Abstract
Strains of the Beijing genotype family of Mycobacterium tuberculosis are a cause of particular concern because of their increasing dissemination in the world and their association with drug resistance. Phylogenetically, this family includes distinct ancient and modern sublineages. The modern strains, contrary to the ancestral counterparts, demonstrated increasing prevalence in many world regions that suggest an enhanced bacterial pathogenicity. We therefore evaluated virulence of modern versus ancient Beijing strains with similar epidemiological and genotype characteristics. For this, we selected six strains that had very similar 24-locus mycobacterial interspersed repetitive-unit–variable-number tandem-repeat (MIRU-VNTR) typing profiles and belonged to the region of difference 181 (RD181) subgroup but differed using markers (mutT2 and mutT4 genes and NTF locus) that discriminate between modern and ancient Beijing sublineages. The strains were isolated from native patients in Brazil and Mozambique, countries with a low prevalence of Beijing strains. The virulence levels of these strains were determined in models of pulmonary infection in mice and in vitro macrophage infection and compared with that of a strain from Russia, part of the epidemic and hypervirulent Beijing clone B0/W148, and of the laboratory strain H37Rv. The results showed that two of the three modern Beijing strains were highly pathogenic, exhibiting levels of virulence comparable with that of the epidemic Russian strain. In contrast, all isolates of the ancient sublineage displayed intermediate or low virulence. The data obtained demonstrate that the strains of the modern Beijing sublineage are more likely to exhibit highly virulent phenotypes than ancient strains and suggest that genetic alterations characteristic of the modern Beijing sublineage favor selection of highly virulent bacteria.
INTRODUCTION
Despite extensive surveillance, tuberculosis (TB) remains a serious public health problem. In different parts of the world, there is concern about TB caused by the East Asian/Beijing lineage of Mycobacterium tuberculosis, demonstrating increasing prevalence in the global M. tuberculosis population (1). Clinical and epidemiological studies demonstrated that emergence of the Beijing strains could be associated with high levels of bacterial resistance to multiple drugs (2, 3) and enhanced pathogenicity of these strains, leading to increased transmissibility (4) and rapid progression from infection to active disease (5). However, the data on evaluation of the virulence of Beijing isolates were inconclusive, demonstrating a wide range of inflammatory and virulence phenotypes, as determined in animal models (6, 7, 8) and in vitro models of macrophage infection (9, 10).
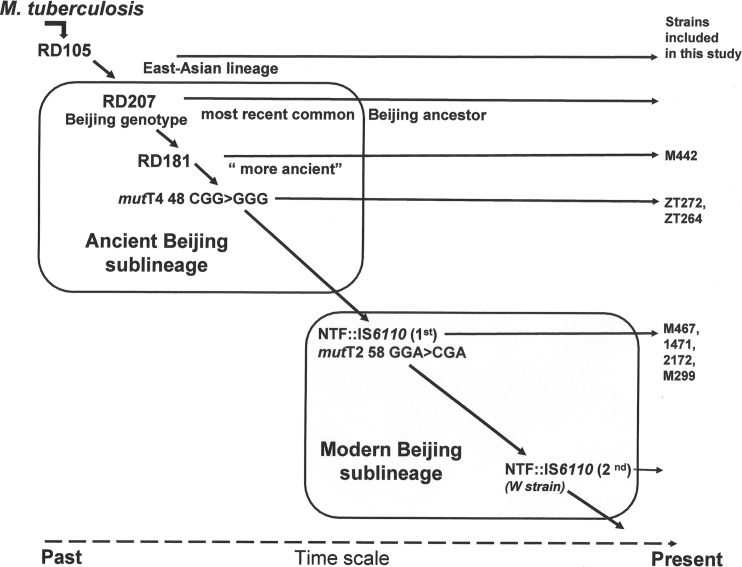
Such differences in the virulence of Beijing strains could be associated with genetic heterogeneity of the Beijing M. tuberculosis lineage. Indeed, bacterial genotyping and sequencing demonstrated that the Beijing lineage, having in common a characteristic spoligotype signature and lack of the region of difference 105 (RD105) and RD207, has evolved into several subgroups that are defined by deletion of other regions, such as RD181, RD150, and RD142 (11, 12). While the large RD181 deletion occurred early in evolution of the Beijing lineage and is found in most of the Beijing strains, the more recent RD150 and RD142 deletions were described in different unrelated strains, demonstrating their homoplasy and hence limited utility for phylogeny (13). Additional subdivision of the Beijing lineage is currently based on the presence of IS6110 insertions in the NTF chromosomal region (14, 15) and on detection of alterations in putative mutator genes, mutT2 and mutT4 (13, 16). The ancient (atypical) sublineage is characterized by an intact NTF region and includes strains with intact RD181 (“more ancient” strains), as well as strains with deleted RD181 (“classical” ancient strains). The strains of modern (typical) Beijing sublineage differ from the ancient strains by presentation of either a single (most of the modern Beijing sublineage) or two IS6110 insertions (W sublineage) in the NTF region (14, 15). Verification of unique missense alterations in mut genes demonstrated that the most ancestral strains possess intact mut genes. The downstream ancient strains were discriminated by detection of a single nucleotide polymorphism (SNP) in codon position 48 of the mutT4 gene, changing CGG (Arg) to GGG (Gly), whereas the modern strains of the RD181 subgroup, and all other descendant branches, were characterized by an additional mutT2 mutation at codon position 58, changing GGA (Gly) to CGA (Arg), in addition to the modified NTF region (16). The summary of main phylogenetic markers discriminating ancient and modern strains of the Beijing lineage is presented in Fig. 1.
FIG 1.
Genetic polymorphisms determining phylogeny of East Asian/Beijing lineage of M. tuberculosis respective to isolates included in this study.
Epidemiological studies evaluating dissemination of different phylogenetic groups of the Beijing M. tuberculosis family in different regions of the world demonstrated that the growing prevalence of Beijing TB is driven by strains of the modern sublineage, not ancient Beijing strains (5, 17, 18). These observations lead us to speculate that the supposed enhanced virulence and transmissibility, promoting dissemination of the Beijing strains, could be associated predominantly with strains of the modern sublineage.
Virulence-associated properties of modern and ancient Beijing strains, comparing different phylogenetic subgroups of the Beijing family, are only starting to be investigated. In a previous study, Aguilar and colleagues investigated Beijing strains from distant phylogenetic groups and observed increased virulence of highly transmissible strains of the recently evolved modern sublineage (sublineage 7, RD150 subgroup), compared with orphan strains of the phylogenetically distant ancient sublineage (8). In the present study, we aimed to study a more homogeneous group of Beijing strains that belonged to the same RD181-defined phylogenetic group and presented considerable similarity in 24 MIRU-VNTR-defined genotypes but differed in genetic markers characteristic for ancient and modern sublineages. In an epidemiological sense, the strains were considered sporadic, as being isolated from unique cases of TB in countries with low prevalence of Beijing strains.
Our results demonstrate that two of the three strains of the modern Beijing sublineage studied, in contrast to the ancient strains, displayed increased levels of virulence comparable with that of the epidemic hypervirulent modern Beijing strain from Russia. These data corroborate the hypothesis that evolution of the Beijing M. tuberculosis lineage led to accumulation of genetic polymorphisms that enhanced bacterial virulence.
MATERIALS AND METHODS
Mycobacterial isolates and genotype classification.
