Abstract
From 2002 to 2013 in Montreal, Quebec, Canada, 38 Campylobacter coli isolates were more frequently erythromycin, tetracycline, and ciprofloxacin resistant than 440 Campylobacter jejuni subsp. jejuni isolates (18.4% versus 1.8%; P = 0.00005), of which the 148 isolates acquired abroad were more frequently erythromycin, tetracycline, and ciprofloxacin resistant than the 292 isolates acquired locally (5.4% versus 0%; P = 0.0001).
TEXT
Campylobacter jejuni subsp. jejuni and Campylobacter coli are major human pathogens representing the first and second most frequent Campylobacter species, respectively, in most countries (1, 2). Macrolides and fluoroquinolones are first- and second-choice agents when antimicrobial treatment is indicated (1, 2). The objectives of this study were to ascertain and compare the erythromycin, tetracycline, and ciprofloxacin resistance rates of human C. jejuni subsp. jejuni and C. coli isolated in 2002 to 2013 in Montreal, Quebec, Canada. The resistance rates of C. jejuni subsp. jejuni and C. coli isolates acquired abroad were compared to the resistance rates of those acquired locally. The multidrug resistance rates of C. jejuni subsp. jejuni and C. coli isolates obtained from 2002 to 2013 to erythromycin and ciprofloxacin or to erythromycin, tetracycline, and ciprofloxacin were compared to one another.
C. jejuni subsp. jejuni and C. coli were isolated from 2002 to 2013 at the Centre Hospitalier de l'Université de Montréal (CHUM)–Hôpital Saint-Luc. The phenotypic identification (2) of 96 C. jejuni subsp. jejuni isolates, including all multidrug-resistant isolates, and all other Campylobacter spp., including C. coli, was confirmed at the genus and species levels by cpn60 gene sequencing (3) at the Laboratoire de Santé Publique du Québec (LSPQ). Susceptibilities to erythromycin, tetracycline, and ciprofloxacin were assessed initially by disk diffusion and later confirmed by agar dilution, Etest (AB Biodisk, Solna, Sweden), or both (2, 4, 5). Clinical and Laboratory Standards Institute Campylobacter susceptibility and resistance breakpoints for erythromycin, tetracycline, and ciprofloxacin were implemented (4). Susceptibilities to amoxicillin-clavulanic acid, gentamicin, and imipenem were determined by the Etest method. The significance of differences was analyzed by the chi-square test, Fisher's exact 2-tailed test, or the chi-square test for linear trend with Epi Info software, version 6.0 (Centers for Disease Control and Prevention). P values of ≤0.05 were considered statistically significant.
Considering a single Campylobacter isolate per patient, 479 C. jejuni subsp. jejuni (86.6%), 38 C. coli (6.9%), and 36 other Campylobacter species (C. fetus, C. lari, C. upsaliensis, and C. hyointestinalis) (6.5%) isolates were obtained at CHUM–Hôpital Saint-Luc from 2002 to 2013. In previous studies, C. coli represented 7 to 15% of the Campylobacter spp. identified (6). Eight of the 479 (1.7%) C. jejuni subsp. jejuni isolates and 1 (2.6%) of the 38 C. coli isolates were isolated from blood (P = 0.5; relative risk [RR] [95% confidence limits {CL}, 0.63 [0.08 to 4.94]); 4 C. jejuni subsp. jejuni bacteremic patients also had stool cultures positive for the same Campylobacter sp. than the one isolated from their blood. Overall, 475 C. jejuni subsp. jejuni and 37 C. coli isolates were obtained from stools, with a 2.25% positive rate for these species in stools during those years. C. jejuni subsp. jejuni and C. coli were isolated more frequently from blood than reported previously (i.e., 0.15% of Campylobacter infections [2]). The automated blood culture systems and patient immune statuses were variables that influenced the bacteremia rates (1, 2). In the week before symptom onset, 148 C. jejuni subsp. jejuni isolates (30.9%) and 21 C. coli (55.3%) isolates were acquired abroad, outside the province of Quebec, and 331 (69.1%) C. jejuni subsp. jejuni and 17 (44.7%) C. coli isolates were acquired locally (P = 0.004; RR [95% CL], 0.56 [0.41 to 0.77]), data similar to those reported previously (6). Among these 479 C. jejuni subsp. jejuni isolates, 3 clusters were documented (7; our unpublished data) and only 1 isolate per outbreak was included in our susceptibility study, for a total of 440 C. jejuni subsp. jejuni. One C. coli isolate was involved in a documented outbreak (8), and all 38 C. coli isolates were included in our susceptibility study.
