Abstract
Cervicitis is a common clinical finding often attributed to sexually transmitted infections (STIs), but no etiologic agent is identified in the majority of cases. In this study, we comparatively assessed inflammation among the common infectious etiologies of cervicitis and assessed the potential value of liquid cytology specimens for predicting STIs. Among 473 Louisiana women at low risk for acquiring STIs, the prevalences of Mycoplasma genitalium, Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis in liquid-based cytology specimens were 1.5, 2.1, 0.6, and 4.4%, respectively. N. gonorrhoeae and human papillomavirus 18 (HPV18) infections were significantly more common among subjects infected with M. genitalium. Using direct microscopy, we observed significant increases in leukocyte infiltrates among subjects with monoinfections with M. genitalium or C. trachomatis compared to women with no detectable STIs. Inflammation was highest among subjects with M. genitalium. Using a threshold of ≥2 leukocytes per epithelial cell per high-powered field, the positive predictive values for M. genitalium, C. trachomatis, N. gonorrhoeae, and T. vaginalis were 100, 70, 67, and 20%, respectively. Several novel M. genitalium genotypes were identified, all of which were predicted to be susceptible to macrolide antibiotics, suggesting that different strains may circulate among low-risk women and that macrolide resistance is substantially lower than in high-risk populations. This study highlights the capacity of M. genitalium to elicit cervical inflammation and, considering the strong epidemiologic associations between M. genitalium and human immunodeficiency virus (HIV), provides a potential mechanism for acquisition and shedding of HIV via chronic leukocyte recruitment to the cervical mucosa.
INTRODUCTION
Inflammation of the uterine cervix, termed cervicitis, is characterized clinically by the presence of either mucopurulent discharge from the cervical os and/or easily induced bleeding after endocervical sampling (cervical friability). Cervicitis appears to be a very common clinical finding (1) and has, in fact, been coined “the ignored counterpart in women of urethritis in men” (2). Classically, cervicitis has been managed “syndromically” by treating for the most likely etiologic agents prior to an accurate diagnosis. This is a common practice in sexually transmitted disease (STD) clinics to manage lower urogenital inflammation in men and women because it is imperative to initiate therapy before sexually transmitted infection (STI) test results are available. CDC guidelines recommend such empirical therapy of cervicitis in patients suspected to be infected with Chlamydia trachomatis or Neisseria gonorrhoeae, particularly in populations with high STI prevalence (3). Although no etiologic agent is identified in the majority of cases, the most common infectious causes of cervicitis are Chlamydia trachomatis and Neisseria gonorrhoeae (2, 4), followed by Mycoplasma genitalium, Trichomonas vaginalis, and herpes simplex viruses 1 and 2 (5).
A diagnosis of cervicitis currently relies upon direct observation of the aforementioned clinical signs during pelvic exam. Unfortunately, algorithms for syndromic management of STIs have proven to be largely ineffective when using vaginal signs to predict cervical infection, which can be detected by less invasive methods (5). Some specialists continue to utilize microscopic signs of cervicitis (elevated number of polymorphonuclear leukocytes per high-powered microscope field [PMN/HPF]) observed on endocervical Gram stains as presumptive evidence for treatment of STIs despite conflicting data on usefulness and being removed from the CDC treatment guidelines in 1993 (6). Over time, the threshold of inflammation to define a positive test has varied among studies from 10 to 30 PMN/HPF, and to date, no consensus or adequately justified definition of microscopically defined cervicitis exists (6). In addition, the positive predictive value (PPV) of inflammation on endocervical smears for identifying C. trachomatis or N. gonorrhoeae is generally less than 50% (7, 8) and declines with age (9), even in populations with high STI prevalence. Data regarding the use of microscopic signs for predicting M. genitalium are comparatively sparse, but a 2013 study showed that endocervical Gram stains have similarly poor utility (10). Collectively, the studies show that endocervical Gram staining appears to have limited usefulness as a point-of-care (POC) procedure for predicting the most commonly identified etiologies of cervicitis. With the assumption that cervicitis is an important pathological condition, either as an independent syndrome or as an identifiable risk factor for upper tract disease, a clear need exists to enhance the POC management of STIs in this context.
M. genitalium is a prevalent and emerging STI linked epidemiologically to pelvic inflammatory disease, tubal-factor infertility, and cervicitis (reviewed in references 11 and 12). Despite solid evidence for this organism as a cause of male nongonococcal urethritis (NGU), additional studies are needed to unequivocally implicate M. genitalium as a cause of cervicitis and other female reproductive tract syndromes. M. genitalium has a remarkable ability to establish chronic infections of the lower genital tract (13–16) in lieu of strong antibody responses to at least two outer membrane antigens (17–27). The data regarding M. genitalium as a cause of cervicitis have been conflicting, with approximately half of published studies showing significant associations (28). Comparative assessments of these studies indicate that M. genitalium is more commonly associated with cervicitis when microscopic criteria are considered independently of nonmicroscopic criteria (11, 28). In contrast to the case with C. trachomatis and N. gonorrhoeae, this suggests that microscopic signs may indeed be useful for predicting M. genitalium infection, and further investigation is warranted.
Very little evidence has been put forth to compare the inflammatory capacity of M. genitalium to that of other STIs, and therefore, its true role as a pathogen has yet to be established. Our primary goal in this study was to comparatively analyze the intensities of inflammation among the common infectious etiologies of cervicitis. Secondarily, we investigated the utility of liquid cytology specimens, routinely used for cervical cancer screening and human papillomavirus (HPV) testing, for predicting M. genitalium infection. Liquid cytology specimens have been FDA approved for the PCR-based diagnosis of HPV, C. trachomatis, N. gonorrhoeae, and T. vaginalis and are ideal to screen older and/or low-risk populations, since they are an integral component of cervical cancer management algorithms (29). This specimen type also uniquely facilitates evaluation of cells present in the cervix and is amendable to POC staining procedures to potentially enhance syndromic management of cervicitis.
MATERIALS AND METHODS
Patient population and specimen collection.
