Abstract
Infections caused by Penicillium species are rare in dogs, and the prognosis in these cases is poor. An unknown species of Penicillium was isolated from a bone lesion in a young dog with osteomyelitis of the right ilium. Extensive diagnostic evaluation did not reveal evidence of dissemination. Resolution of lameness and clinical stability of disease were achieved with intravenous phospholipid-complexed amphotericin B initially, followed by long-term combination therapy with terbinafine and ketoconazole. A detailed morphological and molecular characterization of the mold was undertaken. Sequence analysis of the internal transcribed spacer revealed the isolate to be closely related to Penicillium menonorum and Penicillium pimiteouiense. Additional sequence analysis of β-tubulin, calmodulin, minichromosome maintenance factor, DNA-dependent RNA polymerase, and pre-rRNA processing protein revealed the isolate to be a novel species; the name Penicillium canis sp. nov. is proposed. Morphologically, smooth, ovoid conidia, a greenish gray colony color, slow growth on all media, and a failure to form ascomata distinguish this species from closely related Penicillium species.
INTRODUCTION
Penicillium is a genus of ubiquitous saprobic fungi with >300 known species, but few are recognized as pathogens of dogs or people (1, 2). Penicillium marneffei, the only dimorphic member of the genus, is an emerging pathogen of immunocompromised humans, and infections of immunocompetent patients have also been reported (3, 4). Dogs are potential reservoirs of P. marneffei in some regions (5). Infections caused by other species are exceedingly rare in humans.
Opportunistic filamentous fungal infections of dogs are infrequently reported (2, 6–12). Clinical manifestations often include some combination of bone or back pain, respiratory disease, neurologic abnormalities, renal disease, hepatic disease, draining wounds, and uveitis. Cases incited by Geosmithia argillacea (subsequently characterized in the genus Rasamsonia and reidentified by Houbraken et al. as Rasamsonia piperina [13, 14]), Paecilomyces species, Phialosimplex species, Aspergillus terreus, and other unclassified species of the genera Penicillium and Aspergillus have been reported, with infections caused by aspergilli being the most common (2, 6–12, 15–18). The exact species is questionable in some reports, as isolates were identified on the basis of morphological features without concurrent molecular sequencing (2, 6–9). The majority of infections were reported in German shepherd dogs (GSD), a breed presumed to have a hereditary immunologic defect (2, 7, 11, 12, 18, 19). Both systemic disease and osteomyelitis due to Penicillium species are rare in dogs. P. verruculosum, P. purpurogenum, and several penicillia not identified to the species level have been reported in dogs with fungal osteomyelitis (2, 6, 7, 10–12). The prognosis in these cases is poor, with most dogs succumbing to disease or undergoing euthanasia shortly after diagnosis (2, 7, 9–12). Although clinically stable disease during aggressive combination antifungal therapy has been reported rarely, cures are unlikely (2). Newer antifungals have improved response rates in humans with opportunistic fungal infections; however, limited species-specific information and great expense have limited their use in veterinary medicine (20).
This report documents the clinicopathologic findings, treatment, and outcome of a young Rhodesian ridgeback dog with fungal osteomyelitis caused by a novel Penicillium species, Penicillium canis. The morphological and molecular sequencing features of P. canis are characterized in this report.
CASE REPORT
A 3-year-old, 44-kg, female, spayed Rhodesian ridgeback dog was referred to the Michigan State University (MSU) Veterinary Teaching Hospital (VTH) for further evaluation of a painful, proliferative, bony lesion of the right ilium causing clinically apparent lameness (day 0). The onset of lameness was approximately 2 weeks prior to evaluation at MSU VTH, and the patient was otherwise normal. Empirical therapy with carprofen (100 mg per os every 12 h) and tramadol (100 mg per os every 8 to 12 h) had been initiated for pain control prior to evaluation. On general physical examination, the dog had significant toe-touching lameness of the right hind limb. Palpation of the right ilium elicited severe pain and vocalization.
Initial diagnostic testing included a complete blood count, serum biochemical profile, and urinalysis performed at the MSU Diagnostic Center for Population and Animal Health (DCPAH) Clinical Pathology Laboratory. Hematologic evaluation revealed leukocytosis (14.6 × 103 leukocytes/μl; reference range, 6.1 × 103 to 12.0 × 103 leukocytes/μl) characterized by marked lymphocytosis (6.8 × 103 lymphocytes/μl; reference range, 1.1 × 103 to 3.1 × 103 lymphocytes/μl) with a normal neutrophil concentration. Serum biochemical and urinalysis abnormalities included mild hypoalbuminemia (2.6 g/dl; reference range, 2.8 to 4.0 g/dl), hyperglobulinemia (4.6 g/dl; reference range, 2.2 to 4.1 g/dl), and proteinuria. Three-view thoracic radiographs did not reveal any evidence of pathology. Pelvic radiographs revealed a proliferative bony lesion of the right ilium (Fig. 1A), similar to radiographs obtained by the referring veterinarian. Fine-needle aspirates of the lesion revealed marked neutrophilic and moderate macrophagic inflammation; fungal culture was recommended. A Blastomyces urine antigen test (MiraVista Diagnostics Laboratory, Indianapolis, IN) was negative. Results of flow cytometric evaluation and PCR clonality testing for antigen receptor rearrangements to further characterize the lymphocytosis were supportive of a heterogeneous and reactive lymphoid population.
FIG 1.