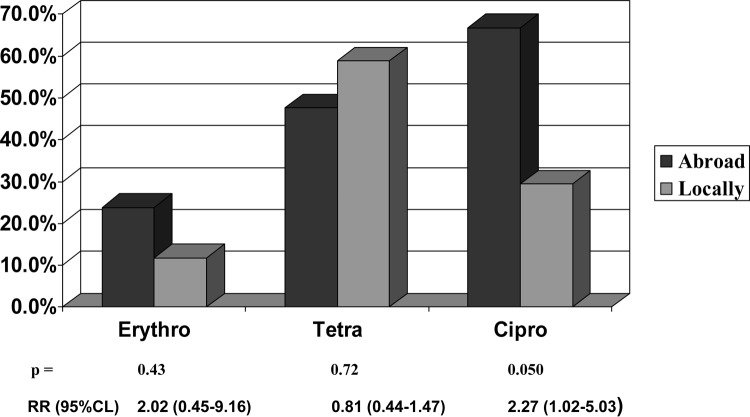
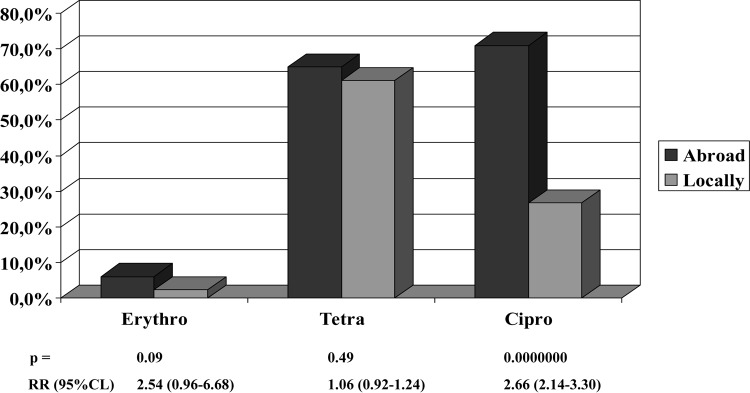
The 38 C. coli isolates were significantly more resistant to erythromycin, but not to tetracycline or ciprofloxacin, than the 440 C. jejuni subsp. jejuni isolates (Table 1), similar to data reported previously (1, 2, 9). The 148 C. jejuni subsp. jejuni and 21 C. coli isolates acquired abroad were more resistant to ciprofloxacin, but not to tetracycline or erythromycin, than the 292 C. jejuni subsp. jejuni and 17 C. coli isolates acquired locally (Fig. 1 and 2). In three 4-year time periods (2002 to 2005, 2006 to 2009, and 2010 to 2013), C. jejuni subsp. jejuni isolates acquired abroad were more resistant to ciprofloxacin than were the C. jejuni subsp. jejuni isolates acquired locally (P ≥ 0.0002) (data not shown). In each of these 12 years, the number of ciprofloxacin-resistant C. jejuni subsp. jejuni isolates acquired abroad did not increase significantly (chi-square for linear trend, P = 0.052), but the number of ciprofloxacin-resistant C. jejuni subsp. jejuni isolates acquired locally increased significantly (chi-square for linear trend, P = 0.00000) (data not shown). In some studies, travel was associated with increased resistance to ciprofloxacin (9, 10, 11). With the availability of fluoroquinolones in veterinary and clinical practice, increased resistance of C. jejuni subsp. jejuni and C. coli to ciprofloxacin has been documented along with decreasing efficacy in the treatment of infections with these pathogens (1, 2, 9, 11).
TABLE 1.
Antimicrobial susceptibility of 440 C. jejuni subsp. jejuni and 38 C. coli isolates from Montreal, Quebec, Canada, 2002 to 2013a
| Organism | Erythromycin (% Rb) | Tetracycline (% R) | Ciprofloxacin (% R) |
|---|---|---|---|
| Campylobacter jejuni subsp. jejuni | 3.6 | 62.3 | 41.6 |
| C. coli | 18.4 | 52.6 | 50.0 |
| P | 0.001 | 0.3 | 0.4 |
| RR (95% CL)c | 0.20 (0.09–0.45) | 1.18 (0.87–1.61) | 0.83 (0.59–1.16) |
One isolate per patient and per outbreak included.
