ABSTRACT
Major histocompatibility complex class II-deficient (MHC-II KO; Aβ−/−) mice were used to assess the roles of MHC-II molecules in inducing protective immune responses to vaccination. After vaccination with influenza A/PR8 virus-like particle (VLP) vaccine, in vivo and in vitro vaccine antigen-specific IgG isotype antibodies were not detected in MHC-II KO mice, which is quite different from CD4 T cell-deficient mice that induced vaccine-specific IgG antibodies. The deficiency in MHC-II did not significantly affect the induction of antigen-specific IgM antibody in sera. MHC-II KO mice that were vaccinated with influenza VLP, whole inactivated influenza virus, or live attenuated influenza virus vaccines were not protected against lethal infection with influenza A/PR8 virus. Adoptive transfer of fractionated spleen cells from wild-type mice to MHC-II KO mice indicated that CD43+ cell populations with MHC-II contributed more significantly to producing vaccine-specific IgG antibodies than CD43− B220+ conventional B cell or CD4 T cell populations, as well as conferring protection against lethal infection. Bone marrow-derived dendritic cells from MHC-II KO mice showed a significant defect in producing interleukin-6 and tumor necrosis factor alpha cytokines. Thus, results indicate that MHC-II molecules play multiple roles in inducing protective immunity to influenza vaccination.
IMPORTANCE Major histocompatibility complex class II (MHC-II) has been known to activate CD4 T helper immune cells. A deficiency in MHC-II was considered to be equivalent to the lack of CD4 T cells in developing host immune responses to pathogens. However, the roles of MHC-II in inducing protective immune responses to vaccination have not been well understood. In the present study, we demonstrate that MHC-II-deficient mice showed much more significant defects in inducing protective antibody responses to influenza vaccination than CD4 T cell-deficient mice. Further analysis showed that CD43 marker-positive immune cells with MHC-II, as well as an innate immunity-simulating adjuvant, could rescue some defects in inducing protective immune responses in MHC-II-deficient mice. These results have important implications for our understanding of host immunity-inducing mechanisms to vaccination, as well as in developing effective vaccines and adjuvants.
INTRODUCTION
Vaccination is the most effective measure for preventing infectious diseases, including influenza, a highly contagious respiratory disease resulting in widespread morbidity and mortality. Most licensed human vaccines are based on their capability to induce protective humoral antibodies that block infection or reduce pathogen loads, although cellular immune responses are also important (1–3). However, mechanisms by which vaccination induces effective protective immunity have not been well understood yet.
A model for producing protein antigen-specific immunoglobulin G (IgG) antibodies initiates with antigen uptake by antigen-presenting cells such as dendritic cells (DCs), macrophages, and B cells. In particular, DCs after antigen uptake migrate to secondary lymphoid tissues from peripheral sites. Antigen-presenting cells present peptide fragments of processed antigens on their surfaces in the context of major histocompatibility complex class II (MHC-II) molecules (4). Specific CD4+ T cells are activated and undergo clonal expansion after recognition of antigenic peptide/MHC-II on antigen-presenting cells via a T cell receptor. In the meantime, naive B cells internalize and process a specific antigen bound by surface immunoglobulin receptors, presenting antigenic peptides in the context of MHC-II molecules. The T cell help to drive the B cell response is initiated by recognizing peptide/MHC-II on the B cell surfaces via T cell receptor through the specific CD4+ T cells. Subsequently, T cell-derived signaling molecules and cytokines initiate B cell proliferation and direct immunoglobulin isotype switching (5–7). In this model, cognate T and B cell interaction is a requirement for B cell IgG responses and isotype switching.
This scenario of cognate T and B cell interactions through the T cell receptor and peptide-MHC complex does not appear to fully explain the strong humoral responses that are rapidly generated against many pathogens probably due to low frequencies of antigen-specific T and B cells at the time of initial antigen encounter. Alternative T cell help for B cell isotype-switched IgG responses might be mediated by secreted cytokines or nonspecific molecular interactions between adjacent cells (8, 9). It is noteworthy that DCs are capable of retaining antigens in a form that is recognized by B cells and also provide signals that direct isotype switching in T cell-dependent humoral responses (10–12).
The normal development of mature T cells needs their interactions with MHC molecules in the thymus. MHC-II-deficient (MHC-II KO) mice were found to be deficient in mature CD4+ T cell-mediated immune responses (13). Previous studies used MHC-II KO mouse models to study the roles of CD4+ T cells and/or MHC-II molecules in inducing host CD8+ cytotoxic T cell immune responses to viral, bacterial, and parasitic infections (14–20). The apparent efficacy of comparable or less control of infecting pathogens was attributed to the intact activity of CD8+ cytotoxic T cells despite the deficiency of CD4+ T cells. Polyomavirus infection of mice with a deficiency of functional αβ+ T cells or αβ+ and γδ+ T cells induced IgM and IgG antiviral antibodies (21, 22). Vesicular stomatitis virus (VSV) infection in αβ+ T cell-deficient mice induced IgG antibody responses (23–26). Our previous studies have shown that mucosal or systemic immunization of CD4+ T cell-deficient mice with inactivated influenza virus can also induce antigen-specific isotype-switched IgG antibody responses, virus neutralizing antibodies, and protection (27, 28).
The potential roles of MHC-II molecules in inducing immune responses to vaccination largely remain unknown. In this study, we investigated host immune responses and protection against lethal challenge after vaccination of MHC-II KO mice with a recombinant influenza A/PR8 virus-like particle (VLP), whole inactivated influenza virus, or 2009 H1N1 pandemic influenza virus live attenuated vaccine. We demonstrate that, despite the presence of naturally occurring IgG antibodies at significant levels, MHC-II KO mice were not able to induce antigen-specific IgG antibody responses to influenza vaccination and thus were not protected. CD43+ cell populations with MHC-II molecules were found to induce protective antibody responses by adoptive-transfer studies, suggesting additional roles for MHC-II in addition to activating CD4 T cells.
MATERIALS AND METHODS
Mice and cells.
MHC-II KO (I-Aβ−/−) and wild-type C57BL/6 (B6 WT) mice were purchased from the Jackson Laboratory (Bar Harbor, ME) and bred at the animal facility at Georgia State University. BALB/c mice were purchased from Harlan Laboratories (Indianapolis, IN). Spodoptera frugiperda Sf9 insect cells (American Type Culture Collection, CRL-1711) for the production of recombinant baculoviruses (rBVs) and influenza VLPs were cultured in SF900-II serum-free medium (Invitrogen, Carlsbad, CA) at 27°C.
Preparation of influenza VLPs and virus.
Preparation of influenza VLP vaccines containing the hemagglutinin (HA) protein as a major protective antigen has been well described in our previous studies (29–33). To produce influenza VLPs, SF9 insect cells were coinfected with rBVs expressing influenza virus M1 gene and HA derived from the strain of A/PR/8/34 H1N1 (A/PR8) virus. Influenza VLPs in the culture supernatants were harvested at 3 days after infection and purified as described previously (29, 34). H1N1 influenza A viruses A/PR8 (29) and A/California/04/2009 (a kind gifts from Richard Webby) were propagated in allantoic cavity of 10-day-old embryonated hen's eggs (32). An att.NL virus is an attenuated 2:6 reassortant containing the surface HA and neuraminidase (NA) genes of A/Netherlands/602/09 (H1N1) (35) and the internal genes from the attenuated A/turkey/Ohio/313053/04 (H3N2) virus. Rg20 A/Texas/5/2009, kindly provided by Ruben Donis, is a reassortant virus containing six A/PR/8/34 internal genes and A/Texas/5/2009 HA and NA (32).
Immunization and viral challenge infection.
MHC-II KO mice (8 to 10 weeks old) and B6 WT mice (8 to 10 weeks old) were intramuscularly immunized with influenza A/PR8 VLPs (3 or 10 μg) at weeks 0 and 4. For in vivo infection, mice were anesthetized with isoflurane (Baxter, Deerfield, IL), intranasally challenged with A/PR8 virus (5× 50% lethal dose [LD50]) at 3 weeks after boost immunization and sacrificed at 4 days after challenge or monitored for 14 days for measuring body weights and survival rates to assess the protective efficacy of vaccination. For the serum protective efficacy, 6- to 7-week-old female naive BALB/c mice were infected with a lethal dose A/PR8 virus mixed with heat-inactivated sera (56°C for 30 min) from each of the indicated groups after incubation at room temperature for 30 min (36, 37).
To understand the protective immune response from live and inactivated whole viral vaccines, B6 WT mice and MHC-II KO mice were intranasally prime-boost inoculated with a live attenuated att.NL virus (∼105 tissue culture infective doses per mouse) or intramuscularly boost immunized with inactivated Rg20 A/Texas/5/2009 (Rg20i; 5 μg/mouse). Mice were challenged with 2009 pandemic H1N1 virus (5× LD50) 4 weeks after boost immunization. All animal studies were approved and conducted under the guidelines by Georgia State University's IACUC (A11026). Mice were euthanized if their body weight loss exceeded 25%.
Flow cytometry analysis of MHC-II KO mice phenotype.