A retrospective case control study was performed in accordance with an approved LSU Health Sciences Center Institutional Review Board (IRB) protocol. Deidentified ThinPrep PreservCyt specimens (n = 482) were received from the Interim LSU Public Hospital's Molecular Pathology Laboratory (New Orleans, LA) from November 2012 to January 2013 for nucleic acid amplification testing (NAAT) for STIs. HPV infection status was available for 347 specimens as determined by Cobas HPV test (Roche Diagnostics, Inc., Indianapolis, IN). HIV infection status was unknown. The median age of the subjects was 42 years (range, 20 to 70 years; mean, 42 years) and was determined using clinical laboratory testing records after deidentification of specimens. Therefore, we were unable to correlate subject age with STI testing results. Any specimen without visible turbidity, a general indicator of specimen collection and cellularity, was excluded from the study (n = 9) in order to reduce the likelihood of false-negative results due to insufficient cellular material.
STI screening and quantification of M. genitalium.
All STI testing was performed using DNA extracts from ThinPrep PreservCyt (Hologic, Inc., Bedford, MA) specimens as the template. The specimens were obtained after Pap cytology and HPV testing with volumes ranging from 4 to 16 ml and stored at room temperature for up to 3 months prior to DNA extraction and STI testing. After swirling, 400 μl was removed and centrifuged at 4,000 × g for 5 min, and the resultant pellet was used as input for DNA purification using the Qiagen DNeasy Blood and Tissue kit (Qiagen, Inc., Valencia, CA). Purified DNA was eluted into 200 μl of the recommended elution buffer and stored at −20°C until PCR was performed.
To detect and enumerate M. genitalium organisms, we employed a real-time quantitative PCR (qPCR) assay targeting a 92-bp region of the MG190 (mgpA) gene (30) with the following reaction conditions: 12.5 μl of iQ Supermix (Bio-Rad Laboratories, Inc., Hercules, CA), 1.0 μl of forward primer (190F; 5 μM stock), 1.0 μl of reverse primer (190R3; 5 μM stock), 1.0 μl of the MG190 TaqMan probe (MG190P; 5 μM stock), and 5 μl of the DNA template. Real-time PCR was performed using the CFX96 real-time PCR detection system (Bio-Rad Laboratories, Inc.) with two-step cycling parameters as described previously (30). Samples with a positive result using the MG190 TaqMan PCR assay were confirmed using a PCR assay targeting a conserved region of mgpB using primers 1F and 1R (16) with SYBR green detection chemistry. Melt curve analysis was used to confirm amplicon specificity.
C. trachomatis testing was performed using a real-time qPCR assay targeting the cryptic plasmid as described previously (31). N. gonorrhoeae testing was performed using a SYBR green qPCR assay targeting a 102-bp region of the porA pseudogene (32). The PCR setup was as follows: 12.5 μl of iQ SYBR green Supermix (Bio-Rad Laboratories, Inc.), 1.0 μl of forward primer (5 μM stock), 1.0 μl of reverse primer (5 μM stock), and 5 μl of the DNA template. The cycling parameters consisted of an initial denaturation at 95°C for 5 min, followed by 50 cycles of 95°C for 1 s and 60°C for 20 s. Melt curve analysis was used to confirm amplicon specificity. T. vaginalis testing was done using a laboratory-developed quantitative real-time PCR assay targeting the single-copy glycine hydroxymethyltransferase gene (TVAG_109540). The PCR setup volumes were identical to those outlined for N. gonorrhoeae using forward primer 5′-CCATCAAGAGCATGCTTAGCTGC-3′, reverse primer 5′-GTTCATCAACGTATTTGGTGCCTCCA-3′ (5 μM stock), and TaqMan probe (5′-AGTATGCGGAAGGATATCCAGGTGCTCGC-3′). Cycling parameters consisted of an initial denaturation at 95°C for 5 min, followed by 35 cycles of 95°C for 10 s, 53°C for 30 s, and 72°C for 30 s.
Cervical leukocyte quantification.
Approximately 100 μl of each ThinPrep PreservCyt specimen was placed onto a glass microscope slide, air dried for 15 min at room temperature, and then stained with Diff-Quik reagents per the manufacturer's instructions (Siemens Healthcare Diagnostics, Ltd., Deerfield, IL). Leukocytes and epithelial cells were quantified in parallel from 5 representative high-powered fields by an American Society for Clinical Pathology (ASCP)-certified cytologist; cell counts were entered into a customized requisition form. First, samples from all subjects monopositive for M. genitalium (n = 5) and from a randomly selected subset of subjects negative for all tested STIs (n = 40) were stained for evaluation of M. genitalium-specific modulation of cervical leukocyte counts. Next, all specimens testing positive for monoinfections with C. trachomatis (n = 9), N. gonorrhoeae (n = 2), and T. vaginalis (n = 18) were processed for comparison to specimens from M. genitalium-positive subjects and those testing negative for all STIs. The prevalence of each STI, stratified by variable thresholds of leukocyte/epithelial cell ratios (≥2 or ≥2.5), was used to calculate PPVs (Table 1). In order to eliminate experimental bias, the cytologist was masked to the NAAT results throughout the study.
TABLE 1.
Value of leukocyte/epithelial cell ratios detected in liquid cytology specimens for predicting M. genitalium and other STIs
| Infection status | Parameter | Value at indicated threshold of leukocyte/EC ratio |
|
|---|---|---|---|
| >2.0 | >2.5 | ||
| M. genitalium positive (n = 7) | No. | 7 | 5 |
| % | 100.0 | 71.4 | |
| M. genitalium negative (n = 70) | No. | 16 | 10 |
| % | 22.9 | 14.3 | |
| P valuea | 0.0001 | 0.0025 | |
| PPVb (%) | 100.0 | 71.4 | |
| C. trachomatis positive (n = 10) | No. | 7 | 5 |
| % | 70.0 | 50.0 | |
| C. trachomatis negative (n = 67) | No. | 16 | 10 |
| % | 23.9 | 14.9 | |
| P valuea | 0.0062 | 0.0205 | |
| PPVb (%) | 70.0 | 50.0 | |
| N. gonorrhoeae positive (n = 3) | No. | 2 | 2 |
| % | 66.7 | 66.7 | |
| N. gonorrhoeae negative (n = 74) | No. | 21 | 13 |
| % | 28.4 | 17.6 | |
| P valuea | 0.211 | 0.0952 | |
| PPVb (%) | 66.7 | 66.7 | |
| T. vaginalis positive (n = 20) | No. | 4 | 3 |
| % | 20.0 | 15.0 | |
| T. vaginalis negative (n = 57) | No. | 19 | 12 |
| % | 33.3 | 21.1 | |
| P valuea | 0.3952 | 0.7466 | |
| PPVb (%) | 20.0 | 15.0 | |
Fisher's exact test.