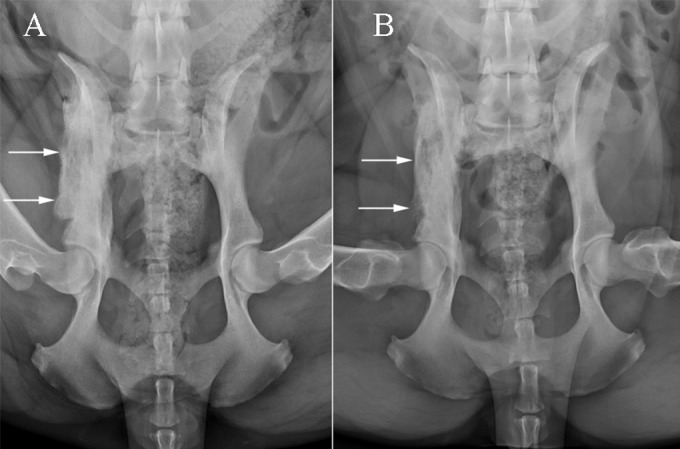
Ventrodorsal projections of the pelvis at presentation (A) and 3 months later (B). There is extensive mixed periosteal proliferation and permeative lysis along the right ilial body and wing in the initial radiograph, which are smoother and less severe in the subsequent image.
Seven days after the initial evaluation, a computed tomography (CT) scan and guided biopsies were performed. The CT scan revealed a mixed proliferative and lytic lesion involving predominantly the right ilial body but also involving the ventral aspect of the adjacent sacrum. A whole-body bone scan was performed with intravenously (i.v.) administered technetium 99m-methylene diphosphonate to further evaluate the dog for systemic involvement. In addition to the known iliac lesion, there was focal uptake at the left fourth costochondral junction. The costochondral region was fine needle aspirated, but samples were hemodiluted and lacked cytologic evidence of disease. Four core biopsy specimens of the ilium were obtained with a trephine bone biopsy needle. Ultrasound-guided fine-needle regional lymph node (sublumbar) aspirates were obtained. Biopsy samples were submitted to the MSU DCPAH Microbiology and Anatomic Pathology laboratories for microbial culture and histopathologic evaluation, respectively. Biopsy samples were routinely prepared and examined via light microscopy (Fig. 2). There were myriad nonpigmented septate fungal hyphae 3 to 4 μm in width and 7 to 40 μm in length with nonparallel walls and occasional 45° angle branching throughout all sections of bone. Cytologic examination of lymph node samples revealed reactive lymphoid hyperplasia with no evidence of fungal hyphae. Routine aerobic and anaerobic bacteriologic cultures were negative for growth. However, mycologic cultures incubated on Sabouraud dextrose (SDA; Remel, Lenexa, KS) and Mycosel (Becton, Dickinson, and Company, Sparks, MD) agars at 25 and 35°C resulted in heavy growth of a nonpigmented mold with white/brown (surface/reverse) colony morphology within 4 to 5 days of the initial incubation. The hyphae were septate and had branched, “Penicillum-like” conidiophores. No other agents were isolated. The conidiogenous cells appeared to be annellated. Although the morphological features resembled those of a moniliaceous (hyaline) hyphomycete, in particular, a Scopulariopsis species, definitive identification was not possible. Partial 28S rDNA sequences of DNA extracted from the sample showed that the organism belonged to the genus Penicillium. Given its uncertain identity, the isolate from the SDA culture was submitted to the Fungus Testing Laboratory at the University of Texas Health Science Center in San Antonio for molecular characterization, identification, and in vitro antifungal susceptibility testing. The isolate was added to the culture collection there as UTHSC DI13-196. Antifungal susceptibility testing was performed by broth microdilution according to the CLSI guidelines for filamentous fungi (M38-A2) (21). The MICs of amphotericin B (AMB), fluconazole, itraconazole, posaconazole, voriconazole, ketoconazole, and terbinafine were determined as the lowest concentrations of these agents that resulted in 100% inhibition of growth compared to the growth control after 48 h of incubation. Posaconazole and terbinafine had the most potent in vitro activity (MIC of both, 0.06 μg/ml), followed by AMB, itraconazole, ketoconazole (MIC of all, 0.125 μg/ml), and voriconazole (MIC, 0.25 μg/ml), while fluconazole was the least active of the agents tested (MIC, 64 μg/ml). The DNA was isolated, and the internal transcribed spacer (ITS), β-tubulin, and calmodulin loci were amplified and sequenced (22–24). Analysis of ITS the sequence showed the best matches with Penicillium menonorum (GenBank accession no. HQ646591, 97.6% identity) and Penicillium pimiteouiense (GenBank accession no. FJ624254, 97.3% identity) (25). Because of the relatively poor sequence matches, the isolate was referred to the Bacterial Foodborne Pathogens and Mycology Research Unit, National Center for Agricultural Utilization Research, United States Department of Agriculture, for further analysis. The methods used to further characterize the isolate and the results are described below.
FIG 2.
