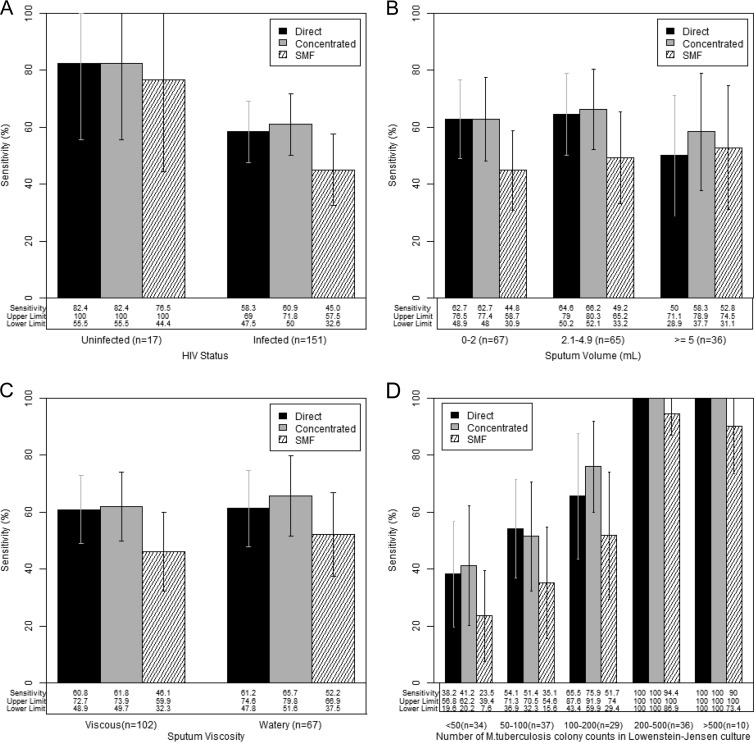
To further understand the effects that specimen volume, viscosity, and bacterial load had on the performance of the SMF method, we performed subgroup analyses by HIV status, specimen volume and viscosity, and semiquantitative LJ culture results, using culture as the reference standard. In HIV-uninfected patients, the sensitivities of the direct (14/17, 82.4%), concentrated (14/17, 82.4%), and SMF (13/17, 76.5%) smear methods were similar (P = 1.0), and the specificities of all three methods (>97%) were comparable (Fig. 3A; see also Table S1 in the supplemental material). In HIV-infected patients, however, the sensitivity of SMF (68/151, 45.0%) was significantly lower than those of both the direct (88/151, 58.3% [P = 0.0003]) and concentrated (92/151, 60.9% [P < 0.0001]) smear methods.
FIG 3.
(A to D) Sensitivity of the SMF method compared to those of direct and concentrated AFB smear microscopy in a per-specimen analysis with MGIT culture as the reference standard. Only MGIT culture-positive specimens were included. Results show sensitivity by HIV infection status (n = 168; one HIV result was missing) (A), sputum volume (n = 168; one volume was missing) (B), sputum viscosity (visual determination) (C), and number of M. tuberculosis CFU in Lowenstein-Jensen (LJ) culture (n = 146; 23 specimens were negative or contaminated on LJ culture) (D).
In a subgroup analysis by volume, we grouped samples into low (≤2 ml), intermediate (2.1 to 4.9 ml), and high (≥5 ml) volumes (Fig. 3B). Whereas the sensitivities of the direct and concentrated smear methods varied slightly across volume categories, the sensitivity of the SMF method decreased with decreasing sputum volumes. The sensitivity of the SMF method was lower in viscous samples than in watery samples (Fig. 3C). Finally, whereas the sensitivity of all three smear methods decreased as the number of M. tuberculosis colonies in LJ culture decreased, the drop in sensitivity of the SMF method was the largest (Fig. 3D).