The PPV is equal to the percentage of subjects identified with >2 or >2.5 leukocytes/epithelial cell/HPF who tested positive for the indicated STI.
M. genitalium genotyping.
DNA specimens from subjects with positive results from both the MG190 TaqMan and MG191 SYBR PCR assays were used as templates for the MG191 (mgpB) genotyping assay (15). Conventional PCR using the MgPa-1 and MgPa-3 primers (33), followed by agarose gel electrophoresis, PCR cleanup (QIAquick PCR purification kit; Qiagen, Inc.), and bidirectional Sanger sequencing, was performed and analyzed using standard methods. Sequences were compared to those from published M. genitalium strains using NCBI's BLASTn program.
Macrolide resistance screening of M. genitalium.
Subjects with positive results from the MG190 TaqMan and MG191 SYBR PCR assays were screened with a third PCR targeting the 23S rRNA gene (rrlA). Mutations in rrlA known to confer macrolide resistance were identified using a PCR assay previously developed by Jensen (34). Amplification was detected by agarose gel electrophoresis followed by PCR cleanup and Sanger sequencing as described above. Sequence data were analyzed using MacVector version 12.0.3 (MacVector, Inc.) to identify M. genitalium nucleotides corresponding to M. genitalium G37 (GenBank accession number NC_000908) positions 173798 and 173799 that indicate macrolide resistance (Escherichia coli positions A2058 and A2059 of the rrlA gene).
Statistical analysis.
Analysis of variance (ANOVA) was used to determine whether STIs were associated significantly with increased ratios of cervical leukocytes to epithelial cells whereby significant differences were noted when the P value was <0.05. Compiled contingency tables and calculated odd ratios (OR) were used for univariate analysis of the associations between M. genitalium infection and other STIs. Fisher's exact test was used to determine whether significant associations exist, and P values are reported in Table 2. Similarly, contingency tables were assembled to determine the PPV of variable leukocyte/epithelial cell ratios among STIs, followed by Fisher's exact test (Table 1). PPV was calculated using Prism (version 6; GraphPad Software, Inc., La Jolla, CA).
TABLE 2.
Prevalence and univariate associations between M. genitalium infection and other STIs
| STI | NAAT prevalence, % (no. positive/no. tested) | No. (%) of samples with result |
Odds ratio (95% CI) | P valuea | |
|---|---|---|---|---|---|
| M. genitalium positive | M. genitalium negative | ||||
| M. genitalium | 1.5 (7/473) | NAe | NA | NA | NA |
| C. trachomatis | 2.1 (10/473) | 0 (0.0) | 10 (2.1) | 2.9 (0.2–54.2) | 1.00 |
| N. gonorrhoeae | 0.6 (3/473) | 1 (14.3) | 2 (0.4) | 38.7 (3.1–486.6) | 0.04 |
| T. vaginalis | 4.4 (21/473) | 1 (14.3) | 20 (4.3) | 3.7 (0.04–32.4) | 0.27 |
| High-risk HPVb,c | 32 (111/347) | 3 (60.0) | 108 (31.6) | 3.2 (0.5–19.7) | 0.33 |
| HPV16c | 5.2 (18/347) | 0 (0.0) | 18 (5.3) | 1.6 (0.1–30.0) | 1.00 |
| HPV18c | 3.5 (12/347) | 2 (40.0) | 10 (2.9) | 22.1 (3.3–147.5) | 0.01 |
| OHR HPV typesc,d | 28.8 (100/347) | 2 (40.0) | 98 (28.7) | 1.6 (0.3–10.0) | 0.63 |
Fisher's exact test.
Combined HPV results for 14 high-risk HPV types (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68).
HPV results were available for 347 subjects.
OHR, combination result for 12 other high-risk HPV types (31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68).
NA, not applicable.
Nucleotide sequence accession numbers.
The nucleotide sequences obtained in this study have been submitted to NCBI with accession numbers KF995739 to KF995744.
RESULTS
Performance of the M. genitalium diagnostic PCR system.
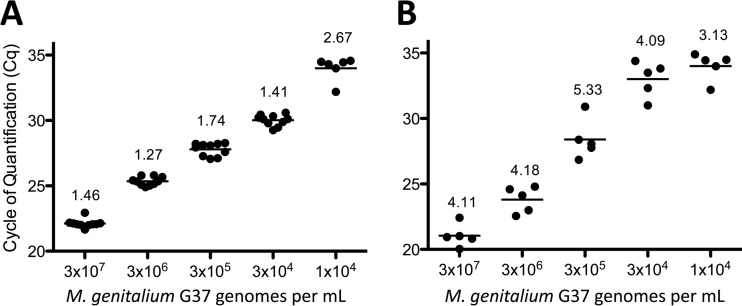
The MG190 TaqMan PCR assay was described previously (30). In the current study, we evaluated performance of the MG191 assay with liquid cytology specimens. Reproducibility of M. genitalium detection was determined by measuring interassay precision among 10 independent PCR runs. Liquid cytology specimens spiked with serial dilutions of M. genitalium G37 organisms showed a linear range of detection from 1 × 104 to 3 × 107 organisms per ml, with coefficients of variation ranging from 1.3 to 2.7% (Fig. 1A). The reproducible limit of detection (LOD) was 3 × 104 organisms per ml, which was detected in 100% of reactions. Concentrations of 1 × 104 organisms per ml were detected in 60% of specimens (Fig. 1A). Spiking M. genitalium organisms into liquid cytology specimens from 5 different subjects showed similar reproducibility of the combined extraction and PCR system, with coefficients of variation between 3.1 and 5.3% (Fig. 1B).
FIG 1.
Reproducibility of the MG190 TaqMan PCR assay. (A) M. genitalium G37 organisms were spiked into ThinPrep PreservCyt specimens at concentrations ranging from 1 × 104 to 3 × 107 organisms per ml. DNA was purified from each spiked specimen and used as the template in 10 replicate PCRs run on separate plates. (B) In a separate study, after spiking M. genitalium G37 organisms at the indicated concentrations, five independent DNA purifications and PCRs were performed once weekly over a 5-week period to evaluate interassay precision of the assay system. Data are presented as cycle of quantification (Cq) with percent coefficient of variation for each group.