Spleen cells were analyzed by fluorescence-activated cell sorting (FACS) to check phenotypes of MHC-II KO and B6 WT mice. Isolated spleen cells were stained with fluorescence-conjugated monoclonal antibodies specific to cell phenotypes, MHC-II–FITC (M5/114.15.2; eBioscience, San Diego, CA), CD19-PE (6D5; eBioscience), B220-PE Cy7 (RA3-6B2; BD Pharmingen), CD3-Pacific Blue (17A2; Biolegend), CD4-APC (GK1.5; eBioscience), and CD8-PerCP (53-6.7; BD Pharmingen). Dead cells are excluded by staining with near-infrared fluorescent reactive dye (Invitrogen, Eugene, OR). Live lymphocytes were gated according to their sizes, and granularity was defined in the forward light scatter (FSC) and side light scatter (SSC) plot. Cell acquisition was performed with a multilaser multiparameter analysis cytometer (LSR-II and LSRFortessa; BD Biosciences, Mountain View, CA), and the data were analyzed using FlowJo software (v7.6.4; Tree Star, Inc., Ashland, OR).
Determination of serum antibody responses.
Blood samples were collected from the retro-orbital plexus puncture using heparinized microcapillaries (Drummond Scientific Company, Broomall, PA) after anesthetization of the animals by isoflurane inhalation. Sera were collected 18 days after prime and 10 days after boost immunization and stored at −20°C until analysis. Influenza virus-specific total IgG, IgM and isotype antibodies (Southern Biotechnology, Birmingham, AL) were determined using standard enzyme-linked immunosorbent assay (ELISA) as described previously (29). Purified mouse IgG, IgM, and isotype antibodies were used as standards to determine the relative antibody concentrations in immune sera from optical spectrophotometer readings at 450 nm (ELx800; BioTek, Winooski, VT).
Virus-specific antibody secreting cell responses.
Freshly isolated spleen and bone marrow cells (5 × 105 cells/well) were incubated for 24 h in the 96-well culture plates coated with either culture medium or A/PR8 VLP (400 ng/well) for in vitro antigenic stimulation. Antibody-secreting cell responses in the spleen and bone marrow were determined after 5 days of in vitro culture. The levels of antibodies specific to A/PR8 VLP vaccine in the culture plates were determined by ELISA as previously described (29, 38).
Determination of T cell responses.
Spleen cells were obtained from the mice sacrificed at 4 days postchallenge. Gamma interferon (IFN-γ)- and interleukin-4 (IL-4)-secreting cell spots were determined on MultiScreen 96-well filter plates (Millipore, Billerica, MA) coated with cytokine-specific capture antibodies as described previously (29). Briefly, 5 × 105 spleen cells (per well) were cultured in anti-mouse IFN-γ or anti-mouse IL-4 (300 ng/100 μl/well) precoated plates with or without A/PR8 VLP (2 μg/ml) as an antigenic stimulator. After 36 h of incubation, the number of IFN-γ- or IL-4-secreting cell spots was counted using a Bioreader 5000-Eβ (Biosys, Miami, FL).
Analysis of lung and BALF samples.
Bronchoalveolar lavage fluids (BALF) were obtained by infusing 1 ml of phosphate-buffered saline (PBS) using a 18Gx11/4 catheter (Exelint International, Tokyo, Japan) into the lungs via the trachea, and lung samples were also collected at day 4 postchallenge. Cells were removed after centrifugation, and inflammatory cytokine (IL-6) in lung extracts and BALF were analyzed by using Ready-Set-Go cytokine kits (eBioscience) according to the manufacturer's protocol. To determine lung virus titers at 4 days postchallenge with A/PR8 virus, 10-day-old embryonated hen's eggs were infected with 10-fold serially diluted lung extracts for 3 days, and lung virus titers were calculated as described previously (38).
Adoptive cell transfer.
The CD4+, CD43+, or CD43− cell fractions were isolated from the spleen cells of naive B6 WT mice by the MACS system using antibody-coated microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer's instructions. Briefly, mouse spleens from the naive B6 WT group were minced using glass slides, passed through a cell strainer (40-μm pore size; Becton Dickinson, Franklin Lakes, NJ), resuspended in 0.5% bovine serum albumin (Sigma, St. Louis, MO) with 2 mM EDTA in PBS, and incubated with CD4 microbeads. Flowthrough cells in a magnetic field were applied to the CD43 microbeads to purify CD43 positive and negative cell fractions. Cells bound or unbound to the column were collected, and nucleated cells were counted by using a hemacytometer after trypan blue dead-cell exclusion. The purification of MACS-isolated cell populations was determined by cell surface staining and flow cytometric analysis prior to adoptive cell transfer to MHC-II KO mice. Enriched CD4+, CD43+, or CD43− cells were intravenously injected via tail vein into recipient naive MHC-II KO mice, which were then immunized with 10 μg of A/PR8 VLPs intramuscularly 3 to 4 h after adoptive cell transfer. Blood samples were collected 2 weeks after immunization, and IgG antigen-specific antibody levels were determined by ELISA.
Generation of BMDCs.
Bone marrow-derived DCs (BMDCs) were obtained from B6 WT and MHC-II KO mice. BM cells were harvested from femur and tibia, and the red blood cells were removed by using red blood cell lysis buffer (Sigma). The cells were cultured in the presence of 10 ng of mouse recombinant granulocyte macrophage-colony-stimulating factor (Invitrogen)/ml to generate DCs. After 6 to 10 days of culture, the generated DCs were harvested and used for experiments. After 2 days of BMDC culture in the presence of influenza (A/PR8) VLP vaccine (10 μg/ml) or lipopolysaccharide (LPS; 0.2 μg/ml), the cultured DCs were harvested and stained with specific antibodies. After blocking Fc receptors with CD16/32 antibodies, the cells were stained with DC surface marker antibodies (eBioscience): phycoerythrin-labeled anti-mouse CD40 (clone 1C1O) and CD86 (clone GL1) and fluorescein isothiocyanate (FITC)-labeled anti-mouse CD80 (clone 16-10A1). To measure the amount of cytokines, mouse IL-12p70-, IL-6-, and tumor necrosis factor alpha (TNF-α)-specific ELISA Ready-Set-Go kits (eBioscience) were used. The culture supernatants were harvested, and cytokine ELISAs were performed according to the manufacturer's protocol.
Statistics.
Unless otherwise stated, all results are presented as means ± the standard error. Statistical analysis was performed using a two-tailed Student t test and one-way analysis of variance when comparing two or more different groups, respectively. The data were analyzed using Prism software (GraphPad Software, Inc., San Diego, CA). Probability values of <0.05 were considered statistically significant.
RESULTS
Naive MHC-II KO mice are able to produce natural IgG antibodies.
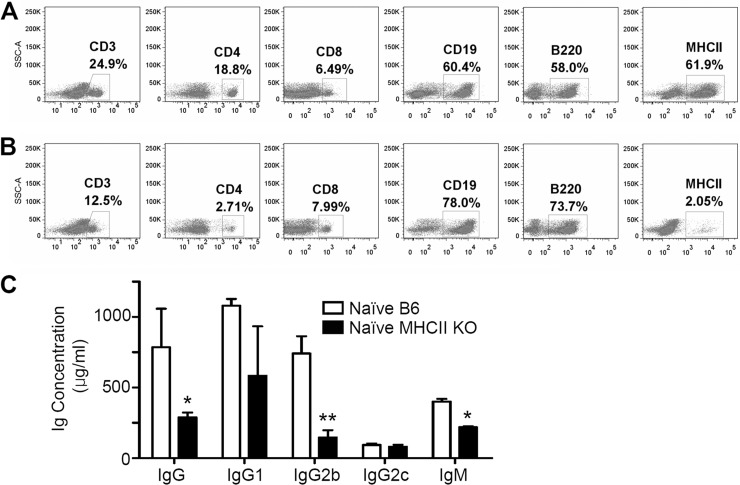
It is important to confirm that MHC-II KO mice have the MHC-II-deficient phenotype. We determined the phenotypes of T cell and B cell populations of spleen cells in MHC-II KO mice compared to B6 WT mice by flow cytometry (Fig. 1). Spleen cells from MHC-II KO mice showed ∼2-fold lower levels of CD3+, significantly lower levels of CD4+ cells (2.7% versus 18.8%) and MHC-II+ cells (2.05% versus 61.9%), and a slightly higher level of CD8, CD19, and B220 expression compared to WT control mice (Fig. 1A and B). Peripheral blood lymphocytes from naive MHC-II KO mice also had a similar deficiency of CD4+ and MHC-II+ cell populations (data not shown). These results indicate that the MHC-II KO mice are an authentic strain of mice with a MHC-II deficiency.
FIG 1.
Immune cell phenotypes and serum antibodies from naive MHC-II KO mice. (A) B6 WT mice; (B) MHC-II KO (MHC-II-deficient) mice. Spleen cells were stained with fluorescence-conjugated antibodies specific to cell surface markers (CD3, CD4, CD8, CD19, B220, and MHC-II), and live lymphocytes were gated based on their size and granularity defined in the forward light scatter (FSC) and side light scatter (SSC) plot. The FACS profiles shown are representative of two independent experiments. (C) Total serum IgG, IgM, and isotype-switched IgG antibodies were measured in naive B6 WT and MHC-II KO mice. Total natural antibodies were captured using goat anti-mouse IgG, IgM, and isotype-switched antibodies. The relative antibody concentrations were determined and calculated from standard curves of purified mouse antibodies. The data shown are the representative of three independent experiments that were consistently reproducible. Naive B6, a pooled serum sample of unimmunized C57BL/6 WT mice (n = 4); naive MHCII KO, a pooled serum sample of unimmunized MHC-II KO mice (n = 4). *, P < 0.05; **, P < 0.01.
