ABSTRACT
The immediate-early protein ICP0 from herpes simplex virus 1 (HSV-1) plays pleiotropic roles in promoting viral lytic replication and reactivation from latency. Most of the known actions of ICP0 occur in the nucleus and are thought to involve the E3 ubiquitin ligase activity of its RING finger domain, which targets proteins for degradation via the proteasome. Although ICP0 translocates to the cytoplasm as the infection progresses, little is known about its activities in this location. Here, we show that cytoplasmic ICP0 has two distinct functions. In primary cell cultures and in an intravaginal mouse model, cytoplasmic ICP0 promotes viral replication in the absence of an intact RING finger domain. Additionally, ICP0 blocks the activation of interferon regulatory factor 3 (IRF3), a key transcription factor of the innate antiviral response, in a mechanism that requires the RING finger domain but not the proteasome. To our knowledge, this is the first observation of a proteasome-independent function of the RING finger domain of ICP0. Collectively, these results underscore the importance of cytoplasm-localized ICP0 and the diverse nature of its activities.
IMPORTANCE Despite ICP0 being a well-studied viral protein, the significance of its cytoplasmic localization has been largely overlooked. This is, in part, because common experimental manipulations result in the restriction of ICP0 to the nucleus. By overcoming this constraint, we both further characterize the ability of cytoplasmic ICP0 to inhibit antiviral signaling and show that ICP0 at this site has unexpected activities in promoting viral replication. This demonstrates the importance of considering location when analyzing protein function and adds a new perspective to our understanding of this multifaceted protein.
INTRODUCTION
Herpes simplex virus 1 (HSV-1) is an enveloped, double-stranded DNA virus that is highly prevalent in human populations. One of the first proteins expressed during HSV-1 infection is infected-cell protein 0 (ICP0). A multifunctional protein, ICP0 is involved in regulating viral gene expression, promoting reactivation from latency, and optimizing the cellular environment to maximize replication (1). For the most part, the mechanisms used by ICP0 to achieve these varied tasks remain unclear. At present, almost all of the assorted actions of ICP0 are thought to require the E3 ubiquitin ligase activity of its RING finger domain, which has been implicated in the proteasome-dependent degradation of multiple cellular proteins (2–4). ICP0 has a nuclear localization signal (NLS) that initially causes it to translocate to the nucleus (5), and much of the work on ICP0 has focused on its roles in this location. However, as the infection progresses, ICP0 moves to the cytoplasm (6–10), and there are accumulating data suggesting that it also has important functions in this compartment (11, 12).
The innate immune response to virus infection is classically described as involving the production of type I interferon (IFN). Detection of conserved viral components by various pattern recognition receptors, including the Toll-like receptors (TLRs) and the retinoic-acid-inducible gene I (RIG-I)-like receptors (RLRs), results in the activation of a number of transcription factors, such as IFN-regulatory factor 3 (IRF3) and nuclear factor kappa B (NF-κB), leading to the production of IFN (13). Autocrine and paracrine signaling activated by IFN culminates in the expression of IFN-stimulated genes (ISGs), the products of which work collectively to limit viral replication and spread (14). Accordingly, viruses have evolved a tremendous diversity of strategies to evade the IFN response (15). For example, ICP0 decreases IRF3 activation and ISG expression (16–20). Initial work suggested this function required both the RING finger domain and the activity of the proteasome (18, 19), yet during a wild-type (WT) HSV-1 infection, IRF3 pathway components are not degraded (12, 18), although some model systems remain contradictory in this regard (20–22). Recently, it has been found that when ICP0 is restricted to the nucleus during infection, by mutation or treatment with proteasome inhibitors, it loses its ability to block IRF3 signaling, while cytoplasmic ICP0 efficiently inhibits the antiviral response, even in the absence of a functional proteasome (12). Similarly, ectopic expression of wild-type ICP0, which causes its retention in the nucleus, fails to prevent IRF3-mediated ISG induction (12, 23). Currently, the function of cytoplasmic ICP0 in opposing IRF3 activation remains unknown.
Given that the proteasome is not required for cytoplasmic ICP0 to impede antiviral signaling, we were interested in determining whether the RING finger itself is involved in this process. Typically, the E3 ubiquitin ligase activity of the RING finger is associated with proteasome-mediated degradation. However, there is mounting evidence to suggest that ubiquitin tagging does not act solely to target a protein for destruction but instead is involved in a variety of proteasome-independent signaling functions (reviewed in references 24 to 29). To investigate the role of the RING domain in the context of cytoplasmic ICP0, we generated a virus in which ICP0 is restricted to the cytoplasm and lacks the RING finger. Intriguingly, we found that despite the ability of cytoplasmic ICP0 to block ISG production in the absence of a functional proteasome, the RING finger is essential for ICP0 to inhibit the antiviral response. Unexpectedly, we also observed that cytoplasmic ICP0 has an important activity in promoting viral replication both in cell culture and in mice, even in the absence of the RING finger domain. These observations highlight two unknown aspects of ICP0: the RING finger can act in a proteasome-independent manner, and ICP0 has RING finger-independent functions in the cytoplasm.
MATERIALS AND METHODS
Reagents.
MG132 (Sigma) or an equal volume of the vehicle control dimethyl sulfoxide (DMSO) was added to the culture medium 30 min prior to infection at a concentration of 5 μM and was maintained in the medium at this concentration throughout the experiment. Poly(I·C) (GE Healthcare) was added directly to the culture medium at a concentration of 100 μg/ml.
Cells and viruses.
Human embryonic lung (HEL) fibroblasts and U2OS and Vero cells were purchased from the American Type Culture Collection (ATCC) and grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% (HEL and U2OS) or 5% (Vero) fetal bovine serum, 2 mM l-glutamine, 100 U/ml penicillin, and 100 mg/ml streptomycin. The virus strains used in this study are described in Table 1. 17 syn and D8/FXE-R were propagated on Vero cells, while all ICP0 mutants were grown on U2OS cells in the presence of 3 mM hexamethylene bisacetamide (HMBA) and purified on a 36% sucrose cushion. Viral infections were performed for 1 h in serum-free medium at 37°C and at a multiplicity of infection (MOI) of 10, unless otherwise stated.
TABLE 1.
ICP0 mutants used in this study
| Virus | Mutation | Phenotype | Reference |
|---|---|---|---|
| 17 syn | None | Wild type | 112 |
| D8 | Deletion of amino acids 475–548 (NLS) | ICP0 restricted to cytoplasm | 30 |
| FXE | Deletion of amino acids 106–150 (RING) | ICP0 lacks a functional RING finger and is mainly nuclear | 30 |
| D8/FXE | Deletion of amino acids 106–150 (RING) and 475–548 (NLS) | ICP0 restricted to cytoplasm; lack of a functional RING finger | This work |
| D8/FXE-R | D8 and FXE mutations are repaired to wild-type sequence | Wild type | This work |
| dl1403 | 2-kb deletion in ICP0 | ICP0 null | 111 |
Construction of recombinant D8/FXE and D8/FXE-R viruses.
To create ICP0 containing the D8 and FXE lesions, a XhoI-KpnI fragment from p110-FXE (9), which contains the FXE lesion, was inserted into XhoI-KpnI-cut p110-D8 (9). The resulting plasmid, p110-D8/FXE, was sequenced to verify the presence of the D8 and FXE lesions. The D8/FXE virus was constructed using homologous recombination following cotransfection of U2OS cells with infectious DNA from 17 syn and the linearized p110-D8/FXE plasmid. Plaques were screened on Vero cells, which are semipermissive only for ICP0-deficient viruses, and small plaques were isolated for further characterization. Viruses containing D8/FXE ICP0 were plaque purified three times, and the presence of the D8 and FXE lesions was confirmed by Western blot analysis and sequencing. The D8/FXE-R revertant was generated by homologous recombination in U2OS cells of infectious DNA from D8/FXE with the p110 plasmid and screened on Vero cells for large plaques. Plaque purification and confirmation were done as with D8/FXE.
Preparation of cell extracts.
For RIPA extracts, cells were washed twice in ice-cold phosphate-buffered saline (PBS) and then scraped into RIPA buffer (50 mM Tris-HCl, pH 7.4, 100 mM NaCl, 5 mM EDTA, 50 mM sodium fluoride, 40 mM β-glycerophosphate, 1% Triton X-100, 0.1% SDS, 1% sodium deoxycholate, 1 mM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride, 2 mM dithiothreitol, and 1× protease inhibitor cocktail [Sigma]), passed through a 25-gauge needle 5 times, and then centrifuged at 14,000 rpm for 20 min at 4°C. For cytoplasmic extracts, cells were washed once with ice-cold 1× PBS and once with ice-cold 0.2× PBS and then scraped into cytoplasmic buffer (10 mM HEPES, pH 7.3, 10 mM potassium chloride, 1.5 mM magnesium chloride, 50 mM sodium fluoride, 1 mM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride, 2 mM dithiothreitol, and 1× protease inhibitor cocktail [Sigma]). The lysates were incubated on ice for 10 min, and then Triton X-100 was added to a final concentration of 1%, and samples were spun at 12,000 × g for 3 min. Extracts were quantified via Bradford assay (Bio-Rad Laboratories).
Western blotting.
Twenty-five micrograms of the indicated extracts were separated via electrophoresis on 10% denaturing polyacrylamide gels, transferred onto polyvinylidene difluoride (Millipore) membranes, and blocked in 5% skim milk. Blots were probed with the following primary antibodies, as indicated, at 1:1,000: anti-ICP0 (Virusys Corporation), anti-actin (Santa Cruz SC-1616), anti-ISG-56 (provided by G. Sen, Cleveland Clinic), and anti-USP7 (R2B2; provided by L. Frappier, University of Toronto). Secondary antibodies conjugated to horseradish peroxidase were used at 1:5,000, and the signal was visualized via chemiluminescence.
Immunoprecipitation.
