Abstract
Streptococcus gallolyticus subsp. gallolyticus (formerly known as S. bovis biotype I) is a commensal of the gastrointestinal tract in animals and in up to 15% of healthy humans. Furthermore, it is a facultative pathogen that can cause infectious endocarditis, mastitis, and septicemia. The number of infections is increasing, but the transmission routes and zoonotic potential remain unknown. To assess the zoonotic potential and characterize the epidemiological structure of S. gallolyticus subsp. gallolyticus, we established a multilocus sequence typing (MLST) scheme. We amplified and sequenced internal fragments of seven housekeeping genes. The resulting sequences were analyzed with BioNumerics software 6.6 by using the unweighted-pair group method using average linkages algorithm. A total of 101 S. gallolyticus subsp. gallolyticus strains isolated from animals, humans, and environmental samples were analyzed and divided into 50 sequence types. Our first results highlight the importance of this MLST scheme for investigating the epidemiology, transmission patterns, and infection chains of S. gallolyticus subsp. gallolyticus.
INTRODUCTION
Streptococcus gallolyticus subsp. gallolyticus is a Gram-positive bacterium belonging to the Lancefield group D streptococci. Traditionally, it was classified as a member of the Streptococcus bovis biotype I group. Depending on the bacterium's ability to ferment mannitol, three biotypes of S. bovis were distinguished, I, II/1, and II/2. The taxonomy of S. bovis underwent several amendments before mannitol-fermenting S. bovis biotype I was reclassified as S. gallolyticus subsp. gallolyticus in 2003 (1).
S. gallolyticus subsp. gallolyticus, a commensal of the gastrointestinal tract, is found in 2.5 to 15% of healthy humans (2). The organism can also act as a pathogen. This opportunistic bacterium may cause septicemia and meningitis in animals, as well as in humans (3, 4). In 24% of cases of streptococcal endocarditis, S. gallolyticus subsp. gallolyticus was identified as the causative agent (5–7). Furthermore, studies have shown a correlation between streptococcal endocarditis and colon cancer (8). Nevertheless, S. gallolyticus subsp. gallolyticus has been found in various animals, especially in pigeons, chickens, and cattle, where it can cause various diseases (4, 9, 10). Particularly in dairy cows, it is often the causative agent of mastitis (11). Further studies have identified this facultative pathogen in milk and raw milk products (12–14). Indirect transmission by contact with a contaminated environment or directly by smear or droplet infection from human to human or from animal to human can be assumed. However, the transmission pathways, as well as the pathogenic mechanisms, remain unexplained. Because of its presence in, e.g., poultry, ruminants, and humans, it is suspected but has not been confirmed to have zoonotic potential (4, 8–10, 15).
Several multilocus sequence typing (MLST) schemes have been successfully developed for various bacterial species. MLST is a portable method based on the sequencing of housekeeping genes that provides accurate and comparable results for analyzing evolutionary structures and infection chains, which help explain the virulence of pathogenic bacteria (16).
Recently, an MLST scheme for the species Streptococcus gallolyticus was established for investigation of its epidemiology and determination of its subspecies. There was no indication of pathogenic groups within the clusters of S. gallolyticus subsp. gallolyticus (17). To explore the zoonotic potential and characterize the epidemiology of S. gallolyticus subsp. gallolyticus, we established a subspecies-specific MLST scheme. This typing method permits the classification of different clusters of S. gallolyticus subsp. gallolyticus isolates. Furthermore, this technique can provide evidence of the zoonotic potential of this facultative pathogen and may provide information about transmission routes.
MATERIALS AND METHODS
Bacterial strains and cultivation.
One hundred one S. gallolyticus subsp. gallolyticus strains (51 from animals, 33 from humans, 1 from an environmental sample, and 17 from unknown sources) were analyzed. Bacterial strains were obtained from the American Type Culture Collection (ATCC, LGC Standards GmbH, Wesel, Germany), the Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH (Braunschweig, Germany), or the Belgian Coordinated Collections of Microorganisms (Ghent, Belgium), or were previously isolated from blood cultures or feces from patients at the Herz- und Diabeteszentrum NRW (Bad Oeynhausen, Germany) or from fecal samples from animals (e.g., poultry) (see Table S1 in the supplemental material). Eight strains were kindly given by the National Reference Center for Streptococci, Institute of Medical Microbiology, University Hospital, Aachen, Germany, and two strains were from LADR GmbH MVZ Dr. Kramer & Colleagues, Geesthacht, Germany (see Table S1 in the supplemental material). All isolates were characterized by matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) and partial sequencing of the manganese-dependent superoxide dismutase (sodA) gene (18, 19). Bacteria were grown on brain heart infusion agar (Oxoid Ltd., Cambridge, United Kingdom) at 37°C.
DNA extraction.