Next, we compared the levels of natural antibodies, IgG, IgM, and isotypes from naive B6 WT and MHC-II KO mice using a standard ELISA method. An approximately 2- to 3-fold-lower but still significant level of IgG antibody and a moderately lower level of IgM antibody were observed in naive MHC-II KO mice compared to wild-type mice (Fig. 1C). Since the B6 mouse strain is to have the gene for isotype IgG2c instead of IgG2a (39), we examined IgG1, IgG2b, and IgG2c antibodies in sera by ELISA. Interestingly, MHC-II KO mice showed a similar level of IgG2c antibody compared to that of B6 WT mice and, on the other hand, approximately 2- to 4-fold-lower levels of IgG1 and IgG2b antibodies (Fig. 1C). Therefore, MHC-II KO mice can generate natural IgG and isotype-switched antibodies not specific to an antigen, although their levels were moderately lower than those in B6 WT mice.
MHC-II KO mice do not induce antigen-specific IgG isotype-switched antibodies.
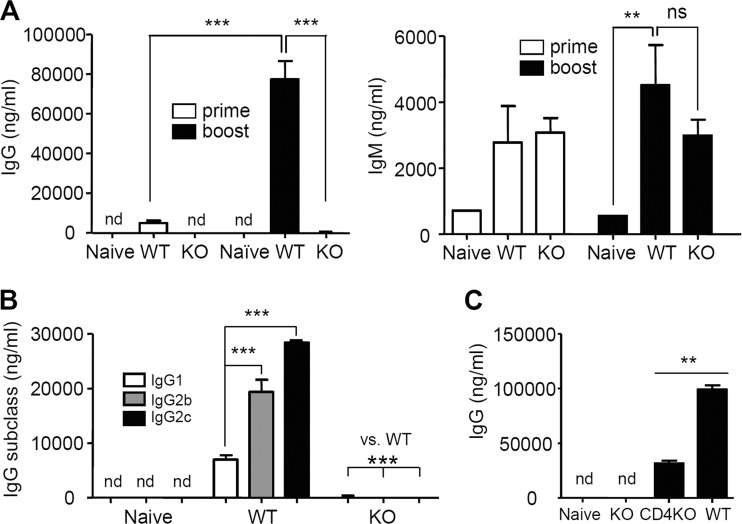
To determine the roles of an MHC-II molecule in inducing protective immune responses to vaccination, B6 WT and MHC-II KO mice were intramuscularly immunized with 3 μg of A/PR8 HA VLPs. WT mice showed significantly increased levels of IgG antibodies after prime and boost immunizations (16-fold higher levels of IgG after boost) (Fig. 2A). In contrast to B6 WT mice, significant levels of IgG antibodies specific to virus were not detected in the sera of MHC-II KO mice after prime and boost immunizations, similar to naive mice (Fig. 2A). However, IgM antibodies specific to the A/PR8 viral antigens were induced at high levels in immunized MHC-II KO mice, and there was no statistical difference in IgM levels between MHC-II KO and B6 WT mice (Fig. 2A). There was no significant boosting effect on IgM antibodies even in wild-type mice, which is different from IgG antibodies. IgG2c isotype antibody showed the highest level among IgG isotypes in B6 WT mice. However, levels of vaccine antigen-specific IgG isotype antibodies were not significantly detected in MHC-II KO mice, which were similar to naive mice (Fig. 2B). These results indicate that MHC-II KO mice have a significant defect in inducing IgG and its IgG isotype antibodies in response to vaccination with influenza VLPs.
FIG 2.
Immunization of MHC-II KO mice does not induce vaccine-specific IgG antibodies. (A) Total IgG and IgM antibodies specific for inactivated influenza (A/PR8) virus antigen on day 18 post-prime and day 10 post-boost immunization. (B) Isotype-switched IgG antibodies specific for inactivated influenza (A/PR8) virus antigen after boost immunization. A/PR8 inactivated virus was used as an ELISA plate-coating antigen (400 ng/100 μl/well). (C) Serum antibody levels in mice after PR8 VLP vaccination. Groups of mice (n = 6) were intramuscularly immunized with 3 μg of influenza PR8 VLP vaccine at weeks 0 and 4. At 4 weeks after boost immunization, A/PR8 virus-specific antibody levels were measured (ng/ml). Relative antibody concentrations were determined using a standard measurement of mouse purified IgG, IgM, and each isotype specific to A/PR8 virus. Naive, unimmunized mice B6 WT mice; WT, B6 WT mice (n = 9); KO, MHC-II KO mice (n = 9); CD4KO, CD4 knockout mice. nd, not detectable or similar to naive controls; ns, not significant; **, P < 0.01; ***, P < 0.001.
To compare vaccine-specific IgG antibody levels in CD4 knockout mice (CD4KO) after PR8 VLP vaccination, we intramuscularly immunized MHC-II KO, CD4KO, and B6 WT mice (n = 6/group) with 3 μg of influenza PR8 VLP vaccine at weeks 0 and 4 (Fig. 2C). At 4 weeks after boost immunization, A/PR8 virus-specific IgG antibody levels were measured (ng/ml) (Fig. 2C). Although vaccine-specific IgG antibody levels of CD4KO were ca. 30 to 40% of those for B6 WT mice, CD4KO mice showed significantly higher IgG antibody levels compared to MHC-II KO or naive mice. These results suggest that MHC-II KO mice have additional defects in inducing vaccine-specific IgG antibody responses compared to CD4KO mice.
MHC-II KO mice induce IgM but not IgG antibody-secreting cell responses.
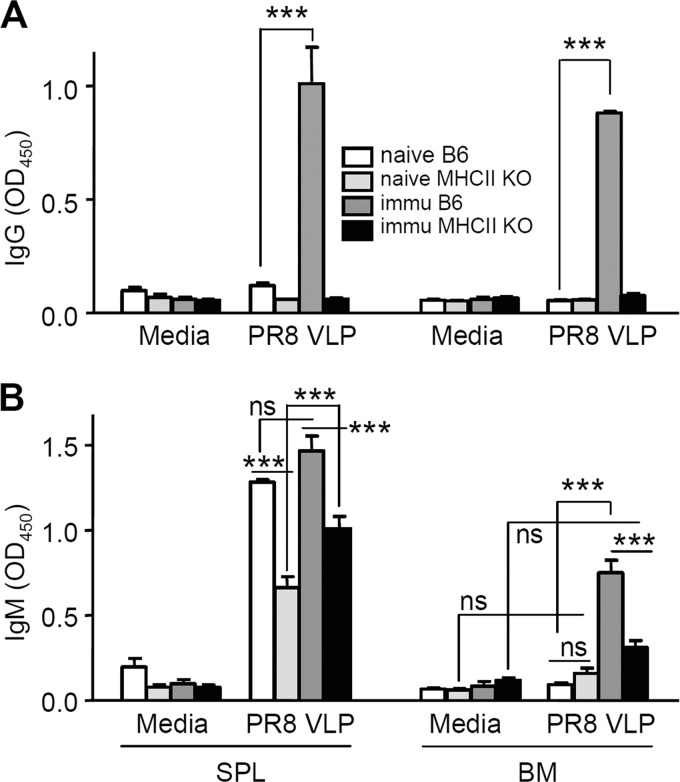
To further confirm the capacity of MHC-II KO mice to induce vaccine-specific humoral responses, we analyzed antibody-secreting cell responses that may detect small numbers of vaccine-specific B cells. Spleen and bone marrow cells were obtained from vaccinated B6 WT and MHC-II KO mice at day 4 postchallenge infection with A/PR8 virus and subjected to in vitro culture plates coated with the same VLP vaccine antigen (Fig. 3). Spleen and bone marrow cells from B6 WT mice showed a similar high level of IgG antibodies captured on vaccine antigen coated plates, but not those cells from MHC-II KO or naive mice (Fig. 3A).
FIG 3.
Immunized MHC-II KO mice do not have antigen-specific IgG antibody-secreting cell responses. In vitro production of IgG (A) and IgM (B) antibodies specific to the vaccine antigen (influenza A/PR8 VLPs). Spleen (SPL) and bone marrow (BM) cells were collected from naive B6 WT (n = 3), naive MHC-II KO (n = 3), boosted B6 WT (n = 5), and boosted MHC-II KO (n = 5) mice at day 4 after challenge with A/PR8 virus (5× LD50). Media, 5-day-old in vitro cultures under medium only; PR8 VLP, 5-day-old in vitro cultures with influenza PR8 VLP vaccines coated on the plates. SPL and BM cells were plated at concentrations of 5 × 105 cells/well. A/PR8 VLP-specific IgG and IgM antibodies in culture supernatants are represented by optical density readings at 450 nm. Each value represents the mean ± the standard error in quadruplicate. ns, not significant; ***, P < 0.001.
Spleen cells from naive, immunized B6 WT and MHC-II KO mice at day 4 postchallenge showed higher levels of IgM antibody production upon vaccine antigen stimulation in vitro compared to medium controls (P < 0.001). Lower but significant levels of IgM antibodies were detected in spleen cell cultures of both naive and immunized MHC-II KO mice (Fig. 3B). In bone marrow cells, VLP vaccine-specific IgM antibodies were detected at a higher level in immunized WT mice compared to those from both naive and immunized MHC-II KO mice (P < 0.001, Fig. 3B). Also, bone marrow cells from immunized B6 WT but not from MHC-II KO mice displayed statistically significant higher levels of IgM antibody production compared to medium controls (P < 0.001, Fig. 3B). Consistent with levels of antibodies secreted into culture supernatants, only immunized WT mice showed antigen-specific IgG antibody-secreting cell spots in spleen and bone marrow cells, and a low level of IgM antibody-secreting spleen cell spots was detected in MHC-II KO mice (data not shown). MHC-II KO mice failed to generate vaccine antigen-specific IgG antibody-secreting cell responses after vaccination despite their capability to produce IgM antibody-secreting splenic B cells in response to vaccination or in vitro stimulation with influenza VLP vaccines.