Anti-ICP0 antibody (5 μg) was incubated with 30 μl of Protein G Plus agarose (Thermo Scientific) for 1 h at 4°C. Beads were washed three times with immunoprecipitation buffer (50 mM Tris, pH 8.0, 150 mM NaCl, and 1% NP-40) and incubated with 500 μg of cytoplasmic extract for an additional 4 h. The beads were washed 5 times and boiled in sample buffer containing SDS and β-mercaptoethanol.
Immunofluorescence.
HEL cells were seeded onto coverslips and infected at 50% confluence. At the indicated times, the cells were fixed with 10% formalin (Sigma); permeabilized with 0.1% Triton X-100; and blocked with 3% goat serum, 3% bovine serum albumin (BSA), and 0.02% Tween 20. The cells were incubated with 1:250 anti-ICP0 and then 1:250 anti-mouse Alexa Fluor 488-conjugated secondary antibody (Invitrogen), and nuclei were stained with 1:10,000 Hoechst 33258 dye. All images were captured with a Leica DM-IRE2 inverted microscope and analyzed using Openlab software (Improvision).
Plaque assays.
HEL cells were infected at the indicated MOIs and grown in 1 ml medium for 24 h, and the cells were scraped into the medium and freeze-thawed three times. Serial dilutions of the resulting samples were used to infect U2OS cells in the presence of HMBA and 2% human serum, and after 3 days, the cells were fixed with methanol and stained with Giemsa (Sigma), and the plaques were counted.
Quantitative reverse transcription (RT)-PCR.
Six hours after infection with the indicated viruses, total RNA from HEL cells was harvested using TRIzol reagent (Invitrogen); 2.5 μg of RNA was DNase treated (DNA-free kit; Ambion), and then, 150 ng of each sample was reverse transcribed using 200 ng of a random 6-mer primer and 200 U of SuperScript II reverse transcriptase (Invitrogen). Samples were then analyzed in triplicate with universal PCR master mix and gene-specific TaqMan primers (Life Technologies): Hs01911452_s1 for IFIT1 and Hs99999905_m1 for glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Gene expression was normalized to GAPDH via the ΔΔCT method and expressed as a fold change relative to the internal control, poly(I·C).
Mice and HSV-1 infection.
Female C57BL/6 mice, 6 to 8 weeks of age, were purchased from Charles River Laboratory (Montreal, Quebec, Canada). The mice were housed under biosafety level 2 conditions in the Central Animal Facility at McMaster University. All mice were housed in level B rooms that followed a 12-h day and 12-h night schedule and were maintained under standard temperature-controlled conditions. All animal experiments were approved by the Animal Research Ethics Board (AREB) of McMaster University. To prepare mice for intravaginal infection, female mice were injected with 50 μl of 50-mg/ml Depo-Provera hormone (Pfizer Canada Inc., Kirkland, Quebec, Canada) subcutaneously 4 days prior to HSV-1 infection. To infect the mice, they were anesthetized by injectable anesthetic (150 μl of ketamine-xylazine [0.75 ml:0.25 ml]) given intraperitoneally and infected intravaginally with 105 PFU of the indicated viruses in 10 μl of PBS. The mice were kept on their backs under the influence of anesthesia for 1 h to allow infection. The infected mice were followed daily for genital pathology and survival. Genital pathology was scored daily as follows: 0, no apparent infection changes; 1, redness of the external vagina; 2, severe redness and swelling of the external vagina; 3, severe redness and swelling of the external vagina and surrounding tissues and hair loss; 4, genital ulceration with severe redness, swelling, and hair loss of genital and surrounding tissues; 5, severe genital ulceration extending to surrounding tissue or any sign of hind-limb paralysis. To examine viral shedding and cytokines in the vaginal mucosa, vaginal lavage fluids were collected on days 1 to 5 postinfection by pipetting 2 doses of 30 μl of PBS into and out of the vagina six to eight times. Virus in the vaginal washes was quantified via plaque assay as described above.
ELISA.
To measure IFN-γ concentrations in the vaginal lumen of infected mice, vaginal lavage fluids from several mice were pooled and assayed for IFN-γ production with the DuoSet ELISA (enzyme-linked immunosorbent assay) kit, according to the manufacturer's protocol (R&D Systems, Minneapolis, MN). The plates were read using the Sapphire ELISA plate reader at 450-nm wavelength.
Statistical analysis.
Analysis was performed using GraphPad Prism. Where necessary, values were first transformed via logarithmic transformation to equalize variance or arcsine-square-root transformation for proportions.
RESULTS
Generation of an HSV mutant expressing cytoplasmic ICP0 lacking the RING finger.
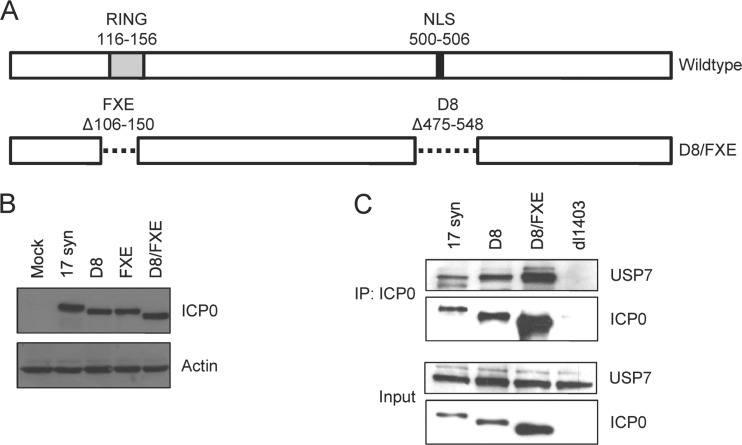
To investigate the role of the RING finger in the cytoplasmic activity of ICP0, we generated an ICP0 construct containing both the FXE deletion (Δ106 to 150), which removes the RING finger domain, and the D8 deletion (Δ475 to 548), which disrupts the NLS (30) (Fig. 1A). This construct was introduced into the 17 syn genome via homologous recombination, and the presence of the expected mutations was verified via DNA sequencing. Western blot analysis confirmed the size reduction of the ICP0 protein expressed by the double-mutant virus, designated D8/FXE (Fig. 1B). To ensure that the presence of two deletions did not result in gross protein misfolding, we confirmed that the D8/FXE ICP0 was capable of binding to USP7, a well-characterized interaction partner whose binding site in the C terminus of ICP0 would not be directly impacted by FXE and D8 deletions (31–33) (Fig. 1C). As expected, the amount of ICP0 recovered in the cytoplasmic extracts differed among the viruses. Due to its exclusive cytoplasmic localization, a greater quantity of D8 ICP0 than of WT ICP0 was present, a portion of which was still nuclear at this time. D8/FXE ICP0 was found at the highest level, in accordance with its increased stability as a result of the loss of the RING finger (see below). However, the amount of USP7 recovered in each coimmunoprecipitation (co-IP) was proportional to the amount of ICP0 in the particular sample, demonstrating that all forms of ICP0 interact with USP7 to comparable degrees.
FIG 1.
Generation of an HSV mutant expressing cytoplasmic ICP0 lacking the RING finger. (A) Schematic of the deletions in the icp0 gene found in the wild-type and D8/FXE viruses. (B and C) HEL cells were infected with the indicated viruses at an MOI of 10 for 8 h. (B) Cells were harvested with RIPA extract and analyzed for ICP0 size and expression relative to actin by Western blotting. (C) Cytoplasmic extracts were obtained, and samples were immunoprecipitated with an αICP0 antibody. The eluant and input extracts were analyzed by Western blotting for ICP0 and USP7.
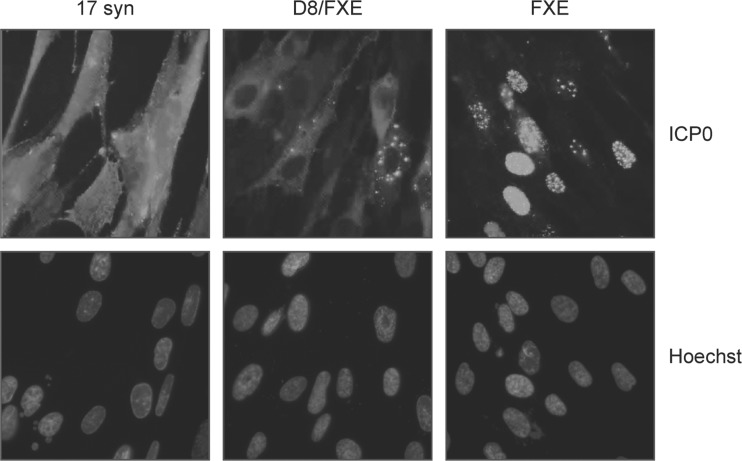
We next investigated the localization of D8/FXE ICP0 via immunofluorescence microscopy (Fig. 2). Unlike the FXE mutant, which was found predominantly in the nucleus, D8/FXE ICP0 was found exclusively in the cytoplasm. This localization pattern was confirmed in primary mouse fibroblasts and primary human genital epithelial cells (data not shown).
FIG 2.
An ICP0 NLS mutant lacking the RING finger localizes to the cytoplasm. HEL cells were infected with the indicated viruses at an MOI of 10 for 8 h, and immunofluorescence microscopy was performed to determine the subcellular location of ICP0. Nuclei were stained with Hoechst dye. Magnification, ×400.
Cytoplasmic ICP0 promotes virus replication in cell culture in the absence of the RING finger.
