ABSTRACT
The virion of dengue virus (DENV) is composed of a viral envelope covering a nucleocapsid formed by a complex of viral genomic RNA and core protein (CP). DENV CP forms a dimer via the internal α2 and α4 helices of each monomer. Pairing of α2-α2′ creates a continuous hydrophobic surface, while the α4-α4′ helix pair joins the homodimer via side-chain interactions of the inner-edge residues. However, the importance of dimer conformation and the α4 helix of DENV CP in relation to its function are poorly understood. Loss of association between CP and lipid droplets (LDs) due to mutation suggests that the CP hydrophobic surface was not exposed, offering a possible explanation for the absence of dimers. Further assays suggest the connection between CP folding and protein stability. Attenuation of full-length RNA-derived virus production is associated with CP mutation, since no significant defects were detected in virus translation and replication. The in vitro characterization assays further highlighted that the α4-α4′ helix pair conformation is critical in preserving the overall α-helical content, thermostability, and dimer formation ability of CP, features correlated with the efficiency of nucleocapsid formation. Addition of Tween 20 improves in vitro nucleocapsid-like particle formation, suggesting the role of the LD in nucleocapsid formation in vivo. This study provides the first direct link between the α4-α4′ helix pair interaction and the CP dimer conformation that is the basis of CP function, particularly in nucleocapsid formation during virion production.
IMPORTANCE Structure-based mutagenesis study of the dengue virus core protein (CP) reveals that the α4-α4′ helix pair is the key to maintaining its dimer conformation, which is the basis of CP function in nucleocapsid formation and virus production. Attenuation of full-length RNA-derived virus production is associated with CP mutation, since no significant defects in virus translation and replication were detected. In vitro inefficiency and size of nucleocapsid-like particle (NLP) formation offer a possible explanation for in vivo virus production inefficiency upon CP mutation. Further, the transition of NLP morphology from an incomplete state to an intact particle shown by α4-α4′ helix pair mutants in the presence of a nonionic detergent suggests the regulatory role of the intracellular lipid droplet (LD) in CP-LD interaction and in promoting nucleocapsid formation. This study provides the first direct link between the α4-α4′ helix pair interaction and CP dimer conformation that is the fundamental requirement of CP function, particularly in nucleocapsid formation during virion production.
INTRODUCTION
Viruses of the Flaviviridae family are enveloped and have a monopartite, linear, and positive-polarity single-stranded RNA genome. A member of this family in the Flavivirus genus, Dengue virus (DENV), is the most important human arthropod-borne viral pathogen, putting 3 billion people at risk and resulting in 50 million to 100 million infections and around 22,000 deaths annually (1). No specific treatment is available, partly due to a lack of detailed understanding of the virus life cycle. The DENV genome is ∼11 kb in length and is flanked by 5′ and 3′ untranslated regions (UTR) with a 5′ cap but no 3′ polyadenylated tail. The genome encodes a polyprotein that is processed co- and posttranslationally by host and viral proteases to generate three structural proteins, including the core, precursor membrane (prM), and envelope (E) proteins, and seven nonstructural proteins, NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5 (2).
Core protein (CP) is the first translated protein generated by the polyprotein. A signal peptide at the C terminus of CP facilitates the translocation of the subsequent prM protein into the lumen of the endoplasmic reticulum (ER) (2). The mature form of CP (100 amino acids [aa]) is liberated from the ER membrane upon viral NS2B/NS3 protease cleavage (3, 4). The N-terminal coding sequence of CP, which overlaps with cis-acting RNA elements, is required for virus replication and translation (5, 6). The DENV virion is ∼50 nm in diameter and contains an outer glycoprotein shell that is composed of M and E proteins on the surface, a host-derived lipid bilayer, and the nucleocapsid embedded within (7, 8). The internal nucleocapsid has a relatively poorly ordered structure compared to its external icosahedral glycoprotein shell (7–9). CP is the building block of the nucleocapsid, and it has been postulated that multiple copies of CP and one copy of the RNA genome form one nucleocapsid. The replication and assembly events of DENV occur within the same compartment of cytoplasmic virus-induced membranous structures (10), and virion assembly appears to occur through the budding of nucleocapsid into the ER lumen, acquiring the membrane-anchored prM and E proteins (10).
The structure of flavivirus CP suggests the function of the structural features (11, 12). The N terminus of DENV CP is unstructured, and the remaining 80% of the protein contains four α-helices (α1 to α4) and forms a dimer in solution (11, 13). The CP dimer is formed by two pairs of antiparallel helices, α2-α2′ and α4-α4′, through extensive hydrophobic interaction (11). The dimer conformation and surface positive charge distribution of DENV CP suggest that nucleocapsid assembly occurs through lipid membrane interaction on the α2-α2′ hydrophobic surface and genome RNA interaction on the other positively charged surface, the α4-α4′ surface (11). CP interacts with RNA nonspecifically, and the RNA chaperone activity displayed by flavivirus CP may play an important role in assisting nucleocapsid formation (14, 15).
The disordered N-terminal region of DENV CP is reported to be important in virus production (16). Several conserved residues within the N-terminal region, the α1 helix, the loop between the α1 and α2 helices, and the α2 helix are involved in the interaction between CP and lipid droplets (LDs), likely via an LD surface protein, perilipin 3 (TIP47) (17, 18). The CP-LD interaction prompts the conformational rearrangement of the CP dimer and enables access of the α2-α2′ hydrophobic surface to the LD (17). The DENV CP-LD association is essential for virus production (19). These studies (17, 19) have suggested that the CP dimer displays a certain degree of plasticity, particularly from the N-terminal region to the α2 region, whereas the α3 and α4 helices do not undergo significant changes upon binding of the LD at the N-terminal region (17). Structural preservation of the α3 and α4 helices might be explained by the extensive hydrophobic interaction among the side chains within the inner edge of α4-α4′ to stabilize the dimer conformation (11). Further, the locations of basic residues on the CP surface, particularly the α4-α4′ surface, were preserved. Collectively, all these findings underlined the role played by α4 in maintaining CP structural integrity to display the features of basic and hydrophobic surfaces.
In the present study, we examined the importance of the α4 coiled-coil-like structure of DENV CP for the function of nucleocapsid formation and virion assembly using a structure-based mutagenesis strategy. In vivo protein stability and in vitro characterization experiments suggested that disruption of the α4-α4′ helix pair affects protein stability and overall conformation. Additionally, the unstable α4-α4′ helix pair CP mutants were unable to interact with the LD, suggesting that their α2-α2′ hydrophobic surface was not exposed and the formation of the dimer was prevented. Attenuation of virus production efficiency upon CP mutation appeared to be associated with less-efficient nucleocapsid formation. To our knowledge, this is the first study providing a direct link between the disruption of α4-α4′ helix pair interaction, leading to overall protein structural changes, and the impairment of protein function. Importantly, the study establishes a strong correlation between CP dimer conformation and its function as the building block for the nucleocapsid.
MATERIALS AND METHODS
Cells and virus.
The baby hamster kidney fibroblast cell line BHK-21 was maintained in Dulbecco's modified Eagle's medium (DMEM) (Invitrogen) supplemented with 10% heat-inactivated fetal bovine serum (FBS) and 2% penicillin-streptomycin. The Aedes albopictus cell clone C6/36 was maintained in RPMI 1640 medium (Invitrogen) supplemented with 10% FBS, 2 mM l-glutamine, 0.1 mM nonessential amino acids, 1 mM sodium pyruvate, and 2% penicillin-streptomycin. All cells were cultured at 37°C (BHK-21) or 28°C (C6/36) with 5% CO2. DENV-2 strain PL046 (GenBank accession number AJ968413.1) was used in this study. In this work, a constructed recombinant full-length infectious clone of DENV-2 PL046 is referred to as the wild type (WT). For in vivo virus characterization study, DENV-2 strain 16681 (GenBank accession no. AJ87411.1) was used.
Plasmids and constructs.
Standard molecular biology techniques were used. DENV genome RNA was obtained from virus stock using the QIAamp viral RNA minikit (Qiagen). Reverse transcription-PCR (RT-PCR) was carried out to obtain DENV cDNA. Primers used for RT-PCR amplification were designed according to the conserved sequences of published DENV-2 sequences. The multiple cloning site of Escherichia coli-yeast shuttle vector pRS424 was engineered to become pRS424-LK3, which has unique restriction enzyme sites in the order SacI, BstEII, XmaI, MluI, and ClaI. Different DENV cDNA fragments were cloned into the pRS424-LK3 vector.
DENV cDNA fragment A, containing a SacI site, the SP6 promoter, the coding sequence of DENV nucleotides (nt) 1 to 3206, and a BstEII site, was generated by multiple steps. Construct pRS424-AΔ, with an in-frame deletion of the prM and E protein coding sequence (DENV nt 462 to 2355), was obtained by overlapping PCR from DENV cDNA followed by cloning into the pRS424-LK3 vector. The retention of the N-terminal 2 amino acids of the prM protein and the C-terminal 26 amino acids of the E protein allowed the viral polyprotein to retain the correct topology across the endoplasmic reticulum for viral polyprotein processing. Construct pRS424-AΔN, with a NotI site inserted into DENV nt 2355 of construct pRS424-AΔ, was obtained by site-directed mutagenesis (SDM). The DENV prM-E gene was cloned into the NotI site of construct pRS424-AΔN to generate construct pRS424-AN. Both NotI sites were then removed via SDM, restoring the DENV-2 strain PL046 genome sequence to obtain construct pRS424-A. The extra SacI and BstEII sites within the prM gene were silenced through SDM, retaining the native amino acid residues, to obtain construct pRS424-AdSdB (fragment A).
DENV cDNA fragment B (BstEII site–DENV nt 3196 to 5481–XmaI site), fragment C (XmaI site–DENV nt 5476 to 8130–MluI site), and fragment F (MluI site–DENV nt 8125 to 10723–ClaI site) were individually cloned into the pGEM-Teasy vector by TA cloning and then subcloned into the pRS424-LK3 vector to generate construct pRS424-B, construct pRS424-C, and construct pRS424-F, respectively. Construct pRS424-Fb was derived from construct pRS424-F by inserting a unique BamHI site into nt 10316 of DENV cDNA via SDM. A hepatitis delta virus cis-cleaving ribozyme coding sequence (RzD) was inserted between the 3′ terminus of the DENV genome and the unique ClaI site. To generate the replication-defective mutant (GDDm), the protein sequence of NS5 662GDD664 was mutated to 662GAA664. Details of amino acid and nucleotide changes upon mutation are listed in Table 1.
TABLE 1.
Mutants and mutated nucleotides of dengue virus CP and NS5
| Original fragment or mutation(s) | Nucleotide (amino acid) sequencea |
|---|---|
| DENV-2 CP α1 | 187ACAAAGAGA195 (30TKR32) |
| K31A/R32A | 187ACggccgcC195 (30TAA32) |
| DENV-2 CP α2 | 232CUGUUCAUGGCCCUGGUGGCGUUCCUU258 (46LFMALVAFL54) |
| L46A/L50A/F53A/L54A | 232gcGUUCAUGGCCgcGGUGGCGgcCgcU258 (46AFMAAVAAA54) |
| DENV-2 CP α2 | 250GCGUUCCUU258 (52AFL54) |
| F53A/L54A | 250GCGgcCgcU258 (52AAA54) |
| DENV-2 CP α4 | 328AUCAACGUCUUGAGA342 (78INVLR82) |
| I78S/L81Sb | 328ucCAACGUCUcuAGA342 (78SNVSR82) |
| DENV-2 CP α4 | 349AGGAAA354 (85RK86) |
| R85A/K86A | 349gcGgcA354 (85AA86) |
| DENV-2 CP α4 | 370CUG372 (L92) |
| L92S | 370ucG372 (S92) |
| DENV-2 | 163GUGUCGACU171//373AACgcgUcGAcCAGG387 (23VST25//93NASTR97) |
| Δ26–96 (ΔC)c | 163GUGUCG168_382AcCAGG387 (23VST25-R97) |
| DENV-3 CP α1 | 185GCGAAGUUC193 (30AKR32) |
| K31A/R32A | 185GCGgcGgca193 (30AAA32) |
| DENV-3 CP α4 | 326AUUAAGGUCUUA337 (78IKVL81) |
| I78S/L81S | 326ucUAAGGUCucA337 (78SKVS81) |
| DENV-3 CP α4 | 368CUG370 (L92) |
| L92S | 368ucG370 (S92) |
| DENV-2 | 9547AUCAGUGGAGAUGAUUGUGUU9567 (660ISGDDCV666) |
| NS5 GDDm | 9547AUCAGUGGAGcUGcUUGUGUU9567 (660ISGAACV666) |
The amino acids encoded by their corresponding nucleotide sequence are shown in parentheses. The bottom line for each pair of sequences represents the coding and amino acid sequence after mutation. The mutated nucleotides are shown in lowercase, and the mutated amino acids are underlined. The superscript and subscript numbers represent the nucleotide numbers of the DENV genome and the amino acid numbers of the DENV CP or NS5 protein, respectively.
