ABSTRACT
The development of immunization strategies to protect against ocular infection with herpes simplex virus 1 (HSV-1) must address the issue of the effects of the strategy on the establishment of latency in the trigeminal ganglia (TG). It is the reactivation of this latent virus that can cause recurrent disease and corneal scarring. CD8+ T cells and dendritic cells (DCs) have been implicated in the establishment and maintenance of latency through several lines of inquiry. The objective of the current study was to use CD8α−/− and CD8β−/− mice to further evaluate the contributions of CD8+ T cells and the CD8α+ and CD8α− subpopulations of DCs to the protection afforded against ocular infection by immunization against HSV-1 and their potential to increase latency. Neutralizing antibody titers were similar in immunized CD8α−/−, CD8β−/−, and wild-type (WT) mice, as was virus replication in the eye. However, on day 3 postinfection (p.i.), the copy number of HSV-1 glycoprotein B (gB) was higher in the corneas and TG of CD8α−/− mice than those of WT mice, whereas on day 5 p.i. it was lower. As would be anticipated, the lack of CD8α+ or CD8β+ cells affected the levels of type I and type II interferon transcripts, but the effects were markedly time dependent and tissue specific. The levels of latent virus in the TG, as estimated by measurement of LAT transcripts and in vitro explant reactivation assays, were lower in the immunized, ocularly challenged CD8α−/− and WT mice than in their CD8β−/− counterparts. Immunization reduced the expression of PD-1, a marker of T-cell exhaustion, in the TG of ocularly challenged mice, and mock-immunized CD8α−/− mice had lower levels of PD-1 expression and latency than mock-immunized WT or CD8β−/− mice. The expansion of the CD8α− subpopulation of DCs through injection of WT mice with granulocyte-macrophage colony-stimulating factor (GM-CSF) DNA reduced the amount of latency and PD-1 expression in the TG of infected mice. In contrast, injection of FMS-like tyrosine kinase 3 ligand (Flt3L) DNA, which expanded both subpopulations, was less effective. Our results suggest that the absence of both CD8α+ T cells and CD8α+ DCs does not reduce vaccine efficacy, either directly or indirectly, in challenged mice and that administration of GM-CSF appears to play a beneficial role in reducing latency and T-cell exhaustion.
IMPORTANCE In the past 2 decades, two large clinical HSV vaccine trials were performed, but both vaccine studies failed to reach their goals. Thus, as an alternative to conventional vaccine studies, we have used a different strategy to manipulate the host immune responses in an effort to induce greater protection against HSV infection. In lieu of the pleiotropic effect of CD8α+ DCs in HSV-1 latency, in this report, we show that the absence of CD8α+ T cells and CD8α+ DCs has no adverse effect on vaccine efficacy. In line with our hypothesis, we found that pushing DC subpopulations from CD8α+ DCs toward CD8α− DCs by injection of GM-CSF reduced the amount of latent virus and T-cell exhaustion in TG. While these studies point to the lack of a role for CD8α+ T cells in vaccine efficacy, they in turn point to a role for GM-CSF in reducing HSV-1 latency.
INTRODUCTION
A hallmark of ocular infection with herpes simplex virus 1 (HSV-1) is the establishment of latency in the trigeminal ganglia (TG) of the infected individual (1, 2). During the life of the latently infected individual, the virus can occasionally reactivate, travel back to the eye, and cause recurrent disease. Indeed, a major cause of corneal scarring (CS), also known as herpes stromal keratitis (HSK), is the scarring induced by HSV-1 following reactivation from latency (3, 4). Thus, the development of immunization strategies to protect against ocular HSV-1 infection must address the effects of the immunization strategy on the elicitation of latency by subsequent ocular exposure to HSV-1 and the maintenance of latency in the immunized mice. Protective immunity induced by a host following infection is mediated by a combination of innate (e.g., macrophage, NK cell) and adaptive (e.g., neutralizing antibody, cytotoxic T-lymphocyte) immune responses (5–13). In terms of adaptive responses, neutralizing antibodies and T-cell-mediated responses are involved in controlling primary ocular HSV-1 infection in naive mice (5, 14, 15). Both CD4+ T-cell-mediated and CD8+ T-cell-mediated immune responses have been implicated in protection against ocular HSV-1 infection in naive mice (16–18), with adoptive transfer and in vivo T-cell-subset depletion studies suggesting variously that CD8+ T cells alone are sufficient (19–22), that CD4+ T cells alone are sufficient (23–26), or that the CD8+ and CD4+ T cells act together (16, 23, 27). However, CD8+ T cells have been implicated in the development of latency of HSV-1 in naive mice (28).
Dendritic cells (DCs) are powerful antigen-presenting cells (APCs) that play a key role in triggering the immune response against infectious agents. Although both macrophages (29) and DCs (30) can cross present antigens to T cells, only DCs are capable of stimulating naive CD8+ T cells (31, 32). DCs can also play an important role in the initiation of NK activation by viruses (33, 34). As DCs play a crucial role in linking innate and adaptive immunity and optimizing responses, there is increasing interest in using signals that are known to activate DCs or stimulate expansion of these cells to improve vaccine efficacy. Although the ability of DCs to stimulate NK cells and naive CD8+ T cells is of interest in the development of vaccines against HSV-1, it is necessary to clearly establish whether the DCs affect latency as well as protect against primary infections. Previously, we found that the levels of latency in mice that had been depleted of DCs using the diphtheria toxin/diphtheria toxin transgenic mouse system were lower than those in their mock-depleted counterparts, which suggested that DCs can promote HSV-1 latency and demonstrated that myeloid DCs regulate this process (35). In mice, CD11c+ DCs can be subdivided on the basis of their expression of CD8α (36–40). The CD8α+ and CD8α− subsets of DCs differ in terms of their expression of other molecules and their functional roles in vivo and in vitro (41–43). Using an adoptive transfer approach, we have found that the CD8α+ subset of DCs plays a key role in the establishment and maintenance of HSV-1 latency in mice (40), which would suggest that approaches that stimulate the development of CD8α-expressing DCs should be avoided in the development of vaccines against ocular HSV-1 infection.
In the current study, we compared the effects of HSV-1 immunization of CD8α−/− mice, CD8β−/− mice, and wild-type (WT) mice on protection against primary infection and latency in the TG. In T cells, CD8 is functional when dimerized as either CD8α-CD8α homodimers or CD8α-CD8β heterodimers and CD8β cannot form homodimers (44–46). Thus, CD8α−/− mice lack both functional CD8+ T cells and CD8α+ DCs, whereas CD8β−/− mice have functional CD8α+ T cells and CD8α+ DCs. We report here that the absence of functional CD8+ T cells and CD8α+ DCs has no effect on the neutralizing antibody titer or virus replication in the eye after immunization or latency reactivation in ocularly infected mice. In addition, we found that treatment of WT mice with FMS-like tyrosine kinase 3 ligand (Flt3L) DNA enhanced the number of CD8α+ DCs and that this heightened the level of latency in the TG of the surviving mice. In contrast, treatment of WT mice with a granulocyte-macrophage colony-stimulating factor (GM-CSF) genetic adjuvant, which pushes the development of CD8α+ DCs to CD8α− DCs, reduced the amount of latent virus in the TG. These studies point to a lack of a key role for CD8α+ T cells in HSV-1 vaccine efficacy and point to a role for GM-CSF in reducing HSV-1 latency.
MATERIALS AND METHODS
Virus and cells.
Plaque-purified HSV-1 strains were grown in rabbit skin (RS) cell monolayers in minimal essential medium (MEM) containing 5% fetal calf serum (47). RS cells (from Steven L. Wechsler) were described previously (47). KOS, a nonneurovirulent nonstromal disease-producing strain, was used as a live virus vaccine. Since its original isolation by Kendal O. Smith at the Baylor College of Medicine, different substrains of KOS with various degrees of virulence in animals have been isolated (48–50). The avirulent KOS strain that we used in this study is also called KOS-63 (51, 52). McKrae, a stromal disease-causing, neurovirulent HSV-1 strain, was used as the ocular challenge virus.
Mice.
WT C57BL/6 and C57BL/6 CD8α−/− mice were purchased from The Jackson Laboratory. C57BL/6 CD8β−/− mice have been described previously (53) and were bred in-house. All animal procedures adhered to the Association for Research in Vision and Ophthalmology (ARVO) statement for the use of animals in ophthalmic and vision research and were conducted according to institutional animal care and use guidelines (Cedars-Sinai Medical Center).
Immunization.
Mice were immunized three times at 2-week intervals by intraperitoneal (i.p.) administration of 1 × 106 PFU of live, avirulent HSV-1 strain KOS in tissue culture medium, as we described previously (54).
Serum neutralizing antibody titers.
Sera were collected 3 weeks after the final immunization, and serum neutralizing antibody titers were determined using 50% plaque reduction assays, as we described previously (55).
Ocular infection.
Mice were challenged 3 weeks after the final immunization by ocular infection (without corneal scarification) with 2 × 105 PFU of HSV-1 strain McKrae per eye in tissue culture medium (2 μl) (56).
Titration of virus in tears.
Tear films were collected from both eyes of 10 mice per group on days 1 to 5 postinfection (p.i.) of the eye using a Dacron-tipped swab. Each swab was placed in 0.5 ml tissue culture medium and squeezed, and the amount of virus was determined using a standard plaque assay on RS cells (56).
In vitro explant reactivation assay.
Mice were sacrificed at 28 days p.i., and individual TG were removed and cultured in 1.5 ml tissue culture medium, as we described previously (35). Briefly, a 10-μl aliquot was removed from each culture daily for 20 days and used to infect RS cell monolayers. The RS cells were monitored daily for 2 days for the appearance of a cytopathic effect (CPE) to determine the time of first appearance of reactivated virus from each TG. As the media from the explanted TG cultures were plated daily, the time at which reactivated virus first appeared in the explanted TG cultures could be determined.
RNA extraction and cDNA synthesis.