The total DNA of S. gallolyticus subsp. gallolyticus strains was isolated with the QIAamp Blood minikit (Qiagen, Hilden, Germany). For extraction, approximately 10 single colonies were inoculated into 180 μl of lysis buffer (containing 20 mg/ml lysozyme). The suspension was incubated at 37°C for 30 min. DNA extraction was performed in accordance with the manufacturer's instructions. DNA was eluted in 50 μl of elution buffer. DNA integrity and concentrations were measured with a NanoDrop 2000 (Thermo Scientific, Wilmington, DE).
Nucleotide sequencing of gene fragments.
To establish an MLST scheme, the published whole-genome sequences of S. gallolyticus subsp. gallolyticus strains UCN 34 (GenBank accession no. FN597254), ATCC 43143 (accession no. AP012053.1), and ATCC BAA-2069 (accession no. FR824043) were compared by using the EDGAR software to identify genes they have in common (20–23). From the resulting gene pool, a set of 22 housekeeping genes were selected on the basis of the variability and length (>500 bp) of DNA sequences or usage in other MLST schemes. For these candidate genes, 22 primer pair systems were designed. Nine strains differing in their genetic characteristics (sodA sequence, DNA fingerprinting profile) were selected to test these primer systems. All of the primers were adjusted to the same annealing temperature. Primers that provided no or too much DNA sequence variation were excluded.
Gene fragments from the chromosomal DNA of 101 S. gallolyticus subsp. gallolyticus strains were amplified with primers for the housekeeping genes aroE (shikimate-5-dehydrogenase), glgB (glycogen branching enzyme), nifS (cysteine desulfurase), p20 (acyl coenzyme A N-acyltransferase), tkt (transketolase), trpD (anthranilate phosphoribosyltransferase), and uvrA (excinuclease ABC subunit A). For the primers used, see Table S2 in the supplemental material.
PCRs were carried out in a 50-μl reaction volume that comprised HotMaster Taq DNA polymerase (5Prime, Hamburg, Germany). For sequencing analyses, 5 μl of the PCR products was purified enzymatically with 1 μl of exonuclease I solution and 1 μl of shrimp alkaline phosphatase (USB, Cleveland, OH).
Each cycle sequencing reaction mixture was prepared with the BigDye Terminator v1.1 Cycle Sequencing kit (Applied Biosystems, Darmstadt, Germany). Excess dye terminators and primers were removed by centrifugation with a spin column prepared with Sephadex G-50 (Amersham, Braunschweig, Germany). Finally, denaturation at 95°C for 120 s was performed. The sequences of both strands were determined with a 3500 Genetic Analyzer DNA sequencer (Applied Biosystems, Darmstadt, Germany). Detailed protocols concerning the PCR, purification of PCR products, and sequencing reactions are available at www.pubmlst.org. All sequences were aligned and analyzed by BioNumerics software 6.6 (Applied Maths, Sint-Martens-Latem, Belgium), START version 2, and eBURST version 3 (www.mlst.net) (24, 25). To investigate the relatedness of the strains, a dendrogram was constructed by the unweighted-pair group method using average linkages (UPGMA; BioNumerics). eBURST (based upon related sequence types [STs]) was used to identify clonal lineages. Clonal complexes were defined as groups when six out of seven alleles were the same (most stringent definition) (24). The program START version 2 (www.mlst.net) was used to determine the numbers of nucleotide alterations causing amino acid changes (nonsynonymous, dN) and silent mutations (synonymous, dS) (dN/dS ratio), the polymorphic sites, and the index of association (IA) (25). IA was calculated to determine the linkage disequilibrium among the alleles of seven housekeeping genes. It was defined as the observed variance (vo) in the distribution of allelic mismatches in all pairwise comparisons of the allelic profiles divided by the expected variance (ve) in a freely recombining population minus 1 (26). The significance of IA was estimated by comparing the vo of the actual data with the maximum variance (vmax) calculated by using 1,000 randomizations of data sets. The linkage disequilibrium was considered significant if the vo was greater than the vmax obtained in 1,000 trials; otherwise, there was no evidence of a departure from the linkage equilibrium (26).
Simpson's index of diversity (SID) was calculated on the basis of the molecular pattern of the seven loci by using the Comparing Partitions website (http://darwin.phyloviz.net/ComparingPartitions). A SID and a 95% confidence interval (CIs) was calculated for a set of 101 strains. A value close to 1 reflects high diversity, and a value close to 0 indicates little diversity.
