Abstract
Dientamoeba fragilis is a common enteropathogen of humans. Recently a cyst stage of the parasite was described in an animal model; however, no cyst stage has been described in detail from clinical samples. We describe both cyst and precystic forms from human clinical samples.
TEXT
Dientamoeba fragilis is an amoeboid trichomonad parasite described in all continents with human habitation (1). The prevalence of D. fragilis varies widely; however, with the advent of molecular diagnostics, rates of infection are reportedly higher than those of Giardia intestinalis (2). These statistics are consistent with other studies using nonmolecular methods (3–6).
The pathogenic potential of Dientamoeba has been suggested by numerous researchers (7–9). Confirmation of virulence and the potential mechanisms of pathogenicity are yet to be determined (10). However, infection of mice with D. fragilis resulted in statistically significant weight loss, gastrointestinal disturbance, and unformed stools compared to the results for controls (11). Levels of calprotectin, a marker of inflammatory disease in the lower gastrointestinal tract (12), in infected animals were found to be twice those in control mice, which is indicative of intestinal inflammation (11).
The development of this animal model also led to the discovery and first description of the cyst form of D. fragilis (11). The discovery of a protozoan cyst in D. fragilis-infected mice was made initially by light microscopy and was confirmed by transmission electron microscopy. The mice were confirmed to be protozoan free by screening for several days by PCR (for D. fragilis DNA) prior to infection and by examination of iron hematoxylin-stained fecal smears for protozoa. The cysts possessed a distinct thick cyst wall, with a membranous, irregular inner cyst wall located directly adjacent to the encysted parasite surrounded by a distinctive peritrophic space. Furthermore, cysts of D. fragilis possessed a characteristic D. fragilis nucleus (11) (Fig. 1). While humans are thought to be the preferred host of D. fragilis (13), a number of animal hosts have been reported, including nonhuman primates, rodents, pigs, and a sheep (11, 14–16). While there have been sporadic reports of both precystic stages, pseudocysts, and true cysts of D. fragilis in human samples, there is a lack of conclusive evidence for these stages (17). In a recent report, Clark et al. (18) raise the point that the existence of a D. fragilis cyst stage would be greatly substantiated if these forms could be identified in human stool specimens also containing D. fragilis trophozoites. This study was carried out to determine if cysts (or precystic stages) identical to those reported in mice could be identified in human clinical specimens.
FIG 1.
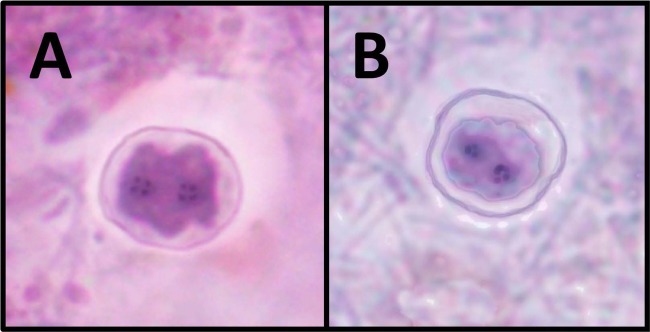
Dientamoeba fragilis cysts from a mouse animal model (A) and a human clinical sample (B).
A retrospective study was performed on all permanently stained smears positive for D. fragilis performed at the Microbiology Department at St. Vincent's Hospital Sydney. All positive slides collected over a 2-year period from 5 July 2012 to 12 December 2014 were included in the study. The slides were stained as previously described (19). A total of 500 slides were examined by oil immersion (1,000×) light microscopy, with approximately 500 fields of view examined for each slide. Special care was taken to identify forms bearing a strong resemblance (or considered identical) to the cysts described by Munasinghe et al. (11). Care was also taken to make note of forms bearing a morphology between that of D. fragilis trophozoites and cysts as these were likely to represent the precystic or pseudocyst forms previously described (and illustrated) by pioneering investigators (20–22). At the same time in the United States, a total of 47 Wheatley's trichrome permanently stained smears positive for D. fragilis trophozoites were reexamined for forms consistent with the prior description of the cyst stage (11). These smears represented 47 different patients.