R, resistant.
RR, relative risk; 95% CL, 95% confidence limits.
FIG 1.
Rates of resistance of 148 and 292 Campylobacter jejuni subsp. jejuni isolates acquired abroad or locally, respectively, in Montreal, Quebec, Canada, 2002 to 2013.
FIG 2.
Rates of resistance of 21 and 17 Campylobacter coli isolates acquired abroad or locally, respectively, in Montreal, Quebec, Canada, 2002 to 2013.
From 2002 to 2013 at CHUM–Hôpital Saint-Luc, 7 of the 38 C. coli (18.4%) and 8 of the 440 C. jejuni subsp. jejuni (1.8%) isolates were erythromycin, tetracycline, and ciprofloxacin resistant (P = 0.00005; RR [95% CL], 10.13 [3.88 to 26.43]), which are data not reported previously, to the best of our knowledge. Eight of the 148 C. jejuni subsp. jejuni (5.4%) isolates acquired abroad and 0% of the 292 C. jejuni subsp. jejuni isolates acquired locally were erythromycin, tetracycline, and ciprofloxacin resistant (P = 0.0001), also data not reported previously; they were isolated in 2006, 2008, and 2011 to 2013. Five of the 21 C. coli (23.8%) isolates acquired abroad and 2 of the 17 C. coli (11.8%) isolates acquired locally were erythromycin, tetracycline, and ciprofloxacin resistant (P = 0.43; RR [95% CL], 2.02 [0.45 to 9.16]); they were isolated in 2006, 2010, and 2012 to 2013. The continent of acquisition was known for 167 of the 169 C. jejuni subsp. jejuni and C. coli isolates acquired abroad, and no C. jejuni subsp. jejuni or C. coli isolates were acquired from Oceania. Erythromycin-, tetracycline-, and ciprofloxacin-resistant isolates represented 0% to 15.9% of C. jejuni subsp. jejuni and C. coli isolates acquired in one or the other of the 4 other continents (P = 0.013) (Table 2). Of the 15 erythromycin-, tetracycline-, and ciprofloxacin-resistant C. jejuni subsp. jejuni/C. coli isolates, 3 and 12 isolates were resistant and susceptible, respectively, to amoxicillin-clavulanic acid (MICs, 64 to 128 and 0.12 to 2 mg/liter, respectively), 1 and 14 isolates were resistant and susceptible, respectively, to gentamicin (MICs, >256 and 0.12 to 2 mg/liter, respectively), and all 15 isolates were susceptible to imipenem (MICs, 0.015 to 0.25 mg/liter). Two of the 292 C. jejuni subsp. jejuni isolates acquired locally, but none of the 148 C. jejuni subsp. jejuni isolates acquired abroad and none of the 38 C. coli isolates, were erythromycin and ciprofloxacin resistant and tetracycline susceptible.
TABLE 2.
Continent of acquisition of erythromycin-, tetracycline-, and ciprofloxacin-resistant (multidrug-resistant) C. jejuni subsp. jejuni and C. coli isolates from Montreal, Quebec, Canada, 2002 to 2013
| Continent of isolate acquisitiona | No. of C. jejuni subsp. jejuni and C. coli isolates | No. (%) of multidrug-resistant C. jejuni subsp. jejuni and C. coli isolatesb |
|---|---|---|
| The Americas | 63 | 10 (15.9) |
| Europe | 24 | 2 (8.3) |
| Asia | 31 | 1 (3.2) |
| Africa | 49 | 0 (0) |
| Total | 167 | 13 |
No C. jejuni subsp. jejuni/C. coli isolates were acquired from Oceania. The continent of acquisition was unknown for 2 C. jejuni subsp. jejuni/C. coli isolates.
P = 0.013.