RESULTS
To establish an MLST scheme, the whole sequences of the three independent S. gallolyticus subsp. gallolyticus strains were compared by using the EDGAR software (23). The seven housekeeping genes (loci) of 101 S. gallolyticus subsp. gallolyticus strains were successfully amplified and sequenced. Each locus was cut with trimming sequences to compare the internal fragments. An allelic profile was assigned to 101 S. gallolyticus subsp. gallolyticus isolates (see Table S1 in the supplemental material). This study made use of the PubMLST website (http://pubmlst.org/) developed by K. Jolley (27). Data for the MLST scheme are available at www.pubmlst.org and in Table S1 in the supplemental material (27). In summary, 14 (glgB) to 22 (p20) different alleles were present at each locus, resulting in an average of 16.9 alleles in each internal fragment. The proportions of polymorphic sites ranged from 2.2% (tkt) to 7.4% (p20) and provided 1.3 × 1010 genotypes. A dN/dS ratio of <1 and an IA value of 2.4 were calculated, resulting in the detection of significant linkage disequilibrium (Table 1) (26).
TABLE 1.
Characteristics of MLST loci used for S. gallolyticus subsp. gallolyticus
| Gene | Locus tag | Gene product | No. of alleles | Simpson's index of diversity | dN/dS ratioa | No. (%) of polymorphic sites |
|---|---|---|---|---|---|---|
| aroE | SGGBAA2069_c13440 | Shikimate 5-dehydrogenase | 15 | 0.830 | 0.2971 | 24 (3.57) |
| glgB | SGGBAA2069_c07540 | Glycogen branching enzyme | 14 | 0.815 | 0.0519 | 27 (3.74) |
| nifS | SGGBAA2069_c13360 | Cysteine desulfurase | 19 | 0.854 | 0.0618 | 23 (4.32) |
| p20 | SGGBAA2069_c04560 | Acyl coenzyme A N-acyltransferase | 22 | 0.911 | 0.1566 | 34 (7.40) |
| tkt | SGGBAA2069_c21090 | Transketolase | 15 | 0.827 | 0.0534 | 15 (2.23) |
| trpD | SGGBAA2069_c05200 | Anthranilate phosphoribosyltransferase | 16 | 0.855 | 0.0758 | 44 (6.90) |
| uvrA | SGGBAA2069_c18560 | Excinuclease ABC subunit A | 17 | 0.798 | 0.0353 | 38 (6.30) |
Ratio of nonsynonymous to synonymous substitutions.
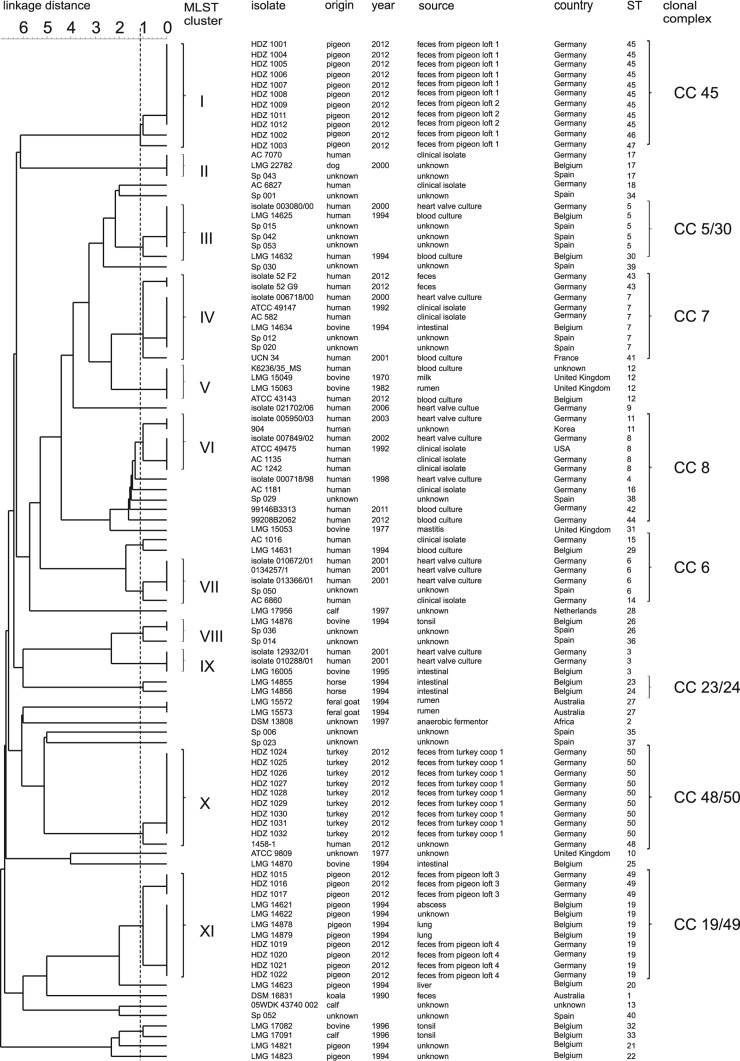
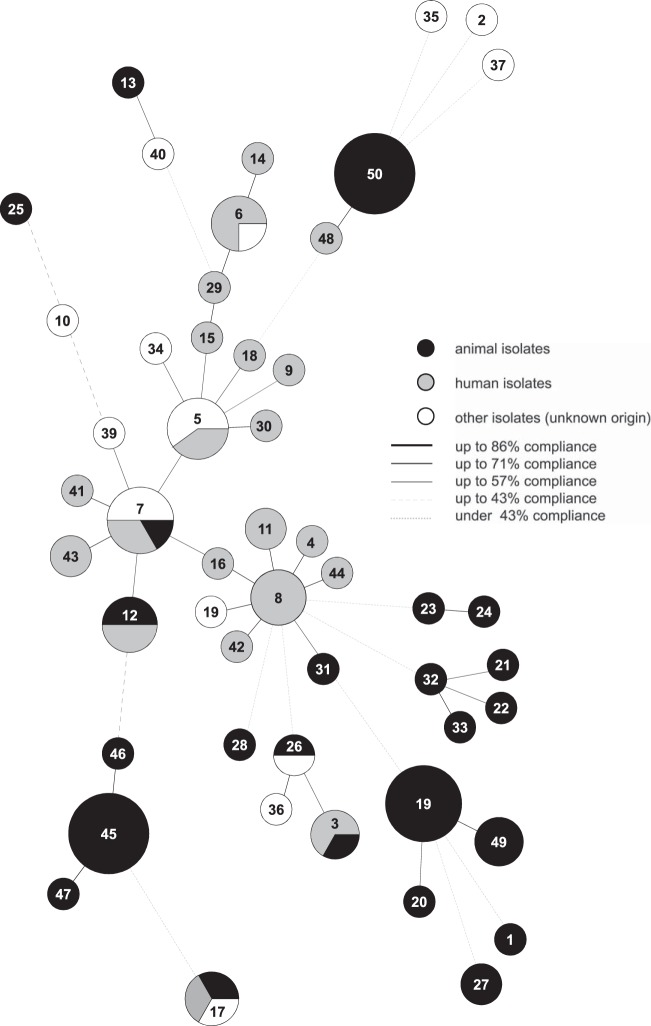
For each strain, the combination of the allelic numbers determines the STs. The isolates were resolved into 50 different STs, and STs 45 and 50 were the most common. Within these two STs, multiple isolates from pigeons (pigeon lofts 1 and 2) and turkeys (turkey coop 1) were found. Thirty-five STs are represented by a single strain (Fig. 1; see Table S1 in the supplemental material). To illustrate the potential relatedness between the strains, a