Immunized MHC-II KO mice show a defect in controlling challenge virus and inducing cytokine-secreting cellular responses.
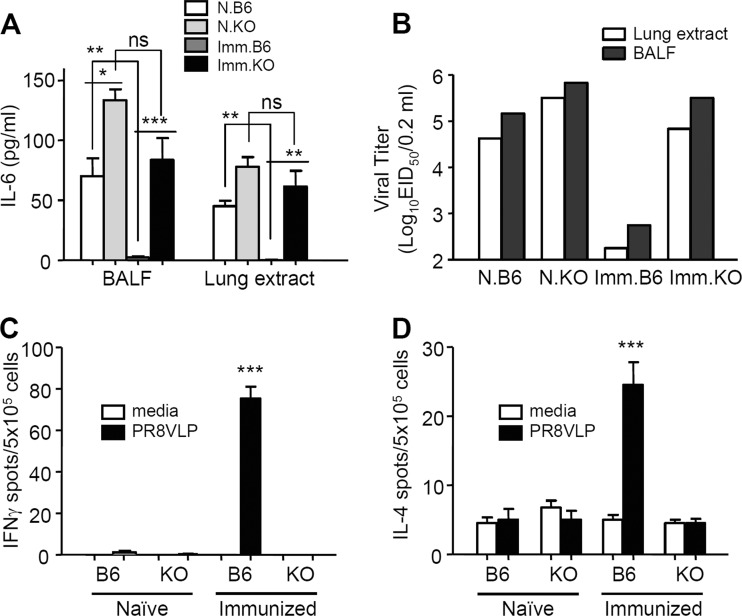
To determine the protective efficacy of vaccinated MHC-II KO mice, the levels of proinflammatory cytokine IL-6 and virus titers were determined at day 4 after A/PR8 virus challenge infection (Fig. 4A and B). The level of IL-6 was low or below the detection limit in both BALF and lung extracts from the B6 WT immunized mice. In contrast, the B6 WT naive and MHC-II KO immunized mice showed similar patterns of high levels of IL-6 in both BALF and lung extract samples (Fig. 4A and B). The levels of the virus titers indicate the efficiency of virus clearance after challenge. Influenza VLP immunized B6 WT mice showed over 100- to 1,000-fold lower levels of virus titers compared to those in immunized MHC-II KO or naive control groups. That is, high viral loads were found in lungs and BALF samples from naive B6 WT and naive and immunized MHC-II KO mice (Fig. 4B). These results indicate that vaccination of MHC-II KO mice failed to control and clear the challenge virus, whereas the vaccinated B6 WT mice were highly effective in clearing the virus after challenge.
FIG 4.
Immunized MHC-II KO mice show high levels of IL-6 cytokine and virus titers upon influenza virus challenge infection. (A) Inflammatory cytokine IL-6 (pg/ml). The levels of IL-6 cytokine and viral loads were determined at day 4 postchallenge with A/PR8 virus. BALF, bronchoalveolar lavage fluids. Each group has five animals (n = 5). (B) Virus titers (50% infectious dose in embryonated chicken eggs). Each group has five animals (n = 5). For lung virus titers, pooled BALF and lung extract samples were 10-fold serially diluted and used to infect chicken eggs. Representative results of two independent experiments are shown. N.B6, naive B6 WT mice infected with virus; N.KO, naive MHC-II KO mice infected with virus; Imm.B6, prime boost-immunized B6 WT mice that were infected with virus; Imm.KO, prime boost-immunized MHC-II KO mice that were infected with virus. (C) IFN-γ ELISPOT assay. (D) IL-4 ELISPOT assay. IFN-γ- and IL-4-secreting spleen cell spots (ELISPOT assays) were determined after stimulation of spleen cells with influenza (A/PR8) VLP. KO, MHC-II KO mice (n = 5 per group). The asterisks indicate a significant difference between immunized B6 WT mice and MHC-II KO mice or between immunized and naive mice of B6 WT and MHC-II KO, as indicated by the brackets. ns, not significant; *, P < 0.05; **, P < 0.01; ***, P < 0.001.
To observe T cell immune responses, freshly isolated spleen cells 4 days after challenge with A/PR8 virus from naive or immunized WT and MHC-II KO mice were cultured on enzyme-linked immunospot (ELISPOT) plates to determine IFN-γ- and IL-4-secreting cell spots using VLPs as an antigenic stimulator (Fig. 4C and D). Immunized WT mice displayed a significant level of IFN-γ-secreting cell spots in responses to A/PR8 influenza VLPs, but none of the other groups—including immunized MHC-II KO mice—showed IFN-γ-producing cell spots (Fig. 4C). Similarly, immunized B6 WT mice showed the highest number of IL-4-secreting cell spots, but only background levels of IL-4 spots were detected in spleen cells from immunized MHC-II KO mice (Fig. 4D). These results suggest that MHC-II KO mice have also a defect in inducing T cell immune responses.
VLP vaccination of MHC-II KO mice do not confer effective protection.
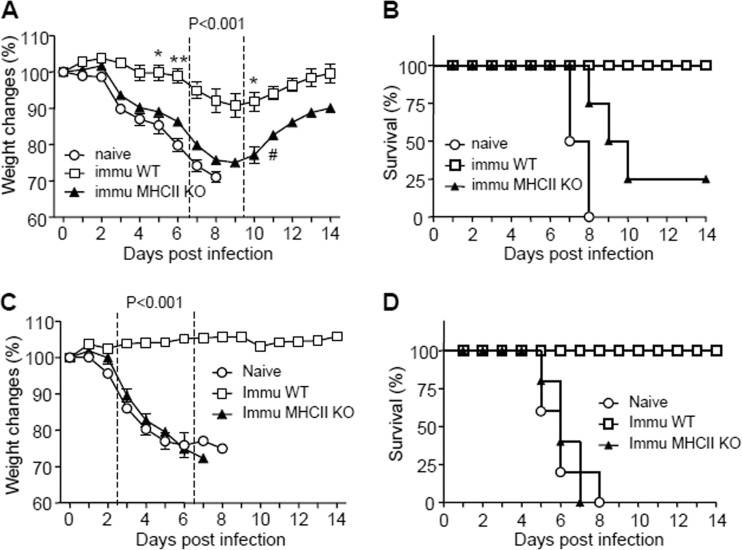
We wanted to determine whether vaccination of MHC-II KO mice would confer protection against viral infection. Naive and vaccinated WT and MHC-II KO mice were infected with a lethal infectious dose of A/PR8 virus at 3 weeks after immunization with A/PR8 VLPs. Naive mice showed severe body weight loss and had to be euthanized by day 8 postchallenge. Similarly, immunized MHC-II KO mice lost, on average, over 20% their body weight and showed partial protection, with 25% survival rates (Fig. 5A and B). All naive mice after influenza virus infection died between days 7 and 8. In contrast, immunized B6 WT mice showed a moderate level of body weight loss, with ca. 10% on average at a peak point, and then all mice fully recovered with 100% survival rates (Fig. 5A and B).
FIG 5.
Protective efficacy of immunized MHC-II KO mice and immune sera from MHC-II KO mice. (A and B) Protective efficacy of immunized MHC-II KO mice. (A) Body weight changes. (B) Survival rates. Naive B6 WT (n = 4), boost-immunized B6 wild-type (immu WT [n = 5]), and boost-immunized MHC-II KO (immu MHC-II KO [n = 4]) mice were intranasally challenged with a lethal infectious dose of A/PR/8/34 virus (5× LD50). #, One mouse survived. (C and D) Protective efficacy of immune sera from MHC-II KO mice. (C) Body weight changes. (D) Survival rates. Naive BALB/c mice (n = 5/group) were intranasally infected with a lethal dose of A/PR/8/34 virus (10× LD50) that had been preincubated with heat-inactivated sera (final 4-fold dilutions). Naive, A/PR/8/34 virus plus sera from naive B6 WT mice; Immu WT, A/PR/8/34 virus plus immune sera from prime boost-immunized B6 WT mice; Immu MHC-II KO, A/PR/8/34 virus plus immune sera from prime boost-immunized MHC-II KO mice. P values indicate significant differences between immu WT and immu MHC-II KO groups. *, P < 0.05; **, P < 0.01.
A goal of vaccination is to induce antibodies capable of conferring protection. To compare the protective efficacy of immune sera, we infected naive mice with mixtures of a lethal dose of A/PR8 virus and immune sera from MHC-II KO or B6 WT mice that had been immunized with influenza VLP vaccines. In conferring protection by immune sera, infected mice that received sera from immunized B6 WT mice did not show any body weight loss. On the other hand, mice that were administered immune sera from immunized MHC-II KO or unimmunized (naive) mice showed a substantial level of body weight loss starting at day 3 and were not protected against A/PR8 virus infection (Fig. 5C and D). Therefore, the virus-specific IgM antibodies induced by vaccinating MHC-II KO mice VLPs were not sufficient for conferring protection in the absence of protective IgG antibodies.
MHC-II KO mice immunized with other forms of viral vaccines fail to induce protection.
