ABSTRACT
Primary attachment to cellular glycans is a critical entry step for most human viruses. Some viruses, such as herpes simplex virus type 1 (HSV-1) and hepatitis C virus (HCV), bind to heparan sulfate, whereas others, such as influenza A virus (IAV), bind to sialic acid. Receptor mimetics that interfere with these interactions are active against viruses that bind to either heparan sulfate or to sialic acid. However, no molecule that inhibits the attachment of viruses in both groups has yet been identified. Epigallocatechin gallate (EGCG), a green tea catechin, is active against many unrelated viruses, including several that bind to heparan sulfate or to sialic acid. We sought to identify the basis for the broad-spectrum activity of EGCG. Here, we show that EGCG inhibits the infectivity of a diverse group of enveloped and nonenveloped human viruses. EGCG acts directly on the virions, without affecting the fluidity or integrity of the virion envelopes. Instead, EGCG interacts with virion surface proteins to inhibit the attachment of HSV-1, HCV, IAV, vaccinia virus, adenovirus, reovirus, and vesicular stomatitis virus (VSV) virions. We further show that EGCG competes with heparan sulfate for binding of HSV-1 and HCV virions and with sialic acid for binding of IAV virions. Therefore, EGCG inhibits unrelated viruses by a common mechanism. Most importantly, we have identified EGCG as the first broad-spectrum attachment inhibitor. Our results open the possibility for the development of small molecule broad-spectrum antivirals targeting virion attachment.
IMPORTANCE This study shows that it is possible to develop a small molecule antiviral or microbicide active against the two largest groups of human viruses: those that bind to glycosaminoglycans and those that bind to sialoglycans. This group includes the vast majority of human viruses, including herpes simplex viruses, cytomegalovirus, influenza virus, poxvirus, hepatitis C virus, HIV, and many others.
INTRODUCTION
Antiviral drugs targeting viral entry offer several advantages. For example, they prevent viruses from infecting cells altogether. They also avoid the need for intracellular drug delivery. Some entry steps, such as primary attachment and fusion, are conserved among many unrelated viruses. Therefore, antiviral drugs targeting these common entry steps could also have broad-spectrum activity against unrelated viruses. Rigid amphipathic fusion inhibitors, for example, inhibit the formation of the negative membrane curvature required for fusion of all enveloped viruses (1, 2) and therefore inhibit the infectivity of multiple enveloped but otherwise unrelated viruses.
Unlike membrane fusion itself, viruses use three different types of primary attachments. The primary attachment of most human viruses requires a low-affinity interaction between basic binding pockets in the virion glycoproteins and negatively charged heparan sulfate moieties in cellular glycosaminoglycans (GAGs) (3–16). Attachment of another group of viruses, including influenza virus, requires similar low-affinity interactions with sialic acid-containing sialoglycans (SGs) (17–19). Another very small group of human viruses binds to neither heparan sulfate nor sialic acid moieties. The primary low-affinity attachment step often serves to concentrate virions on the cell surface to facilitate the higher-affinity interactions with secondary receptors (20). For influenza virus (and other viruses), however, the glycan moieties are the only known receptors.
Attachment to glycan moieties is therefore a step conserved among many unrelated viruses. Molecules that interfere with these low-affinity interactions often have antiviral activities. Such compounds act as receptor mimetics, competing for virion binding to cellular heparan sulfate or sialic acid moieties (21–25). However, such inhibitors are restricted to the viruses that bind to either heparan sulfate or sialic acid. No compound has yet been identified that inhibits the attachment of viruses in both groups, precluding the development of broad-spectrum small molecule inhibitors of attachment.
Polyphenolic compounds from green tea possess many beneficial properties, including antiviral and anticancer activities (26). The most abundant of these polyphenols are the green tea catechins. They are predominantly comprised of four compounds: epicatechin (EC), epigallocatechin (EGC), epicatechin gallate (ECG), and epigallocatechin gallate (EGCG). EGCG is the most active compound, with activity against human immunodeficiency virus (HIV), influenza A virus (IAV), enterovirus 71, adenovirus (AdV), hepatitis B virus (HBV), clinical isolates of herpes simplex viruses 1 and 2 (HSV-1 and -2), and hepatitis C virus (HCV), among others (27–35). Derivatives of EGCG, such as digallate dimers, also have antiviral activities (35). For the most part, EGCG has been shown to inhibit the infectivity of a broad range of unrelated enveloped and nonenveloped viruses. However, the specific antiviral mechanisms of EGCG remain unclear, as do the bases for its broad antiviral spectrum.
EGCG binds to a range of proteins, including virion glycoproteins (32, 36), which likely contributes to its ability to inhibit viral entry. EGCG interacts with the hemagglutinin (HA) envelope glycoprotein of IAV, which binds to a sialic acid terminally linked to a galactose in cell surface glycans (30, 37). EGCG also interacts with the HSV-1 glycoprotein gB, which binds to heparan sulfate residues on cellular glycans, and with gD, which binds to 3-O-sulfated heparan sulfate (and to herpesvirus entry mediator [HVEM] and nectin) (32, 38–40). EGCG inhibits binding of the HIV envelope glycoprotein gp120 to CD4 on cells (36). We have recently shown that EGCG inhibits the infectivity of HCV by inhibiting virion attachment (33). We proposed that the interactions of EGCG with viral glycoproteins might interfere with attachment of unrelated viruses.
Our objectives now are to characterize the antiviral mechanisms of EGCG, in order to identify the basis for its broad-spectrum activity. We selected HCV, vesicular stomatitis virus (VSV), Sindbis virus (SIN), vaccinia virus (VACV), HSV-1, adenovirus (AdV), IAV, murine cytomegalovirus (mCMV), and reovirus (RV) as model unrelated RNA or DNA viruses for this study. HCV, SIN, VACV, HSV-1, mCMV, and AdV bind to heparan sulfate moieties in cellular GAGs (12, 41), whereas IAV and RV bind to sialic acid linked to galactose on cellular glycans (17). The primary attachment of VSV requires electrostatic interactions that likely involve heparan sulfate, although the specific details are not well characterized (4). Although all of these viruses initially attach to glycan moieties, they otherwise differ in the presence or absence of an envelope, their secondary receptors, the membranes they fuse to and the fusion mechanisms, their genome compositions (RNA or DNA), their replication sites (cytoplasmic or nuclear) and replication strategies, and other characteristics.
Here, we show that EGCG acts directly on HCV, VSV, VACV, HSV-1, AdV, IAV, and RV virions to inhibit their primary attachment to cells. Surprisingly, we found that EGCG acts against these unrelated viruses through a common mechanism, by competing with both heparan sulfate and sialic acid for virion binding. Such inhibition of attachment of both types of viruses by a single molecule is unprecedented in the public literature. Our findings demonstrate that it is possible for a single small molecule to inhibit the binding of viruses that depend on heparan sulfate or on sialic acid for attachment, which opens the possibility to develop truly broad-spectrum antivirals targeting primary attachment.
MATERIALS AND METHODS
Compounds and reagents.
Epigallocatechin-3-gallate (EGCG), epicatechin (EC), heparin, N-acetylneuraminic acid, l-1-tosylamide-2-phenylmethyl chloromethyl ketone (TPCK)-treated trypsin, and 1,6-diphenyl-1,3,5-hexatriene (DPH) were purchased from Sigma-Aldrich (Oakville, Ontario, Canada). Suspensions of chicken erythrocytes (10%) were obtained from Lampire Biological Laboratories (Pipersville, PA).
Cells and viruses.
African green monkey Vero fibroblast cells and Madin-Darby canine kidney (MDCK) cells were cultured in Dulbecco's minimal essential medium (DMEM) supplemented with 5% fetal bovine serum (FBS), 50 U/ml penicillin, and 50 μg/ml streptomycin at 37°C in 5% CO2. Huh7.5, NIH 3T3, and HEK293 cells were cultured in DMEM with 10% FBS, 50 U/ml penicillin, and 50 μg/ml streptomycin at 37°C in 5% CO2. Herpes simplex virus 1 and 2 (HSV-1 and -2), murine cytomegalovirus (mCMV), vaccinia virus (VACV) strains Western Reserve (WR) and International Health Department-white (IHD-W), adenovirus type 5 (AdV), vesicular stomatitis virus (VSV), influenza A virus (IAV) strains A/Puerto Rico/8/1934 H1N1 (PR8), A/USSR/90/77 H1N1 (USSR), A/Aichi/2/1968 H3N2 (Aichi), and A/Port Chalmers/1/73 H3N2 (PC), hepatitis C virus strain JFH-1 (HCV), Sindbis virus (SIN), poliovirus (PV), and reovirus (RV) stocks were prepared according to standard procedures as described previously (2).
For VACV, Vero cells were infected with 0.05 PFU/cell and incubated at 33°C in 5% CO2 until full cytopathic effects were observed (∼48 h postinfection [hpi]). Cells were then harvested by scraping with sterile disposable lifters. The resulting suspensions were centrifuged at 4,000 rpm for 30 min at 4°C. The supernatants were collected, and virions were pelleted by centrifugation at 10,000 × g for 2 h at 4°C. Meanwhile, cell pellets were resuspended and lysed by 3 freeze-thaw cycles followed by sonication. Cellular debris was pelleted by centrifugation at 4,000 rpm for 30 min at 4°C, and the resulting supernatant was used to resuspend the virion pellet obtained from the initial supernatant. The viral stocks were then aliquoted and stored at −80°C.