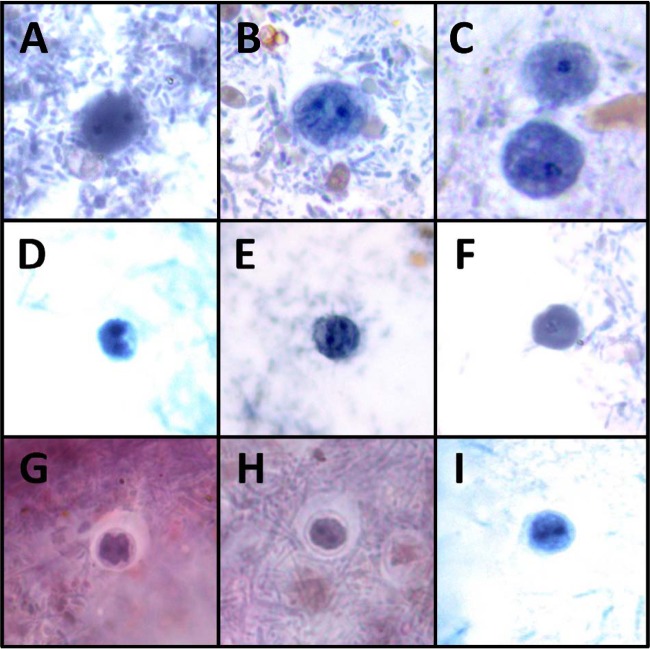
A total of 5 true cysts (as per reference 11) were detected from 4 patient samples (Fig. 2), giving a prevalence of 0.01% cyst per patient sample. The cysts were detected independently in 2 different laboratories from different locations (Australia and the United States).
FIG 2.
Dientamoeba trophozoites (A to C), precysts (D to F), and cysts (G to I) stained with a modified iron hematoxylin stain at ×1,000 magnification.
Putative precystic or pseudocyst forms (as per the studies by Wenrich [21], Kofoid [20], and Kudo [22]) were found in a total of 163 of the 500 slides, giving a prevalence of 32.6% per patient sample.
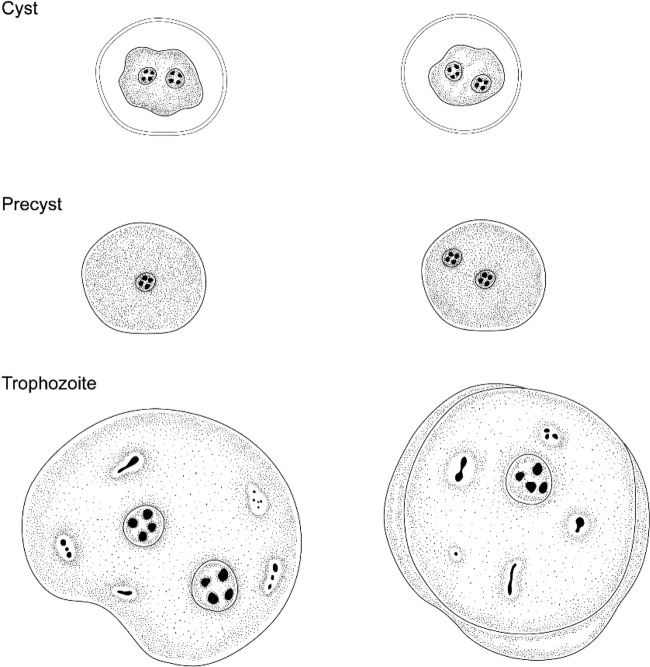
In this article, we describe D. fragilis cysts and what appear to be precystic forms of D. fragilis from human clinical samples (Fig. 2 and 3). The role these forms play in the transmission of human dientamoebiasis is yet to be confirmed. However, the cyst forms described here are morphologically indistinguishable from the D. fragilis cysts recently described in detail by Munasinghe et al. (11) in rodents (Fig. 1). The cysts are comprised of a distinct cyst wall (∼5 μm in diameter) with a zone of clearance around the cyst. A peritrophic space is present between the cyst wall and the amoebic parasite enclosed within. The nuclear structure is characteristically identical to what is found in D. fragilis trophozoites. All cysts seen were binucleate, with each nucleus containing a large central karyosome with a delicate nuclear membrane. No chromatin is visible on the nuclear membrane, and the nucleus is often fragmented into distinct granules of chromatin, often referred to as “chromatin packets.” According to these findings, these “true” cysts are rarely encountered in clinical samples, which probably accounts for the limited number of reports describing these structures. In contrast, the precystic forms of D. fragilis were more frequently encountered, with a prevalence of up to 5% in clinical samples. This precystic stage is characterized by a compact spherical shape with a reduction in size of up to 50% from “normal” trophozoites. These forms range in size from 4 to 5 μm. The cytoplasm is darkly staining, indicating a denser structure than what is found in normal trophozoites. The cytoplasm is homogeneous and rarely contains any inclusions (Fig. 3).