Mycobacterium tuberculosis strains of the Beijing genotype were collected over a 6-year period (2001 to 2007) from native TB patients attending primary health care clinics in Rio de Janeiro and Sao Paulo in Brazil (19) and in Maputo and the Maputo province in Mozambique (20) as a part of molecular epidemiology studies in these countries. The Russian M. tuberculosis isolate 1471 was isolated from a patient with pulmonary TB in the St. Petersburg Research Institute of Phthisiopulmonology, Russia, and kindly provided by B. Vishnevsky. This isolate was assigned to the Beijing genotype B0/W148 clone by IS6110 restriction fragment length polymorphism (RFLP) typing as described in our previous publication (19). The laboratory M. tuberculosis strain H37Rv (ATCC) was obtained from the Laboratory of Molecular Biology Applied to Mycobacteria, FIOCRUZ, Rio de Janeiro, Brazil. The M. tuberculosis isolates were obtained as Lowenstein-Jensen slants of low-passage-number cultures and stored frozen at −80°C as aliquots of 108 bacilli/ml in complete 7H9 Middlebrook medium supplemented with 10% albumin-dextrose-catalase (ADC) enrichment (Difco) and 0.05% Tween 80. The number of further culture passages was kept to a minimum.
The Beijing genotype of all isolates was confirmed by spoligotyping, and all presented the Beijing shared international type 1 (SIT 1). Additionally, the strains were tested for regions of difference (RDs), RD105, RD142, RD150, and RD181 by multiplex PCR and analysis of amplicon size on agarose gels (5). The strains were submitted to mycobacterial interspersed repetitive-unit–variable-number tandem-repeat (MIRU-VNTR) analysis, using standardized 24-locus MIRU-VNTR typing (21). Verification of the presence of SNPs in the mutT2 and mutT4 genes was performed by direct sequencing of PCR product generated as described previously (22). Further characterization of the modern/typical and ancestral/atypical sublineages was done following the NTF locus-based evolutionary framework of the Beijing genotype (15) and using primers MDR6-F (MDR stands for multidrug resistance, and F stands for forward), MDR6-R (R stands for reverse), MDR7-F, and MDR7-R to detect IS6110 insertions in the NTF region (14).
Mouse infections.
Specific-pathogen-free C57BL/6 mice (8 to 10 weeks old) were purchased from the Biotério do Instituto de Ciências Biomédicas at the University of Sao Paulo (USP). The mice were maintained in microisolators in a biosafety level 3 animal facility at the Universidade Estadual do Norte Fluminense (UENF). All experimental protocols were approved and performed according to the guidelines set by the Institutional Animal Care and Use Committee of the UENF.
The M. tuberculosis strains were thawed and cultured for 5 days in complete Middlebrook 7H9 broth to optimize viability of the thawed bacteria. Suspensions were sonicated, vortexed, and kept for 10 min for sedimentation of eventual clumps. The culture supernatant was microscopically verified for the absence of clumps larger than five bacteria and adjusted to an optical density at 600 nm (OD600) of 0.1 using densitometry. Bacteria were inoculated in the mouse intratracheally (i.t.), either at low dose (102 bacilli) or high dose (2.5 × 103 bacilli) in 60 μl of phosphate-buffered saline (PBS), while the control group was inoculated with sterile PBS. Infection experiments were performed in triplicate. The efficiency of targeting of the inoculum was confirmed by culture of lung homogenates, obtained 18 h postinfection (p.i.) (day 0), on plates with complete Middlebrook 7H10 agar supplemented with oleic acid-albumin-dextrose-catalase (OADC) enrichment (Becton, Dickinson, USA), and the numbers of CFU were enumerated 21 days later.
Animal survival and lung bacillus loads.
For evaluation of animal survival, groups of 10 mice were infected with either low or high doses of bacilli and observed during 350 or 150 days postinfection, respectively. All mice were monitored twice per week until moribund and then sacrificed. For quantification of the bacterial loads in the lungs, three animals from each group infected with the low dose of bacilli were sacrificed at 28 and 120 days after infection. The entire lungs and separated upper lobes of the right lungs, indicative for bacterial burdens, were weighed. The lobes were homogenized, and serial dilutions of the homogenates were plated on Middlebrook 7H10 agar for the CFU test. Results were expressed as log10 CFU per organ.
Lung pathology.
The left lungs were fixed in 10% buffered formalin, photographed to evaluate morphological changes, and subsequently embedded in paraffin. For histopathological studies, serial 4- to 5-μm sections were stained with hematoxylin and eosin (H&E) to visualize tissue alterations and by the Ziehl-Neelsen method to detect the presence of acid-fast bacteria (BAAR). The samples were examined with an Axioplan 2 microscope (Carl Zeiss, Inc.), and the images of lung sections from four mice per group were captured by Coolpix P995 (Nikon)-coupled device camera. For the morphometric analysis, photographs were taken using a magnification of ×10, and the Image J program (NIH, Bethesda, MD) was utilized to objectively assess the area of inflammation. Color images were converted to white/black images, and a contrast cutoff was established to allow software identification of aerated areas (in black) and nonaerated areas, including both inflamed and noninflamed tissue (in white). To quantify the percentage of nonaerated area, we determined the mean percent white area for 10 lung sections of control uninfected mice and each of the different infected groups. To quantify the percentage of inflamed tissue (area of pneumonia), the mean percent tissue area of control mice was subtracted from that of each infected group.
The numbers of lung-infiltrating cells were determined by quantification of the cells obtained from enzymatically digested lung tissue. Two lobes of the right lungs were mechanically dissected, resuspended in RPMI 1640 medium (Gibco, USA) complemented with Liberase (Sigma-Aldrich; 2 μg/ml) and type IV bovine pancreatic DNase (Roche Diagnostic; 1 μg/ml), and incubated at 37°C for 45 min. Cell concentrations were determined by Neubauer chamber cell counting.
Cell culture of bone marrow-derived macrophages and macrophage infection.
The bone marrow-derived macrophages (BMDM) were obtained through the cultivation of bone marrow cells of C57BL/6 mice in Dulbecco's modified Eagle medium–nutrient mixture F-12 (DMEM/F12) complemented with 20% L929 cell-conditioned medium (as a source of macrophage colony-stimulating factor [M-CSF] to induce macrophage differentiation), as previously described (23). The cells were infected with the mycobacterial strains at a multiplicity of infection (MOI) of 1:1 bacterium/macrophage. After 3-h incubation, the cultures were washed with PBS and cultured in DMEM-F12 medium containing 2% fetal bovine serum (FBS) for 6 days. The CFU test was employed to quantify intracellular growth of mycobacteria. For this, the infected cultures were lysed on day 0 and day 6 by adding 0.1% saponin for 10 min, and homogenates were cultured in triplicate by plating onto complete Middlebrook 7H10 agar. Induction of necrotic cell death was evaluated in the BMDM cultures infected at an MOI of 10:1 by the lactate dehydrogenase (LDH) test, using LDH cytotoxicity assay kit (Doles, Goiânia, Brazil). Cell lysates obtained via treatment with 1% Triton X-100 were used as a positive control, and the rate of LDH release was calculated using the formula: (supernatant value − blank value)/(lysate value − blank value) × 100%.
Statistical analysis.
Statistical analysis was performed using Prism4 GraphPad software. Survival Kaplan-Meier curves were compared by log rank test. To compare multiple groups, one-way analysis of variance (ANOVA) was used, followed by Bonferroni's multiple-comparison test.
RESULTS
Genetic characteristics of the Beijing strains selected for virulence evaluation.