STI prevalence and coinfections in low-risk Louisiana women.
Among 473 subjects, 35 were positive by the MG190 TaqMan PCR assay, of whom 7 were confirmed positive using the MG191 SYBR assay (1.5%; 7/473). The titers from confirmed M. genitalium-positive specimens ranged from 2 × 102 to 6 × 104 genomes per ml of ThinPrep PreservCyt fluid (mean ± standard error of the mean [SEM], 1.8 × 104 ± 1.0 × 104). The prevalences of C. trachomatis, N. gonorrhoeae, and T. vaginalis were 2.1 (10/473), 0.6 (3/473), and 4.4% (21/473), respectively. The titers of T. vaginalis ranged from 4 × 105 to 1 × 107 genomes per ml (mean ± SEM, 3.1 × 106 ± 8.4 × 105). N. gonorrhoeae infection was significantly more common among subjects with M. genitalium, but this was not the case for T. vaginalis or C. trachomatis (Table 2). No coinfections with C. trachomatis were identified.
Human papillomavirus (HPV) results were available for a subset of subjects in this study (n = 347). The prevalence of high-risk HPV infection was determined to be 32% (111/347), with HPV16 and HPV18 present at 5.2% (18/347) and 3.5% (12/347), respectively. The combined prevalence of 12 other high-risk HPV types (31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68) was 28.8% (100/347). No associations were observed between M. genitalium and high-risk HPV infection (OR, 3.2; 95% confidence interval [CI], 0.5 to 19.7), M. genitalium and HPV16 (OR, 1.6; 95% CI, 0.1 to 30.0), or M. genitalium with a combination result of 12 high-risk HPV types other than types 16 and 18 (OR, 1.7; 95% CI, 0.3 to 10.0). However, HPV18 detection was associated significantly (P = 0.01; Fisher's exact test) with M. genitalium infection (OR, 22.1; 95% CI, 3.3 to 147.5). With exception of HPV infections, all subjects with coinfections were excluded from these calculations for purposes of enumerating pathogen-specific modulation of cervical leukocytes.
M. genitalium and C. trachomatis infections were associated with microscopic signs of cervical inflammation.
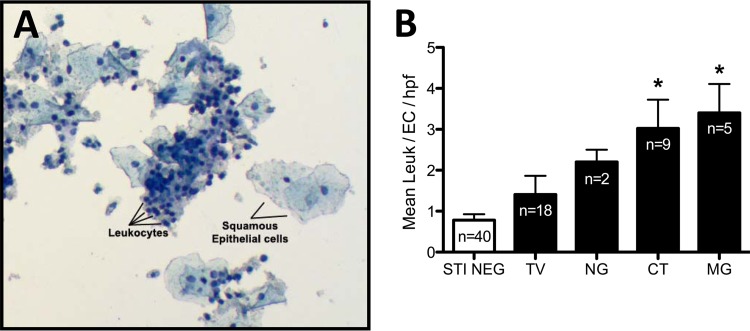
We evaluated whether M. genitalium and other STIs are associated with cervical inflammation using a ratio of leukocytes to epithelial cells quantified from liquid cytology specimens. A representative stained preparation is shown in Fig. 2A. Subjects with monoinfections with M. genitalium (3.4 ± 0.7 leukocytes/epithelial cell/HPF; n = 5) and C. trachomatis (3.0 ± 0.7; n = 9) had significantly higher (P < 0.05) mean leukocyte/epithelial cell ratios than did a representative subset of women without STIs (0.8 ± 0.15; n = 40) (Fig. 2B). The largest ratio of leukocytes to epithelial cells was observed in subjects with M. genitalium infection. Subjects with N. gonorrhoeae (2.2 ± 0.3; n = 2) or T. vaginalis (1.4 ± 0.5; n = 18) infections also showed increased leukocyte/epithelial cell ratios, but these increases were not significantly different than for subjects with no detectable STIs (P > 0.05; ANOVA).
FIG 2.
Quantification of cervical leukocytes from liquid cytology specimens. ThinPrep PreservCyt specimens from low-risk Louisiana women were screened for M. genitalium using the TaqMan PCR system described herein, as well as for C. trachomatis, N. gonorrhoeae, and T. vaginalis. All subjects with coinfections among those positive for M. genitalium, C. trachomatis, N. gonorrhoeae, and T. vaginalis were excluded from the calculation of leukocyte modulation. Those specimens that were monopositive for each STI and a randomly selected subset of specimens negative for all tested STIs (n = 40) were Diff-Quik stained, followed by quantification of cervical epithelial cells and leukocytes. (A) Representative microscope field from a stained ThinPrep PreservCyt specimen illustrating squamous epithelial cells and cervical leukocytes. Differing ratios of leukocytes to epithelial cells are readily observed using the preparation and staining paradigm. (B) Comparison of cervical leukocyte counts among women with and without STIs presented as the ratio of leukocytes to epithelial cells per high-powered field (HPF) compiled from five representative microscope fields. Significant differences between leukocyte/epithelial cell ratios among subjects are indicated with an asterisk (P < 0.05; ANOVA).
The PPV of measuring leukocyte/epithelial cell ratios from stained smears of liquid cytology specimens ranged from 20 to 100% among STIs using a threshold of positivity of >2.0 leukocytes/epithelial cell/HPF (Table 1). As expected, the PPV decreased if the threshold was raised to the more stringent cutoff of >2.5 leukocytes/epithelial cell/HPF. Using either threshold of positivity, the prevalence of M. genitalium or C. trachomatis infection was significantly higher in subjects with these inflammatory signs than in those without signs. Similar trends were not observed for subjects with N. gonorrhoeae or T. vaginalis infections (Table 1).
Macrolide resistance-mediating mutations and novel M. genitalium genotypes.
Several recent studies have highlighted that azithromycin (AZM)-resistant M. genitalium strains are emerging worldwide (35–42), two of which provide molecular evidence that resistance can be induced with 1 g of STAT dosing (38, 42). Using conventional PCR and Sanger sequencing, analysis of the M. genitalium-positive subjects in this study showed that none of their infecting strains possessed mutations that confer macrolide resistance (data not shown). Using a previously validated genotyping system that targets a relatively conserved region of the MG191 gene (mgpB) (15), we observed only a single infecting strain to be 100% identical to a previously defined strain (M6283; Miyazaki, Japan). The remaining six genotypes had unique MG191 sequences differing from all previously published sequences in the NCBI database, as well as among each other (Fig. 3).