Corneas, TG, and spleens from immunized mice were collected on days 3 and 5 p.i. Additionally, in some immunized mice, TG were isolated on day 28 p.i. Isolated tissues were immersed in RNAlater RNA stabilization reagent and stored at −80°C until processing. The corneas, TG, or spleen from each animal was processed for RNA extraction using the TRIzol reagent (Invitrogen, Carlsbad, CA) and RNeasy column cleanup (Qiagen Inc., Valencia, CA). Briefly, frozen tissue was resuspended in TRIzol and homogenized, followed by addition of chloroform and subsequent precipitation using isopropanol. The RNA was then treated with DNase I to degrade any contaminating genomic DNA, followed by cleanup using a Qiagen RNeasy column as described in the manufacturer's instructions. The RNA yield from all samples was determined by spectroscopy (NanoDrop ND-1000; NanoDrop Technologies, Inc., Wilmington, DE). Isolated total RNA was reverse transcribed with random hexamer primers and the murine leukemia virus (MuLV) reverse transcriptase contained in the High Capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA), according to the manufacturer's recommendations. All isolated corneas used for quantitative reverse transcription-PCR (qRT-PCR), as described below, were free of contamination from other parts of the mouse eye, vitreous fluid, and tears.
TaqMan real-time PCR.
The expression levels of various target genes, as well as the expression of the endogenous control gene, GAPDH (glyceraldehyde-3-phosphate dehydrogenase), were evaluated using commercially available TaqMan gene expression assays (Applied Biosystems, Foster City, CA) with reaction mixtures containing optimized primer and probe concentrations. Primer-probe sets consisted of two unlabeled PCR primers and the 6-carboxyfluorescein (FAM) dye-labeled TaqMan MGB probe formulated into a single mixture. Additionally, all probes used to measure expression of cellular transcripts were designed to overlay an intron-exon junction to eliminate the signal from any potential genomic DNA contamination. The assays used in this study were as follows: (i) CD8 (α-chain) ABI assay Mn01182108_m1 (amplicon length, 67 bp), (ii) programmed death 1 (PD-1; also known as CD279) ABI assay Mm00435532_m1 (amplicon size, 65 bp), (iii) alpha interferon (IFN-α) ABI assay Mm00833961_s1 (amplicon length, 158 bp), (iv) IFN-β ABI assay Mm00439552_s1 (amplicon length, 69 bp), (v) IFN-γ ABI assay Mm00801778_m1 (amplicon length, 101 bp), and (vi) GAPDH ABI assay Mm999999.15_G1 (amplicon length, 107 bp).
The custom-made primers and probe set used in this study were as follows: (i) LAT-specific primers (forward primer, 5′-GGGTGGGCTCGTGTTACAG-3′; reverse primer, 5′-GGACGGGTAAGTAACAGAGTCTCTA-3′; probe, 5′-FAM-ACACCAGCCCGTTCTTT-3′; amplicon length, 81 bp, corresponding to LAT nucleotides 119553 to 119634) and (ii) glycoprotein B (gB)-specific primers (forward primer, 5′-AACGCGACGCACATCAAG-3′; reverse primer, 5′-CTGGTACGCGATCAGAAAGC-3′; probe, 5′-FAM-CAGCCGCAGTACTACC-3′). qRT-PCR was performed using an ABI ViiA7 sequence detection system (Applied Biosystems) in 384-well plates. The threshold cycle (CT) values, which represent the PCR cycles at which there is a noticeable increase in the reporter fluorescence above the baseline, were determined using ViiA7 RUO software.
DNA injection.
The complete open reading frames (ORFs) for murine GM-CSF and Flt3L were purchased from InvivoGen (San Diego, CA). Plasmid DNA was purified using a cesium chloride gradient. In each experiment, 10 female WT mice per group were injected intramuscularly (in the quadriceps), using a 27-gauge needle, with 10 μg of cesium chloride-purified DNA in a total volume of 50 μl of phosphate-buffered saline three times (14 days, 7 days, and 4 h before ocular infection), as we described previously (39, 55). The efficacy of GM-CSF injection compared with that of Flt3L injection to stimulate the increase of CD11c+ CD4+ cells is shown in Fig. 9.
FIG 9.
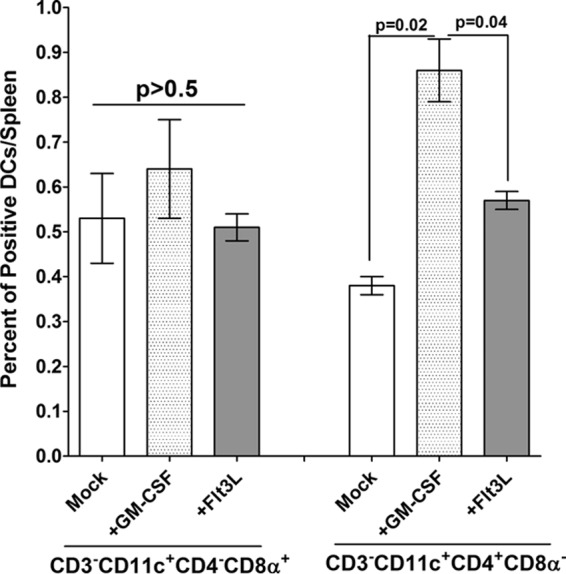
Expansion of CD11c+ CD4+ cells after injection of mice with GM-CSF. WT mice were injected once with GM-CSF DNA or Flt3L DNA or mock injected, as described in Materials and Methods. At 2 weeks postinjection and prior to HSV-1 infection, the spleens of some of the injected mice were harvested, and single cells were prepared, stained with anti-CD3, anti-CD4, anti-CD8α, and anti-CD11c MAbs, and analyzed by flow cytometry. CD3-negative/CD11c-positive cells were gated on expression of CD8α and CD4 cells. A minimum of 104 events was acquired on a gate including viable cells. The mean percentages of CD3− CD11c+ CD4− CD8α+ or CD3− CD11c+ CD4+ CD8α− cells are shown for each treatment from two experiments.
Flow cytometric analysis.
Spleens were isolated from mice injected with GM-CSF or Flt3L or mock-injected mice 2 weeks after the first injection, and single cells were prepared. Isolated lymphocytes were stained with anti-CD3, anti-CD4, anti-CD8α, and anti-CD11c obtained from BD Pharmingen (San Diego, CA) and BioLegend (San Diego, CA) and then analyzed by flow cytometry.
Statistical analysis.
Student's t test and analysis of variance were performed using the computer program Instat (GraphPad, San Diego, CA) to analyze protective parameters. Results were considered statistically significant when the P value was <0.05.
RESULTS
Effects of HSV-1 immunization on levels of neutralizing antibody and replication of HSV-1 in the eyes of challenged mice.
CD8α−/−, CD8β−/−, and WT mice were immunized three times with avirulent HSV-1 strain KOS (n = 8 to 16), and the titers of neutralizing antibody in sera collected 3 weeks after the final immunization were determined. The titers of neutralizing antibody were high in the immunized CD8α−/−, CD8β−/−, and WT mice, and there was no statistically significant difference in the titers among the groups of immunized mice (Fig. 1, P > 0.5, Student's t test).
FIG 1.
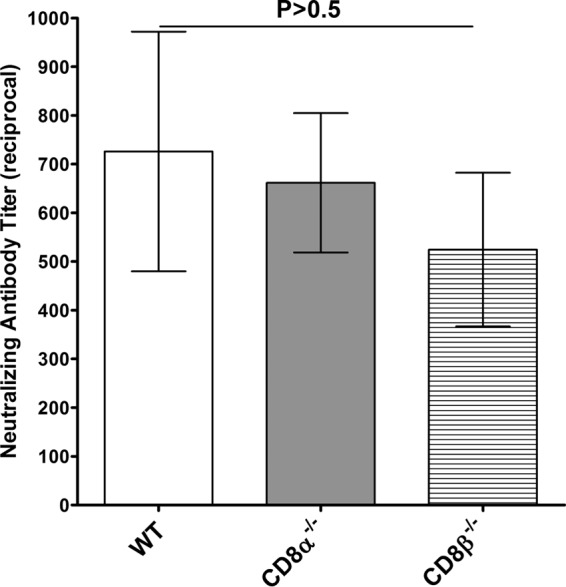
Neutralizing antibody titers in immunized mice. CD8α−/−, CD8β−/−, and WT mice were immunized i.p. with avirulent HSV-1 strain KOS as described in Materials and Methods. Three weeks after the third immunization, mice were bled and neutralizing antibody titers were determined by plaque reduction assays. Each bar represents the average neutralizing antibody titer from 10 serum samples for WT mice, 16 serum samples for CD8α−/− mice, and 8 serum samples for CD8β−/− mice. The error bars indicate the standard errors.
To assess the effects of immunization on primary infection in the different groups, the mice were challenged by ocular infection with HSV-1 and tear films were collected daily on days 1 through 5 p.i. (n = 20 to 32 eyes per group). The amount of infectious virus, determined using a plaque assay, was similar in the immunized CD8α−/−, CD8β−/−, and WT mice during this time period (Fig. 2). Thus, the absence of CD8α+ or CD8β+ T cells or CD8α-expressing DCs did not appear to affect the ability of the immunization strategy to promote the clearance of HSV-1 from the eyes of infected mice.
FIG 2.
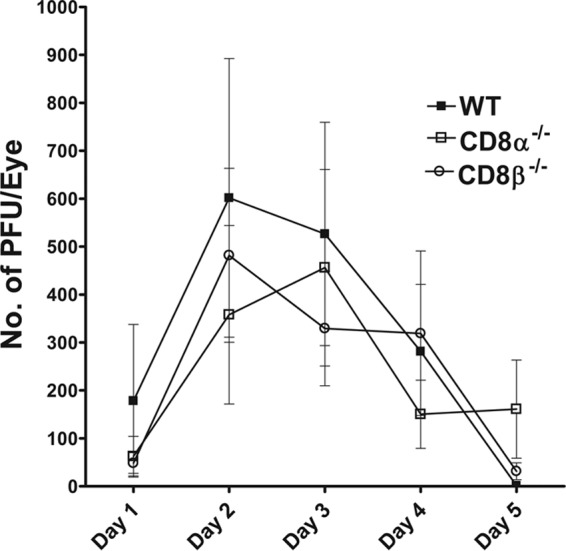
Virus titers in mouse eyes following ocular infection of immunized mice. The immunized mice described in the legend to Fig. 1 were ocularly infected with 2 × 105 PFU/eye of virulent HSV-1 strain McKrae. The presence of infectious virus in the eyes of immunized mice was monitored daily by collecting tear films from 20 eyes for WT mice, 32 eyes for CD8α−/− mice, and 28 eyes for CD8β−/− mice, as described in Materials and Methods. The error bars indicate the standard errors.