In our previous study, we performed genotyping of 28 Beijing M. tuberculosis strains isolated from native TB patients in Brazil (n = 10) and Mozambique (n = 18) from native patients with TB (L. L. Gomes, S. Vasconcellos, H. M. Gomes, A. Elias, A. Rocha, S. Ribeiro, A. Panunto, L. Ferrazoli, M. Telles, M. Ivens de Araujo, A. Kritski, I. Mokrousov, O. Manicheva, E. Lasunskaia, and P. Suffys, submitted for publication). For this work, we selected three ancient strains and three modern strains, determined by SNP analysis in the mutT2 and mutT4 genes and IS6110 insertions in the NTF region, with similar genotype characteristics (Table 1). These strains belonged to the RD181 Beijing subgroup, characterized by deletion of the RD181 region, and presented related genotypes determined by 24-locus MIRU-VNTR genotyping (triple locus variation at most). The laboratory M. tuberculosis strain H37Rv was included as a reference strain with a moderate level of virulence, and the Beijing strain 1471, representing Russian epidemic clone B0/W148 (17), was included as a reference strain with high virulence. Genetic analysis demonstrated that strain 1471 differed in three, four, or five MIRU-VNTR loci from the modern isolates selected for this study (Table 1).
TABLE 1.
Genetic characteristics of the M. tuberculosis strains included in this study
| Strain | Family | Geographic origin | 24-locus MIRU-VNTRa | Insertion of IS6110 in NTF regions | mutT2 | mutT4 | Sublineage |
|---|---|---|---|---|---|---|---|
| H37RV | ATCC | ||||||
| M442 | Beijing | Mozambique | 223325173543424354433427 | No insertion | Free of mutation | Free of mutation | Ancient |
| zt272 | Beijing | Brazil | 223325173533424444433637 | No insertion | Free of mutation | Mutation codon 48 | Ancient |
| zt264 | Beijing | Brazil | 223325173533424444433737 | No insertion | Free of mutation | Mutation codon 48 | Ancient |
| M467 | Beijing | Mozambique | 223325173543424354433427 | One insertion | Mutation codon 58 | Mutation codon 48 | Modern |
| 1471 | Beijing | Russia | 223325173533424454443627 | One insertion | Mutation codon 58 | Mutation codon 48 | Modern |
| 2172 | Beijing | Brazil | 223326173433425454433623 | One insertion | Mutation codon 58 | Mutation codon 48 | Modern |
| M299 | Beijing | Mozambique | 223325173533424354433427 | One insertion | NDb | ND | Modern |
For the MIRU-VNTR data, the loci differed in repeat numbers are underlined.
ND, not defined.
Survival times of mice infected with M. tuberculosis strains at low and high doses of bacilli.
To determine the relative virulence of the M. tuberculosis strains, we employed an established model of pulmonary infection of C57BL/6 mice. In animals inoculated i.t. with 102 CFU of each strain, three patterns of animal survival were observed (Fig. 2A). Eighty percent of mice of the groups infected with modern Beijing strains 1471, 2172, and M299 succumbed to death within a 260-day period (pattern III), while mice infected by strain 1471 started to die earlier, at the end of the acute phase of infection, 27 days p.i. In contrast, all mice infected with the H37Rv strain and ancient Beijing strain M442 were alive after 1 year, demonstrating relatively low virulence of these strains (pattern I). The animals infected with other Beijing strains survived up to the late phase of chronic infection of 200 days p.i., starting to die later on (pattern II). The mice infected with the modern Beijing strains generally succumbed more rapidly than those infected with the strains of ancient Beijing sublineage; however, only two groups of infected animals (strains 1471 and M299) presented significant differences in mortality rate compared with the group of H37Rv-infected mice (P < 0.05).
FIG 2.
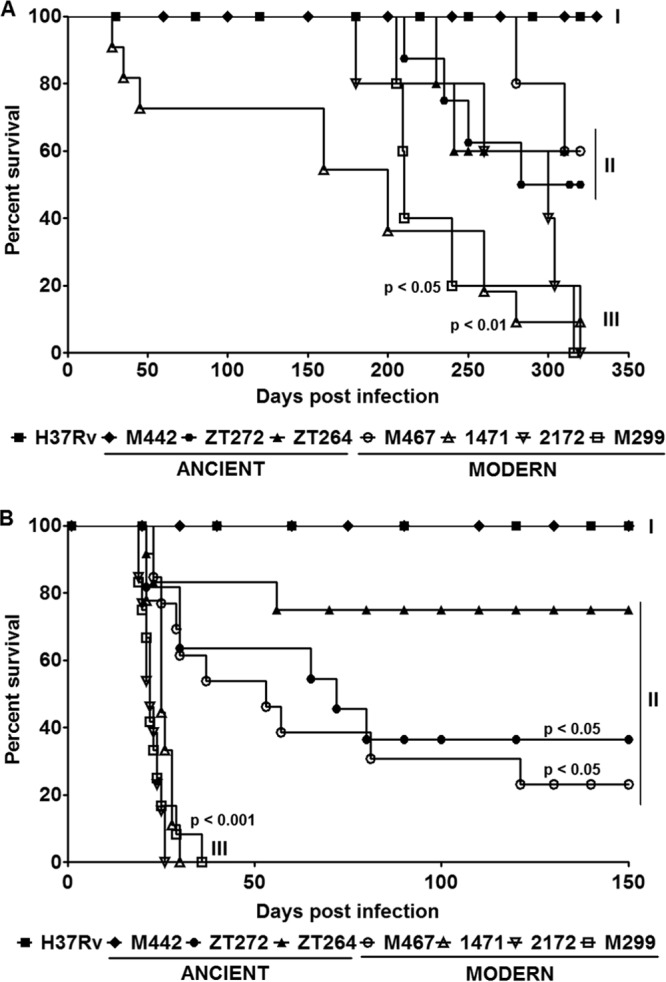
Survival of mice after infection with M. tuberculosis Beijing strains of modern and ancient sublineages. C57BL/6 mice were i.t. infected with low doses (102 CFU) (A) and high doses (2.5 × 103 CFU) (B) of each strain and observed for 320 days and 150 days, respectively. The data were obtained in three independent experiments with 10 to 15 mice in each group. Kaplan-Meier curves and log rank test were used to evaluate statistical significance. Statistically significant differences between each group infected with the individual clinical isolate and the group infected with strain H37Rv are presented. Three different patterns of animal survival are indicated (pattern I, II, and III).
For better discrimination of the virulence of studied Beijing strains, the mice were also infected with a higher dose of 2.5 × 103 bacilli/mouse (Fig. 2B). While three of the modern Beijing strains (strains 1471, M299, and 2172) caused death of all infected animals within 35 days p.i. (pattern III), the animals infected with strains H37Rv and M442 maintained viability during the whole period of observation of 150 days (pattern I) (P < 0.001). The mortality rate of mice in other groups was variable. The animals infected with strains M467, zt272, and zt264 started to die at the acute stage of infection, but a considerable numbers (between 30% and 70%) were able to survive within the period of observation (pattern II). Our data (Fig. 2A and B) demonstrate that three of the four strains of the modern Beijing sublineage (strains 1471, M299, and 2172) were highly virulent, whereas one modern strain (M467) and two strains of the ancient sublineage displayed intermediate levels of virulence. The reference strain H37Rv and strain M442 of the ancient sublineage demonstrated relatively low virulence in our experimental model.
Bacterial loads in the lungs of mice infected with low bacterial dose.
At the acute stage of infection, all isolates except strain M442 grew faster in the lungs than strain H37Rv (Fig. 3). Bacterial loads of the modern strains measured at 28 days p.i. varied on average between log 7.0 and log 8.0, whereas that of the strain H37Rv and ancient strain M442 was lower than log 6.0 (Fig. 3A). The growth of the modern strains was on average higher than that of the ancient strains; however, the difference was not statistically significant.