FIG 3.
Forms of Dientamoeba fragilis: cysts, precysts, and trophozoites.
Despite recent reports that suggest that a cyst has never been reported in humans previously (18), this is not the case. The present study is one of several to report a D. fragilis cyst in clinical specimens. Charles Atwood Kofoid was the first protozoologist to describe an encysted stage of D. fragilis in 1923. He also described a precystic form at the same time (20). Kudo some 3 years later while unable to find any cyst-like structures did, however, discover forms approximately 4 μm in diameter that he described as “small spherical amoeba without food particles,” which he concluded were precystic forms of the parasite (22). Cyst forms of D. fragilis were also described from Argentina in 1928 (23). Another study conducted in 1936 on the morphological observations of the parasite from 70 infected patients reported the absence of cysts (21). While no true cyst forms were described in this study, the author did describe what he thought was a precystic or pseudocyst stage of Dientamoeba. These precystic forms were small (3.5 to 5 μm) and both mononuclear and binucleate, in which the cytoplasm becomes finely and uniformly granular and exhibits intense staining. Wenrich ruled out these being a degenerate form of the parasite as the nuclear structure was “fairly normal,” a condition that is not usually seen in true degenerated trophozoites. It is also noted in a later study that these forms “appear no more common in 24-hour old feces than in fresher material,” once again not supporting the theory of these being a degenerated form (24). Piekarski reported a number of D. fragilis cysts from several patients; however, this work has been underrepresented in the peer-reviewed literature to date and should be appropriately considered (25).
In contrast to these findings, some researchers have dismissed the notion of D. fragilis having either a true cyst form or a pseudocyst. Wenrich dismissed the report of cysts by Kofoid by attributing the forms as “animals with thin clear ectosarcs” (24). The most vocal opponent of cyst formation in D. fragilis was Clifford Dobell, the parasitologist who first described Dientamoeba in the scientific literature. He dismissed these reports of both cyst and pseudocyst formation as a misinterpretation of degenerate individuals (16). Dobell also stated that it was inconceivable that D. fragilis would have a cyst stage while “Histomonas—its closest relative—also has no cysts.” However, recent reports describe precystic and cyst forms of Histomonas meleagridis (26, 27). These stages are completely spherical compact structures with a size range of 4 to 7 μm in diameter, have been demonstrated to survive adverse conditions, including extreme acid conditions (pH 2) (27), and are morphologically similar to the precystic form of D. fragilis described herein. A recent study by our group utilizing cultured D. fragilis has demonstrated that Dientamoeba is not as fragile as first proposed as it can survive extreme acid conditions of pH 1 to 2 for approximately 4 h (unpublished data). It is probable that the D. fragilis precystic/cyst stages facilitate its survival under these extreme conditions. It should also be noted that Dobell described small forms that are identical to the forms that other researchers and our group believe are a precystic form of the parasite (16). In his report (16), Dobell describes “dwarfs, with diameters of only 3–4 μm are also to be found in cultures. They appear to be formed by rapid division, without intermediate growth, of normal individuals. Even the smallest often contain 2 nuclei. Most of these very small organisms also degenerate and die. They appear to be unable to feed, as their cytoplasm is usually free from all food-inclusions when their nuclei are breaking up—unlike giants, which remain gorged with ingesta long after their nuclei have disintegrated.”
Further definitive studies are required to fully elucidate the role of D. fragilis cysts in transmission. In situ hybridization studies should be performed to allow visual correlation with the cyst structures identified by light microscopy. Studies utilizing both rodent and pig animal models should also be undertaken, and given the ease of establishing short-term Dientamoeba cultures, cyst formation experiments could also be undertaken on trophozoite cultures to establish what environmental conditions are needed to induce encystations. Next-generation sequencing of both the transciptome and whole genome could also provide detailed information on cyst wall formation pathways and should also be undertaken.