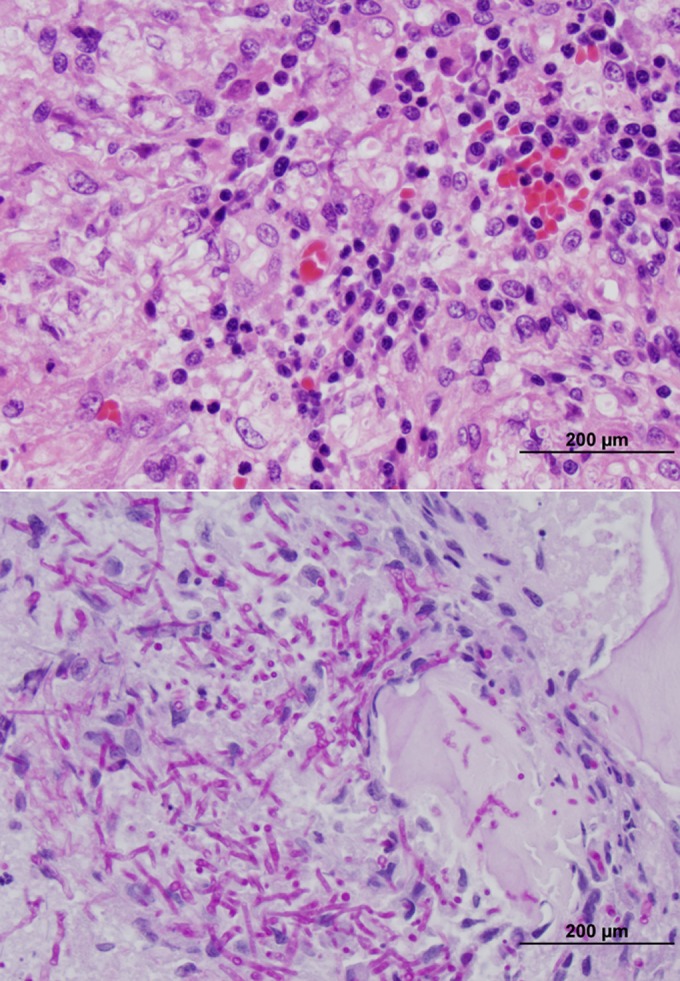
Photomicrographs of sections from the bone biopsy specimen. (A) Hematoxylin-and-eosin staining; magnification, ×40. Bone segments were surrounded by marked granulomatous inflammation and small regions of necrosis. Intrahistiocytic and extracellular nonpigmented fungal organisms were documented throughout the section. Small numbers of lymphocytes, plasma cells, and neutrophils were also present throughout the section. (B) Periodic acid-Schiff staining; magnification, ×40. Note the large number of organisms within the cytoplasm of macrophages and free throughout the section. Intracellular and extracellular fungal organisms were nonpigmented and septate (3 to 4 μm in diameter by 10 to 20 μm in length) and exhibited occasional dichotomous branching. There were also round, 5- to 7-μm-diameter fungal structures that likely represent cross sections of conidia.
Multiple treatment options, including aggressive surgical resection followed by medical therapy, oral antifungal therapy with an azole agent, i.v. antifungal therapy with AMB, and a combination of these therapies, were discussed with the owners. Given the possibility of systemic disease combined with the challenging and invasive nature of surgical resection (due to sacral involvement), the owners elected aggressive multimodal medical therapy, and carprofen therapy was discontinued (day 19).
The results of hematologic and biochemical reevaluations on day 23 were similar to the initial findings. Therapy was initiated with 75 mg of phospholipid-complexed AMB (Abelcet; Sigma-Tau Pharmaceuticals, Gaithersburg, MD) administered i.v. over 1 h. A 2-h diuresis with 0.9% saline i.v. was administered before and after the AMB infusion. A cumulative dose of 975 mg (23 mg/kg over 4 weeks) was given at a frequency of 75 mg three times per week. During the second week of AMB therapy, combination oral antifungal therapy consisting of ketoconazole (1 g/day) and terbinafine (1 g/day) was initiated. Therapy with posaconazole was recommended but declined because of financial restrictions. Therapy with a combination product of S-adenosylmethionine and silymarin (Denamarin; Nutramax Laboratories, Lancaster, SC) was also initiated to minimize the potential hepatotoxicity of high-dose ketoconazole. Upon cessation of AMB therapy (day 51), the dog was clinically sound and not in pain. Physical examination findings and owner-reported activity levels were normal.
During the second month of ketoconazole and terbinafine therapy, the dog developed diffuse, progressive hyperpigmentation and truncal alopecia. Skin cytology samples were obtained and reported to show no cytologic abnormalities. Therapy with oral cephalexin (100 mg every 12 h) and topical chlorhexidine shampoo failed to improve the pigmentation changes and alopecia. An adverse drug reaction was considered but was not pursued, as dose reductions or drug withdrawal could have led to a relapse and progression of clinical disease. Aside from dermatologic changes, the dog remained normal.
On day 83, repeat pelvic radiographs documented remodeling of the right ilial lesion with overall improvement (Fig. 1B). Periodic reevaluations were performed at MSU VTH over the subsequent 8 months. Although serum globulin concentrations returned to normal, only a mild improvement in reactive lymphocytosis (5.8 × 103 lymphocytes/μl; reference range, 1.1 × 103 to 3.1 × 103 lymphocytes/μl) was observed. Repeat pelvic radiographs on day 150 revealed further mild improvements of the right ilial lesion. The ketoconazole dose was reduced to 800 mg/day, while the terbinafine dose remained unchanged. At the time this report was submitted for publication (day 250), the dog was continuing to receive ketoconazole and terbinafine and was clinically normal. Both drugs were continued for long-term maintenance with no intent to modify therapy unless side effects or disease progression were observed.
MATERIALS AND METHODS
For the cultures used in this study and the corresponding GenBank accession numbers, see Table S1 in the supplemental material. P. canis z-384 was isolated and sequenced in 2008 but not characterized (S. W. Peterson, unpublished data), and the culture died during the intervening period.
Isolates were grown on Czapek's yeast autolysate agar (CYA; 26), Blakeslee's malt extract agar (MEA; 26), CYA amended with 5% NaCl, CYA amended with 20% sucrose (26), and mixed-grain baby food agar (MGA) composed of 50 g of Gerber mixed-grain baby cereal and 15 g of agar in 1 liter of deionized water. All of the media were prepared in house. Nine-centimeter-diameter petri plates of each medium were inoculated at three points and incubated at 25°C; CYA cultures were also prepared and incubated at 5 and 37°C for 7 days in darkness. Mature colonies were photographed with a digital camera, and bits of mycelium were teased apart in a drop of 1% Triton X-100 for microscopy and digital photography. Images were cropped, sized, and positioned into plates with Adobe Photoshop LE ver. 10.