FIG 3.
Nucleotide alignment of M. genitalium genotypes. Using a previously described genotyping system (15), we PCR amplified and sequenced the specific genotyping region of MG191 from all specimens confirmed to be positive for M. genitalium infection (n = 7). Sequencing reads were trimmed using Sequencher version 4.10.1 and aligned using Clustal W in MacVector version 12.0.3. Sequences were compared to those for previously described M. genitalium strains using NCBI's BLASTn program. M. genitalium strain G37 was processed in parallel to confirm accuracy of the system and is presented as a reference. Nucleotide reference numbers are relative to that for G37 (GenBank accession number NC_000908).
DISCUSSION
In this study, approximately 1 in 65 women presenting to women's clinics in Louisiana for cervical cancer screening tested positive for M. genitalium infection (1.5%). As expected, this prevalence was in between those of N. gonorrhoeae (0.6%) and C. trachomatis (2.1%) and substantially lower than previous observations of high-risk subjects, including STD clinic attendees, subjects attending family planning clinics for termination of pregnancy, or commercial sex workers (11). On average, urogenital M. genitalium infections occur in approximately 2% of low-risk women recruited from fertility clinics, otherwise healthy subjects enrolled in population-based studies, or subjects enrolled in studies of adverse pregnancy outcomes (11). In a study by Manhart and colleagues, 1.3% of otherwise healthy young adults aged 18 to 27 years in the southern region of the United States tested positive for M. genitalium infection (43). Our observed prevalence of 1.5% from Louisiana women's clinics was on par with these studies; however, women enrolled in our study were older (median age, 42 years), and samples were obtained during routine cervical cancer screening visits to either rural or urban women's clinics. The finding that M. genitalium is more common than N. gonorrhoeae and similar to C. trachomatis in this population highlights the potential need to screen older populations such as the case for T. vaginalis.
The convention of categorically defining cervicitis based on elevated levels of PMN/HPF on endocervical Gram stains has prevented an accurate assessment of inflammation by distilling the findings into a qualitative endpoint of cervicitis/no cervicitis. Perhaps even more troubling with this method is the lack of an adequate control for sampling efficiency. Using liquid cytology specimens in the current study, direct leukocyte quantification as a ratio to epithelial cells accounts for sampling efficiency and highlights the observation that women with M. genitalium have approximately three times more leukocytes in the cervix than do women without STIs. The significance of this finding is somewhat unclear since no pathological threshold of cervical leukocytes has been established. Previous studies have utilized varied microscopic definitions of cervical inflammation (12), and those that employed high thresholds of inflammation (>20 or >30 PMN/HPF) showed fewer associations between M. genitalium and cervicitis (11). Combined with results of the 2010 Swedish study in which no (0/6) M. genitalium-positive subjects had >30 PMN/HPF (44), cumulative evidence suggests that M. genitalium infections may be characterized by relatively low-intensity inflammation. Therefore, the concept of utilizing a defined microscopic threshold of leukocyte infiltrates without a measure of sampling efficiency is inadequate for detecting inflammation and/or predicting infection. Importantly, increases in cervical leukocytes among M. genitalium-positive women in our study were highest among the STIs and substantiate the role of M. genitalium as an inflammatory pathogen. Using a cutoff ratio of >2, the PPV of the leukocyte/epithelial cell ratio for predicting M. genitalium was very high (100%), but this and the PPVs for other STIs should be interpreted with caution since the STI prevalence was very low. Collectively, despite screening almost 500 low-risk women, expansion of the study to include high-risk women and differing age groups is warranted to better understand the utility of this method for predicting STIs.
Previous studies have shown that vaginal swabs may have a greater relative sensitivity for M. genitalium detection than cervical swabs (45, 46), and a combination of these sites yields a sensitivity of more than 95% (45). Liquid cytology specimens predominately contain squamous cells of the ectocervix, but because the anatomical target of sampling is the transition zone between the ectocervix and endocervix, columnar cells of the endocervix are also present. We routinely observed vaginal microflora suggesting that this specimen type may be ideal for M. genitalium detection, as it offers a composite sampling of the lower urogenital tract. Regarding analytical sensitivity, the defined lower threshold of reproducible detection was 3 × 104 organisms/ml of ThinPrep PreservCyt fluid and represents a potential limitation to the current study. We hypothesize that this is due to the relatively small (5 μl) template used in the PCR and that additional optimization of the system would likely enhance sensitivity. With this potential limitation, it is possible that subjects with low M. genitalium titers were not identified, and therefore, the observed microscopic signs of inflammation are representative of subjects with high titers. Additionally, several specimens identified as positive with the MG190 screening test were not confirmed with a second test, suggesting that specificity of the MG190 PCR is subpar and should be enhanced to reduce false-positive results. Additional investigation of this specimen type and detection system is warranted considering the widespread use of liquid cytology specimens for cervical cancer screening and HPV testing in women aged 21 to 65 years (29).
We identified six previously undiscovered genotypes, all of which were susceptible to macrolides, suggesting that M. genitalium strains circulating in low-risk women may differ from those in high-risk populations. This study, to our knowledge, is the first to investigate macrolide susceptibility among low-risk women and suggests that M. genitalium strains circulating in this population are more susceptible to these antibiotics than those in higher-risk populations such that AZM is less effective (12, 47). AZM treatment failures using the 1-g dose have ranged from 15 to 60% (47–49). Other studies have highlighted the hazard of STAT 1-g AZM therapy by describing the molecular mechanisms of induced resistance and subsequent treatment failure (35, 38, 42). Since AZM is commonly used to treat male NGU and is also included in the current CDC treatment guidelines for gonorrheae, it remains imperative to monitor resistance patterns for M. genitalium and other bacterial STIs since virtually all men with urethritis receive AZM. In patients with AZM-resistant M. genitalium infections, moxifloxacin is the recommended and most commonly used antibiotic (12, 47), but reports of resistance are emerging (50).