Infectivity assays.
Vero or MDCK cells (5 × 105 cells/well in 6-well plates) were infected with approximately 200 PFU of HSV-1/2, VACV WR or IHD-W, VSV, SIN, PV, or RV for Vero cells or with IAV for MDCK cells preexposed for 10 min at 37°C to EGCG, EC, or dimethyl sulfoxide (DMSO) vehicle in DMEM (pH 7.2). Inocula were removed 1 h later, and monolayers were washed and overlaid with 2% methylcellulose in DMEM containing 5% FBS, with the following exceptions. For IAV, monolayers were overlaid with 0.8% agarose in DMEM containing 2 μg/ml TPCK-trypsin. For VACV, monolayers were overlaid with DMEM–5% FBS. Infected cells were incubated at 37°C in 5% CO2 until well-defined plaques developed (typically, 1 to 3 days postinfection). Cells were then fixed and stained with 1% (wt/vol) crystal violet–17% (vol/vol) methanol in H2O.
NIH 3T3 cells (3 × 105 cells/well in 12-well plates) were infected with approximately 100 PFU of mCMV RM427+, preexposed to EGCG, EC, or DMSO vehicle for 10 min at 37°C in DMEM (pH 7.2). Foci of infected cells were detected after 24 h using a LacZ cell detection kit (InvivoGen) as described previously (2).
Huh7.5 cells (9 × 104 cells/well in 12-well plates) were infected with approximately 200 focus-forming units (FFU) of HCV JFH-1, preexposed to EGCG, EC, or DMSO vehicle for 10 min at 37°C in DMEM (pH 7.2). Inocula were removed 4 h later, and monolayers were washed and overlaid with fresh DMEM–10% FBS containing no drug. Infected cells were fixed with cold methanol-acetone (1:1) after 72 h and processed for immunocytochemistry for HCV core protein as described previously (2).
Green fluorescent protein-expressing AdV (AdV-GFP) virions preexposed to EGCG or the DMSO vehicle control for 10 min at 37°C in DMEM (pH 7.2) were used to infect HEK293 cell monolayers as described previously (1). Infected cells expressing GFP were detected 24 h later using a fluorescence microscope (Leica DM IRB, Itzlar, Germany).
In all experiments, 50% effective concentrations (EC50s) were calculated by nonlinear regression analysis (unrestrained fit) using GraphPad Prism (version 5.0; GraphPad Software, Inc.).
Time-of-addition and dilution experiments.
Near-confluent Vero cell monolayers in 6-well plates were pretreated with EGCG or DMSO vehicle for 1 h at 37°C in DMEM (pH 7.2). Cells were washed with DMEM and infected with 200 PFU of HSV-1 or VSV for 1 h at 37°C. Infectivity was assessed by plaquing efficiency. Alternatively, Vero cells were first infected with 200 PFU of HSV-1 or VSV for 1 h at 37°C. After removing the inocula and washing the cells with DMEM, the infected cells were overlaid with EGCG in DMEM–5% FBS for either 4 or 48 h (after which fresh medium containing no EGCG was added). Infectivity was assessed after 48 h by plaquing efficiency.
For dilution experiments, approximately 2,000 PFU of HSV-1 or VSV was exposed to EGCG or DMSO vehicle for 10 min at 37°C. Virions were then diluted 10-fold prior to being added to Vero cell monolayers. The inocula were removed after 1 h, and the infected cells were overlaid with 2% methylcellulose in DMEM containing 5% FBS.
Labeling of VSV, HCV, IAV, HSV-1, and VACV with octadecyl rhodamine B (R18).
VSV, HCV, and IAV virions were labeled with octadecyl rhodamine B chloride (R18; Invitrogen) at self-quenching concentrations as described previously (2). HSV-1 (108 PFU) and VACV (106 PFU) virions were labeled with 1.8 μM (HSV-1) or 2.7 μM (VACV) R18 and otherwise prepared as described for VSV, HCV, and IAV (2).
Labeling of HSV-1, VSV, RV, AdV, and PV with [35S]methionine.
Round dishes (60-cm2) were seeded with Vero, HEK293T, or L929 cells (2.5 × 106 cells/dish) and incubated at 37°C in 5% CO2 for approximately 16 h. Vero cells were infected with HSV-1 (2.5 PFU/cell), VSV (5 PFU/cell), or PV (5 PFU/cell). HEK293T or L929 cells were infected with AdV (5 PFU/cell) or RV (5 PFU/cell). For each cell type, a mock-infected sample was also included. For HSV-1, VSV, PV, and RV, the plates were rocked every 10 min, and inocula were removed after 1 h at 37°C. For AdV, the plates were rocked every 10 min and the inoculum was removed after 30 min at 37°C. The infected cells were then washed twice with cold DMEM and overlaid with DMEM–5% FBS. Infected cells were methionine starved at 3 hpi (HSV-1, VSV, PV, AdV, and RV) by replacing the media with methionine-free DMEM–5% FBS. At 5 hpi, the cells were washed twice with warmed DMEM and overlaid with 4 ml (HSV-1 or VSV) or 5 ml (AdV, PV, or RV) of methionine-free DMEM–5% FBS supplemented with 42 μCi/ml l-[35S]methinione (PerkinElmer, Boston, MA). Supernatants were recovered when full cytopathic effects were observed, ranging from 6 to 48 hpi. The supernatants were centrifuged at 3,200 × g at 4°C for 30 min to remove cell debris. The resulting supernatant was collected and centrifuged at 10,000 × g for 2 h at 4°C. The viral pellet was resuspended in 100 μl of serum-free DMEM.
Titers of 35S-labeled viruses were determined by standard plaquing assays. To test incorporation of 35S, 1 μl of the labeled virions or mock preparation was fixed in 100 μl of 100% ethanol and added to scintillation vials containing 4 ml of aqueous scintillant. 35S radioactivity was measured using a Beckman Coulter LS 6500 scintillation counter. Virions were labeled to approximately 0.02 cpm/PFU (HSV-1 and VSV), 1 cpm/PFU (PV), 14 cpm/PFU (RV), or 7 cpm/PFU (AdV).
Lysis assay.
R18-labeled VSV or HCV virions were exposed for 10 min at 37°C to EGCG or DMSO vehicle in DMEM (pH 7.2). The virions so exposed were then added to polymethacrylate cuvettes (Sigma) containing 2.5 ml of 180 mM Na2HPO4–10 mM citric acid (pH 7.4) buffer. Fluorescence was excited at 560 nm and detected at 590 nm using a QuantaMaster 40 scanning spectrofluorometer (Photon Technology International) equipped with a 75-W xenon lamp. Emitted light was detected at 590 nm using a model 814 switchable photon-counting/analog photomultiplier detection unit with an R1527 photomultiplier tube, and data were collected using FeliX32 software (Photon Technology International). After establishing the basal signal for approximately 100 s, membranes were lysed with Triton X-100 (0.1%), and the total fluorescence was measured for the 100% lysis control.
Membrane fluidity assays.
DPH was dissolved in tetrahydrofuran and then added to 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC)-cholesterol liposomes or HCV virions (105 FFU) to a final concentration of 2 μM for 10 min at 37°C as described previously (2, 42). The DPH-labeled liposomes or virions were then incubated with EGCG, cholesterol, or DMSO vehicle for a further 10 min at 37°C in 200 mM sodium phosphate buffer (pH 7.4). DPH fluorescence was excited at 350 nm, and emission was measured at 450 nm. Fluorescence polarization (P) was calculated by P = (IVV – GIVH)/(IVV + 2GIVH), where IVV and IVH are the intensities (I) obtained with polarizers aligned vertically (V) and horizontally (H) relative to the vertically polarized excitation beam. G is the instrument grating correction factor, which is the intensity ratio of the vertical to horizontal emitted fluorescence (G = IHV/IHH) obtained when the sample is excited with horizontally polarized light.
Fusion assays.
R18-labeled HCV, IAV, or VSV virions (1 × 104 PFU or FFU) were exposed to 100 μM EGCG or DMSO vehicle for 10 min at 37°C in phenol red-free DMEM (pH 7.2), chilled on ice, and then adsorbed onto Huh7.5, MDCK, or Vero cells for 1 h at 4°C. Alternatively, virions were first adsorbed onto cells for 1 h at 4°C and then exposed to EGCG or DMSO vehicle. Fusion was triggered by lowering the pH to 5 and evaluated by R18 fluorescence dequenching as described previously (2).
Binding assays.
