Abstract
Clinical laboratories are constantly facing challenges to do more with less, enhance quality, improve test turnaround time, and reduce operational expenses. Experience with adopting and applying lean concepts and tools used extensively in the manufacturing industry is described for a high-volume clinical molecular microbiology laboratory, illustrating how operational success and benefits can be achieved.
TEXT
Molecular diagnostics has been a part of the clinical microbiology laboratory for more than 25 years. Such testing has become the standard for diagnosis and monitoring of patients receiving treatment for various infectious diseases. In the early days of molecular diagnostics, multiple instruments and workspaces or rooms were necessary for nucleic acid extraction, amplification, detection, and sequencing procedures. Testing might take up to 3 days for completion and require multiple manual checks for result entry into the laboratory information system (LIS). Laboratory staff were involved in all activities associated with preanalytic, analytic, and postanalytic steps. The time associated with each of these critical steps varied according to the experience and expertise of the staff. In addition, the use and manipulation of reagents that contained hazardous material had to be considered. Clinical laboratories nowadays face increasing test volumes, a demand for shorter test turnaround time, and a decreasing supply of skilled laboratory staff. Together with changes in reimbursement and health care practices, these demands place increased economic pressure on the operation of clinical laboratories.
As a U.S. reference testing laboratory supporting a large network of hospital laboratories, the Mayo Medical Laboratories in Rochester, MN, has been performing molecular diagnostic tests since 1993. Specifically, the Hepatitis/HIV Molecular Laboratory within the Mayo Medical Laboratories offers molecular HIV and viral hepatitis tests utilizing PCR, branched-chain DNA (bDNA), and sequencing methods. Concurrent with the evolution of molecular test methods and instrument platforms, the laboratory has had to manage and balance clinical care demands and the constraints imposed by quality assurance, laboratory accreditations and regulations, and limited qualified laboratory personnel. Aware of the success and benefits of lean processes adopted widely in the manufacturing industry (1–3), our laboratory staff learned to adopt lean processes for daily operation of the laboratory to adjust to these new and ongoing challenges. Herein, we describe our 2-year experience in implementing lean processes as well as the benefits and limitations of this approach to improve efficiency of a high-volume clinical molecular microbiology laboratory.
Prior to the adoption of lean principles, clinical serum, plasma, and whole-blood specimens sent to our laboratory for various qualitative and quantitative molecular diagnostic assays arrived at one central location. They were placed randomly in tube racks and stored at specified holding temperatures for 8 to 24 h until they were retrieved randomly (not in any specific order) for testing. There were no specific rules or order for storing, retrieving, and processing these specimens according to the order of receipt or arrival in the laboratory. All test results obtained were manually entered into the laboratory information system by the bench technologists and reviewed and verified for accuracy by a second technologist before release of the results.
To begin the lean approach, several compulsory educational sessions on the concept of lean principles were provided to our laboratory staff over a 2-month period. These sessions emphasized the basic principles of the lean approach: (i) focus on creating value from the perspective of the customer (e.g., patient, care provider, and reference laboratory client); (ii) understand the business by fully mapping the system, including laboratory processes and how they are linked; (iii) create continuous flow that allows the needs of customers to be met and eliminate waste; and (iv) recognize optimally that the lean approach is a way of being, and as such, it is a journey and not a destination (1–3). During these sessions, laboratory staff were challenged on their current workflow and laboratory practices and encouraged to identify nonproductive or wasteful operational activities (e.g., unnecessary movement of specimens and laboratory personnel, assay run failures, time delay to report test results), imbalances in workload, and variations in various laboratory practices. Web-based tools (e.g., sample throughput by batch testing versus continuous flow) and hands-on games (e.g., manipulation of and building of Lego structures) were used to illustrate multiple features of the lean approach, demonstrate how minor operational changes can improve an entire process, and provide examples of immediate success from adoption of lean processes (4–8).
Next, laboratory staff volunteers formed a kaizen team to conduct value stream map exercises, identifying opportunities or kaizen initiatives (i.e., quick changes of current processes) that could potentially decrease or eliminate wasted efforts and improve workflow. These initiatives included continuous testing of clinical specimens in smaller batch sizes throughout the work shift with the “first-in, first-out” (FIFO) rule, relocation of instruments within the laboratory space to minimize travel for personnel, consolidation of instrument platforms to maximize the number of assays that can be performed with a given instrument system, the use of instrument-ready reagents, avoidance of hazardous reagents, minimization of generation of waste material, implementation of electronic interfaces between the laboratory information system and the instrument systems for direct test ordering and result entry and minimizing human errors, an increase in the frequency of preventative maintenance measures performed by manufacturers on high-frequency-use instruments to maintain “up time,” and elimination of third (overnight) and Sunday work shifts, as workload was being completed efficiently within the first two work shifts of the day. Desirable outcome goals included a reduction in test turnaround time, a reduction in operational expenses, the capacity to accommodate increased test volume without increasing personnel and/or space, level loading (even distribution) of activities among bench technologists and across work shifts, and improved work-life balance for laboratory personnel.