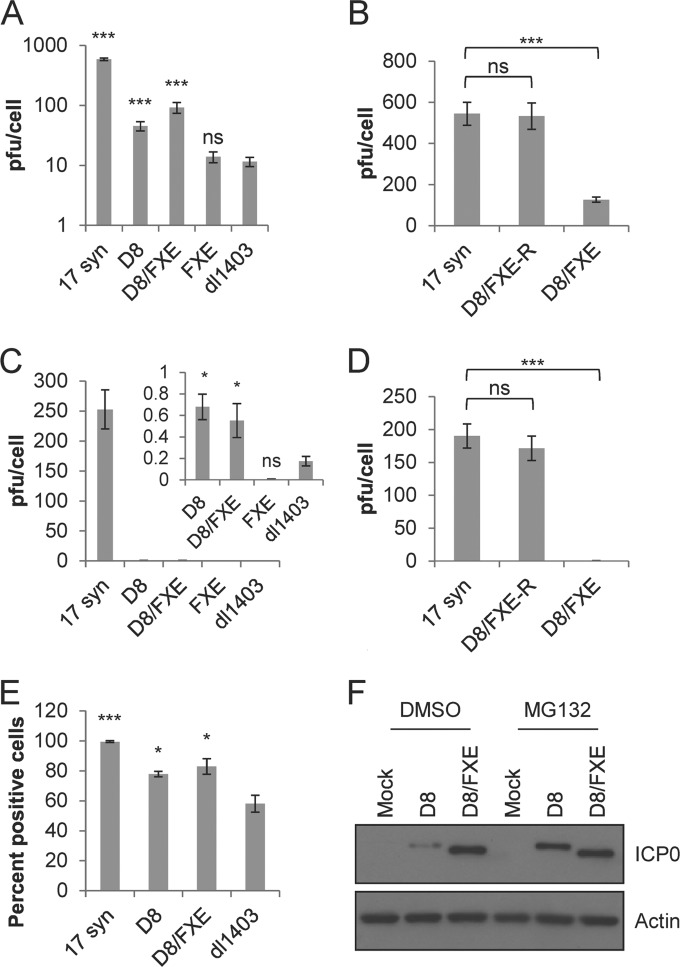
Since most of the known roles of ICP0 are thought to occur in the nucleus and require an intact RING finger domain, we expected the D8/FXE virus to be highly attenuated. HEL fibroblast cells were infected with D8/FXE, the single mutant D8 or FXE, the ICP0-null dl1403, and the wild-type parent strain 17 syn at both high (10) and low (0.1) MOIs for 24 h. The titer of the virus on U2OS cells in the presence of HMBA was then determined. Remarkably, the D8/FXE virus, as well as the D8 virus, grew significantly better than either the dl1403 or the FXE mutant at both high and low MOIs (Fig. 3A and C). Although neither D8/FXE nor D8 replicated as well as 17 syn, both viruses caused a productive infection at an MOI of 10, reaching titers approximately 8-fold higher than that of dl1403, which produced little more than the input virus. A similar pattern was observed even at the low MOI of 0.1, indicating that cytoplasmic ICP0 is still able to promote viral replication, even in the absence of a functional RING finger domain. The particle-to-PFU ratios of D8, D8/FXE, and dl1403 were comparable to, though higher than, those of 17 syn, as expected (data not shown). Therefore, differences in particle numbers cannot explain these results. To confirm that the increased growth of D8/FXE was not due to secondary site mutations, we generated the revertant virus D8/FXE-R, in which mutant ICP0 is replaced with WT ICP0. As expected, D8/FXE-R replicated to titers similar to those of 17 syn at both high and low MOIs (Fig. 3B and D).
FIG 3.
Cytoplasmic ICP0 promotes viral replication in the absence of the RING finger domain in primary fibroblasts. (A to D) HEL cells were infected with the indicated viruses at a high MOI of 10 (A and B) or a low MOI of 0.1 (C and D) for 24 h. Cells and supernatant were harvested and sent through 3 freeze-thaw cycles, and the titer of U20S cells in the presence of HMBA was determined. (E) HEL cells were infected with the indicated viruses at an MOI of 10 for 12 h, and immunofluorescence microscopy was performed to determine the number of cells expressing ICP4 relative to the total number of cells, as determined by staining nuclei with Hoechst dye. The data are the averages of three independent experiments ± standard errors of the mean (SEM). Statistical analysis was performed using one-way analysis of variance (ANOVA) and Tukey's posttest (B and D) or Dunnett's posttest (A and C) relative to dl1403. *, P < 0.05; ***, P < 0.001; ns, not significant. (F) HEL cells were infected with the indicated viruses at an MOI of 10 for 8 h in the presence of MG132 or the carrier DMSO and then harvested via cytoplasmic extract. Western blot analysis was performed to determine the levels of ICP0 and actin.
To further characterize the point in the replication cycle affected by cytoplasmic ICP0, the expression of the immediate-early protein ICP4 was determined via immunofluorescence microscopy after infection with 17syn, D8, D8/FXE, or dl1403. The number of cells positive for ICP4 was determined relative to the total number of cells in each field of view (Fig. 3E). The pattern of ICP4 expression mirrored the observations for viral replication, with both D8 and D8/FXE showing a significantly greater number of cells expressing ICP4 than dl1403.
In terms of both titer and ICP4 expression, D8/FXE showed a slight but reproducible improvement in replication over D8. This may be explained by ICP0 autoubiquitination and subsequent degradation (34), which would be expected to occur in D8 but not the RING-deficient D8/FXE. In support of this possibility, D8/FXE ICP0 was found to accumulate to higher levels than D8, but the addition of MG132 normalized the levels of the two cytoplasmic ICP0 mutants (Fig. 3F). Together, these data suggest that cytoplasmic ICP0 stimulates the replication of HSV-1 in cell culture through a mechanism independent of the RING finger.
Cytoplasmic ICP0 cannot block antiviral signaling in the absence of the RING finger.
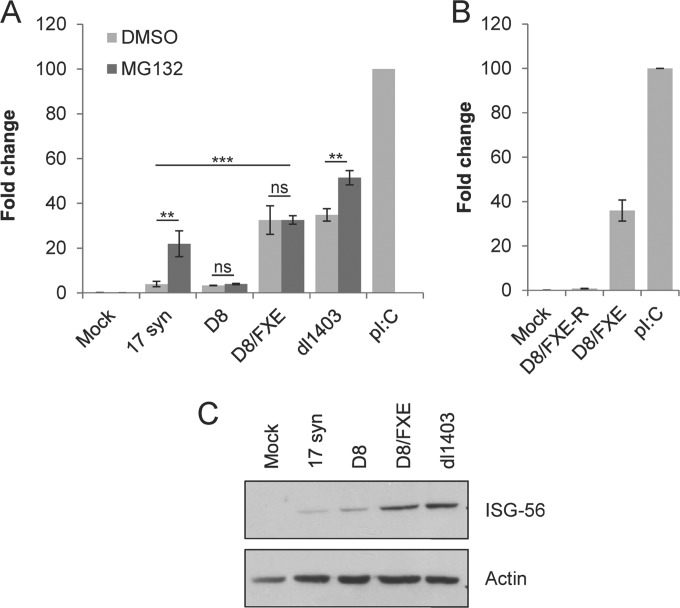
We next investigated whether the increase in viral replication observed with cytoplasmic mutants of ICP0 could be explained by their ability to block antiviral signaling. HEL cells were infected with various HSV-1 mutants in the presence or absence of the proteasome inhibitor MG132, and antiviral state induction was monitored by determining the accumulation of ISG-56 message at 8 h postinfection via quantitative RT-PCR (Fig. 4A). 17 syn efficiently blocked the accumulation of ISG-56 message when the proteasome was active but lost the ability after treatment with MG132, while D8 blocked ISG-56 accumulation even when the proteasome was inhibited, in accordance with previous results (12). Intriguingly, D8/FXE was unable to prevent ISG-56 accumulation, regardless of the status of the proteasome, suggesting that cytoplasmic ICP0 requires the RING finger, but not the proteasome, for its ability to block antiviral signaling. We confirmed that the inability of D8/FXE to impede ISG-56 induction was due to the absence of the ICP0 RING finger domain, as the repaired strain, D8/FXE-R, efficiently prevented the activation of innate signaling (Fig. 4B). Similarly, examining ISG-56 protein levels via Western blot analysis confirmed that D8 efficiently blocked the activation of the antiviral response, while D8/FXE could not (Fig. 4C). Collectively, these data suggest that cytoplasmic ICP0 plays distinct roles in viral replication and antiviral state inhibition.
FIG 4.
Cytoplasmic ICP0 requires the RING finger but not the proteasome to block ISG expression. HEL cells were infected with the indicated viruses at an MOI of 10. (A) Infections were performed in the presence or absence of the proteasome inhibitor MG132, as indicated. (A and B) RNA was harvested after 6 h, and the expression of ISG-56 relative to GAPDH was determined using the TaqMan system of quantitative RT-PCR. Values are reported relative to poly(I·C) treated cells, whose fold change was set to 100. The data are the averages of three independent experiments ± SEM. Statistical analysis was performed using one-way ANOVA and Tukey's posttest. **, P < 0.01; ***, P < 0.001; ns, not significant. (C) Protein was harvested after 8 h and analyzed for ISG-56 and actin expression by Western blotting.
Cytoplasmic ICP0 does not require the RING finger to promote virus replication in vivo.