Effects of expression of CD8 on establishment of latency in ocularly challenged immunized mice.
To assess the establishment of latency in the immunized, ocularly challenged CD8α−/−, CD8β−/−, and WT mice, we determined the amounts of LAT transcript in individual TG isolated on day 28 p.i. TaqMan RT-PCR was carried out using total RNA isolated from the individual TG. The amount of LAT mRNA detected in the TG of CD8β−/− mice was significantly higher than that detected in the TG of WT or CD8α−/− mice (Fig. 3, P = 0.03). Thus, an absence of CD8β is associated with an increase in HSV-1 latency in the TG of immunized mice that have been challenged by ocular infection, whereas an absence of CD8α does not affect latency. These results are consistent with those in our recent report showing that in naive mice, the absence of CD8α+ T cells correlated with a reduction in viral latency (40). Similarly, it has been shown that the antiviral immune response after acute and chronic lymphocytic choriomeningitis virus (LCMV) infection is enhanced by genetic disruption of Qa-1-restricted CD8+ T-regulatory (Treg) cell activity (57). In marked contrast, there was no significant difference in the amount of LAT RNA in the TG of CD8α−/− mice and WT mice (Fig. 3, P = 0.9), suggesting that the absence of CD8α in immunized mice did not affect the level of latency compared with that detected in immunized WT mice.
FIG 3.
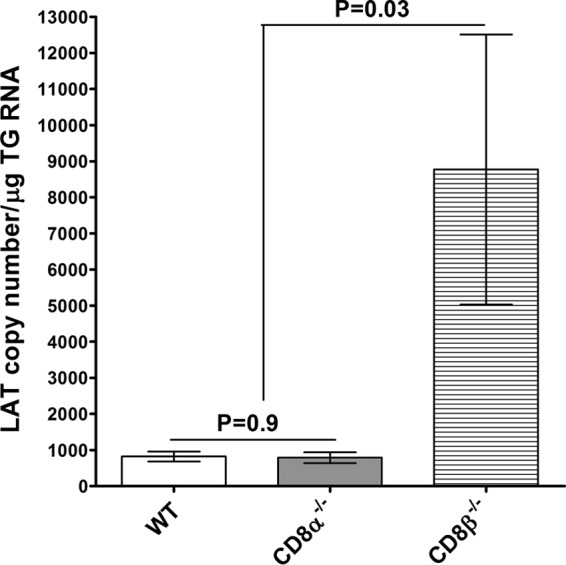
Quantitation of LAT RNA in TG of immunized mice. TG from the mice immunized as described in the legend to Fig. 1 were harvested on day 28 p.i. Quantitative RT-PCR was performed on the TG from each mouse. In each experiment, an estimated relative copy number of the HSV-1 LAT was calculated using standard curves generated from pGem5317. Briefly, DNA template was serially diluted 10-fold such that 5 μl contained from 103 to 1011 copies of LAT and then subjected to TaqMan PCR with the same set of primers. By comparing the normalized threshold cycle of each sample to the threshold cycle of the standard, the copy number for each reaction was determined. GAPDH expression was used to normalize the relative expression of viral LAT RNA in the TG. Each bar represents the mean ± SEM from 18 TG for WT mice, 32 TG for CD8α−/− mice, and 16 TG for CD8β−/− mice.
As it is well established that lower levels of LAT are associated with less reactivation (35, 39, 58, 59), we tested whether this reduction in the levels of LAT in the TG of immunized CD8α−/− mice and WT mice correlated with a reduction in latent virus reactivation. For this purpose, TG were harvested from immunized, ocularly infected CD8α−/− and WT mice on day 28 p.i., and the kinetics of virus reactivation in the explanted TG were measured. During the 20-day monitoring period, reactivation was detected in only 5 of 22 TG from CD8α−/− mice and was not detected in any of the TG from WT mice (Table 1). Although we detected higher levels of reactivation in the CD8α−/− mice than the WT mice, these differences were not statistically significant (Table 1, P = 0.3, Fisher exact test). Collectively, these results suggested that although the immunized CD8α−/−, CD8β−/−, and WT mice had similar neutralizing antibody titers and levels of virus replication, they exhibited differences in the establishment of latency, with the immunized CD8β−/− mice exhibiting higher latency after challenge than CD8α−/− or WT mice. Our results demonstrate that the absence of CD8+ T cells did not increase the level of HSV-1 reactivation from latency in CD8α−/− mice compared with that in WT mice.
TABLE 1.
Effect of CD8α on kinetics of induced reactivation in explanted TG from latently infected immunized micea
| Mouse strain | No. of reactivated TG/total no. of TGb | Days of reactivationc |
|---|---|---|
| CD8α−/− | 5/22 | 4, 5, 6, 7, 9 |
| WT | 0/8 | NAd |
CD8α−/− and WT mice were immunized as described in the legend to Fig. 1. Three weeks after the third immunization, mice were ocularly challenged with HSV-1 strain McKrae, as described in Materials and Methods. On day 28 p.i., individual TG were harvested from infected mice. Each individual TG was incubated in 1.5 ml of tissue culture medium at 37°C. Aliquots of medium were removed from each culture daily for up to 20 days and plated on indicator cells (RS cells) to assay for the appearance of reactivated virus.
Number of TG showing CPEs/total number of TG. Five of the 22 isolated TG showed a CPE on the indicator cells by 20 days after the monitoring period.
The days that the CPE was detected on RS cells.
NA, not applicable.
Levels of PD-1 mRNA in the TG of ocularly challenged immunized mice.
We have shown previously that higher levels of latency of HSV-1 in the TG are associated with higher levels of expression of PD-1, which is a marker of T-cell exhaustion (60). We therefore compared the relative levels of PD-1 in the TG of ocularly challenged immunized mice using RT-PCR. The results are presented in Fig. 4 as the fold increase compared to the baseline mRNA levels in TG from unchallenged, mock-immunized mice for each group. The levels of PD-1 mRNA in the TG of the mock-immunized CD8α−/− mice were significantly lower than the levels of PD-1 mRNA in the TG of mock-immunized WT or CD8β−/− mice (Fig. 4, P < 0.0001). In WT and CD8β−/− mice, immunization with HSV-1 significantly reduced the levels of PD-1 in the TG compared with those in the TG of their mock-immunized counterparts (Fig. 4, P < 0.0001), whereas the levels of PD-1 mRNA in the TG of CD8α−/− mice was not affected by immunization (Fig. 4, CD8α−/−, P = 0.4). However, the level of PD-1 mRNA was higher in the TG of immunized CD8β−/− mice than in the TG of immunized CD8α−/− and WT mice, but the differences were not statistically significant (Fig. 4, P > 0.05). Although the level of PD-1 in immunized CD8α−/− mice was somewhat lower than that in WT mice, this difference was not statistically significant (Fig. 4, P > 0.05). These results suggest that immunization reduces T-cell exhaustion in ocularly challenged mice and that the absence of CD8α is associated with less T-cell exhaustion in immunized mice. As the levels of PD-1 expression have been shown to correlate with the levels of LAT (60), these results provide further evidence that the absence of CD8α is associated with less latency in immunized mice and suggest that this effect may be linked to the levels of T-cell exhaustion in the immunized mice.
FIG 4.
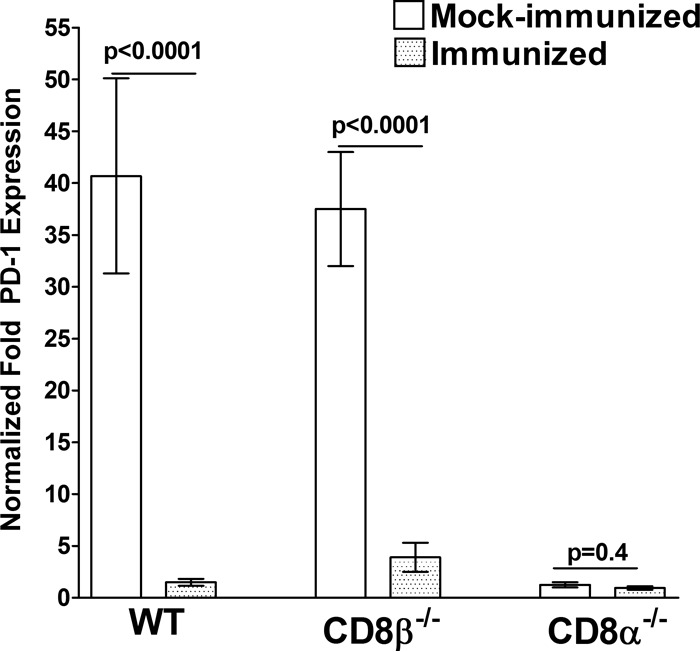
qRT-PCR analyses of the PD-1 transcript in the TG of latently infected mice. WT, CD8α−/−, and CD8β−/− mice were immunized i.p. with avirulent HSV-1 strain KOS or mock immunized as described in the legend to Fig. 1. Total RNA was isolated from each individual TG and used to estimate the relative expression of the PD-1 transcript in the TG of WT, CD8α−/−, or CD8β−/− mice. GAPDH expression was used to normalize the relative expression of each transcript in the TG of immunized mice. For mock-immunized mice, each bar represents the mean ± SEM from 20 TG, while for immunized mice, each bar represents the mean ± SEM from 18, 32, and 16 TG for WT, CD8α−/−, and CD8β−/− mice, respectively.
Effect of CD8α on gB and IFN expression during primary ocular challenge.