UPGMA dendrogram was constructed from the allelic distances of each strain. It showed a highly divergent population (Fig. 1). An average SID of 0.84 (95% CI, 0.735 to 0.931) was defined as demonstrating the discriminatory power of the S. gallolyticus subsp. gallolyticus-specific MLST scheme (Table 1) (28, 29). Therefore, 11 clusters (containing at least three isolates) are formed in the dendrogram based on a linkage distance of 1.12 (conforms SID = 0.84) (Fig. 1; Table 1). STs 45, 46, 47, and 50 can be associated with cluster I or X and contain isolates from only one animal species. Clusters I and XI are the biggest groups and comprise 11 isolates from poultry, followed by cluster X with 10 isolates from pigeons (with one exception). In clusters I and XI, fecal isolates from different pigeon lofts were found (Fig. 1). Furthermore, strains of fecal samples from a turkey coop are in cluster X. Clusters VI and VII comprise many isolates from humans. Several clusters (e.g., IV, V, and IX) comprise isolates from cattle and humans (Fig. 1). For a better characterization of the relatedness between strains and for more conclusive information about infection chains, geography, or host specificity, a minimum spanning tree (MST) based on the MLST data set of 101 strains was generated (Fig. 2). Besides the UPGMA dendrogram and the MST, clonal complexes were defined. The 50 STs can be divided into 10 clonal complexes, and a total of 29 STs are included. Furthermore, 21 singletons were identified. The snapshot of the eBURST (zero out of seven alleles are in common) proposes ST 8 as the predicted primary founder of the S. gallolyticus subsp. gallolyticus strain collection (data not shown). ST 8 belongs to clonal complex 8. This complex includes STs 4, 11, 16, 38, 42, and 48 (Fig. 1 and 2). With one exception, this lineage comprises only isolates from humans from different time points and countries.
FIG 1.
UPGMA dendrogram of 101 S. gallolyticus subsp. gallolyticus strains. The phylogenetic tree shown was calculated with the allelic profile by using the UPGMA algorithm. The dashed line symbolizes the border defining the clusters. A linkage distance of 1.12 contributes to 11 clusters.
FIG 2.
Relatedness of 50 STs of 101 S. gallolyticus subsp. gallolyticus strains in an MST. The results were calculated by BioNumerics software on the basis of MLST data. Each ST is shown as a circle whose size is proportional to the number of strains included. Shading shows the origins of the isolates, and the lines represent the compliance levels of the strains.