Cultures were grown for 2 to 3 days on malt extract broth in gently agitated flasks to accumulate 1 to 2 g of biomass. Mycelia were harvested by vacuum filtration over Whatman no. 1 paper, placed in microcentrifuge tubes, frozen, and freeze-dried. Dry mycelium was ground to powder and rehydrated with cetyltrimethylammonium bromide buffer, proteins were extracted with chloroform, and nucleic acids were precipitated with isopropanol (27).
β-Tubulin (BT2), calmodulin (CF), the nuclear ITS region, minichromosome maintenance factor (Mcm7), DNA-dependent RNA polymerase (RPB2), and pre-rRNA processing protein (Tsr1) were amplified with the primers and under the conditions specified by Peterson and Jurjevic (27). Amplified DNA was prepared for sequencing with ExoSapit (Affymetrix), sequencing reactions were carried out with BigDye ver. 3.2 (Applied Biosystems), and sequences were read on an ABI 3730 genetic analyzer (Applied Biosystems). Sequencing was bidirectional. Raw sequences were compared and corrected with Sequencher 5.1 (Gene Codes).
Sequences were aligned by using ClustalW or Muscle as implemented in MEGA 5.2 (28). Data sets containing sequences from each single locus were aligned and analyzed under MEGA5.2 by using the maximum-likelihood estimation and the GTR+G+I model, as suggested by ModelTest (29). Bootstrapping was performed with 500 iterations of the data and the criterion detailed above. The trees were formatted for publication with CorelDraw.
Accession numbers.
The MycoBank accession number of the sequence determined in this study is MB 807056. The type strain (NRRL 62798) has been deposited into the culture collection of the University of Texas Health Science Center Fungus Testing Laboratory under accession number UTHSC DI13-196 and into the University of Alberta Microfungus Collection and Herbarium under accession number UAMH 11811.
RESULTS
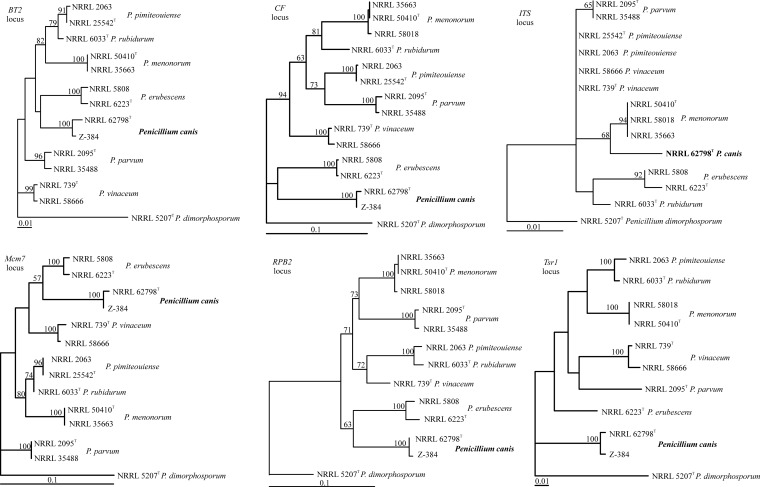
The maximum-likelihood trees obtained (Fig. 3) were based on 442 aligned BT2 nucleotides, 750 aligned CF nucleotides, 565 aligned ITS nucleotides, 616 aligned Mcm7 nucleotides, 1,014 aligned RPB2 nucleotides, and 817 aligned Tsr1 nucleotides. In the initial sequence analysis, Penicillium lassenii was used as the outgroup (30). In the tree, P. canis is basal among the species examined and a sister group to Penicillium erubescens, although with a low bootstrap proportion. The same isolates formed terminal groups at each locus (Fig. 3), but the relative positions of the taxa in the tree differed, with low bootstrap support for some of the branches. In the ITS barcode analysis, Penicillium parvum and P. pimiteouiense are indistinguishable from each other but other species in this clade can be identified.
FIG 3.
Phylogenetic trees showing the relationship of P. canis with closely related Penicillium species. The trees were calculated for each locus sequenced by using maximum likelihood in MEGA5.2. Bootstrap support above 50% is shown at the tree nodes; a superscript T indicates the type strain. Branch lengths are proportional to phylogenetic distances.
Taxonomy.
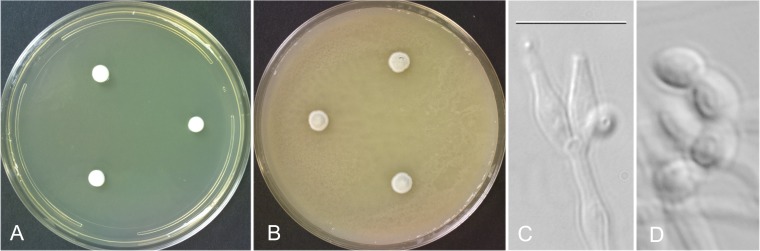
The isolate described in this report has been named Penicillium canis sp. nov. S. W. Peterson (Fig. 4).
FIG 4.
P. canis NRRL 62798. (A) Seven-day-old colonies grown on CYA at 25°C. (B) Seven-day-old colonies grown on MGA at 25°C. (C) Typical conidiophore with two apical phialides. (D) Characteristic elongate-ovoid, smooth-walled conidia. Bar = 10 μm for panels C and D.
Etymology.
The name canis refers to the isolation of the fungus from the ilium of a dog.
Holotype.