An XbaI site (underlined) was introduced into the mutation site by site-directed mutagenesis.
A SalI site was introduced into the WT sequence by site-directed mutagenesis. The SalI site sequences are underlined. The double slash represents a discontinuous sequence.
To generate a DENV full-length infectious (FL) clone, DNA fragments A, B, C, and F from the pRS424-AdSdB, pRS424-B, pRS424-C, and pRS424-F constructs, respectively, were sequentially assembled. CP mutants were generated through SDM of construct pRS424-AdSdB, and the changes in the CP sequence are listed in Table 1. Internal deletion of CP aa 26 to 96 (ΔC, or Δ26–96) was created by introducing a new SalI site at nt 378 followed by self-ligation with another SalI site at nt 165 to remove the internal region. The manipulated A fragments were cloned into the WT FL replicon with the aid of SacI and BstEII. The mini-expression cassette for mature CP is a truncated form of the viral genome that encodes only a mature form of CP (DENV-2 nt 97 to 396) and two copies of the hemagglutinin (HA) tag, flanked by the 5′ and 3′ untranslated regions (UTRs), DENV-2 nt 1 to 96 and nt 10270 to 10723, respectively. The translation of HA fusion CP was driven by the native DENV-2 RNA elements on the 5′ and 3′ terminal regions of the DENV genome. All constructs were cloned and propagated in E. coli strain JM101. Sequences of all clones were validated using primers covering various regions of the DENV genome. The sequence of the DENV-2 PL046 strain infectious clone has been deposited in GenBank (see below), and the infectious clone is available to qualified researchers upon request.
In vitro RNA synthesis and RNA transfection.
Plasmid templates to be used for in vitro transcription were linearized with ClaI at 37°C overnight. The 5′-capped transcripts were synthesized in vitro from ClaI-linearized plasmids using the AmpliCap SP6 High Yield Message Maker kit (Cellscript) according to the manufacturer's protocol. The amount of transcribed RNA was quantified by using a spectrophotometer, and the RNA integrity was examined using formaldehyde-agarose gel (1.5% agarose) electrophoresis. A total of 10 μg of FL RNA or 5 μg of mini-CP RNA with 5 μg of BHK-21 total cell RNA was electroporated into 2 × 106 BHK-21 cells using the Gene Pulser Xcell electroporation system (Bio-Rad) according to the manufacturer's instructions (140 V, 25 ms, 1 pulse). The transfected cells were resuspended in DMEM with 10% FBS and incubated at 37°C with 5% CO2.
Immunofluorescence assay.
Cells grown on glass coverslips were fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS) for 15 min and washed with PBS. For the lipid droplet association assay, fixed cells were permeated with 0.02% TX-100 for 5 min and washed in PBS. Permeated cells were blocked with 5% bovine serum albumin (BSA) in PBS and incubated with mouse monoclonal anti-HA antibody (1:300) overnight at 4°C. Antibody-labeled cells were examined by using Cy5-conjugated goat anti-mouse IgG (1:400) for 2 h at room temperature. Cells were counterpermeated with 0.1% TX-100 for 5 min before staining with Nile Red (1:1,000) for 30 min at room temperature. Alternatively, fixed cells were permeated with 0.1% Triton X-100 (TX-100) for 5 min and a wash in PBS. After blocking with 5% BSA in PBS, cells were incubated with rabbit polyclonal anti-NS3 (1:300), mouse monoclonal anti-E (3H5-1) (1:300), mouse monoclonal anti-CP (1:100), or mouse monoclonal anti-double-stranded RNA (dsRNA) (J2; Biological Research Center of the Hungarian Academy of Sciences) (1:1,000) antibody for 4 h at room temperature or overnight at 4°C. Antibody-labeled cells were detected by cyanine 5 (Cy5)- or fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit or mouse IgG (1:400 for Cy5 and 1:300 for FITC) for 2 h at room temperature. Cells were then stained with 4′,6-diamidino-2-phenylindole (DAPI) prior to PBS washing before mounting. Cells were visualized using a Zeiss 510 LSM confocal microscope. Stacking of three-dimensional (3D) images and 3D surfaces were generated using Imaris 7.6.5 software from multiple continuous sections (0.35 μm/section) from Z-section analysis. Further, the intensity and volume of CP staining of each cell that contained at least 8 LDs were determined, and the LD within the cells that had a CP density of staining intensity per volume (voxel) of at least 65 arbitrary units (AU) was analyzed using Imaris 7.6.5 software to confirm the CP-LD interaction. Colocalization of CP and LD was analyzed by determining the surface area of LD (%) colocalized with CP.
Western blot analysis.
Cell lysate was prepared using passive lysis buffer (Promega) according to the manufacturer's protocols. Total protein was resolved by SDS-PAGE. Western transfer to nitrocellulose membranes was performed. After blocking with 5% skim milk, the blot was incubated with mouse monoclonal anti-HA (8G5F) antibody (1:5,000), rabbit polyclonal anti-NS3 antibody (1:5,000), rabbit polyclonal anti-DENV-3 CP antibody (1:1,000), or anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (1:5,000) followed by horseradish peroxidase (HRP)-conjugated goat anti-mouse or rabbit IgG (1:5,000). The blot was processed for detection with a commercial enhanced chemiluminescence (ECL) detection system. For cycloheximide (CHX) treatment, mini-CP RNA-electroporated BHK-21 cells were divided equally, with 2 × 106 cells were seeded for each time point and incubated at 37°C. Culture medium was replaced with culture medium containing 1 mg/ml of CHX at 4 h posttransfection (hpt). Cell lysates were harvested at the indicated time points and subjected to SDS-PAGE for Western detection using mouse monoclonal anti-HA antibody.
Expression and purification of recombinant CP.
For recombinant CP purification, a plasmid expressing the mature form of DENV-3 (strain Philippines/H87/1956) CP was constructed in a modified pET21b vector in which a stop codon was inserted upstream of the His tag coding sequence (15). Vectors for the expression of CP mutants were generated using site-directed mutagenesis (SDM) (Table 1). CP was expressed in E. coli 41(DE3) cells and purified as described below. Several bacterial colonies were precultured in 45 ml of LB broth containing 100 mg/ml ampicillin overnight at 37°C. A third of the overnight culture was subcultured to 1.5 liter of culturing medium at 37°C for ∼3 h until the A600 reached 0.5 to 0.7. CP expression was induced by application of 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) at 37°C for 5.5 h. Bacterial pellets were harvested by centrifugation and disrupted by using a Microfluidizer in buffer A (50 mM HEPES, pH 7.9, 1 mM EDTA, 15% sucrose, and 10 mM phenylmethanesulfonyl fluoride [PMSF]). Lysate was then pelleted, and the supernatant was filtered through a 0.45-μm filter. NaCl was added to the filtered bacterial cell lysate to a final concentration of 0.4 M NaCl. A phosphocellulose (PC) column was preequilibrated with 0.4 M NaCl-buffer C (50 mM HEPES, pH 8.0, 1 mM EDTA, and 10% glycerol) before bacterial cell lysate loading. The PC column was washed with 0.4 M NaCl-buffer C (without urea) followed by 0.4 M NaCl-buffer C with 6 M urea. Multiple washing steps were carried out by washing the column with decreasing urea concentrations of 4 M, 2 M, and 1 M in 0.4 M NaCl-buffer C. Another two washing steps were conducted using 0.8 M NaCl-buffer C and 0.9 M NaCl-buffer C in the absence of urea. Purified CP was eluted with 1.5 M NaCl-buffer C containing no urea. Fractions containing CP were snap-frozen in liquid nitrogen and stored for further characterization work. Protein concentrations were determined by Bradford assay.
Size exclusion chromatography.
The oligomeric state of CP in solution was analyzed by a Superdex 75 10/300 GL high-performance column using an Äkta fast-protein liquid chromatograph (FPLC) system (GE Healthcare) according to the manufacturer's instructions. A total of 30 μg purified CP in a 180-μl total volume was applied to the column in buffer containing 50 mM Tris (pH 7.5) and 0.2 M NaCl at a flow rate of 0.5 ml/min. Protein elution profiles at 280 nm UV absorption were recorded. Collected fractions (400 μl/fraction) were applied to nitrocellulose paper by slot blotting. CP was detected using rabbit polyclonal anti-DENV-3 CP antibody and HRP-conjugated anti-rabbit IgG antibody.
Cross-linking of CP.
For the cross-linking study, recombinant CP was purified as described previously (15). For cross-linking of purified protein, a volume of 10 μl containing the purified CP dissolved in 50 mM HEPES, pH 7.9, 0.1 M NaCl, 1 mM EDTA, and 2% glycerol was preincubated for 15 min at 25°C. The cross-linking reaction was initiated by the addition of 2.5 μl glutaraldehyde (electron microscopy grade). After incubation for 5 min at 25°C, the reaction was terminated by the addition of 2.5 μl of 1 M Tris-HCl (pH 8.0). Samples were subsequently resolved by 15% SDS-PAGE and transferred to nitrocellulose paper for Western detection using rabbit polyclonal anti-DENV-3 CP antibody.
CD spectroscopy.
The circular dichroism (CD) spectrum of CP (10 μM) in 5 mM HEPES buffer (pH 7.9), 0.2 M NaCl, and 1% glycerol was recorded on an Aviv model 400 CD spectrometer (Aviv Biomedical, Inc.). Measurements were taken in a rectangular quartz cuvette of 2-mm path length (AVIV Biomedical, Inc.). Spectra were recorded in the 190- to 250-nm-wavelength range with 1-nm-increment intervals at 25°C. Ten scans were performed to obtain the average spectra, and the data were smoothed for baseline correction using the software provided by the manufacturer. Melting curves were recorded at 222 nm from 25°C to 90°C with a heating rate of 1°C/min; measurements were obtained at one-degree intervals.
Extracellular and intracellular virus preparation.
A total of 1 × 106 FL RNA-transfected BHK-21 cells were seeded on 6-cm dishes with 4 ml of 2% FBS DMEM and incubated at 37°C. For cell lysate harvest at 4 hpt, 1 × 106 cells/well were seeded in 12-well plates. At 24 hpt, the culture dish was switched to 30°C for further incubation until 96 hpt. Cultural fluid (CF) of FL RNA-transfected cells was filtered and stored at −80°C. Transfected cells were trypsinized and washed twice with PBS via centrifugation. Cell pellets were resuspended in 1 ml complete culture medium and subjected to multiple freeze-thaw cycles. Cell lysate fluid (CLF) and cell debris were separated by centrifugation at 5,000 × g for 5 min. Collected CF and CLF were subjected to focus-forming assay (FFA) or plaque assay as described below.
FFA and plaque assay.