This study highlights the importance of M. genitalium as an etiology of cervicitis and the potential utility of liquid cytology specimens for diagnosis and measuring inflammation. The major limitations to interpreting the significance of the findings are the low prevalence of M. genitalium and other STIs and the inability to stratify our results by age. A larger cohort will be required to corroborate the results in both high- and low-risk populations, particularly with regard to the PPV of this method as a POC procedure. Since the prevalence of STIs would be substantially higher in high-risk populations, our findings may not be generalizable to these women since coinfections, frequent reinfection, bacterial vaginosis, and other factors may complicate the validity of measuring leukocyte/epithelial cell ratios. Importantly, cervicitis has been associated with increased HIV shedding, and treatment of the condition reduces both viral shedding and the presence of virus-infected cells in cervical secretions (51–53). Having been shown to enhance susceptibility to HIV infection (54, 55), and with cross-sectional associations with HIV infection in more than 20 studies (56), continued investigation of M. genitalium remains important, as antibiotic-resistant strains continue to emerge worldwide.
ACKNOWLEDGMENTS
We are grateful to Sue Favaloro from the Interim LSU Public Hospital in New Orleans, LA, for collaborative efforts in acquiring liquid cytology specimens for this study. We thank David Martin, Alison Quayle, and Lyndsey Buckner from LSU Health Sciences Center in New Orleans for their scientific insight and critical evaluation of the manuscript.
None of the authors have any commercial interests, financial holdings, professional affiliations, advisory board positions, board memberships, patent holdings, or any other associations that pose a conflict of interest related to the subject matter presented in this article.
Footnotes
Published ahead of print 23 April 2014
REFERENCES
- 1. Gaydos C, Maldeis NE, Hardick A, Hardick J, Quinn TC. 2009. Mycoplasma genitalium as a contributor to the multiple etiologies of cervicitis in women attending sexually transmitted disease clinics. Sex. Transm. Dis. 36:598–606. 10.1097/OLQ.0b013e3181b01948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brunham RC, Paavonen J, Stevens CE, Kiviat N, Kuo CC, Critchlow CW, Holmes KK. 1984. Mucopurulent cervicitis—the ignored counterpart in women of urethritis in men. N. Engl. J. Med. 311:1–6. 10.1056/NEJM198407053110101 [DOI] [PubMed] [Google Scholar]
- 3. Workowski KA, Berman S. 2010. Sexually transmitted diseases treatment guidelines, 2010. MMWR Morb. Mortal. Wkly. Rep. 59:1–11020075837 [Google Scholar]
- 4. Paavonen J, Wolner-Hanssen P. 1986. Chlamydia infection and infertility. Lakartidningen 83:2916–2917 [PubMed] [Google Scholar]
- 5. Holmes KK. 1999. Sexually transmitted diseases, 3rd ed. McGraw-Hill Press, New York, NY [Google Scholar]
- 6. Marrazzo JM, Martin DH. 2007. Management of women with cervicitis. Clin. Infect. Dis. 44(Suppl 3):S102–S110. 10.1086/511423 [DOI] [PubMed] [Google Scholar]
- 7. Ryan CA, Courtois BN, Hawes SE, Stevens CE, Eschenbach DA, Holmes KK. 1998. Risk assessment, symptoms, and signs as predictors of vulvovaginal and cervical infections in an urban US STD clinic: implications for use of STD algorithms. Sex. Transm. Infect. 74(Suppl 1):S59–S76 [PubMed] [Google Scholar]
- 8. Tyndall MW, Kidula N, Sande J, Ombette J, Temmerman M. 1999. Predicting Neisseria gonorrhoeae and Chlamydia trachomatis infection using risk scores, physical examination, microscopy, and leukocyte esterase urine dipsticks among asymptomatic women attending a family planning clinic in Kenya. Sex. Transm. Dis. 26:476–482. 10.1097/00007435-199909000-00010 [DOI] [PubMed] [Google Scholar]
- 9. Marrazzo JM, Handsfield HH, Whittington WL. 2002. Predicting chlamydial and gonococcal cervical infection: implications for management of cervicitis. Obstet. Gynecol. 100:579–584. 10.1016/S0029-7844(02)02140-3 [DOI] [PubMed] [Google Scholar]
- 10. Oliphant J, Azariah S. 2013. Cervicitis: limited clinical utility for the detection of Mycoplasma genitalium in a cross-sectional study of women attending a New Zealand sexual health clinic. Sex. Health 10:263–267. 10.1071/SH12168 [DOI] [PubMed] [Google Scholar]
- 11. McGowin CL, Anderson-Smits C. 2011. Mycoplasma genitalium: an emerging cause of sexually transmitted disease in women. PLoS Pathog. 7:e1001324. 10.1371/journal.ppat.1001324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Taylor-Robinson D, Jensen JS. 2011. Mycoplasma genitalium: from chrysalis to multicolored butterfly. Clin. Microbiol. Rev. 24:498–514. 10.1128/CMR.00006-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bradshaw CS, Chen MY, Fairley CK. 2008. Persistence of Mycoplasma genitalium following azithromycin therapy. PLoS One 3:e3618. 10.1371/journal.pone.0003618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cohen CR, Nosek M, Meier A, Astete SG, Iverson-Cabral S, Mugo NR, Totten PA. 2007. Mycoplasma genitalium infection and persistence in a cohort of female sex workers in Nairobi, Kenya. Sex. Transm. Dis. 34:274–279. 10.1097/01.olq.0000237860.61298.54 [DOI] [PubMed] [Google Scholar]