FIG 3.
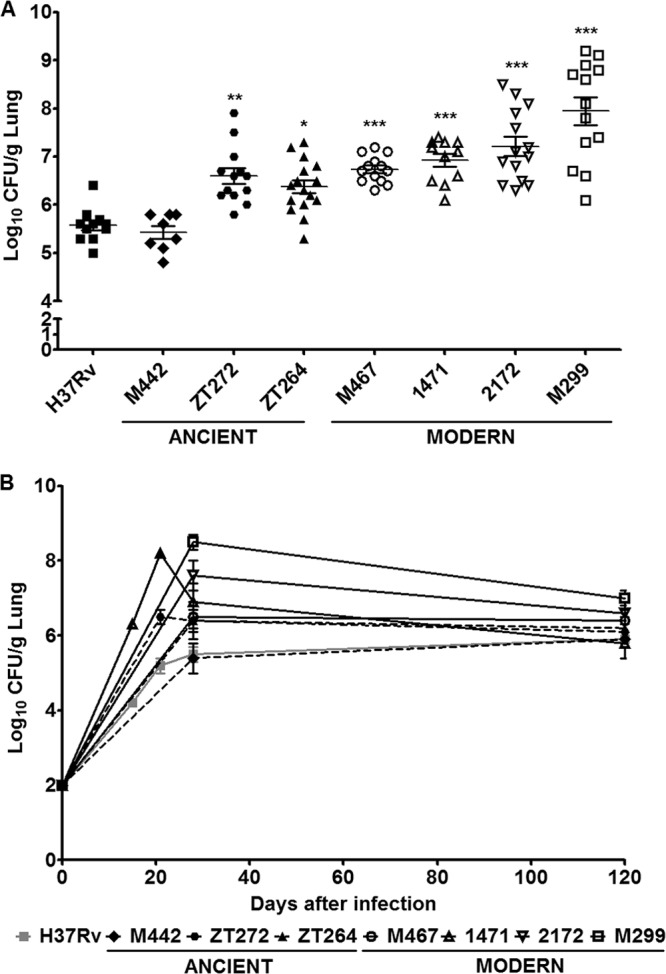
Bacterial growth in the lungs of mice infected with M. tuberculosis Beijing strains of modern and ancient sublineages. C57BL/6 mice were i.t. inoculated with 102 bacilli of each strain, and lungs were examined for the bacterial growth by CFU test. (A) Bacterial burdens in the lungs determined 28 days p.i. Each symbol represents the value for an individual mouse. (B) Bacterial curves within 120 days p.i. Results of at least three experiments (three mice in each group at each time point) are expressed logarithmically as the mean log10 CFU ± standard deviation (SD) (error bars). Mean values that were significantly different from the mean value of the group infected by H37Rv strain are indicated by asterisks as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Growth curves of the bacteria studied were monitored up to day 120 p.i., with more-detailed analysis of growth kinetics of strains 1471, zt272, and H37Rv. The data presented in Fig. 3B demonstrated that the bacterial loads increased up to day 21 p.i. and was then contained, suggesting inhibitory effect of acquired immunity established 3 to 4 weeks after infection. At the chronic phase of lung infection, the levels of viable bacteria were maintained without significant reduction of the CFU numbers and were similar for all studied strains (Fig. 3B).
Pathological alterations in the lungs of mice infected with low M. tuberculosis dose.
To further define the virulence of the studied strains, we compared their ability to induce lung pathology, evaluating macropathological (Fig. 4) and histopathological changes (Fig. 5).
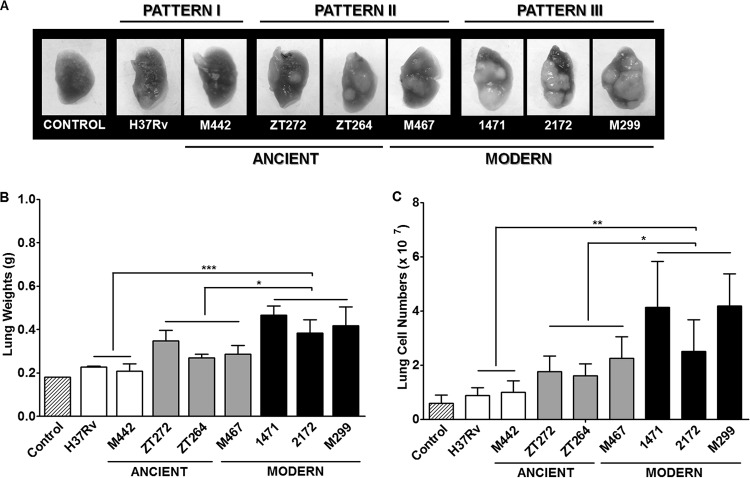
FIG 4.
Macropathological changes in the lungs of M. tuberculosis-infected mice. C57BL/6 mice were i.t. infected with 102 bacilli of each strain, and lungs were examined 28 days p.i. (A) Representative macroscopic lung images demonstrating gross pathology, observed as numerous giant inflammatory lesions (white nodes of different size). (B and C) Lung weights (B) and numbers of cells obtained from lungs after mechanical and enzymatic tissue disruption (C). Results of at least three experiments (three mice in each group in each experiment). Data represent means plus standard deviations (SD) (error bars). Significant differences were determined between the groups of pattern III strains (black columns), pattern II strains (gray columns), and pattern I strains (white columns). Values that are significantly different for the different groups of strains (groups with different patterns) are indicated by asterisks as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
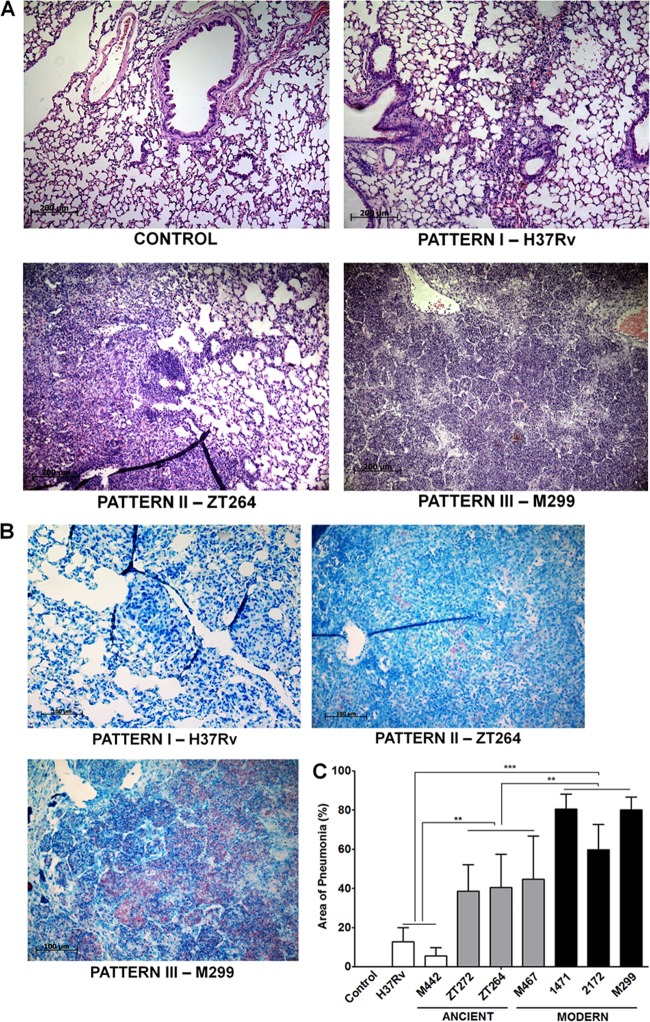
FIG 5.