All of the analysis methods used, MLST, MST, and eBURST, demonstrated the high diversity of our random collection of S. gallolyticus subsp. gallolyticus strains. Hence, no evidence of host or geographic specificity was found (Fig. 1 and 2; eBURST data not shown).
DISCUSSION
The host specificities, pathogenic mechanisms, and biochemical characteristics of S. gallolyticus subsp. macedonicus, S. gallolyticus subsp. pasteurianus, S. gallolyticus subsp. gallolyticus differ widely. S. gallolyticus subsp. gallolyticus and S. gallolyticus subsp. pasteurianus are commensals of the gastrointestinal system and can cause endocarditis and meningitis (3, 5–7, 30–32). However, S. gallolyticus subsp. pasteurianus can cause septicemia and is often associated with, e.g., chronic liver disease or cirrhosis, especially in immunocompromised patients (33, 34). Besides human infections, to date there have been only two reported cases of septicemia due to S. gallolyticus subsp. pasteurianus in animals (ducklings, goslings) (35, 36). S. gallolyticus subsp. macedonicus is often isolated from dairy products, e.g., cheese, and sour mash and is nonpathogenic (12). In summary, S. gallolyticus subsp. gallolyticus shows a broader host range and differs in the spectrum of diseases. On the basis of the different characteristics of these three subspecies, we established a subspecies-specific MLST scheme for S. gallolyticus subsp. gallolyticus. This scheme offers the opportunity to characterize the phylogenetic structure, the zoonotic potential, and the transmission routes of this subspecies, as well as to assess its risks. We used bacteria from several strain collections and isolates from patients (e.g., blood cultures, feces) and from animal fecal samples. Housekeeping genes were chosen with a low dN/dS ratio of <1 and a high SID of 0.84 (95% CI, 0.735 to 0.931) to characterize 101 S. gallolyticus subsp. gallolyticus isolates. A dN/dS ratio of <1 was also calculated by Shibata et al. (17). The number of alleles in the subspecies-specific MLST scheme varies from 14 (glgB) to 22 (p20), which distinguishes more than 3.6 × 108 STs. This range is comparable to those reported in other publications. For group B streptococci, a 1.2 to 2.5% range of allelic variation was identified (37). A comparable range of allelic variation (1.4 to 6.1%) was observed in the MLST scheme developed for group A streptococci (38). A comparison with the recently published MLST scheme presents from 15 (parC) to 24 (rpoD) allelic variations by using other genes (dpr, gmk, rpoD, parC, pta, pyrC, and recN) for S. gallolyticus. However, in contrast to our work, the publication of Shibata et al. supports no SID calculations. Therefore, the description of the divergent structure is based only on the calculated number of STs (17).
The distribution of the STs can be illustrated by calculating clusters or can be presented in the MST. The MST shows accumulations of S. gallolyticus subsp. gallolyticus strains isolated from various animals. Otherwise, there are groups consisting of bacterial isolates from animals and humans but there is no evidence of host specificity and no suggestion of geographic-region-related occurrence. Remarkably, clonal complex 8 is dominated by human isolates. The occurrence of the ST 8 lineage especially in human isolates may indicate that the STs involved are associated with human hosts. To gain better insights into the epidemiologic structure of S. gallolyticus subsp. gallolyticus, cluster borders were based on the calculated average SID. One must acknowledge that the clusters of Shibata et al. are different. The S. gallolyticus MLST scheme shows clusters based on the roots of the UPGMA dendrogram. The clusters presented also contain S. gallolyticus subsp. gallolyticus isolates from animals or humans, as well as from both animals and humans (17).
Moreover, on the basis of the IA, no epidemiological population structure can be observed referring to the detection of significant linkage disequilibrium, which can be interpreted as a bacterial population with low rates of recombination. Recombination events were tested by using Sawyer's run test in the MLST scheme for all three subspecies, whereby two genes with evidence of recombination (rpoC, parC) were identified (17). However, this test is less sensitive for detecting recombination (39). Therefore, we calculated the IA for our strain collection. For the comparison of population structures for the S. gallolyticus MLST scheme, an IA was not calculated (17). To answer questions concerning epidemiology, host specificity, and virulence, Shibata et al. defined clonal complexes. For this purpose, a more relaxed group definition (five out of seven alleles are in common) was used and the 57 STs of 63 strains were divided into four lineages and 31 singletons (17).
Nevertheless, the established MLST schemes differ in focus. In addition to the epidemiologic application, Shibata et al. included all three S. gallolyticus subspecies and strived to simplify subspecies classification (17). The 57 STs identified form five clusters (A to E), and three of these (A, B, and D) contain 41 STs of S. gallolyticus subsp. gallolyticus strains. Consequently, it is presumed that two of the three S. gallolyticus subsp. gallolyticus clusters belong to a novel subspecies (17). In 2011, Hinse et al. published a reliable method for identifying isolates to the subspecies level by MALDI-TOF MS and sodA DNA sequencing, which was used in our research to identify S. gallolyticus subsp. gallolyticus isolates (19).