R18-labeled HSV-1, VSV, HCV, IAV, or VACV virions (∼1 × 104 PFU or FFU) were exposed for 10 min at 37°C to EGCG, DMSO, vehicle or 100 μg/ml heparin in phenol red-free DMEM (pH 7.2). The exposed virions were then chilled at 4°C for 15 min prior to being adsorbed onto prechilled Vero (HSV-1, VSV, and VACV), Huh7.5 (HCV), or MDCK (IAV) cells for 1 h at 4°C. After three washes with cold phosphate-buffered saline (PBS), the cells and attached virions were lysed with 0.1% Triton X-100 to fully dequench R18 fluorescence. Fluorescence was excited at 560 nm and detected at 590 nm using a QuantaMaster 40 spectrofluorometer. Fluorescence still attached to cells after the washes was normalized to the fluorescence of the input virions. The percentage of binding was then expressed relative to binding of virions treated with the vehicle control.
[35S]methionine-labeled virions (∼1 × 104 PFU) were exposed to EGCG, DMSO vehicle, or 100 μg/ml heparin for 10 min at 37°C in DMEM (pH 7.2). Next, the preexposed [35S]methionine-labeled virions were adsorbed onto Vero (HSV-1, VSV, and PV) or L929 (RV) cells for 1 h at 4°C before being washed three times with ice-cold PBS. For AdV, preexposed virions were mixed with 293T cells (1 × 106 cells) in suspension and incubated in Eppendorf tubes on ice for 1 h while being mixed by gentle pipetting every 10 min. AdV virion-cell complexes were washed three times by centrifugation at 300 × g. Radioactivity still attached to cells was measured using a Beckman Coulter LS 6500 scintillation counter. Binding was calculated as cpm bound to cells divided by total cpm (in the input, washes, and cells) and expressed as a percentage. The background cpm of the mock sample was subtracted for all samples. The percentage of binding was then expressed relative to binding of virions exposed to the vehicle control.
Heparin column chromatography.
R18-HSV-1 virions (105 PFU) were loaded onto a 1-ml HiTrap heparin Sepharose column (GE Healthcare Life Sciences, Westborough, MA) in 10 mM sodium phosphate (pH 7.4) containing 0.3 M NaCl (loading buffer). The column was then washed with 10 ml of loading buffer to remove unbound virions. We then eluted the heparin-bound virions with soluble heparin or equivalent concentrations of EGCG, EC, or sialic acid in loading buffer. Virions that were still bound after the elution were further eluted with 2 M NaCl in 10 mM sodium phosphate (pH 7.4). The flow rate was 1 ml/min for washes and elution. Eluted virions were detected by R18 fluorescence after lysis by 0.1% Triton X-100.
HCV virions (105 FFU) were loaded onto the heparin column in 10 mM sodium phosphate (pH 7.4). The column was washed with 10 ml of the same buffer and eluted with heparin, EGCG, EC, or sialic acid in the same buffer. Still-bound virions were then eluted with 2 M NaCl in 10 mM sodium phosphate (pH 7.4). Fractions were concentrated using Amicon 100,000 (100K) ultrafiltration tubes and analyzed for HCV RNA. Results are expressed as the percentage of the bound virions that were eluted by the compounds.
HCV RNA isolation and quantitation.
Viral RNA from each fraction was eluted using the Roche High Pure viral nucleic acid kit (Roche, Laval, QC, Canada), according to the manufacturer's instructions. The RNA was transcribed to cDNA using Superscript III reverse transcriptase (Invitrogen, Burlington, ON, Canada) and an HCV-specific primer (5′-GTG TTT CTT TTG GTT TTT CTT TGA GGT TTA GG-3′) (43). Quantitative real-time PCR was performed using the 7900HT Fast real-time PCR system (Applied Biosystems) and TaqMan universal PCR master mix (Applied Biosystems). We used primers amplifying the conserved 5′-untranslated region of the HCV genome (5′-TCT GCG GAA CCG GTG AGT A-3′ and 5′-GTG TTT CTT TTG GTT TTT CTT TGA GGT TTA GG-3′) and 5′–6-carboxyfluorescein (6-FAM)-CAC GGT CTA CGA GAC CTC CCG GGG CAC–6-carboxytetramethylrhodamine (TAMRA)-3′ as the HCV-specific detection probe (43, 44). Ten-fold dilutions from 101 to 106 copies of a linearized plasmid containing the sequence of HCV JFH-1 were used to generate a standard curve for quantitation.
Hemagglutination assays.
IAV virions (PR8, USSR, Aichi, and PC strains) were exposed to 100 μM EGCG or EC, 50 μg/ml heparin, and 50 μg/ml sialic acid or the equivalent volume of DMSO in DMEM for 15 min at 37°C. The virions were then serially diluted 2-fold in DMEM in 96-well round-bottom plates. Fifty microliters of a 0.5% suspension of chicken erythrocytes was added to each well and mixed by pipetting up and down. The plates were incubated for 1 to 2 h at room temperature before scanning.
RESULTS
EGCG inhibits the infectivity of unrelated viruses.
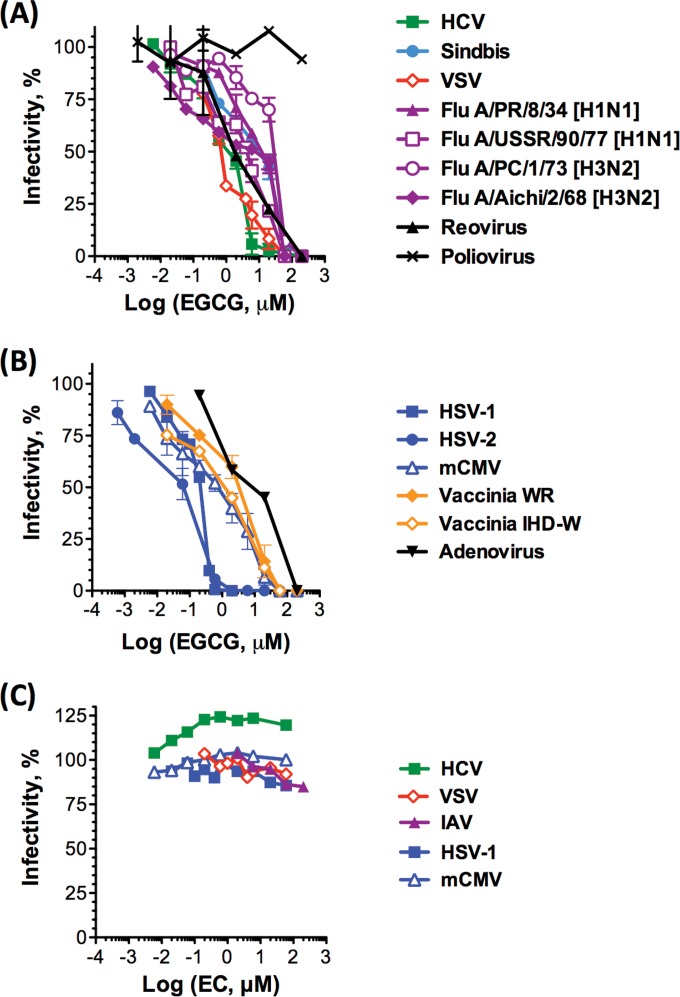
We first tested the effects of EGCG (Fig. 1) on the infectivity of unrelated enveloped or nonenveloped RNA or DNA viruses (Fig. 2). VSV, IAV, HCV, SIN, RV, PV, HSV-1/2, VACV, mCMV, or AdV virions (∼200 PFU) were exposed to EGCG or DMSO vehicle in DMEM (pH 7.2) for 10 min at 37°C prior to infecting monolayers of susceptible cells. Inocula were removed 1 h later, and the monolayers were overlaid with appropriate semisolid or liquid medium. Infected cells were incubated at 37°C in 5% CO2 until well-defined plaques developed (VSV, SIN, RV, PV, IAV, HSV-1/2, and VACV). Alternatively, foci of infected cells were identified by immunocytochemistry after 72 h (HCV) or LacZ or GFP expression after 24 h (for mCMV or AdV, respectively).
FIG 1.

Structures of epigallocatechin gallate (EGCG), epicatechin (EC), epicatechin gallate (ECG), and epigallocatechin (EGC).
FIG 2.
EGCG, but not EC, inhibits the infectivity of several unrelated viruses, including important human pathogens. Cell monolayers were infected with RNA (A and B) or DNA (B and C) virions preexposed to EGCG (A and B) or EC (C) for 10 min at 37°C. Infectivity was assessed by plaquing or focus-forming efficiency and is expressed as a percentage relative to that of the DMSO-treated control. Dose-response curves are shown as average ± standard deviation (SD) (n = 3).
EGCG inhibited the infectivity of VSV, four IAV strains (H1N1 or H3N2), HCV, SIN, RV, HSV-1, HSV-2, mCMV, two VACV strains (WR and IHD-W), and AdV at low micromolar concentrations (EC50s, 3.3, 7.3 to 40.1, 2.6, 15.8, 4.3, 0.1, 2.6, 5.4, 7.1 to 7.7, and 17.7 μM, respectively) (Fig. 2A and B). In contrast, EGCG did not inhibit the infectivity of PV even at 200 μM (Fig. 2A).
We next tested epicatechin (EC) (Fig. 1), a catechin chemically related to EGCG, against selected unrelated enveloped RNA and DNA viruses. In contrast to EGCG, EC did not inhibit the infectivity of HCV, VSV, HSV-1, mCMV, or IAV (PR8) at concentrations up to 200 μM (Fig. 2C).