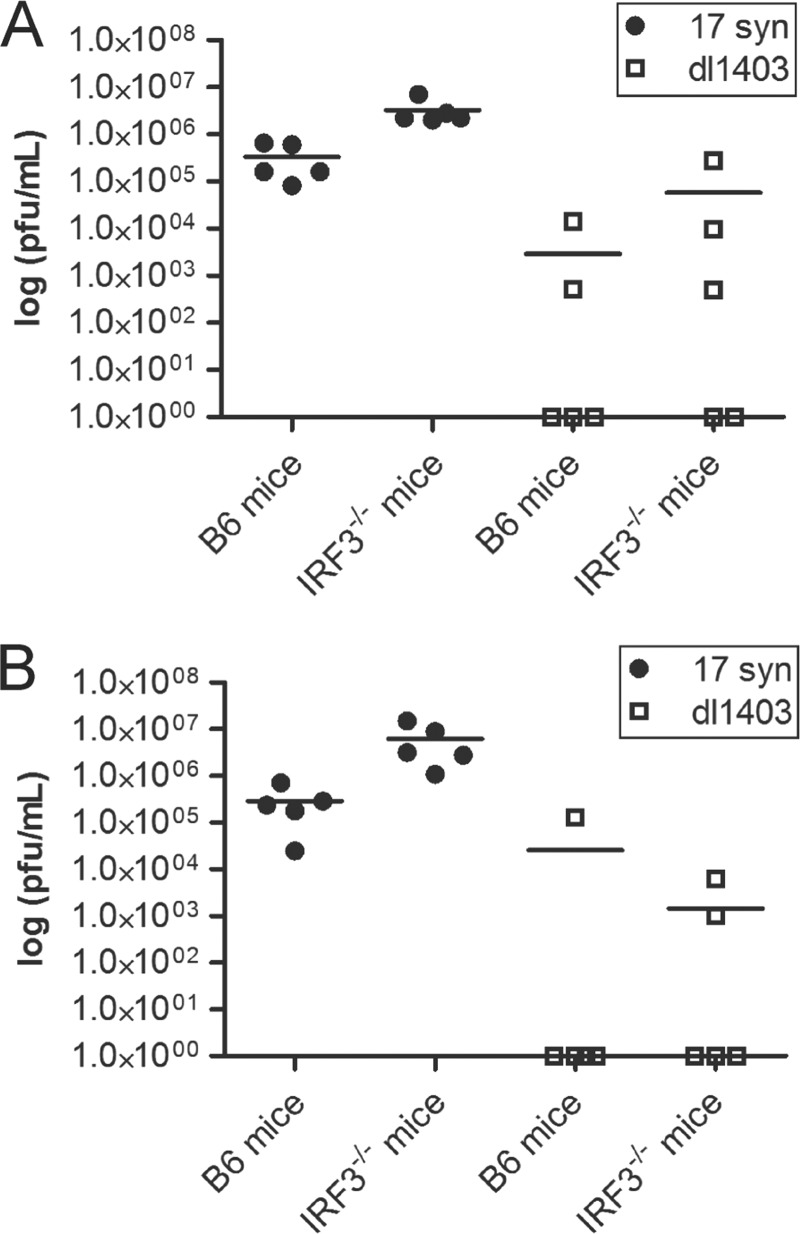
To determine whether the differences in viral replication observed in cell culture were reproducible in vivo, a mouse intravaginal model was used. Despite increasing clinical data linking HSV-1 with genital infections, few murine studies utilize HSV-1 in intravaginal inoculation. Thus, in preliminary studies, 17 syn and dl1403 were tested in parental C57BL/6 (B6) and IRF3−/− mice (Fig. 5). One day postinfection (Fig. 5A), there was a trend toward increased viral titers in the vaginal washes for both 17 syn and dl1403 in IRF3−/− mice compared to B6-infected mice, suggesting that IRF3 contributes to the host response to vaginal HSV challenge. While this general trend was maintained at day 2 postinfection (Fig. 5B), we found that dl1403 was being cleared in both strains of mice. Of note, the ICP0-null virus did not reach wild-type titers in IRF3-deficient mice, indicating that ICP0 contributes important activities in addition to blocking IRF3.
FIG 5.
IRF3 deficiency cannot compensate for the loss of ICP0 in a mouse model of genital HSV infection. Wild-type B6 or irf3−/− mice were intravaginally infected with 1 × 105 PFU of the indicated viruses. Vaginal washes were collected after 1 day (A) and 2 days (B), and the titer on U2OS cells was determined in the presence of HMBA. Scatter plot and mean values are shown.
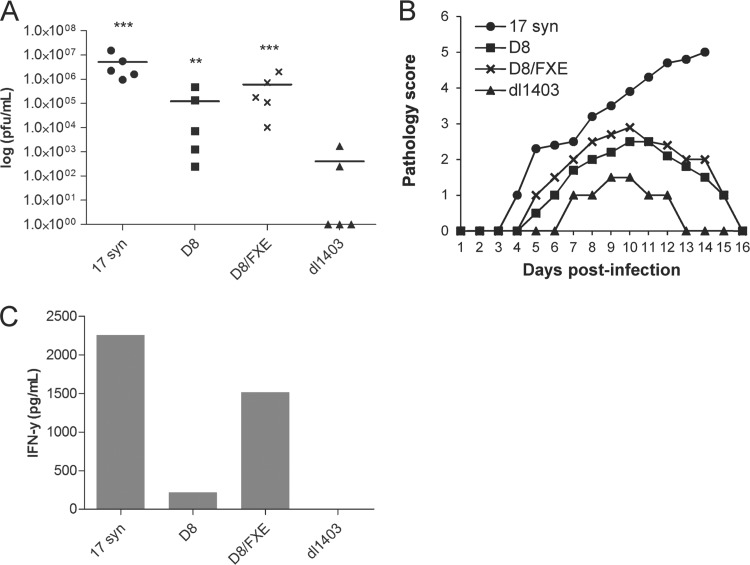
From our in vitro data, we were particularly intrigued to observe that cytoplasmic ICP0 contributes to HSV replication in the absence of a RING finger. Since D8/FXE cannot block the IRF3-mediated antiviral response, while D8 can (Fig. 4), we chose to assess its ability to support virus replication in vivo in mice deficient for IRF3. Thus, we challenged IRF3-deficient mice with 17 syn, D8, D8/FXE, or dl1403, and viral titers in the vaginal washes were determined 2 days postinfection (Fig. 6A). Similar to in vitro findings, both D8 and D8/FXE replicated to significantly higher titers than dl1403, with the enhanced stability of ICP0 in D8/FXE likely responsible for the increase in titers over D8. Vaginal pathology results correlated closely with viral titers (Fig. 6B), and a similar pattern was also observed when levels of the cytokine IFN-γ were quantified in the vaginal washes (Fig. 6C). Collectively, these data show that in vivo, as well as in cell culture, cytoplasmic ICP0 has a growth-promoting activity that is distinct from its ability to block antiviral signaling and that does not require the RING finger.
FIG 6.
Cytoplasmic ICP0 promotes viral replication in the absence of the RING finger domain in a mouse genital model of HSV infection. The indicated viruses (1 × 105 PFU) were used to intravaginally infect irf3−/− mice. (A) Vaginal washes were collected after 2 days, and the titer on U2OS cells was determined in the presence of HMBA. Statistical analysis was performed using one-way ANOVA and Dunnett's posttest relative to dl1403. **, P < 0.01; ***, P < 0.001. Scatter plot and mean values are shown. (B) Vaginal pathology was monitored daily and scored on a 5-point scale. (C) Vaginal washes collected after 2 days were pooled, and ELISA was used to measure IFN-γ levels.
DISCUSSION
This study demonstrates that while ICP0 mediates significant functions in the nucleus, its cytoplasmic roles also have a largely unappreciated importance in viral replication. We show that ICP0 restricted to the cytoplasm can promote viral growth both in cell culture and in mice and, surprisingly, that it can achieve this equally well in the presence or absence of a functional RING finger domain. We also demonstrate that the RING finger contributes to blocking antiviral signaling in a proteasome-independent fashion.
Partial or complete restriction of ICP0 to the nucleus results from a wide variety of experimental manipulations (6, 35–40), including commonly used strategies, such as DNA transfection prior to infection, the use of proteasome inhibitors, or ICP0 expression in the absence of other viral proteins. Thus, unintentionally, many studies have exclusively considered the roles of nuclear ICP0. Similarly, as shown here (Fig. 2) and elsewhere (37), RING finger mutations cause ICP0 to be largely restricted to the nucleus. This may be explained by the previously suggested hypothesis that ICP0 must complete its nuclear functions before it can translocate to the cytoplasm (35), so that preventing ICP0 from performing these activities by disrupting its RING finger results in its nuclear retention. Our results are consistent with the vast amount of literature demonstrating that disrupting the RING finger of ICP0 results in a virus as attenuated as an ICP0-null strain (30, 41–45) and suggest that ICP0 absolutely requires the RING finger to perform its functions specifically within the nucleus. However, most studies using RING finger mutants conclude that ICP0 requires a RING finger for all of its biological roles, without taking the effect of ICP0 localization into consideration. By including the additional D8 deletion to remove the NLS and create D8/FXE, we have overcome the nuclear restriction of FXE, allowing us to investigate the activity of RING-deficient ICP0 in the cytoplasm. It is important to note that although immunofluorescence studies cannot conclusively demonstrate that a small amount of our ICP0 with the NLS deleted is not reaching the nucleus, we have previously shown that the D8 virus does not degrade nuclear PML (12). Since it is well established that even a small amount of nuclear ICP0 leads to the loss of PML (43), it can be concluded that this mutation prevents a biologically relevant amount of ICP0 from localizing to the nucleus. Additionally, since FXE is mainly nuclear and yet is inactive, a small amount of D8/FXE reaching the nucleus cannot explain our results.
How cytoplasmic ICP0 induces virus replication, particularly in the absence of the RING finger domain, is currently unclear. ICP0 has been found to bind to EF-1δ, a cytoplasmic elongation factor involved in translation (7), although little is known about the significance of this interaction. Other potential cytoplasmic activities identified for ICP0, such as the degradation of IκBα (46) or the disruption of the cellular microtubule network (11), appear to require RING-dependent ubiquitination. It has also been found that ICP0 must be cytoplasmic in order to be packaged into the viral tegument (47, 48), and capsids from viruses lacking tegument ICP0 have been found to have disrupted transport to the nucleus (49). Therefore, the cytoplasmic localization of D8 and D8/FXE may allow their packaging into the tegument, resulting in more direct capsid transport and greater efficiency of replication. Finally, ICP0 has been found to bind the cellular deubiquitinating enzyme USP7 (31–33) and to transport it from the nucleus to the cytoplasm in a RING-independent but NLS-dependent manner (50). Conversely, USP7 translocation can occur after TLR stimulation in the absence of ICP0 (50), and we observed that ICP0 restricted to the cytoplasm can still interact with USP7. Loss of USP7 binding by ICP0 has been found by some groups to decrease viral growth (51, 52), while others have found that such viruses show increased gene expression, though decreased cell-to-cell spread (53). While USP7 binding has been found to stabilize ICP0 (34), the exact function of this interaction in viral replication is unknown.