BPI 892763 is a dried colony of strain NRRL 62798 grown for 10 days on mixed-grain baby food agar. The culture was isolated from an ilial bone lesion in a Rhodesian ridgeback dog residing in Ann Arbor, MI, on 3 April 2013 by Daniel K. Langlois.
Barcode sequence.
The barcode sequence of the isolate described here is ITS-KJ511291. Additional identifying sequences are BT-KF900167, calmodulin-KF900177, and RPB2-KF900196.
Phenotypic diagnosis.
Its smooth, ovoid conidia; greenish gray colony color; and slow growth on all media distinguish this species from P. pimiteouiense and P. menonorum.
Description.
Colonies on CYA attained diameters of 6 to 7 mm after 7 days of incubation at 25°C, were white, and formed a 2-mm-high cushion of loose vegetative hyphae. Sporulation was sparse and basal, with no exudate, soluble pigments, sclerotia, or ascomata. The reverse was yellowish near chamois in color. Colonies on MEA attained diameters of 7 to 8 mm after 7 days of incubation at 25°C, were white, and formed a 2- to 3-mm raised cushion of largely vegetative hyphae. Sporulation was sparse, with no exudate or soluble pigments and no sclerotia or ascomata, and the reverse was a pale drab. Colonies on MGA incubated for 7 days at 25°C attained diameters of 5 to 6 mm, formed a 2- to 3-mm raised cushion, and sporulated heavily in a greenish gray color with no exudate, soluble pigments, sclerotia, or ascomata. The colony reverse was not visible on this medium. There was no growth on CYA at 5°C; at 37°C, small, white, 2- to 3-mm-diameter colonies were formed after 7 days. Colonies grown at 25°C on CYA—5% NaCl or CYA–20% sucrose were 2 or 5 mm in diameter, respectively.
Conidiophores were simple, arising from basal and aerial hyphae, smooth walled, hyaline, 5 to 25 by 1.5 to 2.5 μm, nonvesiculate, bearing an apical whorl of two to five ampuliform phialides 5 to 7 (uncommonly, up to 10) by 2 to 3 μm with a 1- to 2-μm collula, bearing ovoid, smooth-walled conidia 4 to 5 by 2 to 3 μm.
Phenotypically, P. canis resembles P. pimiteouiense and P. menonorum at the microscopic level, with these species all possessing short, unbranched, nonvesiculate conidiophores with a whorl of two to five phialides. Conidia of P. pimiteouiense are spherical and finely roughened, and conidia of P. menonorum are spherical to subspherical and rugose, while conidia of P. canis are elongate-ovoid and smooth. Colony diameters on CYA at 25°C after 7 days are 5 to 6 mm for P. canis, 17 to 18 mm for P. pimiteouiense, and 17 to 20 mm for P. menonorum, and conidial colors en masse are greenish gray, olive gray, and bluish gray, respectively. P. canis produces CYA colonies 2 to 3 mm in diameter when grown for 7 days at 37°C, while the colony diameters of P. pimiteouiense and P. menonorum are 20 to 21 and 29 to 32 mm, respectively. P. canis is easily distinguished from P. erubescens, P. parvum, and Penicillium rubidurum by its failure to form ascomata as the latter three species do. Penicillium vinaceum produces vinaceous purple pigments in the growth agar, which is quite distinct from the appearance of P. canis. Penicillium dimorphosporum produces conidia with two quite distinct phenotypes, aiding differentiation from P. canis.
DISCUSSION
Similar to the present case, lameness and pain are common signs in dogs with fungal osteomyelitis and typically the primary reason for evaluation (10, 11). However, most dogs with osteomyelitis caused by opportunistic molds have evidence of disseminated disease and often present with additional clinical signs, including anorexia, weight loss, pyrexia, lameness, back pain, lymphadenomegaly, ocular disease, epistaxis, and neurologic signs (2, 10, 16, 18, 19, 31, 32). Rapid dissemination commonly occurs, and the short-term prognosis is grave. Reported cases of infections with Penicillium species have involved the appendicular and axial skeletal systems, liver, spleen, kidney, pancreas, heart, lungs, and lymph nodes (2, 7, 11, 12). Although a thorough systemic evaluation of the dog in our report was performed (radiographs, ultrasound, CT scan, bone scan, and hematologic evaluations), only evidence of osteomyelitis was found. Whether the costochondral junction lesion observed in the bone scan represented fungal disease or another lesion, such as a healing fracture, was unknown. Radiopharmaceutical uptake was modest, and cytological examination of aspirates was inconclusive.
The possibility of sample contamination must be considered for fungal cultures obtained on a single occasion because penicillia are common laboratory contaminants. Fungal hyphae clearly documented within histopathological lesions from the present case had morphological features similar to those of cultures of these samples, and bacterial cultures were negative. Fungal cultures and preliminary molecular characterization performed at the initial institution resulted in the growth of a solitary identical fungus on multiple plates that belonged to the genus Penicillium. Original material and subsamples thereof exhibited pure growth of the same single fungus in all three laboratories. Moreover, contamination of multiple plates with the same previously uncharacterized fungus is highly improbable. In fact, of >500 fungal cultures performed at the initial laboratory in 2013, only 5 resulted in the growth of a Penicillium species and none were identified within 4 months of the isolate in our report. Given the above evidence, we believe that P. canis sp. nov. was the cause of osteomyelitis in the dog reported here.