The 12-well plate was seeded with BHK-21 cells at a density of 1 × 105 cells per well and incubated overnight at 37°C to produce a confluent monolayer. The cell monolayers were inoculated with 100 μl of serially diluted inoculums, CF or CLF. Viral adsorption was carried out for 2 h at 37°C. Cells were washed with PBS at the conclusion of adsorption, followed by overlaying the monolayer with culture containing 2% FBS and 1.2% Avicel RC-591 (FMC BioPolymer). After 72 h of incubation at 37°C, the overlay medium was removed from the wells and fixed for 15 min in 4% formaldehyde in PBS, followed by PBS washing and blocking with 5% BSA for 1 h at room temperature. Cells were then incubated with rabbit polyclonal anti-NS3 antibody (1:300) for 4 h at room temperature or overnight at 4°C. Antibody-labeled cells were detected by incubation of the cells for 2 h with Cy3-conjugated goat anti-rabbit IgG (1:300). Fluorescent foci comprising more than 5 cells were counted for each well, and the viral titers are expressed as fluorescent focus-forming units (FFU) per μg of electroporated RNA. For plaque assay, an ∼80% confluent monolayer was cultivated on 6-well plates inoculated with 200 μl of serially diluted inoculums as described above. After virus adsorption for 2 h at 37°C, cells were washed with PBS, followed by overlaying with culture medium containing 2% FBS and 1% low-melting agarose. After 7 days of incubation at 30°C, 10% formaldehyde was added to the well, and the plate was rocked for at least 4 h until the agarose layer detached from the well. The agarose layer was removed, and the cell monolayer was stained with crystal violet.
Determination of viral growth kinetics.
A monolayer of BHK-21 cells in a 6-well dish was infected with virus at a multiplicity of infection (MOI) of 0.1 and cultured for 96 h in 2 ml of 2% FBS DMEM. Every 12 h, 500 μl of CF was removed and replaced with fresh 2% FBS DMEM. Collected CF was subjected to titer determination by FFA.
In vitro NLP assembly.
Different DENV RNA fragments were synthesized in vitro for the nucleocapsid-like particle (NLP) assembly experiment. FL RNA corresponds to the full-length DENV genome, which has a size of 10.7 kb; +180 RNA and +374 RNA contain nt 1 to 180 and nt 1 to 374 of DENV genome, respectively; −393 RNA contains nt 1 to 393 of the minus sense of the DENV genome; +374/−393 is the preannealed dsRNA of +374 RNA and −393 RNA. Purified DENV-3 CP (1 or 2 μM) was mixed with RNA (30 ng/μl) in 10 μl reaction buffer containing 10 mM HEPES (pH 7.5), 0.2 M NaCl, and 0% to 0.02% Tween 20 and incubated at room temperature for 1 h. Reaction mixtures were applied to a 400-mesh Formvar/carbon film nickel grid (Electron Microscopy Sciences) and incubated for 2 min, followed by staining with 2% uranyl acetate (UA) for 45 s. NLP were observed using a Tecnai G2 Spirit Twin electron microscope (FEI) and were photographed with a charge-coupled-device (CCD) Camera (Gatan). Several independent experiments were carried out, and electron micrographs of at least 100 particles were collected from each experiment. To assess NLP-forming efficiency, the number of NLP counted from 20 to 45 2.5-μm2 grid areas was determined. For immunogold staining, the grid with NLP was stained with rabbit polyclonal anti-DENV-3 CP antibody (1:150) or rabbit polyclonal anti-NS3 antibody (1:150), followed by application of 12-nm gold particle-conjugated anti-rabbit IgG (1:40) prior to UA staining.
For sucrose gradient centrifugation, the reaction mixture was loaded on top of 5 to 40% discontinuous sucrose gradients and ultracentrifuged at 38,000 rpm for 90 min at 4°C using a SW 41Ti rotor (Beckman). Sucrose solutions were prepared in TNE buffer (50 mm Tris–HCl, pH 7.5, 0.1 M NaCl, and 0.1 mM EDTA). Fractions of 500 μl (each) were collected from the top to the bottom of the gradient, and 50 μl of the fractions were subjected to immuno-slot blot hybridization to determine the NLP distribution using anti-DENV-3 CP antibody. The remaining fractions (∼400 μl) were concentrated using a Vivaspin 500 concentrator (GE Healthcare), followed by electron microscopy (EM) examination.
Virus preparation and characterization.
C6/36 cells were inoculated with DENV-2 strain 16681 at an MOI of 0.02 and cultured in 2% FBS. CF were harvested at day 8 postinoculation and replaced with new cultural medium. At day 16 postinoculation, CF were filtered and subjected to titer determination by FFA. Filtered CF was precipitated with 8% polyethylene glycol (PEG) (molecular mass, 8,000 kDa) and 0.4 M NaCl at 4°C overnight. The mixture was subjected to centrifugation at 10,000 × g at 4°C for 30 min. Virus pellet was resuspended in 50 to 120 μl of TNE buffer. Virus (∼2 × 106 FFU) was subjected to 0.02% TX-100 treatment or mock treated for 5 min at 25°C for EM examination. Particles were identified in single-blinded fashion in at least three independent experiments, and electron micrographs of at least 50 particles were collected in each experiment. Measurement of particle size, the average of perpendicular diameters of each particle, was performed for virion-size or nucleocapsid-size particles in multiple fields. Immunogold detection was performed using anti-E (HB46) monoclonal antibody to identify the particle of virus.
For sedimentation analysis ∼5 × 106 FFU of virus, with or without 0.02% TX-100 treatment, was subjected to 5 to 40% discontinuous sucrose gradient centrifugation as described above. Fractions were collected from the top to the bottom of the gradient, and 20 μl out of 500 μl of the collected fractions were subjected to immuno-slot blot hybridization using anti-E (4G2) monoclonal antibody. Collected fractions were further diluted to a ∼12.5% sucrose concentration using TNE buffer, followed by centrifugation at 100,000 rpm for 2.5 h at 4°C using a TLA 100.2 rotor (Beckman). Pellet was resuspended in 10 μl of TNE buffer, followed by EM examination.
Nucleotide sequence accession number.
The sequence of the DENV-2 PL046 strain infectious clone has been deposited in GenBank under accession number KJ734727.
RESULTS
Design of CP mutants.
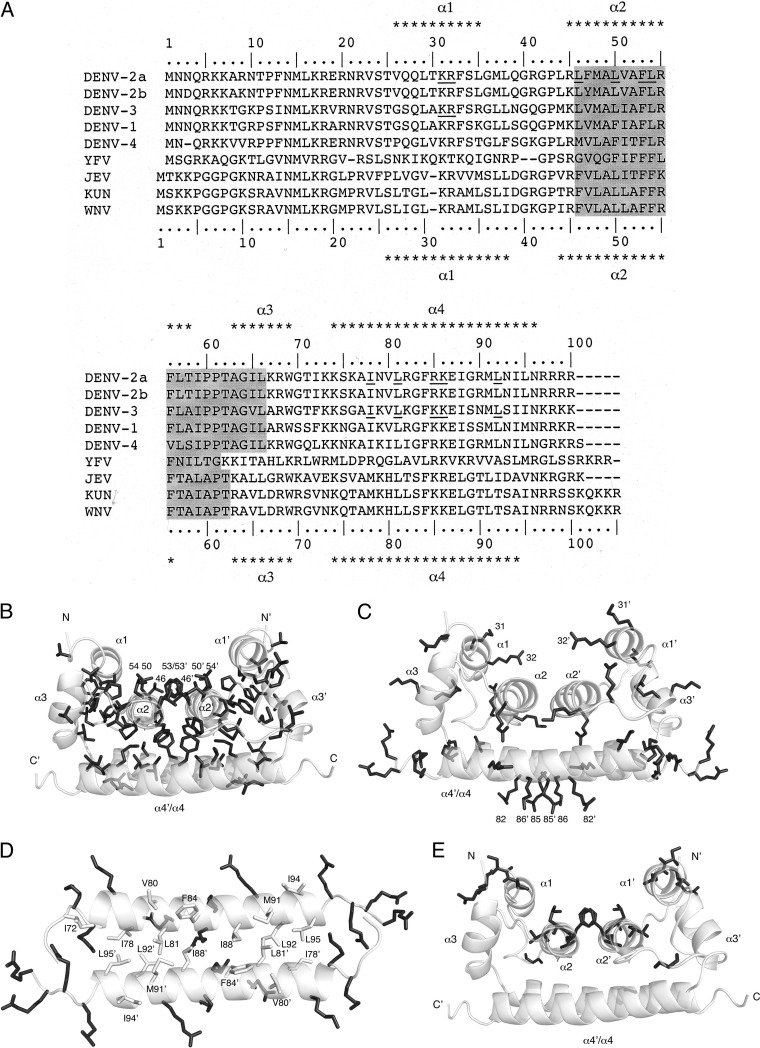
The structural properties of flavivirus CP are highly conserved, particularly the distribution of charged amino acids and the internal hydrophobic sequence (Fig. 1A). DENV CP contains four α-helices (α1 to α4) and exists as a dimer in solution (11, 13). The N-terminal region of DENV CP is unstructured, and the remaining 80% of the protein is responsible for the formation of homodimers (Fig. 1B, C, and D). The interaction between the α2 and α4 helices of two monomers in an antiparallel orientation via α2-α2′ and α4-α4′ interactions is necessary for dimerization (11) and ultimately result in exposure of hydrophobic and basic surfaces. The exposure of these surfaces on the CP dimer, in addition to the membrane and genomic RNA interactions, has suggested it functions as the building block of the nucleocapsid. The joining of the α2-α2′ helix pair forms a hydrophobic cleft (Fig. 1B), and the rest of the hydrophobic residues of CP serve to stabilize the overall protein conformation. Meanwhile, the basic residues of CP are scattered on the surface (Fig. 1C), and it has been previously shown that the highest density of positive charges resides on the α4-α4′ surface (11). Although joining of α2 from each monomer forms a continuous α2-α2′ hydrophobic surface, the antiparallel homodimer structure is mainly joined by the side chains of hydrophobic residues I78, L81, I88, L92, and L95 within the inner edge of helix α4 of one monomer with their counterparts in another monomer to form the α4-α4′ coiled-coil-like structure (Fig. 1D) (11).
FIG 1.
Structural analysis of DENV-2 CP. (A) Alignment of mosquito-borne flavivirus CP sequences. Multiple sequence alignments of CP generated using the BioEdit software program (37) are shown. The α-helical structures α1 to α4 of DENV-2 CP (11) and WNV CP (12) are indicated above and below the amino acid sequences, respectively. The conserved internal hydrophobic region of flavivirus CP is shaded in gray. The conserved residues examined here are underlined. DENV-2, dengue type 2 (a, strain PL046; b, strain 16681); DENV-3, dengue type 3 (strain Philippines/H87/1956); DENV-1, dengue type 1 (strain BR/97-111); DENV-4, dengue type 4 (strain 814669); YFV, yellow fever virus (strain 17D); JEV, Japanese encephalitis virus (strain 826306); KUN, Kunjin (strain MRM61C); WNV, West Nile virus (strain NY99). (B, C, D, and E) Structure of DENV-2 CP. The N-terminal (aa 1 to 20) region of DENV CP is unstructured, while the remaining (aa 21 to 100) residues compose four α-helices (α1, α2, α3, and α4) connected by short loops, resulting in the dimer observed in solution. Contact between α2 and α4 of each monomer forming α2-α2′ and α4-α4′ helix pairs contributes to the antiparallel dimer conformation of DENV CP (11). Side chains of hydrophobic residues L, V, I, M, P, and F within the structure are highlighted, and residues 46, 50, 53, and 54 are marked in panel B. Side chains of basic residues R and K within the structure are highlighted, and the basic residues 31, 32, 82, 85, and 86 are marked in panel C. Residues 71 to 100 of the CP dimer show the hydrophobic side-chain interactions that form the α4-α4′ interface and the high density of positive charge on the surface of the two edges of α4-α4′ dimer. The R and K side chains are shown in black sticks, while the side chains of the L, V, I, M, and F residues are shown in gray sticks in panel D. The structural view in panel D is a 90° rotation about the horizontal axis of the structures in panels B, C, and E. CP dimer structure and residues, including R5, K6, R9, and R18 in the N-terminal region (not shown), undergo chemical shift perturbations upon interaction with a lipid droplet. Highlighted in panel E are R22, S24, and T25 in α1, G40 in the loop between α1 and α2, and R49, V51, F53, and K54 in α2, which undergo chemical shift perturbation upon interaction with a lipid droplet (17). Labels for dimer subunits are designated by primes (′). N and C represent two termini, and α1, α2, α3, and α4 are four alpha helices. All pictures were reproduced from the data under PDB ID code 1R6R using the PyMOL software program.