Notwithstanding the fact that the typing schemes have different aims, both suggest zoonotic potential. In the S. gallolyticus MLST scheme, it is suggested by S. gallolyticus subsp. gallolyticus cluster A, which includes animal and human isolates (17). Furthermore, only virulent cluster C of S. gallolyticus subsp. pasteurianus, which includes exclusively human patient isolates of ST 14, could be identified (17).
A comparison of these two different schemes shows that the MLST method described here is focused explicitly on the zoonotic and epidemiological investigation of the distinct subspecies of S. gallolyticus and does not aim at subspecies identification or determination of virulence. The subspecies-specific focus on the epidemiological structure and risk assessment is confirmed by the specific primer binding sites for the housekeeping genes for S. gallolyticus subsp. gallolyticus. On the basis of the observation of identical allelic profiles of S. gallolyticus subsp. gallolyticus isolates and different species, we propose that there is zoonotic potential. Additionally, the transferability to other subspecies is quite limited because of the specificity of our MLST scheme for S. gallolyticus subsp. gallolyticus.
To prove the zoonotic potential of S. gallolyticus subsp. gallolyticus, further studies are being performed. We are examining isolates from livestock in cooperation with the respective livestock owners to examine the transmission pattern of this bacterium and to try to assess the risk associated with this facultative pathogen. Our first results suggest that S. gallolyticus subsp. gallolyticus might act as a zoonotic agent. In summary, the S. gallolyticus subsp. gallolyticus-specific MLST scheme developed can be used for molecular genetic characterization aiming for insight into its zoonotic potential, epidemiology, and potential infection chains.
Supplementary Material
ACKNOWLEDGMENTS
We thank Jochen Schulz from the University of Veterinary Medicine, Hannover, Germany, for several S. gallolyticus subsp. gallolyticus isolates from poultry. We also thank M. D. Fred Splittgerber for his linguistic advice.
This work was supported by the Ruhr-Universität Bochum Medizinische Fakultät (FoRUM).
Footnotes
Published ahead of print 30 April 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JCM.03329-13.
REFERENCES
- 1.Schlegel L, Grimont F, Ageron E, Grimont PA, Bouvet A. 2003. Reappraisal of the taxonomy of the Streptococcus bovis/Streptococcus equinus complex and related species: description of Streptococcus gallolyticus subsp. gallolyticus subsp. nov., S. gallolyticus subsp. macedonicus subsp. nov. and S. gallolyticus subsp. pasteurianus subsp. nov. Int. J. Syst. Evol. Microbiol. 53:631–645. 10.1099/ijs.0.02361-0 [DOI] [PubMed] [Google Scholar]
- 2.Sillanpää J, Nallapareddy SR, Qin X, Singh KV, Muzny DM, Kovar CL, Nazareth LV, Gibbs RA, Ferraro MJ, Steckelberg JM. 2009. A collagen-binding adhesin, Acb, and ten other putative MSCRAMM and pilus family proteins of Streptococcus gallolyticus subsp. gallolyticus (Streptococcus bovis group, biotype I). J. Bacteriol. 191:6643–6653. 10.1128/JB.00909-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Headings DL, Herrera A, Mazzi E, Bergman MA. 1978. Fulminant neonatal septicemia caused by Streptococcus bovis. J. Pediatr. 92:282–283. 10.1016/S0022-3476(78)80026-2 [DOI] [PubMed] [Google Scholar]
- 4.Sekizaki T, Nishiya H, Nakajima S, Nishizono M, Kawano M, Okura M, Takamatsu D, Nishino H, Ishiji T, Osawa R. 2008. Endocarditis in chickens caused by subclinical infection of Streptococcus gallolyticus subsp. gallolyticus. Avian Dis. 52:183–186. 10.1637/8048-070307-Case [DOI] [PubMed] [Google Scholar]
- 5.Sillanpää J, Nallapareddy SR, Singh KV, Ferraro MJ, Murray BE. 2008. Adherence characteristics of endocarditis-derived Streptococcus gallolyticus ssp. gallolyticus (Streptococcus bovis biotype I) isolates to host extracellular matrix proteins. FEMS Microbiol. Lett. 289:104–109. 10.1111/j.1574-6968.2008.01378.x [DOI] [PubMed] [Google Scholar]