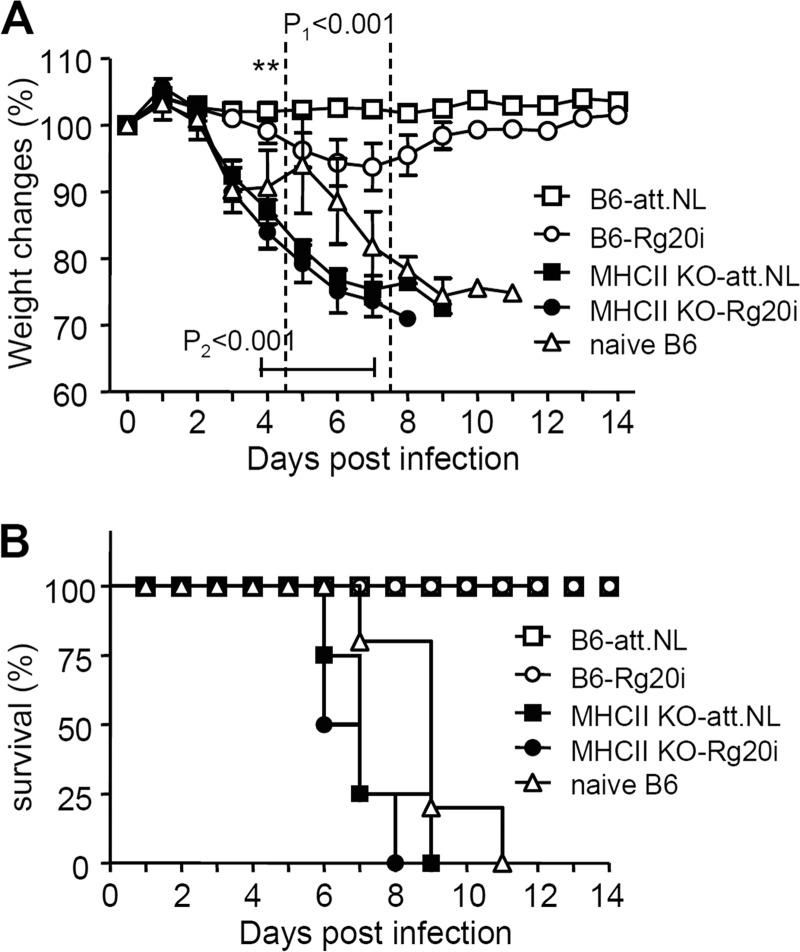
We further tested the critical roles of MHC class II in inducing protective immunity by immunizing B6 WT and MHC-II KO mice with replicating live attenuated influenza virus or inactivated whole viral vaccines. MHC-II KO and B6 WT mice were intramuscularly immunized with whole inactivated 2009 H1N1 pandemic virus (10 μg of Rg20i) or intranasally inoculated with live attenuated 2009 H1N1 virus vaccine (att.NL) (35). At 4 weeks after the boost immunization, groups of mice were challenged with a lethal dose A/California/04/2009 pandemic H1N1 virus as described previously (32). B6 WT mice that were intranasally immunized with live attenuated influenza viral vaccine prevented body weight loss and were 100% protected against 2009 H1N1 pandemic virus challenge (Fig. 6). Also, B6 WT mice that received whole inactivated viral vaccine (Rg20) were 100% protected against lethal challenge with 2009 H1N1 pandemic virus, despite showing a moderate body weight loss of ca. 5 to 10% (Fig. 6). Consistent with the results of influenza VLP vaccination, none of MHC-II KO mice that were immunized with live attenuated viral vaccine or inactivated whole viral vaccine were protected against 2009 H1N1 pandemic virus challenge (Fig. 6). These results further support evidence that MHC-II molecule is critically important for inducing protective immunity after vaccination.
FIG 6.
MHC-II KO mice immunized with inactivated or live attenuated influenza vaccines are not protected against lethal infection. (A) Body weight changes. (B) Survival rates. B6 WT and MHC-II KO mice that were either intranasally two times inoculated with live attenuated att.NL virus vaccine or intramuscularly boost immunized with inactivated Rg20 (Rg20i, 5 μg/mouse) were challenged with a lethal dose (5× LD50) of 2009 H1N1 virus (A/California/04/2009). B6-att.NL, B6 WT mice (n = 5) intranasally inoculated with live attenuated virus (att.NL); B6-Rg20i, B6 WT mice (n = 5) intramuscularly immunized with whole inactivated 2009 H1N1 virus (Rg20i); MHCII KO-att.NL, MHC-II KO mice (n = 4) intranasally inoculated with live attenuated virus (att.NL); MHCII KO-Rg20i, MHC-II KO mice (n = 5) intramuscularly immunized with whole inactivated 2009 H1N1 virus (Rg20i). P1 is between B6 and MHC-II KO-att.NL; P2 is between B6 and MHC-II KO-Rg20i. **, P < 0.01.
CD43+ cell populations contribute to antigen-specific antibody production and confer protection.
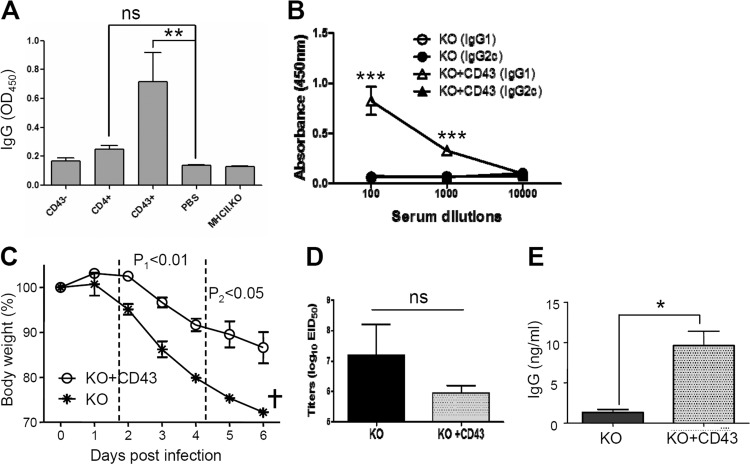
To identify phenotypes of cells contributing to the defect observed in MHC-II KO mice, we carried out an adoptive-transfer study of CD43− cells (conventional B220+ B cells) as previously described (40), CD43+ cell populations, and CD4+ T cells. The purity of separated cell fractions derived from naive B6 WT mice was 97% for CD4+ T cells, and more than 90% each for CD43− (81% B220+ phenotype cells) and CD43+ cells (93% B220− phenotype) (Fig. 7A). MHC-II KO mice received adoptive transfer of 1.5 × 107 enriched CD4+ T cells, 2.2 × 107 enriched CD43+ cells, or 3.3 × 107 enriched CD43− cells derived from wild-type mice (Fig. 7A). The groups of MHC-II KO mice that received adoptive transfer with a fraction of CD4+, CD43+, or CD43− cells from B6 WT mice were subsequently vaccinated with influenza VLPs. Vaccine antigen-specific IgG antibodies in sera were determined 14 days after adoptive transfer (Fig. 7A). The transfer of wild-type CD43− cells or PBS to MHC-II KO mice did not induce detectable levels of antigen-specific IgG antibodies similar to those in sera from immunized MHC-II KO mice (MHC-II KO, Fig. 7A). In contrast, MHC-II KO mice that received an adoptive transfer of wild-type CD43+ cells produced the largest amount of antigen-specific IgG antibodies (P < 0.01 compared to PBS or MHC-II KO, Fig. 7A). The group of MHC-II KO mice with a transfer of CD4+ cells induced a marginal level of antigen-specific IgG antibodies, which is not statistically significant. We determined IgG isotypes in immune sera of MHC-II KO mice that received CD43+ fractionated cells and showed the highest IgG antibody levels. IgG1 isotype antibody was dominantly detected (Fig. 7B). Interestingly, this is different from immunized B6 WT mice that induced predominantly IgG2c isotype antibody. Overall, these results indicate that CD43+ phenotypic cell populations mainly contribute to the defect of lacking antigen-specific IgG antibody production, together with a marginal role for CD4+ T cells that are also deficient in MHC-II KO mice.
FIG 7.
Adoptive transfer of B6 WT fractionated CD43+ cells to MHC-II KO mice confers protective immunity. (A) Vaccine-specific IgG antibody responses in MHC-II KO mice with adoptive transfer. Naive MHC-II KO mice (n = 4 for each group) received CD43−, CD4+, and CD43+ cell populations fractionated from spleens of naive B6 WT mice. CD43−, 3.3 × 107 enriched CD43− cells per MHC-II KO mouse; CD4+, 1.5 × 107 enriched CD4+ T cells per MHC-II KO mouse; CD43+, 2.2 × 107 enriched CD43+ cells per MHC-II KO mouse; PBS, no B6 WT cells (a negative control); MHCII.KO, a serum ELISA control from MHC-II KO mice immunized with influenza VLP vaccine. (B) IgG isotypes (IgG1 and IgG2c) in immune sera of MHC-II KO mice with adoptive transfer of naive wild-type CD43+ cells after influenza VLP immunization. MHC-II KO mice with or without CD43+ adoptive transfer were intramuscularly immunized with influenza A/PR8 VLPs. KO, MHC-II KO mice without adoptive transfer prior to immunization; KO+CD43, MHC-II KO mice with CD43+ cell adoptive transfer prior to immunization. (C to E) Protective roles of wild-type CD43+ cells in MHC-II KO mice. MHC-II KO mice with (KO+CD43) or without (KO) CD43+ adoptive transfer were intramuscularly immunized with influenza A/PR8 VLPs and then challenged with influenza virus 2 weeks later. (C) Body weight changes. (D) Lung viral loads. (E) Virus-specific IgG antibodies in BALF. ns, not significant; †, all mice were euthanized. Statistical significances were compared between KO and KO+CD43: *, P < 0.05; **, P < 0.01; ***, P < 0.0001.