EGCG acts on virions, not cells.
The inhibitory effect of EGCG could result from effects on the target cells, rather than direct effects on the virions. To test this model, we used VSV and HSV-1 as model RNA and DNA viruses to perform time-of-addition assays. We treated cells with EGCG or DMSO vehicle prior to infecting them with VSV or HSV-1 virions. Alternatively, cells were first infected with VSV or HSV-1 virions and then exposed to EGCG or DMSO vehicle for either 4 or 48 h. EGCG did not inhibit VSV or HSV-1 plaquing under any of these conditions (Fig. 3A and B). To test for the possibility that EGCG may act on cellular factors exposed only when virions are added, we performed a dilution assay. VSV or HSV-1 virions were treated with EGCG and then diluted 10-fold prior to infecting cells, such that the cells were exposed to 10-fold-lower concentrations of EGCG than the virions. This dilution did not affect the inhibitory activity of EGCG, further supporting the conclusion that EGCG acts directly on the virions (Fig. 3C).
FIG 3.
EGCG does not inhibit infectivity by acting on cellular factors. Vero cell monolayers were infected with ∼200 PFU of HSV-1 or VSV, preexposed to EGCG or DMSO vehicle for 10 min at 37°C (A). Alternatively, cells were treated with EGCG or DMSO for 1 h at 37°C prior to infection or for 4 or 48 h after infection (B), or virions were treated with EGCG and then diluted 10-fold prior to infecting cells (C). Dose-response curves are shown as average ± range (n = 2).
EGCG has no effects on the integrity or fluidity of virion envelopes.
EGCG inhibited the infectivity of all enveloped viruses we tested (as well as the nonenveloped adenovirus and reovirus). EGCG can undergo chemical changes, such as oxidation and dimerization in solution (45). One possibility, therefore, was that EGCG or its reaction products could disrupt the integrity of virion envelopes or modulate their fluidity, which are both critical for virion infectivity (42, 46). To test for envelope lysis, VSV, HCV, or IAV virions labeled at self-quenching concentrations with R18 were exposed for 10 min at 37°C to EGCG or DMSO vehicle in DMEM (pH 7.2). EGCG did not induce the increase in R18 fluorescence that would result from dequenching if the envelopes were disrupted. In contrast, and as expected, 0.1% Triton X-100 (which lyses the envelopes) did increase the fluorescence (Fig. 4A).
FIG 4.
EGCG does not disrupt the integrity or fluidity of virion envelopes. (A) Self-quenched R18-labeled VSV, HCV, or IAV virions were exposed for 10 min at 37°C to EGCG or DMSO vehicle. R18 fluorescence was then measured. As a lysis control, 0.1% Triton X-100 was then added. EGCG did not induce the increase in R18 fluorescence that would result from the release of self-quenched R18 if the envelopes were disrupted. (B) DPH-labeled liposomes or HCV virions were treated with EGCG for 10 min at 37°C. DPH fluorescence polarization was measured.
We next used the DPH fluorescence polarization assay to test envelope fluidity (42, 47). In this assay, decreases in membrane fluidity increase the polarization of DPH fluorescence. DPH-labeled liposomes or HCV virions were treated with EGCG or cholesterol for 10 min at 37°C. In contrast to cholesterol, which decreases membrane fluidity and was therefore used as a control, EGCG did not induce any increase in DPH polarization. Therefore, EGCG has no major effects on membrane fluidity (Fig. 4B).
EGCG interacts with VSV, HSV-1, RV, and AdV surface proteins.
We next tested whether EGCG interacted with the surface proteins of VSV, HSV-1, RV, or AdV. We obtained the fluorescence spectra of the virions in the absence and presence of EGCG, using a wavelength of 280 nm to excite the tryptophan residues (including those in the virion surface proteins). The fluorescence intensity of the virions was quenched upon addition of EGCG (Fig. 5A), indicating an interaction between EGCG and tryptophan residues in virion surface proteins.
FIG 5.
EGCG interacts with HSV-1, VSV, RV, and AdV surface proteins. Fluorescence emission spectra of HSV-1, VSV, RV, or AdV virions in the absence or presence of EGCG. The excitation wavelength was 280 nm (to excite the tryptophan residues). EGCG quenched the emitted fluorescence of the tryptophan residues.
EGCG inhibits attachment of VSV, IAV, HCV, HSV-1, and VACV to cells.
One of the critical functions of virion surface (glyco)proteins is attachment to cellular receptors. We therefore tested whether the interaction of EGCG with the virion surface proteins interfered with the primary attachment of virions to cells. To test this model, R18-labeled VSV, IAV, HCV, HSV-1, or VACV virions preexposed to EGCG or DMSO vehicle in DMEM at pH 7.2 were adsorbed onto Vero (VSV, HSV-1, and VACV), MDCK (IAV), or Huh7.5 (HCV) cell monolayers at 4°C, to allow binding but not fusion. EGCG inhibited the attachment of VSV, IAV, HCV, HSV-1, and VACV to their target cells (EC50s, 3.0, 22.9, 48.3, 4.0, and 23.8 μM, respectively) (Fig. 6A).
FIG 6.
EGCG inhibits the attachment of enveloped and nonenveloped virions to cells. EGCG inhibits the binding of R18-labeled VSV, HSV-1, HCV, IAV, and VACV (A). EGCG also inhibits attachment of [35S]methionine-labeled VSV and HSV-1 (B) and [35S]methionine-labeled RV and AdV (C). However, EGCG did not affect the binding of [35S]methionine-labeled PV at the active concentrations for the other viruses (D). Virions preexposed to EGCG were adsorbed onto target cells for 1 h at 4°C. The radioactivity or fluorescence attached to the cells was then measured and normalized to total input and is presented as a percentage relative to attachment of DMSO vehicle-treated control virions (average ± range; n = 2). Several error bars are too small to be seen at this scale.
R18 attachment assays had not been used before. We therefore validated these assays by further testing the effects of EGCG on binding using conventional attachment assays with [35S]methionine-labeled virions. [35S]methionine-labeled VSV and HSV-1 virions preexposed to EGCG or DMSO were adsorbed onto Vero cells at 4°C. EGCG inhibited binding of 35S-labeled VSV and HSV-1 to cells to a similar extent to the heparin control (EC50s, 19.6 and 15.0 μM, respectively) (Fig. 6B). We also used the [35S]methionine binding assay to test nonenveloped viruses. EGCG inhibited the binding of RV and AdV (EC50s, 395.1 and 3.7 μM, respectively) (Fig. 6C). In contrast, EGCG slightly but reproducibly enhanced PV binding (∼110% compared to the vehicle control) at concentrations up to 200 μM (Fig. 6D). Only at 600 μM did EGCG inhibit PV binding to some extent (by ∼25%) (Fig. 6D).
Most unexpectedly, therefore, EGCG inhibited the attachment of virions that bind to either heparan sulfate (HSV-1, HCV, VSV, VACV, and AdV) or sialic acid (IAV and RV). EGCG is thus the first example of a small molecule that inhibits attachment of virions that bind to heparan sulfate or to sialic acid.
EGCG does not directly inhibit HCV, IAV, or VSV fusion.
If EGCG inhibits attachment, then it should also inhibit fusion, which occurs after attachment. We tested fusion of HCV, IAV, and VSV, which is triggered by low pH to fuse to endosomal membranes after clathrin-mediated endocytosis. These three viruses represent different classes of fusion proteins. Virions labeled with self-quenching concentrations of R18 were exposed to EGCG or DMSO vehicle in phenol red-free DMEM at pH 7.2 prior to mixing with Huh7.5 (HCV), MDCK (IAV), or Vero (VSV) cells. Fluorescence was dequenched by approximately 18% (HCV) or 10% (IAV and VSV) for virions treated with DMSO vehicle, as expected (2), but dequenching was inhibited to background levels when virions were treated with EGCG prior to adding them to cells (Fig. 7). These results could indicate that EGCG inhibits fusion as well as attachment. Therefore, we next tested whether EGCG directly inhibited fusion itself, independently of its effects on virion attachment. Virions were first allowed to attach to cells at 4°C prior to treatment with EGCG. Under these conditions, EGCG did not inhibit fusion (Fig. 7), in contrast to compounds that inhibit fusion directly (2). The effects of EGCG on entry, therefore, are at a step prior to fusion (such as binding).
FIG 7.

EGCG inhibits HCV, IAV, and VSV fusion only if virions are treated prior to attachment. R18-labeled HCV (A), IAV (B), or VSV (C) virions preexposed to EGCG were adsorbed onto target cells for 1 h at 4°C. Alternatively, virions were first adsorbed onto target cells for 1 h at 4°C, and then virion-cell complexes were treated with EGCG. Fusion was triggered by increasing the temperature to 37°C and lowering the pH to 5. Fusion was evaluated by fluorescence dequenching of R18.
EGCG competes with heparin for virion binding.