- 15. Hjorth SV, Bjornelius E, Lidbrink P, Falk L, Dohn B, Berthelsen L, Ma L, Martin DH, Jensen JS. 2006. Sequence-based typing of Mycoplasma genitalium reveals sexual transmission. J. Clin. Microbiol. 44:2078–2083. 10.1128/JCM.00003-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Iverson-Cabral SL, Astete SG, Cohen CR, Rocha EP, Totten PA. 2006. Intrastrain heterogeneity of the mgpB gene in Mycoplasma genitalium is extensive in vitro and in vivo and suggests that variation is generated via recombination with repetitive chromosomal sequences. Infect. Immun. 74:3715–3726. 10.1128/IAI.00239-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Baseman JB, Cagle M, Korte JE, Herrera C, Rasmussen WG, Baseman JG, Shain R, Piper JM. 2004. Diagnostic assessment of Mycoplasma genitalium in culture-positive women. J. Clin. Microbiol. 42:203–211. 10.1128/JCM.42.1.203-211.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Clausen HF, Fedder J, Drasbek M, Nielsen PK, Toft B, Ingerslev HJ, Birkelund S, Christiansen G. 2001. Serological investigation of Mycoplasma genitalium in infertile women. Hum. Reprod. 16:1866–1874. 10.1093/humrep/16.9.1866 [DOI] [PubMed] [Google Scholar]
- 19. Furr PM, Taylor-Robinson D. 1984. Microimmunofluorescence technique for detection of antibody to Mycoplasma genitalium. J. Clin. Pathol. 37:1072–1074. 10.1136/jcp.37.9.1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Iverson-Cabral SL, Manhart LE, Totten PA. 2011. Detection of Mycoplasma genitalium-reactive cervicovaginal antibodies among infected women. Clin. Vaccine Immunol. 18:1783–1786. 10.1128/CVI.05174-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McGowin CL, Spagnuolo RA, Pyles RB. 2010. Mycoplasma genitalium rapidly disseminates to the upper reproductive tracts and knees of female mice following vaginal inoculation. Infect. Immun. 78:726–736. 10.1128/IAI.00840-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Møller BR, Taylor-Robinson D, Furr PM, Freundt EA. 1985. Acute upper genital-tract disease in female monkeys provoked experimentally by Mycoplasma genitalium. Br. J. Exp. Pathol. 66:417–426 [PMC free article] [PubMed] [Google Scholar]
- 23. Svenstrup HF, Fedder J, Kristoffersen SE, Trolle B, Birkelund S, Christiansen G. 2008. Mycoplasma genitalium, Chlamydia trachomatis, and tubal factor infertility—a prospective study. Fertil. Steril. 90:513–520. 10.1016/j.fertnstert.2006.12.056 [DOI] [PubMed] [Google Scholar]
- 24. Svenstrup HF, Jensen JS, Gevaert K, Birkelund S, Christiansen G. 2006. Identification and characterization of immunogenic proteins of Mycoplasma genitalium. Clin. Vaccine Immunol. 13:913–922. 10.1128/CVI.00048-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Taylor-Robinson D, Furr PM, Hanna NF. 1985. Microbiological and serological study of non-gonococcal urethritis with special reference to Mycoplasma genitalium. Genitourin. Med. 61:319–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Taylor-Robinson D, Furr PM, Tully JG, Barile MF, Moller BR. 1987. Animal models of Mycoplasma genitalium urogenital infection. Isr. J. Med. Sci. 23:561–564 [PubMed] [Google Scholar]
- 27. Tully JG, Taylor-Robinson D, Rose DL, Furr PM, Graham CE, Barile MF. 1986. Urogenital challenge of primate species with Mycoplasma genitalium and characteristics of infection induced in chimpanzees. J. Infect. Dis. 153:1046–1054. 10.1093/infdis/153.6.1046 [DOI] [PubMed] [Google Scholar]
- 28. Manhart LE. 2013. Mycoplasma genitalium: an emergent sexually transmitted disease? Infect. Dis. Clin. North Am. 27:779–792. 10.1016/j.idc.2013.08.003 [DOI] [PubMed] [Google Scholar]
- 29. Massad LS. 2012. New guidelines on cervical cancer screening: more than just the end of annual Pap testing. J. Low. Genit. Tract Dis. 16:172–174. 10.1097/LGT.0b013e31824bc178 [DOI] [PubMed] [Google Scholar]
- 30. McGowin CL, Annan RS, Quayle AJ, Greene SJ, Ma L, Mancuso MM, Adegboye D, Martin DH. 2012. Persistent Mycoplasma genitalium infection of human endocervical epithelial cells elicits chronic inflammatory cytokine secretion. Infect. Immun. 80:3842–3849. 10.1128/IAI.00819-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jalal H, Stephen H, Curran MD, Burton J, Bradley M, Carne C. 2006. Development and validation of a rotor-gene real-time PCR assay for detection, identification, and quantification of Chlamydia trachomatis in a single reaction. J. Clin. Microbiol. 44:206–213. 10.1128/JCM.44.1.206-213.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hjelmevoll SO, Olsen ME, Sollid JU, Haaheim H, Unemo M, Skogen V. 2006. A fast real-time polymerase chain reaction method for sensitive and specific detection of the Neisseria gonorrhoeae porA pseudogene. J. Mol. Diagn. 8:574–581. 10.2353/jmoldx.2006.060024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jensen JS, Uldum SA, Sondergard-Andersen J, Vuust J, Lind K. 1991. Polymerase chain reaction for detection of Mycoplasma genitalium in clinical samples. J. Clin. Microbiol. 29:46–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jensen JS. 2012. Protocol for the detection of Mycoplasma genitalium by PCR from clinical specimens and subsequent detection of macrolide resistance-mediating mutations in region V of the 23S rRNA gene. Methods Mol. Biol. 903:129–139. 10.1007/978-1-61779-937-2_8 [DOI] [PubMed] [Google Scholar]
- 35. Anagrius C, Lore B, Jensen JS. 2013. Treatment of Mycoplasma genitalium. Observations from a Swedish STD clinic. PLoS One 8:e61481. 10.1371/journal.pone.0061481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chrisment D, Charron A, Cazanave C, Pereyre S, Bebear C. 2012. Detection of macrolide resistance in Mycoplasma genitalium in France. J. Antimicrob. Chemother. 67:2598–2601. 10.1093/jac/dks263 [DOI] [PubMed] [Google Scholar]
- 37. Ito S, Shimada Y, Yamaguchi Y, Yasuda M, Yokoi S, Nakano M, Ishiko H, Deguchi T. 2011. Selection of Mycoplasma genitalium strains harbouring macrolide resistance-associated 23S rRNA mutations by treatment with a single 1 g dose of azithromycin. Sex. Transm. Infect. 87:412–414. 10.1136/sextrans-2011-050035 [DOI] [PubMed] [Google Scholar]
