ABSTRACT
The results of a clinical trial of a subunit vaccine against genital herpes were recently reported (R. B. Belshe, P. A. Leone, D. I. Bernstein, A. Wald, M. J. Levin, J. T. Stapleton, I. Gorfinkel, R. L. Morrow, M. G. Ewell, A. Stokes-Riner, G. Dubin, T. C. Heineman, J. M. Schulte, C. D. Deal, N. Engl. J. Med. 366:34–43, 2012, doi:10.1056/NEJMoa1103151). The vaccine consisted of a soluble form of herpes simplex virus 2 (HSV-2) glycoprotein D (gD2) with adjuvant. The goal of the current study was to examine the composition of the humoral response to gD2 within a selected subset of vaccinated individuals. Serum samples from 30 vaccine recipients were selected based upon relative enzyme-linked immunosorbent assay (ELISA) titers against gD2; 10 samples had high titers, 10 had medium titers, and the remaining 10 had low ELISA titers. We employed a novel, biosensor-based monoclonal antibody (MAb)-blocking assay to determine whether gD2 vaccination elicited IgG responses against epitopes overlapping those of well-characterized MAbs. Importantly, IgGs from the majority of gD2-immunized subjects competed for gD binding with four antigenically distinct virus-neutralizing MAbs (MC2, MC5, MC23, and DL11). Screening of patient IgGs against overlapping peptides spanning the gD2 ectodomain revealed that about half of the samples contained antibodies against linear epitopes within the N and C termini of gD2. We found that the virus-neutralizing abilities of the 10 most potent samples correlated with overall gD-binding activity and to an even greater extent with the combined content of IgGs against the epitopes of MAbs MC2, MC5, MC23, and DL11. This suggests that optimal virus-neutralizing activity is achieved by strong and balanced responses to the four major discontinuous neutralizing epitopes of gD2.
IMPORTANCE Several herpes simplex virus 2 (HSV-2) subunit vaccine studies have been conducted in human subjects using a recombinant form of HSV-2 glycoprotein D (gD2). Although several distinct, well-characterized virus-neutralizing epitopes on gD2 are targeted by murine monoclonal antibodies, it is not known whether the same epitopes are targeted by the humoral response to gD2 in humans. We have developed a novel, biosensor-based competition assay to directly address this important question. Using this approach, we identified epitopes that elicit strong humoral responses in humans, as well as other epitopes that elicit much weaker responses. These data provide new insight into the human response to known neutralizing gD2 epitopes and reveal characteristics of this response that may guide future vaccine development.
INTRODUCTION
Genital herpes, caused by herpes simplex virus 1 (HSV-1) and HSV-2, is the second most common sexually transmitted infection in the United States (after human papillomavirus [HPV]), with HSV-2 affecting 16.5% of the population between the ages of 15 and 49 years (1). Within this age group, women are almost twice as likely to be affected as men (21.7% versus 11.3% seropositive, respectively) (1). Importantly, recent data suggest that in North America and Europe, HSV-1 is becoming an increasingly common cause of newly acquired genital herpes infections and now is more common than HSV-2 (2). Primary infection of the genital epithelium with HSV leads to lifelong latent infection of neurons of the sacral ganglia. Periodic reactivation from latently infected neurons leads to the production and shedding of infectious virus at genital mucosal sites. Reactivation and associated virus shedding may be accompanied by symptoms such as pain and ulceration at the site of virus replication. However, reactivation events frequently occur asymptomatically and may thus allow spread of virus without the knowledge of the infected individual (2, 3). Several studies have also provided evidence that prior genital herpes infection increases the risk of acquiring sexually transmitted HIV (4, 5). Thus, a safe and effective vaccine against genital herpes would be an important tool in reducing both the incidence and severity of genital HSV infections, and possibly HIV transmission.
In addition to virus transmission between sexual partners, genital HSV can also be transmitted from mother to child at birth. Although rare (about 1,500 cases annually in the United States), neonatal HSV infections are associated with relatively high mortality (29% for disseminated infection and 4% for central nervous system [CNS] infection) (6). Because of the disproportionately high disease burden in women and the risk of transmitting potentially life-threatening HSV infections to newborns, it is particularly important that a genital HSV vaccine induce potent, durable protection in women.
Early clinical trials showed that glycoprotein D (gD)-based subunit vaccines were safe and induced virus-neutralizing antibodies in humans (7, 8). Modest vaccine efficacy in protecting against HSV-2 in seronegative individuals was observed in some studies but not others (9, 10). In the most recently published clinical trial, HSV-2 gD (gD2) was tested for efficacy in over 8,000 HSV-1- and HSV-2-negative women (11). Although the gD2 vaccine induced immune responses, vaccinated women were just as likely to acquire primary HSV-2 infection as those who had received a control vaccine. Somewhat surprisingly, despite the lack of protection against HSV-2, vaccinated women showed a significant level of protection against acquisition of HSV-1 (82% protection against culture-positive genital disease caused by HSV-1 and 35% protection against HSV-1 infection). Consistent with this unexpected outcome, Awasthi et al. (12) observed increased sensitivity of low-passage-number HSV-1 clinical isolates to neutralization by sera from gD2-vaccinated individuals compared to HSV-2 clinical isolates. They also showed that deletion of gC or gE from HSV-2 increased its susceptibility to neutralization by gD antibodies and suggested that differential effects of gC and gE in the two HSV serotypes may account for the observed differences in neutralization. Separately, Belshe et al. (13) reported that among the gD2 vaccine trial participants, serum antibodies against gD2, but not cell-mediated immunity, correlated with protection against HSV-1 genital disease. Given the documented relevance of gD-specific serum antibodies to virus neutralization and protection from disease, our goal in the current study was to dissect the humoral response to gD2 epitopes elicited by the vaccine. An abbreviated monoclonal antibody (MAb) tree of our collection of gD-specific MAbs is shown in Fig. 1A. Key sites discussed in this paper are shown on the structure of gD (Fig. 1A and B).
FIG 1.

