ABSTRACT
CD8 and CD4 T cells are each critically important for immune control of murine gammaherpesvirus 68 (γHV68) infection. In immunocompetent mice, acute γHV68 infection results in lifelong latency, but in the absence of CD4 T cell help, mice succumb to viral recrudescence and disease. However, the requirements for CD4 T cell help in the generation and maintenance of antiviral CD8 T cell responses are incompletely understood, and it is unclear whether there are epitope-specific differences in the requirement of CD8 T cells for CD4 help. In this report, we characterized the CD8 T cell response to γHV68 in major histocompatibility complex (MHC) class II−/− mice, which lack CD4 T cells, or after antibody-mediated depletion of CD4 T cells. All antiviral CD8 T cells exhibited marked upregulation of surface expression of the inhibitory receptor programmed death-1 (PD-1), but surprisingly, while the immunodominant memory response appeared to be functionally impaired, helpless CD8 T cells of a subdominant specificity had increased numbers and enhanced functionality. Thus, we demonstrate differential requirements for CD4 help in the antiviral CD8 T cell response to a latent gammaherpesvirus.
IMPORTANCE γHV68 is a mouse pathogen closely related to the oncogenic human γHVs, which infect a majority of the world's population. Reactivation of these viruses from latency can lead to complications, disease, and even death. CD4 T cells are required for complete immune control of long-term infection, in part by providing key signals to dendritic cells that in turn instruct optimal antiviral CD8 T cell responses. We have investigated multiple virus-specific CD8 T cell responses during infection and identified a subdominant CD8 T cell response that is numerically and functionally enhanced in the absence of CD4 T cell help. This occurs in spite of high surface expression of an inhibitory receptor and in contrast to the immunodominant response, which is impaired. Our data suggest that signals from CD4 T cells are important in maintaining the CD8 T cell hierarchy during γHV infections.
INTRODUCTION
The human gammaherpesviruses (γHVs) are ubiquitous and present a considerable public health risk by establishing lifelong latent infections. Under conditions of immunosuppression, such as HIV coinfection, γHVs can reactivate from latency, leading to recurrent disease, transplantation complications, and cancers (1). The human γHVs are tightly species specific, making studies of their in vivo pathogenesis and of virus-specific immunity difficult. Intranasal (i.n.) infection of mice with the natural rodent pathogen murine γHV68 provides a tractable small-animal model of human γHV pathogenesis and immunity.
CD8 and CD4 T cells are critically important for long-term survival of γHV68-infected mice. Mice deficient in either CD8 or CD4 T cells succumb to infection, albeit with different kinetics, and both CD8 and CD4 T cells contribute to the long-term control of viral latency (2–5). In some models of viral infection, CD4 T cells are critically important for helping CD8 T cells generate optimal memory cells. In the absence of CD4 help, the resulting “helpless” CD8 T cells are often impaired in the ability to mount recall responses (6). When CD4 T cells are absent during the priming of γHV68-specific CD8 T cells, whether by antibody (Ab)-mediated depletion or in major histocompatibility complex (MHC) class II knockout (I-Ab−/−) mice, the initial acute infection is cleared, but control of viral latency is disrupted, resulting in viral recrudescence and mortality (3, 7, 8).
Early studies of γHV68-infected mice suggested that the lack of viral control in CD4-deficient mice was not due to gross CD8 T cell dysfunction, as helpless CD8 T cell numbers, gamma interferon (IFN-γ) production, and cytotoxicity were not decreased (3, 7, 8). However, helpless γHV68-specific CD8 T cells exhibited impaired recall responses, and boosting helpless CD8 T cell numbers by postexposure vaccination did not improve survival, likely due to impaired functionality of the responding T cells (9, 10). Thus, the quality of the helpless CD8 T cells was called into question. Indeed, more recent studies have demonstrated that helpless CD8 T cells express the B7 family inhibitory receptor programmed death-1 (PD-1), and blocking the interaction of PD-1 with its ligand PD-L1 (also called B7-H1) reduces the viral burden in helpless mice (11, 12). Additionally, a small population of CD8 T cells develops in helpless γHV68-infected mice that has been shown to produce interleukin-10 (IL-10) and suppress viral control (13).
Not all CD8 T cell responses need CD4 help for their optimal generation, and different epitope-specific requirements have been noted for responses to a single viral infection (14, 15). Our laboratory and others have recently identified new γHV68-specific CD8 T cell epitopes (16, 17) and demonstrated that the epitope-specific CD8 T cell responses are differentially regulated during viral infection and reactivation from latency, suggesting they may also have differential requirements for CD4 help (18). Therefore, we revisited the role for CD4 help in the priming of CD8 T cell responses during γHV68 infection. We found that the immunodominant ORF61524Kb-specific CD8 T cell response showed profound PD-1 expression but lower expression of the α-subunit of the IL-7 receptor (CD127) and a marker of terminal differentiation, killer cell lectin-like receptor G-1 (KLRG-1). ORF61524Kb-specific CD8 T cells also had reduced numbers during latency and exhibited functional deficiencies. Surprisingly, the subdominant ORF8604Kb-specific response showed similar surface receptor expression but had elevated numbers and improved functionality. Our study has identified added complexity in the regulation of CD8 T cell responses during γHV infections and provides potential therapeutic vaccination targets to consider. These findings also may be relevant to understanding the generation and maintenance of γHV-specific T cell responses in HIV-coinfected, CD4-deficient patients.
MATERIALS AND METHODS
Mice and viruses.
Female 8- to 12-week-old C57BL/6 (B6; CD45.2+), B6.SJL-PtprcaPepcb/Boy (CD45.1+), and B6.129S-H2dlAb1-Ea (I-Ab−/−; CD45.2+) mice were obtained from the Trudeau Institute animal facility and maintained under specific-pathogen-free conditions. Mice were anesthetized with 2,2,2-tribromoethanol and infected intranasally (i.n.) with 400 PFU γHV68 (strain WUMS). All studies were approved by the Trudeau Institute Animal Care and Use Committee.