The results presented above and those from our previously published studies using adoptive transfer approaches (40) indicate that the loss of CD8α+ cells has a beneficial effect in immunized mice in that it reduces latency (40). We therefore undertook a more detailed analysis of the effects of ocular challenge in immunized CD8α−/− mice. We first assessed the levels of the viral glycoprotein gB as an indicator of virus infection. The corneas, TG, and spleens were harvested on days 3 and 5 p.i., RNA was isolated, and the levels of transcripts for gB in the cornea and TG were determined using qRT-PCR. On day 3 p.i., the levels of gB mRNA in the cornea (Fig. 5A, P = 0.2) and TG (Fig. 5B, P = 0.09) were higher in CD8α−/− mice than in WT mice, but these differences were not statistically significant. This effect was reversed by day 5, at which time the levels of gB mRNA in the cornea (Fig. 5A, P = 0.03) and TG (Fig. 5B, P = 0.02) were significantly lower in CD8α−/− mice than in WT mice. On day 5 p.i., the differences in the levels of gB expression between CD8α−/− and WT mice were statistically significant, which is in marked contrast to the similarity in the titers of replicating virus in the tears of immunized CD8α−/− and WT mice after ocular challenge. It is thus reasonable to speculate that the absence of CD8α in CD8α−/− mice may interfere with the posttranscriptional aspect of virus replication but not at the level of virus production.
FIG 5.
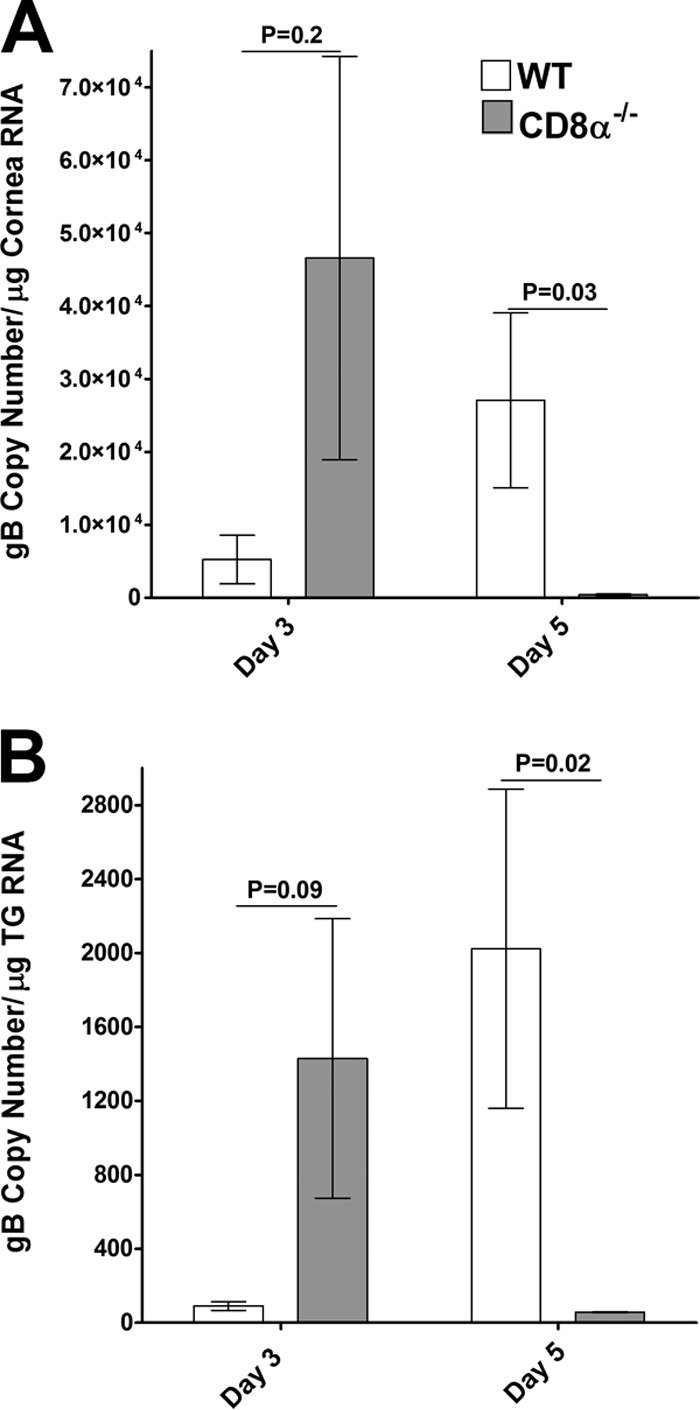
Expression of gB in the corneas (A) and TG (B) of infected mice. CD8α−/− and WT mice were immunized as described in the legend to Fig. 1 and ocularly infected as described in the legend to Fig. 2. gB expression in the cornea and TG was determined on days 3 and 5 p.i. In each experiment, an estimated relative copy number of HSV-1 gB was calculated using standard curves generated from pAC-gB1. Briefly, the DNA template was serially diluted 10-fold such that 5 μl contained from 103 to 1011 copies of LAT and then subjected to TaqMan PCR with the same set of primers. By comparing the normalized threshold cycle of each sample to the threshold cycle of the standard, the copy number for each reaction was determined. GAPDH expression was used to normalize the relative expression of each transcript in the cornea and TG of infected mice. Each bar represents the mean ± SEM from 6 corneas or TG.
We also determined the levels of type I IFN (IFN-α, IFN-β) or type II IFN (IFN-γ) transcripts by qRT-PCR using the same total RNA used in the experiments described above. In this case, RNA isolated from the spleens of the same mice was used as a control. The levels of IFN-α mRNA were significantly lower in the corneas of CD8α−/− mice than in the corneas of WT mice on day 3 p.i. (Fig. 6A, P = 0.004) and day 5 p.i. (Fig. 6A, P = 0.0003). In contrast, the levels of IFN-α in the TG of CD8α−/− mice on day 3 (Fig. 6B, P = 0.09) and day 5 (Fig. 6B, P = 0.08) were not statistically significantly higher than those in the TG of WT mice, while they were significantly higher in the spleens of CD8α−/− mice than in the spleens of WT mice on days 3 and 5 p.i. (Fig. 6C). There was no significant difference in the levels of IFN-β mRNA in the corneas of the CD8α−/− and WT mice on day 3 p.i. (Fig. 7A, P = 0.4), whereas on day 5, the levels of IFN-β were significantly lower in the corneas of CD8α−/− mice than in the corneas of WT mice (Fig. 7A, P = 0.04). Similarly, there were no significant differences between the levels of IFN-β mRNA in the TG (Fig. 7B, P = 0.6) and spleens (Fig. 7C, P = 0.1) of CD8α−/− mice and the levels of IFN-β mRNA in the TG and spleens of WT mice on day 3. However, on day 5 the levels of IFN-β mRNA in the TG (Fig. 7B, P = 0.04) and spleen (Fig. 7C, P = 0.0003) were higher in the CD8α−/− mice than in the WT mice. Although there was no significant difference in the levels of IFN-γ mRNA in the corneas (Fig. 8A, P = 0.2), TG (Fig. 8B, P = 0.7), and spleens (Fig. 8C, P = 0.07) of CD8α−/− and WT mice on day 3 p.i., the levels of IFN-γ mRNA expression in the corneas (Fig. 8A, P = 0.01), TG (Fig. 8B, P = 0.03), and spleens (Fig. 8C, P = 0.02) were significantly lower in the CD8α−/− mice than in the WT mice on day 5 p.i. Taken together, these data demonstrate that the absence of CD8α cells was associated with the downregulation of induction of type I IFNs in the cornea, whereas the absence of CD8α cells did not downregulate the induction of type I IFNs in the TG on challenge after HSV-1 immunization. In contrast, the absence of CD8α cells was associated with a significant reduction in the levels of type II IFNs in both the corneas and TG. Collectively, these results suggest that type I IFN plays a protective role against establishment of latency; i.e., higher levels of the gB transcript in the cornea occurred in the context of lower levels of type I IFN and lower levels of the gB transcript in the TG occurred in the context of higher levels of type I IFN. In contrast, type II IFN did not appear to play a major protective role against HSV-1 replication or establishment of latency.
FIG 6.
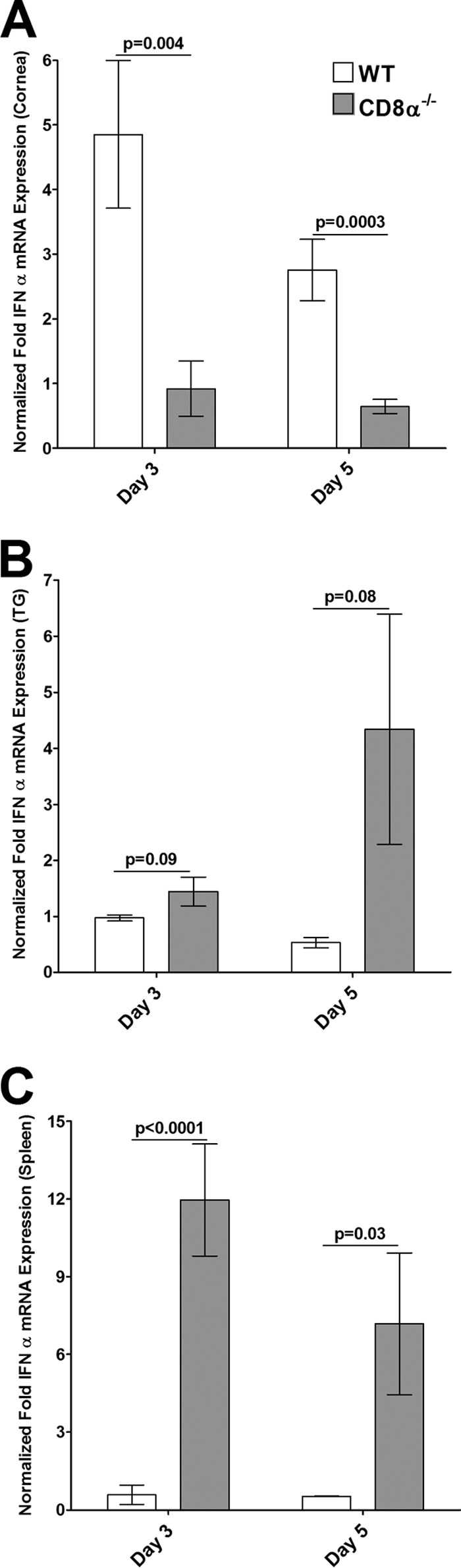
Expression of IFN-α in corneas (A), TG (B), and spleens (C) of infected mice. Total RNA isolated from individual mouse corneas and TG as described in the legend to Fig. 5 as well as RNA from the spleens of the same mice was used to estimate the relative levels of expression of IFN-α transcripts in WT and CD8α−/− immunized mice. IFN-α expression in the cornea, TG, and spleen was determined on days 3 and 5 p.i. GAPDH expression was used to normalize the relative expression of each transcript in the cornea, TG, or spleen in each group. Each bar represents the mean ± SEM from 6 corneas or TG and 3 spleens.