Penicillium is a genus of ascomycetous fungi that are saprobic, filamentous, and typically monomorphic. Penicillium species have septate hyphae (2 to 5 μm in diameter) that give rise to branched or unbranched conidiophores with secondary branches that give Penicillium a brush-like appearance (2, 33). Scopulariopsis species also have septate hyphae with either single unbranched conidiophores or branched “Penicillium-like” conidiophores, and their colony morphology can be similar. Although Scopulariopsis species may resemble Penicillium species, they generally have shorter and sometimes simpler conidiophores. In addition, their conidium-bearing (conidiogenous) cells are annellidic rather than phialidic (34). On the basis of morphology alone, the isolate was originally thought to be a Scopulariopsis species. However, initial sequencing of the ITS revealed the isolate reported here to be a novel Penicillium species closely related to but not conspecific with P. menonorum and P. pimiteouiense (25). These sequencing results revealed only 76% similarity to a Scopulariopsis species. As definitive identification to the species level was not possible with the above target, the isolate was subsequently referred to and characterized as a new species by one of us (S.W.P.).
Fungal systematics most commonly involve phylogenetic species concepts (35) and analysis of multiple loci in order to apply the genealogical concordance test of species boundaries (36). In this case, the five protein-coding loci used are in complete agreement (Fig. 3) about the strongly supported terminal grouping of the isolates, leading to clear identification to the species level, including P. canis. The multilocus analysis validates the species concept and allows confident use of the barcode locus (ITS) in future investigations. These findings emphasize the importance of using the genealogical concordance phylogenetic species concept to define species and understand intraspecific ITS variability and thus barcode species identification. ITS barcode sequence analysis can point out potential cryptic species even when a presumed morphological diagnosis is possible.
The pathogenic potential of the newly discovered species P. canis is unknown. Overall, Penicillium species are rare opportunists, affecting individuals with compromised immunologic function (1). However, there is recent evidence that some species may be more pathogenic than others. P. marneffei is a well-recognized emerging public health concern endemic in southeastern Asia (3). P. marneffei infection is considered a disease-defining illness of HIV-infected patients, with mortality rates as high as 50% (4). The isolate in our report caused disease in a seemingly immunocompetent host. The long-term survival of the dog reported here was surprising, compared to previously reported infections of dogs with Penicillium species.
Antifungal susceptibility testing was performed to guide treatment; however, some reports suggest that in vitro activity does not always correlate with in vivo activity (37, 38). Nonetheless, these results were used to support therapeutic decision making. AMB was chosen as the initial therapeutic agent for its broad-spectrum antifungal activity and in vitro activity, as well as its documented efficacy in treating human P. marneffei infections (3, 39). Posaconazole was the recommended treatment of choice to follow AMB therapy, but financial limitations precluded its use. Terbinafine and ketoconazole were chosen as alternatives for long-term maintenance therapy. It is well recognized that combination antifungal therapy has potential synergistic effects (40–43). Furthermore, the MICs of both drugs were suggestive of drug efficacy, as these concentrations should be readily achieved on the basis of canine pharmacokinetic studies (44, 45). Clinical and radiographic improvements observed in the dog in our report substantiated the efficacy of this multimodal therapy.
It is noteworthy that the dog in the present report was not a GSD, as the majority of disseminated opportunistic fungal infections have been reported in this breed, possibly because of a heritable immune deficiency (2, 7, 11, 12, 15, 18, 21). Similar to opportunistic fungal infections in non-GSD breeds, the Rhodesian ridgeback in our report did not have evidence of underlying immune dysfunction and had been healthy prior to the onset of lameness (10). There was no evidence of dissemination during the follow-up period. Nonetheless, lymphocyte proliferation assays and measurement of immunoglobulin levels would be needed to provide more information on the immune competence of this dog.
This report characterizes the morphological and histopathological features of a newly discovered species of Penicillium with the potential to cause disease in otherwise apparently healthy dogs. Identification of future cases will provide additional insight into the ecological niche and pathogenic potential of this species. Although the overall prognosis of opportunistic fungal infections of dogs is poor, aggressive multimodal antifungal therapy provided long-term disease stability in the present case of a dog infected with P. canis.
Supplementary Material
ACKNOWLEDGMENTS
The medical care of the dog reported herein was supported in part by the MSU College of Veterinary Medicine Trinket Fund.
We thank Lori Moon for her microbiologic assistance.
We have no conflict of interest to declare.
Footnotes
Published ahead of print 30 April 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JCM.03602-13.