From the CP dimer structure data, we suspected the α4-α4′ helix pair to be the key element of CP structure and function. Apart from stabilizing the dimer conformation through inner-helix α4-α4′ conformation, the high basic density on the α4-α4′ surface implicates it as the major RNA binding region (11). To examine the association of dimer conformation and CP function, several CP mutants were created via site-directed mutagenesis. In brief, the first two nucleotides of each codon were modified, leaving the third nucleotide unchanged, to create mutants with single, double, or triple codon mutations (Table 1). The conserved hydrophobic residues I78, L81, and L92 along the α4-α4′ inner edge were replaced with the hydrophilic residue serine to weaken the α4-α4′ hydrophobic interaction, resulting in the I78S/L81S/L92S, I78S/L81S, and L92S mutants. To examine the importance of the basic charge on the α4-α4′ surface, the dibasic residues R85 and K86 at the center of the α4-α4′ surface were mutated to alanine to create an R85A/K86A mutant. The dibasic residues of K31 and R32 on the opposite surface (α1 surface) were mutated to create the K31A/R32A mutant to determine the importance of these two basic residues in CP function. Since the α2-α2′ hydrophobic surface is important for CP-LD interaction and virus production (19), several residues located on the α2-α2′ surface were mutated to alanine to reduce the hydrophobicity, to generate F53A/L54A and L46A/L50A/F53A/L54A mutants. These identified structural features of CP were manipulated to gain a better understanding of CP function.
LD association provides insight into CP conformation.
First, we sought to obtain more insight on DENV CP structural features in relation to their functions. CP dimer structure revealed a fold in which the hydrophobic and basic surfaces were exposed (11). Besides possessing membrane association ability (20), the internal hydrophobic region of DENV CP, particularly the surfaces of the α2-α2′ helices, has been shown to possess lipid droplet (LD) association ability (19). In addition to playing an important role in virus production (16), the disordered and basic residue-rich N-terminal region of CP has been reported to bind to the LD, leading to a local conformation change of some basic residues in the N terminus and several residues residing in α1, in the loop linking α1 and α2, and in the central hydrophobic region. This conformational change ultimately permits the LD access to the α2-α2′ hydrophobic region (Fig. 1E) (17).
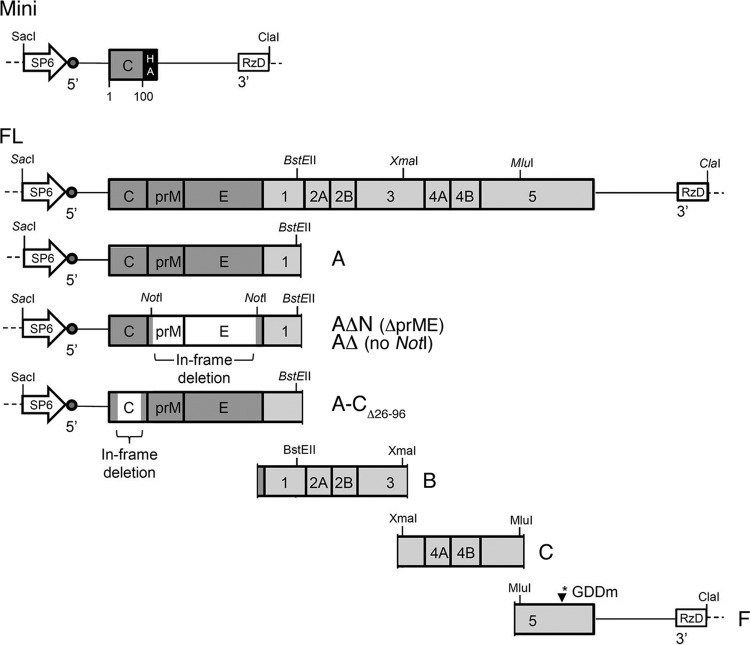
We exploited the interaction with LDs to gain insight into DENV CP conformation upon mutation, focusing on evaluating the CP-LD association to indicate the exposure of the α2-α2′ hydrophobic surface. The expression of CP was induced using an engineered mini-expression cassette to represent the mature form of CP (1 to 100 aa) in BHK-21 cells (Fig. 2). Since our available anti-CP antibodies were not suitable for Western detection, two copies of the HA tag were inserted at the C terminus of CP to create an HA fusion CP. The construct is a truncated form of the viral genome that encodes only a HA fusion CP, and the translation was driven by the native DENV-2 RNA elements in the 5′ and 3′ terminal regions of the DENV genome. Detection of intracellular mature CP was possible using both anti-CP and anti-HA antibodies. Mutations in DENV-2 CP listed in Table 1 were introduced into the mini-HA fusion CP coding sequence through site-directed mutagenesis. To mimic intracellular virus translation, the in vitro-transcribed mini-RNA encoding the HA fusion CP was electroporated into BHK-21 cells and fixed at 8 h posttransfection (hpt). The designed L46A/L50A/F53A/L54A quadruple α2 mutant was used as a non-LD association control. CP staining was detected mainly in the nuclear compartment by 0.1% TX-100 permeation (not shown). To detect the CP located at the same cytoplasmic compartment with LD, mild permeation was performed. CP was detected in the cytoplasm under 0.02% TX-100 permeation conditions using anti-HA antibody. Additional permeation was performed with 0.1% TX-100 after antibody probing but prior to Nile Red staining to avoid smearing of the LD stain under 0.02% TX-100 permeation. LD association was characterized by colocalization of the HA signal of CP surrounding or overlapping with the Nile Red stain of LD.
FIG 2.
Constructs used in this study. The mini-expression cassette (Mini) of CP is a truncated form of the viral genome that encodes the mature CP (100 aa) fused with two copies of the HA tag at its C terminus. The HA fusion CP coding sequence is flanked by viral 5′ and 3′ UTRs. The translation was initiated by the genome RNA elements on the UTRs and its own initiation codon. The full-length infectious clone (FL) was constructed by assembly of different intermediate cDNA fragments (A, B, C, and F) with the aid of unique restriction sites, as indicated (see Materials and Methods for details). An SP6 promoter was placed upstream of the 5′ UTR to drive in vitro transcription, and a hepatitis delta virus cis-cleaving ribozyme coding sequence (RzD) was inserted at the 3′ terminal sequence of the genome to produce a precise 3′ end of synthetic RNA. To mimic the DENV intracellular translation, RNA transcripts were synthesized in vitro from ClaI-linearized plasmids, followed by electroporation.
The interaction between CP and LD was examined in a single confocal microscopy plane (Fig. 3, panel 1) and using Z sectioning (0.35 μm/section) (Fig. 3, panel 2). Most of the cytoplasmic LDs were associated with CP, showing their intracellular colocalizations in cells expressing the WT, the K31A/R32A and R85A/K86A mutants, and the α4-α4′ helix pair L92S single mutant (Fig. 3, panels 1 and 2). It is likely that these CP mutations retained the integrity of α2-α2′ hydrophobic surface for LD interaction. Similar LD association was not observed in the I78S/L81S and I78S/L81S/L92S α4-α4′ helix pair double and triple mutants, respectively (Fig. 3, panels 1 and 2), suggesting that disruption of the α4-α4′ helix pair affects the exposure of α2-α2′ hydrophobic surfaces and CP-LD interaction. The F53A/L54A double hydrophobic mutant retained its LD association ability, while no CP-LD association was observed for the L46A/L50A/F53A/L54A quadruple hydrophobic mutant, indicating that these four residues are important for LD association (Fig. 3, panels 1 and 2).
FIG 3.
The lipid droplet (LD) association ability of CP suggests the exposure of the α2-α2′ hydrophobic region. CP mini-RNA (5 μg) was electroporated into BHK-21 cells (2 × 106 cells) and cultured at 37°C with 5% CO2. Approximately 4 × 105 cells were seeded on glass coverslips in 12-well plates and were fixed at 8 hpt. Fixed cells were permeated with 0.02% TX-100, followed by probing with mouse monoclonal anti-HA antibody (for CP). After secondary fluorescein Cy5-conjugated goat anti-mouse IgG probing, cells were counterpermeated with 0.1% TX-100 prior to Nile Red staining (for LD). Nuclei were stained with DAPI (blue). Cells were visualized using a Zeiss 510 LSM confocal microscope. Panel 1, colocalization of CP-LD in a single plane; panel 2, stacking of 3D images reconstructed from multiple continuous images from Z sectioning (0.35 μm/section); panel 3, 3D surface derived from 3D image of panel 2; sectioning of CP signal revealed the CP-LD interaction (left) and the original image before undergoing sectioning (right); panels 2 and 3 were generated using the Imaris 7.6.5 software; panel 4, CP staining intensity and volume (voxel) of each cell that contains at least 8 LDs were determined, and cells with a CP density of at least 65 AU were selected to determine the surface area of each LD (%) colocalized with CP using Imaris 7.6.5 software. The average surface area of each LD (%) colocalized with CP and the CP density per cell were indicated.
To gain a better understanding of the interaction between CP and LD, we next generated 3D images by stacking the continuous Z-sectioned images of Fig. 3, panel 2, to view the HA tag and Nile Red signals as surface using the Imaris 7.6.5 software program. Partial sectioning of the CP surface covering the LD surface revealed the mode of interaction between CP and the LD (Fig. 3, panel 3). The 3D images revealed that CP of the WT and the K31A/R32A, R85A/K86A, L92S, and F53A/L54A mutants was distributed in clusters within the cytoplasm, colocalizing with a LD by enveloping it fully or partially, whereas CP of the L46A/L50A/F53A/L54A, I78S/L81S/L92S, and I78S/L81S mutants was dispersed throughout the cytoplasmic compartment and was located proximally to the LD without covering it. To perform objective quantification of the CP-LD interaction, the CP staining intensity and volume (voxel) of each cell that contained at least 8 LDs were determined using the Imaris 7.6.5 software program. To avoid low expression of CP that might affect the evaluation of CP-LD interaction, cells with a CP density of staining intensity per volume (voxel) of at least 65 arbitrary units (AU) were selected, and all the LD within the cells was analyzed by determining the surface area of LD (%) colocalized with CP. The surface area of LD in cells expressing CP of the WT and K31A/R32A, R85A/K86A, L92S, and F53A/L54A mutants had more than 50% of CP colocalization, whereas the surface area of LD in cells expressing I78S/L81S/L92S, I78S/L81S, and L46A/L50A/F53A/L54A mutants had less than 12% of CP colocalization (Fig. 3, panel 4). The CP-LD association ability suggests that the exposed hydrophobic surface of CP is associated with its dimer conformation in vivo.
Since mutations of significant residues may affect protein stability, we next examined protein stability in vivo. Mini-expression cassettes containing full-length and mutant CP RNA was electroporated into BHK-21 cells, followed by cycloheximide (CHX) treatment 4 h later. Cell lysates were harvested at different time points post-CHX treatment, followed by Western blotting with anti-HA antibody. The WT and other CP mutants carrying substitutions for selected dibasic surface residues (K31A/R32A and R85A/K86A) and surface hydrophobic residues (F53A/L54A and L46A/L50A/F53A/L54A) were relatively stable (Fig. 4). Among the mutations of the α4 helix pair interacting residues, the single mutant L92S was relatively stable, while the double (I78S/L81S) and triple (I78S/L81S/L92S) mutants were vulnerable to protein degradation (Fig. 4). This suggests that the interaction of residues along the α4 helix pair correlates with protein stability, and protein instability suggested that CP conformation might be affected upon mutation. Collectively, these findings pointed out the association of protein stability and CP folding.
FIG 4.

In vivo stability of CP. CP mini-RNA-transfected cells were treated with cycloheximide (CHX) at 4 hpt and harvested at the indicated time points post-CHX treatment. Cell lysate from approximate 4 × 105 cells at each time point was subjected to 15% SDS-PAGE and Western detection. CP was probed with mouse monoclonal anti-HA antibody followed by HRP-conjugated anti-mouse antibody. GAPDH detection served as the loading control. The results are representative of at least three independent experiments.
Dimer conformation and α-helical configuration of CP.