(A) gD2 MAb tree. The diagram shows gD antigenic sites and associated MAbs used in this study. The defining properties of MAbs within each branch of the tree are indicated. Antigenic sites are defined by groups of MAbs that compete with each other for gD binding. MAb group designations are shown in boxes. gD residues implicated in MAb binding are in parentheses below the group names. Representative MAbs are shown in large type below the group names. Virus-neutralizing MAbs are shown in boldface. Receptor-blocking MAbs are indicated by asterisks. (B) Two views of the gD structure rotated 180°. The locations of the epitopes of the MAbs listed in panel A are indicated.
gD is an essential glycoprotein of HSV. Its role in virus entry is 2-fold: it binds to one of two major cellular receptors (HVEM and nectin-1) and activates the downstream components of the viral fusion machinery, gH/gL and gB (reviewed in reference 14). The atomic structure of gD alone (Fig. 1B) and in complex with both HVEM and nectin-1 has been solved (15–17). In addition, because of its ability to induce potent virus-neutralizing antibodies, the antigenic structure of gD has been extensively studied (18, 19) using a large panel of anti-gD MAbs (Fig. 1A). Structural and antigenic studies have provided insight into the mechanism of neutralization by certain gD MAbs. MAbs targeting three antigenic sites (groups Ia, Ib, and VII) neutralize virus infectivity by blocking the interaction of gD with one or both of its receptors (14, 20–23). Neutralizing antibodies in two other groups (XVI and XVII) do not block receptor binding but instead block gD-mediated activation of gH/gL, which in turn activates gB, the fusion protein (14, 19, 24). Thus, there are at least five distinct gD2 epitopes capable of inducing virus-neutralizing antibodies. In addition, MC14, an antibody to a 6th epitope (group IIa) near the C terminus of the gD ectodomain, enhances the neutralizing ability of MC2 (group XVII) antibodies (19). One would expect that a protective humoral response to gD2 vaccination would vigorously target as many of these neutralizing and ancillary epitopes as possible.
In this study, we used several approaches to dissect the antibody response to gD within 35 serum samples from a selected subgroup of participants in the most recent gD vaccine efficacy trial. Thirty of these samples were supplied as groups of 10 based on previously determined enzyme-linked immunosorbent assay (ELISA) titers (high, medium, or low). Five additional samples came from individuals who had been immunized with a hepatitis A vaccine rather than gD. These samples were coded so that their vaccination status was unknown when we began our study. In addition, we were supplied with serum specimens taken from each subject prior to immunization. After determining which samples came from control-vaccinated subjects, we focused on the 30 gD-immunized samples. For the remaining studies, we used purified IgG so that the gD epitope-specific antibody content could be compared between samples and related to HSV-2-neutralizing activity. We found that IgGs from the majority of samples contained appreciable, but varying, levels of antibodies directed at neutralizing gD epitopes. A smaller number of samples contained antibodies to linear epitopes within the N and C termini of gD. Within the 10 samples with the most potent neutralizing activities, the strongest correlates of neutralization were the overall gD-specific IgG content, as well as the combined response to group Ia, Ib, XVI, and XVII epitopes. This suggests that optimal virus neutralization, and presumably protection, would be achieved by strong and balanced responses to these four major neutralizing epitopes of gD.
MATERIALS AND METHODS
Cells and soluble proteins.
African green monkey kidney (Vero) cells were grown in Dulbecco's modified Eagle medium (DMEM) (Gibco Life Technologies, Grand Island, NY) supplemented with 5% fetal bovine serum (FBS). Soluble gD1 (285t) and gD2 (285t) were produced from baculovirus-infected insect (Sf9) cells as described previously (19).
Antibodies.
IgG was purified from human sera by protein G chromatography (HiTrap; GE Healthcare) according to the manufacturer's instructions. Following purification, the IgGs were dialyzed against phosphate-buffered saline (PBS). gD-specific MAbs 1D3, DL6, DL11, MC2, MC5, MC14, and MC23 were characterized previously (19, 25–27).
ELISA.
The wells of a 96-well microtiter plate were coated with 50 μl of soluble protein (10 μg/ml in PBS) for 2 h. The plates were then washed 3 times with PBS-0.05% Tween 20 (PBS-T) and then blocked with 5% milk in PBS-T (5% milk) for 30 min, followed by addition of diluted sera or IgGs to be tested for 1 h. Bound IgG was detected by incubation with horseradish peroxidase (HRP)-conjugated goat anti-human IgG (KPL, Gaithersburg, MD) for 30 min at a dilution of 1:200 in 5% milk, followed by the addition of ABTS [2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid)] substrate (Rockland Imunochemicals, Gilbertsville, PA). Absorbance values were read at 405 nm. For each IgG, the signal generated from control wells containing no protein was subtracted from the final reading.
Western blotting.
Purified gD2 (285t) was separated by electrophoresis on a 10% sodium dodecyl sulfate-polyacrylamide gel under denaturing or “native” (25) conditions. Proteins were transferred to nitrocellulose and probed with IgG (1 μg/ml) purified from human sera. The anti-gD polyclonal antibody (PAb) R8 was used as a positive control.
Biosensor/surface plasmon resonance (SPR) experiments.
Experiments were performed using a Biacore 3000 optical biosensor (GE Healthcare, Biacore Life Sciences) at 25°C. Filtered and degassed HBS-EP buffer (10 mM HEPES, pH 7.4, 150 mM NaCl, 3 mM EDTA, 0.005% surfactant P20) was used in all experiments. An anti-His antibody (Qiagen, Inc., Valencia, CA) was covalently coupled to a CM5 sensor chip (GE Healthcare Bio-Sciences, Pittsburgh, PA) following our previous protocol (28). Next, 200 resonance units (RU) of purified gD2 (285t) was captured by the anti-His antibody via its C-terminal His tag. Purified IgGs (100 μg/ml) from human sera were then injected for 240 s, followed by IgGs from mouse MAbs (75 to 150 μg/ml), also for 240 s. After each experiment, the chip surface was treated with brief pulses of 0.2 M Na2CO3 (pH 11) until the RU signal returned to baseline, and then a new cycle was started. All injections were performed at a flow rate of 5 μl/min. For MAb-blocking experiments, the blocking activity of each human IgG was calculated for each MAb as a percentage using the following formula: [1 − (RU MAb binding to human IgG-coated chip/RU MAb binding to control chip)] × 100. Two samples (samples 36 and 41) were tested for blocking activity against 5 different MAbs in two independent experiments to assess reproducibility. For sample 36, blocking activities differed by an average of 2.6% (range, 1% to 4%). For sample 41, blocking activities differed by an average of 2.2% (range, 1% to 5%). The remaining samples were tested once.