FIG 7.
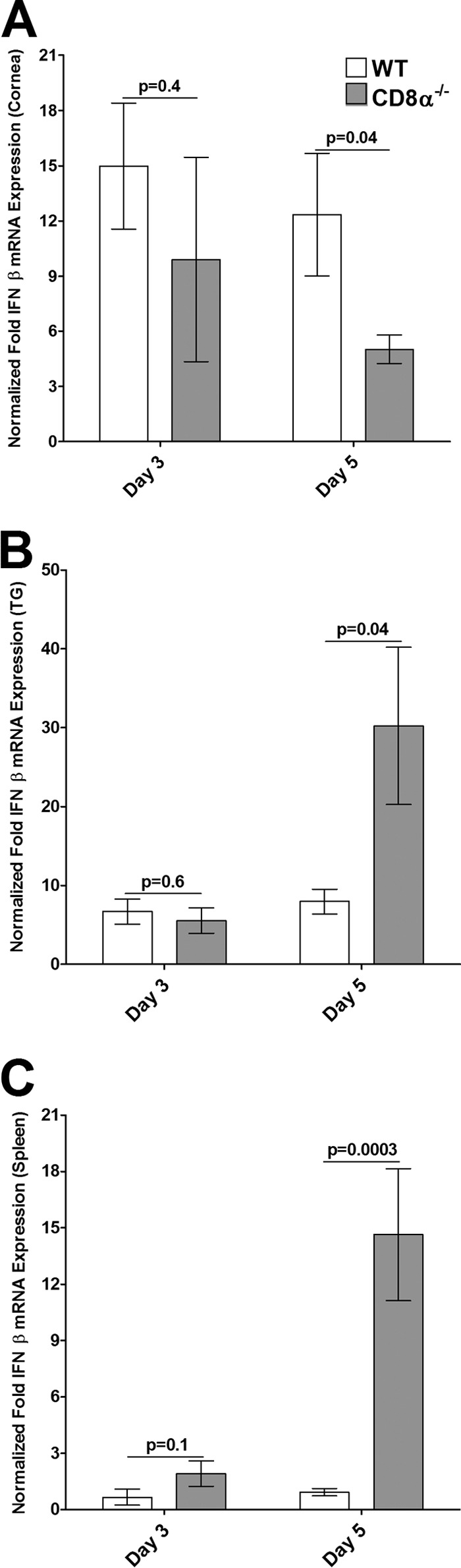
Expression of IFN-β in corneas (A), TG (B), and spleens (C) of infected mice. Total RNA isolated from individual mouse corneas and TG as described in the legend to Fig. 5 as well as RNA from the spleens of the same mice was used to estimate the relative levels of expression of IFN-β transcripts in WT and CD8α−/− immunized mice. IFN-β expression in the cornea, TG, and spleen was determined on days 3 and 5 p.i. GAPDH expression was used to normalize the relative expression of each transcript in the cornea, TG, or spleen in each group. Each bar represents the mean ± SEM from 6 corneas or TG and 3 spleens.
FIG 8.
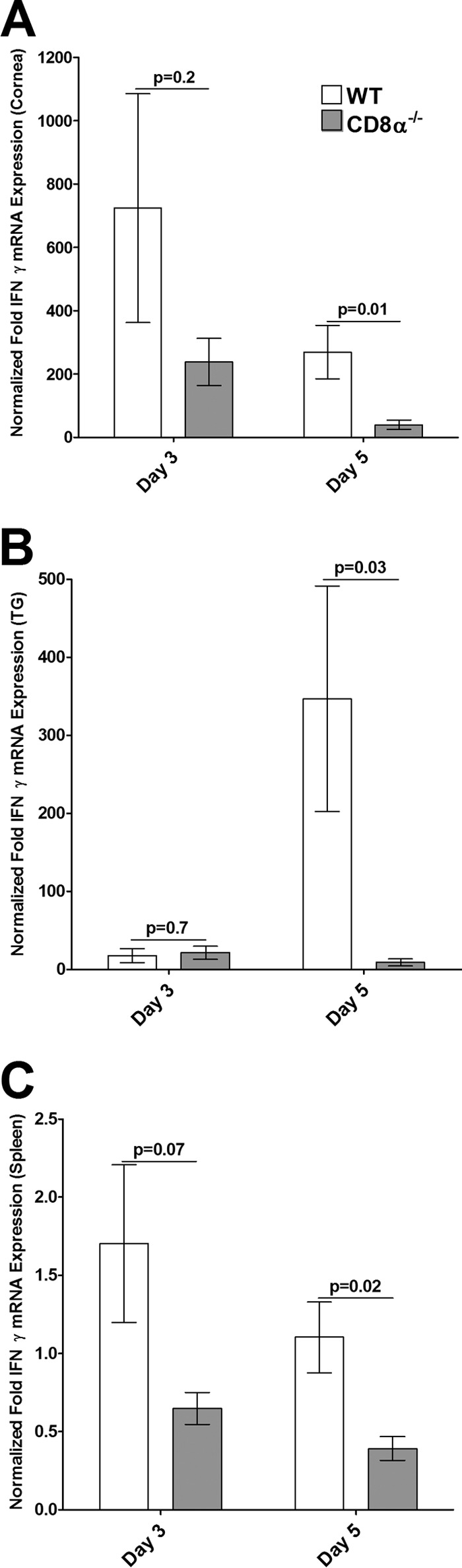
Expression of IFN-γ in corneas (A), TG (B), and spleens (C) of infected mice. Total RNA isolated from individual mouse corneas and TG as described in the legend to Fig. 5 as well as RNA from the spleens of the same mice was used to estimate the relative expressions of IFN-γ transcripts in WT and CD8α−/− immunized mice. IFN-γ expression in the cornea, TG, and spleen was determined on days 3 and 5 p.i. GAPDH expression was used to normalize the relative expression of each transcript in the cornea, TG, or spleen in each group. Each bar represents the mean ± SEM from 6 corneas or TG and 3 spleens.
Contribution of CD8α-positive cells to latency and T-cell exhaustion.
It has previously been reported that CD8+ T cells play a dominant role in maintaining HSV-1 latency (28), while we have recently shown that the absence of CD8α+ DCs, rather than CD8α+ T cells, is associated with a significant reduction of latency in the TG of infected mice (40). In the current study, we found that immunized CD8α−/− mice, despite their lack of both CD8α+ T cells and CD8α+ DCs, exhibit protection against ocular HSV-1 infection that was the same as or better than that of their immunized WT counterparts. This is consistent with our previous observation that transfer of CD11c+ CD8α+ cells to recipient mice that have been depleted of their DCs results in significantly enhanced latency in the TG of infected mice, whereas transfer of CD11c+ CD8α− cells results in a reduction in latency (39). The number of DCs in mice can be manipulated by administration of DNA adjuvants. Flt3L-deficient mice have reduced levels of both myeloid-related (CD11c+ CD8α−) and lymphoid-related (CD11c+ CD8α+) DCs (61), and injection of Flt3L results in the expansion of both these DC subpopulations (39). In contrast, injection of GM-CSF results in expansion of the CD8α− DC population but not the CD8α+ DC population in vivo (62, 63). As a proof of principle that an increase in the numbers of CD8α+ DCs contributes to higher latency and, consequently, T-cell exhaustion, WT mice were injected with GM-CSF or Flt3L DNA or mock injected. In these experiments, the mice were not immunized with the avirulent HSV-1 strain KOS to permit analysis of fine differences between the effects of injection of GM-CSF versus the effects of injection of Flt3L. WT mice rather than CD8α−/− mice were used in this experiment to compare the effects of expansion of CD4+ DCs compared to the effects of expansion of CD8α+ DCs in the context of a WT C57BL6 background. The results of fluorescence-activated cell sorting (FACS) analysis following the first injection of mice with GM-CSF and Flt3L DNA are shown in Fig. 9. The results of FACS analysis suggested that the injection of mice with GM-CSF DNA significantly increased the CD11c+ CD4+ CD8α− population in the spleens of injected mice compared with that in the spleens of mice injected with Flt3L DNA (Fig. 9, P = 0.04, right side) and mock-injected mice (Fig. 9, P = 0.02, right side), while the levels of CD11c+ CD4− CD8α+ cells stayed the same among the three groups (Fig. 9, P > 0.5, left). These results are consistent with those of previous studies showing that GM-CSF expands the CD11c+ CD4+ CD8α− population but not the CD8α+ DC population when administered to mice (62–66).
Following the third injection of GM-CSF or Flt3L DNA, mice were challenged ocularly, and on day 28 p.i., the levels of LAT mRNA in the TG of the infected mice were determined. The levels of LAT mRNA in the TG of latently infected mice were 4-fold lower in the GM-CSF-injected mice than in the mock-injected control mice (Fig. 10A, P = <0.04 for GM-CSF-injected compared to mock-injected mice). Similarly, the levels of LAT mRNA in the TG of latently infected mice were 3-fold lower in the Flt3L-injected mice than the mock-injected control mice (Fig. 10A, P < 0.04 for Flt3L-injected compared to mock-injected mice). Although the levels of LAT in the Flt3L-injected mice were higher than those in the GM-CSF-injected mice, this difference was not statistically significant (Fig. 10A, P = 0.2). These results indicate that latency in the TG is reduced in mice injected with either GM-CSF or Fl3tL DNA and further suggest that GM-CSF may be somewhat more effective.
FIG 10.