Histopathological changes in the lungs of M. tuberculosis-infected mice. The lungs were obtained from infected mice as described in the legend to Fig. 3. (A) Representative hematoxylin-and-eosin-stained lung sections, demonstrating three main pathological patterns. Bars, 200 μm. (B) Representative lung sections stained by Ziehl-Neelsen method. In pattern I, induced by the strains H37Rv and M442, small and medium-sized peribronchial and perivascular granulomas, consisting of macrophages and lymphocytes, are observed. Small numbers of intracellular bacteria are seen. In pattern II, induced by strains zt264, zt272, and M467, moderate, multifocal granulomatous pneumonia is observed. Increasing numbers of intracellular bacteria are seen. In pattern III, induced by strains M299, 2172, and 1471, extensive diffuse granulomatous pneumonia with areas of necrosis and alveolitis is observed. Large numbers of intracellular and extracellular bacteria are seen. Bars, 200 μm. (C) Morphometric analysis of the inflammatory lung area. Results of two experiments (three mice in each group in each experiment). Data represent means plus SD. Significant differences were determined between the groups of pattern III strains (black columns), pattern II strains (gray columns), and pattern I strains (white columns) and indicated by asterisks as follows: **, P < 0.01; ***, P < 0.001.
The lungs of mice infected with the clinical isolates presented clearly visible large or small white tuberculous nodules, hardly detectable in mice infected with the laboratory H37Rv strain and strain M442, defined as pattern I strains (Fig. 4A). Particularly large and coalescing masses of the inflammatory nodes were observed in the case of modern strains M299, 2172, and 1471 (pattern III strains), and lung weights in these cases were 1.5- to 2.0-fold higher than those of mice infected with the pattern I strains (P < 0.001) and 1.3-fold higher (P < 0.05) compared with the pattern II strains (Fig. 4B). The increase in lung mass was associated with a rise in the number of cells infiltrating infected lungs (Fig. 4C).
According to the gross pathology data, three distinct histopathological patterns that differed in the severity of lesions were observed 28 days p.i. In the lungs of mice infected with the ancient Beijing strain M442 and strain H37Rv, exhibiting minimal signs of gross pathology (pattern I), the tissue lesions were presented by small numbers of granulomas, formed by recruited macrophages and lymphocytes (Fig. 5A). Ziehl-Neelsen staining revealed few intracellular mycobacteria in macrophages (Fig. 5B). In mice infected with the more virulent pattern II strains (modern strain M467 and ancient strains zt272 and zt264), pulmonary granulomas were larger and coalescing, and the cellular lymphohistiocytic infiltrates occupied significantly larger areas (up to 40%) of the lung on average (Fig. 5C). The most striking differences were observed in mice infected with the modern pattern III strains. These strains induced rapidly expanding lesions leading to occupation of an average of 70% of the lung by inflammatory cells (Fig. 5C), also presenting extensive areas of alveolitis, with thickened alveolar walls and alveoli filled with histiocytic macrophages, lymphocytes, and neutrophils. Some of the pneumonic lesions progressed to necrosis, presenting acellular areas with disrupted alveoli (Fig. 5A) and numerous intracellular and extracellular bacteria in necrotic areas (Fig. 5B). Intrabronchial cellular exudates with large numbers of bacteria were observed only in the mice infected by highly virulent strains (data not shown).
Evaluation of virulence-associated properties of modern and ancient Beijing strains in the in vitro model of macrophage infection.
To study bacterial properties determining differential growth of the M. tuberculosis strains in lungs at the initial stage of infection, we compared the capacity of the studied strains to replicate in cultured macrophages, as well as their capacity to induce cell necrosis, measured by the LDH test.
Bone marrow-derived macrophages from C57BL/6 mice cultured in vitro were infected, and bacterial intracellular growth within a 6-day period, as well as macrophage death, were evaluated. The data presented in Fig. 6 demonstrate that the modern Beijing strains 1471, 2172, and M299 presented faster multiplication in macrophages than other studied strains (P < 0.01). At a higher dose of infection (MOI of 10), strains 1471, 2172, and M299 and the ancient strain zt264 induced higher levels of necrotic macrophage death. The release of LDH in cultures infected by these strains was significantly higher than that in cultures infected by strain H37Rv (P < 0.05). These data demonstrate that modern Beijing strains (pattern III strains 1471, 2172, and M299) displayed increased virulence in the macrophage model of infection, suggesting that macrophage inhibition could contribute to the pathogenicity of these bacterial strains.
FIG 6.
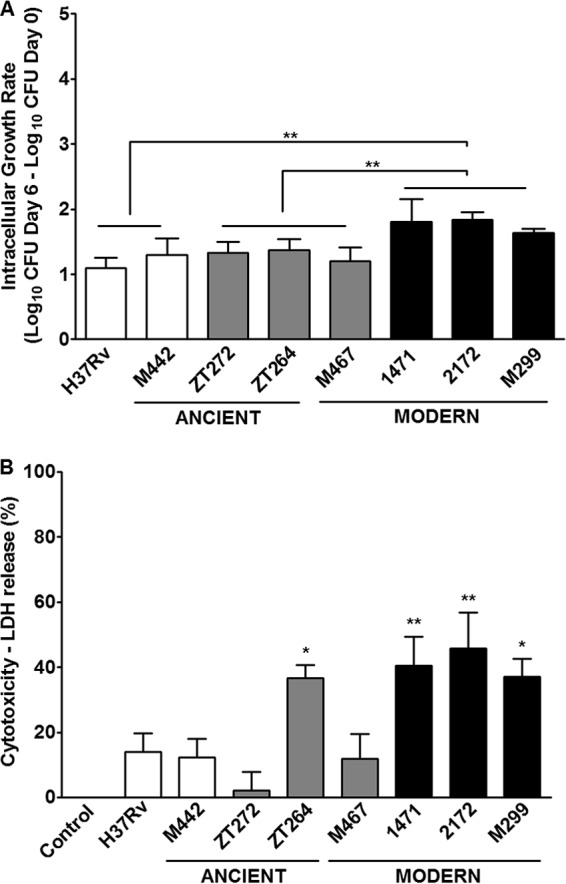
Virulence-associated properties of the ancient and modern Beijing strains evaluated in the macrophage-based model of infection. Bone marrow-derived macrophages obtained from C57BL/6 mice were infected with different mycobacterial strains at an MOI of 1:1 and incubated for 6 days. (A) Intracellular growth of mycobacteria, measured as a fold increase in the numbers of CFU recovered from macrophages on days 0 and 6 after infection. The bacterial growth rate is presented as mean plus SD of log increases in the numbers of intracellular CFU measured in two separate experiments. (B) Induction of necrotic cell death in the macrophage cultures infected at an MOI of 10:1. LDH release from the dead cells was measured in the culture supernatants on day 5 after infection. Asterisks indicate the isolates for which the values of growth or cytotoxicity differed significantly from those of the H37Rv strain or between the indicated groups: *, P < 0.05; **, P < 0.01.
DISCUSSION
In order to understand better the recent worldwide dissemination and increasing prevalence of the modern sublineage of Beijing/East Asian M. tuberculosis lineage, we verified the pathogenicity of modern and ancient Beijing strains with related genotypes and similar epidemiological characteristics. For this, we focused our study on the strains of the major RD181 phylogenetic subgroup of the Beijing family, characterized by the deletion of RD181 and without phylogenetically ambiguous deletions RD150 and RD142. Strains of the RD181 subgroup, which includes strains of both the ancient and modern sublineages (13), were isolated more frequently than strains of other Beijing subgroups in non-East Asian regions, such as the United States (24), Peru (18), and Mozambique (25).