- 6.Macneal WJ, Blevins A. 1945. Bacteriological studies in endocarditis. J. Bacteriol. 49:603–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ballet M, Gevigney G, Gare J, Delahaye F, Etienne J, Delahaye J. 1995. Infective endocarditis due to Streptococcus bovis. A report of 53 cases. Eur. Heart J. 16:1975–1980 [DOI] [PubMed] [Google Scholar]
- 8.Klein RS, Recco RA, Catalano MT, Edberg SC, Casey JI, Steigbigel NH. 1977. Association of Streptococcus bovis with carcinoma of the colon. N. Engl. J. Med. 297:800–802. 10.1056/NEJM197710132971503 [DOI] [PubMed] [Google Scholar]
- 9.Devriese L, Uyttebroek E, Gevaert D, Vandekerckhove P, Ceyssens K. 1990. Streptococcus bovis infections in pigeons. Avian Pathol. 19:429–434. 10.1080/03079459008418697 [DOI] [PubMed] [Google Scholar]
- 10.Devriese LA, Vandamme P, Pot B, Vanrobaeys M, Kersters K, Haesebrouck F. 1998. Differentiation between Streptococcus gallolyticus strains of human clinical and veterinary origins and Streptococcus bovis strains from the intestinal tracts of ruminants. J. Clin. Microbiol. 36:3520–3523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sasaki E, Osawa R, Nishitani Y, Whiley RA. 2004. ARDRA and RAPD analyses of human and animal isolates of Streptococcus gallolyticus. J. Vet. Med. Sci. 66:1467–1470. 10.1292/jvms.66.1467 [DOI] [PubMed] [Google Scholar]
- 12.Tsakalidou E, Zoidou E, Pot B, Wassill L, Ludwig W, Devriese L, Kalantzopoulos G, Schleifer K, Kersters K. 1998. Identification of streptococci from Greek Kasseri cheese and description of Streptococcus macedonicus sp. nov. Int. J. Syst. Bacteriol. 48:519–527. 10.1099/00207713-48-2-519 [DOI] [PubMed] [Google Scholar]
- 13.Randazzo CL, Vaughan EE, Caggia C. 2006. Artisanal and experimental Pecorino Siciliano cheese: microbial dynamics during manufacture assessed by culturing and PCR-DGGE analyses. Int. J. Food Microbiol. 109:1–8. 10.1016/j.ijfoodmicro.2005.11.002 [DOI] [PubMed] [Google Scholar]
- 14.Fortin M, Messier S, Paré J, Higgins R. 2003. Identification of catalase-negative, non-beta-hemolytic, gram-positive cocci isolated from milk samples. J. Clin. Microbiol. 41:106–109. 10.1128/JCM.41.1.106-109.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garvie EI, Bramley A. 1979. Streptococcus bovis—an approach to its classification and its importance as a cause of bovine mastitis. J. Appl. Microbiol. 46:557–566 [DOI] [PubMed] [Google Scholar]
- 16.Urwin R, Maiden MC. 2003. Multi-locus sequence typing: a tool for global epidemiology. Trends Microbiol. 11:479–487. 10.1016/j.tim.2003.08.006 [DOI] [PubMed] [Google Scholar]
- 17.Shibata Y, Tien LHT, Nomoto R, Osawa R. 2014. Development of a multilocus sequence typing scheme for Streptococcus gallolyticus. Microbiology 160:113–122. 10.1099/mic.0.071605-0 [DOI] [PubMed] [Google Scholar]
- 18.Poyart C, Quesne G, Coulon S, Berche P, Trieu-Cuot P. 1998. Identification of streptococci to species level by sequencing the gene encoding the manganese-dependent superoxide dismutase. J. Clin. Microbiol. 36:41–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hinse D, Vollmer T, Erhard M, Welker M, Moore E, Kleesiek K, Dreier J. 2011. Differentiation of species of the Streptococcus bovis/equinus complex by MALDI-TOF mass spectrometry in comparison to sodA sequence analyses. Syst. Appl. Microbiol. 34:52–57. 10.1016/j.syapm.2010.11.010 [DOI] [PubMed] [Google Scholar]
- 20.Rusniok C, Couvé E, Da Cunha V, El Gana R, Zidane N, Bouchier C, Poyart C, Leclercq R, Trieu-Cuot P, Glaser P. 2010. Genome sequence of Streptococcus gallolyticus: insights into its adaptation to the bovine rumen and its ability to cause endocarditis. J. Bacteriol. 192:2266–2276. 10.1128/JB.01659-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin I-H, Liu T-T, Teng Y-T, Wu H-L, Liu Y-M, Wu K-M, Chang C-H, Hsu M-T. 2011. Sequencing and comparative genome analysis of two pathogenic Streptococcus gallolyticus subspecies: genome plasticity, adaptation and virulence. PLoS One 6:e20519. 10.1371/journal.pone.0020519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hinse D, Vollmer T, Rückert C, Blom J, Kalinowski J, Knabbe C, Dreier J. 2011. Complete genome and comparative analysis of Streptococcus gallolyticus subsp. gallolyticus, an emerging pathogen of infective endocarditis. BMC Genomics 12:400. 