Tetramers and flow cytometry.
Allophycocyanin-conjugated MHC class I-restricted tetramers specific for γHV68 epitopes ORF6487-495Db (AGPHNDMEI), ORF8604-612Kb (KNYIFEEKL), ORF39167-174Kb (LVLFYRPI), ORF48148-155Kb (TNYKFSLV), ORF54253-260Kb (AVVQFIRV), ORF61524-531Kb (TSINFVKI), ORF75c176-184Db (SAIENYETF), and ORF75c940-947Kb (KSLTYYKL) were obtained from the Trudeau Institute Molecular Biology Core Facility. Splenocytes were treated with Fc block (BD Biosciences) and then stained with tetramers for 1 h at room temperature, followed by incubation with appropriate antibodies. For intracellular cytokine staining, cells were incubated with congenic splenocytes, 10 μg/ml appropriate peptide, and anti-CD107a antibody for 5 h at 37°C in the presence of brefeldin A and recombinant human IL-2 and then washed, labeled, and permeabilized using the BD Cytofix/Cytoperm kit. Fluorochrome-conjugated antibodies to CD4, CD8α, CD44, CD45.1, CD45.2, CD62L, CD107a, CD127, IL-2, IFN-γ, KLRG-1, PD-1, and tumor necrosis factor alpha (TNF-α) were purchased from BioLegend, BD Biosciences, or eBioscience as needed. Samples were acquired on a BD FACSCanto II cytometer and analyzed using FlowJo software (TreeStar).
Anti-CD4 MAb treatment.
Mice were administered anti-CD4 monoclonal Ab (MAb; clone GK1.5; BioXcell) intraperitoneally (i.p.) at days −2, 0, and 2 (0.5 mg/injection) and at days 7, 14, 21, 28, and 34 (0.25 mg/injection). Control mice were treated with phosphate-buffered saline (PBS).
Statistical analysis.
Data were analyzed using the parametric Student t test or one-way analysis of variance (ANOVA) where appropriate using Prism 5 software (GraphPad). Differences were considered significant at P values of less than 0.05.
RESULTS
CD8 T cell responses without CD4 T cell help.
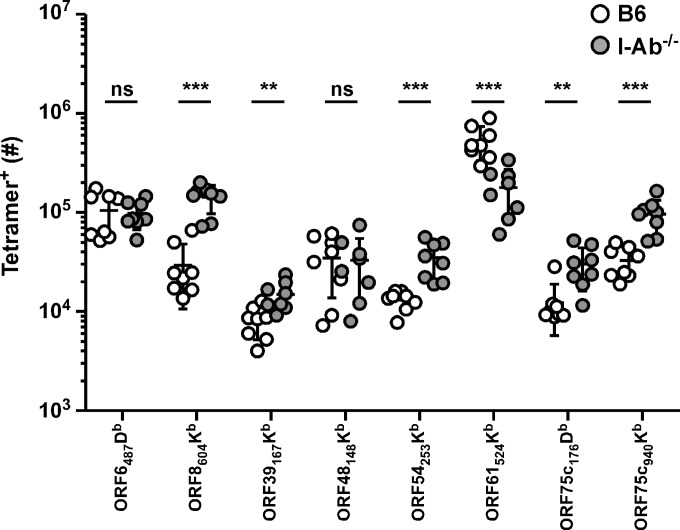
The CD8 T cell response to γHV68 is comprised of numerous specificities. These individual viral epitope-specific responses appear to be differentially regulated during acute infection and latency, likely due to kinetic differences in γHV68 epitope expression (16, 17, 19). Whether these responses are similarly dependent on CD4 T cell help during infection is unknown. We investigated the number of memory CD8 T cells specific for 8 of these epitopes 1 month after infection in either wild-type C57BL/6 (B6) or MHC class II-deficient (I-Ab−/−) mice, which lack CD4 T cells (Fig. 1). Surprisingly, the individual responses appeared to be differentially susceptible to the lack of CD4 help in I-Ab−/− mice, with many subdominant responses seemingly enhanced in the absence of CD4 T cells.
FIG 1.
Numbers of γHV68 epitope-specific CD8 T cells in I-Ab−/− mice. One month after γHV68 infection of B6 or I-Ab−/− mice, spleens were harvested and numbers of tetramer+ CD8 T cells were enumerated by flow cytometry (n = 8/group). **, P ≤ 0.01; ***, P ≤ 0.001; ns, not significant (Student's t test).