To gain first-hand experience with lean practices, our laboratory staff first elected a small process change that was easy and quick to implement—reducing the test batch size from 24 to 12 clinical specimens per assay run for 4 molecular diagnostic microbiology tests: Cobas Amplicor HCV Test, version 2.0 (CAM HCV v2; Roche Molecular Systems, Inc.), used as 2 separate assays (stand-alone qualitative detection and amplification of hepatitis C virus [HCV] RNA for genotype test); Cobas Amplicor HIV-1 Monitor, version 1.5 (CAM HIV v1.5; Roche Molecular Systems, Inc.), using the ultrasensitive specimen preparation procedure; and the HCV RNA detection and quantification assay using TaqMan HCV analyte-specific reagents (TaqMan HCV ASR; Roche Molecular Systems, Inc.). The effect of this intervention on first-time success (defined as a valid assay run on the first attempt of testing with a given assay) rates of these assays was determined. The assumption was that small batch size would increase the frequency of assay runs throughout the work shifts, allowing bench technologists to remain focused on the tasks at hand and reducing or eliminating opportunities for human errors. Table 1 shows the improvement in first-time assay run success rates obtained with this initial process change. Improvement in overall average success rates ranged from 0.3% to 9.9% for the 4 tests during the 3 months after lean practices were implemented. The improvement in success rates was statistically significant for 3 of the 4 assays and was sustained during a monitoring period of 7 months after the adoption of the lean approach, with lowest success rates of 91.2%, 94.9%, 85.1%, and 92.2% for assays A, B, C, and D, respectively. These lowest rates achieved during the period after the switch to the lean approach were higher than the lowest rates observed for the corresponding assays before the lean approach was adopted.
TABLE 1.
First-time success rates of 4 molecular diagnostic tests before and after initial implementation of lean practices
| Molecular test | Assay run success rate (%) (no. of successful runs/total) |
Pa | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Before lean practices |
After lean practices |
||||||||||
| Month 1 | Month 2 | Month 3 | Month 1 | Month 2 | Month 3 | Month 4 | Month 5 | Month 6 | Month 7 | ||
| A | 95.1 (58/61) | 90.9 (70/77) | 90.1 (119/121) | 97.5 (115/118) | 97.5 (117/120) | 93.0 (132/142) | 95.8 (136/142) | 91.2 (124/136) | 96.3 (104/108) | 97.2 (103/106) | <0.05 |
| B | 91.7 (55/60) | 91.3 (73/80) | 92.1 (105/114) | 99.0 (99/100) | 99.1 (115/116) | 96.0 (121/126) | 97.0 (128/132) | 94.9 (112/118) | 97.2 (106/109) | 96.3 (103/107) | <0.05 |
| C | 78.5 (146/186) | 80.2 (142/177) | 83.8 (145/173) | 93.7 (148/158) | 93.6 (161/172) | 87.1 (135/155) | 91.0 (159/175) | 89.5 (162/181) | 85.1 (131/154) | 89.2 (149/167) | <0.05 |
| D | 92.2 (47/51) | 93.9 (62/66) | 91.9 (91/99) | 96.0 (97/101) | 92.9 (91/98) | 93.1 (81/87) | 92.2 (94/102) | 94.9 (94/99) | 94.9 (93/98) | 92.7 (101/109) | 0.58 |
Determined by the chi-square test for comparing data before and after implementation of lean practices.