We next attempted to express and purify recombinant protein. However, relatively low levels of CP expression in E. coli cells made the purification work unfeasible. Previously, the RNA chaperone activity of CP was characterized using purified recombinant CP of DENV-3 (15). Besides having 70% sequence identity and 80% sequence similarity between DENV-2 and DENV-3 CP (Fig. 1A), the amino acid sequence is conserved across the studied mutations. Hence, site-directed mutagenesis was performed to generate several DENV-3 CP mutants (Table 1), and recombinant DENV-3 CP was expressed and purified from E. coli. To characterize the impact of mutations on the α4-α4′ helix pair inner edge on protein conformation, the WT and L92S, I78S/L81S, and I78S/L81S/L92S mutant proteins were purified. The dibasic mutants of K31A/R32A and R85A/K86A CP, which are located at the opposite surface from α1 and α4, respectively, were included since they displayed LD association abilities and were relatively stable in vivo. The purification of the R85A/K86A mutant, however, was not possible due to the low expression level (not shown).
Recombinant CP was purified to near-homogeneity (Fig. 5A). To determine the oligomeric state of CP in its native form, size exclusion column chromatography was performed. A total of 30 μg of purified protein was subjected to column separation, and fractions were collected. After applying the eluate to a nitrocellulose filter by slot blotting, CP in each fraction was detected using the anti-DENV-3 CP antibody. The chromatograms of eluted protein showed that each of the WT, K31A/R32A, and L92S CP forms was eluted in the same fraction, confirming that their protein sizes and shapes were similar (Fig. 5B and C). Additionally, these CP proteins were eluted between the reference fractions of BSA and trypsinogen, suggesting that the WT and the K31A/R32A and L92S mutants exist in dimer form (Fig. 5B and C). However, I78S/L81S CP and I78S/L81S/L92S CP were detected in almost all the fractions (Fig. 5C), suggesting that these CP mutants were nonuniform in size. From their elution profile, they may exist in various conformations ranging from monomers to large aggregates.
FIG 5.
In vitro characterization of recombinant CP from DENV-3. (A) Purified recombinant DENV-3 CP (1.5 μg) resolved by 15% SDS-PAGE and stained with Coomassie blue. (B and C) Chromatogram of purified CP (30 μg) run on a Superdex 75 10/300 GL size exclusion column. Protein elution profiles at 280-nm UV absorption were recorded, and bovine serum albumin (BSA) (66 kDa), trypsinogen (24 kDa), and lysozyme (14.3 kDa) served as protein molecular mass standards (B). Collected fractions from size exclusion chromatography were blotted on a nitrocellulose membrane, and CP was probed with rabbit polyclonal anti-DENV-3 CP antibody followed by HRP-conjugated anti-rabbit antibody (C). (D) Cross-linking to determine the oligomeric state of purified CP. CP (2 μg) was treated with glutaraldehyde at the indicated concentrations for 5 min at 25°C. Proteins were resolved by 15% SDS-PAGE for Western detection. (E) CP CD spectrum. Spectra were measured with 10 μM purified at 25°C. The observed ellipticity value was plotted against wavelength. (F) CP melting curve. The thermal transition of purified CP was recorded at 222 nm, and the observed ellipticity value at 222 nm as a function of temperature was plotted.
To verify the oligomeric state of CP, cross-linking was performed using purified CP (Fig. 5D). The results suggested that WT, K31A/R32A, and L92S CP exist primarily as dimers (Fig. 5D). However, the I78S/L81S and I78S/L81S/L92S proteins tended to form large aggregates in the presence of very low concentrations of cross-linker (Fig. 5D). The dimer conformation appeared to be one of the intermediate forms prior to aggregate formation, which was nondetectable using anti-DENV-3 CP antibody (Fig. 5D). The cross-linking and antibody detection profile of the I78S/L81S and I78S/L81S/L92S forms suggested that their protein conformations varied from those of the WT and the K31A/R32A and L92S mutants, underscoring the importance of the inner-edge hydrophobic side chains of α4-α4′ helix pair for CP dimer formation.
We next examined the structure and thermostability of purified recombinant CP by determining their far-UV circular dichroism (CD) spectrum and midpoint of thermal transition (Tm). WT, K31A/R32A, and L92S CP showed double minima at 208 nm and 222 nm, indicating they are α-helical proteins (Fig. 5E). Both the I78S/L81S and I78S/L81S/L92S mutants lost the double minima profile, suggesting the overall folding of the protein was changed (Fig. 5E). The denaturation curve derived from the ellipticity changes at 222 nm upon heating indicated that WT and K31A/R32A CP had a broad thermal transition profile with a midpoint of thermal transition (Tm) above 65°C (Fig. 5F), suggesting that their α-helical content and protein stability are similar. The Tm of L92S CP was decreased to around 50°C (Fig. 5F). This suggested that CP dimer integrity can be affected by a single residue change in the inner edge of the α4-α4′ helix pair. These findings further confirmed that the pairing of α4 is the key element for CP dimer conformation that appears to be associated with protein folding and stability in vivo.
CP mutations attenuate infectious virus production.
The mutational analysis indicated that single mutations could dramatically alter the conformation of the CP, which led us to examine the effect of these changes on the virus life cycle. A full-length infectious clone (FL) of DENV-2 strain PL046, the WT, was constructed (Fig. 2) (see above for the GenBank accession number of the FL infectious clone). To examine the role of CP conformation on infectious virus production, the designed mutations were introduced into the CP open reading frame (ORF) in the FL context. Transcripts of FL RNA were then electroporated into BHK-21 cells to characterize their virus life cycle events. Viral components of the E protein, NS3 protein, and dsRNA were detectable in the cytoplasm by immunofluorescence 48 hpt in all but the GDDm replication-defective NS5 mutant (Fig. 6A). This indicated that FL of WT and all the CP mutants were successfully replicating within the cells, since the presence of dsRNA is a hallmark of viral RNA replication. Further, the presence of dsRNA in in-frame-deleted CP and prM-E replicons (Fig. 6A) indicated that DENV structural proteins are not required for virus replication.
FIG 6.
Characterization of FL RNA in BHK-21 cells. (A) Immunofluorescence detection of intracellular viral components of FL RNA-electroporated cells at 48 h posttransfection. Viral components in infected cells were detected using rabbit polyclonal anti-NS3 antibody, mouse monoclonal dsRNA antibody, mouse monoclonal anti-E antibody, or mouse monoclonal anti-DENV-2 CP antibody. Protein staining was performed with FITC-conjugated goat anti-rabbit antibody for NS3 or Cy5-conjugated goat anti-mouse antibody for dsRNA, E, and CP. (B) Translation of transfected FL RNA. Cell lysate from approximately 2 × 105 cells were prepared at 4 hpt for Western detection of viral NS3 protein expression. GAPDH detection served as the loading control. (C) Extracellular and intracellular virus production. Viral titers in CF (extracellular fraction) and cell lysate (intracellular fraction) at 96 h post-FL RNA transfection were determined by focus-forming assay. Viral titers are expressed in FFU/μg of electroporated RNA. Data are a representative result of titer determination in triplicates from one transfection experiment. (D) Plaque morphology (upper panel) and focus morphology (lower panel) of virus-producing FL RNAs. Collected CF at 96 h post-FL RNA transfection was subjected to plaque assay and focus-forming assay. (E) Growth kinetics of FL WT-derived virus and DENV-2 PL046. A monolayer of BHK-21 cells in a 6-well dish was infected with virus at an MOI of 0.1 and cultured for 96 h. Titer of virus in cultural fluid (CF) at the indicated period postinfection was determined by FFA. The viral titer is expressed in FFU/ml of CF. Data were obtained from average results of titer determination in triplicates from two independent infections. (F) Immunofluorescence detection of intracellular viral components at 24 h post-virus infection in BHK-21 cells. Cells were infected with FL WT-derived virus and DENV-2 PL046 at an MOI of 1 for 24 h. Viral components in infected cells were detected by immunofluorescence assay (IFA) as described above. (G) Plaque (upper panel) and focus (lower panel) morphologies of FL WT-derived and DENV-2 PL046 viruses. Collected CF at 96 h post-virus infection was subjected to plaque assay and focus-forming assay.
Virus life cycle events require the orchestration of different viral components and are highly correlated with viral protein levels under replication-dependent translation. Since CP is the first translated protein of the genome, viral protein NS3 detection was performed at early time points postelectroporation to examine the translation among different FL replicons. Similar NS3 protein levels were detected at 4 hpt, indicating that there was no significant defect in translation efficiency and protein processing among these FL replicons (Fig. 6B). The processing of CP in cells was not studied because our CP antibody was unsuitable for Western detection (data not shown).
To investigate the effect of engineered CP mutants in infectious virus production, extracellular virus of FL RNA-transfected cells was determined at 96 hpt by immunofluorescent focus-forming assay (FFA). Extracellular viruses produced by WT and K31A/R32A, R85A/K86A, L92S, and F53A/L54A mutants were detected (Fig. 6C). The virus production efficiency of these CP mutants was attenuated, although all of them showed no significant defect in polyprotein processing, translation, and replication. Intracellular viruses were detected in extracellular virus-producing CP mutants (Fig. 6C). No detectable infectious virus was formed in I78S/L81S, I78S/L81S/L92S, and L40A/L50A/F53A/L54A mutants (Fig. 6C), suggesting that these mutations impaired virus production. The results observed for the I78S/L81S and I78S/L81S/L92S mutants underscore the importance of the α4-α4′ helix pair in CP function, while the defect seen in the L40A/L50A/F53A/L54A mutant further emphasized the importance of the hydrophobic surface in virus production. The titer of extracellular virus was higher than that of intracellular virus, suggesting no significant defect in virus release. Since no significant differences were revealed in the tested virus life cycle events as well as the characterization approaches above, attenuation of virus production of CP mutants was most likely due to modifications of its structure that are essential for virus production.
Sizes and morphologies of plaques and immunofluorescent foci were then examined. A significant degree of variability suggested that virus production efficiency and infectivity of these viruses also varied (Fig. 6D). The mutants that formed plaques also induced regional cell death, suggesting that their virus production and infectivity are relatively more efficient. However, the K31A/R32A and R85A/K86A mutants produced foci but not plaques, suggesting reduced infectivity.
Using our CP antibody, we next examined the localization of CP, looking for clues to its structural changes. No significant differences were observed in CP localization among WT, R85A/K86A, and L92S clones (Fig. 6A). Under normal permeation conditions, CP was detected within the nucleus, and cytoplasmic CP was not detected even under mild permeation conditions (not shown). Unfortunately this prevented observation of the interaction between the CP and LD in the FL clone. While slight structural changes in CP following amino acid mutation might not be revealed by the above approaches, failure of the antibody to detect any level of CP suggested that the antibody recognition site might be altered or completely abolished by mutation. In addition, the impairment in virus production might correlate with the diminished availability of functional CP for nucleocapsid formation.
To further characterize the FL WT virus, the FL WT RNA-derived virus from BHK-21 cells and DENV-2 PL046 virus were amplified in C6/36 mosquito cells for 10 days. Harvested cultural fluid (CF) was subjected to BHK-21 cells infection at a multiplicity of infection (MOI) of 0.1 and cultured for 96 h. To determine the growth kinetics of both viruses, harvested CF was subjected to FFA every 12 h for titer determination. The growth curve revealed that the growth kinetics of FL WT-derived virus is similar to that of DENV-2 PL046 (Fig. 6E). Further characterization on immunofluorescent detection of viral components (NS3 protein, dsRNA, E protein, and CP) (Fig. 6F) and plaque and immunofluorescent focus morphology determination (Fig. 6G) indicated that their replication efficiency and infectivity were similar. Hence, the constructed FL WT is applicable for further characterization work to study DENV-2 PL046.
In vitro assembly of NLP.