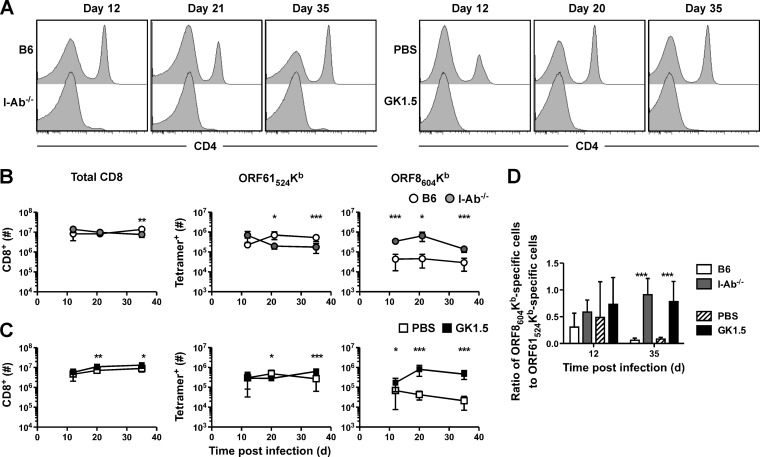
We next examined the requirement for CD4 T cell help in more detail for cells specific for two of the epitopes, the immunodominant epitope ORF61524Kb and the subdominant ORF8604Kb epitope, using I-Ab−/− mice and another well-characterized model, treatment of B6 mice with the anti-CD4 MAb GK1.5. In both models, CD4 T cells remained deficient at all times tested (Fig. 2A). First, B6 or I-Ab−/− mice were infected intranasally with γHV68, and total CD8 T cells were enumerated in the spleen at various times after infection (Fig. 2B). Overall, there was little difference in total CD8 T cell numbers between B6 and I-Ab−/− mice, but ORF61524Kb-specific responses had slightly decreased numbers at 21 and 35 days postinfection (p.i.). At all time points tested, there were more CD8 T cells specific for the ORF8604Kb epitope. Interestingly, we observed a small increase in total and ORF61524Kb-specific cell numbers following anti-CD4 MAb treatment (Fig. 2C). Consistent with the data from I-Ab−/− mice, GK1.5-treated mice had an increase in ORF8604Kb-specific cells at all times tested. The change in immune dominance hierarchy is clearly shown as a ratio of the number of ORF8604Kb-specific cells to ORF61524Kb-specific cells (Fig. 2D). Whereas the memory CD8 T cell pool in B6 mice is highly skewed toward an ORF61524Kb-specific response, in I-Ab−/− or GK1.5-treated mice, the two responses are nearly codominant.
FIG 2.
Enhancement of the subdominant ORF8604Kb-specific response in the absence of CD4 T cell help. At indicated times after γHV68 infection of B6 or I-Ab−/− mice (A and B) or of B6 mice treated with PBS or GK1.5 MAb (A and C), spleens were harvested and CD4 expression on T cells (A) and numbers of total and tetramer+ CD8 T cells (B and C) were enumerated by flow cytometry. (D) The ratio of the number of ORF8604Kb-specific to ORF61524Kb-specific CD8 T cells is shown (n = 5 to 10/group, representative of 2 to 3 experiments). *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001 (Student's t test).
PD-1 expression on helpless CD8 T cell responses.
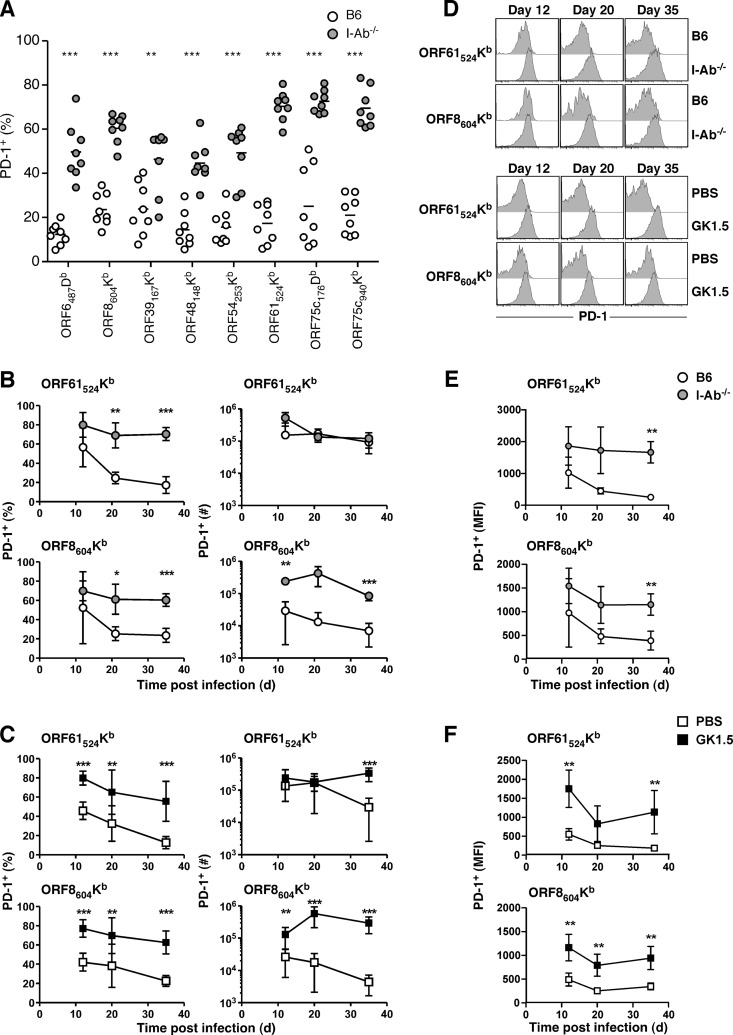
In several infection models, including γHV68, CD8 T cells primed in the absence of CD4 T cells have been shown to upregulate the B7 costimulatory family molecule programmed death-1 (PD-1) (20). Expression of PD-1 is correlated with impaired cytokine production and reduced recall potential. We measured PD-1 expression on CD8 T cells specific for multiple epitopes 35 days p.i. in B6 or I-Ab−/− mice (Fig. 3A). Consistent with previous reports (11, 12), all epitope-specific responses tested showed significant upregulation of PD-1 expression. We examined the kinetics of PD-1 expression in I-Ab−/− mice (Fig. 3B and D) or after GK1.5 treatment (Fig. 3C and D) for ORF61524Kb-specific and ORF8604Kb-specific responses. Both helpless responses exhibited high PD-1 expression (Fig. 3D), and the higher proportion of PD-1 positivity combined with increased numbers resulted in significantly higher numbers of PD-1+ ORF8604Kb-specific CD8 T cells in the absence of CD4 help at all times tested (Fig. 3B and C). Similarly, the level of PD-1 expression was high early but declined over time on virus-specific cells, but helpless CD8 T cells retained elevated PD-1 expression (Fig. 3E and F). These data suggest that all virus-specific CD8 T cell responses are functionally impaired in the absence of CD4 T cell help, even though some responses are numerically enhanced. Notably, GK1.5 MAb-treated mice had markedly more PD-1+ ORF61524Kb-specific cells than controls at day 35 p.i. (Fig. 3C), in contrast to I-Ab−/− mice, which maintained numbers similar to those of B6 mice (Fig. 3B). This discrepancy was due to the presence of more overall ORF61524Kb-specific CD8 T cells in GK1.5 MAb-treated mice than PBS-treated controls compared to fewer overall ORF61524Kb-specific cells than controls in I-Ab−/− mice (Fig. 1). Such differences reinforce the importance of using two distinct model systems in these studies.