REFERENCES
- 1. Pitt JI. 1994. The current role of Aspergillus and Penicillium in human and animal health. J. Vet. Med. Mycol. 32(Suppl 1):17–32. 10.1080/02681219480000701 [DOI] [PubMed] [Google Scholar]
- 2. Watt PR, Robins GM, Galloway AM, O'Boyle DA. 1995. Disseminated opportunistic fungal disease in dogs: 10 cases (1982-1990). J. Am. Vet. Med. Assoc. 207:67–70 [PubMed] [Google Scholar]
- 3. Duong TA. 1996. Infection due to Penicillium marneffei, an emerging pathogen: review of 155 reported cases. Clin. Infect. Dis. 23:125–130. 10.1093/clinids/23.1.125 [DOI] [PubMed] [Google Scholar]
- 4. Hu Y, Zhang J, Li X, Yang Y, Zhang Y, Ma J, Xi L. 2013. Penicillium marneffei infection: an emerging disease in mainland China. Mycopathologia 175:57–67. 10.1007/s11046-012-9577-0 [DOI] [PubMed] [Google Scholar]
- 5. Chaiwun B, Vanittanakom N, Jiviriyawat Y, Rojanasthien S, Thorner P. 2011. Investigation of dogs as a reservoir for Penicillium marneffei in northern Thailand. Int. J. Infect. Dis. 15:e236–239. 10.1016/j.ijid.2010.12.001 [DOI] [PubMed] [Google Scholar]
- 6. Caro-Vadillo A, Paya-Vicens MJ, Martinez-Merlo E, Garcia-Real I, Martin-Espada C. 2007. Fungal pneumonia caused by Penicillium brevicompactum in a young Staffordshire bull terrier. Vet. Rec. 160:595–596. 10.1136/vr.160.17.595 [DOI] [PubMed] [Google Scholar]
- 7. Foley JE, Norris CR, Jang SS. 2002. Paecilomycosis in dogs and horses and a review of the literature. J. Vet. Intern. Med. 16:238–243. 10.1111/j.1939-1676.2002.tb02363.x [DOI] [PubMed] [Google Scholar]
- 8. Harvey CE. 1984. Nasal aspergillosis and penicilliosis in dogs: results of treatment with thiabendazole. J. Am. Vet. Med. Assoc. 184:48–50 [PubMed] [Google Scholar]
- 9. Holahan ML, Loft KE, Swenson CL, Martinez-Ruzafa I. 2008. Generalized calcinosis cutis associated with disseminated paecilomycosis in a dog. Vet. Dermatol. 19:368–372. 10.1111/j.1365-3164.2008.00706.x [DOI] [PubMed] [Google Scholar]
- 10. Miyakawa K, Swenson CL, Mendoza L, Boyle MH, Steficek BA. 2011. Pathology in practice. J. Am. Vet. Med. Assoc. 238:51–53. 10.2460/javma.238.1.51 [DOI] [PubMed] [Google Scholar]
- 11. Wigney D, Allan G, Hay L, Hocking A. 1990. Osteomyelitis associated with Penicillium verruculosum in a German shepherd dog. J. Small Anim. Pract. 31:449–452. 10.1111/j.1748-5827.1990.tb00512.x [DOI] [Google Scholar]
- 12. Zanatta R, Miniscalco B, Guarro J, Gene J, Capucchio M, Gallo M, Mikulicich B, Peano A. 2006. A case of disseminated mycosis in a German shepherd dog due to Penicillium purpurogenum. Med. Mycol. 44:93–97. 10.1080/13693780500302726 [DOI] [PubMed] [Google Scholar]
- 13. Houbraken J, Giraud S, Meijer M, Bertout S, Frisvad JC, Meis JF, Bouchara JP, Samson RA. 2013. Taxonomy and antifungal susceptibility of clinically important Rasamsonia species. J. Clin. Microbiol. 51:22–30. 10.1128/JCM.02147-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Houbraken J, Spierenburg H, Frisvad JC. 2012. Rasamsonia, a new genus comprising thermotolerant and thermophilic Talaromyces and Geosmithia species. Antonie Van Leeuwenhoek 101:403–421. 10.1007/s10482-011-9647-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Day MJ, Penhale WJ, Eger CE, Shaw SE, Kabay MJ, Robinson WF, Huxtable CR, Mills JN, Wyburn RS. 1986. Disseminated aspergillosis in dogs. Aust. Vet. J. 63:55–59. 10.1111/j.1751-0813.1986.tb02924.x [DOI] [PubMed] [Google Scholar]
- 16. Grant DC, Sutton DA, Sandberg CA, Tyler RD, Jr, Thompson EH, Romanelli AM, Wickes BL. 2009. Disseminated Geosmithia argillacea infection in a German shepherd dog. Med. Mycol. 47:221–226. 10.1080/13693780802559023 [DOI] [PubMed] [Google Scholar]
- 17. Sigler L, Sutton DA, Gibas CF, Summerbell RC, Noel RK, Iwen PC. 2010. Phialosimplex, a new anamorphic genus associated with infections in dogs and having phylogenetic affinity to the Trichocomaceae. Med. Mycol. 48:335–345. 10.3109/13693780903225805 [DOI] [PubMed] [Google Scholar]
- 18. Schultz RM, Johnson EG, Wisner ER, Brown NA, Byrne BA, Sykes JE. 2008. Clinicopathologic and diagnostic imaging characteristics of systemic aspergillosis in 30 dogs. J. Vet. Intern. Med. 22:851–859. 10.1111/j.1939-1676.2008.0125.x [DOI] [PubMed] [Google Scholar]
- 19. Bruchim Y, Elad D, Klainbart S. 2006. Disseminated aspergillosis in two dogs in Israel. Mycoses 49:130–133. 10.1111/j.1439-0507.2006.01168.x [DOI] [PubMed] [Google Scholar]
- 20. Diekema DJ, Messer SA, Hollis RJ, Jones RN, Pfaller MA. 2003. Activities of caspofungin, itraconazole, posaconazole, ravuconazole, voriconazole, and amphotericin B against 448 recent clinical isolates of filamentous fungi. J. Clin. Microbiol. 41:3623–3626. 10.1128/JCM.41.8.3623-3626.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Committee for Clinical and Laboratory Standards (CLSI). 2008. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi: approved standard—second edition. Document M38-A2. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 22. Peterson SW. 2008. Phylogenetic analysis of Aspergillus species using DNA sequences from four loci. Mycologia 100:205–226. 10.3852/mycologia.100.2.205 [DOI] [PubMed] [Google Scholar]
- 23. Peterson SW, Orchard SS, Menon S. 2011. Penicillium menonorum, a new species related to P. pimiteouiense. IMA Fungus 2:121–125. 10.5598/imafungus.2011.02.02.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Peterson SW, Corneli S, Hjelle JT, Miller-Hjelle MA, Nowak DM, Bonneau PA. 1999. Penicillium pimiteouiense: a new species isolated from polycystic kidney cell cultures. Mycologia 91:269–277. 10.2307/3761372 [DOI] [Google Scholar]