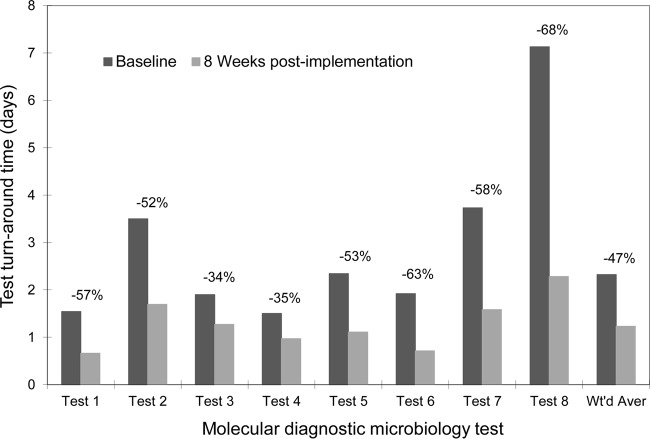
With the above initial success resulting from the reduction in assay run batch size, our laboratory staff added 2 changes simultaneously to the workflow process by establishing a FIFO order of testing clinical specimens (i.e., in the order that they were received in our laboratory) and installing electronic interfaces between the test instruments and the LIS for result reporting for 8 tests: CAM HCV v2, used as 2 separate assays (stand-alone qualitative detection and amplification of HCV RNA for genotype test); CAM HIV-1 v1.5, used as 2 assays (standard and ultrasensitive specimen preparation procedures); TaqMan HCV ASR; a Versant HCV RNA 3.0 (bDNA) assay (Siemens Healthcare Diagnostics Inc.); Trugene HCV 5′NC Genotyping Kit (Siemens Healthcare Diagnostics Inc.); and Trugene HIV-1 Genotyping Kit (Siemens Healthcare Diagnostics Inc.). The overall impact of the combined interventions implemented was analyzed by determining the percentage of test results reported within the desirable test turnaround time (TAT; defined as ≤24 h from receiving the clinical specimens in the laboratory). The laboratory's goal was to achieve desirable TAT for ≥85% of the specimens tested. Figure 1 shows the successful reduction in TAT for these 8 tests as a result of these changes to our laboratory workflow. Since the FIFO order and instrument interfaces were implemented simultaneously, we were unable to determine the proportion of the reduction of TAT that attributable separately to each of these two interventions.
FIG 1.
Decreases in average test turnaround time (TAT) for 8 molecular diagnostic microbiology tests during the pre-kaizen and post-kaizen periods. Each bar represents average TAT over 1 week for the corresponding test. Values above the bars represent decreases in TAT between the two periods. Wt'd Aver, weighted average.
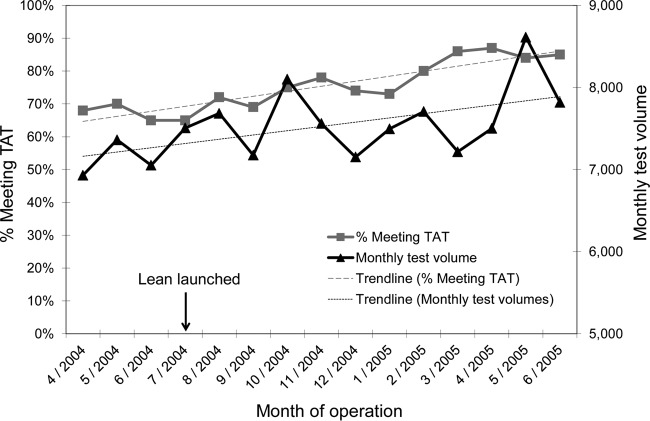
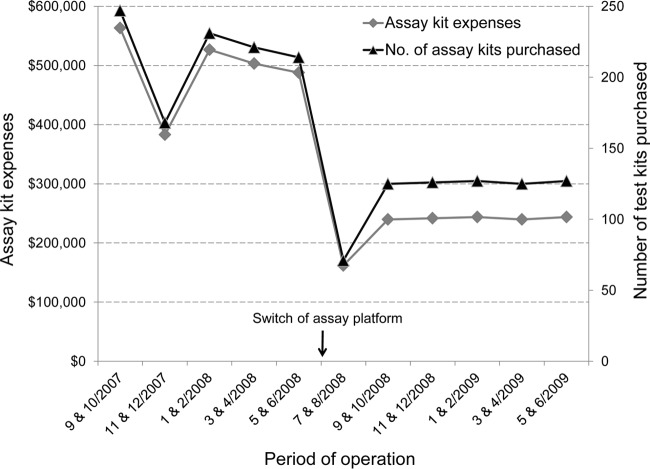
Over a 2-year period, our laboratory implemented various changes and enhancements to the workflow and operational processes, as the use of lean practices became an established culture to guide continuous improvement initiatives in the laboratory. To gain efficiency of testing clinical specimens and achieve desirable TAT, work shift schedules of laboratory staff were adjusted and optimized to match arrival times of clinical specimens in the laboratory (e.g., a work shift start time was changed from 6:00 a.m. to 7:00 a.m.). At the same time, in our favor was the introduction of semiautomated diagnostic molecular assays to replace some of the manual assays, allowing eventual reduction of work shift hours and personnel (e.g., from a 10-hour to an 8-hour work shift). Incorporating lean concepts into our laboratory operation enabled us to attain and maintain the desirable TAT for all of the tests performed despite increasing test volumes (Fig. 2), and reduce assay run failure rates and reagent purchase expenses (Fig. 3). Prior to the change in assay platform, less frequent first-time assay run failures due to smaller assay run batch sizes also contributed to assay kit expense reduction. Other savings were observed from reduction in personnel resources (salaries and benefits), instrument maintenance effort, and vendor service contracts due to a switch from multiple semiautomated assay instruments to fewer automated assay instruments. Specific financial data were not easily separated and attributed to individual factors in our current analyses.