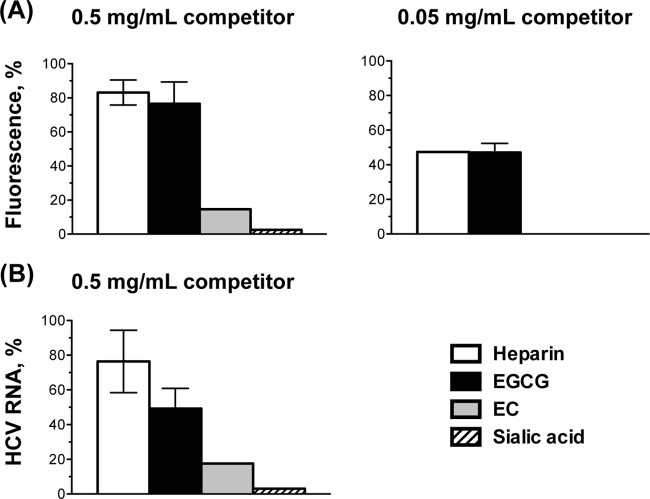
The primary, low-affinity attachment of many viruses, including all those we found to be inhibited by EGCG, is to heparan sulfate or sialic acid moieties in cellular glycans. We proposed as a model that EGCG might, most unexpectedly, compete with both heparan sulfate and sialic acid moieties for virion binding. To test this model, we first used heparin affinity chromatography. R18-labeled HSV-1 (105 PFU) or unlabeled HCV (105 FFU) was loaded onto a heparin column. The column was then washed to remove unbound virions. Bound virions were eluted with equivalent concentrations of soluble heparin, sialic acid, EGCG, or EC. The eluted virions were detected by R18 fluorescence (HSV-1) or RNA (HCV). EGCG eluted the HSV-1 virions from the heparin column very much like the heparin control. Approximately 75% of bound virions were eluted by 0.5 mg/ml EGCG or heparin and 50% by 0.05 mg/ml (Fig. 8A). In contrast, neither sialic acid nor the inactive catechin EC (at the highest concentration, 0.5 mg/ml) eluted the HSV-1 virions (Fig. 8A). EGCG (0.5 mg/ml) also eluted HCV virions bound to the heparin column, as did heparin (Fig. 8B), whereas neither sialic acid nor EC did (Fig. 8B).
FIG 8.
EGCG elutes HSV-1 and HCV virions bound to a heparin column with approximately equal efficiency to heparin. (A) R18-labeled HSV-1 (105 PFU) or (B) HCV (105 FFU) virions were loaded onto a heparin column. Bound virions were eluted with soluble heparin (as a positive control) or equivalent concentrations of EGCG, EC, and sialic acid. Eluted virions were detected by R18 fluorescence (average ± range; n = 2) (A) or viral RNA (average ± SD; n = 3) (B).
EGCG competes with sialic acid to inhibit hemagglutination.
The binding of IAV hemagglutinin to sialic acid agglutinates red blood cells. If EGCG inhibits IAV binding to sialic acid moieties in cellular glycans, then it should inhibit hemagglutination. We exposed IAV virions (H1N1 and H3N2 strains) to 100 μM (50 μg/ml) EGCG or EC and 50 μg/ml heparin or an equivalent volume of DMSO in DMEM for 15 min at 37°C. The treated virions were then tested for their ability to hemagglutinate chicken erythrocytes. Treatment with EGCG inhibited hemagglutination by all four IAV strains by 4-fold (Table 1 and Fig. 9). As expected, treatment with heparin or EC had no effects on hemagglutination (Table 1 and Fig. 9). Treatment with monomeric sialic acid only partially inhibited hemagglutination, as expected (24, 25, 48–50), and not to the same extent as EGCG (Fig. 9).
TABLE 1.
EGCG inhibits hemagglutination by influenza virusa
| Compound | Hemagglutination for straina: |
|||||||
|---|---|---|---|---|---|---|---|---|
| A/PR8 |
A/USSR |
A/Aichi |
A/PC |
|||||
| Titer | Ratio | Titer | Ratio | Titer | Ratio | Titer | Ratio | |
| DMSO | 6,400 | 1 | 3,200 | 1 | 16,000 | 1 | 16 | 1 |
| EGCG | 1,600 | 1/4 | 800 | 1/4 | 4,000 | 1/4 | 4 | 1/4 |
| EC | 6,400 | 1 | 3,200 | 1 | 16,000 | 1 | 16 | 1 |
| Heparin | 6,400 | 1 | 3,200 | 1 | 16,000 | 1 | 16 | 1 |
| Sialic acid | 3,200 | 1/2 | 3,200 | 1 | 16,000 | 1 | 8 | 1/2 |
Shown are hemagglutination titers for IAV virions (PR8, USSR, Aichi, and PC) exposed to 50 μg/ml (∼100 μM) EGCG or EC, 50 μg/ml heparin, or the equivalent volume of DMSO for 15 min at 37°C. The ratios shown represent the ratio of the titer with compound to the titer with DMSO.
FIG 9.

EGCG inhibits hemagglutination of erythrocytes by influenza virus. IAV virions (PR8, USSR, Aichi, and PC) were exposed to 50 μg/ml (∼100 μM) EGCG, EC, heparin, or sialic acid or an equivalent volume of DMSO for 15 min at 37°C. The treated virions were then added to chicken erythrocytes.
DISCUSSION
EGCG inhibits the infectivity of many unrelated viruses, including important human pathogens (28–34, 51–54). Here, we show that EGCG acts directly on virions to inhibit their entry into cells, without affecting the integrity or fluidity of virion envelopes. EGCG interacts with virion surface (glyco)proteins to inhibit virion attachment, but it does not affect fusion or other postbinding steps. EGCG inhibited the attachment of several unrelated enveloped or nonenveloped DNA or RNA viruses that bind to either heparan sulfate or sialic acid (HCV, VSV, HSV-1, VACV, AdV, IAV, and RV). Although these viruses differ in their secondary receptors, internalization pathways, sites and mechanisms of fusion, genome compositions, replication strategies, and replication sites, they do have in common their primary attachment to modified saccharide moieties in cellular glycans.
The primary attachment of many unrelated viruses (including HCV, HSV-1, and others we tested) is to heparan sulfate moieties in cellular glycans. Another group of viruses, including IAV, RV, rotavirus, enteroviruses, and Sendai virus, depend on binding to sialic acid-containing glycans (14, 17–19, 55–58). Treatment with heparin or related molecules inhibits the attachment of viruses that bind to heparan sulfate moieties, such as HCV and HSV-1 (12, 23, 41). Treatment with sialyl mimetics inhibits the attachment of viruses that bind to sialic acid moieties, such as IAV (48). Many such receptor mimetics with antiviral properties have been described. For example, heparin and other sulfated polysaccharides and polysulfonated compounds inhibit the adsorption of viruses that bind to heparan sulfate moieties (21–23). Sialyl mimetics inhibit the adsorption of IAV and other viruses that bind sialic acid (24, 25, 48–50). Such compounds act as receptor mimics, competing with cellular heparan sulfate or sialic acid moieties for virion binding. The different binding specificities of the two groups of viruses has so far precluded the development of broad-spectrum primary attachment inhibitors active against viruses that bind to both heparan sulfate and sialic acid.
EGCG, however, most uniquely inhibits the primary attachment of viruses with both types of primary attachment. EGCG (but not sialic acid) competes with heparin for HSV-1 and HCV virion binding, shown by heparin column chromatography. EGCG (but not heparin) inhibited IAV hemagglutination, which requires interaction of IAV HA with sialic acid residues on erythrocytes (59). In contrast, EGCG did not inhibit the infectivity of PV, which does not require binding to heparan sulfate or sialic acid moieties. These findings support the model that EGCG unexpectedly competes with both heparin and sialic acid moieties for virion binding. EGCG is the first molecule shown to inhibit similarly the attachment of viruses that bind to heparan sulfate or sialic acid.
EGCG has been reported to inhibit the infectivity of many unrelated viruses, including the heparan sulfate-binding HIV, adenovirus, hepatitis B virus, HSV-1/2, and HCV and the sialic acid-binding IAV and enterovirus 71. The specific antiviral mechanisms of EGCG have been unclear, and different mechanisms have even been proposed for different viruses (reviewed in reference (27). Most of the previously reported activities of EGCG, however, are consistent with the mechanism proposed here. EGCG was shown to be a strong inhibitor of HIV replication in cultured peripheral blood cells (60), although the mechanisms were not investigated. EGCG was later shown to inhibit recombinant gp120 binding to CD4+ T cells (36). Yamaguchi et al. also observed that EGCG inhibited HIV-1 binding to cells, although at concentrations 100 μM or higher (28). Specific effects of EGCG on HIV-1 gp120 binding to cellular heparan sulfate (61) were not tested. EGCG was shown to interact with the HSV-1 glycoproteins gB and gD to inhibit viral infectivity (32). gB interacts with heparan sulfate and gD with 3-O-sulfated heparan sulfate (38, 62). The effects of EGCG on gC, which also binds to heparan sulfate (62), were not investigated.