Six sporadic Beijing strains of the RD181 group, including three modern strains and three ancient strains with relatively similar 24-locus MIRU-VNTR genotypes, were evaluated in a well-established model of pulmonary infection of C57BL/6 mice. Three distinct virulence patterns were observed, the first of relatively low virulence (pattern I), the second of intermediate virulence (pattern II), and the third defined by highly virulent strains (pattern III). High virulence was exhibited by two of the three strains with the modern genotype that were isolated in Mozambique (strain M299) and Brazil (strain 2172), and their level of virulence was similar to that of the modern strain 1471 representing the successful Russian clone of the Beijing genotype (17). The pattern II strains included isolates of both sublineages (modern strain M467 and ancient strains zt272 and zt264), while group I strains were represented by the “more ancient” strain M442 (characterized by wild-type mutT2 and mutT4 alleles [Fig. 1]) and the laboratory strain H37Rv. The difference between the highly virulent modern strains (pattern III) and the ancient strains, particularly strain M442, was striking. Survival of mice infected with pattern III bacteria at a high dose of infection was limited to 25 to 35 days p.i., whereas a large proportion of mice infected with moderately virulent strains maintained viability up to 120 days p.i.; no reduction of animal viability was observed in the group infected with pattern I strains, including strain H37Rv. It should be noted, however, that the laboratory strain H37Rv used in our experiments presented a lower level of virulence compared to the virulence of this strain reported in other studies using a similar murine model of infection (26, 27). This could be due to genetic variability of independent sublineages of this strain that evolved in different laboratories and possibly is linked to phenotype variability (28).
The higher virulence of modern Beijing strains observed in this study was based on the ability of these bacteria to induce severe lung pathology, rather than on increased bacterial growth in lungs that was only slightly higher than that of the ancient strains. Extensive pneumonia with areas of necrosis occupying up to 80% of the organ was associated with enhanced inflammatory cell recruitment to the lungs. The results of macrophage infection in vitro demonstrated higher ability of these strains to induce necrotic macrophage death known to lead to liberation of mycobacteria and a variety of cellular components called the “danger” signals. Release of these components enhances recruitment of phagocytic leukocytes, including neutrophils, that promote, on one hand, elimination of the bacteria by oxidative burst, but aggravate pathology by collateral tissue damage contributing to lung necrosis (29).
The main limitation of this study, as well as other virulence challenge studies comparing different phylogenetic groups of M. tuberculosis strains in animal models of infection (8, 30), is a relatively small number of strains that can be investigated in each experimental setting. Combining analysis of published reports determining virulence of the Beijing strains, that were further discriminated as strains of modern or ancient Beijing sublineages, allows comparison of larger samples of the characterized strains of each sublineage. The strains determined in earlier studies as highly virulent, including strains HN878, W10, 210, and SA161 (31, 32, 33, 34) were further identified as strains of the modern Beijing sublineage (35), whereas the less virulent strains, such as strains NHN5 and N4 (31, 34), belonged to the ancient sublineage (35, 36). In accordance with these observations, some isolates of a recently evolved modern Beijing sublineage (sublineage 7, RD150 group) were more virulent in mice than the ancient strains (8). It should be noted that most of these modern strains came from environments where they caused large numbers of clustered secondary cases of TB in immunocompetent individuals, therefore demonstrating high transmissibility, whereas the ancient strains were isolated from unique TB cases or groups of immunocompromised patients. The data obtained in the present study show that sporadic strains of the modern Beijing sublineage isolated in regions with low prevalence of Beijing strains, but not the ancient strains, could exhibit high levels of virulence comparable with those of the epidemic modern strains in the emerging regions. These data, however, do not exclude the possibility that some strains of the ancient Beijing sublineage display high virulence. A previous study (30) in San Francisco, CA, demonstrated that some of the ancient Beijing strains (the RD207 subgroup) exhibited levels of virulence similar to or even higher than those of the modern Beijing strains isolated in that region and were more transmissible (24). On the other hand, in a region where TB is endemic and caused by ancient Beijing strains, such as Japan, the recently transmitted TB cases were more frequently caused by modern Beijing strains, whereas isolates of the ancient sublineage were associated with cases of disease reactivation in older patients and not with ongoing transmission (37). All these data together demonstrate that strains of the modern Beijing sublineage are more likely to exhibit high virulence than strains of the ancient Beijing sublineage. Although both ancient and modern Beijing strains can exhibit highly virulent phenotypes, the proportion of circulating strains with increased virulence is higher in the modern Beijing subgroup, suggesting a higher propensity of these bacteria to attain more pronounced virulence.
The reasons for higher virulence and general competitiveness of the modern Beijing strains have not been fully determined, but whole-genome sequencing of strains belonging to different Beijing subgroups defined by the presence of RD deletions demonstrated that these strains also displayed distinct mutations in virulence-associated genes (30). Some of these SNPs are shared by the most recent common ancestor and all of its descendants, whereas others could be detected only in the descendant strains. The specific mutations in the mutT genes are typical for the descendant modern Beijing strains and lacking in ancestral counterparts (16); as a consequence, the resulting weakness of DNA repair functions in the modern strains could promote acquisition of mutations increasing bacterial adaptability to the immunocompetent host. Additionally, the accelerated general mutation rate and its link to bacterial adaptability, leading to multifold increase in acquisition of multidrug-resistant (MDR) and extensively drug-resistant (XDR) mutants, were also demonstrated in strains of East Asian/Beijing lineage, compared with those of the Euro-American lineage (38; but see opposite opinion in reference 39) or of the East African-Indian lineage (40). This could favor selection of virulence-associated mutants as well, particularly in patients treated with antituberculosis drugs. Highly virulent MDR mutants, expressing compensatory mutations for bacterial fitness and able to enhanced multiplication in treated host organisms, may be selected under such conditions at a higher rate than the low-virulence mutants. Studies in Singapore (41) and Russia (3) demonstrated a higher frequency of MDR isolates and higher transmission rates of drug-resistant TB among Beijing genotype strains than non-Beijing strains, suggesting increased virulence of the MDR Beijing strains circulating in these regions.
In conclusion, highly virulent modern Beijing strains were identified in countries characterized by low prevalence of the East Asian/Beijing M. tuberculosis lineage in the local population structures of M. tuberculosis strains (Brazil and Mozambique). The data obtained in animal models of infection provide the evidence that modern Beijing strains, epidemic as well as sporadic ones, are more likely to display phenotypes associated with increased virulence than strains of the ancestral Beijing sublineage. These data give new insight on the recent success of this competitive family of strains that appears to contribute to recurrence of TB in particular of multidrug-resistant TB in some parts of the world and demonstrate the importance of continuous monitoring of the populations of Beijing strains in different geographic regions.
ACKNOWLEDGMENTS
This work was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and the Fundação de Amparo à Pesquisa do Rio de Janeiro (FAPERJ), Brazil.
We gratefully acknowledge M. A. Telles at the Instituto Adolfo Lutz, Sao Paulo, Brazil, and A. Panunto at the Unicamp, Sao Paulo, Brazil, for providing the strains and epidemiological data. We thank Fernando C. Lopes for excellent technical assistance and Rodrigo S. Oliveira for support in animal experimentation.
We declare that we have no conflicts of interest.