DISCUSSION
This study is the first prospective evaluation of the SMF smear microscopy method using a new, multitest, semiautomated manifold and a new laboratory protocol. In the population studied, comprised of patients suspected to have pulmonary TB, most of whom had advanced HIV-related immunosuppression, the sensitivity of the SMF method was significantly lower than those of the direct and the concentrated AFB smear microscopy techniques. This finding was consistent across per-specimen and per-participant analyses and whether culture or the Xpert MTB/RIF test was considered the gold standard. Our results suggest that the new laboratory SMF protocol, which was developed to minimize the filtration failure rate by diluting the clinical sample, may have compromised the performance of the method in specimens with a low bacterial load (or low volume) where the concentrating capacity of the SMF method would be most useful.
Prior studies in both HIV-uninfected and HIV-infected patients with pulmonary TB had shown promising results with previous versions of the SMF method (8, 9). In a study of 313 HIV-uninfected TB suspects using solid Ogawa culture as the reference standard, the mean sensitivity of the SMF method on the first sputum specimen using either light microscopy (LM) or fluorescence microscopy (FM) was 89% (95% CI, 80 to 94), compared to sensitivities of 60% (95% CI, 49 to 70) for centrifuged smears and 56.1% (95% CI, 40 to 71) for direct smears. In a subset of 55 subjects with two specimens each, the sensitivities were 97% (95% CI, 83 to 99) for the SMF method and 70% (95% CI, 53 to 83) for the centrifuged smear method. The incremental yield (41%) of the SMF method was largest in specimens with a low concentration of bacilli on culture (1 to 200 CFU) (8). Among smear-negative/culture-positive patients, the sensitivity of the SMF method using FM was 73% (95% CI, 56 to 85), similar to the sensitivity of the Xpert MTB/RIF test for traditionally smear-negative specimens. However, this proof-of-concept study used a time-consuming manual version of the SMF method (version 1.0), and approximately 5% of samples failed to filter because the SMF membrane became clogged with debris (personal communication, M. Palaci). In a second study in Manaus, Brazil, that included 432 patients (60% HIV positive), the SMF method also performed well. In HIV-uninfected patients, the sensitivity of the SMF method (81.8%) was lower than that of the initial study but significantly higher than those for centrifugation of sputum samples with or without NALC treatment (63.6% and 57.5%, respectively). In HIV-infected TB patients, the sensitivity of the SMF method (61.9%) was significantly higher than the sensitivities achieved by centrifugation of sputum samples with or without NALC treatment (47.6% and 45.2%, respectively). However, the sample filtration failure rate was as high as 20% using an earlier version (version 4.0) of the semiautomated manifold evaluated in this study and a different sample preparation protocol (9).
In this study, the SMF method performed particularly poorly in viscous sputum samples with either a low specimen volume or low bacterial load (as measured by M. tuberculosis growth in semiquantitative Lowenstein-Jensen culture) where the concentrating capacity of the SMF method would be most useful. The importance of sputum quality, specimen volume, and bacterial load content in the performance of AFB smear microscopy methods is well known (3, 5). In a large, prospective study by Warren et al., the sensitivity of smears during a 39-month period (n = 1,849) using ≥5.0 ml of sputum was 92.0%, significantly greater (P < 0.001) than the sensitivity of 72.5% in a previous 24-month period (n = 3,486) when all specimens were processed regardless of volume (14). In a recent study, sputum gross appearance and volume were associated with direct smear positivity (15). A related issue in TB diagnostic studies is the difficulty in accurately evaluating low-volume samples, as specimen splitting is likely to provide a more robust comparison of diagnostic performance than testing sister samples from the same patient (16). In this study, our specimen processing and splitting protocol may have unexpectedly improved the performance of the direct smear (17) and, therefore, further masked the performance of the SMF method. However, our specimen-splitting protocol would have similarly masked the performance of concentrated smears and both types of culture. In an effort to assess the resulting bacillary load present in the three specimen aliquots that were tested, we performed a theoretical analysis that included various specimen volume and AFB/ml scenarios (data not shown) and found that the expected total bacillary load available upon inoculation of each aliquot was comparable.