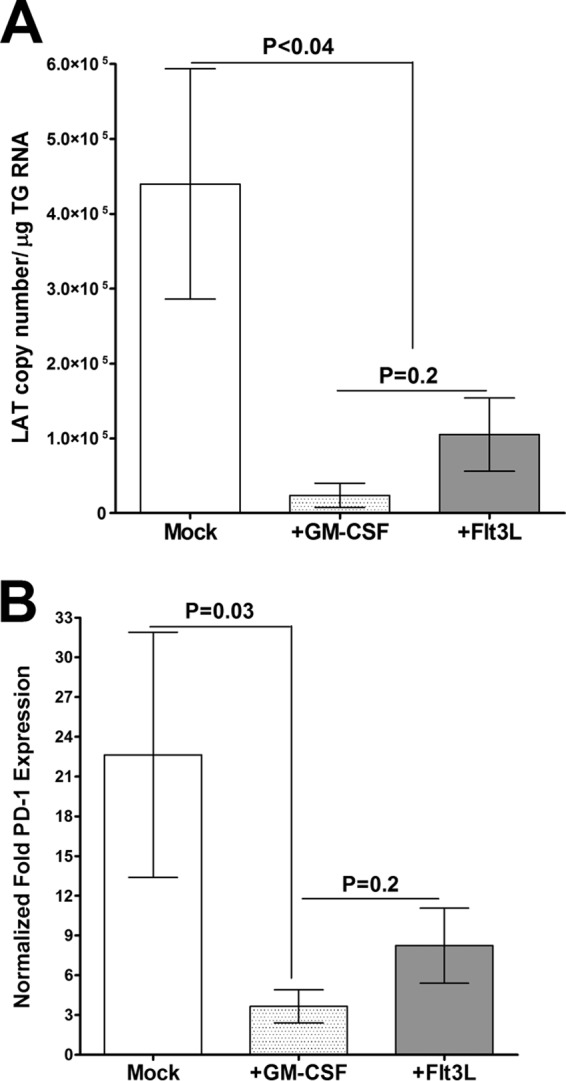
Detection of LAT (A) and PD-1 (B) following GM-CSF or Flt3L injection. WT mice were injected 3 times with GM-CSF or Flt3L DNA prior to ocular HSV-1 infection. Injected mice were ocularly infected with HSV-1 strain McKrae, and quantitative RT-PCR was performed to assay LAT and PD-1 expression. Each point represents the mean ± SEM from 10 TG.
As PD-1 is a marker of T-cell exhaustion and higher LAT expression correlates with higher PD-1 expression (60), we also investigated the effects of injection of GM-CSF or Fl3tL on the expression of PD-1 in the TG of latently infected mice. For these purposes, the relative level of PD-1 was determined by RT-PCR of the total TG RNA extracts used to generate the data shown in Fig. 10A. The results are presented as the fold increase compared to the baseline mRNA level in TG from uninfected naive mice (Fig. 10B). The levels of PD-1 mRNA in the TG of the GM-CSF-injected mice were significantly lower than those in the TG of the mock-injected group (Fig. 10B, P = 0.03 for GM-CSF-injected mice compared to mock-injected mice). Although the level of PD-1 mRNA was lower in Flt3L-injected mice than the mock-injected control group, this difference was not statistically significant (Fig. 10B, P = 0.5). Similarly, although the levels of PD-1 in the TG of GM-CSF-injected mice were lower than those in the TG of Flt3L-injected mice, this difference was not significantly different (Fig. 10B, P > 0.2). These results provide further evidence of a direct correlation between a reduction in latency and a reduction in PD-1 expression.
DISCUSSION
The most efficient way to decrease latency and, thus, subsequent recurrent infections and a loss of vision associated with HSV-1 infection is to reduce latency in the TG. Our previously published studies provide several lines of evidence that CD8α+ DCs contribute to latency in the TG of infected mice. We have shown that CD11c+ CD8α+ DCs are involved in the enhancement of latency in the TG of ocularly infected mice (35, 39), that increased latency is correlated with increased levels of CD8+ PD-1-poitive T cells in the TG of latently infected mice (60), that CD11c+ CD8α+ DCs are involved in the exhaustion of T cells, and that this T-cell exhaustion is involved in enhanced latency (35, 39). Most recently, we used an adoptive transfer approach to demonstrate that CD8α+ DCs, rather than CD8+ T cells, are responsible for enhanced viral latency and recurrences (40). In the current study, we tested the overall hypothesis that the absence of both CD8 T cells and myeloid DCs has no negative effect on vaccine efficacy and may be beneficial in terms of a reduction in the establishment of latency. In addition, as a proof of principle, we tested if pushing DCs toward myeloid-related DCs (CD11c+ CD8α−) and away from lymphoid-related DCs (CD11c+ CD8α+) would reduce latency in infected mice and that if it would do so by reducing T-cell exhaustion in the TG of infected mice.
In early studies, we found that naive mice in which the cornea was scarified prior to ocular infection with 1 × 106 PFU/eye of HSV-1 strain McKrae needed both CD4+ and CD8+ T cells for protection against death and CS (16). However, more recently we found that CD8α−/−, CD8β−/−, and WT mice with a C57BL/6 background are refractory to death and CS following ocular infection with 2 × 105 PFU/eye of HSV-1 strain McKrae in the absence of corneal scarification (40). In the current study, the absence of CD8α+ or CD8β+ cells in immunized mice did not adversely affect the generation of neutralizing antibodies or protection against virus replication in the eyes of ocularly infected mice. However, the absence of CD8β+ cells was associated with enhanced latency in the TG of challenged mice compared with that in the TG of either CD8α−/− or WT mice. Immunization completely protected immunized mice against HSV-1-induced eye disease (not shown). In WT mice and CD8β−/− mice, immunization significantly reduced the levels of PD-1 mRNA. The levels of PD-1 mRNA in unimmunized CD8α−/− mice were lower than those in WT mice and CD8β−/− mice, and the levels of PD-1 mRNA in immunized CD8α−/− mice remained lower than those in immunized WT mice and CD8β−/− mice. This is consistent with the results of our recent studies that showed that CD8α+ DCs enhance both latency and T-cell exhaustion, as determined by elevation of PD-1 expression (40). The higher latency in CD8β−/− mice was unexpected and may be due to the unaltered function of CD8α+ DCs in these mice, as CD8 consists of either CD8α-CD8α homodimers or CD8α-CD8β heterodimers but not CD8β-CD8β homodimers (44–46). Thus, CD8α-CD8α homodimers in the absence of CD8α-CD8β may contribute to higher LAT expression and, consequently, higher PD-1 expression. Taken together with the findings of our previously published studies, the current studies establish that an effective vaccine against ocular HSV-1 infection does not require the presence of CD8α+ T cells and CD8α+ DCs; indeed, the results suggest that a lack of CD8α+ DCs plays a beneficial role in reducing latency and T-cell exhaustion in mice (35, 39, 40, 60, 67). This aspect of the present work and results from our previously published study (40) are consistent with the report that the absence of CD8+ Treg cell activity enhances the immune response to viral infection of mice (57).
Although the CD8α−/− mice used in this study lacked both CD8α+ T cells and CD8α+ DCs, we found that their absence did not affect several parameters associated with protection against challenge of the immunized mice. Recently, we reported that in naive mice the presence of CD8α+ DCs but not CD8α+ T cells contributes to higher HSV-1 latency in the TG and that bone marrow (BM)-derived CD8α+ DCs harbor more virus than CD8α− DCs (40). Both Flt3L and GM-CSF are known to be potent stimulatory factors for DCs in vivo (68–73) and have been considered a means of augmenting the efficacy of various vaccines. Flt3L and GM-CSF differ, however, in terms of their effects on the development of DCs (74, 75). It has been shown that GM-CSF expands the CD8α− population but not the CD8α+ population, whereas Flt3L enhances the populations of both myeloid- and lymphoid-related DCs when administered to mice (62–66). We have shown previously that transfer of CD11c+ CD8α+ cells significantly enhances latency in the TG of HSV-1-infected mice, whereas transfer of CD11c+ CD8α− cells reduces latency (39). Thus, in this study we injected WT mice with GM-CSF or Flt3L DNA and looked at the level of latency and T-cell exhaustion in ocularly infected mice. Our results suggested that the mice administered GM-CSF exhibited both reduced latency and reduced PD-1 expression compared to the levels of latency and PD-1 expression in mice administered Flt3L DNA. This result is in line with our previous observations that latency is increased by >1,000-fold in BALB/c mice (rather than the C57BL/6 mice that were used in this study) that are injected with human Flt3L DNA (35, 39). The results of the current study suggest that GM-CSF treatment may be more effective than Flt3L treatment in reducing HSV-1 latency in mice. The results of the current study are consistent with those of our previously published studies that have shown that shifting the DC population from CD8α+ to CD8α− reduces the establishment of latent infection in the TG of ocularly infected mice without having a deleterious effect on the prevention of eye disease or primary virus replication in the eyes and TG of ocularly infected mice (35, 39). The concept that CD8α+ DCs may act to exacerbate HSV-1 latency rather than play a protective role in mice is consistent with the reports of the deleterious effects of certain populations of DCs in the control of vaccinia virus (76), HIV-1 (77, 78), and dengue virus (79).
The results of the current study further suggest a mechanism by which CD8α+ DCs may promote latency. We found that CD8α−/− mice, whether they were immunized or not, had very low levels of PD-1 mRNA compared with the levels in naive CD8β−/− and WT mice and that immunization significantly reduced the levels of PD-1 mRNA in CD8β−/− and WT mice. These observations are consistent with our recent report that CD8α+ DCs contribute to higher PD-1 expression in T cells and, thus, may promote T-cell exhaustion, thereby enhancing latency (80). It has been shown that polyethylene glycol-modified GM-CSF [PEG-(GM-CSF)] expands the CD8α− population but not the CD8α+ population in mice in vivo (62, 63). Our future plans include investigation of whether administration of PEG-(GM-CSF) with the vaccine results in a greater reduction in latency compared to that achieved in mice administered GM-CSF DNA with the vaccine. It was recently shown that lymphotoxin-β receptor (LTβR) is a key growth signal for self-renewal of CD8α− DCs (81), and the use of an agonist LTβR monoclonal antibody (MAb) in vivo led to increases in the population of CD8α− DCs (82). We have tested whether the use of an LTβR agonist MAb in vivo affects the level of latency and T-cell exhaustion in recipient mice compared to that in mice administered an irrelevant MAb. However, in contrast to GM-CSF injection, mice administered LTβR MAb exhibited no reduction in the level of latency or T-cell exhaustion compared to that in mice administered the irrelevant MAb (not shown).