To better understand the possible roles of WT CD43+ cells in conferring protection against influenza virus challenge infection, MHC-II KO mice with or without adoptive transfer of naive WT CD43+ cells were challenged with a lethal dose of influenza virus (Fig. 7C). Influenza VLP-immunized MHC-II KO mice without adoptive transfer showed severe weight loss, were all below the endpoint (25% weight loss), and had to be sacrificed (Fig. 7C). Antibodies induced by adoptive transfer of CD43+ cells into MHC-II KO mice were found to be protective, as evidenced by significantly lower levels (ca. 12%) of body weight losses (Fig. 7C). Also, the lung virus titers were ∼15-fold lower in the CD43+ group than in the PBS control of MHC-II KO mice, although there was no significant difference due to high variations in the control group (Fig. 7D). Higher levels of virus-specific IgG antibodies in BALF upon challenge infection were induced in the CD43+ group than in the PBS control of MHC-II KO mice (Fig. 7E). These results indicate that CD43+ cell populations with MHC-II molecules contribute to antigen-specific antibody production and confer effective protection against influenza virus infection in MHC-II KO mice.
MHC-II-deficient DCs have a defect in secreting inflammatory cytokines upon stimulation.
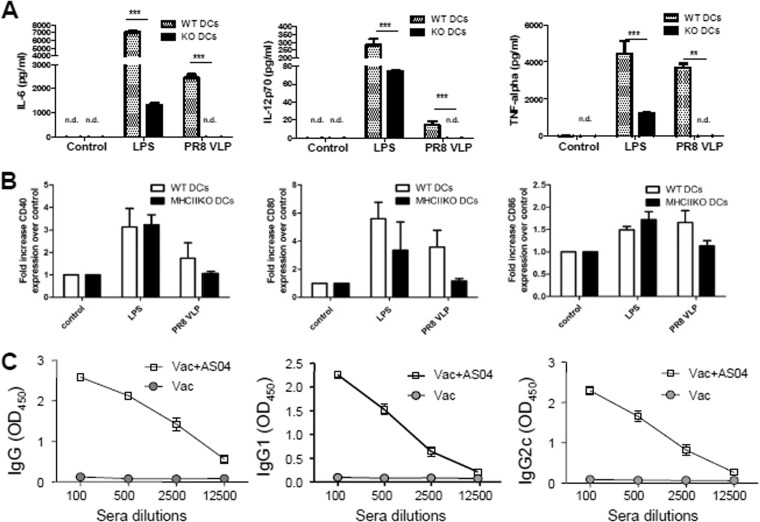
Since DCs play an important role in activating T and B cells, cytokine secretion and activation marker expression of MHC-II-deficient DCs from MHC-II KO mice were compared to those of wild-type DCs (Fig. 8A and B). DCs from MHC-II KO mice showed a significant defect in secreting inflammatory cytokines (IL-6, IL-12, and TNF-α) in respond to stimulation with influenza VLPs (A/PR8 VLP) or LPS (Fig. 8A). Also, the activation markers (CD40, CD80, and CD86) on MHC-II-deficient DCs displayed a trend of lower expression compared to wild-type DCs upon influenza VLP stimulation, as determined from the mean fluorescence intensity. However, there was no statistical significance in expressing activation markers between WT and MHC-II-deficient DCs (Fig. 8B). Here, the results suggest that DCs from MHC-II KO mice have a significant defect in producing inflammatory cytokines upon vaccine antigen stimulation.
FIG 8.
Defective DCs from MHC-II KO mice and the effects of AS04 adjuvant on inducing IgG isotype-switched antibodies in MHC-II KO mice. (A) Cytokines produced by BM-derived DCs. (B) Activation marker expression levels of BMDCs. In vitro enriched BMDCs were treated with influenza virus PR8 VLP vaccines (10 μg/ml). WT DCs, B6 WT mouse BMDCs; KO DCs or MHC-II KO DCs, MHC-II KO mouse BMDCs. LPS was used as a positive control. Mean values of each triplicate are shown out of two independent experiments. nd, not detectable or similar to medium controls; **, P < 0.01; ***, P < 0.001 compared to WT DCs. Fold increase in activation markers is determined by mean fluorescence intensity. (C) IgG and isotype antibodies specific for vaccine strain (A/PR8 virus) were measured in immune sera from MHC-II KO mice immunized with vaccine only (Vac) or AS04-adjuvanted vaccines (Vac+AS04; A/PR8 VLP [10 μg] with alum [100 μg] plus monophosphoryl lipid A [10 μg per mouse]).
It is possible that DCs without MHC-II molecules may need strong stimulatory signals such as that of AS04 adjuvant containing the Toll-like receptor-4 (TLR4) ligand of MPL (monophosphoryl lipid A) component. We tested whether AS04 adjuvanted-influenza VLP vaccine could overcome a defect in MHC-II KO mice in generating vaccine antigen-specific IgG isotype-switched antibodies (Fig. 8C). MHC-II KO mice that were intramuscularly immunized with AS04 adjuvanted-influenza A/PR8 VLP vaccine were found to induce both IgG1 and IgG2c isotype antibodies specific for vaccine strain (A/PR8 virus) at high levels (Fig. 8C). Therefore, the addition of TLR4 stimulating adjuvant (AS04) might play a role in overcoming a defect in MHC-II deficiency for inducing IgG isotype-switched antibodies.
DISCUSSION
This study demonstrates critical roles of MHC-II molecules in inducing protective immune responses to vaccination using an MHC-II KO mouse model. The MHC-II KO mice that were immunized with influenza VLPs, inactivated whole virus, or live attenuated virus vaccine did not induce IgG antibodies and were not protected against lethal challenge. In naive MHC-II KO mice, the CD8+ T cell and CD19+ and B220+ B cell populations were comparable or higher, but the CD4+ T cell and MHC-II+ cell populations were significantly lower, indicating the authentic deficiency of MHC-II molecule (13). Interestingly, the naive MHC-II KO mice were found to maintain substantial amounts of antigen-nonspecific natural IgG and isotype antibodies, which is consistent with a pattern reported in a previous study (41). In particular, antigen-nonspecific IgG2c antibodies in MHC-II KO mice were comparable to those in WT mice, indicating that B cells in MHC-II KO mice have the intrinsic capacity to produce isotype-switched IgG antibodies. The results from the present study provide evidence that the lack of MHC-II molecules, but not the CD4 deficiency, causes a significant defect in inducing vaccine antigen-specific IgG antibody responses after vaccination, which could be overcome by TLR4-activating adjuvant. In addition, CD43+ cell populations with MHC-II molecules could play a critical role in producing vaccine-specific IgG antibodies and conferring protection to MHC-II KO mice.
The roles of MHC-II molecule have been investigated in the context of infection models, relating to the study of CD4+ T cells for controlling pathogens. MHC-II molecules were required for the development, maturation, and function of CD4+ T cells (13). Previous studies demonstrated that MHC-II KO mice were not effective in controlling infection with the parasites Schistosoma mansoni (16), Leishmania major (42), Mycobacterium bovis bacilli Calmette-Guerin (18), Strongyloides venezuelensis (43), and Theiler's virus (20). Meanwhile, other studies reported few or no obvious defects on virus clearance of Sendai virus or influenza virus in mice lacking the MHC-II molecule, after sublethal infection, indicating that virus-specific CD8+ cytotoxic T lymphocyte precursors are intact in MHC-II KO mice (14, 19). It is also known that CD40 or CD4+ T cell-deficient mice produce detectable titers of influenza virus-specific IgG antibodies and recover from influenza virus infection in a manner similar to that of normal mice (44). Therefore, MHC-II and CD4 (or CD40) have been considered to have a similar role of providing CD4 T cell help in controlling infection. This might be due to the fact that some viral infections in naive hosts at a sublethal dose are controlled before developing the full scale of humoral adoptive immunity. In the present study, we found that MHC-II molecules play a critical role in inducing vaccine-mediated protective immune responses, including virus antigen-specific IgG antibodies, antibody-secreting cells, and IFN-γ- and IL-4-secreting T cell responses.
The traditional concept is that the main role of MHC-II molecules is to initiate the maturation and activation of CD4+ helper T cells, which is now being challenged by new findings. Previous studies demonstrated the production of CD4+ T cell-independent antigen-specific IgG antibodies in response to infection or vaccination. Infection of CD4+ T cell-deficient mice with vesicular stomatitis virus (VSV) or recombinant vaccinia virus expressing the VSV glycoprotein induced IgG responses (23–26). CD4+ T cell knockout mice were able to induce isotype-switched IgG antibodies after systemic or mucosal immunization with inactivated influenza virus (27, 28) or VLP vaccines (45). Hou et al. reported a defect in inducing antigen-specific IgG antibody-forming cell responses in MHC-II KO mice after infection with Sendai virus, due to the absence of CD4+ T cell help (19). In the present study, MHC-II KO mice can induce antigen-specific IgM antibody-secreting cells in spleens after influenza VLP vaccination but not antigen-specific IgG antibodies. Virus antigen-specific IgM antibodies in MHC-II KO mice were not sufficient for conferring protection against lethal challenge infection. Inability of MHC-II KO mice to induce antigen-specific IgG antibodies was similarly observed after vaccination with whole inactivated or live attenuated viral vaccines (data not shown). Developing protective immunity to vaccination may be different from CD8+ T cell-dependent virus clearance in MHC-II KO mice after sublethal infection (14, 19). Immunization of CD4+ T cell knockout mice with inactivated whole virus was shown to induce protective immunity against lethal infection of influenza A/PR8 virus (27, 28). We also found that immunization of CD4+ T knockout mice with influenza A/PR8 VLP vaccines induced virus-specific IgG antibodies at substantial levels compared to those in wild-type mice (Fig. 2C). Therefore, it is speculated that an MHC-II molecule may have other additional roles that are not well defined in inducing antigen-specific IgG antibodies, which is different from its role in activating CD4+ T helper cells after vaccination or infection.