- 25. Schoch CL, Seifert KA, Huhndorf S, Robert V, Souge JL, Levesque CA, Chen W, Fungal Barcoding Consortium 2012. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for fungi. Proc. Natl. Acad. Sci. U. S. A 109:6241–6246. 10.1073/pnas.1117018109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pitt JI. 1979. The genus Penicillium and its teleomorphic states Eupenicillium and Talaromyces. Academic Press, London, United Kingdom [Google Scholar]
- 27. Peterson SW, Jurjevic Z. 2013. Talaromyces columbinus sp. nov., and genealogical concordance analysis in Talaromyces clade 2a. PLoS One 8:e78084. 10.1371/journal.pone.0078084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28:2731–2739. 10.1093/molbev/msr121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Posada D, Crandall KA. 1998. MODELTEST: testing the model of DNA substitution. Bioinformatics 14:817–818. 10.1093/bioinformatics/14.9.817 [DOI] [PubMed] [Google Scholar]
- 30. Houbraken J, Samson RA. 2011. Phylogeny of Penicillium and the segregation of Trichocomaceae into three families. Stud. Mycol. 70:1–51. 10.3114/sim.2011.70.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Brockus CW, Myers RK, Crandell JM, Sutton DA, Wickes BL, Nakasone KK. 2009. Disseminated Oxyporus corticola infection in a German shepherd dog. Med. Mycol. 47:862–868. 10.3109/13693780902962267 [DOI] [PubMed] [Google Scholar]
- 32. Hugnet C, Marrou B, Dally C, Guillot J. 2009. Osteomyelitis and discospondylitis due to Scedosporium apiospermum in a dog. J. Vet. Diagn. Invest. 21:120–123. 10.1177/104063870902100119 [DOI] [PubMed] [Google Scholar]
- 33. Sutton DA, Fothergill AW, Rinaldi MG. 1998. Guide to clinically important fungi, 1st ed. Williams & Wilkins, Baltimore, MD [Google Scholar]
- 34. Sandoval-Denis M, Sutton DA, Fothergill AW, Cano-Lira J, Gen.é Decock JCA, de Hoog GS, Guarro J. 2013. Scopulariopsis, a poorly known opportunistic fungus: spectrum of species in clinical samples and in vitro responses to antifungal drugs. J. Clin. Microbiol. 51:3937–3943. 10.1128/JCM.01927-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Taylor JW, Jacobson DJ, Kroken S, Kasuga T, Geiser DM, Hibbett DS, Fisher MC. 2000. Phylogenetic species recognition and species concepts in fungi. Fungal Genet. Biol. 31:21–32. 10.1006/fgbi.2000.1228 [DOI] [PubMed] [Google Scholar]
- 36. Dettman JR, Jacobson DJ, Taylor JW. 2006. Multilocus sequence data reveal extensive phylogenetic species diversity within the Neurospora discreta complex. Mycologia 98:36–446. 10.3852/mycologia.98.3.436 [DOI] [PubMed] [Google Scholar]
- 37. Rex JH, Pfaller M, Barry A, Nelson P, Webb C. 1995. Antifungal susceptibility testing of isolates from a randomized, multicenter trial of fluconazole versus amphotericin B as treatment of nonneutropenic patients with candidemia. NIAID Mycoses Study Group and the Candidemia Study Group. Antimicrob. Agents Chemother. 39:40–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Johnson EM, Oakley KL, Radford SA, Moore CB, Warn P, Warnock DW, Denning DW. 2000. Lack of correlation of in vitro amphotericin B susceptibility testing with outcome in a murine model of Aspergillus infection. J. Antimicrob. Chemother. 45:85–93. 10.1093/jac/45.1.85 [DOI] [PubMed] [Google Scholar]
- 39. Ellis D. 2002. Amphotericin B: spectrum and resistance. J. Antimicrob. Chemother. 49:7–10. 10.1093/jac/49.suppl_1.7 [DOI] [PubMed] [Google Scholar]
- 40. Perea S, Gonzalez G, Fothergill AW, Sutton DA, Rinaldi MG. 2002. In vitro activities of terbinafine in combination with fluconazole, itraconazole, voriconazole, and posaconazole against clinical isolates of Candida glabrata with decreased susceptibility to azoles. J. Clin. Microbiol. 40:1831–1833. 10.1128/JCM.40.5.1831-1833.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ryder NS. 1999. Activity of terbinafine against serious fungal pathogens. Mycoses 42:115. [PubMed] [Google Scholar]
- 42. Supparatpinyo K, Nelson KE, Merz WG, Breslin BJ, Cooper CR, Jr, Kamwan C, Sirisanthana T. 1993. Response to antifungal therapy by human immunodeficiency virus-infected patients with disseminated Penicillium marneffei infections and in vitro susceptibilities of isolates from clinical specimens. Antimicrob. Agents Chemother. 37:2407–2411. 10.1128/AAC.37.11.2407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rangel H, Dagger F, Hernandez A, Liendo A, Urbina JA. 1996. Naturally azole-resistant Leishmania braziliensis promastigotes are rendered susceptible in the presence of terbinafine: comparative study with azole-susceptible Leishmania mexicana promastigotes. Antimicrob. Agents Chemother. 40:2785–2791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Baxter JG, Brass C, Schentag JJ, Slaughter RL. 1986. Pharmacokinetics of ketoconazole administered intravenously to dogs and orally as tablet and solution to humans and dogs. J. Pharm. Sci. 75:443–447. 10.1002/jps.2600750504 [DOI] [PubMed] [Google Scholar]
- 45. Sakai MR, May ER, Imerman PM, Felz C, Day TA, Carlson SA, Noxon JO. 2011. Terbinafine pharmacokinetics after single oral dose administration in the dog. Vet. Dermatol. 22:528–534. 10.1111/j.1365-3164.2011.00985.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.