A role for cytoplasmic ICP0 in promoting viral replication via increased translation, capsid transport, or USP7 binding would be a departure from the current paradigm in ICP0 biology, which suggests that ICP0 stimulates virus replication by increasing the probability that an incoming genome will launch a productive infection (reviewed in reference 1). In the absence of ICP0, the viral genome is more likely to remain in a quiescent state, suggestive of latency, though in the infrequent cells where a productive infection is launched, the replication cycle proceeds normally (30). ICP0 is thought to overcome the intrinsic cellular resistance provided by several constituents of specific subnuclear domains known as ND10, including PML, which are degraded by ICP0 (54–56). ICP0 has also been suggested to act as a DNA template remodeler (57), decreasing repressive and increasing active histone modifications and counteracting the formation of heterochromatin (58–61). The mechanism behind this activity is currently unclear (62) and may involve a variety of factors (63–67), including the disruption of the coREST-REST repressor complex (68, 69). However, it is difficult to understand how cytoplasmic ICP0 could affect such nuclear components. Interestingly, our results are supported by a recent study demonstrating that a virus expressing a RING finger mutant of ICP0 that was retained exclusively at ND10 domains was more highly attenuated than a virus encoding a RING domain mutant with additional deletions allowing it to disperse away from ND10, suggesting that RING-independent functions of ICP0, in a location-dependent context, can promote viral replication (70).
Although D8/FXE was as capable as D8 in promoting viral replication, cytoplasmic ICP0 failed to block antiviral signaling in the absence of the RING domain. The mechanism through which ICP0 blocks IRF3 activation remains controversial. We have consistently found that cytoplasmic ICP0 can prevent IRF3 signaling in the absence of a functional proteasome (12), and others have demonstrated that cytoplasmic bICP0 from the related bovine herpesvirus 1 also inhibits IRF3 activation (71). In contrast, ICP0 is unable to block IRF3 activation within the nucleus (12, 23), although it plays a role in inhibiting cellular responsiveness to IFN (23), likely via its disruption of PML (72). However, it has been alternatively reported in a Sendai virus coinfection model that wild-type HSV-1 can both enhance IRF3 degradation and sequester nuclear IRF3 away from its target genes in comparison to an ICP0-null virus (21, 22). It has also been suggested that nuclear ICP0 blocks IRF3 activation by degrading the nuclear DNA sensor IFI16 in a RING- and proteasome-dependent manner (20). However, IFI16 may be more important in epigenetic regulation of DNA expression as opposed to IFN production (67, 73). Importantly, under conditions allowing efficient viral replication, IFI16 has been found to be degraded during infection with an ICP0-null virus (74). This suggests that ICP0 simply promotes the expression of an additional viral product that is directly responsible for the loss of IFI16, though ICP0 does appear to block the recruitment of IFI16 to the viral genome even without directing the degradation of IFI16 (74).
At first glance, it is difficult to understand how the RING finger, with its ubiquitin ligase activity, could be required for the ability of ICP0 to prevent antiviral signaling while the proteasome itself is dispensable. This observation is consistent with our previous work demonstrating the requirement for the RING finger in antiviral inhibition (18) and that no factor involved in IRF3 activation has been unequivocally identified as being degraded by ICP0 during HSV-1 infection (12, 18), though some have been suggested (20–22). In recent years, there has been a tremendous increase in our understanding of the proteasome-independent activities of ubiquitin modifications (reviewed in references 24 to 29 and 75). The traditional signal for proteasomal degradation consists of ubiquitin moieties conjugated into chains via their lysine residues at position 48 (Lys 48), yet ubiquitin can also be linked in a variety of atypical manners via other lysine residues, and these alternative chains have been associated with nondegradative signaling roles. Atypical linkages result in chains that adopt a variety of conformations, which can be recognized by different ubiquitin-binding domains (UBDs), found in a wide diversity of proteins, in a linkage-specific manner. Thus, ubiquitin modification can control protein-protein interactions and is therefore involved in cellular processes ranging from receptor endocytosis to DNA repair. Of particular interest is the fact that Lys 63 linkage, one of the best characterized of these alternative chains, plays an extensive role in antiviral signaling (76–80). For example, Lys 63-linked chains, but not Lys 48-linked chains, are essential for the activation of IRF3 (76); Lys 63 chains are important in RLR activation (reviewed in reference 81); and NF-κB activation is extensively regulated by atypical ubiquitin modification of signal transduction proteins (reviewed in reference 82).
Though the activity of the RING finger of ICP0 as a ubiquitin ligase has been confirmed in vitro (2, 83), as well as in vivo (3), and the RING domain has been implicated in the proteasome-dependent degradation of a tremendous variety of targets (20, 46, 51, 66, 83–92), to our knowledge, the linkage type found in the ubiquitin chains produced by ICP0 has never been determined. The loss of ICP0-targeted proteins from the cell implicates Lys 48 linkages and proteasomal degradation; however, the RING finger may also generate alternatively linked chains involved in proteasome-independent signaling roles, a possibility that we are currently investigating. Alternatively, the RING finger may act in a ubiquitin-independent manner. Recently, it has been found that the cellular retrovirus restriction factor TRIM-CypA is able to inhibit virus infection by a mechanism that requires its RING domain but neither the proteasome nor ubiquitin conjugation (93). Finally, we cannot rule out the possibility that HUL-1, the second region of ICP0 with E3 ubiquitin ligase activity (94), plays a role in the replication-promoting activities of D8/FXE in the absence of the RING, though to date only one target of HUL-1 has been identified. We are currently investigating potential proteasome-independent functions of the ICP0 RING finger domain.
While unable to control the antiviral response, D8/FXE grows as well as D8 in cell culture. This observation is consistent with previous studies demonstrating that the depletion of neither IRF3 nor STAT-1 in cultured cells could improve the replication of an ICP0-null virus (95). In contrast, the type I IFN response is crucial in controlling HSV replication in mouse models (96–100). Conversely, IRF3-deficient mice survive intravenous infection with wild-type HSV-1 (101) and show no increased viral replication in peripheral tissues (101–103), though augmented replication was observed in the central nervous system (102, 103). While ICP0-null mutants are attenuated in wild-type mice (96, 104, 105), viruses lacking ICP0 have not been studied in IRF3-deficient mice. Here, we used IRF3−/− mice to investigate the replication of our ICP0 mutants, using an intravaginal model of infection, as HSV-1 is now responsible for at least 50% of new genital herpes episodes in developed countries (reviewed in reference 106). We found that while there was a trend toward higher titers in IRF3−/− mice for both 17 syn and dl1403, it was not statistically significant. It is probable that this results from the compensatory role of IRF7 in IRF3−/− mice, as these mice continue to express IFN-α via IRF7 in plasmacytoid dendritic cells and thus survive infection with wild-type HSV-1, while IRF7−/− mice lack IFN-α production and succumb (101). This is analogous to previous work demonstrating that ICP0-null viruses show augmented replication in STAT1−/− and IFNAR−/− mice (96, 97) but remain attenuated in PML−/− mice (96). Therefore, IRF3−/− mice are useful for these studies, allowing us to compare our two cytoplasmic ICP0 mutants without the confounding effects of IRF3 activation, which D8 controls and D8/FXE cannot. However, these mice are not so deficient as to eliminate the requirement for ICP0 to achieve maximal viral replication.
Our observations in IRF3−/− mice confirm the ability of cytoplasmic ICP0 to support virus growth in a RING-independent manner. Virus growth is mirrored by the observed pathology in these mice and further confirmed by measurement of levels of IFN-γ, a cytokine produced by NK cells that represents a characteristic feature of the innate immune response to replicating genital HSV (107–109). Consistent with our data, previous work in lymphocyte-deficient rag2−/− mice demonstrated that an HSV-2 virus expressing an NLS mutant of ICP0 was lethal in 80% of mice compared to only 20% infected with a RING finger mutant (110). Surprisingly, D8/FXE reaches titers even higher than those of D8 and also induces higher levels of IFN-γ. This may be explained by the increased stability of RING finger mutants (34, 41), as ICP0 undergoes RING-dependent autoubiquitination. Indeed, we observed that ICP0 accumulates to higher levels in D8/FXE than in D8.
In conclusion, our work demonstrates that cytoplasmic ICP0 has two independent activities: blocking ISG production in a mechanism that involves the RING finger but not the proteasome and promoting virus replication in a RING-independent manner. These observations underscore the importance of cytoplasmic ICP0 and suggest alternative functions for the RING finger domain, opening new avenues for the investigation of this multifaceted viral protein.
ACKNOWLEDGMENTS
This work is supported by Canadian Institutes of Health Research (CIHR) grant MOP-57669. K.T. was supported by a Natural Sciences and Engineering Research Council (NSERC) Alexander Graham Bell Canada graduate doctoral scholarship.
We thank S. Dizzell for technical assistance.