The nucleocapsid is formed in a near-neutral environment within the cytoplasmic membranous compartment, and the maturation of the virion occurs along the secretory pathway for egress in the gradually-reduced-pH environment (21). It is speculated that nucleocapsid formation requires the binding of viral RNA to the basic surface of the CP dimer. To test the RNA binding affinity of the CP mutant, an electrophoretic mobility shift assay (EMSA) was performed as described previously (15). No significant difference was observed in the RNA binding affinities of different CP mutants (not shown). Recombinant DENV-3 CP was then subjected to in vitro nucleocapsid formation to more closely examine the interaction between CP and RNA. Our experiments analyzing the formation of nucleocapsid-like particles (NLP) were carried out in the absence of membranes, similar to the condition of a previous study of DENV-2 CP (22).
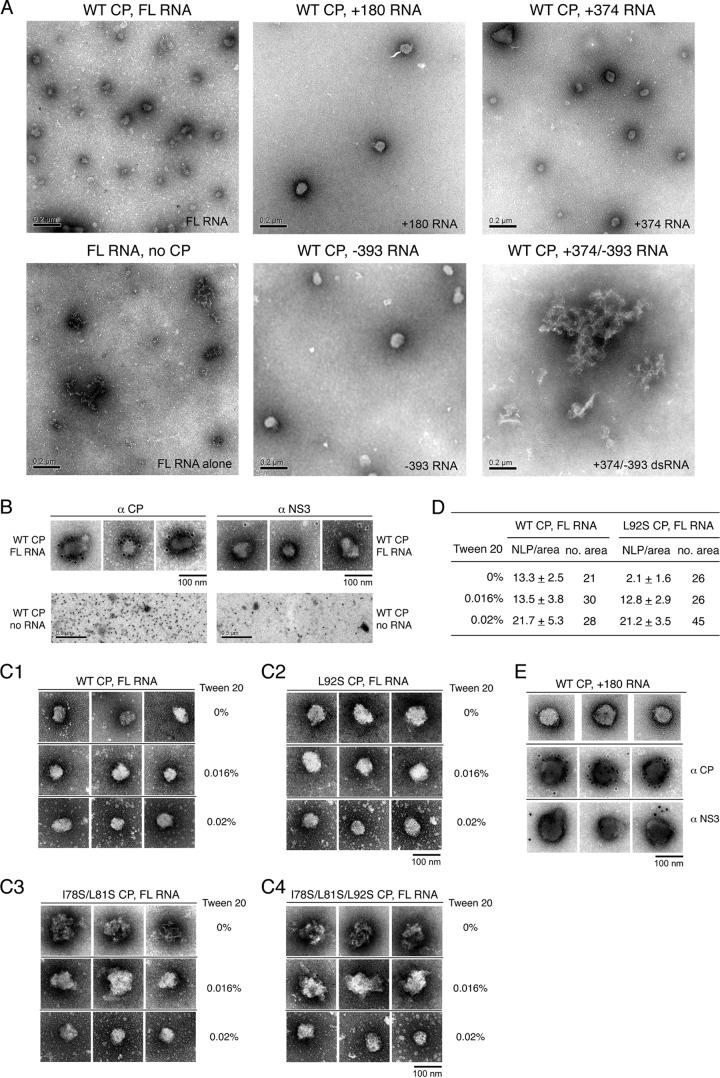
To determine the specificity and optimal conditions for in vitro assembly, recombinant CP (WT) was reacted with different species of RNA to permit NLP formation and then examined by electron microscopy (Fig. 7A). CP was able to form NLP with different lengths of positive-sense DENV RNA, which were 10,723 nt (FL RNA), +180 nt, and +374 nt. Formation of NLP with the negative-sense −393 RNA suggested that DENV CP has no specificity toward the positive or negative strands of DENV RNA (Fig. 7A). No NLP was detected in the absence of CP, suggesting that CP and RNA are cooperating with each other to form the NLP. To determine NLP formation using dsRNA, the +374 and −393 RNAs were preannealed prior to the in vitro assembly reaction. No NLP was detected in the presence of dsRNA (Fig. 7A), suggesting that CP assembles only single-stranded RNA (ssRNA). Further, immunogold staining was performed to identify the complex component of the NLP (Fig. 7B). The anti-DENV-3 CP antibody but not the anti-DENV NS3 antibody recognized the NLP surface, revealing that the NLP contains CP. No NLP was detected in the absence of RNA, and the DENV-3 CP immunogold signal was scattered all over the grid (Fig. 7B). This implies that the formation of a CP-only particle is not possible due to the high positive charge of CP (11).
FIG 7.
In vitro assembly of nucleocapsid-like particles (NLP). (A) Representative electron micrograph of NLP assembled from wild-type (WT) CP and different RNAs. The in vitro assembly reaction of WT CP (2 μM) and FL RNA, +180 RNA, +394 RNA, −393 RNA, or +374/−393 dsRNA (30 ng/μl each) was performed in the standard assembly buffer containing 10 mM HEPES (pH 7.5), 0.2 M NaCl, and 0.02% Tween 20. The no-CP control contained FL RNA alone. The grid was stained with 2% UA. Scale bar, 200 nm. (B) Immunogold staining of in vitro-assembled NLP. The assembly reaction of WT CP (2 μM) plus FL RNA (30 ng/μl each) (top panel) or WT CP (2 μM) alone (bottom panel) was performed in the standard assembly buffer. The grid was probed with rabbit polyclonal anti-DENV-3 CP antibody or rabbit polyclonal anti-DENV NS3 polyclonal antibody and 12-nm gold particle-conjugated anti-rabbit IgG prior to UA staining. Scale bar: 100 nm, top panel; 500 nm, bottom panel. (C1 to C4) Representative electron micrographs of NLP, showing the impact of the nonionic detergent Tween 20 and CP α4 mutation on NLP assembly with FL RNA. The assembly reaction of WT CP (C1), L92S CP (C2), I78S/L81S CP (C3), or I78S/L81S/L92S CP (C4) (1 μM each) and FL RNA (30 ng/μl) was performed in the presence of the indicated concentration of Tween 20. Scale bar, 100 nm. (D) Summary of the effect of Tween 20 on NLP assembly of WT CP and L92S mutant CP (1 μM each) with the FL RNA (30 ng/μl). The average value ± the standard deviation of NLP number per grid area (2.5 μm2) and the total number of examined grid areas are shown. (E) Representative images and immunogold staining of NLP formed from WT CP (2 μM) with the +180 RNA (30 ng/μl) in the presence of 0.02% Tween 20. Scale bar, 100 nm.
Since nonionic detergent has been previously reported to improve in vitro CP oligomerization (13), Tween 20 was applied to our NLP assembly reaction. Proceeding with in vitro assembly reactions in the presence of Tween 20, we observed that FL RNA and WT CP, as well as the L92S, I78S/L81S, and I78S/L81S/L92S α4 mutant CPs, were able to form a complete NLP in the presence of 0.02% Tween 20 (Fig. 7C1 to 7C4, 0.02% Tween 20).
Further, although no significant differences in the NLP morphology of WT or L92S CP was observed in the absence or presence of 0.02% Tween 20 (Fig. 7C1 and 7C2), the L92S mutant exhibited attenuated in vivo virus production (Fig. 6C). To compare NLP formation efficiency between WT CP and L92S CP, the total amounts of NLP within a number of grid areas (NLP/area) were compared. In the absence of Tween 20, the number of NLP formed by the L92S mutant was relatively lower than that for the WT (Fig. 7D). However, in the presence of 0.016% and 0.02% Tween 20, NLP formation efficiency between WT CP and L92S CP as evaluated by NLP/area was similar (Fig. 7D). Although application of Tween 20 was observed to restore NLP formation efficiency for L92S CP, similar efficiency was observed for WT CP in the absence of Tween 20 (Fig. 7D). This finding underscores that the native conformation of WT CP is optimal for NLP formation, whereas L92S CP requires the assistance of Tween 20 to restore its function, suggesting its native conformation is altered.
Interestingly, there was a gradual improvement in NLP formation in the I78S/L81S and I78S/L81S/L92S CP mutants in the presence of Tween 20. More complete NLP were formed in the presence of 0.02% Tween 20, while NLP were incomplete or arrested in an intermediate form at 0% and 0.016% of Tween 20 (Fig. 7C3 and C4). Taken together with the results of the biochemical assays demonstrating that both the I78S/L81S and I78S/L81S/L92S CP mutants lost the native CP conformations necessary for both stability and dimer formation (Fig. 4 and 5B to E), this suggests that NLP formation efficiency is also impacted by the conformation of CP. Further, electron micrographs of the different stages of NLP formation of I78S/L81S CP and I78S/L81S/L92S CP (Fig. 7C3 and C4) also suggest that Tween 20 is improving in vitro NLP formation, resulting in more intact and uniformly sized NLP. All of the CP characterization work described above provides an explanation for the defect in virion production efficiency exhibited by the α4 mutant CPs. These findings further conclude that α4-α4′ helix-pair formation is critical for CP function.
The NLP formation ability of WT CP was further characterized using short +180 RNA (Fig. 7E). No NLP was detected in the absence of Tween 20 (not shown). Interestingly, in the presence of Tween 20, the NLP formed by +180 RNA were larger than the NLP formed by FL RNA, with an average diameter of 67.6 ± 17.7 nm (average for 198 NLP) and 49.5 ± 11.7 nm (average for 277 NLP), respectively. This suggested that genome-length RNA is able to efficiently coordinate the binding of CP and neutralize CP surface charge, resulting in smaller NLP. WT CP was able to form NLP with FL RNA but not short +180 RNA in the absence of Tween 20 (Fig. 7C1 and E). This further implied that genome-length RNA is much more efficient in NLP assembly than short RNA.
Characterization of NLP and envelope-free nucleocapsid.
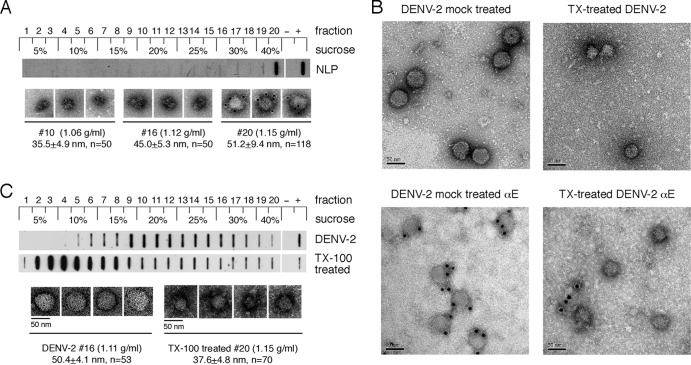
In order to gain insight into the property of NLP, in vitro-assembled NLP was subjected to 5 to 40% discontinuous sucrose gradient centrifugation. Fractions from top to bottom were collected for slot blotting followed by NLP detection using anti-DENV-3 CP antibody. Interestingly, NLP was detected at fraction 20 of the sucrose gradient, which corresponded with the buoyant density of 1.15 g/ml in sucrose, with an average size of 51.2 ± 9.4 nm (n = 117) (Fig. 8A). Selected fractions 10, 16, and 20 were concentrated using a Vivaspin 500 concentrator (GE Healthcare). Electron microscopy and immunogold detection using anti-DENV-3 CP antibody confirmed that the NLP in fraction 20 contained CP (Fig. 8A).
FIG 8.
Characterization of NLP and envelope-free nucleocapsid. (A) Analysis of in vitro-assembled NLP by sucrose gradients. The NLP of WT CP and FL RNA was assembled as described in the legend to Fig. 7 and subjected to 5 to 40% discontinuous sucrose gradient centrifugation. Fractions were collected for slot blotting, followed by NLP detection using anti-DENV-3 CP antibody. Sucrose gradient concentrations with the corresponding fraction numbers are indicated. Concentrated fractions 10, 16, and 20 were subjected to EM and immunogold detection using anti-DENV-3 CP polyclonal antibody followed by 12-nm gold particle-conjugated anti-rabbit IgG prior to UA staining. The “−” control is the 40% sucrose in TNE buffer, and the “+” control is input of assembled NLP. The buoyant density for the fractions and average size of total examined particles are indicated. (B) Representative electron micrographs of DENV-2 particle. Top panel, mock-treated or 0.02% TX-100-treated DENV-2 16681 virus stained by 1% UA average particle size is indicated. Bottom panel, immunogold-stained mock-treated or 0.02% TX-100-treated DENV-2 16681 virus probed with mouse monoclonal anti-E (HB46) antibody followed by 12-nm gold particle-conjugated anti-mouse IgG prior to UA staining. Scale bar, 50 nm. (C) Analysis of DENV-2 particles by sucrose gradient. Top panel, mock-treated or 0.02% TX-100-treated DENV-2 16681 virus was subjected to 5 to 40% discontinuous sucrose gradient centrifugation followed by slot blot analysis using anti-E (4G2) antibody. The “−” control is 40% sucrose in TNE buffer, and the “+” control is DENV. Bottom panel, representative electron micrographs of particles contained in fractions 16 and 20 precipitated down by sucrose cushion centrifugation. The buoyant density and average size of total examined particles are indicated. Scale bar, 50 nm.