As interferons have potent immunomodulatory functions (83, 84), we also looked at the contribution of type I IFNs (IFN-α and IFN-β) and type II IFN (IFN-γ) to vaccine efficacy. Type I IFNs are produced by nucleated cells and mediate antiviral effects (85). Type II IFN (IFN-γ) is produced by NK cells, NK T cells, and T-cell populations (86). Our results suggest that the absence of CD8α T cells and CD8α DCs affected IFN-α and IFN-β expression in a tissue-specific manner, whereas it reduced IFN-γ production in all the tissues tested. However, although differences in the levels of IFN-α, IFN-β, and IFN-γ expression were observed between WT and CD8α-deficient mice, these differences did not affect the level of virus replication in the eye, the duration of virus replication in the eye, or the duration of explant reactivation or LAT expression in the TG of latently infected mice.
In conclusion, our results suggest that the absence of CD8α+ T cells and CD8α+ DCs does not negatively affect the efficacy of vaccines against ocular HSV-1 infection in mice and that the absence of the CD8α+ DCs may be beneficial, as this results in a failure of DC stimulation of LAT and PD-1 expression. The higher level of latency in CD8β−/− mice can most likely be attributed to the presence of CD8α+ DCs that are capable of stimulating LAT and PD-1 expression. In addition, injection of mice with GM-CSF but not Flt3L either directly or indirectly resulted in a reduction in latency and a reduction in T-cell exhaustion. Although DCs in humans cannot be distinguished by the expression of CD8α, human BDCA3-positive conventional DCs (cDCs; CD141) have been proposed to be human homolog CD8α cDCs on the basis of the expression of other molecules and functional activity (87–89).
Our results demonstrate not only that HSV-1 latency-reactivation is not dependent on the presence of CD8+ T cells but also that shifting CD8α+ DCs to CD8α− DCs prophylactically might have the potential to reduce or eliminate HSV-1 latency-reactivation. Thus, using GM-CSF as a vaccine adjuvant, which would push DCs toward the phenotype associated with CD8α− DCs and away from the phenotype of CD8α+ DCs in mice, may improve the overall efficacy of vaccines against HSV infection.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grants AI093941 and EY13615.
We thank Dan Littman (New York University) for gifting the CD8β-knockout mice.
Footnotes
Published ahead of print 7 May 2014
REFERENCES
- 1.Jordan MC, Jordan GW, Stevens JG, Miller G. 1984. Latent herpesviruses of humans. Ann. Intern. Med. 100:866–880. 10.7326/0003-4819-100-6-866 [DOI] [PubMed] [Google Scholar]
- 2.Whitley RJ, Kimberlin DW, Roizman B. 1998. Herpes simplex viruses. Clin. Infect. Dis. 26:541–553. 10.1086/514600 [DOI] [PubMed] [Google Scholar]
- 3.Dawson CR. 1984. Ocular herpes simplex virus infections. Clin. Dermatol. 2:56–66. 10.1016/0738-081X(84)90066-X [DOI] [PubMed] [Google Scholar]
- 4.Barron BA, Gee L, Hauck WW, Kurinij N, Dawson CR, Jones DB, Wilhelmus KR, Kaufman HE, Sugar J, Hyndiuk RA, Laibson PR, Stulting RD, Asbell PA, for the Herpetic Eye Disease Study Group. 1994. Herpetic Eye Disease Study. A controlled trial of oral acyclovir for herpes simplex stromal keratitis. Ophthalmology 101:1871–1882 [DOI] [PubMed] [Google Scholar]
- 5.Ghiasi H, Kaiwar R, Nesburn AB, Slanina S, Wechsler SL. 1994. Expression of seven herpes simplex virus type 1 glycoproteins (gB, gC, gD, gE, gG, gH, and gI): comparative protection against lethal challenge in mice. J. Virol. 68:2118–2126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lausch RN, Monteiro C, Kleinschrodt WR, Oakes JE. 1987. Superiority of antibody versus delayed hypersensitivity in clearance of HSV-1 from eye. Invest. Ophthalmol. Vis. Sci. 28:565–570 [PubMed] [Google Scholar]
- 7.Bonina L, Nash AA, Arena A, Leung KN, Wildy P. 1984. T cell-macrophage interaction in arginase-mediated resistance to herpes simplex virus. Virus Res. 1:501–505. 10.1016/0168-1702(84)90007-8 [DOI] [PubMed] [Google Scholar]
- 8.Kunder SC, Wu L, Morahan PS. 1993. Role of NK cells in immunomodulator-mediated resistance to herpesvirus infection. Antiviral Res. 21:103–118. 10.1016/0166-3542(93)90047-M [DOI] [PubMed] [Google Scholar]
- 9.Ghiasi H, Roopenian DC, Slanina S, Cai S, Nesburn AB, Wechsler SL. 1997. The importance of MHC-I and MHC-II responses in vaccine efficacy against lethal herpes simplex virus type 1 challenge. Immunology 91:430–435. 10.1046/j.1365-2567.1997.00261.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghiasi H, Cai S, Nesburn AB, Wechsler SL. 1997. MHC-II but not MHC-I responses are required for vaccine-induced protection against ocular challenge with HSV-1. Curr. Eye Res. 16:1152–1158. 10.1076/ceyr.16.11.1152.5104 [DOI] [PubMed] [Google Scholar]
- 11.Nash AA, Field HJ, Quartey-Papafio R. 1980. Cell-mediated immunity in herpes simplex virus-infected mice: induction, characterization and antiviral effects of delayed type hypersensitivity. J. Gen. Virol. 48:351–357. 10.1099/0022-1317-48-2-351 [DOI] [PubMed] [Google Scholar]
- 12.Kohl S, Loo LS, Drath DB, Cox P. 1989. Interleukin-2 protects neonatal mice from lethal herpes simplex virus infection: a macrophage-mediated, gamma interferon-induced mechanism. J. Infect. Dis. 159:239–247. 10.1093/infdis/159.2.239 [DOI] [PubMed] [Google Scholar]
- 13.Kohl S. 1992. The role of antibody in herpes simplex virus infection in humans. Curr. Top. Microbiol. Immunol. 179:75–88 [DOI] [PubMed] [Google Scholar]
- 14.Ghiasi H, Bahri S, Nesburn AB, Wechsler SL. 1995. Protection against herpes simplex virus-induced eye disease after vaccination with seven individually expressed herpes simplex virus 1 glycoproteins. Invest. Ophthalmol. Vis. Sci. 36:1352–1360 [PubMed] [Google Scholar]
- 15.Ghiasi H, Wechsler SL, Kaiwar R, Nesburn AB, Hofman FM. 1995. Local expression of tumor necrosis factor alpha and interleukin-2 correlates with protection against corneal scarring after ocular challenge of vaccinated mice with herpes simplex virus type 1. J. Virol. 69:334–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghiasi H, Cai S, Perng GC, Nesburn AB, Wechsler SL. 2000. Both CD4+ and CD8+ T cells are involved in protection against HSV-1 induced corneal scarring. Br. J. Ophthalmol. 84:408–412. 10.1136/bjo.84.4.408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Erlich KS, Wofsy D, Dix RD, Mills J. 1989. Effects of selective depletion of L3T4+ T-lymphocytes on herpes simplex virus encephalitis. Clin. Immunol. Immunopathol. 52:190–201. 10.1016/0090-1229(89)90171-2 [DOI] [PubMed] [Google Scholar]
- 18.Dix RD. 1987. Prospects for a vaccine against herpes simplex virus types 1 and 2. Prog. Med. Virol. 34:89–128 [PubMed] [Google Scholar]
- 19.Oakes JE, Rector JT, Lausch RN. 1984. Lyt-1+ T cells participate in recovery from ocular herpes simplex virus type 1 infection. Invest. Ophthalmol. Vis. Sci. 25:188–194 [PubMed] [Google Scholar]
- 20.Nagafuchi S, Hayashida I, Higa K, Wada T, Mori R. 1982. Role of Lyt-1 positive immune T cells in recovery from herpes simplex virus infection in mice. Microbiol. Immunol. 26:359–362. 10.1111/j.1348-0421.1982.tb00186.x [DOI] [PubMed] [Google Scholar]
- 21.Sethi KK, Omata Y, Schneweis KE. 1983. Protection of mice from fatal herpes simplex virus type 1 infection by adoptive transfer of cloned virus-specific and H-2-restricted cytotoxic T lymphocytes. J. Gen. Virol. 64:443–447. 10.1099/0022-1317-64-2-443 [DOI] [PubMed] [Google Scholar]
- 22.Hendricks RL, Tumpey TM. 1990. Contribution of virus and immune factors to herpes simplex virus type I-induced corneal pathology. Invest. Ophthalmol. Vis. Sci. 31:1929–1939 [PubMed] [Google Scholar]
- 23.Staats HF, Oakes JE, Lausch RN. 1991. Anti-glycoprotein D monoclonal antibody protects against herpes simplex virus type 1-induced diseases in mice functionally depleted of selected T-cell subsets or asialo GM1+ cells. J. Virol. 65:6008–6014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manickan E, Rouse BT. 1995. Roles of different T-cell subsets in control of herpes simplex virus infection determined by using T-cell-deficient mouse-models. J. Virol. 69:8178–8179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manickan E, Francotte M, Kuklin N, Dewerchin M, Molitor C, Gheysen D, Slaoui M, Rouse BT. 1995. Vaccination with recombinant vaccinia viruses expressing ICP27 induces protective immunity against herpes simplex virus through CD4+ Th1+ T cells. J. Virol. 69:4711–4716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Newell CK, Martin S, Sendele D, Mercadal CM, Rouse BT. 1989. Herpes simplex virus-induced stromal keratitis: role of T-lymphocyte subsets in immunopathology. J. Virol. 63:769–775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nash AA, Jayasuriya A, Phelan J, Cobbold SP, Waldmann H, Prospero T. 1987. Different roles for L3T4+ and Lyt 2+ T cell subsets in the control of an acute herpes simplex virus infection of the skin and nervous system. J. Gen. Virol. 68:825–833. 10.1099/0022-1317-68-3-825 [DOI] [PubMed] [Google Scholar]