In a recent review of the literature, it was suggested that while cysts of D. fragilis have been reported recently in mice (28), their absence in humans represented a major downfall of this work, casting doubt on the identity of these structures (18). This study describes in detail both precystic forms and cysts of D. fragilis found in human clinical samples which are morphologically indistinguishable from the cysts reported by Minasinghe et al. (28). Moreover, we report the finding of these structures in two different diagnostic laboratories in geographically distant locations. What roles these distinct structures play in transmission is yet to be determined, and further study is needed on the life cycle of this peculiar parasite.
Footnotes
Published ahead of print 7 May 2014
REFERENCES
- 1.Stark DJ, Beebe N, Marriott D, Ellis JT, Harkness J. 2006. Dientamoebiasis: clinical importance and recent advances. Trends Parasitol. 22:92–96. 10.1016/j.pt.2005.12.001 [DOI] [PubMed] [Google Scholar]
- 2.Roser D, Simonsen J, Nielsen HV, Stensvold CR, Molbak K. 2013. Dientamoeba fragilis in Denmark: epidemiological experience derived from four years of routine real-time PCR. Eur. J. Clin. Microbiol. Infect. Dis. 32:1303–1310. 10.1007/s10096-013-1880-2 [DOI] [PubMed] [Google Scholar]
- 3.Lagace-Wiens PR, VanCaeseele PG, Koschik C. 2006. Dientamoeba fragilis: an emerging role in intestinal disease. CMAJ 175:468–469. 10.1503/cmaj.060265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crotti D, D'Annibale ML, Fonzo G, Lalle M, Caccio SM, Pozio E. 2005. Dientamoeba fragilis is more prevalent than Giardia duodenalis in children and adults attending a day care centre in Central Italy. Parasite 12:165–170. 10.1051/parasite/2005122165 [DOI] [PubMed] [Google Scholar]
- 5.Grendon JH, DiGiacomo RF, Frost FJ. 1995. Descriptive features of Dientamoeba fragilis infections. J. Trop. Med. Hyg. 98:309–315 [PubMed] [Google Scholar]
- 6.Gijsbers CF, Schweizer JJ, Buller HA. 2013. Protozoa as a cause of recurrent abdominal pain in children. J. Pediatr. Gastroenterol. Nutr. 57:603–606. 10.1097/MPG.0b013e31829f1bc0 [DOI] [PubMed] [Google Scholar]
- 7.Gray TJ, Kwan YL, Phan T, Robertson G, Cheong EY, Gottlieb T. 2013. Dientamoeba fragilis: a family cluster of disease associated with marked peripheral eosinophilia. Clin. Infect. Dis. 57:845–848. 10.1093/cid/cit389 [DOI] [PubMed] [Google Scholar]
- 8.Sarafraz S, Farajnia S, Jamali J, Khodabakhsh F, Khanipour F. 2013. Detection of Dientamoeba fragilis among diarrheal patients referred to Tabriz health care centers by nested PCR. Trop. Biomed. 30:113–118 http://www.msptm.org/files/113_-_118_Farajnia_S.pdf [PubMed] [Google Scholar]
- 9.Stark D, Barratt J, Roberts T, Marriott D, Harkness J, Ellis J. 2010. A review of the clinical presentation of dientamoebiasis. Am. J. Trop. Med. Hyg. 82:614–619. 10.4269/ajtmh.2010.09-0478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barratt JL, Harkness J, Marriott D, Ellis JT, Stark D. 2011. A review of Dientamoeba fragilis carriage in humans: several reasons why this organism should be considered in the diagnosis of gastrointestinal illness. Gut Microbes 2:3–12. 10.4161/gmic.2.1.14755 [DOI] [PubMed] [Google Scholar]
- 11.Munasinghe VS, Vella NG, Ellis JT, Windsor PA, Stark D. 2013. Cyst formation and faecal-oral transmission of Dientamoeba fragilis—the missing link in the life cycle of an emerging pathogen. Int. J. Parasitol. 43:879–883. 10.1016/j.ijpara.2013.06.003 [DOI] [PubMed] [Google Scholar]