The activity of EGCG against influenza virus, a sialic acid-binding virus, has been described in several publications. EGCG inhibited the infectivity of influenza A and B viruses to MDCK cells (37). EGCG prevented influenza virus virions from adsorbing to MDCK cells and inhibited hemagglutination (30, 37) (Fig. 9). EGCG was shown to directly inhibit neuraminidase and viral RNA synthesis (30), as well as to agglutinate virions (37), but only at millimolar concentrations, far higher than the concentrations required to inhibit virion binding. Recently, EGCG was proposed to interfere with IAV fusion, but not adsorption or hemagglutination, by affecting the integrity of the viral envelope (34). In these experiments, IAV virions were not pretreated with EGCG, which is necessary for inhibition of binding and hemagglutination (our data) (30). Moreover, in contrast to Kim et al. (34), we did not observe any major effects of EGCG on IAV fusion (when virions were treated after attachment) or IAV envelope integrity at the concentrations required to inhibit infectivity and binding (Fig. 4).
EGCG was also active against nonenveloped viruses, through apparently similar mechanisms. EGCG inhibited AdV infection by targeting multiple steps of the viral replication cycle. A direct inhibition of the virion particles, by unknown mechanisms, was observed (29). It may well be that EGCG interacts with AdV fiber protein to inhibit its binding to cellular GAGs (63). EGCG also inhibited the infectivity of rotavirus and enteroviruses, which was attributed to interference with virion adsorption to cells (64). However, the specific targets were not tested. All of these activities are consistent with our proposed mechanism for EGCG, although of course other mechanisms are also possible for other viruses.
Isaacs et al. tested EGCG (mostly as a control for several oxidative dimerization products of EGCG) against a panel of enveloped and nonenveloped viruses (35). EGCG was active against the heparan sulfate-binding respiratory syncytial virus and Semliki Forest virus, but not against PV (which does not bind heparan sulfate or sialic acid), consistent with our results and model. In those experiments, EGCG failed to inhibit infectivity of measles virus, coxsackie A9 virus, coxsackie B4 virus, and echovirus 6 (35). Although many of these viruses are thought to bind to heparan sulfate, this binding is actually strain dependent. Some strains of these viruses require heparan sulfate for binding, and some do not (65–68). Isaacs et al. did not specify which strains were tested. Surprisingly, Isaacs et al. reported that EGCG was not active against VSV (35). In contrast, we found that EGCG inhibits VSV infectivity (Fig. 2) (EC50, 3.3 μM) and attachment (Fig. 6A and B) (EC50s, 3.0 and 19.6 μM, respectively) and prevents fusion (Fig. 7C). Differences in experimental conditions (buffer and incubation time) may explain these apparent discrepancies. Most of the experiments described by Isaacs et al. were performed in phosphate-buffered saline at pH 8.0 (35), whereas we used DMEM at pH ∼7.2. pH may well affect the ionization of the hydroxyl groups in EGCG and affect its activity, and the different cations can help form or disrupt polar interactions.
There are several possible mechanisms whereby EGCG may inhibit the attachment of viruses that bind to either heparin or sialic acid. A moiety of EGCG may resemble heparan sulfate, whereas another may resemble sialic acid-linked galactose. However, this simple mechanism appears unlikely. The catechins EC and epigallocatechin (EGC), which together contain two of the three moieties of EGCG, did not inhibit the infectivity of either heparin- or sialic acid-binding virions (our data) (30, 32, 33). On the other hand, epicatechin gallate (ECG) has a similar overall shape and polarity distribution to EGCG and has similar activity to EGCG against many viruses (30, 32). Another possibility is that EGCG may unexpectedly be able to interact with basic residues in the binding pockets of both heparan sulfate- and sialic acid-binding virion glycoproteins. We are now exploring these possible mechanisms.
EGCG, like other green tea polyphenols, is relatively unstable, poorly absorbed, and undergoes metabolic alterations (69). The serum concentration obtained after oral ingestion of pure EGCG (2 mg/kg in 100 ml of water, which is approximately the equivalent of two cups of green tea) was 0.17 μM (70), below the antiviral EC50. Although EGCG itself is therefore not likely to become a clinical antiviral drug, our findings show that it is indeed possible for a single molecule to have broad-spectrum activity against virion attachment. Other molecules with appropriate shape and polarity distribution may be designed to inhibit the attachment of unrelated viruses that bind to either heparin or sialic acid moieties.
In conclusion, EGCG inhibits the infectivity of many unrelated viruses, including important human pathogens. EGCG blocks the primary low-affinity attachment of unrelated virions to cells, including virions that bind to heparan sulfate or to terminal sialic acid in glycans. Our results show that EGCG competes with heparan sulfate or sialic acid moieties in cellular glycans for virion binding. In summary, we provide the first proof of principle that a single molecule can inhibit binding of viruses with both types of attachment. This most unexpected finding opens the possibility for the development of small molecule compounds with broad-spectrum activity against viral attachment.
ACKNOWLEDGMENTS
This research was supported by a Canadian Institute of Health Research grant to L.M.S. (MOP 13033) and the Burroughs Wellcome Fund (L.M.S.). L.M.S. is a Burroughs Wellcome Investigator in the Pathogenesis of Infectious Disease. C.C.C. acknowledges the Natural Sciences and Engineering Research Council of Canada and Alberta Innovates—Health Solutions for graduate scholarships.
We are grateful to Takaji Wakita, Charles Rice, Veronika von Messling, Edward Mocarski, Maya Shmulevitz, and David Evans for the kind gifts of HCV JFH-1, Huh7.5 cells, influenza virus strains, mCMV, reovirus T3, and vaccinia virus strains, respectively. We thank Justin Shields (in Lorne Tyrrell's group) and Abdullah Awadh for assistance with HCV RNA quantitation and Gary Eitzen for the use of his fluorometer. We are grateful to the Li Ka Shing Institute of Virology for continuous support.
Footnotes
Published ahead of print 30 April 2014
REFERENCES
- 1.St Vincent MR, Colpitts CC, Ustinov AV, Muqadas M, Joyce MA, Barsby NL, Epand RF, Epand RM, Khramyshev SA, Valueva OA, Korshun VA, Tyrrell DL, Schang LM. 2010. Rigid amphipathic fusion inhibitors, small molecule antiviral compounds against enveloped viruses. Proc. Natl. Acad. Sci. U. S. A. 107:17339–17344. 10.1073/pnas.1010026107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colpitts CC, Ustinov AV, Epand RF, Epand RM, Korshun VA, Schang LM. 2013. 5-(Perylen-3-yl)ethynyl-arabino-uridine (aUY11), an arabino-based rigid amphipathic fusion inhibitor, targets virion envelope lipids to inhibit fusion of influenza virus, hepatitis C virus, and other enveloped viruses. J. Virol. 87:3640–3654. 10.1128/JVI.02882-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Compton T, Nowlin DM, Cooper NR. 1993. Initiation of human cytomegalovirus infection requires initial interaction with cell surface heparan sulfate. Virology 193:834–841. 10.1006/viro.1993.1192 [DOI] [PubMed] [Google Scholar]
- 4.Conti C, Mastromarino P, Riccioli A, Orsi N. 1991. Electrostatic interactions in the early events of VSV infection. Res. Virol. 142:17–24. 10.1016/0923-2516(91)90023-V [DOI] [PubMed] [Google Scholar]
- 5.Dechecchi MC, Melotti P, Bonizzato A, Santacatterina M, Chilosi M, Cabrini G. 2001. Heparan sulfate glycosaminoglycans are receptors sufficient to mediate the initial binding of adenovirus types 2 and 5. J. Virol. 75:8772–8780. 10.1128/JVI.75.18.8772-8780.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Germi R, Crance JM, Garin D, Guimet J, Lortat-Jacob H, Ruigrok RW, Zarski JP, Drouet E. 2002. Cellular glycosaminoglycans and low density lipoprotein receptor are involved in hepatitis C virus adsorption. J. Med. Virol. 68:206–215. 10.1002/jmv.10196 [DOI] [PubMed] [Google Scholar]