This study had several limitations. The study population was restricted to hospitalized patients suspected to have TB (most with advanced HIV/AIDS), and thus, we do not know if the results of this study are applicable to TB patients in outpatient settings. However, because we did not exclude patients with low-volume sputum samples or those with missing samples, the results of this study are broadly applicable to the intended study population—namely, patients suspected to have TB, with advanced HIV/AIDS, producing challenging, low-quality sputum samples for diagnostic purposes. The sputum samples submitted for study were manipulated (volume top-off if the volume was <5 ml, dilution, and homogenization with glass beads) in an effort to generate comparable aliquots for parallel testing. These laboratory modifications, which would not typically be performed in real-world specimens, may have modified our study results; in particular, the direct smear technique we employed may not be representative of the standard smear microscopy used in field laboratories. However, the standard microbiological results (MGIT culture, LJ culture, and Xpert test) from this well-conducted study (minimal losses and protocol-specified, quality-controlled laboratory methods) were consistent with those of other diagnostic studies in similar settings.
In conclusion, the results from this study show that the SMF smear microscopy method using a new, multitest, semiautomated manifold and a new laboratory protocol did not improve sensitivity using either culture or Xpert MTB/RIF as the reference standard. Our results suggest that the SMF method is sensitive to sample preparation techniques and that refinements in specimen processing are required to minimize filtration failures and yet preserve recovery of bacilli on the SMF membrane.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Kevin Fennelly for his help in developing the SMF laboratory protocol and to Stephanie Moine (BMC research and regulatory coordinator). We acknowledge the invaluable contributions from Gloria Lubega, Joy Kisa Lwanga, Teddy Nalwoga, Shiphrah Birungi, Francis Kakooza, and Olive Mbabazi in Kampala and the staff of the Mycobacteriology (BSL-3) laboratory, Department of Medical Microbiology at Makerere University, Francis Mumbowa, Namaganda Carolyn, Maria Nasoolo, Happy Edward, Mboowa Gerald, Kiyimba Anthony, Germine Nakayita, and Bugumirwa Eric. We also wish to thank the study participants for their willingness to be involved in the study.
The authors' contributions were as follows: conception and design, E.J.-L., Y.C.M., M.P., D. Armstrong, P.A.J., D. Alland, R.D., M.J., J.J.E., and S.E.D.; acquisition of data, Y.C.M., C.K., D. Armstrong, L.N., W.S., M.G., R.K., and M.J.; analysis and interpretation, E.J.-L., M.G., R.K., and S.E.D. All authors contributed to either drafting or revising the manuscript and gave final approval.
This study was supported by NIH-NIAID award N01AI90500C (Tuberculosis Clinical Diagnostics Research Consortium [TBCDRC]).
Footnotes
Published ahead of print 7 May 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JCM.00642-14.