FIG 3.
Elevated PD-1 expression without CD4 T cell help. One month (A) or at the indicated times after γHV68 infection of B6 or I-Ab−/− mice (B, D, and E) or of B6 mice treated with PBS or GK1.5 MAb (C, D, and F), spleens were harvested and the percentage of tetramer+ cells expressing PD-1, the total numbers of tetramer+ PD-1+ CD8 T cells, or the mean fluorescence intensity (MFI) of PD-1 expression was enumerated by flow cytometry (n = 8 to 10/group, representative of at least 2 independent experiments). *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001 (Student's t test).
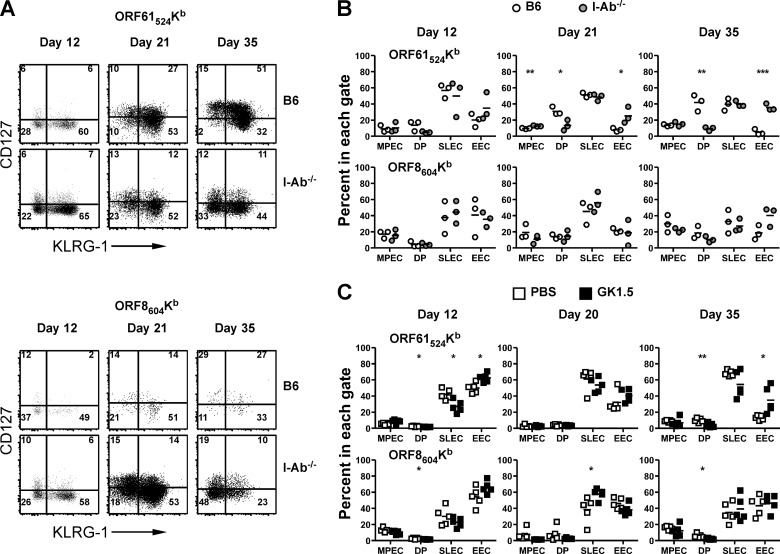
We next investigated whether CD4 T cell help affected the development of memory CD8 T cell responses. Which CD8 T cells will develop into memory cells can be identified at the peak of the effector immune response based upon differential expression of CD127 and a marker of terminal differentiation, KLRG-1 (21). Expression of KLRG-1 without CD127 marks a population of short-lived effector cells (SLECs). Cells that are negative for both receptors have been termed early effector cells (EECs), and their development is regulated by IL-2 (22). Cells that are CD127+ and KLRG-1− have been termed memory precursor effector cells (MPECs) and are thought to survive throughout the response and develop into memory cells in acute infections. Double-positive (DP) cells are less well characterized, but they have been noted in models of chronic infection.
We examined expression of CD127 and KLRG-1 on ORF61524Kb-specific or ORF8604Kb-specific CD8 T cell responses with and without CD4 T cell help. At day 12 p.i., the peak of the effector response, we observed no differences in the expression of either marker between B6 and I-Ab−/− mice for either response (Fig. 4A). For ORF61524Kb-specific cells in B6 mice, there was an accumulation of DP cells over time, as CD127 is expressed on KLRG-1+ cells. In I-Ab−/− mice, however, CD127 was suboptimally expressed at late times, resulting in a decrease in DP cells and a corresponding increase in double-negative EECs (Fig. 4B). Accordingly, there were no significant differences in the proportions of MPEC (CD127+ KLRG1−) or SLEC (CD127− KLRG1+) cells but rather a significant increase in the percentage of EECs (CD127− KLRG1−). Interestingly, these findings were not applicable to ORF8604Kb-specific cells, as there was not as much accumulation of DP cells over time for this specificity (Fig. 4A and B). Similar results were seen for B6 mice treated with GK1.5 MAb (Fig. 4C), demonstrating that a lack of CD4 T cells during priming differentially affects memory T cell development for ORF61524Kb-specific and ORF8604Kb-specific responses.
FIG 4.
Impaired memory cell generation in the absence of CD4 T cell help. At the indicated times after γHV68 infection of B6 or I-Ab−/− mice (A and B) or B6 mice treated with PBS or GK1.5 MAb (C), spleens were harvested, stained with anti-CD127 and anti-KLRG-1, and then analyzed by flow cytometry. (B and C) The percentages of tetramer+ CD8 T cells that are in each quadrant are enumerated. MPEC, memory precursor effector cells (CD127+ KLRG-1−); DP, double-positive cells (CD127+ KLRG-1+); SLEC, short-lived effector cells (CD127− KLRG-1+); EEC, early effector cells (CD127− KLRG-1−) (n = 3 to 5/group, representative of 2 experiments). *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001 (Student's t test).