Peptide mapping.
Epitope mapping by ELISA using peptides was performed as described previously (29) with some modifications. Synthetic 20-mer peptides spanning the gD2 (strain G) ectodomain with biotin at the N terminus were purchased from Mimotopes (Australia). The peptides were captured on 96-well streptavidin-coated microtiter plates (Thermo Fisher Scientific, Waltham, MA) and reacted with patient IgG (25 μg/ml) for 1 h at 25°C. The plates were then washed and incubated for 30 min with peroxidase-conjugated goat anti-human IgG. The plates were washed again and incubated with ABTS until a signal was observed, and then absorbance readings at 405 nm were measured and recorded.
Virus neutralization assays.
Serial 2-fold dilutions of IgG were combined with HSV-1 (KOS) or HSV-2 (333), and the mixture was incubated at 37°C for 1 h. Monolayers of Vero cells grown in 48-well plates were then incubated with the IgG-virus mixture for 24 h. The cells were fixed with methanol-acetone (2:1), and the plaques were visualized by the black plaque assay (30). The data are plotted as percent neutralization versus control wells, where no IgG was added.
RESULTS
History of samples.
A set of samples consisting of paired sera from each of 35 vaccine study (11) participants were selected for analysis; one sample was obtained prior to vaccination (month 0; preimmune), and the other was obtained after the third dose of gD2 (or control) vaccine (month 7). The study participants received 3 vaccinations (at 0, 1, and 6 months) with 20 μg of truncated gD2 (284t) (30 patients) or with inactivated hepatitis A virus (5 patients) combined with adjuvant. The subjects were selected for having met the protocol conditions (all vaccinations and serum samples were obtained on time) and for absence of concurrent HSV infection. The latter condition was independently confirmed by Western blotting (data not shown), in which none of the patient sera reacted against HSV-2 gB or gC. In order to represent the range of antibody responses to vaccination, samples from the gD vaccine recipients were originally selected from 3 categories of serum ELISA responses (10 samples from each category): high (the top 1/3 of subjects; ELISA titers of >8,061), medium (the middle 1/3 of subjects; ELISA titers between 2,463 and 8,061), and low (the bottom 1/3 of subjects; ELISA titers of <2,463). At the outset of the study, the vaccination status of the samples (control versus gD vaccinated) was intentionally withheld so that there would be no bias in their treatment.
Serum ELISA.
All 70 serum samples were tested for reactivity with gD2 (285t) by ELISA. In order to compare the serum responses, the ELISA readings generated by each serum at 1:640 dilution were plotted on a bar graph (Fig. 2). At this dilution, none of the serum samples exhibited saturation of gD binding. From these data, the strongest and weakest serum antibody responses to gD vaccination could be readily determined. In addition to preimmune samples (not shown), 5 samples from vaccinated individuals failed to react with gD (samples 42, 46, 55, 56, and 59; shaded in Fig. 2). Once the samples were unblinded, we were able to confirm that they were obtained from patients who had received the control vaccine. We noted some minor differences in ELISA binding ranks between the current and the original studies. However, in both instances, the strongest and weakest gD-binding samples grouped at opposite ends of the observed range. Thus, these differences seem likely to reflect experimental variability. Prior to all subsequent analyses, we purified IgG from each human serum sample using protein G Sepharose. IgG yields ranged from 2 mg/ml to 11 mg/ml.
FIG 2.
Reactivities of high, medium, and low ELISA titer subject sera with gD2. Fourfold dilutions of sera prepared from human subject sera were reacted with gD2 (285t) by ELISA. Absorbance values (at 405 nm) from the 1:640 dilution of serum are plotted in the bar graph. The samples are ranked from highest to lowest absorbance readings. The fill patterns of the bars indicate samples from the 3 groups (based on ELISA titers) that were initially supplied for this study: open bars, high ELISA titers; hatched bars, medium ELISA titers; solid bars, low ELISA titers (and control samples). The control samples (42, 46, 55, 56, and 59) appear in the shaded area on the right in the bar graph.
MAb competition.
A major goal of the study was to investigate the repertoire of neutralizing gD epitopes recognized by serum IgGs from gD2-vaccinated human subjects. We have characterized a large number of murine MAbs against gD and have categorized them according to their antigenic and biologic properties (18, 19). Figure 1 shows an abbreviated version of a more extensive tree of >100 MAbs. We identified 5 distinct virus-neutralizing epitopes on HSV gD and prototype MAbs that bind these epitopes (groups Ia [MAb MC23], Ib [MAb DL11], VII [MAb 1D3], XVI [MAb MC5], and XVII [MAb MC2]) (Fig. 1A). Two additional epitopes of interest are bound by MAbs MC14 (group IIa) and DL6 (group IIb). The MAbs in these two groups bind adjacent linear epitopes near the C terminus of the gD ectodomain (19, 26, 31). MC14 does not neutralize virus by itself but enhances neutralization by the group XVII MAb, MC2 (19). DL6 neither neutralizes virus nor enhances neutralization by other gD MAbs. However, it dramatically reduces the size of HSV plaques in cell culture (unpublished data). With the exception of MC2, each of these MAbs is capable of binding gD from both HSV-1 and HSV-2. MC2 is HSV-2 specific.
In order to quantify the responses to these 7 epitopes in vaccinated individuals, we employed a novel binding competition assay. The approach was to determine whether and to what extent the binding of these MAbs to gD is diminished by preincubation of gD with IgGs from the gD-immunized human subjects. Reduced MAb binding would indicate the presence, within the human IgG, of antibodies to an epitope that overlaps that of the neutralizing MAb. In this assay, we first captured gD2 (285t) on the surface of a biosensor chip via its 6-His tag. In contrast to standard ELISA, where antigen presentation can be quite heterogeneous, His tag capture allows the protein to be uniformly oriented on the binding surface. This helps to ensure consistent antigen presentation from one experiment to the next. A fixed amount of each human IgG was then made to flow across the chip surface to allow binding of gD-specific antibodies present in the total IgG (Fig. 3). As a control, a separate gD chip surface was exposed, in parallel, to buffer alone (no human IgG). Immediately after binding of the human IgG to the gD-coated chip, individual MAb IgGs were made to flow across both the test (Fig. 4A, red and gray lines) and control (Fig. 4A, black lines) chip surfaces. If the human IgG contained antibodies to an epitope similar to the test MAb, that MAb would be unable to bind gD. However, if antibodies against that epitope were not represented in the human IgG, the mouse MAb would be able to bind (compare the red and black curves in Fig. 4A). This analysis was done for every combination of MAb and human IgG, thereby providing a comparison of the gD MAb-blocking activity of each of the IgG samples relative to one another.