FIG 2.
Trends of monthly test volume and percentage of test results meeting the desired turnaround time before and after launching of lean initiatives. Standard linear regression analysis showed a coefficient of correlation of 0.802 for the trendline of the percentage meeting TAT.
FIG 3.
Significant reduction in assay reagent kit usage and purchase expenses (P = 0.0059 by Wilcoxon rank sum test for both parameters) after the switch from a semiautomated to a fully automated assay platform for HIV-1 viral load testing. Several lean processes had already been in place for 1 year prior to this change in assay platform.
Clinical laboratories in the United States face many challenges these days, such as limited availability and turnover of skilled laboratory personnel, an increasing number of molecular diagnostic laboratory tests and associated instrument platforms, demand for rapid test results for patient care, and operating budgetary constraints. Laboratory leadership has the complex tasks of cost-effectively managing the staff work schedules, training, test menus, and instrument systems while meeting the needs of patient care providers. The concept and tools associated with the lean approach have allowed our laboratory to examine and organize workflow processes to best meet these challenges to achieve an efficient and successful operation. Demonstrating immediate successes from simple and easy-to-implement process changes was important to gain the confidence and enthusiasm of our laboratory staff with lean activities, thereby increasing their work satisfaction and decreasing the desire or risk to revert to the previous processes. We were able to cross-train laboratory staff, optimize work shift schedules to match staff availability to workload, establish staff recognition programs to encourage staff to identify errors to improve workflow processes, decrease instrument down-times, request “value-add” features from commercial assay and instrument manufacturers (e.g., technical support, increased frequency of preventative maintenance, test kit ordering behavior, back-up instruments, instrument-to-LIS interfaces, workflow analyses, improvements to reagent kit configuration, and instrument hardware and software applications), and prepare the staff for future opportunities in quality improvement and workflow enhancement (“future state mapping”). Desirable results were reached and maintained, while increased test volumes were accommodated and the laboratory operated within budgetary constraints. One of our lean initiatives even led to the discovery of an instrument operational option of which the instrument manufacturer was unaware.
Initial attempts to implement lean principles in the clinical laboratory may encounter setbacks or failures of varying degree. Such setbacks that delayed our progress included miscalculations of the duration of various laboratory workflow processes, erroneous assumptions of actual operation and features of several instrument systems, and unavailability of sophisticated information technology tools to provide real-time data and metrics to monitor workflow processes. These impediments point to the need for instrument platforms and software applications that enable clinical laboratories to easily apply lean initiatives in their clinical specimen testing operations with workflow processes that are flexible, efficient, automated, and rapid, in order to meet future demands. Challenges to commercial manufacturers of future clinical diagnostic assays and instrument systems are to incorporate lean concepts in the design and use of their future products for clinical laboratories. Applying lean principles in the laboratory workflow and maintaining a culture of lean practices among the staff personnel will become increasingly important for clinical laboratories to achieve and maintain cost-effective and successful operation.
Footnotes
Published ahead of print 14 May 2014
REFERENCES
- 1.Liker J. 2004. The Toyota way: 14 management principles from the world's greatest manufacturer. McGraw-Hill, New York, NY [Google Scholar]
- 2.Liker J, Meier D. 2006. The Toyota way fieldbook. McGraw-Hill, New York, NY [Google Scholar]
- 3.Womack JP, Jones DT. 2003. Lean thinking: banish waste and create wealth in your corporation, revised and updated. Free Press, New York, NY [Google Scholar]
- 4.Murman E, Weigel A, Haggerty A, McManus H. 2008. Introduction to lean six sigma methods. http://www.youtube.com/watch?v=Swo3Lvw7ivg YouTube video, 34:12, posted by MIT Open CourseWare Accessed 4 February 2014
- 5.Ruiz C. 2011. One piece flow versus batch production—lean manufacturing. http://www.youtube.com/watch?v=JoLHKSE8sfU YouTube video, 0:46, posted by carloruiz Accessed February 4, 2014.
- 6.Pereira R. 2012. Lean manufacturing (TPS): One piece flow simulation. http://www.youtube.com/watch?v=Bi9R1Hqr8dI YouTube video, 9:46, posted by Lean Six Sigma Academy Accessed February 4, 2014
- 7.Jacobson JM, Johnson ME. 2006. Lean and six sigma: not for amateurs (part 1). Lab. Med. 37:78–83. 10.1309/N4THYEQCHCGWQ3ND [DOI] [Google Scholar]
- 8.Jacobson JM, Johnson ME. 2006. Lean and six sigma: not for amateurs (part 2). Lab. Med. 37:140–145. 10.1309/9LHB-9G96-AHMT-9XG2 [DOI] [Google Scholar]