In an attempt to better understand the defective mechanism in MHC-II KO mice incapable of inducing antigen-specific IgG antibody responses, an adoptive-transfer experiment of wild-type cell fractions with MHC-II molecules was performed (Fig. 7). The transfer of CD43− fraction (conventional B220+ B cells) did not produce detectable levels of antigen-specific IgG antibodies in MHC-II KO mice after vaccination. Provision of wild-type CD4+ T cells to MHC-II KO mice produced antigen-specific IgG only at a marginal level. Unexpectedly, the adoptive transfer of CD43+ populations led to inducing significant levels of antigen-specific IgG and IgG1 isotype antibodies, as well as protective immunity to lethal challenge infection in MHC-II KO mice after influenza VLP vaccination. Nonetheless, after influenza VLP vaccination, IgG2b and IgG2c isotype antibodies were not detected at significant levels in MHC-II KO mice with CD43+ cell adoptive transfer from naive B6 WT mice (data not shown). In B6 WT mice, IgG2b and IgG2c antibodies were induced dominantly after influenza VLP vaccination. Thus, naive CD43+ B220− cells (from naive wild-type mice) alone would have limited capacity to fully restore other IgG isotypes in a deficiency of CD4 T cells in MHC-II KO mice. Importantly, addition of AS04 adjuvant to influenza VLP vaccines could overcome the MHC-II defects in inducing isotype-switched IgG antibodies in MHC-II KO mice after influenza vaccination. This is highly significant in understanding possible action mechanisms of adjuvant and vaccines.
CD43 was shown to be expressed on plasma cells and nonconventional splenic B-1 cells, as well as some granulocytes, including monocytes, macrophages, and DCs (46, 47), but not on conventional mature B (B-2) cells (48). B220+ B cells in the CD43− spleen cell fraction from wild-type mice were found to be 81%, whereas the majority (93%) of the CD43+ fraction was B220− cells (data not shown). In wild-type adult spleens, conventional B cells with CD43− (B-2) are predominantly resting follicular B cells and marginal and immature B cells. CD43− fractions were used to determine the roles of B cells by adoptive transfer, together with cognate CD4+ T cells (40). Thus, the roles of combined conventional B cells and CD4+ T cells remain to be determined in the context of MHC-II KO mice. It might be possible that CD43+ B220− phenotypic populations are plasmablasts and/or plasma-like cells contributing to the production of antigen-specific IgG antibodies without CD4+ T cell help despite their small populations. DCs were reported to retain antigens in a form that is recognized by B cells and enhance the differentiation of naive B cells into plasma cells (10, 11, 49). Alternatively, a small fraction of CD43+ CD11c+ and CD43+ CD11b+ cells present in the CD43+ fraction from wild-type spleen cells might have contributed to IgG-producing B cell responses without CD4+ T cell help in adopted MHC-II KO mice. In support of this idea, CD11c+ and CD11b+ cells were found to be ca. 12 and 14%, respectively, in the CD43+ fraction (data not shown). Also, we found that MHC-II-deficient BMDCs showed a significant defect in secreting inflammatory cytokines in response to stimulation with a VLP vaccine antigen, indicating that MHC-II deficiency might have resulted in a microenvironment not sufficient to effectively initiate antigen-specific IgG antibody immune responses. MHC-II KO mice would not have an intrinsic defect in producing inflammatory cytokines since higher levels of IL-6 were detected in naive and immunized MHC-II KO mice after lethal influenza virus infection due to high viral replication. It is possible that DCs (and/or B cells) without MHC-II molecules may need strong stimulatory signals, such as TLR4-activating AS04 adjuvant, compared to wild-type antigen-presenting cells. This hypothesis is supported by the induction of vaccine-specific IgG isotype-switched antibodies in MHC-II KO mice that were immunized with AS04-adjuvanted influenza VLP vaccination. Taken together, the results presented here show that MHC-II molecules have additional roles other than simply stimulating the development and activation of CD4+ T cells.
In summary, we found that MHC-II was a critical molecule in inducing antigen-specific IgG antibody responses and protective immunity against lethal challenge infection. CD43+ (B220−/low) populations with MHC-II were found to play an essential role in inducing IgG isotype-switched antibody responses to vaccination and conferring protection in MHC-II KO mice. Antigen-presenting cells, such as DCs from MHC-II KO mice, were defective in producing inflammatory cytokines. The addition of innate immunity-stimulating adjuvants could overcome the defect of MHC-II KO mice in inducing IgG isotype-switched antibody responses to vaccination. Further studies are needed to further define these mechanisms by which the CD43+ populations, MHC-II molecules, and adjuvants contribute to inducing antigen-specific IgG antibodies and protective immunity after vaccination.
ACKNOWLEDGMENTS
This study was in part supported by NIH/NIAID grants AI105170 (S.-M.K.), AI093772 (S.-M.K.), and P30AI050409.
We thank T. Kang for careful reading of the manuscript.
Footnotes
Published ahead of print 23 April 2014
REFERENCES
- 1.Plotkin SA. 2005. Vaccines: past, present and future. Nat. Med. 11:S5–S11. 10.1038/nm1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Plotkin SA. 2010. Correlates of protection induced by vaccination. Clin. Vaccine Immunol. 17:1055–1065. 10.1128/CVI.00131-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Plotkin SA. 2008. Vaccines: correlates of vaccine-induced immunity. Clin. Infect. Dis. 47:401–409. 10.1086/589862 [DOI] [PubMed] [Google Scholar]
- 4.Banchereau J, Steinman RM. 1998. Dendritic cells and the control of immunity. Nature 392:245–252. 10.1038/32588 [DOI] [PubMed] [Google Scholar]
- 5.Bishop GA, Hostager BS. 2001. B lymphocyte activation by contact-mediated interactions with T lymphocytes. Curr. Opin. Immunol. 13:278–285. 10.1016/S0952-7915(00)00216-8 [DOI] [PubMed] [Google Scholar]
- 6.Clark EA, Ledbetter JA. 1994. How B and T cells talk to each other. Nature 367:425–428. 10.1038/367425a0 [DOI] [PubMed] [Google Scholar]
- 7.Stavnezer J. 2000. Immunology: a touch of antibody class. Science 288:984–985 [DOI] [PubMed] [Google Scholar]
- 8.Marshall D, Sealy R, Sangster M, Coleclough C. 1999. TH cells primed during influenza virus infection provide help for qualitatively distinct antibody responses to subsequent immunization. J. Immunol. 163:4673–4682 [PubMed] [Google Scholar]
- 9.Snapper CM, Mond JJ. 1993. Towards a comprehensive view of immunoglobulin class switching. Immunol. Today 14:15–17. 10.1016/0167-5699(93)90318-F [DOI] [PubMed] [Google Scholar]
- 10.Wykes M, Pombo A, Jenkins C, MacPherson GG. 1998. Dendritic cells interact directly with naive B lymphocytes to transfer antigen and initiate class switching in a primary T-dependent response. J. Immunol. 161:1313–1319 [PubMed] [Google Scholar]
- 11.Fayette J, Dubois B, Vandenabeele S, Bridon JM, Vanbervliet B, Durand I, Banchereau J, Caux C, Briere F. 1997. Human dendritic cells skew isotype switching of CD40-activated naive B cells toward IgA1 and IgA2. J. Exp. Med. 185:1909–1918. 10.1084/jem.185.11.1909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dubois B, Vanbervliet B, Fayette J, Massacrier C, Van Kooten C, Briere F, Banchereau J, Caux C. 1997. Dendritic cells enhance growth and differentiation of CD40-activated B lymphocytes. J. Exp. Med. 185:941–951. 10.1084/jem.185.5.941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grusby MJ, Johnson RS, Papaioannou VE, Glimcher LH. 1991. Depletion of CD4+ T cells in major histocompatibility complex class II-deficient mice. Science 253:1417–1420. 10.1126/science.1910207 [DOI] [PubMed] [Google Scholar]
- 14.Tripp RA, Sarawar SR, Doherty PC. 1995. Characteristics of the influenza virus-specific CD8+ T cell response in mice homozygous for disruption of the H-2lAb gene. J. Immunol. 155:2955–2959 [PubMed] [Google Scholar]
- 15.Rahemtulla A, Fung-Leung WP, Schilham MW, Kundig TM, Sambhara SR, Narendran A, Arabian A, Wakeham A, Paige CJ, Zinkernagel RM. 1991. Normal development and function of CD8+ cells but markedly decreased helper cell activity in mice lacking CD4. Nature 353:180–184. 10.1038/353180a0 [DOI] [PubMed] [Google Scholar]
- 16.Angyalosi G, Pancre V, Herno J, Auriault C. 1998. Immunological response of major histocompatibility complex class II-deficient (Aβ°) mice infected by the parasite Schistosoma mansoni. Scand. J. Immunol. 48:159–169. 10.1046/j.1365-3083.1998.00372.x [DOI] [PubMed] [Google Scholar]