- 7.Leistner CM, Gruen-Bernhard S, Glebe D. 2008. Role of glycosaminoglycans for binding and infection of hepatitis B virus. Cell. Microbiol. 10:122–133. 10.1111/j.1462-5822.2007.01023.x [DOI] [PubMed] [Google Scholar]
- 8.Lycke E, Johansson M, Svennerholm B, Lindahl U. 1991. Binding of herpes simplex virus to cellular heparan sulphate, an initial step in the adsorption process. J. Gen. Virol. 72:1131–1137. 10.1099/0022-1317-72-5-1131 [DOI] [PubMed] [Google Scholar]
- 9.Morikawa K, Zhao Z, Date T, Miyamoto M, Murayama A, Akazawa D, Tanabe J, Sone S, Wakita T. 2007. The roles of CD81 and glycosaminoglycans in the adsorption and uptake of infectious HCV particles. J. Med. Virol. 79:714–723. 10.1002/jmv.20842 [DOI] [PubMed] [Google Scholar]
- 10.Patel M, Yanagishita M, Roderiquez G, Bou-Habib DC, Oravecz T, Hascall VC, Norcross MA. 1993. Cell-surface heparan sulfate proteoglycan mediates HIV-1 infection of T-cell lines. AIDS Res. Hum. Retroviruses 9:167–174. 10.1089/aid.1993.9.167 [DOI] [PubMed] [Google Scholar]
- 11.Zhu Z, Gershon MD, Ambron R, Gabel C, Gershon AA. 1995. Infection of cells by varicella zoster virus: inhibition of viral entry by mannose 6-phosphate and heparin. Proc. Natl. Acad. Sci. U. S. A. 92:3546–3550. 10.1073/pnas.92.8.3546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.WuDunn D, Spear PG. 1989. Initial interaction of herpes simplex virus with cells is binding to heparan sulfate. J. Virol. 63:52–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bengali Z, Townsley AC, Moss B. 2009. Vaccinia virus strain differences in cell attachment and entry. Virology 389:132–140. 10.1016/j.virol.2009.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gentsch JR, Pacitti AF. 1985. Effect of neuraminidase treatment of cells and effect of soluble glycoproteins on type 3 reovirus attachment to murine L cells. J. Virol. 56:356–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Byrnes AP, Griffin DE. 1998. Binding of Sindbis virus to cell surface heparan sulfate. J. Virol. 72:7349–7356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neu U, Bauer J, Stehle T. 2011. Viruses and sialic acids: rules of engagement. Curr. Opin. Struct. Biol. 21:610–618. 10.1016/j.sbi.2011.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weis W, Brown JH, Cusack S, Paulson JC, Skehel JJ, Wiley DC. 1988. Structure of the influenza virus haemagglutinin complexed with its receptor, sialic acid. Nature 333:426–431. 10.1038/333426a0 [DOI] [PubMed] [Google Scholar]
- 18.Nilsson EC, Jamshidi F, Johansson SM, Oberste MS, Arnberg N. 2008. Sialic acid is a cellular receptor for coxsackievirus A24 variant, an emerging virus with pandemic potential. J. Virol. 82:3061–3068. 10.1128/JVI.02470-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reiter DM, Frierson JM, Halvorson EE, Kobayashi T, Dermody TS, Stehle T. 2011. Crystal structure of reovirus attachment protein sigma1 in complex with sialylated oligosaccharides. PLoS Pathog. 7:e1002166. 10.1371/journal.ppat.1002166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barton ES, Forrest JC, Connolly JL, Chappell JD, Liu Y, Schnell FJ, Nusrat A, Parkos CA, Dermody TS. 2001. Junction adhesion molecule is a receptor for reovirus. Cell 104:441–451. 10.1016/S0092-8674(01)00231-8 [DOI] [PubMed] [Google Scholar]
- 21.Baba M, Snoeck R, Pauwels R, de Clercq E. 1988. Sulfated polysaccharides are potent and selective inhibitors of various enveloped viruses, including herpes simplex virus, cytomegalovirus, vesicular stomatitis virus, and human immunodeficiency virus. Antimicrob. Agents Chemother. 32:1742–1745. 10.1128/AAC.32.11.1742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aguilar JS, Rice M, Wagner EK. 1999. The polysulfonated compound suramin blocks adsorption and lateral difusion of herpes simplex virus type-1 in Vero cells. Virology 258:141–151. 10.1006/viro.1999.9723 [DOI] [PubMed] [Google Scholar]
- 23.Lin LT, Chen TY, Lin SC, Chung CY, Lin TC, Wang GH, Anderson R, Lin CC, Richardson CD. 2013. Broad-spectrum antiviral activity of chebulagic acid and punicalagin against viruses that use glycosaminoglycans for entry. BMC Microbiol. 13:187. 10.1186/1471-2180-13-187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fazli A, Bradley SJ, Kiefel MJ, Jolly C, Holmes IH, von Itzstein M. 2001. Synthesis and biological evaluation of sialylmimetics as rotavirus inhibitors. J. Med. Chem. 44:3292–3301. 10.1021/jm0100887 [DOI] [PubMed] [Google Scholar]
- 25.Matsubara T, Onishi A, Saito T, Shimada A, Inoue H, Taki T, Nagata K, Okahata Y, Sato T. 2010. Sialic acid-mimic peptides as hemagglutinin inhibitors for anti-influenza therapy. J. Med. Chem. 53:4441–4449. 10.1021/jm1002183 [DOI] [PubMed] [Google Scholar]
- 26.Cabrera C, Artacho R, Gimenez R. 2006. Beneficial effects of green tea—a review. J. Am. Coll. Nutr. 25:79–99. 10.1080/07315724.2006.10719518 [DOI] [PubMed] [Google Scholar]
- 27.Steinmann J, Buer J, Pietschmann T, Steinmann E. 2013. Anti-infective properties of epigallocatechin-3-gallate (EGCG), a component of green tea. Br. J. Pharmacol. 168:1059–1073. 10.1111/bph.12009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamaguchi K, Honda M, Ikigai H, Hara Y, Shimamura T. 2002. Inhibitory effects of (−)-epigallocatechin gallate on the life cycle of human immunodeficiency virus type 1 (HIV-1). Antiviral Res. 53:19–34. 10.1016/S0166-3542(01)00189-9 [DOI] [PubMed] [Google Scholar]
- 29.Weber JM, Ruzindana-Umunyana A, Imbeault L, Sircar S. 2003. Inhibition of adenovirus infection and adenain by green tea catechins. Antiviral Res. 58:167–173. 10.1016/S0166-3542(02)00212-7 [DOI] [PubMed] [Google Scholar]
- 30.Song JM, Lee KH, Seong BL. 2005. Antiviral effect of catechins in green tea on influenza virus. Antiviral Res. 68:66–74. 10.1016/j.antiviral.2005.06.010 [DOI] [PubMed] [Google Scholar]
- 31.Xu J, Wang J, Deng F, Hu Z, Wang H. 2008. Green tea extract and its major component epigallocatechin gallate inhibits hepatitis B virus in vitro. Antiviral Res. 78:242–249. 10.1016/j.antiviral.2007.11.011 [DOI] [PubMed] [Google Scholar]
- 32.Isaacs CE, Wen GY, Xu W, Jia JH, Rohan L, Corbo C, Di Maggio V, Jenkins ECJ, Hillier S. 2008. Epigallocatechin gallate inactivates clinical isolates of herpes simplex virus. Antimicrob. Agents Chemother. 52:962–970. 10.1128/AAC.00825-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ciesek S, von Hahn T, Colpitts CC, Schang LM, Friesland M, Steinmann J, Manns MP, Ott M, Wedemeyer H, Meuleman P, Pietschmann T, Steinmann E. 2011. The green tea polyphenol epigallocatechin-3-gallate (EGCG) inhibits hepatitis C virus (HCV) entry. Hepatology 54:1947–1955. 10.1002/hep.24610 [DOI] [PubMed] [Google Scholar]
- 34.Kim M, Kim SY, Lee HW, Shin JS, Kim P, Jung YS, Jeong HS, Hyun JK, Lee CK. 2013. Inhibition of influenza virus internalization by (−)-epigallocatechin-3-gallate. Antiviral Res. 100:460–472. 10.1016/j.antiviral.2013.08.002 [DOI] [PubMed] [Google Scholar]
- 35.Isaacs CE, Xu W, Merz G, Hillier S, Rohan L, Wen GY. 2011. Digallate dimers of (−)-epigallocatechin gallate inactivate herpes simplex virus. Antimicrob. Agents Chemother. 55:5646–5653. 10.1128/AAC.05531-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kawai K, Tsuno NH, Kitayama J, Okaji Y, Yazawa K, Asakage M, Hori N, Watanabe T, Takahashi K, Nagawa H. 2003. Epigallocatechin gallate, the main component of tea polyphenol, binds to CD4 and interferes with gp120 binding. J. Allergy Clin. Immunol. 112:951–957. 10.1016/S0091-6749(03)02007-4 [DOI] [PubMed] [Google Scholar]
- 37.Nakayama M, Suzuki K, Toda M, Okubo S, Hara Y, Shimamura T. 1993. Inhibition of the infectivity of influenza virus by tea polyphenols. Antiviral Res. 21:289–299. 10.1016/0166-3542(93)90008-7 [DOI] [PubMed] [Google Scholar]