In order to study the nucleocapsid of DENV, PEG-purified virus of DENV-2 strain 16681 was subjected to TX-100 treatment to remove the viral membrane (23). The virus preparation was treated with 0.02% TX-100 for 5 min at 25°C and loaded on a mesh nickel grid, followed by negative staining. EM of the mock-treated virus showed spherical particles with an average diameter of 49.0 ± 3.4 nm (n = 119), whereas the particles of 0.02% TX-100-treated virus were relatively smaller, with an average diameter of 32.5 ± 2.2 nm (n = 99) (Fig. 8B, top). Envelope-free nucleocapsid-like particles were recognized by irregular and relatively smaller particles than the virion. Immunogold staining was performed using anti-E (HB46) antibody to identify the observed particles (Fig. 8B, bottom). However, our CP antibody was unsuitable for immunogold staining to recognize the envelope-free nucleocapsid-like particles or CP-RNA complex under EM. Hence, in vitro assembly using envelope-free nucleocapsid isolated from DENV was not performed. No particle was observed for virus treated with TX-100 for 15 min (not shown), suggesting dissociation of envelope-free nucleocapsid.
To further characterize the property of DENV particles, TX-100-treated virus as mentioned above was subjected to 5 to 40% discontinuous sucrose gradient centrifugation followed by slot blot hybridization using anti-E (4G2) antibody. A shift of E protein distribution to the top of the gradient indicated the removal of E protein-anchored viral membrane (Fig. 8C, top). CP distribution in the gradient remained elusive, since our CP antibody was unsuitable for immunoblot detection. Particles contained in fractions 16 and 20, with buoyant densities of 1.11 g/ml and 1.15 g/ml in sucrose, respectively, were pelleted down by sucrose cushion centrifugation and examined under EM (Fig. 8C, bottom). Virion-sized particles were detected in fraction 16 of mock-treated virus with an average size of 50.4 ± 4.1 nm (n = 53), and nucleocapsid-size particles were detected in fraction 20 of TX-100 treated virus, with an average size of 37.6 ± 4.8 nm (n = 70). Sedimentation of nucleocapsid-sized particles at a 40% sucrose concentration suggested the presence of envelope-free nucleocapsid in fraction 20, since the in vitro-assembled NLP (Fig. 8A) was detected in the same sucrose concentration. Further, the two particles share similar morphology. However, the DENV envelope-free nucleocapsid-like particle is relatively smaller than the in vitro-assembled NLP, albeit irregular in shape. Thus, involvement of other proteins or host factors, which are absent in the in vitro assembly reaction, during in vivo nucleocapsid formation to produce the optimal-size nucleocapsid offers a possible explanation for the slightly larger size of in vitro-assembled NLP.
DISCUSSION
Truncation studies of flavivirus CP suggested that the protein exhibits a certain degree of flexibility in virus production, provided its membrane association and RNA binding abilities are retained (24–28). However, truncations altering the structure of CP obscure the precise importance of specific elements of the internal structure. Therefore, point mutations through base substitutions offer an alternative approach to study the role of conserved internal structural features of CP while preserving the overall protein conformation. To determine the global influence of the α4-α4′ helix pair in CP function and in particular while maintaining the dimerization ability, selected α4-α4′ inner-edge residues were replaced. To our knowledge, this is the first study that correlates the α4-α4′ helix pair of DENV CP with its function in nucleocapsid formation and virus production. In this study, we found that the C-terminal, α4 region of CP is important for maintaining the CP dimer conformation that is the base of CP stability and function.
Structure-based mutagenesis in this study shed light on the function of CP structural features in virus production. Mutation of I78, L81, and L92 impaired the CP dimer formation ability and was found to be associated with the issue of CP integrity. This indicates that the inner-edge hydrophobic residues of α4-α4′ significantly contribute to DENV CP dimer formation. The charge distribution of the DENV CP dimer is not uniform, and the solvent-exposed α4-α4′ surface has the highest basic charge density (11). In this study, mutation of basic residues K31/R32 and R85/K86 on the surface of α1 and α4, respectively, attenuated virus production. Although only 4 out of 52 basic residues within the CP dimer were replaced by nonbasic residues, the reduction in virus production efficiency suggested that these residues are playing an important role in coordinating nucleocapsid formation.
Currently, there is no mechanistic explanation describing how CP regulates incorporation of RNA during assembly. The oligomerization mechanism of DENV CP, possibly on the basis of dimerization, has remained elusive since there are no distinct structures in nucleocapsid organization within an infectious virus particle (7–9), and no packaging signal in the viral genome has been identified. The RNA binding of CP is electrostatic and nonspecific (15). This implies that the CP dimer could bind to either positive or negative strands of genomic RNA during viral replication. The nonspecific RNA binding feature of CP can be overcome by the microenvironment provided by the membranous compartments for virus replication and nucleocapsid formation that are induced upon virus infection (10). Positive-strand RNA appeared to be relatively more accessible than negative-strand RNA, since most of the negative-strand RNA existed in the replicative-intermediate form, hybridizing with positive-strand RNA (29). Additionally, our in vitro nucleocapsid formation study showed no interaction between CP and dsRNA to form a nucleocapsid-like particle (NLP) (Fig. 7A), which shed light on the specificity of cytosolic viral proteins. This suggested that although CP and NS5 (the viral RNA-dependent RNA polymerase) are located within the same compartments, CP particles are able to avoid competing with NS5 for the same genome template by discriminating against the single-stranded genome for virion assembly from the replicative-form (double-stranded) RNA for replication. In this study, the in vitro assembly reaction occurred at pH 7.5, close to the intracellular pH value suitable for nucleocapsid formation. However, no NLP was observed in an acidic pH 6.0 environment (not shown).
Due to the large positive charge on the CP dimer, oligomerization of CP dimers into a protein-only core is unlikely (11). We found no NLP in vitro in the absence of RNA (Fig. 7B). Moreover, the sites of RNA replication and virion assembly are adjacent to each other in virus-infected cells, implying that the orchestration of events involving genome packaging and RNA replication occurred rapidly, since full virions but no intermediate particles were detected within the ER vesicles (10). However, the in vitro-assembled NLP appeared to be larger than the ordinary DENV envelope-free nucleocapsid (Fig. 7), suggesting that other cellular or viral components might be necessary for the formation of a standard-sized nucleocapsid in vivo. Hence, the glycoprotein envelope of the prM-E complex, which has a distinct icosahedral structure (7–9), might restrict the size of the nucleocapsid to be embedded within the virion. This suggests that the oversized nucleocapsid, which was unable to fit into the envelope shell, might lead to reduce infectious virion production. It can be further extrapolated that encapsidation of a shorter or truncated viral genome into the virion can be prevented, since a standard nucleocapsid size is required to fit inside the virion. Besides providing insight into charge neutralization as a suggested mode for nucleocapsid formation, the in vitro NLP assembly experiment further implies that efficiency and formation of an optimal-sized nucleocapsid are the important issues during nucleocapsid formation. However, the nucleation events, including the copackaging of prM and E proteins during virion assembly, have yet to be determined.
DENV CP is a small basic protein that has nuclear localization ability (30, 31). When CP is liberated from the membrane after NS2B/NS3 cleavage, initial binding to an LD might serve to retain the availability of CP in the cytoplasm, although CP tends to be localized to and retained in the nucleus due to its size and basic charge-rich feature, respectively (31). Previously, the internal hydrophobic surface of CP was reported to be important for LD association and virus production, whereas the unstructured N-terminal region appears to be important for LD binding and local conformation rearrangement upon LD binding (17, 19). Since no modification of the N-terminal region was done for all CP mutants, the loss of CP-LD interaction in the quadruple hydrophobic mutant (L46A/L50A/F53A/L54A), which includes the two previously reported hydrophobic residues (19), suggested that the initial CP-LD interaction in the N-terminal region is transient and the interaction on the α2-α2′ hydrophobic surface is relatively stable. In addition, CP-LD interaction contributed by the exposure of the α2-α2′ hydrophobic surface suggested CP exists as a dimer in the cell. Further in vitro assays revealed that pairing of α4 is the basis for CP folding and stability. So far, no direct evidence is available to support the notion that the LD plays an important role in DENV virion assembly, as shown in hepatitis C virus (HCV) (32–34). Recent reports showing the interaction of the unstructured N terminus and central hydrophobic regions of CP with LD surface proteins suggest that the LD acts as a scaffold to regulate the availability of CP in the cytoplasmic compartment (17, 18). Hence, it appeared that the impairment of CP-LD association ability might be caused by a more global defect during nucleocapsid formation rather than membrane association or LD targeting alone (35). Although the exact mechanism is yet unclear, the in vitro experiments showing enhanced NLP formation in the presence of Tween 20 provide evidence of the importance of nonionic detergent for in vitro assembly. One possibility is that Tween 20 might be mimicking the role of an intracellular LD. This further suggests that the LD assists the CP-RNA complex in forming an optimally sized nucleocapsid that will precisely fit into the core of the prM-E complex during virion formation. This adds further support for our conjecture that the CP-LD interaction, and by necessity the α2-α2′ hydrophobic surface, are essential for virus production (19).
Collectively, a model for nucleocapsid formation and virion assembly was proposed. Binding of the N-terminal region of CP to LD triggers local conformational rearrangement, exposing the concave hydrophobic surface for optimal CP-LD interaction. CP binds to the LD through interaction with the LD surface protein (e.g., perilipin 3, TIP47), leading to a positively charged surface on the LD (18). The LD regulates the availability of CP for binding with positive-sense and single-stranded viral genome RNA released from the replication complex (RC) within the nearby virus-induced vesicles to form the CP-RNA complex. This event happens within the microenvironment of LD and virus-induced ER membrane-derived vesicles (10) in the cytoplasm. CP might recruit more CP to neutralize the viral RNA negative charge, leading to the collapse of the RNA within the CP-RNA complex. Binding of RNA to CP on the LD surface during the charge-neutralizing process would increase the local phosphate group concentration on the LD surface, and consequently the gradient molecular equilibrium switch will decrease CP-LD binding, leading to the detachment of the CP-RNA complex from the LD (18). Further, improvement of NLP formation in the presence of a nonionic detergent, as observed in our in vitro study, suggests a possible additional role for LD in vivo of making the CP-RNA complex more intact and uniform in size. The two membrane-anchored prM and E proteins aggregate and undergo structural organization at the luminal site of the ER to form a spherical virion envelope. During this process, the membrane-interacting CP-RNA complex on the other cytosolic site of the ER became more packed and condensed approaching the lipid bilayer of the ER. Eventually, the CP-RNA complex or nucleocapsid buds into the ER lumen and is packaged within the virion. Hence, it is suggested that the process of CP-RNA complex formation toward producing a stable nucleocapsid particle is a rapid and continuous event. An optimal-size nucleocapsid will fit into the virion core for infectious virion production, or else empty virus-like particles will be produced. The details describing the nucleocapsid formation mechanism might be revealed when higher-resolution structures of DENV or flavivirus CP-RNA complex are available. Recently, it was reported that DENV CP binds specifically to very low density lipoproteins suggesting the formation of lipoviroparticles, which may be a novel step in the DENV life cycle (36). Finally, this study provides the first direct link between the α4-α4′ helix pair interaction and the CP dimer conformation that is the basic requirement of CP function and in particular contribute to nucleocapsid formation during virion production.