FIG 3.
Biosensor binding of subject IgGs to gD2. A fixed amount of each IgG was injected across a chip surface coated with gD2. The levels of IgG binding (RU) for each gD-immunized subject sample are shown. The samples are arranged according to their binding activities (highest to lowest). Sample 37 failed to detectably bind to the chip surface and was omitted from subsequent biosensor analyses. The error bars indicate percent error from a minimum of 2 independent experiments.
FIG 4.
MAb blocking (biosensor). (A) Raw data on blocking of neutralizing gD MAbs by human subject IgGs. Binding curves for the association of test MAbs (MC2, MC5, DL11, and MC23) with gD are shown. The black lines indicate MAb binding to gD that was not exposed to human subject IgGs. The red and gray lines indicate MAb binding to gD following exposure to human subject IgGs. The red lines indicate samples where MAb binding was reduced by at least 20%. The gray lines indicate samples where MAb binding was reduced by less than 20%. The human subject number is shown at the left of each group under the curves for MC2 blocking. Test MAbs are indicated above and refer to the columns of graphs below. The samples are ordered as in Fig. 3 (starting at the upper left and reading down within the first column, then shifting to the top of the next column). (B to E) Samples 36 and 41 were tested in two independent experiments. Differences averaged 2.6% and 2.2%, respectively (range, 1% to 5%). The remaining samples were tested once. (B) Percent blocking activity against MAb MC23. The samples are ordered as in Fig. 3. (C) As in panel B, except that the bars indicate blocking activity against DL11. (D) As in panel B, except that the bars indicate blocking activity against MC5. (E) As in panel B, except that bars indicate blocking activity against MC2.
Several pieces of data were extracted from this analysis. First, the binding activity (RU) of each human IgG to the gD-coated chip surface was measured. The binding data are plotted, from strongest to weakest, in Fig. 3. This provided a comparison of the gD-specific binding activity of each of the IgG samples relative to one another using this system. For reference, the sample order established in Fig. 3 is maintained in Fig. 4. One sample (sample 37) failed to detectably bind gD via the biosensor and was therefore omitted from competition analyses. Second, binding curves were generated for the binding of each gD MAb to the IgG-occupied and control chip surfaces (Fig. 4A). From these data, the blocking activity of each human IgG was calculated for each MAb as a percentage using the following formula: [1 − (RU MAb binding to human IgG-coated chip/RU MAb binding to control chip)] × 100. We consider 20% the minimum level of blocking activity required for a human IgG to be scored as positive for MAb blocking. Competition values below 20% are too weak to be confidently distinguished from background. Samples 36 and 41 were tested for blocking activity against 5 different MAbs in two independent experiments to assess reproducibility. For sample 36, the blocking activities differed by an average of 2.6% (range, 1% to 4%). For sample 41, the blocking activities differed by an average of 2.2% (range, 1% to 5%). The remaining samples were tested once.
Three MAbs (1D3, MC23, and DL11) chosen for biosensor competition studies block the association of gD with one or both of its principal receptors (HVEM and nectin-1). 1D3 blocks the gD-HVEM interaction (20) and neutralizes HSV-2 (50% plaque reduction) at a concentration of 6.2 μg/ml (19). Within the panel of gD2-vaccinated patient sera, IgG capable of blocking 1D3 was either absent or present at very low levels (less than 20% blocking) (data not shown). Thus, gD vaccination failed to elicit a strong humoral response to this important neutralizing epitope. The group Ia MAb, MC23, blocks association of gD with nectin-1 (21) and neutralizes HSV-2 at a concentration of 0.26 μg/ml (19). In contrast to 1D3, IgGs from the majority (20/29) of gD2-vaccinated patient sera were capable of blocking MC23 (Fig. 4B). The group Ib MAb, DL11, blocks the binding of gD to both HVEM and nectin-1 (21–23) and neutralizes HSV-2 at 0.312 μg/ml (19). DL11 binding was blocked by at least 20% in 25/29 samples (Fig. 4C). From these data, we conclude that vaccination with gD2 (285t) was able to elicit humoral responses to epitopes overlapping those of the potent group I HSV-neutralizing MAbs, MC23 and DL11.
Four other MAbs used for biosensor competition studies, MC2, MC5, MC14, and DL6, do not block receptor binding. We have hypothesized that MC2 and MC5 neutralize by blocking the interaction of gD with gH/gL, a downstream component of the HSV fusion cascade (14, 19, 24). MC2 and MC5 neutralize HSV-2 at 0.781 μg/ml and 6.2 μg/ml, respectively. Although MC14 is unable to neutralize HSV on its own, it potentiates the neutralizing activity of MC2 (19). DL6 does not neutralize virus but limits cell-to-cell spread of HSV in tissue culture. Among gD2 vaccine recipients, the response to the MC5 epitope was relatively broad (21/29 samples were blocked by greater than 20%) (Fig. 4D). gD2 vaccination also induced responses to the MC2 epitope in the vast majority of patients (22/29) (Fig. 4E). In contrast, the MC14 and DL6 epitopes induced weak or undetectable responses (not shown) among gD2-vaccinated subjects. Thus, in general, responses to the discontinuous epitopes of gD represented by MAbs MC23, DL11, MC2, and MC5 were readily detected in the majority of vaccinated subjects. However, the linear epitopes represented by MAbs 1D3, MC14, and DL6 elicited much weaker responses.
Peptide ELISA.