- 17.Oliveira SC, Splitter GA. 1995. CD8+ type 1 CD44hi CD45 RBlo T lymphocytes control intracellular Brucella abortus infection as demonstrated in major histocompatibility complex class I- and class II-deficient mice. Eur. J. Immunol. 25:2551–2557. 10.1002/eji.1830250922 [DOI] [PubMed] [Google Scholar]
- 18.Ladel CH, Daugelat S, Kaufmann SH. 1995. Immune response to Mycobacterium bovis bacille Calmette-Guerin infection in major histocompatibility complex class I- and II-deficient knockout mice: contribution of CD4 and CD8 T cells to acquired resistance. Eur. J. Immunol. 25:377–384. 10.1002/eji.1830250211 [DOI] [PubMed] [Google Scholar]
- 19.Hou S, Mo XY, Hyland L, Doherty PC. 1995. Host response to Sendai virus in mice lacking class II major histocompatibility complex glycoproteins. J. Virol. 69:1429–1434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Njenga MK, Pavelko KD, Baisch J, Lin X, David C, Leibowitz J, Rodriguez M. 1996. Theiler's virus persistence and demyelination in major histocompatibility complex class II-deficient mice. J. Virol. 70:1729–1737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Szomolanyi-Tsuda E, Le QP, Garcea RL, Welsh RM. 1998. T-Cell-independent immunoglobulin G responses in vivo are elicited by live-virus infection but not by immunization with viral proteins or virus-like particles. J. Virol. 72:6665–6670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Szomolanyi-Tsuda E, Welsh RM. 1996. T cell-independent antibody-mediated clearance of polyoma virus in T cell-deficient mice. J. Exp. Med. 183:403–411. 10.1084/jem.183.2.403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bachmann MF, Kundig TM, Kalberer CP, Hengartner H, Zinkernagel RM. 1993. Formalin inactivation of vesicular stomatitis virus impairs T-cell- but not T-help-independent B-cell responses. J. Virol. 67:3917–3922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bachmann MF, Bast C, Hengartner H, Zinkernagel RM. 1994. Immunogenicity of a viral model vaccine after different inactivation procedures. Med. Microbiol. Immunol. 183:95–104. 10.1007/BF00277160 [DOI] [PubMed] [Google Scholar]
- 25.Bachmann MF, Hengartner H, Zinkernagel RM. 1995. T helper cell-independent neutralizing B cell response against vesicular stomatitis virus: role of antigen patterns in B cell induction? Eur. J. Immunol. 25:3445–3451. 10.1002/eji.1830251236 [DOI] [PubMed] [Google Scholar]
- 26.Maloy KJ, Odermatt B, Hengartner H, Zinkernagel RM. 1998. Interferon gamma-producing γδ T cell-dependent antibody isotype switching in the absence of germinal center formation during virus infection. Proc. Natl. Acad. Sci. U. S. A. 95:1160–1165. 10.1073/pnas.95.3.1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sha Z, Kang SM, Compans RW. 2005. Mucosal immunization of CD4(+) T cell-deficient mice with an inactivated virus induces IgG and IgA responses in serum and mucosal secretions. Virology 331:387–395. 10.1016/j.virol.2004.10.014 [DOI] [PubMed] [Google Scholar]
- 28.Sha Z, Compans RW. 2000. Induction of CD4+ T-cell-independent immunoglobulin responses by inactivated influenza virus. J. Virol. 74:4999–5005. 10.1128/JVI.74.11.4999-5005.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quan FS, Huang C, Compans RW, Kang SM. 2007. Virus-like particle vaccine induces protective immunity against homologous and heterologous strains of influenza virus. J. Virol. 81:3514–3524. 10.1128/JVI.02052-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quan FS, Kim YC, Vunnava A, Yoo DG, Song JM, Prausnitz MR, Compans RW, Kang SM. 2010. Intradermal vaccination with influenza virus-like particles by using microneedles induces protection superior to that with intramuscular immunization. J. Virol. 84:7760–7769. 10.1128/JVI.01849-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quan FS, Sailaja G, Skountzou I, Huang C, Vzorov A, Compans RW, Kang SM. 2007. Immunogenicity of virus-like particles containing modified human immunodeficiency virus envelope proteins. Vaccine 25:3841–3850. 10.1016/j.vaccine.2007.01.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quan FS, Vunnava A, Compans RW, Kang SM. 2010. Virus-like particle vaccine protects against 2009 H1N1 pandemic influenza virus in mice. PLoS One 5:e9161. 10.1371/journal.pone.0009161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Quan FS, Yoo DG, Song JM, Clements JD, Compans RW, Kang SM. 2009. Kinetics of immune responses to influenza virus-like particles and dose-dependence of protection with a single vaccination. J. Virol. 83:4489–4497. 10.1128/JVI.02035-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quan FS, Steinhauer D, Huang C, Ross TM, Compans RW, Kang SM. 2008. A bivalent influenza VLP vaccine confers complete inhibition of virus replication in lungs. Vaccine 26:3352–3361. 10.1016/j.vaccine.2008.03.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pena L, Vincent AL, Ye J, Ciacci-Zanella JR, Angel M, Lorusso A, Gauger PC, Janke BH, Loving CL, Perez DR. 2011. Modifications in the polymerase genes of a swine-like triple-reassortant influenza virus to generate live attenuated vaccines against 2009 pandemic H1N1 viruses. J. Virol. 85:456–469. 10.1128/JVI.01503-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Song JM, Van Rooijen N, Bozja J, Compans RW, Kang SM. 2011. Vaccination inducing broad and improved cross protection against multiple subtypes of influenza A virus. Proc. Natl. Acad. Sci. U. S. A. 108:757–761. 10.1073/pnas.1012199108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Song JM, Wang BZ, Park KM, Van Rooijen N, Quan FS, Kim MC, Jin HT, Pekosz A, Compans RW, Kang SM. 2011. Influenza virus-like particles containing M2 induce broadly cross protective immunity. PLoS One 6:e14538. 10.1371/journal.pone.0014538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Song JM, Hossain J, Yoo DG, Lipatov AS, Davis CT, Quan FS, Chen LM, Hogan RJ, Donis RO, Compans RW, Kang SM. 2010. Protective immunity against H5N1 influenza virus by a single dose vaccination with virus-like particles. Virology 405:165–175. 10.1016/j.virol.2010.05.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gray D, Dullforce P, Jainandunsing S. 1994. Memory B cell development but not germinal center formation is impaired by in vivo blockade of CD40-CD40 ligand interaction. J. Exp. Med. 180:141–155. 10.1084/jem.180.1.141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jegerlehner A, Maurer P, Bessa J, Hinton HJ, Kopf M, Bachmann MF. 2007. TLR9 signaling in B cells determines class switch recombination to IgG2a. J. Immunol. 178:2415–2420. 10.4049/jimmunol.178.4.2415 [DOI] [PubMed] [Google Scholar]
- 41.Madsen L, Labrecque N, Engberg J, Dierich A, Svejgaard A, Benoist C, Mathis D, Fugger L. 1999. Mice lacking all conventional MHC class II genes. Proc. Natl. Acad. Sci. U. S. A. 96:10338–10343. 10.1073/pnas.96.18.10338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Erb K, Blank C, Ritter U, Bluethmann H, Moll H. 1996. Leishmania major infection in major histocompatibility complex class II-deficient mice: CD8+ T cells do not mediate a protective immune response. Immunobiology 195:243–260. 10.1016/S0171-2985(96)80043-X [DOI] [PubMed] [Google Scholar]
- 43.Rodrigues RM, Silva NM, Goncalves AL, Cardoso CR, Alves R, Goncalves FA, Beletti ME, Ueta MT, Silva JS, Costa-Cruz JM. 2009. Major histocompatibility complex (MHC) class II but not MHC class I molecules are required for efficient control of Strongyloides venezuelensis infection in mice. Immunology 128:e432–e441. 10.1111/j.1365-2567.2008.02995.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee BO, Rangel-Moreno J, Moyron-Quiroz JE, Hartson L, Makris M, Sprague F, Lund FE, Randall TD. 2005. CD4 T cell-independent antibody response promotes resolution of primary influenza infection and helps to prevent reinfection. J. Immunol. 175:5827–5838. 10.4049/jimmunol.175.9.5827 [DOI] [PubMed] [Google Scholar]
- 45.Yao Q, Zhang R, Guo L, Li M, Chen C. 2004. Th cell-independent immune responses to chimeric hemagglutinin/simian human immunodeficiency virus-like particles vaccine. J. Immunol. 173:1951–1958. 10.4049/jimmunol.173.3.1951 [DOI] [PubMed] [Google Scholar]
- 46.Rosenstein Y, Santana A, Pedraza-Alva G. 1999. CD43, a molecule with multiple functions. Immunol. Res. 20:89–99. 10.1007/BF02786465 [DOI] [PubMed] [Google Scholar]
- 47.Yedidia Y, Ben-Neriah Y, Jung S. 1998. Primary B cells essentially lack constitutive NF-κB activity. Eur. J. Immunol. 28:30–36 [DOI] [PubMed] [Google Scholar]
- 48.Wells SM, Kantor AB, Stall AM. 1994. CD43 (S7) expression identifies peripheral B cell subsets. J. Immunol. 153:5503–5515 [PubMed] [Google Scholar]
- 49.Fayette J, Durand I, Bridon JM, Arpin C, Dubois B, Caux C, Liu YJ, Banchereau J, Briere F. 1998. Dendritic cells enhance the differentiation of naive B cells into plasma cells in vitro. Scand. J. Immunol. 48:563–570. 10.1046/j.1365-3083.1998.00471.x [DOI] [PubMed] [Google Scholar]