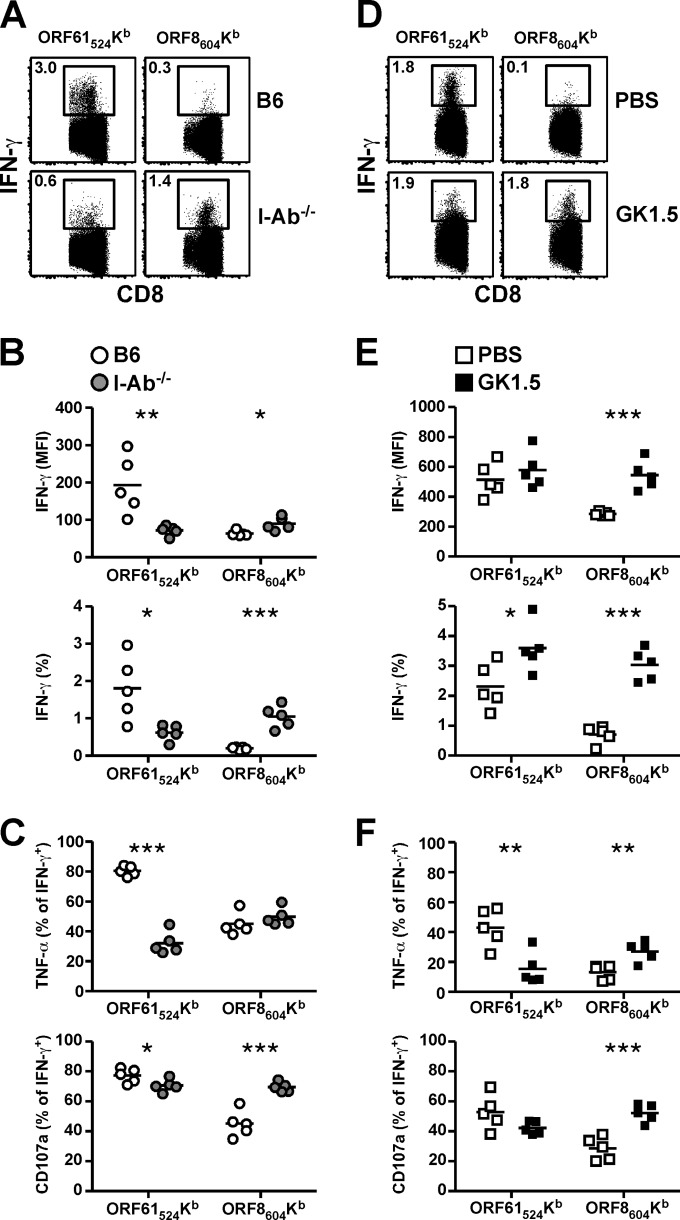
In order to determine the outcome of increased PD-1 expression and differential memory T cell phenotype, we investigated the ability of virus-specific CD8 T cells to synthesize IFN-γ following ex vivo antigen stimulation with or without CD4 T cell help (Fig. 5). In I-Ab−/− mice, we observed decreased IFN-γ production by ORF61524Kb-specific cells and increased IFN-γ production by ORF8604Kb-specific cells compared to B6 mice (Fig. 5A). These differences were clear when we compared overall IFN-γ mean fluorescence intensity and the percentage of cells that were IFN-γ+ (Fig. 5B). We then measured the proportion of IFN-γ+ cells that produced TNF-α or exhibited surface expression of CD107a, a marker of degranulation (Fig. 5C). There was a significant reduction in the percentage of IFN-γ+ ORF61524Kb-specific cells that coexpressed TNF-α or upregulated CD107a during stimulation. Remarkably, there was no change in the percentage of IFN-γ+ ORF8604Kb-specific cells that expressed TNF-α, and there was a significant increase in the percentage that exhibited CD107a surface expression (Fig. 5C). We observed similar results for ORF8604Kb-specific cells when we tested mice that had been treated with GK1.5 MAb: IFN-γ expression was higher, and a greater proportion of IFN-γ+ cells produced TNF-α and expressed CD107a (Fig. 5D to F). The only discrepancy between I-Ab−/− and GK1.5 MAb-treated mice was the difference in the percentage of ORF61524Kb-specific cells that produced IFN-γ (Fig. 5E). In the GK1.5 MAb-treated mice there was a small increase in ORF61524Kb-specific cells that produced IFN-γ, whereas in I-Ab−/− mice the percentage of IFN-γ+ cells that were TNF-α+ after stimulation was reduced (Fig. 5F). Although the reason for the discrepancy is unclear, overall it is evident that the functionality of ORF8604Kb-specific CD8 T cells is enhanced and the functionality of ORF61524Kb-specific CD8 T cells is impaired in the absence of CD4 T cell help.
FIG 5.
Enhanced functionality of ORF8604Kb-specific CD8 T cells. One month after infection, spleens were harvested and stimulated with cognate peptide in the presence of anti-CD107a antibody and brefeldin A. Samples then were stained for intracellular accumulation of IFN-γ and TNF-α. (A and D) Representative dot plots show IFN-γ production in B6 or I-Ab−/− mice (A) or in B6 mice treated with PBS or GK1.5 MAb (D). (B and E) Enumeration of the mean fluorescence intensity (MFI) and percentage of tetramer+ cells that are IFN-γ+. (C and F) The percentage of tetramer+ IFN-γ+ CD8 T cells that are TNF-α+ (top graph) or exhibit surface CD107a expression (bottom graph) (n = 5/group, representative of at least 2 experiments). *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001 (Student's t test).
DISCUSSION
Mucosal infection of mice with γHV68 leads to acute replication at the sites of inoculation and concomitant establishment of latency in dendritic cells, macrophages, and B cells mainly in the spleen (23). In the absence of either B cells or T cells, the virus is never fully controlled, and latent virus reactivates, leading to progressive disease and eventually death (3, 24). CD4 T cells are particularly important for full protection; they are key orchestrators of anti-γHV68 immunity by providing necessary signals for CD8 T cells and B cells to each function properly, and CD4 T cells themselves provide direct antiviral functions. Without signals from CD4 T cells to provide help, CD8 T cell responses have been shown to be impaired in several ways, including cell survival, cytokine production, and secondary recall efficacy.