REFERENCES
- 1.WHO. 2013. Global tuberculosis report 2013. No. WHO/HTM/TB/2013.11. World Health Organization, Geneva, Switzerland: http://www.who.int/tb/publications/global_report/en/ [Google Scholar]
- 2.Dorman SE. 2010. New diagnostic tests for tuberculosis: bench, bedside, and beyond. Clin. Infect. Dis. 50(Suppl 3):S173–S177. 10.1086/651488 [DOI] [PubMed] [Google Scholar]
- 3.Steingart KR, Ng V, Henry M, Hopewell PC, Ramsay A, Cunningham J, Urbanczik R, Perkins MD, Aziz MA, Pai M. 2006. Sputum processing methods to improve the sensitivity of smear microscopy for tuberculosis: a systematic review. Lancet Infect. Dis. 6:664–674. 10.1016/S1473-3099(06)70602-8 [DOI] [PubMed] [Google Scholar]
- 4.Getahun H, Harrington M, O'Brien R, Nunn P. 2007. Diagnosis of smear-negative pulmonary tuberculosis in people with HIV infection or AIDS in resource-constrained settings: informing urgent policy changes. Lancet 369:2042–2049. 10.1016/S0140-6736(07)60284-0 [DOI] [PubMed] [Google Scholar]
- 5.Anonymous. 2000. Diagnostic standards and classification of tuberculosis in adults and children. Am. J. Respir. Crit. Care Med. 161:1376–1395. 10.1164/ajrccm.161.4.16141 [DOI] [PubMed] [Google Scholar]
- 6.Davies PD, Pai M. 2008. The diagnosis and misdiagnosis of tuberculosis. Int. J. Tuberc. Lung Dis. 12:1226–1234 [PubMed] [Google Scholar]
- 7.Best M, Sattar SA, Springthorpe VS, Kennedy ME. 1990. Efficacies of selected disinfectants against Mycobacterium tuberculosis. J. Clin. Microbiol. 28:2234–2239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fennelly KP, Morais CG, Hadad DJ, Vinhas S, Dietze R, Palaci M. 2012. The small membrane filter method of microscopy to diagnose pulmonary tuberculosis. J. Clin. Microbiol. 50:2096–2099. 10.1128/JCM.00572-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quinco P, Buhrer-Sekula S, Brandao W, Monte R, Souza SL, Saraceni V, Palaci M, Dietze R, Cordeiro-Santos M. 2013. Increased sensitivity in diagnosis of tuberculosis in HIV-positive patients through the small-membrane-filter method of microscopy. J. Clin. Microbiol. 51:2921–2925. 10.1128/JCM.00683-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO. 1998. Laboratory services in tuberculosis control: microscopy, part II. World Health Organization, Geneva, Switzerland: http://whqlibdoc.who.int/hq/1998/WHO_TB_98.258_(part2).pdf [Google Scholar]
- 11.Kent PT, Kubica GP. 1985. Public health mycobacteriology—a guide for the level III laboratory. U. S. Department of Health and Human Services, Public Health Service, Centers for Disease Control, Atlanta, GA [Google Scholar]
- 12.Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig LM, Lijmer JG, Moher D, Rennie D, de Vet HC. 2003. Towards complete and accurate reporting of studies of diagnostic accuracy: The STARD Initiative. Ann. Intern. Med. 138:40–44. 10.7326/0003-4819-138-1-200301070-00010 [DOI] [PubMed] [Google Scholar]
- 13.Fontela PS, Pant Pai N, Schiller I, Dendukuri N, Ramsay A, Pai M. 2009. Quality and reporting of diagnostic accuracy studies in TB, HIV and malaria: evaluation using QUADAS and STARD standards. PLoS One 4:e7753. 10.1371/journal.pone.0007753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Warren JR, Bhattacharya M, De Almeida KN, Trakas K, Peterson LR. 2000. A minimum 5.0 ml of sputum improves the sensitivity of acid-fast smear for Mycobacterium tuberculosis. Am. J. Respir. Crit. Care Med. 161:1559–1562. 10.1164/ajrccm.161.5.9908063 [DOI] [PubMed] [Google Scholar]
- 15.Yoon SH, Lee NK, Yim JJ. 2012. Impact of sputum gross appearance and volume on smear positivity of pulmonary tuberculosis: a prospective cohort study. BMC Infect. Dis. 12:172. 10.1186/1471-2334-12-172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hadad DJ, Morais CG, Vinhas SA, Fennelly KP, Dietze R, Nascimento CP, Palaci M. 2012. Evaluation of processing methods to equitably aliquot sputa for mycobacterial testing. J. Clin. Microbiol. 50:1440–1442. 10.1128/JCM.05835-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cattamanchi A, Dowdy DW, Davis JL, Worodria W, Yoo S, Joloba M, Matovu J, Hopewell PC, Huang L. 2009. Sensitivity of direct versus concentrated sputum smear microscopy in HIV-infected patients suspected of having pulmonary tuberculosis. BMC Infect. Dis. 9:53. 10.1186/1471-2334-9-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.