The humoral response to vaccination with truncated and full-length forms of gD in rabbits and mice typically includes antibodies directed against linear epitopes near the N and C termini of the gD ectodomain (residues 1 to 30 and residues 260 to 316, respectively). This is consistent with structural data showing or suggesting that the N and C termini of gD are solvent exposed and largely devoid of secondary structure (Fig. 5C and D). Indeed, these regions of gD are believed to be flexible and to play roles in regulating the receptor-binding (N and C termini) and fusion activation (C terminus) functions of gD (15–17, 32, 33). Although IgGs from vaccinated individuals did not appreciably block the binding of 1D3, MC14, and DL6 to gD, it is possible that this method was not ideally suited for the detection of Abs against linear epitopes. Therefore, we next screened for Abs directed against linear epitopes using overlapping synthetic peptides encompassing the gD ectodomain amino acid sequence. To do this, we utilized a peptide library consisting of overlapping (11 amino acids) biotinylated 20-mers. They were captured on streptavidin-coated ELISA plates and incubated with IgGs prepared from all gD2-vaccinated individuals (as well as control IgG). Signals generated by preimmune IgGs were subtracted from those of the matching gD- or control-immunized samples. Consistent with the notion that the N and C termini of gD contain linear epitopes, several patient samples reacted against peptides consisting of gD residues 1 to 20 (N terminus) (Fig. 5A), 10 to 29, and 19 to 38 (not shown), as well as residues 262 to 281 (C terminus) (Fig. 5B). Thus, despite the inability to block the 1D3, MC14, and DL6 MAbs, a subset of patient samples did contain antibodies directed against linear epitopes within the N and C termini of gD.
FIG 5.
Peptide ELISA. Binding of human subject IgGs to peptides mimicking portions of the gD2 ectodomain. Absorbance values generated by matching preimmune IgGs were subtracted from the data plotted. (A) Reactivity with a peptide representing the N terminus of gD (residues 1 to 20). (B) Reactivity with a peptide representing the C terminus of gD (residues 262 to 281). (C) Structure of gD (space-filling model) in complex with HVEM (green ribbon structure) and nectin-1 (purple ribbon structure). The N-terminal gD peptide (residues 1 to 20) is colored red. (D) Same structures as in panel C rotated 90° to more clearly show the locations of gD residues 262 to 281 (colored green).
Virus neutralization.
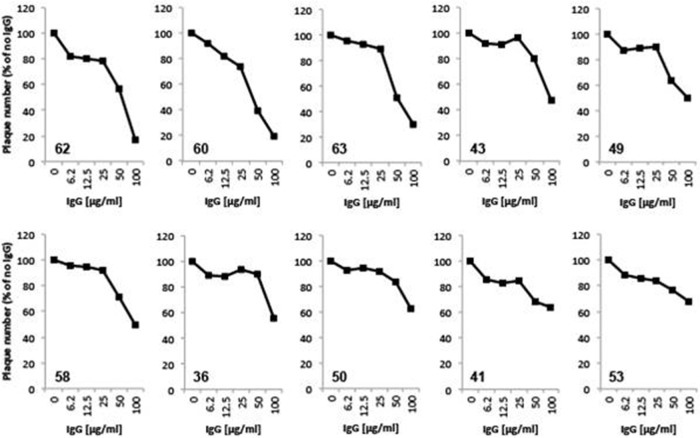
IgG from gD-immunized patients was screened for neutralizing activity against HSV-2 (333) via plaque reduction assay. Twofold dilutions of IgG, from 6.2 μg/ml through 100 μg/ml, were tested. Of the 30 samples tested, only 16 were able to neutralize more than 20% of input virus at the highest IgG concentration used. The remaining 13 samples neutralized very weakly or not at all and could not be reliably distinguished from background. Neutralization curves for the 10 most potent samples are shown in Fig. 6.
FIG 6.
Virus neutralization. Sample IgGs were tested for the ability to neutralize HSV-2. Plaque numbers were determined for each sample and plotted as a percentage of the plaques obtained in the absence of human IgG. Sample numbers are indicated within each individual plot (lower left). The samples are ordered according to neutralizing activity at 100 μg/ml.
DISCUSSION
Numerous studies have demonstrated the efficacy of gD-based subunit vaccination for protection against HSV-2 infection and establishment of latency in female guinea pigs (34–37), a model believed to closely replicate the pathology of natural HSV-2 infection in humans (38, 39). The promising outcomes of these studies prompted several recent gD-based vaccine trials in humans (7–11, 40, 41). In two small earlier studies, some vaccine efficacy was observed in women with sexual partners having confirmed genital HSV-2 disease (10). In contrast, the most recent study, in a population of doubly seronegative women, did not show efficacy against HSV-2 disease or acquisition (11). Our goal in the studies reported here was to characterize the humoral response to known neutralizing epitopes and antigenic regions of gD within vaccinated human subjects, a likely component of vaccine success.
We began the study with a selected subset of sera from 35 patients who had been vaccinated, per protocol, with either the gD2 subunit vaccine (30 samples) (11) or a control vaccine (hepatitis A; 5 samples). None of the samples came from HSV-infected individuals. In addition, samples from the gD vaccine recipients were selected to represent 3 categories of ELISA responses (10 samples from each category): high, medium, and low titers. As anticipated, all 30 samples from gD-vaccinated individuals bound to gD2 by ELISA, and none reacted with either gB2 or gC2, confirming that none of the individuals had been exposed to other HSV antigens. Furthermore, the ELISA responses varied over a wide range, reflecting the intended goal of analyzing IgGs representing strong, medium, and weak responses to gD2 vaccination.
As mentioned previously, gD induces a strong neutralizing humoral response in vaccinated laboratory animals. Importantly, gD presents multiple protective/neutralizing epitopes to the host immune system. We have identified at least 6 distinct epitopes that contribute to virus neutralization, as well as a 7th epitope that affects the spread of HSV in tissue culture. These sites target at least 2 functions of gD, namely, receptor binding and activation of the downstream membrane fusion cascade (14, 19, 21–24). The fact that multiple gD epitopes are capable of independently inducing neutralizing antibodies means that the virus is unlikely to escape vaccine protection via point mutation within a single epitope. In addition to neutralizing extracellular virus, gD antibodies may also provide protection at later stages of HSV replication by binding to infected cells and initiating antibiotic-dependent cell-mediated cytotoxicity (ADCC) and complement-mediated lysis.