The functional exhaustion that results from a lack of adequate CD4 T cell help during persistent or chronic infections can lead to impaired control and often death. However, it is still unclear exactly how CD4 T cells provide help to CD8 T cells. Recent evidence suggests that CD4 help is indirectly transmitted to CD8 T cells through an antigen-presenting cell (APC) intermediate via interactions of CD40 and its ligand, CD154 (11, 25). These signals then instruct the APC to regulate CD8 T cell responses via CD27 and CD70 interactions (26, 27). The CD8 T cells that arise exhibit increased expression of TNF-related apoptosis-inducing ligand (TRAIL) and PD-1, thereby becoming susceptible to increased apoptosis and severe functional and proliferative impairment (12, 28).
In the γHV68 infection model, supporting CD40 signaling with an agonist antibody restores functionality to γHV68-specific CD8 T cells in I-Ab−/− mice (11, 25). However, the lack of helper signals can be overcome in other ways. In HSV-1 infection, for example, antiviral CD8 T cells in the trigeminal ganglion initially exhibit profound impairment, but over time only the most functional cells are retained (29). Therefore, on a population level, CD4 help is only transiently required. In other models, such as acute lymphocytic choriomeningitis virus (LCMV)-Armstrong infection, CD4 help during the primary response is dispensable for effector cell generation but required for secondary recall responses (6, 30). However, chronic functional exhaustion and depletion of helpless CD8 T cell responses occurs following persistent LCMV-clone 13 infection (31, 32). Thus, persistent antigen has a profound impact on the actions of CD4 T cell help. Indeed, high viral loads might contribute to the shift in CD8 T cell epitope hierarchy we see here, as mice deficient in CD4 T cells have been shown to exhibit high viral loads (3). Interestingly, numbers of ORF61524Kb-specific cells, which are reduced late after infection in I-Ab−/− mice, are also lower in mice following infection with a latency-null virus (17). Thus, more work is needed to fully understand the influence of antigenic load on the virus-specific CD8 T cell response.
I-Ab−/− mice lack CD4 T cells because they cannot be selected in the thymus. CD8 T cells in these mice mature in the absence of CD4 T cells; thus, they never receive signals from CD4 T cells. γHV68 infection in I-Ab−/− mice leads to a progressive loss of CD8 T cell control of viral latency, and the mice ultimately succumb to the infection (3). The antiviral CD8 T cells that develop after γHV68 infection in I-Ab−/− mice are characterized by profound expression of the inhibitory receptor PD-1, and blocking PD-1/PD-L1 interactions with monoclonal antibodies reduced viral reactivation (11). However, helpless CD8 T cell functionality in I-Ab−/− mice is less defined, as some studies have demonstrated direct functional deficiency and others have failed to find such defects (8, 11). Part of this discrepancy may be due to the methods used to measure functions or to the functions measured. Indeed, as T cell polyfunctionality has become a recognized measure of potency, determining the extent of functional impairment of helpless CD8 T cells requires more specific measurements. In addition, CD8 T cells that mature in an environment deficient in peptide-MHC-II interactions have recently been shown to be hyperactive in their proliferative capacity in lymphopenic conditions (33). Due to these concerns, we also examined the role of CD4 help in B6 mice treated with GK1.5 MAb, which depletes CD4 T cells. In these mice, the CD8 T cells develop in a normal environment and CD4 T cells are depleted just prior to infection. In both situations subdominant ORF8604Kb-specific CD8 T cells are promoted within the response hierarchy and exhibit improved effector functionality in spite of increased PD-1 expression and concurrent decreased functionality of the immunodominant ORF61524Kb-specific responses.
To our knowledge, this is the first study to examine immunodominant and subdominant γHV68-specific CD8 T cell responses in the absence of CD4 T cell help. However, it is not the first study to investigate the role of help on different CD8 specificities in persistent infections. We have previously shown that the infectious mononucleosis-like expansion of T cell receptor Vβ4+ CD8 T cells in γHV68 infection is dependent on the presence of CD4 T cells (14). These cells likely are stimulated to expand by actions of the viral M1 protein (34), and it remains unclear how CD4 T cell help regulates this process. In chronic murine cytomegalovirus (MCMV) infection, the IE3-specific CD8 T cell response greatly expands well after the establishment of viral latency (35, 36). This inflationary response has been shown to have a differential requirement for CD4 T cell help than the noninflationary responses; in the absence of CD4 T cell help, there is no inflation of the IE3-specific response, and the noninflationary responses are not functionally affected under helpless conditions (15). Thus, in these two infectious model systems, responses that greatly expand appear more dependent on signals provided by CD4 T cells than their noninflationary counterparts. In addition, in polyomavirus (PyV) infections, PyV-specific CD8 T cells are differentially dependent on CD4 T cell help in different allogeneic strains of inbred mice as well as differentially dependent on CD4 T cell help during the acute and persistent phases of infection (37), as we observe here.
Our data call into question the inhibitory activity of PD-1. Although subdominant ORF8604Kb-specific CD8 T cells highly upregulate PD-1 expression, they are actually numerically and functionally enhanced in the absence of CD4 help. The immunodominant ORF61524Kb-specific response, however, is impaired without CD4 T cells. How ORF8604Kb-specific CD8 T cell function is maintained in the face of high PD-1 expression is still unclear. As these responses occur within the same animal, the overall latent viral loads and reactivations must be similar under these conditions, and we can eliminate the overall inflammatory milieu in mediating these epitope-specific differences. Thus, we can exclude PD-1 expression, gross inflammation, and latent viral loads in driving these different effects, although we cannot rule out subtle differences in the presentation of the viral epitopes. Interestingly, we did observe significant increases in CD127− KLRG1− EECs in ORF61524Kb-specific, but not ORF8604Kb-specific, CD8 T cells at day 35 p.i. in both models of CD4 T cell deficiency. Therefore, our data suggest that impaired T cell function is related to impaired conversion of effector cells to CD127+ effector memory cells. Why cells of subdominant specificity would react differently is still an open question.