Footnotes
Published ahead of print 14 May 2014
REFERENCES
- 1.Parwati I, van Crevel R, van Soolingen D. 2010. Possible underlying mechanisms for successful emergence of the Mycobacterium tuberculosis Beijing genotype strains. Lancet Infect. Dis. 10:103–111. 10.1016/S1473-3099(09)70330-5 [DOI] [PubMed] [Google Scholar]
- 2.European Concerted Action on New Generation Genetic Markers and Techniques for the Epidemiology and Control of Tuberculosis. 2006. Beijing/W genotype Mycobacterium tuberculosis and drug resistance. Emerg. Infect. Dis. 12:736–743. 10.3201/eid1205.050400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Casali N, Nikolayevskyy V, Balabanova Y, Harris SR, Ignatyeva O, Kontsevaya I, Corander J, Bryant J, Parkhill J, Nejentsev S, Horstmann RD, Brown T, Drobniewski F. 2014. Evolution and transmission of drug-resistant tuberculosis in a Russian population. Nat. Genet. 46:279–286. 10.1038/ng.2878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buu TN, Huyen MMN, Lan NT, Quy HT, Hen NV, Zignol M, Borgdorff MW, Cobelens FG, van Soolingen D. 2009. The Beijing genotype is associated with young age and multidrug-resistant tuberculosis in rural Vietnam. Int. J. Tuberc. Lung Dis. 13:900–906 [PubMed] [Google Scholar]
- 5.Hanekom M, van der Spuy GD, Streicher E, Ndabambi SL, McEvoy CR, Kidd M, Beyers N, Victor TC, van Helden PD, Warren RM. 2007. A recently evolved sublineage of the Mycobacterium tuberculosis Beijing strain family is associated with an increased ability to spread and cause disease. J. Clin. Microbiol. 45:1483–1490. 10.1128/JCM.02191-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.López B, Aguilar D, Orozco H, Burger M, Espitia C, Ritacco V, Barrera L, Kremer K, Hernandez-Pando R, Huygen K, van Soolingen D. 2003. A marked difference in pathogenesis and immune response induced by different Mycobacterium tuberculosis genotypes. Clin. Exp. Immunol. 133:30–37. 10.1046/j.1365-2249.2003.02171.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abebe F, Bjune G. 2006. The emergence of Beijing family genotypes of Mycobacterium tuberculosis and low level protection by bacille Calmette-Guérin (BCG) vaccines: is there a link? Clin. Exp. Immunol. 145:389–397. 10.1111/j.1365-2249.2006.03162.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aguilar D, Hanekom M, Mata D, Gey van Pittius NC, van Helden PD, Warren RM, Hernandez-Pando R. 2010. Virulence Mycobacterium tuberculosis strains with the Beijing genotype demonstrate variability in virulence associated with transmission. Tuberculosis (Edinb.) 90:319–325. 10.1016/j.tube.2010.08.004 [DOI] [PubMed] [Google Scholar]
- 9.Li Q, Whalen CC, Albert JM, Larkin R, Zukowski L, Cave MD, Silver RF. 2002. Differences in rate and variability of intracellular growth of a panel of Mycobacterium tuberculosis clinical isolates within a human monocyte model. Infect. Immun. 70:6489–6493. 10.1128/IAI.70.11.6489-6493.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Theus SA, Cave MD, Eisenach KD. 2005. Intracellular macrophage growth rates and cytokine profiles of Mycobacterium tuberculosis strains with different transmission dynamics. J. Infect. Dis. 191:453–460. 10.1086/425936 [DOI] [PubMed] [Google Scholar]
- 11.Tsolaki AG, Gagneux S, Pym AS, Goguet de la Salmoniere YO, Kreiswirth BN, Van Soolingen D, Small PM. 2005. Genomic deletions classify the Beijing/W strains as a distinct genetic lineage of Mycobacterium tuberculosis. J. Clin. Microbiol. 43:3185–3191. 10.1128/JCM.43.7.3185-3191.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gagneux S, Small PM. 2007. Global phylogeography of Mycobacterium tuberculosis and implications for tuberculosis product development. Lancet Infect. Dis. 7:328–337. 10.1016/S1473-3099(07)70108-1 [DOI] [PubMed] [Google Scholar]
- 13.Faksri K, Drobniewski F, Nikolayevskyy V, Brown T, Prammananan T, Palittapongarnpim P, Prayoonwiwat N, Chaiprasert A. 2011. Genetic diversity of the Mycobacterium tuberculosis Beijing family based on IS6110, SNP, LSP and VNTR profiles from Thailand. Infect. Genet. Evol. 11:1142–1149. 10.1016/j.meegid.2011.04.007 [DOI] [PubMed] [Google Scholar]
- 14.Plikaytis BB, Marden JL, Crawford JT, Woodley CL, Butler WR, Shinnick TM. 1994. Multiplex PCR assay specific for the multidrug-resistant strain W of Mycobacterium tuberculosis. J. Clin. Microbiol. 32:1542–1546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mokrousov I, Ly HM, Otten T, Lan NN, Vyshnevskyi B, Hoffner S, Narvskaya O. 2005. Origin and primary dispersal of the Mycobacterium tuberculosis Beijing genotype: clues from human phylogeography. Genome Res. 15:1357–1364. 10.1101/gr.3840605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ebrahimi-Rad M, Bifani P, Martin C, Kremer K, Samper S, Rauzier J, Kreiswirth B, Blazquez J, Jouan M, van Soolingen D, Gicquel B. 2003. Mutations in putative mutator genes of Mycobacterium tuberculosis strains of the W-Beijing family. Emerg. Infect. Dis. 9:838–845. 10.3201/eid0907.020803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mokrousov I. 2013. Insights into the origin, emergence, and current spread of a successful Russian clone of Mycobacterium tuberculosis. Clin. Microbiol. Rev. 26:342–360. 10.1128/CMR.00087-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iwamoto T, Grandjean L, Arikawa K, Nakanishi N, Caviedes L, Coronel J, Sheen P, Wada T, Taype CA, Shaw MA, Moore DA, Gilman RH. 2012. Genetic diversity and transmission characteristics of Beijing family strains of Mycobacterium tuberculosis in Peru. PLoS One 7:e49651. 10.1371/journal.pone.0049651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lasunskaia E, Ribeiro SC, Manicheva O, Gomes LL, Suffys PN, Mokrousov I, Ferrazoli L, Andrade MR, Kritski A, Otten T, Kipnis TL, da Silva WD, Vishnevsky B, Oliveira MM, Gomes HM, Baptista IF, Narvskaya O. 2010. Emerging multidrug resistant Mycobacterium tuberculosis strains of the Beijing genotype circulating in Russia express a pattern of biological properties associated with enhanced virulence. Microbes Infect. 12:467–475. 10.1016/j.micinf.2010.02.008 [DOI] [PubMed] [Google Scholar]
- 20.Nunes EA, De Capitani EM, Coelho E, Joaquim OA, Figueiredo IR, Cossa AM, Panunto AC, Carvalho-Ramos M. 2005. Patterns of anti-tuberculosis drug resistance among HIV-infected patients in Maputo, Mozambique, 2002–2003. Int. J. Tuberc. Lung Dis. 9:494–500 [PubMed] [Google Scholar]