We adopted two strategies to assess the breadth of the humoral response to gD in human vaccine recipients. First, we used a novel biosensor-based binding competition assay that allowed us to determine the Ab response to individual, well-characterized, discontinuous and continuous gD epitopes. Deficiencies in the response to one or more key epitopes would suggest that the gD2 used for vaccination was either damaged or altered in some way. Thus, this technique could prove to be a valuable and more general tool for the assessment of subunit vaccine quality and efficacy. Second, we screened serum IgGs from vaccinated human subjects for reactivity with a set of overlapping peptides spanning the gD ectodomain. This provided a means to examine the Ab response to all potential continuous epitopes within gD without bias toward those recognized by previously characterized murine MAbs. From these studies, we were able to determine that all key epitopes/regions of gD induced detectable antibody responses in at least a subset of individuals. We also infer from these studies that the human IgG response to gD vaccination is similar to that induced in laboratory animals.
Somewhat surprisingly, we observed weak or negligible blocking of MAbs directed against epitopes in the N terminus (1D3) and C terminus (MC14 and DL6) by gD-immunized patient IgGs. Despite the poor blocking activity against these epitopes, we did detect measurable binding of some IgG samples to peptides containing sequences mimicking the N and C termini of gD. There are several possible explanations for the inability of these samples to block 1D3, MC14, and DL6 binding in our competition assay. Most likely, the responses to these epitopes were relatively weak. As such, IgG capable of blocking these MAbs may have been present, but at levels too low to appreciably block MAb binding under the conditions used in our blocking assay. It is also possible, although less likely, that vaccine recipients responded to epitopes within the N and C termini of gD that did not overlap the binding sites of MAbs 1D3, MC14, and DL6. Because of their importance in virus neutralization and limiting cell-to-cell spread, it will be of interest to examine in more detail the strength and composition of the human response to these antigenic regions of gD.
Once we had completed the studies detailed in this paper, it was of interest to determine which IgG properties/characteristics most closely correlated with virus neutralization. To address this, we first arranged the 10 best neutralizers in order from strongest to weakest (Fig. 6). We then plotted other measured characteristics of these 10 samples against their neutralization scores and calculated R2 values for each plot. Among the best correlates of neutralization was overall gD-binding activity as measured by ELISA (not shown) or biosensor (Fig. 7A). Interestingly, when the blocking activities against MAbs MC2, MC5, MC23, and DL11 were combined into a single score, the value correlated remarkably well with HSV-2 neutralization (R2 = 0.859) (Fig. 7B). This does not mean that all MAb-blocking antibodies possess neutralizing activity. Indeed, it may be possible for antibodies directed at nearby nonneutralizing epitopes to interfere with the binding (and activity) of neutralizing antibodies. Our data also do not exclude the possibility of additional HSV-neutralizing epitopes. However, despite these caveats, our data suggest that the combined response to the MC2, MC5, MC23, and DL11 epitopes is of key importance to neutralization and, by extension, protection from disease. Furthermore, these data imply that the breadth of the response to gD epitopes is a crucial aspect of vaccine efficacy. This is consistent with our previous data showing that MAbs MC2, MC5, MC23, and DL11 target at least 2 distinct aspects of gD function (19).
FIG 7.
Correlation plots. Neutralizing activities (percent HSV-2 neutralization [Neut.] at 100 μg/ml IgG) of the 10 most potent IgG samples plotted against other properties of the same IgG samples. Percent neutralization is shown on the left scale of each graph. (A) Neutralization versus IgG binding to gD on the biosensor. The R2 value for the correlation of the two variables is 0.75657 (right graph). (B) Neutralization versus average MAb (MC2 plus MC5 plus DL11 plus MC23) blocking activity. Individual MAb blocking values were added together, and the sum was then divided by 4. The R2 value for the correlation of the two variables is 0.8591 (right graph).
Our overall conclusions from this study are that vaccination of HSV-seronegative human subjects with a gD subunit vaccine induced detectable antibody responses in all samples examined. As expected, based on the selection criteria, the gD-binding activities of the samples varied widely. The majority of IgG samples contained appreciable levels of antibodies directed at known virus-neutralizing gD epitopes. A smaller number of samples responded to linear epitopes within the N and C termini of gD. However, the vast majority of vaccinated-patient IgGs failed to appreciably block the binding of prototype MAbs with epitopes in these regions, suggesting that the response to these portions of gD was relatively weak. At least five patients also responded to a peptide consisting of gD residues 154 to 173. Consistent with the observed wide range of antibody responses to gD vaccination, IgG samples exhibited a broad range of HSV-neutralizing abilities. Among the 10 most potent samples, the strongest correlate of neutralization was the content of antibodies whose epitopes overlap those of 4 antigenically distinct virus-neutralizing MAbs (MC2, MC5, MC23, and DL11). These results suggest that potent virus-neutralizing activity, and possibly protection from infection, can be achieved by a strong and balanced response to the four major discontinuous neutralizing epitopes of gD examined in this study.
ACKNOWLEDGMENTS
This research was supported by a contract with the National Institute of Allergy and Infectious Diseases (HHSN272200800003C), by GlaxoSmithKline, and by NIH grants AI-076231 and AI-056045 (to R.J.E.) and AI-18289 (to G.H.C.).