Taken together, our data demonstrate complex epitope-specific differences in the requirement for antiviral CD8 T cells to receive CD4 T cell-driven help. These findings may be particularly relevant in understanding the generation, function, and maintenance of γHV-specific CD8 T cell responses in HIV+ patients with reduced CD4 T cell numbers, especially those who fail to reconstitute CD4 T cell numbers following antiretroviral treatment (38).
ACKNOWLEDGMENTS
We thank Kathleen Lanzer and Tres Cookenham for excellent technical assistance.
This work was supported by NIH grants AI084327 (M.L.F.), AI042927, AI082919, and CA148250 (M.A.B.) and funding from the Trudeau Institute.
Footnotes
Published ahead of print 30 April 2014
REFERENCES
- 1.Doherty PC, Christensen JP, Belz GT, Stevenson PG, Sangster MY. 2001. Dissecting the host response to a gamma-herpesvirus. Philos. Trans. R. Soc. Lond. B Biol. Sci. 356:581–593. 10.1098/rstb.2000.0786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Usherwood EJ, Roy DJ, Ward K, Surman SL, Dutia BM, Blackman MA, Stewart JP, Woodland DL. 2000. Control of gammaherpesvirus latency by latent antigen-specific CD8(+) T cells. J. Exp. Med. 192:943–952. 10.1084/jem.192.7.943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cardin RD, Brooks JW, Sarawar SR, Doherty PC. 1996. Progressive loss of CD8+ T cell-mediated control of a gamma-herpesvirus in the absence of CD4+ T cells. J. Exp. Med. 184:863–871. 10.1084/jem.184.3.863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu L, Usherwood EJ, Blackman MA, Woodland DL. 1999. T-cell vaccination alters the course of murine herpesvirus 68 infection and the establishment of viral latency in mice. J. Virol. 73:9849–9857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freeman ML, Burkum CE, Yager EJ, Woodland DL, Blackman MA. 2011. De novo infection of B cells during murine gammaherpesvirus 68 latency. J. Virol. 85:10920–10925. 10.1128/JVI.05027-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun JC, Bevan MJ. 2003. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science 300:339–342. 10.1126/science.1083317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stevenson PG, Belz GT, Altman JD, Doherty PC. 1998. Virus-specific CD8(+) T cell numbers are maintained during gamma-herpesvirus reactivation in CD4-deficient mice. Proc. Natl. Acad. Sci. U. S. A. 95:15565–15570. 10.1073/pnas.95.26.15565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Belz GT, Liu H, Andreansky S, Doherty PC, Stevenson PG. 2003. Absence of a functional defect in CD8+ T cells during primary murine gammaherpesvirus-68 infection of I-A(b−/−) mice. J. Gen. Virol. 84:337–341. 10.1099/vir.0.18821-0 [DOI] [PubMed] [Google Scholar]
- 9.Belz GT, Stevenson PG, Castrucci MR, Altman JD, Doherty PC. 2000. Postexposure vaccination massively increases the prevalence of gamma-herpesvirus-specific CD8+ T cells but confers minimal survival advantage on CD4-deficient mice. Proc. Natl. Acad. Sci. U. S. A. 97:2725–2730. 10.1073/pnas.040575197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu H, Andreansky S, Diaz G, Hogg T, Doherty PC. 2002. Reduced functional capacity of CD8+ T cells expanded by post-exposure vaccination of gamma-herpesvirus-infected CD4-deficient mice. J. Immunol. 168:3477–3483. 10.4049/jimmunol.168.7.3477 [DOI] [PubMed] [Google Scholar]
- 11.Dias P, Giannoni F, Lee LN, Han D, Yoon S, Yagita H, Azuma M, Sarawar SR. 2010. CD4 T-cell help programs a change in CD8 T-cell function enabling effective long-term control of murine gammaherpesvirus 68: role of PD-1-PD-L1 interactions. J. Virol. 84:8241–8249. 10.1128/JVI.00784-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fuse S, Tsai CY, Molloy MJ, Allie SR, Zhang W, Yagita H, Usherwood EJ. 2009. Recall responses by helpless memory CD8+ T cells are restricted by the up-regulation of PD-1. J. Immunol. 182:4244–4254. 10.4049/jimmunol.0802041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Molloy MJ, Zhang W, Usherwood EJ. 2011. Suppressive CD8+ T cells arise in the absence of CD4 help and compromise control of persistent virus. J. Immunol. 186:6218–6226. 10.4049/jimmunol.1003812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flano E, Woodland DL, Blackman MA. 1999. Requirement for CD4+ T cells in V beta 4+CD8+ T cell activation associated with latent murine gammaherpesvirus infection. J. Immunol. 163:3403–3408 [PubMed] [Google Scholar]
- 15.Snyder CM, Loewendorf A, Bonnett EL, Croft M, Benedict CA, Hill AB. 2009. CD4+ T cell help has an epitope-dependent impact on CD8+ T cell memory inflation during murine cytomegalovirus infection. J. Immunol. 183:3932–3941. 10.4049/jimmunol.0900227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gredmark-Russ S, Cheung EJ, Isaacson MK, Ploegh HL, Grotenbreg GM. 2008. The CD8 T-cell response against murine gammaherpesvirus 68 is directed toward a broad repertoire of epitopes from both early and late antigens. J. Virol. 82:12205–12212. 10.1128/JVI.01463-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Freeman ML, Lanzer KG, Cookenham T, Peters B, Sidney J, Wu TT, Sun R, Woodland DL, Sette A, Blackman MA. 2010. Two kinetic patterns of epitope-specific CD8 T-cell responses following murine gammaherpesvirus 68 infection. J. Virol. 84:2881–2892. 10.1128/JVI.02229-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freeman ML, Burkum CE, Jensen MK, Woodland DL, Blackman MA. 2012. Gamma-herpesvirus reactivation differentially stimulates epitope-specific CD8 T cell responses. J. Immunol. 188:3812–3819. 10.4049/jimmunol.1102787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu L, Flano E, Usherwood EJ, Surman S, Blackman MA, Woodland DL. 1999. Lytic cycle T cell epitopes are expressed in two distinct phases during MHV-68 infection. J. Immunol. 163:868–874 [PubMed] [Google Scholar]