Footnotes
Published ahead of print 7 May 2014
REFERENCES
- 1.Everett RD. 2000. ICP0, a regulator of herpes simplex virus during lytic and latent infection. Bioessays 22:761–770. [DOI] [PubMed] [Google Scholar]
- 2.Boutell C, Sadis S, Everett RD. 2002. Herpes simplex virus type 1 immediate-early protein ICP0 and is isolated RING finger domain act as ubiquitin E3 ligases in vitro. J. Virol. 76:841–850. 10.1128/JVI.76.2.841-850.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Everett RD. 2000. ICP0 induces the accumulation of colocalizing conjugated ubiquitin. J. Virol. 74:9994–10005. 10.1128/JVI.74.21.9994-10005.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Everett RD, Orr A, Preston CM. 1998. A viral activator of gene expression functions via the ubiquitin-proteasome pathway. EMBO J. 17:7161–7169. 10.1093/emboj/17.24.7161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mullen MA, Ciufo DM, Hayward GS. 1994. Mapping of intracellular localization domains and evidence for colocalization interactions between the IE110 and IE175 nuclear transactivator proteins of herpes simplex virus. J. Virol. 68:3250–3266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lopez P, Van Sant C, Roizman B. 2001. Requirements for the nuclear-cytoplasmic translocation of infected-cell protein 0 of herpes simplex virus 1. J. Virol. 75:3832–3840. 10.1128/JVI.75.8.3832-3840.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kawaguchi Y, Bruni R, Roizman B. 1997. Interaction of herpes simplex virus 1 alpha regulatory protein ICP0 with elongation factor 1delta: ICP0 affects translational machinery. J. Virol. 71:1019–1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maul GG, Everett RD. 1994. The nuclear location of PML, a cellular member of the C3HC4 zinc-binding domain protein family, is rearranged during herpes simplex virus infection by the C3HC4 viral protein ICP0. J. Gen. Virol. 75:1223–1233. 10.1099/0022-1317-75-6-1223 [DOI] [PubMed] [Google Scholar]
- 9.Everett RD. 1988. Analysis of the functional domains of herpes simplex virus type 1 immediate-early polypeptide Vmw110. J. Mol. Biol. 202:87–96. 10.1016/0022-2836(88)90521-9 [DOI] [PubMed] [Google Scholar]
- 10.Everett RD, Maul GG. 1994. HSV-1 IE protein Vmw110 causes redistribution of PML. EMBO J. 13:5062–5069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu M, Schmidt EE, Halford WP. 2010. ICP0 dismantles microtubule networks in herpes simplex virus-infected cells. PLoS One 5:e10975. 10.1371/journal.pone.0010975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paladino P, Collins SE, Mossman KL. 2010. Cellular localization of the herpes simplex virus ICP0 protein dictates its ability to block IRF3-mediated innate immune responses. PLoS One 5:e10428. 10.1371/journal.pone.0010428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kawai T, Akira S. 2011. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 34:637–650. 10.1016/j.immuni.2011.05.006 [DOI] [PubMed] [Google Scholar]
- 14.Sen GC, Sarkar SN. 2007. The interferon-stimulated genes: targets of direct signaling by interferons, double-stranded RNA, and viruses. Curr. Top. Microbiol. Immunol. 316:233–250. 10.1007/978-3-540-71329-6_12 [DOI] [PubMed] [Google Scholar]
- 15.Taylor KE, Mossman KL. 2013. Recent advances in understanding viral evasion of type I interferon. Immunology 138:190–197. 10.1111/imm.12038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mossman KL, Saffran HA, Smiley JR. 2000. Herpes simplex virus ICP0 mutants are hypersensitive to interferon. J. Virol. 74:2052–2056. 10.1128/JVI.74.4.2052-2056.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mossman KL, Macgregor PF, Rozmus JJ, Goryachev AB, Edwards AM, Smiley JR. 2001. Herpes simplex virus triggers and then disarms a host antiviral response. J. Virol. 75:750–758. 10.1128/JVI.75.2.750-758.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin R, Noyce RS, Collins SE, Everett RD, Mossman KL. 2004. The herpes simplex virus ICP0 RING finger domain inhibits IRF3- and IRF7-mediated activation of interferon-stimulated genes. J. Virol. 78:1675–1684. 10.1128/JVI.78.4.1675-1684.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eidson KM, Hobbs WE, Manning BJ, Carlson P, DeLuca NA. 2002. Expression of herpes simplex virus ICP0 inhibits the induction of interferon-stimulated genes by viral infection. J. Virol. 76:2180–2191. 10.1128/jvi.76.5.2180-2191.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orzalli MH, DeLuca NA, Knipe DM. 2012. Nuclear IFI16 induction of IRF-3 signaling during herpesviral infection and degradation of IFI16 by the viral ICP0 protein. Proc. Natl. Acad. Sci. U. S. A. 109:E3008–E3017. 10.1073/pnas.1211302109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Melroe GT, DeLuca NA, Knipe DM. 2004. Herpes simplex virus 1 has multiple mechanisms for blocking virus-induced interferon production. J. Virol. 78:8411–8420. 10.1128/JVI.78.16.8411-8420.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Melroe GT, Silva L, Schaffer PA, Knipe DM. 2007. Recruitment of activated IRF-3 and CBP/p300 to herpes simplex virus ICP0 nuclear foci: potential role in blocking IFN-beta induction. Virology 360:305–321. 10.1016/j.virol.2006.10.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Everett RD, Orr A. 2009. Herpes simplex virus type 1 regulatory protein ICP0 aids infection in cells with a preinduced interferon response but does not impede interferon-induced gene induction. J. Virol. 83:4978–4983. 10.1128/JVI.02595-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hicke L. 2001. Protein regulation by monoubiquitin. Nat. Rev. Mol. Cell. Biol. 2:195–201. 10.1038/35056583 [DOI] [PubMed] [Google Scholar]
- 25.Conaway RC, Brower CS, Conaway JW. 2002. Emerging roles of ubiquitin in transcription regulation. Science 296:1254–1258. 10.1126/science.1067466 [DOI] [PubMed] [Google Scholar]
- 26.Ikeda F, Dikic I. 2008. Atypical ubiquitin chains: new molecular signals. ‘Protein modifications: beyond the usual suspects' review series. EMBO Rep. 9:536–542. 10.1038/embor.2008.93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schnell JD, Hicke L. 2003. Non-traditional functions of ubiquitin and ubiquitin-binding proteins. J. Biol. Chem. 278:35857–35860. 10.1074/jbc.R300018200 [DOI] [PubMed] [Google Scholar]
- 28.Behrends C, Harper JW. 2011. Constructing and decoding unconventional ubiquitin chains. Nat. Struct. Mol. Biol. 18:520–528. 10.1038/nsmb.2066 [DOI] [PubMed] [Google Scholar]
- 29.Bhoj VG, Chen ZJ. 2009. Ubiquitylation in innate and adaptive immunity. Nature 458:430–437. 10.1038/nature07959 [DOI] [PubMed] [Google Scholar]
- 30.Everett RD. 1989. Construction and characterization of herpes simplex virus type 1 mutants with defined lesions in immediate early gene 1. J. Gen. Virol. 70:1185–1202. 10.1099/0022-1317-70-5-1185 [DOI] [PubMed] [Google Scholar]
- 31.Meredith M, Orr A, Everett R. 1994. Herpes simplex virus type 1 immediate-early protein Vmw110 binds strongly and specifically to a 135-kDa cellular protein. Virology 200:457–469. 10.1006/viro.1994.1209 [DOI] [PubMed] [Google Scholar]
- 32.Meredith M, Orr A, Elliott M, Everett R. 1995. Separation of sequence requirements for HSV-1 Vmw110 multimerisation and interaction with a 135-kDa cellular protein. Virology 209:174–187. 10.1006/viro.1995.1241 [DOI] [PubMed] [Google Scholar]
- 33.Everett RD, Meredith M, Orr A, Cross A, Kathoria M, Parkinson J. 1997. A novel ubiquitin-specific protease is dynamically associated with the PML nuclear domain and binds to a herpesvirus regulatory protein. EMBO J. 16:1519–1530. 10.1093/emboj/16.7.1519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Canning M, Boutell C, Parkinson J, Everett RD. 2004. A RING finger ubiquitin ligase is protected from autocatalyzed ubiquitination and degradation by binding to ubiquitin-specific protease USP7. J. Biol. Chem. 279:38160–38168. 10.1074/jbc.M402885200 [DOI] [PubMed] [Google Scholar]
- 35.Kalamvoki M, Roizman B. 2008. Nuclear retention of ICP0 in cells exposed to HDAC inhibitor or transfected with DNA before infection with herpes simplex virus 1. Proc. Natl. Acad. Sci. U. S. A. 105:20488–20493. 10.1073/pnas.0810879105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kalamvoki M, Roizman B. 2009. ICP0 enables and monitors the function of D cyclins in herpes simplex virus 1 infected cells. Proc. Natl. Acad. Sci. U. S. A. 106:14576–14580. 10.1073/pnas.0906905106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gu H, Roizman B. 2009. The two functions of herpes simplex virus 1 ICP0, inhibition of silencing by the CoREST/REST/HDAC complex and degradation of PML, are executed in tandem. J. Virol. 83:181–187. 10.1128/JVI.01940-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu Z, Cai W, Schaffer PA. 1994. Cooperativity among herpes simplex virus type 1 immediate-early regulatory proteins: ICP4 and ICP27 affect the intracellular localization of ICP0. J. Virol. 68:3027–3040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van Sant C, Lopez P, Advani SJ, Roizman B. 2001. Role of cyclin D3 in the biology of herpes simplex virus 1 ICPO. J. Virol. 75:1888–1898. 10.1128/JVI.75.4.1888-1898.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Potel C, Elliott G. 2005. Phosphorylation of the herpes simplex virus tegument protein VP22 has no effect on incorporation of VP22 into the virus but is involved in optimal expression and virion packaging of ICP0. J. Virol. 79:14057–14068. 10.1128/JVI.79.22.14057-14068.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lium EK, Silverstein S. 1997. Mutational analysis of the herpes simplex virus type 1 ICP0 C3HC4 zinc ring finger reveals a requirement for ICP0 in the expression of the essential alpha27 gene. J. Virol. 71:8602–8614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Everett R, O'Hare P, O'Rourke D, Barlow P, Orr A. 1995. Point mutations in the herpes simplex virus type 1 Vmw110 RING finger helix affect activation of gene expression, viral growth, and interaction with PML-containing nuclear structures. J. Virol. 69:7339–7344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Everett RD, Parsy ML, Orr A. 2009. Analysis of the functions of herpes simplex virus type 1 regulatory protein ICP0 that are critical for lytic infection and derepression of quiescent viral genomes. J. Virol. 83:4963–4977. 10.1128/JVI.02593-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grant K, Grant L, Tong L, Boutell C. 2012. Depletion of intracellular zinc inhibits the ubiquitin ligase activity of viral regulatory protein ICP0 and restricts herpes simplex virus 1 replication in cell culture. J. Virol. 86:4029–4033. 10.1128/JVI.06962-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harris RA, Everett RD, Zhu XX, Silverstein S, Preston CM. 1989. Herpes simplex virus type 1 immediate-early protein Vmw110 reactivates latent herpes simplex virus type 2 in an in vitro latency system. J. Virol. 63:3513–3515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Diao L, Zhang B, Fan J, Gao X, Sun S, Yang K, Xin D, Jin N, Geng Y, Wang C. 2005. Herpes virus proteins ICP0 and BICP0 can activate NF-kappaB by catalyzing IkappaBalpha ubiquitination. Cell Signal. 17:217–229. 10.1016/j.cellsig.2004.07.003 [DOI] [PubMed] [Google Scholar]