- 12.Fagerberg UL, Loof L, Merzoug RD, Hansson LO, Finkel Y. 2003. Fecal calprotectin levels in healthy children studied with an improved assay. J. Pediatr. Gastroenterol. Nutr. 37:468–472. 10.1097/00005176-200310000-00013 [DOI] [PubMed] [Google Scholar]
- 13.Johnson EH, Windsor JJ, Clark CG. 2004. Emerging from obscurity: biological, clinical, and diagnostic aspects of Dientamoeba fragilis. Clin. Microbiol. Rev. 17:553–570. 10.1128/CMR.17.3.553-570.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caccio SM, Sannella AR, Manuali E, Tosini F, Sensi M, Crotti D, Pozio E. 2012. Pigs as natural hosts of Dientamoeba fragilis genotypes found in humans. Emerg. Infect. Dis. 18:838–841. 10.3201/eid1805.111093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stark D, Phillips O, Peckett D, Munro U, Marriott D, Harkness J, Ellis J. 2008. Gorillas are a host for Dientamoeba fragilis: an update on the life cycle and host distribution. Vet. Parasitol. 151:21–26. 10.1016/j.vetpar.2007.10.002 [DOI] [PubMed] [Google Scholar]
- 16.Dobell C. 1940. Researches on the intestinal protozoa of monkeys and man. X. The life-history of Dientamoeba fragilis: observations, experiments, and speculations. Parasitol. Res. 32:417–461 [Google Scholar]
- 17.Barratt JL, Harkness J, Marriott D, Ellis JT, Stark D. 2011. The ambiguous life of Dientamoeba fragilis: the need to investigate current hypotheses on transmission. Parasitology 138:557–572. 10.1017/S0031182010001733 [DOI] [PubMed] [Google Scholar]
- 18.Clark CG, Roser D, Stensvold CR. 2014. Transmission of Dientamoeba fragilis: pinworm or cysts? Trends Parasitol. 30:136–140. 10.1016/j.pt.2014.01.005 [DOI] [PubMed] [Google Scholar]
- 19.Stark D, Roberts T, Marriott D, Harkness J, Ellis JT. 2012. Detection and transmission of Dientamoeba fragilis from environmental and household samples. Am. J. Trop. Med. Hyg. 86:233–236. 10.4269/ajtmh.2012.11-0526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kofoid CA. 1923. Amoeba and man. University of California Press, Berkeley, CA [Google Scholar]
- 21.Wenrich DH. 1936. Studies on Dientamoeba fragilis (protozoa). I. Observations with special reference to nuclear structure. J. Parasitol. 22:76–83 [Google Scholar]
- 22.Kudo R. 1926. Observation on Dientamoeba fragilis. Am. J. Trop. Med. Hyg. 6:299–305 [Google Scholar]
- 23.Greenway D. 1928. Dientamoeba fragilis en la Argentina. Arch. Argent. Enferm. Apar. Dig. 3:897 [Google Scholar]
- 24.Wenrich DH. 1944. Studies on Dientamoeba fragilis (protozoa). IV. Further observations, with an outline of present-day knowledge of this species. J. Parasitol. 30:322–338 [Google Scholar]
- 25.Piekarski G. 1948. Zur Frage der Cystenbildung bei Dientamoeba fragilis. Z. Hyg. Infektionskr. 127:496–500. 10.1007/BF02284873 [DOI] [PubMed] [Google Scholar]
- 26.Munsch M, Lotfi A, Hafez HM, Al-Quraishy S, Mehlhorn H. 2009. Light and transmission electron microscopic studies on trophozoites and cyst-like stages of Histomonas meleagridis from cultures. Parasitol. Res. 104:683–689. 10.1007/s00436-008-1246-3 [DOI] [PubMed] [Google Scholar]
- 27.Zaragatzki E, Hess M, Grabensteiner E, Abdel-Ghaffar F, Al-Rasheid KA, Mehlhorn H. 2010. Light and transmission electron microscopic studies on the encystation of Histomonas meleagridis. Parasitol. Res. 106:977–983. 10.1007/s00436-010-1777-2 [DOI] [PubMed] [Google Scholar]
- 28.Munasinghe VS, Stark D, Ellis JT. 2012. New advances in the in-vitro culture of Dientamoeba fragilis. Parasitology 139:864–869. 10.1017/S0031182012000145 [DOI] [PubMed] [Google Scholar]