- 21.Supply P, Allix C, Lesjean S, Cardoso-Oelemann M, Rüsch-Gerdes S, Willery E, Savine E, de Haas P, van Deutekom H, Roring S, Bifani P, Kurepina N, Kreiswirth B, Sola C, Rastogi N, Vatin V, Gutierrez MC, Fauville M, Niemann S, Skuce R, Kremer K, Locht C, van Soolingen D. 2006. Proposal for standardization of optimized mycobacterial interspersed repetitive unit-variable number tandem repeat typing of Mycobacterium tuberculosis. J. Clin. Microbiol. 44:4498–4510. 10.1128/JCM.01392-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lari N, Rindi L, Bonanni D, Tortoli E, Garzelli C. 2006. Mutations in mutT genes of Mycobacterium tuberculosis isolates of Beijing genotype. J. Med. Microbiol. 55:599–603. 10.1099/jmm.0.46261-0 [DOI] [PubMed] [Google Scholar]
- 23.Andrade MR, Amaral EP, Ribeiro SC, Almeida FM, Peres TV, Lanes V, D'Império-Lima MR, Lasunskaia EB. 2012. Pathogenic Mycobacterium bovis strains differ in their ability to modulate the proinflammatory activation phenotype of macrophages. BMC Microbiol. 12:166. 10.1186/1471-2180-12-166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kato-Maeda M, Kim EY, Flores L, Jarlsberg LG, Osmond D, Hopewell PC. 2010. Differences among sublineages of the East-Asian lineage of Mycobacterium tuberculosis in genotypic clustering. Int. J. Tuberc. Lung Dis. 14:538–544 [PMC free article] [PubMed] [Google Scholar]
- 25.Viegas SO, Machado A, Groenheit R, Ghebremichael S, Pennhag A, Gudo PS, Cuna Z, Langa E, Miotto P, Cirillo DM, Rastogi N, Warren RM, van Helden PD, Koivula T, Källenius G. 2013. Mycobacterium tuberculosis Beijing genotype is associated with HIV infection in Mozambique. PLoS One 8:e71999. 10.1371/journal.pone.0071999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Senaratne RH, Sidders B, Sequeira P, Saunders G, Dunphy K, Marjanovic O, Reader JR, Lima P, Chan S, Kendall S, McFadden J, Riley LW. 2008. Mycobacterium tuberculosis strains disrupted in mce3 and mce4 operons are attenuated in mice. J. Med. Microbiol. 57:164–170. 10.1099/jmm.0.47454-0 [DOI] [PubMed] [Google Scholar]
- 27.Kozak RA, Alexander DC, Liao R, Sherman DR, Behr MA. 2011. Region of difference 2 contributes to virulence of Mycobacterium tuberculosis. Infect. Immun. 79:59–66. 10.1128/IAI.00824-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ioerger TR, Feng Y, Ganesula K, Chen X, Dobos KM, Fortune S, Jacobs WR, Jr, Mizrahi V, Parish T, Rubin E, Sassetti C, Sacchettini JC. 2010. Variation among genome sequences of H37Rv strains of Mycobacterium tuberculosis from multiple laboratories. J. Bacteriol. 192:3645–3653. 10.1128/JB.00166-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang CT, Cambier CJ, Davis JM, Hall CJ, Crosier PS, Ramakrishnan L. 2012. Neutrophils exert protection in the early tuberculous granuloma by oxidative killing of mycobacteria phagocytosed from infected macrophages. Cell Host Microbe 12:301–312. 10.1016/j.chom.2012.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kato-Maeda M, Shanley CA, Ackart D, Jarlsberg LG, Shang S, Obregon-Henao A, Harton M, Basaraba RJ, Henao-Tamayo M, Barrozo JC, Rose J, Kawamura LM, Coscolla M, Fofanov VY, Koshinsky H, Gagneux S, Hopewell PC, Ordway DJ, Orme IM. 2012. Beijing sublineages of Mycobacterium tuberculosis differ in pathogenicity in the guinea pig. Clin. Vaccine Immunol. 19:1227–1237. 10.1128/CVI.00250-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manca C, Tsenova L, Bergtold A, Freeman S, Tovey M, Musser JM, Barry CE, III, Freedman VH, Kaplan G. 2001. Virulence of a Mycobacterium tuberculosis clinical isolate in mice is determined by failure to induce Th1 type immunity and is associated with induction of IFN-alpha/beta. Proc. Natl. Acad. Sci. U. S. A. 98:5752–5757. 10.1073/pnas.091096998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ordway D, Henao-Tamayo M, Harton M, Palanisamy G, Troudt J, Shanley C, Basaraba RJ, Orme IM. 2007. The hypervirulent Mycobacterium tuberculosis strain HN878 induces a potent TH1 response followed by rapid down-regulation. J. Immunol. 179:522–531. 10.4049/jimmunol.179.1.522 [DOI] [PubMed] [Google Scholar]
- 33.Palanisamy GS, Smith EE, Shanley CA, Ordway DJ, Orme IM, Basaraba RJ. 2008. Disseminated disease severity as a measure of virulence of Mycobacterium tuberculosis in the guinea pig model. Tuberculosis (Edinb.) 88:295–306. 10.1016/j.tube.2007.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jeon BY, Derrick SC, Lim J, Kolibab K, Dheenadhayalan V, Yang AL, Kreiswirth B, Morris SL. 2008. Mycobacterium bovis BCG immunization induces protective immunity against nine different Mycobacterium tuberculosis strains in mice. Infect. Immun. 76:5173–5180. 10.1128/IAI.00019-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mestre O, Luo T, Dos Vultos T, Kremer K, Murray A, Namouchi A, Jackson C, Rauzier J, Bifani P, Warren R, Rasolofo V, Mei J, Gao Q, Gicquel B. 2011. Phylogeny of Mycobacterium tuberculosis Beijing strains constructed from polymorphisms in genes involved in DNA replication, recombination and repair. PLoS One 6:e16020. 10.1371/journal.pone.0016020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alonso H, Samper S, Martín C, Otal I. 2013. Mapping IS6110 in high-copy number Mycobacterium tuberculosis strains shows specific insertion points in the Beijing genotype. BMC Genomics 14:422. 10.1186/1471-2164-14-422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iwamoto T, Fujiyama R, Yoshida S, Wada T, Shirai C, Kawakami Y. 2009. Population structure dynamics of Mycobacterium tuberculosis Beijing strains during past decades in Japan. J. Clin. Microbiol. 47:3340–3343. 10.1128/JCM.01061-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ford CB, Shah RR, Maeda MK, Gagneux S, Murray MB, Cohen T, Johnston JC, Gardy J, Lipsitch M, Fortune SM. 2013. Mycobacterium tuberculosis mutation rate estimates from different lineages predict substantial differences in the emergence of drug-resistant tuberculosis. Nat. Genet. 45:784–790. 10.1038/ng.2656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mokrousov I. 2014. Widely-used laboratory and clinical Mycobacterium tuberculosis strains: to what extent they are representative of their phylogenetic lineages? Tuberculosis (Edinb.) 94:355–356. 10.1016/j.tube.2014.03.010 [DOI] [PubMed] [Google Scholar]
- 40.de Steenwinkel JE, ten Kate MT, de Knegt GJ, Kremer K, Aarnoutse RE, Boeree MJ, Verbrugh HA, van Soolingen D, Bakker-Woudenberg IA. 2012. Drug susceptibility of Mycobacterium tuberculosis Beijing genotype and association with MDR TB. Emerg. Infect. Dis. 18:660–663. 10.3201/eid1804.110912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun YJ, Lee AS, Wong SY, Heersma H, Kremer K, van Soolingen D, Paton NI. 2007. Genotype and phenotype relationships and transmission analysis of drug-resistant tuberculosis in Singapore. Int. J. Tuberc. Lung Dis. 11:436–442 [PubMed] [Google Scholar]