- 47.Elliott G, Hafezi W, Whiteley A, Bernard E. 2005. Deletion of the herpes simplex virus VP22-encoding gene (UL49) alters the expression, localization, and virion incorporation of ICP0. J. Virol. 79:9735–9745. 10.1128/JVI.79.15.9735-9745.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sedlackova L, Rice SA. 2008. Herpes simplex virus type 1 immediate-early protein ICP27 is required for efficient incorporation of ICP0 and ICP4 into virions. J. Virol. 82:268–277. 10.1128/JVI.01588-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Delboy MG, Siekavizza-Robles CR, Nicola AV. 2010. Herpes simplex virus tegument ICP0 is capsid associated, and its E3 ubiquitin ligase domain is important for incorporation into virions. J. Virol. 84:1637–1640. 10.1128/JVI.02041-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Daubeuf S, Singh D, Tan Y, Liu H, Federoff HJ, Bowers WJ, Tolba K. 2009. HSV ICP0 recruits USP7 to modulate TLR-mediated innate response. Blood 113:3264–3275. 10.1182/blood-2008-07-168203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boutell C, Canning M, Orr A, Everett RD. 2005. Reciprocal activities between herpes simplex virus type 1 regulatory protein ICP0, a ubiquitin E3 ligase, and ubiquitin-specific protease USP7. J. Virol. 79:12342–12354. 10.1128/JVI.79.19.12342-12354.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Everett RD, Meredith M, Orr A. 1999. The ability of herpes simplex virus type 1 immediate-early protein Vmw110 to bind to a ubiquitin-specific protease contributes to its roles in the activation of gene expression and stimulation of virus replication. J. Virol. 73:417–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kalamvoki M, Gu H, Roizman B. 2012. Overexpression of the ubiquitin-specific protease 7 resulting from transfection or mutations in the ICP0 binding site accelerates rather than depresses herpes simplex virus 1 gene expression. J. Virol. 86:12871–12878. 10.1128/JVI.01981-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Everett RD, Rechter S, Papior P, Tavalai N, Stamminger T, Orr A. 2006. PML contributes to a cellular mechanism of repression of herpes simplex virus type 1 infection that is inactivated by ICP0. J. Virol. 80:7995–8005. 10.1128/JVI.00734-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lukashchuk V, Everett RD. 2010. Regulation of ICP0-null mutant herpes simplex virus type 1 infection by ND10 components ATRX and hDaxx. J. Virol. 84:4026–4040. 10.1128/JVI.02597-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Everett RD, Parada C, Gripon P, Sirma H, Orr A. 2008. Replication of ICP0-null mutant herpes simplex virus type 1 is restricted by both PML and Sp100. J. Virol. 82:2661–2672. 10.1128/JVI.02308-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kalamvoki M, Roizman B. 2010. Role of herpes simplex virus ICP0 in the transactivation of genes introduced by infection or transfection: a reappraisal. J. Virol. 84:4222–4228. 10.1128/JVI.02585-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ferenczy MW, DeLuca NA. 2009. Epigenetic modulation of gene expression from quiescent herpes simplex virus genomes. J. Virol. 83:8514–8524. 10.1128/JVI.00785-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ferenczy MW, DeLuca NA. 2011. Reversal of heterochromatic silencing of quiescent herpes simplex virus type 1 by ICP0. J. Virol. 85:3424–3435. 10.1128/JVI.02263-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ferenczy MW, Ranayhossaini DJ, Deluca NA. 2011. Activities of ICP0 involved in the reversal of silencing of quiescent herpes simplex virus 1. J. Virol. 85:4993–5002. 10.1128/JVI.02265-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Coleman HM, Connor V, Cheng ZS, Grey F, Preston CM, Efstathiou S. 2008. Histone modifications associated with herpes simplex virus type 1 genomes during quiescence and following ICP0-mediated de-repression. J. Gen. Virol. 89:68–77. 10.1099/vir.0.83272-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Boutell C, Everett RD. 2013. Regulation of alphaherpesvirus infections by the ICP0 family of proteins. J. Gen. Virol. 94:465–481. 10.1099/vir.0.048900-0 [DOI] [PubMed] [Google Scholar]
- 63.Lomonte P, Thomas J, Texier P, Caron C, Khochbin S, Epstein AL. 2004. Functional interaction between class II histone deacetylases and ICP0 of herpes simplex virus type 1. J. Virol. 78:6744–6757. 10.1128/JVI.78.13.6744-6757.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kawaguchi Y, Tanaka M, Yokoymama A, Matsuda G, Kato K, Kagawa H, Hirai K, Roizman B. 2001. Herpes simplex virus 1 alpha regulatory protein ICP0 functionally interacts with cellular transcription factor BMAL1. Proc. Natl. Acad. Sci. U. S. A. 98:1877–1882. 10.1073/pnas.041592598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kalamvoki M, Roizman B. 2010. Circadian CLOCK histone acetyl transferase localizes at ND10 nuclear bodies and enables herpes simplex virus gene expression. Proc. Natl. Acad. Sci. U. S. A. 107:17721–17726. 10.1073/pnas.1012991107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lomonte P, Sullivan KF, Everett RD. 2001. Degradation of nucleosome-associated centromeric histone H3-like protein CENP-A induced by herpes simplex virus type 1 protein ICP0. J. Biol. Chem. 276:5829–5835. 10.1074/jbc.M008547200 [DOI] [PubMed] [Google Scholar]
- 67.Orzalli MH, Conwell SE, Berrios C, DeCaprio JA, Knipe DM. 2013. Nuclear interferon-inducible protein 16 promotes silencing of herpesviral and transfected DNA. Proc. Natl. Acad. Sci. U. S. A. 110:E4492–E4501. 10.1073/pnas.1316194110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gu H, Liang Y, Mandel G, Roizman B. 2005. Components of the REST/CoREST/histone deacetylase repressor complex are disrupted, modified, and translocated in HSV-1-infected cells. Proc. Natl. Acad. Sci. U. S. A. 102:7571–7576. 10.1073/pnas.0502658102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gu H, Roizman B. 2007. Herpes simplex virus-infected cell protein 0 blocks the silencing of viral DNA by dissociating histone deacetylases from the CoREST-REST complex. Proc. Natl. Acad. Sci. U. S. A. 104:17134–17139. 10.1073/pnas.0707266104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gu H, Zheng Y, Roizman B. 2013. Interaction of herpes simplex virus ICP0 with ND10 bodies: a sequential process of adhesion, fusion, and retention. J. Virol. 87:10244–10254. 10.1128/JVI.01487-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.da Silva LF, Gaudreault N, Jones C. 2011. Cytoplasmic localized infected cell protein 0 (bICP0) encoded by bovine herpesvirus 1 inhibits beta interferon promoter activity and reduces IRF3 (interferon response factor 3) protein levels. Virus Res. 160:143–149. 10.1016/j.virusres.2011.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chee AV, Lopez P, Pandolfi PP, Roizman B. 2003. Promyelocytic leukemia protein mediates interferon-based anti-herpes simplex virus 1 effects. J. Virol. 77:7101–7105. 10.1128/JVI.77.12.7101-7105.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gariano GR, Dell'Oste V, Bronzini M, Gatti D, Luganini A, De Andrea M, Gribaudo G, Gariglio M, Landolfo S. 2012. The intracellular DNA sensor IFI16 gene acts as restriction factor for human cytomegalovirus replication. PLoS Pathog. 8:e1002498. 10.1371/journal.ppat.1002498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cuchet-Lourenco D, Anderson G, Sloan E, Orr A, Everett RD. 2013. The viral ubiquitin ligase ICP0 is neither sufficient nor necessary for degradation of the cellular DNA sensor IFI16 during herpes simplex virus 1 infection. J. Virol. 87:13422–13432. 10.1128/JVI.02474-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kulathu Y, Komander D. 2012. Atypical ubiquitylation: the unexplored world of polyubiquitin beyond Lys48 and Lys63 linkages. Nat. Rev. Mol. Cell. Biol. 13:508–523. 10.1038/nrm3394 [DOI] [PubMed] [Google Scholar]