Erythromycin, tetracycline, and ciprofloxacin susceptibilities were epidemiological markers of C. jejuni subsp. jejuni and C. coli isolates obtained in our hospital (7, 8; this study). In the United States, Campylobacter isolates obtained from 2009 to 2011 had resistance rates of 2% to azithromycin, 23% to ciprofloxacin, and 24% to one or the other (11). Patients who were infected with resistant Campylobacter may have a longer disease duration, increased morbidity, and higher costs (1, 2, 11). Erythromycin and ciprofloxacin susceptibility should at least be assessed routinely for all C. jejuni subsp. jejuni and C. coli isolates (4). Education on how to prevent Campylobacter infections and surveillance of their antibiotic resistance levels are recommended (1, 2, 11). New oral drugs are needed for the treatment of enteric Campylobacter infections.
ACKNOWLEDGMENTS
We thank Ovid M. Da Silva for editing the manuscript and Jean Vincelette for assistance with statistical data analysis.
Huguette Gilbert is retired.
We have no conflicts of interest to declare.
Footnotes
Published ahead of print 23 April 2014
REFERENCES
- 1.Allos BM, Blaser MJ. 2010. Campylobacter jejuni and related species, p 2793–2802 In Mandell GL, Bennett JE, Dolin R. (ed), Principles and practice of infectious diseases, 7th ed. Elsevier Churchill Livingstone, Philadelphia, PA [Google Scholar]
- 2.Fitzgerald C, Nachamkin I. 2011. Campylobacter and Arcobacter, p 885–899 In Versalovic J, Carroll KC, Funke G, Jorgensen JH, Landry ML, Warnock DW. (ed), Manual of clinical microbiology, 10th ed. American Society for Microbiology, Washington, DC [Google Scholar]
- 3.Hill JE, Paccagnella A, Law K, Melito PL, Woodward DL, Price L, Leung AH, Ng LK, Hemmingsen SM, Goh SH. 2006. Identification of Campylobacter spp. and discrimination from Helicobacter and Arcobacter spp. by direct sequencing of PCR-amplified cpn60 sequences and comparison to cpnDB, a chaperonin reference sequence database. J. Med. Microbiol. 55:393–399. 10.1099/jmm.0.46282-0 [DOI] [PubMed] [Google Scholar]
- 4.Clinical and Laboratory Standards Institute. 2010. Methods for antimicrobial dilution and disk susceptibility testing for infrequently-isolated or fastidious bacteria: approved guidelines. CLSI M45-A2M, vol 30 Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 5.Gaudreau C, Girouard Y, Gilbert H, Gagnon J, Bekal S. 2008. Comparison of disk diffusion and agar dilution methods for erythromycin, ciprofloxacin and tetracycline susceptibility testing of Campylobacter coli and for tetracycline for Campylobacter jejuni subsp. jejuni. Antimicrob. Agents Chemother. 52:4475–4477. 10.1128/AAC.00767-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bessede E, Lehours P, Labadi L, Bakiri S, Megraud F. 2014. Comparison of characteristics of patients infected by Campylobacter jejuni, Campylobacter coli, and Campylobacter fetus. J. Clin. Microbiol. 52:328–330. 10.1128/JCM.03029-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gaudreau C, Michaud S. 2003. Cluster of erythromycin- and ciprofloxacin-resistant Campylobacter jejuni subsp. jejuni from 1999 to 2001 in men who have sex with men, Québec, Canada. Clin. Infect. Dis. 37:131–136. 10.1086/375221 [DOI] [PubMed] [Google Scholar]
- 8.Gaudreau C, Helferty M, Sylvestre JL, Allard R, Pilon PA, Poisson M, Bekal S. 2013. Campylobacter coli outbreak in men who have sex with men, Quebec, Canada, 2010-2011. Emerg. Infect. Dis. 19:764–767. 10.3201/eid1905.121344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Engberg J, Aarestrup FM, Taylor DE, Gerner-Smidt P, Nachamkin I. 2001. Quinolone and macrolide resistance in Campylobacter jejuni and C. coli: resistance mechanisms and trends in human isolates. Emerg. Infect. Dis. 7:24–34. 10.3201/eid0701.010104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaudreau C, Gilbert H. 2003. Antimicrobial resistance of Campylobacter jejuni subsp. jejuni strains isolated from humans in 1998 to 2001 in Montréal, Canada. Antimicrob. Agents Chemother. 47:2027–2029. 10.1128/AAC.47.6.2027-2029.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention. 2013. Antibiotic resistance threats in the United States, 2013. Centers for Disease Control and Prevention, Atlanta, GA: http://www.cdc.gov/drugresistance/threat-report-2013/pdf/ar-threats-2013-508.pdf [Google Scholar]