- 38. Jensen JS, Bradshaw CS, Tabrizi SN, Fairley CK, Hamasuna R. 2008. Azithromycin treatment failure in Mycoplasma genitalium-positive patients with nongonococcal urethritis is associated with induced macrolide resistance. Clin. Infect. Dis. 47:1546–1553. 10.1086/593188 [DOI] [PubMed] [Google Scholar]
- 39. Shimada Y, Deguchi T, Nakane K, Yasuda M, Yokoi S, Ito S, Nakano M, Ishiko H. 2011. Macrolide resistance-associated 23S rRNA mutation in Mycoplasma genitalium, Japan. Emerg. Infect. Dis. 17:1148–1150. 10.3201/eid1706.101055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Twin J, Jensen JS, Bradshaw CS, Garland SM, Fairley CK, Min LY, Tabrizi SN. 2012. Transmission and selection of macrolide resistant Mycoplasma genitalium infections detected by rapid high resolution melt analysis. PLoS One 7:e35593. 10.1371/journal.pone.0035593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Walker J, Fairley CK, Bradshaw CS, Tabrizi SN, Twin J, Chen MY, Taylor N, Donovan B, Kaldor JM, McNamee K, Urban E, Walker S, Currie M, Birden H, Bowden FJ, Gunn J, Pirotta M, Gurrin L, Harindra V, Garland SM, Hocking JS. 2013. Mycoplasma genitalium incidence, organism load, and treatment failure in a cohort of young Australian women. Clin. Infect. Dis. 56:1094–1100. 10.1093/cid/cis1210 [DOI] [PubMed] [Google Scholar]
- 42. Yew HS, Anderson T, Coughlan E, Werno A. 2011. Induced macrolide resistance in Mycoplasma genitalium isolates from patients with recurrent nongonococcal urethritis. J. Clin. Microbiol. 49:1695–1696. 10.1128/JCM.02475-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Manhart LE, Holmes KK, Hughes JP, Houston LS, Totten PA. 2007. Mycoplasma genitalium among young adults in the United States: an emerging sexually transmitted infection. Am. J. Public Health 97:1118–1125. 10.2105/AJPH.2005.074062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Falk L. 2010. The overall agreement of proposed definitions of mucopurulent cervicitis in women at high risk of Chlamydia infection. Acta Derm. Venereol. 90:506–511. 10.2340/00015555-0924 [DOI] [PubMed] [Google Scholar]
- 45. Lillis RA, Nsuami MJ, Myers L, Martin DH. 2011. Utility of urine, vaginal, cervical, and rectal specimens for detection of Mycoplasma genitalium in women. J. Clin. Microbiol. 49:1990–1992. 10.1128/JCM.00129-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mobley VL, Hobbs MM, Lau K, Weinbaum BS, Getman DK, Sena AC. 2012. Mycoplasma genitalium infection in women attending a sexually transmitted infection clinic: diagnostic specimen type, coinfections, and predictors. Sex. Transm. Dis. 39:706–709. 10.1097/OLQ.0b013e318255de03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Manhart LE, Gillespie CW, Lowens MS, Khosropour CM, Colombara DV, Golden MR, Hakhu NR, Thomas KK, Hughes JP, Jensen NL, Totten PA. 2013. Standard treatment regimens for nongonococcal urethritis have similar but declining cure rates: a randomized controlled trial. Clin. Infect. Dis. 56:934–942. 10.1093/cid/cis1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mena LA, Mroczkowski TF, Nsuami M, Martin DH. 2009. A randomized comparison of azithromycin and doxycycline for the treatment of Mycoplasma genitalium-positive urethritis in men. Clin. Infect. Dis. 48:1649–1654. 10.1086/599033 [DOI] [PubMed] [Google Scholar]
- 49. Schwebke JR, Rompalo A, Taylor S, Sena AC, Martin DH, Lopez LM, Lensing S, Lee JY. 2011. Re-evaluating the treatment of nongonococcal urethritis: emphasizing emerging pathogens—a randomized clinical trial. Clin. Infect. Dis. 52:163–170. 10.1093/cid/ciq074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Couldwell DL, Tagg KA, Jeoffreys NJ, Gilbert GL. 2013. Failure of moxifloxacin treatment in Mycoplasma genitalium infections due to macrolide and fluoroquinolone resistance. Int. J. STD AIDS 24:822–828. 10.1177/0956462413502008 [DOI] [PubMed] [Google Scholar]
- 51. Coleman JS, Hitti J, Bukusi EA, Mwachari C, Muliro A, Nguti R, Gausman R, Jensen S, Patton D, Lockhart D, Coombs R, Cohen CR. 2007. Infectious correlates of HIV-1 shedding in the female upper and lower genital tracts. AIDS 21:755–759. 10.1097/QAD.0b013e328012b838 [DOI] [PubMed] [Google Scholar]
- 52. Johnson LF, Lewis DA. 2008. The effect of genital tract infections on HIV-1 shedding in the genital tract: a systematic review and meta-analysis. Sex. Transm. Dis. 35:946–959. 10.1097/OLQ.0b013e3181812d15 [DOI] [PubMed] [Google Scholar]
- 53. McClelland RS, Wang CC, Mandaliya K, Overbaugh J, Reiner MT, Panteleeff DD, Lavreys L, Ndinya-Achola J, Bwayo JJ, Kreiss JK. 2001. Treatment of cervicitis is associated with decreased cervical shedding of HIV-1. AIDS 15:105–110. 10.1097/00002030-200101050-00015 [DOI] [PubMed] [Google Scholar]
- 54. Mavedzenge SN, Van Der Pol B, Weiss HA, Kwok C, Mambo F, Chipato T, Van der Straten A, Salata R, Morrison C. 2012. The association between Mycoplasma genitalium and HIV-1 acquisition in African women. AIDS 26:617–624. 10.1097/QAD.0b013e32834ff690 [DOI] [PubMed] [Google Scholar]
- 55. Vandepitte J, Weiss HA, Bukenya J, Nakubulwa S, Mayanja Y, Matovu G, Kyakuwa N, Hughes P, Hayes R, Grosskurth H. 2013. Alcohol use, Mycoplasma genitalium, and other STIs associated With HIV incidence among women at high risk in Kampala, Uganda. J. Acquir. Immune Defic. Syndr. 62:119–126. 10.1097/QAI.0b013e3182777167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Napierala Mavedzenge S, Weiss HA. 2009. Association of Mycoplasma genitalium and HIV infection: a systematic review and meta-analysis. AIDS 23:611–620. 10.1097/QAD.0b013e328323da3e [DOI] [PubMed] [Google Scholar]