- 76.Zeng W, Xu M, Liu S, Sun L, Chen ZJ. 2009. Key role of Ubc5 and lysine-63 polyubiquitination in viral activation of IRF3. Mol. Cell 36:315–325. 10.1016/j.molcel.2009.09.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ning S, Campos AD, Darnay BG, Bentz GL, Pagano JS. 2008. TRAF6 and the three C-terminal lysine sites on IRF7 are required for its ubiquitination-mediated activation by the tumor necrosis factor receptor family member latent membrane protein 1. Mol. Cell. Biol. 28:6536–6546. 10.1128/MCB.00785-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zeng W, Sun L, Jiang X, Chen X, Hou F, Adhikari A, Xu M, Chen ZJ. 2010. Reconstitution of the RIG-I pathway reveals a signaling role of unanchored polyubiquitin chains in innate immunity. Cell 141:315–330. 10.1016/j.cell.2010.03.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jiang X, Kinch LN, Brautigam CA, Chen X, Du F, Grishin NV, Chen ZJ. 2012. Ubiquitin-induced oligomerization of the RNA sensors RIG-I and MDA5 activates antiviral innate immune response. Immunity 36:959–973. 10.1016/j.immuni.2012.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kayagaki N, Phung Q, Chan S, Chaudhari R, Quan C, O'Rourke KM, Eby M, Pietras E, Cheng G, Bazan JF, Zhang Z, Arnott D, Dixit VM. 2007. DUBA: a deubiquitinase that regulates type I interferon production. Science 318:1628–1632. 10.1126/science.1145918 [DOI] [PubMed] [Google Scholar]
- 81.Maelfait J, Beyaert R. 2012. Emerging role of ubiquitination in antiviral RIG-I signaling. Microbiol. Mol. Biol. Rev. 76:33–45. 10.1128/MMBR.05012-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tokunaga F, Iwai K. 2012. Linear ubiquitination: a novel NF-kappaB regulatory mechanism for inflammatory and immune responses by the LUBAC ubiquitin ligase complex. Endocr. J. 59:641–652. 10.1507/endocrj.EJ12-0148 [DOI] [PubMed] [Google Scholar]
- 83.Boutell C, Everett RD. 2003. The herpes simplex virus type 1 (HSV-1) regulatory protein ICP0 interacts with and ubiquitinates p53. J. Biol. Chem. 278:36596–36602. 10.1074/jbc.M300776200 [DOI] [PubMed] [Google Scholar]
- 84.Everett RD, Freemont P, Saitoh H, Dasso M, Orr A, Kathoria M, Parkinson J. 1998. The disruption of ND10 during herpes simplex virus infection correlates with the Vmw110- and proteasome-dependent loss of several PML isoforms. J. Virol. 72:6581–6591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Parkinson J, Everett RD. 2000. Alphaherpesvirus proteins related to herpes simplex virus type 1 ICP0 affect cellular structures and proteins. J. Virol. 74:10006–10017. 10.1128/JVI.74.21.10006-10017.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Everett RD, Earnshaw WC, Findlay J, Lomonte P. 1999. Specific destruction of kinetochore protein CENP-C and disruption of cell division by herpes simplex virus immediate-early protein Vmw110. EMBO J. 18:1526–1538. 10.1093/emboj/18.6.1526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Parkinson J, Lees-Miller SP, Everett RD. 1999. Herpes simplex virus type 1 immediate-early protein vmw110 induces the proteasome-dependent degradation of the catalytic subunit of DNA-dependent protein kinase. J. Virol. 73:650–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kummer M, Turza NM, Muhl-Zurbes P, Lechmann M, Boutell C, Coffin RS, Everett RD, Steinkasserer A, Prechtel AT. 2007. Herpes simplex virus type 1 induces CD83 degradation in mature dendritic cells with immediate-early kinetics via the cellular proteasome. J. Virol. 81:6326–6338. 10.1128/JVI.02327-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.van Lint AL, Murawski MR, Goodbody RE, Severa M, Fitzgerald KA, Finberg RW, Knipe DM, Kurt-Jones EA. 2010. Herpes simplex virus immediate-early ICP0 protein inhibits Toll-like receptor 2-dependent inflammatory responses and NF-kappaB signaling. J. Virol. 84:10802–10811. 10.1128/JVI.00063-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fukuyo Y, Horikoshi N, Ishov AM, Silverstein SJ, Nakajima T. 2011. The herpes simplex virus immediate-early ubiquitin ligase ICP0 induces degradation of the ICP0 repressor protein E2FBP1. J. Virol. 85:3356–3366. 10.1128/JVI.02105-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lilley CE, Chaurushiya MS, Boutell C, Landry S, Suh J, Panier S, Everett RD, Stewart GS, Durocher D, Weitzman MD. 2010. A viral E3 ligase targets RNF8 and RNF168 to control histone ubiquitination and DNA damage responses. EMBO J. 29:943–955. 10.1038/emboj.2009.400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lin AE, Greco TM, Dohner K, Sodeik B, Cristea IM. 2013. A proteomic perspective of inbuilt viral protein regulation: pUL46 tegument protein is targeted for degradation by ICP0 during herpes simplex virus type 1 infection. Mol. Cell. Proteomics 12:3237–3252. 10.1074/mcp.M113.030866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Perez-Caballero D, Hatziioannou T, Zhang F, Cowan S, Bieniasz PD. 2005. Restriction of human immunodeficiency virus type 1 by TRIM-CypA occurs with rapid kinetics and independently of cytoplasmic bodies, ubiquitin, and proteasome activity. J. Virol. 79:15567–15572. 10.1128/JVI.79.24.15567-15572.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hagglund R, Roizman B. 2003. Herpes simplex virus 1 mutant in which the ICP0 HUL-1 E3 ubiquitin ligase site is disrupted stabilizes cdc34 but degrades D-type cyclins and exhibits diminished neurotoxicity. J. Virol. 77:13194–13202. 10.1128/JVI.77.24.13194-13202.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Everett RD, Young DF, Randall RE, Orr A. 2008. STAT-1- and IRF-3-dependent pathways are not essential for repression of ICP0-null mutant herpes simplex virus type 1 in human fibroblasts. J. Virol. 82:8871–8881. 10.1128/JVI.00613-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Halford WP, Weisend C, Grace J, Soboleski M, Carr DJ, Balliet JW, Imai Y, Margolis TP, Gebhardt BM. 2006. ICP0 antagonizes Stat 1-dependent repression of herpes simplex virus: implications for the regulation of viral latency. Virol. J. 3:44. 10.1186/1743-422X-3-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Leib DA, Harrison TE, Laslo KM, Machalek MA, Moorman NJ, Virgin HW. 1999. Interferons regulate the phenotype of wild-type and mutant herpes simplex viruses in vivo. J. Exp. Med. 189:663–672. 10.1084/jem.189.4.663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Svensson A, Bellner L, Magnusson M, Eriksson K. 2007. Role of IFN-alpha/beta signaling in the prevention of genital herpes virus type 2 infection. J. Reprod. Immunol. 74:114–123. 10.1016/j.jri.2006.09.002 [DOI] [PubMed] [Google Scholar]
- 99.Vollstedt S, Arnold S, Schwerdel C, Franchini M, Alber G, Di Santo JP, Ackermann M, Suter M. 2004. Interplay between alpha/beta and gamma interferons with B, T, and natural killer cells in the defense against herpes simplex virus type 1. J. Virol. 78:3846–3850. 10.1128/JVI.78.8.3846-3850.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang JP, Bowen GN, Zhou S, Cerny A, Zacharia A, Knipe DM, Finberg RW, Kurt-Jones EA. 2012. Role of specific innate immune responses in herpes simplex virus infection of the central nervous system. J. Virol. 86:2273–2281. 10.1128/JVI.06010-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Honda K, Yanai H, Negishi H, Asagiri M, Sato M, Mizutani T, Shimada N, Ohba Y, Takaoka A, Yoshida N, Taniguchi T. 2005. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature 434:772–777. 10.1038/nature03464 [DOI] [PubMed] [Google Scholar]
- 102.Menachery VD, Pasieka TJ, Leib DA. 2010. Interferon regulatory factor 3-dependent pathways are critical for control of herpes simplex virus type 1 central nervous system infection. J. Virol. 84:9685–9694. 10.1128/JVI.00706-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Murphy AA, Rosato PC, Parker ZM, Khalenkov A, Leib DA. 2013. Synergistic control of herpes simplex virus pathogenesis by IRF-3, and IRF-7 revealed through non-invasive bioluminescence imaging. Virology 444:71–79. 10.1016/j.virol.2013.05.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Clements GB, Stow ND. 1989. A herpes simplex virus type 1 mutant containing a deletion within immediate early gene 1 is latency-competent in mice. J. Gen. Virol. 70:2501–2506. 10.1099/0022-1317-70-9-2501 [DOI] [PubMed] [Google Scholar]
- 105.Leib DA, Coen DM, Bogard CL, Hicks KA, Yager DR, Knipe DM, Tyler KL, Schaffer PA. 1989. Immediate-early regulatory gene mutants define different stages in the establishment and reactivation of herpes simplex virus latency. J. Virol. 63:759–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gupta R, Warren T, Wald A. 2007. Genital herpes. Lancet 370:2127–2137. 10.1016/S0140-6736(07)61908-4 [DOI] [PubMed] [Google Scholar]
- 107.Ashkar AA, Rosenthal KL. 2003. Interleukin-15 and natural killer and NKT cells play a critical role in innate protection against genital herpes simplex virus type 2 infection. J. Virol. 77:10168–10171. 10.1128/JVI.77.18.10168-10171.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gill N, Ashkar AA. 2007. Adaptive immune responses fail to provide protection against genital HSV-2 infection in the absence of IL-15. Eur. J. Immunol. 37:2529–2538. 10.1002/eji.200636997 [DOI] [PubMed] [Google Scholar]
- 109.Gill N, Ashkar AA. 2009. Overexpression of interleukin-15 compromises CD4-dependent adaptive immune responses against herpes simplex virus 2. J. Virol. 83:918–926. 10.1128/JVI.01282-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Halford WP, Puschel R, Rakowski B. 2010. Herpes simplex virus 2 ICP0 mutant viruses are avirulent and immunogenic: implications for a genital herpes vaccine. PLoS One 5:e12251. 10.1371/journal.pone.0012251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Stow ND, Stow EC. 1986. Isolation and characterization of a herpes simplex virus type 1 mutant containing a deletion within the gene encoding the immediate early polypeptide Vmw110. J. Gen. Virol. 67:2571–2585. 10.1099/0022-1317-67-12-2571 [DOI] [PubMed] [Google Scholar]
- 112.Brown SM, Ritchie DA, Subak-Sharpe JH. 1973. Genetic studies with herpes simplex virus type 1. The isolation of temperature-sensitive mutants, their arrangement into complementation groups and recombination analysis leading to a linkage map. J. Gen. Virol. 18:329–346 [DOI] [PubMed] [Google Scholar]