- 20.Jin HT, Ahmed R, Okazaki T. 2011. Role of PD-1 in regulating T-cell immunity. Curr. Top. Microbiol. Immunol. 350:17–37. 10.1007/82_2010_116 [DOI] [PubMed] [Google Scholar]
- 21.Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, Gapin L, Kaech SM. 2007. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity 27:281–295. 10.1016/j.immuni.2007.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Obar JJ, Molloy MJ, Jellison ER, Stoklasek TA, Zhang W, Usherwood EJ, Lefrancois L. 2010. CD4+ T cell regulation of CD25 expression controls development of short-lived effector CD8+ T cells in primary and secondary responses. Proc. Natl. Acad. Sci. U. S. A. 107:193–198. 10.1073/pnas.0909945107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flano E, Husain SM, Sample JT, Woodland DL, Blackman MA. 2000. Latent murine gamma-herpesvirus infection is established in activated B cells, dendritic cells, and macrophages. J. Immunol. 165:1074–1081. 10.4049/jimmunol.165.2.1074 [DOI] [PubMed] [Google Scholar]
- 24.Weck KE, Kim SS, Virgin HW, Speck SH. 1999. B cells regulate murine gammaherpesvirus 68 latency. J. Virol. 73:4651–4661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sarawar SR, Lee BJ, Reiter SK, Schoenberger SP. 2001. Stimulation via CD40 can substitute for CD4 T cell function in preventing reactivation of a latent herpesvirus. Proc. Natl. Acad. Sci. U. S. A. 98:6325–6329. 10.1073/pnas.101136898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feau S, Garcia Z, Arens R, Yagita H, Borst J, Schoenberger SP. 2012. The CD4(+) T-cell help signal is transmitted from APC to CD8(+) T-cells via CD27-CD70 interactions. Nat. Commun. 3:948. 10.1038/ncomms1948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matter MS, Claus C, Ochsenbein AF. 2008. CD4+ T cell help improves CD8+ T cell memory by retained CD27 expression. Eur. J. Immunol. 38:1847–1856. 10.1002/eji.200737824 [DOI] [PubMed] [Google Scholar]
- 28.Janssen EM, Droin NM, Lemmens EE, Pinkoski MJ, Bensinger SJ, Ehst BD, Griffith TS, Green DR, Schoenberger SP. 2005. CD4+ T-cell help controls CD8+ T-cell memory via TRAIL-mediated activation-induced cell death. Nature 434:88–93. 10.1038/nature03337 [DOI] [PubMed] [Google Scholar]
- 29.Frank GM, Lepisto AJ, Freeman ML, Sheridan BS, Cherpes TL, Hendricks RL. 2010. Early CD4(+) T cell help prevents partial CD8(+) T cell exhaustion and promotes maintenance of herpes simplex virus 1 latency. J. Immunol. 184:277–286. 10.4049/jimmunol.0902373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun JC, Williams MA, Bevan MJ. 2004. CD4+ T cells are required for the maintenance, not programming, of memory CD8+ T cells after acute infection. Nat. Immunol. 5:927–933. 10.1038/ni1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matloubian M, Concepcion RJ, Ahmed R. 1994. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J. Virol. 68:8056–8063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. 2006. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 439:682–687. 10.1038/nature04444 [DOI] [PubMed] [Google Scholar]
- 33.Do JS, Valujskikh A, Vignali DA, Fairchild RL, Min B. 2012. Unexpected role for MHC II-peptide complexes in shaping CD8 T-cell expansion and differentiation in vivo. Proc. Natl. Acad. Sci. U. S. A. 109:12698–12703. 10.1073/pnas.1207219109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Evans AG, Moser JM, Krug LT, Pozharskaya V, Mora AL, Speck SH. 2008. A gammaherpesvirus-secreted activator of Vbeta4+ CD8+ T cells regulates chronic infection and immunopathology. J. Exp. Med. 205:669–684. 10.1084/jem.20071135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Munks MW, Cho KS, Pinto AK, Sierro S, Klenerman P, Hill AB. 2006. Four distinct patterns of memory CD8 T cell responses to chronic murine cytomegalovirus infection. J. Immunol. 177:450–458. 10.4049/jimmunol.177.1.450 [DOI] [PubMed] [Google Scholar]
- 36.Snyder CM, Cho KS, Bonnett EL, van Dommelen S, Shellam GR, Hill AB. 2008. Memory inflation during chronic viral infection is maintained by continuous production of short-lived, functional T cells. Immunity 29:650–659. 10.1016/j.immuni.2008.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kemball CC, Lee ED, Vezys V, Pearson TC, Larsen CP, Lukacher AE. 2005. Late priming and variability of epitope-specific CD8+ T cell responses during a persistent virus infection. J. Immunol. 174:7950–7960. 10.4049/jimmunol.174.12.7950 [DOI] [PubMed] [Google Scholar]
- 38.Lederman MM, Funderburg NT, Sekaly RP, Klatt NR, Hunt PW. 2013. Residual immune dysregulation syndrome in treated HIV infection. Adv. Immunol. 119:51–83. 10.1016/B978-0-12-407707-2.00002-3 [DOI] [PMC free article] [PubMed] [Google Scholar]