Footnotes
Published ahead of print 30 April 2014
REFERENCES
- 1.Satterwhite CL, Torrone E, Meites E, Dunne EF, Mahajan R, Ocfemia MC, Su J, Xu F, Weinstock H. 2013. Sexually transmitted infections among us women and men: prevalence and incidence estimates, 2008. Sex. Transm. Dis. 40:187–193. 10.1097/OLQ.0b013e318286bb53 [DOI] [PubMed] [Google Scholar]
- 2.Schiffer JT, Corey L. 2009. New concepts in understanding genital herpes. Curr. Infect. Dis. Rep. 11:457–464. 10.1007/s11908-009-0066-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whitley RJ, Roizman B. 2001. Herpes simplex virus infections. Lancet 357:1513–1518. 10.1016/S0140-6736(00)04638-9 [DOI] [PubMed] [Google Scholar]
- 4.Freeman EE, Weiss HA, Glynn JR, Cross PL, Whitworth JA, Hayes RJ. 2006. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS 20:73–83. 10.1097/01.aids.0000198081.09337.a7 [DOI] [PubMed] [Google Scholar]
- 5.Abu-Raddad LJ, Magaret AS, Celum C, Wald A, Longini IM, Jr, Self SG, Corey L. 2008. Genital herpes has played a more important role than any other sexually transmitted infection in driving HIV prevalence in Africa. PLoS One 3:e2230. 10.1371/journal.pone.0002230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pinninti SG, Kimberlin DW. 2013. Neonatal herpes simplex virus infections. Pediatr. Clin. N. Am. 60:351–365. 10.1016/j.pcl.2012.12.005 [DOI] [PubMed] [Google Scholar]
- 7.Straus SE, Savarese B, Tigges M, Freifeld AG, Krause PR, Margolis DM, Meier JL, Paar DP, Adair SF, Dina D, Dekker C, Burke RL. 1993. Induction and enhancement of immune responses to herpes simplex virus type 2 in humans by use of a recombinant glycoprotein d vaccine. J. Infect. Dis. 167:1045–1052. 10.1093/infdis/167.5.1045 [DOI] [PubMed] [Google Scholar]
- 8.Langenberg AG, Burke RL, Adair SF, Sekulovich R, Tigges M, Dekker CL, Corey L. 1995. A recombinant glycoprotein vaccine for herpes simplex virus type 2: safety and immunogenicity. Ann. Intern. Med. 122:889–898. 10.7326/0003-4819-122-12-199506150-00001 [DOI] [PubMed] [Google Scholar]
- 9.Corey L, Langenberg AG, Ashley R, Sekulovich RE, Izu AE, Douglas JM, Jr, Handsfield HH, Warren T, Marr L, Tyring S, DiCarlo R, Adimora AA, Leone P, Dekker CL, Burke RL, Leong WP, Straus SE. 1999. Recombinant glycoprotein vaccine for the prevention of genital HSV-2 infection: two randomized controlled trials. Chiron HSV Vaccine Study Group. JAMA 282:331–340 [DOI] [PubMed] [Google Scholar]
- 10.Stanberry LR, Spruance SL, Cunningham AL, Bernstein DI, Mindel A, Sacks S, Tyring S, Aoki FY, Slaoui M, Denis M, Vandepapeliere P, Dubin G. 2002. Glycoprotein-d-adjuvant vaccine to prevent genital herpes. N. Engl. J. Med. 347:1652–1661. 10.1056/NEJMoa011915 [DOI] [PubMed] [Google Scholar]
- 11.Belshe RB, Leone PA, Bernstein DI, Wald A, Levin MJ, Stapleton JT, Gorfinkel I, Morrow RL, Ewell MG, Stokes-Riner A, Dubin G, Heineman TC, Schulte JM, Deal CD. 2012. Efficacy results of a trial of a herpes simplex vaccine. N. Engl. J. Med. 366:34–43. 10.1056/NEJMoa1103151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Awasthi S, Belshe RB, Friedman HM. 24 April 2014. Better neutralization of HSV-1 than HSV-2 by antibody from subjects immunized with the GlaxoSmithKline gd2 vaccine. J. Infect. Dis. 10.1093/infdis/jiu177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Belshe RB, Heineman TC, Bernstein DI, Bellamy AR, Ewell M, van der Most R, Deal CD. 2014. Correlate of immune protection against HSV-1 genital disease in vaccinated women. J. Infect. Dis. 209:828–836. 10.1093/infdis/jit651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eisenberg RJ, Atanasiu D, Cairns TM, Gallagher JR, Krummenacher C, Cohen GH. 2012. Herpes virus fusion and entry: a story with many characters. Viruses 4:800–832. 10.3390/v4050800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carfi A, Willis SH, Whitbeck JC, Krummenacher C, Cohen GH, Eisenberg RJ, Wiley DC. 2001. Herpes simplex virus glycoprotein d bound to the human receptor hvea. Mol. Cell 8:169–179. 10.1016/S1097-2765(01)00298-2 [DOI] [PubMed] [Google Scholar]
- 16.Krummenacher C, Supekar VM, Whitbeck JC, Lazear E, Connolly SA, Eisenberg RJ, Cohen GH, Wiley DC, Carfi A. 2005. Structure of unliganded HSV gd reveals a mechanism for receptor-mediated activation of virus entry. EMBO J. 24:4144–4153. 10.1038/sj.emboj.7600875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Di Giovine P, Settembre EC, Bhargava AK, Luftig MA, Lou H, Cohen GH, Eisenberg RJ, Krummenacher C, Carfi A. 2011. Structure of herpes simplex virus glycoprotein d bound to the human receptor nectin-1. PLoS Pathog. 7:e1002277. 10.1371/journal.ppat.1002277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen GH, Muggeridge MI, Long D, Sodora DA, Eisenberg RJ. 1992. Structural and functional studies of herpes simplex virus glycoprotein d. Adv. Exp. Med. Biol. 327:217–228. 10.1007/978-1-4615-3410-5_24 [DOI] [PubMed] [Google Scholar]
- 19.Lazear E, Whitbeck JC, Ponce-de-Leon M, Cairns TM, Willis SH, Zuo Y, Krummenacher C, Cohen GH, Eisenberg RJ. 2012. Antibody-induced conformational changes in herpes simplex virus glycoprotein gd reveal new targets for virus neutralization. J. Virol. 86:1563–1576. 10.1128/JVI.06480-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nicola AV, Ponce de Leon M, Xu R, Hou W, Whitbeck JC, Krummenacher C, Montgomery RI, Spear PG, Eisenberg RJ, Cohen GH. 1998. Monoclonal antibodies to distinct sites on herpes simplex virus (HSV) glycoprotein d block HSV binding to hvem. J. Virol. 72:3595–3601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krummenacher C, Nicola AV, Whitbeck JC, Lou H, Hou W, Lambris JD, Geraghty RJ, Spear PG, Cohen GH, Eisenberg RJ. 1998. Herpes simplex virus glycoprotein d can bind to poliovirus receptor-related protein 1 or herpesvirus entry mediator, two structurally unrelated mediators of virus entry. J. Virol. 