ACKNOWLEDGMENTS
We thank C. C. King of the College of Public Health, National Taiwan University, Taiwan, for DENV-2 (strain PL046), Y. L. Yang of the Department of Biological Science and Technology, National Chiao Tung University, Taiwan, for DENV-3 (strain Philippines/H87/1956), the late H. Y. Lei of the College of Medicine, National Cheng-Kung University, Taiwan, for mouse monoclonal anti-DENV-2 CP antibody, S. C. Cheng of the Institute of Molecular Biology, Academia Sinica, Taiwan, for anti-HA antibody, and H. C. Wu of the Institute of Cellular and Organismic Biology, Academia Sinica, Taiwan, and H. W. Chen of the National Health Research Institute, Taiwan, for the anti-E (4G2) and anti-E (HB46) antibodies, respectively. We extend our gratitude for technical assistance with immunofluorescence analysis and EM offered by the Imaging Core Facility of the Institute of Molecular Biology, Academia Sinica, Taiwan, and to Pao-Yin Chiang for CP structure generation. We are grateful to AndreAna Pena and N. Gopal Naik for English editing.
This work was supported by a grant from the Ministry of Science and Technology, Taiwan, and Academia Sinica, Taiwan.
Footnotes
Published ahead of print 7 May 2014
REFERENCES
- 1.Guzman A, Istúriz RE. 2010. Update on the global spread of dengue. Int. J. Antimicrob. Agents 36(Suppl 1):S40–S42. 10.1016/j.ijantimicag.2010.06.018 [DOI] [PubMed] [Google Scholar]
- 2.Mukhopadhyay S, Kuhn RJ, Rossmann MG. 2005. A structural perspective of the flavivirus life cycle. Nat. Rev. Microbiol. 3:13–22. 10.1038/nrmicro1067 [DOI] [PubMed] [Google Scholar]
- 3.Lobigs M. 1993. Flavivirus premembrane protein cleavage and spike heterodimer secretion require the function of the viral proteinase NS3. Proc. Natl. Acad. Sci. U. S. A. 90:6218–6222. 10.1073/pnas.90.13.6218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amberg SM, Nestorowicz A, McCourt DW, Rice CM. 1994. NS2B-3 proteinase-mediated processing in the yellow fever virus structural region: in vitro and in vivo studies. J. Virol. 68:3794–3802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alvarez DE, de Lella Ezcurra AL, Fucito S, Gamarnik AV. 2005. Role of RNA structures present at the 3′UTR of dengue virus on translation, RNA synthesis, and viral replication. Virology 339:200–212. 10.1016/j.virol.2005.06.009 [DOI] [PubMed] [Google Scholar]
- 6.Clyde K, Barrera J, Harris E. 2008. The capsid-coding region hairpin element (cHP) is a critical determinant of dengue virus and West Nile virus RNA synthesis. Virology 379:314–323. 10.1016/j.virol.2008.06.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang W, Chipman PR, Corver J, Johnson PR, Zhang Y, Mukhopadhyay S, Baker TS, Strauss JH, Rossmann MG, Kuhn RJ. 2003. Visualization of membrane protein domains by cryo-electron microscopy of dengue virus. Nat. Struct. Biol. 10:907–912. 10.1038/nsb990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang X, Ge P, Yu X, Brannan JM, Bi G, Zhang Q, Schein S, Zhou ZH. 2013. Cryo-EM structure of the mature dengue virus at 3.5-A resolution. Nat. Struct. Mol. Biol. 20:105–110. 10.1038/nsmb.2463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuhn RJ, Zhang W, Rossmann MG, Pletnev SV, Corver J, Lenches E, Jones CT, Mukhopadhyay S, Chipman PR, Strauss EG, Baker TS, Strauss JH. 2002. Structure of dengue virus: implications for flavivirus organization, maturation, and fusion. Cell 108:717–725. 10.1016/S0092-8674(02)00660-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Welsch S, Miller S, Romero-Brey I, Merz A, Bleck CKE, Walther P, Fuller SD, Antony C, Krijnse-Locker J, Bartenschlager R. 2009. Composition and three-dimensional architecture of the dengue virus replication and assembly sites. Cell Host Microbe 5:365–375. 10.1016/j.chom.2009.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma L, Jones CT, Groesch TD, Kuhn RJ, Post CB. 2004. Solution structure of dengue virus capsid protein reveals another fold. Proc. Natl. Acad. Sci. U. S. A. 101:3414–3419. 10.1073/pnas.0305892101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dokland T, Walsh M, Mackenzie JM, Khromykh AA, Ee KH, Wang S. 2004. West Nile virus core protein: tetramer structure and ribbon formation. Structure 12:1157–1163. 10.1016/j.str.2004.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones CT, Ma LX, Burgner JW, Groesch TD, Post CB, Kuhn RT. 2003. Flavivirus capsid is a dimeric alpha-helical protein. J. Virol. 77:7143–7149. 10.1128/JVI.77.12.7143-7149.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ivanyi-Nagy R, Lavergne JP, Gabus C, Ficheux D, Darlix JL. 2008. RNA chaperoning and intrinsic disorder in the core proteins of Flaviviridae. Nucleic Acids Res. 36:712–725. 10.1093/nar/gkm1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pong WL, Huang ZS, Teoh PG, Wang CC, Wu HN. 2011. RNA binding property and RNA chaperone activity of dengue virus core protein and other viral RNA-interacting proteins. FEBS Lett. 585:2575–2581. 10.1016/j.febslet.2011.06.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Samsa MM, Mondotte JA, Caramelo JJ, Garmanik AV. 2011. Uncoupling cis-acting RNA elements from coding sequences revealed a requirement of the N-terminal region of dengue virus capsid protein in virus particle formation. J. Virol. 86:1046–1058. 10.1128/JVI.05431-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martins IC, Gomes-Neto F, Faustino AF, Carvalho FA, Carneiro FA, Bozza PT, Mohana-Borges R, Castanho MARB, Almeida FCL, Santos NC, Da Poian AT. 2012. The disordered N-terminal region of dengue virus capsid protein contains a lipid-droplet-binding motif. Biochem. J. 444:405–415. 10.1042/BJ20112219 [DOI] [PubMed] [Google Scholar]
- 18.Carvalho FA, Carneiro FA, Martins IC, Assunção-Miranda I, Faustino AF, Pereira RM, Bozza PT, Castanho MARB, Mohana-Borges R, Da Poian AT, Santos C. 2012. Dengue virus capsid protein binding to hepatic lipid droplets (LD) is potassium ion dependent and is mediated by LD surface proteins. J. Virol. 86:2096–2108. 10.1128/JVI.06796-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Samsa MM, Mondotte JA, Iglesias NG, Assunção-Miranda I, Barbosa-Lima G, Da Poian AT, Bozza PT, Gamarnik AV. 2009. Dengue virus capsid protein usurps lipid droplets for viral particle formation. PLoS Pathog. 5:e1000632. 10.1371/journal.ppat.1000632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Markoff L, Falgout B, Chang A. 1997. A conserved internal hydrophobic domain mediates the stable membrane integration of the dengue virus capsid protein. Virology 233:105–117. 10.1006/viro.1997.8608 [DOI] [PubMed] [Google Scholar]
- 21.Yu IM, Zhang W, Holdway HA, Li L, Kostyuchenko VA, Chipman PR, Kuhn RJ, Rossmann MG, Chen J. 2008. Structure of the immature dengue virus at low pH primes proteolytic maturation. Science 319:1834–1837. 10.1126/science.1153264 [DOI] [PubMed] [Google Scholar]
- 22.López C, Gil L, Lazo L, Menéndez I, Marcos E, Sánchez J, Valdés I, Falcón V, de la Rosa M, Márquez G, Guillén G, Hermida L. 2009. In vitro assembly of nucleocapsid-like particles from purified recombinant capsid protein of dengue-2 virus. Arch. Virol. 154:695–698. 10.1007/s00705-009-0350-8 [DOI] [PubMed] [Google Scholar]
- 23.Kiermayr S, Kofler RM, Mandl CW, Messner P, Heinz FX. 2004. Isolation of capsid protein dimers from the tick-borne encephalitis flavivirus and in vitro assembly of capsid-like particles. J. Virol. 78:8078–8084. 10.1128/JVI.78.15.8078-8084.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kofler RM, Heinz FX, Mandl CW. 2002. Capsid protein C of tick-borne encephalitis virus tolerates large internal deletions and is a favourable target for attenuation of virulence. J. Virol. 76:3534–3543. 10.1128/JVI.76.7.3534-3543.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kofler RM, Leitner A, O'Riordain G, Heinz FX, Mandl CW. 2003. Spontaneous mutations restore the viability of tick-borne encephalitis virus mutants with large deletions in protein C. J. Virol. 77:443–451. 10.1128/JVI.77.1.443-451.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schlick P, Taucher C, Schittl B, Tran JL, Kofler RM, Schueler W, von Gabain A, Meinke A, Mandl CW. 2009. Helices alpha2 and alpha3 of West Nile virus capsid protein are dispensable for assembly of infectious virions. J. Virol. 83:5581–5591. 10.1128/JVI.02653-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu W, Qin C, Chen S, Jiang T, Yu M, Yu X, Qin E. 2007. Attenuated dengue 2 viruses with deletions in capsid protein derived from an infectious full-length cDNA clone. Virus Res. 126:226–232. 10.1016/j.virusres.2007.03.004 [DOI] [PubMed] [Google Scholar]
- 28.Patkar CG, Jones CT, Chang YH, Warrier R, Kuhn RJ. 2007. Functional requirements of the yellow fever virus capsid protein. J. Virol. 81:6471–6481. 10.1128/JVI.02120-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Westaway EG. 1987. Flavivirus replication strategy. Adv. Virus Res. 33:45–90. 10.1016/S0065-3527(08)60316-4 [DOI] [PubMed] [Google Scholar]
- 30.Wang SH, Syu WJ, Huang KJ, Lei HY, Yao CW, King CC, Hu ST. 2002. Intracellular localization and determination of a nuclear localization signal of the core protein of dengue virus. J. Gen. Virol. 83:3093–3102 [DOI] [PubMed] [Google Scholar]
- 31.Sangiambut S, Keelapang P, Aaskov J, Puttikhunt C, Kasinrerk W, Malasit P, Sittisombut N. 2008. Multiple regions in dengue virus capsid protein contribute to nuclear localization during virus infection. J. Gen. Virol. 89:1254–1264. 10.1099/vir.0.83264-0 [DOI] [PubMed] [Google Scholar]
- 32.Boulant S, Montserret R, Hope RG, Ratinier M, Targett-Adams P, Lavergne JP, Penin F, McLauchlan J. 2006. Structural determinants that target the hepatitis C virus core protein to lipid droplets. J. Biol. Chem. 281:22236–22247. 10.1074/jbc.M601031200 [DOI] [PubMed] [Google Scholar]
- 33.Boulant S, Targett-Adams P, McLauchlan J. 2007. Disrupting the association of hepatitis C virus core protein with lipid droplets correlates with a loss in production of infectious virus. J. Gen. Virol. 88:2204–2213. 10.1099/vir.0.82898-0 [DOI] [PubMed] [Google Scholar]
- 34.Miyanari Y, Atsuzawa K, Usuda N, Watashi IK, Hishiki T, Zayas M, Bartenschlager R, Wakita T, Hijikata M, Shimitohno K. 2007. The lipid droplet is an important organelle for hepatitis C virus production. Nat. Cell Biol. 9:1089–1097. 10.1038/ncb1631 [DOI] [PubMed] [Google Scholar]
- 35.Fischl W, Bartenschlager R. 2011. Exploitation of cellular pathways by Dengue virus. Curr. Opin. Microbiol. 14:470–475. 10.1016/j.mib.2011.07.012 [DOI] [PubMed] [Google Scholar]
- 36.Faustino AF, Carvalho FA, Martins IC, Castanho MARB, Mohana-Borges R, Almeida FCL, Da Poian AT, Santos NC. 2014. Dengue virus capsid protein interacts specifically with very low-density lipoproteins. Nanomedicine 10:247–255. 10.1016/j.nano.2013.06.004 [DOI] [PubMed] [Google Scholar]
- 37.Hall TA. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 41:95–98 [Google Scholar]