10.1186/1471-2164-12-400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blom J, Albaum S, Doppmeier D, Pühler A, Vorhölter F-J, Zakrzewski M, Goesmann A. 2009. EDGAR: a software framework for the comparative analysis of prokaryotic genomes. BMC Bioinformatics 10:154. 10.1186/1471-2105-10-154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feil EJ, Li BC, Aanensen DM, Hanage WP, Spratt BG. 2004. eBURST: inferring patterns of evolutionary descent among clusters of related bacterial genotypes from multilocus sequence typing data. J. Bacteriol. 186:1518–1530. 10.1128/JB.186.5.1518-1530.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jolley KA, Feil E, Chan M-S, Maiden MCJ. 2001. Sequence type analysis and recombinational tests (START). Bioinformatics 17:1230–1231. 10.1093/bioinformatics/17.12.1230 [DOI] [PubMed] [Google Scholar]
- 26.Smith JM, Smith NH, O'Rourke M, Spratt BG. 1993. How clonal are bacteria? Proc. Natl. Acad. Sci. U. S. A. 90:4384–4388. 10.1073/pnas.90.10.4384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jolley KA, Maiden MJ. 2010. BIGSdb: scalable analysis of bacterial genome variation at the population level. BMC Bioinformatics 11:595. 10.1186/1471-2105-11-595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grundmann H, Hori S, Tanner G. 2001. Determining confidence intervals when measuring genetic diversity and the discriminatory abilities of typing methods for microorganisms. J. Clin. Microbiol. 39:4190–4192. 10.1128/JCM.39.11.4190-4192.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simpson EH. 1949. Measurement of diversity. Nature 163:688 (Letter.) 10.1038/163688a0 [DOI] [Google Scholar]
- 30.Onoyama S, Ogata R, Wada A, Saito M, Okada K, Harada T. 2009. Neonatal bacterial meningitis caused by Streptococcus gallolyticus subsp. pasteurianus. J. Med. Microbiol. 58:1252–1254. 10.1099/jmm.0.006551-0 [DOI] [PubMed] [Google Scholar]
- 31.Sturt AS, Yang L, Sandhu K, Pei Z, Cassai N, Blaser MJ. 2010. Streptococcus gallolyticus subspecies pasteurianus (biotype II/2), a newly reported cause of adult meningitis. J. Clin. Microbiol. 48:2247–2249. 10.1128/JCM.00081-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grant RJ, Whitehead TR, Orr JE. 2000. Streptococcus bovis meningitis in an infant. J. Clin. Microbiol. 38:462–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alex D, Garvin D, Peters S. 2013. Streptococcus pasteurianus septicemia. Indian J. Med. Microbiol. 31:310–312. 10.4103/0255-0857.115668 [DOI] [PubMed] [Google Scholar]
- 34.Gonzlez-Quintela A, Martínez-Rey C, Castroagudín J, Rajo-Iglesias M, Domínguez-Santalla M. 2001. Prevalence of liver disease in patients with Streptococcus bovis bacteraemia. J. Infect. 42:116–119. 10.1053/jinf.2001.0799 [DOI] [PubMed] [Google Scholar]
- 35.Barnett J, Ainsworth H, Boon J, Twomey D. 2008. Streptococcus gallolyticus subsp. pasteurianus septicaemia in goslings. Vet. J. 176:251–253. 10.1016/j.tvjl.2007.02.011 [DOI] [PubMed] [Google Scholar]
- 36.Li M, Gu C, Zhang W, Li S, Liu J, Qin C, Su J, Cheng G, Hu X. 2013. Isolation and characterization of S. gallolyticus subsp. pasteurianus causing meningitis in ducklings. Vet. Microbiol. 162:930–936. 10.1016/j.vetmic.2012.11.038 [DOI] [PubMed] [Google Scholar]
- 37.Jones N, Bohnsack JF, Takahashi S, Oliver KA, Chan M-S, Kunst F, Glaser P, Rusniok C, Crook DW, Harding RM, Bisharat N, Spratt BG. 2003. Multilocus sequence typing system for group B streptococcus. J. Clin. Microbiol. 41:2530–2536. 10.1128/JCM.41.6.2530-2536.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Enright MC, Spratt BG, Kalia A, Cross JH, Bessen DE. 2001. Multilocus sequence typing of Streptococcus pyogenes and the relationships between emm type and clone. Infect. Immun. 69:2416–2427. 10.1128/IAI.69.4.2416-2427.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meinersmann RJ, Phillips RW, Wiedmann M, Berrang ME. 2004. Multilocus sequence typing of Listeria monocytogenes by use of hypervariable genes reveals clonal and recombination histories of three lineages. Appl. Environ. Microbiol. 70:2193–2203. 10.1128/AEM.70.4.2193-2203.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.