72:7064–7074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Whitbeck JC, Muggeridge MI, Rux AH, Hou W, Krummenacher C, Lou H, van Geelen A, Eisenberg RJ, Cohen GH. 1999. The major neutralizing antigenic site on herpes simplex virus glycoprotein d overlaps a receptor-binding domain. J. Virol. 73:9879–9890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee CC, Lin LL, Chan WE, Ko TP, Lai JS, Wang AH. 2013. Structural basis for the antibody neutralization of herpes simplex virus. Acta Crystallogr. D Biol. Crystallogr. 69:1935–1945. 10.1107/S0907444913016776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Atanasiu D, Saw WT, Cohen GH, Eisenberg RJ. 2010. Cascade of events governing cell-cell fusion induced by herpes simplex virus glycoproteins gd, gh/gl, and gb. J. Virol. 84:12292–12299. 10.1128/JVI.01700-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohen GH, Isola VJ, Kuhns J, Berman PW, Eisenberg RJ. 1986. Localization of discontinuous epitopes of herpes simplex virus glycoprotein d: use of a nondenaturing (“native” gel) system of polyacrylamide gel electrophoresis coupled with Western blotting. J. Virol. 60:157–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eisenberg RJ, Long D, Ponce de Leon M, Matthews JT, Spear PG, Gibson MG, Lasky LA, Berman P, Golub E, Cohen GH. 1985. Localization of epitopes of herpes simplex virus type 1 glycoprotein d. J. Virol. 53:634–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muggeridge MI, Isola VJ, Byrn RA, Tucker TJ, Minson AC, Glorioso JC, Cohen GH, Eisenberg RJ. 1988. Antigenic analysis of a major neutralization site of herpes simpelx virus glycoprotein d using deletion mutants and monoclonal antibody-resistant mutants. J. Virol. 62:3274–3280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krummenacher C, Baribaud I, Ponce de Leon M, Whitbeck JC, Lou H, Cohen GH, Eisenberg RJ. 2000. Localization of a binding site for herpes simplex virus glycoprotein d on herpesvirus entry mediator c by using antireceptor monoclonal antibodies. J. Virol. 74:10863–10872. 10.1128/JVI.74.23.10863-10872.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aldaz-Carroll L, Whitbeck JC, Ponce de Leon M, Lou H, Hirao L, Isaacs SN, Moss B, Eisenberg RJ, Cohen GH. 2005. Epitope-mapping studies define two major neutralization sites on the vaccinia virus extracellular enveloped virus glycoprotein b5r. J. Virol. 79:6260–6271. 10.1128/JVI.79.10.6260-6271.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tal-Singer R, Peng C, Ponce De Leon M, Abrams WR, Banfield BW, Tufaro F, Cohen GH, Eisenberg RJ. 1995. Interaction of herpes simplex virus glycoprotein gc with mammalian cell surface molecules. J. Virol. 69:4471–4483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Isola VJ, Eisenberg RJ, Siebert GR, Heilman CJ, Wilcox WC, Cohen GH. 1989. Fine mapping of antigenic site ii of herpes simplex virus glycoprotein d. J. Virol. 63:2325–2334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lazear E, Carfi A, Whitbeck JC, Cairns TM, Krummenacher C, Cohen GH, Eisenberg RJ. 2008. Engineered disulfide bonds in herpes simplex virus type 1 gd separate receptor binding from fusion initiation and viral entry. J. Virol. 82:700–709. 10.1128/JVI.02192-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gallagher JR, Saw WT, Atanasiu D, Lou H, Eisenberg RJ, Cohen GH. 2013. Displacement of the c-terminus of herpes simplex virus gd is sufficient to expose the fusion activating interfaces on gd. J. Virol. 87:12656–12666. 10.1128/JVI.01727-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berman PW, Gregory T, Crase D, Lasky LA. 1985. Protection from genital herpes simplex virus type 2 infection by vaccination with cloned type 1 glycoprotein d. Science 227:1490–1492. 10.1126/science.2983428 [DOI] [PubMed] [Google Scholar]
- 35.Berman PW, Vogt PE, Gregory T, Lasky LA, Kern ER. 1988. Efficacy of recombinant glycoprotein d subunit vaccines on the development of primary, recurrent, and latent genital infections with herpes simplex virus type 2 in guinea pigs. J. Infect. Dis. 157:897–902. 10.1093/infdis/157.5.897 [DOI] [PubMed] [Google Scholar]
- 36.Bourne N, Bravo FJ, Francotte M, Bernstein DI, Myers MG, Slaoui M, Stanberry LR. 2003. Herpes simplex virus (HSV) type 2 glycoprotein d subunit vaccines and protection against genital HSV-1 or HSV-2 disease in guinea pigs. J. Infect. Dis. 187:542–549. 10.1086/374002 [DOI] [PubMed] [Google Scholar]
- 37.Skoberne M, Cardin R, Lee A, Kazimirova A, Zielinski V, Garvie D, Lundberg A, Larson S, Bravo FJ, Bernstein DI, Flechtner JB, Long D. 2013. An adjuvanted herpes simplex virus 2 subunit vaccine elicits a t cell response in mice and is an effective therapeutic vaccine in guinea pigs. J. Virol. 87:3930–3942. 10.1128/JVI.02745-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stanberry LR, Bernstein DI, Kit S, Myers MG. 1986. Genital reinfection after recovery from initial genital infection with herpes simplex virus type 2 in guinea pigs. J. Infect. Dis. 153:1055–1061. 10.1093/infdis/153.6.1055 [DOI] [PubMed] [Google Scholar]
- 39.Stanberry LR, Burke R, Myers MG. 1988. Herpes simplex virus glycoprotein treatment of recurrent genital herpes. J. Infect. Dis. 157:156–163. 10.1093/infdis/157.1.156 [DOI] [PubMed] [Google Scholar]
- 40.Straus SE, Corey L, Burke RL, Savarese B, Barnum G, Krause PR, Kost RG, Meier JL, Sekulovich R, Adair SF, Dekker CL. 1994. Placebo-controlled trial of vaccination with recombinant glycoprotein d of herpes simplex virus type 2 for immunotherapy of genital herpes. Lancet 343:1460–1463. 10.1016/S0140-6736(94)92581-X [DOI] [PubMed] [Google Scholar]
- 41.Straus SE, Wald A, Kost RG, McKenzie R, Langenberg AG, Hohman P, Lekstrom J, Cox E, Nakamura M, Sekulovich R, Izu A, Dekker C, Corey L. 1997. Immunotherapy of recurrent genital herpes with recombinant herpes simplex virus type 2 glycoproteins d and b: results of a placebo-controlled vaccine trial. J. Infect. Dis. 176:1129–1134. 10.1086/514103 [DOI] [PubMed] [Google Scholar]