- 38.O'Donnell CD, Kovacs M, Akhtar J, Valyi-Nagy T, Shukla D. 2010. Expanding the role of 3-O sulfated heparan sulfate in herpes simplex virus type-1 entry. Virology 397:389–398. 10.1016/j.virol.2009.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Whitbeck JC, Peng C, Lou H, Xu R, Willis SH, Ponce de Leon M, Peng T, Nicola AV, Montgomery RI, Warner MS, Soulika AM, Spruce LA, Moore WT, Lambris JD, Spear PG, Cohen GH, Eisenberg RJ. 1997. Glycoprotein D of herpes simplex virus (HSV) binds directly to HVEM, a member of the tumor necrosis factor receptor superfamily and a mediator of HSV entry. J. Virol. 71:6083–6093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Di Giovine P, Settembre EC, Bhargava AK, Luftig MA, Lou H, Cohen GH, Eisenberg RJ, Krummenacher C, Carfi A. 2011. Structure of herpes simplex virus glycoprotein D bound to the human receptor nectin-1. PLoS Pathog. 7:e1002277. 10.1371/journal.ppat.1002277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barth H, Schnober EK, Zhang F, Linhardt RJ, Depla E, Boson B, Cosset FL, Patel AH, Blum HE, Baumert TF. 2006. Viral and cellular determinants of the hepatitis C virus envelope-heparan sulfate interaction. J. Virol. 80:10579–10590. 10.1128/JVI.00941-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Anggakusuma , Colpitts CC, Schang LM, Rachmawati H, Frentzen A, Pfaender S, Behrendt P, Brown RJ, Bankwitz D, Steinmann J, Ott M, Meuleman P, Rice CM, Ploss A, Pietschmann T, Steinmann E. Turmeric curcumin inhibits entry of all hepatitis C virus genotypes into human liver cells. Gut. Jul 31, 2013. [DOI] [PubMed] [Google Scholar]
- 43.Santer DM, Ma MM, Hockman D, Landi A, Tyrrell DL, Houghton M. 2013. Enhanced activation of memory, but not naive, B cells in chronic hepatitis C virus-infected patients with cryoglobulinemia and advanced liver fibrosis. PLoS One 8:e68308. 10.1371/journal.pone.0068308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li C, Candotti D, Allain JP. 2001. Production and characterization of monoclonal antibodies specific for a conserved epitope within hepatitis C virus hypervariable region 1. J. Virol. 75:12412–12420. 10.1128/JVI.75.24.12412-12420.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sang S, Lee MJ, Hou Z, Ho CT, Yang CS. 2005. Stability of tea polyphenol (−)-epigallocatechin-3-gallate and formation of dimers and epimers under common experimental conditions. J. Agric. Food Chem. 53:9478–9484. 10.1021/jf0519055 [DOI] [PubMed] [Google Scholar]
- 46.Harada S. 2005. The broad anti-viral agent glycyrrhizin directly modulates the fluidity of plasma membrane and HIV-1 envelope. Biochem. J. 392:191–199. 10.1042/BJ20051069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shinitzky M, Inbar M. 1976. Microviscosity parameters and protein mobility in biological membranes. Biochim. Biophys. Acta 433:133–149. 10.1016/0005-2736(76)90183-8 [DOI] [PubMed] [Google Scholar]
- 48.Matrosovich M, Klenk HD. 2003. Natural and synthetic sialic acid-containing inhibitors of influenza virus receptor binding. Rev. Med. Virol. 13:85–97. 10.1002/rmv.372 [DOI] [PubMed] [Google Scholar]
- 49.Sun XL. 2007. Recent anti-influenza strategies in multivalent sialyloligosaccharides and sialylmimetics approaches. Curr. Med. Chem. 14:2304–2313. 10.2174/092986707781696582 [DOI] [PubMed] [Google Scholar]
- 50.Reuter JD, Myc A, Hayes MM, Gan Z, Roy R, Qin D, Yin R, Piehler LT, Esfand R, Tomalia DA, Baker JRJ. 1999. Inhibition of viral adhesion and infection by sialic-acid-conjugated dendritic polymers. Bioconjug. Chem. 10:271–278. 10.1021/bc980099n [DOI] [PubMed] [Google Scholar]
- 51.Calland N, Albecka A, Belouzard S, Wychowski C, Duverlie G, Descamps V, Hober D, Dubuisson J, Rouille Y, Seron K. 2012. (−)-Epigallocatechin-3-gallate is a new inhibitor of hepatitis C virus entry. Hepatology 55:720–729. 10.1002/hep.24803 [DOI] [PubMed] [Google Scholar]
- 52.Chang LK, Wei TT, Chiu YF, Tung CP, Chuang JY, Hung SK, Li C, Liu ST. 2003. Inhibition of Epstein-Barr virus lytic cycle by (−)-epigallocatechin gallate. Biochem. Biophys. Res. Commun. 301:1062–1068. 10.1016/S0006-291X(03)00067-6 [DOI] [PubMed] [Google Scholar]
- 53.He W, Li LX, Liao QJ, Liu CL, Chen XL. 2011. Epigallocatechin gallate inhibits HBV DNA synthesis in a viral replication-inducible cell line. World J. Gastroenterol. 17:1507–1514. 10.3748/wjg.v17.i11.1507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ho HY, Cheng ML, Weng SF, Leu YL, Chiu DT. 2009. Antiviral effect of epigallocatechin gallate on enterovirus 71. J. Agric. Food Chem. 57:6140–6147. 10.1021/jf901128u [DOI] [PubMed] [Google Scholar]
- 55.Nokhbeh MR, Hazra S, Alexander DA, Khan A, McAllister M, Suuronen EJ, Griffith M, Dimock K. 2005. Enterovirus 70 binds to different glycoconjugates containing alpha2,3-linked sialic acid on different cell lines. J. Virol. 79:7087–7094. 10.1128/JVI.79.11.7087-7094.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Isa P, Arias CF, Lopez S. 2006. Role of sialic acids in rotavirus infection. Glycoconj. J. 23:27–37. 10.1007/s10719-006-5435-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Villar E, Barroso IM. 2006. Role of sialic acid-containing molecules in paramyxovirus entry into the host cell: a minireview. Glycoconj. J. 23:5–17. 10.1007/s10719-006-5433-0 [DOI] [PubMed] [Google Scholar]
- 58.Scott SA, Holloway G, Coulson BS, Szyczew AJ, Kiefel MJ, von Itzstein M, Blanchard H. 2005. Crystallization and preliminary X-ray diffraction analysis of the sialic acid-binding domain (VP8*) of porcine rotavirus strain CRW-8. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 61:617–620. 10.1107/S1744309105013849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Haff RF, Stewart RC. 1965. Role of sialic acid receptors in adsorption of influenza virus to chick embryo cells. J. Immunol. 94:842–851 [PubMed] [Google Scholar]
- 60.Fassina G, Buffa A, Benelli R, Varnier OE, Noonan DM, Albini A. 2002. Polyphenolic antioxidant (−)-epigallocatechin-3-gallate from green tea as a candidate anti-HIV agent. AIDS 16:939–941. 10.1097/00002030-200204120-00020 [DOI] [PubMed] [Google Scholar]
- 61.Vives RR, Imberty A, Sattentau QJ, Lortat-Jacob H. 2005. Heparan sulfate targets the HIV-1 envelope glycoprotein gp120 coreceptor binding site. J. Biol. Chem. 280:21353–21357. 10.1074/jbc.M500911200 [DOI] [PubMed] [Google Scholar]
- 62.Herold BC, WuDunn D, Soltys N, Spear PG. 1991. Glycoprotein C of herpes simplex virus type 1 plays a principal role in the adsorption of virus to cells and in infectivity. J. Virol. 65:1090–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dechecchi MC, Tamanini A, Bonizzato A, Cabrini G. 2000. Heparan sulfate glycosaminoglycans are involved in adenovirus type 5 and 2-host cell interactions. Virology 268:382–390. 10.1006/viro.1999.0171 [DOI] [PubMed] [Google Scholar]
- 64.Mukoyama A, Ushijima H, Nishimura S, Koike H, Toda M, Hara Y, Shimamura T. 1991. Inhibition of rotavirus and enterovirus infections by tea extracts. Jpn. J. Med. Sci. Biol. 44:181–186. 10.7883/yoken1952.44.181 [DOI] [PubMed] [Google Scholar]
- 65.Goodfellow IG, Sioofy AB, Powell RM, Evans DJ. 2001. Echoviruses bind heparan sulfate at the cell surface. J. Virol. 75:4918–4921. 10.1128/JVI.75.10.4918-4921.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McLeish NJ, Williams CH, Kaloudas D, Roivainen MM, Stanway G. 2012. Symmetry-related clustering of positive charges is a common mechanism for heparan sulfate binding in enteroviruses. J. Virol. 86:11163–11170. 10.1128/JVI.00640-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Feldman SA, Audet S, Beeler JA. 2000. The fusion glycoprotein of human respiratory syncytial virus facilitates virus attachment and infectivity via an interaction with cellular heparan sulfate. J. Virol. 74:6442–6447. 10.1128/JVI.74.14.6442-6447.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Terao-Muto Y, Yoneda M, Seki T, Watanabe A, Tsukiyama-Kohara K, Fujita K, Kai C. 2008. Heparin-like glycosaminoglycans prevent the infection of measles virus in SLAM-negative cell lines. Antiviral Res. 80:370–376. 10.1016/j.antiviral.2008.08.006 [DOI] [PubMed] [Google Scholar]
- 69.Smith TJ. 2011. Green tea polyphenols in drug discovery—a success or failure? Expert Opin. Drug Discov. 6:589–595. 10.1517/17460441.2011.570750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee MJ, Maliakal P, Chen L, Meng X, Bondoc FY, Prabhu S, Lambert G, Mohr S, Yang CS. 2002. Pharmacokinetics of tea catechins after ingestion of green tea and (−)-epigallocatechin-3-gallate by humans. Cancer Epidemiol. Biomarkers Prev. 11:1025–1032 http://cebp.aacrjournals.org/content/11/10/1025.long [PubMed] [Google Scholar]