- 28.Liu T, Khanna KM, Chen X, Fink DJ, Hendricks RL. 2000. CD8(+) T cells can block herpes simplex virus type 1 (HSV-1) reactivation from latency in sensory neurons. J. Exp. Med. 191:1459–1466. 10.1084/jem.191.9.1459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bellone M, Iezzi G, Rovere P, Galati G, Ronchetti A, Protti MP, Davoust J, Rugarli C, Manfredi AA. 1997. Processing of engulfed apoptotic bodies yields T cell epitopes. J. Immunol. 159:5391–5399 [PubMed] [Google Scholar]
- 30.Albert ML, Sauter B, Bhardwaj N. 1998. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature 392:86–89. 10.1038/32183 [DOI] [PubMed] [Google Scholar]
- 31.Ronchetti A, Rovere P, Iezzi G, Galati G, Heltai S, Protti MP, Garancini MP, Manfredi AA, Rugarli C, Bellone M. 1999. Immunogenicity of apoptotic cells in vivo: role of antigen load, antigen-presenting cells, and cytokines. J. Immunol. 163:130–136 [PubMed] [Google Scholar]
- 32.Banchereau J, Steinman RM. 1998. Dendritic cells and the control of immunity. Nature 392:245–252. 10.1038/32588 [DOI] [PubMed] [Google Scholar]
- 33.Piccioli D, Sbrana S, Melandri E, Valiante NM. 2002. Contact-dependent stimulation and inhibition of dendritic cells by natural killer cells. J. Exp. Med. 195:335–341. 10.1084/jem.20010934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferlazzo G, Tsang ML, Moretta L, Melioli G, Steinman RM, Munz C. 2002. Human dendritic cells activate resting natural killer (NK) cells and are recognized via the NKp30 receptor by activated NK cells. J. Exp. Med. 195:343–351. 10.1084/jem.20011149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mott KR, Ghiasi H. 2008. Role of dendritic cells in enhancement of herpes simplex virus type 1 latency and reactivation in vaccinated mice. Clin. Vaccine Immunol. 15:1859–1867. 10.1128/CVI.00318-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vremec D, Zorbas M, Scollay R, Saunders DJ, Ardavin CF, Wu L, Shortman K. 1992. The surface phenotype of dendritic cells purified from mouse thymus and spleen: investigation of the CD8 expression by a subpopulation of dendritic cells. J. Exp. Med. 176:47–58. 10.1084/jem.176.1.47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anjuere F, Martin P, Ferrero I, Fraga ML, del Hoyo GM, Wright N, Ardavin C. 1999. Definition of dendritic cell subpopulations present in the spleen, Peyer's patches, lymph nodes, and skin of the mouse. Blood 93:590–598 [PubMed] [Google Scholar]
- 38.Becker Y. 2003. Milestones in the research on skin epidermal Langerhans/dendritic cells (LCs/DCs) from the discovery of Paul Langerhans 1868-1989. Virus Genes 26:131–134. 10.1023/A:1023479212095 [DOI] [PubMed] [Google Scholar]
- 39.Mott KR, UnderHill D, Wechsler SL, Ghiasi H. 2008. Lymphoid-related CD11c+ CD8a+ dendritic cells are involved in enhancing HSV-1 latency. J. Virol. 82:9870–9879. 10.1128/JVI.00566-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mott KR, Allen SJ, Zandian M, Konda B, Sharifi BG, Jones C, Wechsler SL, Town T, Ghiasi H. 2014. CD8a dendritic cells drive establishment of HSV-1 latency. PLoS One 9:e93444. 10.1371/journal.pone.0093444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moron G, Rueda P, Casal I, Leclerc C. 2002. CD8alpha- CD11b+ dendritic cells present exogenous virus-like particles to CD8+ T cells and subsequently express CD8alpha and CD205 molecules. J. Exp. Med. 195:1233–1245. 10.1084/jem.20011930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grabbe S, Kampgen E, Schuler G. 2000. Dendritic cells: multi-lineal and multi-functional. Immunol. Today 21:431–433. 10.1016/S0167-5699(00)01694-7 [DOI] [PubMed] [Google Scholar]
- 43.Vremec D, Pooley J, Hochrein H, Wu L, Shortman K. 2000. CD4 and CD8 expression by dendritic cell subtypes in mouse thymus and spleen. J. Immunol. 164:2978–2986. 10.4049/jimmunol.164.6.2978 [DOI] [PubMed] [Google Scholar]
- 44.Zamoyska R. 1994. The CD8 coreceptor revisited: one chain good, two chains better. Immunity 1:243–246. 10.1016/1074-7613(94)90075-2 [DOI] [PubMed] [Google Scholar]
- 45.Devine L, Kieffer LJ, Aitken V, Kavathas PB. 2000. Human CD8 beta, but not mouse CD8 beta, can be expressed in the absence of CD8 alpha as a beta beta homodimer. J. Immunol. 164:833–838. 10.4049/jimmunol.164.2.833 [DOI] [PubMed] [Google Scholar]
- 46.Gorman SD, Sun YH, Zamoyska R, Parnes JR. 1988. Molecular linkage of the Ly-3 and Ly-2 genes. Requirement of Ly-2 for Ly-3 surface expression. J. Immunol. 140:3646–3653 [PubMed] [Google Scholar]
- 47.Perng GC, Dunkel EC, Geary PA, Slanina SM, Ghiasi H, Kaiwar R, Nesburn AB, Wechsler SL. 1994. The latency-associated transcript gene of herpes simplex virus type 1 (HSV-1) is required for efficient in vivo spontaneous reactivation of HSV-1 from latency. J. Virol. 68:8045–8055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thompson RL, Cook ML, Devi-Rao GB, Wagner EK, Stevens JG. 1986. Functional and molecular analyses of the avirulent wild-type herpes simplex virus type 1 strain KOS. J. Virol. 58:203–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thompson RL, Devi-Rao GV, Stevens JG, Wagner EK. 1985. Rescue of a herpes simplex virus type 1 neurovirulence function with a cloned DNA fragment. J. Virol. 55:504–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dix RD, McKendall RR, Baringer JR. 1983. Comparative neurovirulence of herpes simplex virus type 1 strains after peripheral or intracerebral inoculation of BALB/c mice. Infect. Immun. 40:103–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stroop WG, Banks MC. 1992. The weakly virulent herpes simplex virus type 1 strain KOS-63 establishes peripheral and central nervous system latency following intranasal infection of rabbits, but poorly reactivates in vivo. J. Neuropathol. Exp. Neurol. 51:550–559. 10.1097/00005072-199209000-00010 [DOI] [PubMed] [Google Scholar]
- 52.Mizota A, Dix RD, Hamasaki DI. 1993. Bilateral electroretinographic changes induced by unilateral intra-visual cortex inoculation of herpes simplex virus type 1 in BALB/c mice. Doc. Ophthalmol. 84:213–230. 10.1007/BF01203654 [DOI] [PubMed] [Google Scholar]
- 53.Crooks ME, Littman DR. 1994. Disruption of T lymphocyte positive and negative selection in mice lacking the CD8 beta chain. Immunity 1:277–285. 10.1016/1074-7613(94)90079-5 [DOI] [PubMed] [Google Scholar]
- 54.Mott K, Brick DJ, van Rooijen N, Ghiasi H. 2007. Macrophages are important determinants of acute ocular HSV-1 infection in immunized mice. Invest. Ophthalmol. Vis. Sci. 48:5605–5615. 10.1167/iovs.07-0894 [DOI] [PubMed] [Google Scholar]
- 55.Osorio Y, Cohen J, Ghiasi H. 2004. Improved protection from primary ocular HSV-1 infection and establishment of latency using multigenic DNA vaccines. Invest. Ophthalmol. Vis. Sci. 45:506–514. 10.1167/iovs.03-0828 [DOI] [PubMed] [Google Scholar]
- 56.Ghiasi H, Cai S, Slanina SM, Perng GC, Nesburn AB, Wechsler SL. 1999. The role of interleukin (IL)-2 and IL-4 in herpes simplex virus type 1 ocular replication and eye disease. J. Infect. Dis. 179:1086–1093. 10.1086/314736 [DOI] [PubMed] [Google Scholar]
- 57.Holderried TA, Lang PA, Kim HJ, Cantor H. 2013. Genetic disruption of CD8+ Treg activity enhances the immune response to viral infection. Proc. Natl. Acad. Sci. U. S. A. 110:21089–21094. 10.1073/pnas.1320999110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Allen SJ, Mott KR, Ljubimov AV, Ghiasi H. 2010. Exacerbation of corneal scarring in HSV-1 gK-immunized mice correlates with elevation of CD8+CD25+ T cells in corneas of ocularly infected mice. Virology 399:11–22. 10.1016/j.virol.2009.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Allen SJ, Mott KR, Zandian M, Ghiasi H. 2010. Immunization with different viral antigens alters the pattern of T cell exhaustion and latency in HSV-1 infected mice. J. Virol. 84:12315–12324. 10.1128/JVI.01600-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Allen SJ, Hamrah P, Gate DM, Mott KR, Mantopoulos D, Zheng L, Town T, Jones C, von Andrian UH, Freeman GJ, Sharpe AH, Benmohamed L, Ahmed R, Wechsler SL, Ghiasi H. 2011. The role of LAT in increased CD8+ T cell exhaustion in trigeminal ganglia of mice latently infected with herpes simplex virus type 1. J. Virol. 85:4184–4197. 10.1128/JVI.02290-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McKenna HJ, Stocking KL, Miller RE, Brasel K, De Smedt T, Maraskovsky E, Maliszewski CR, Lynch DH, Smith J, Pulendran B, Roux ER, Teepe M, Lyman SD, Peschon JJ. 2000. Mice lacking flt3 ligand have deficient hematopoiesis affecting hematopoietic progenitor cells, dendritic cells, and natural killer cells. Blood 95:3489–3497 [PubMed] [Google Scholar]
- 62.Daro E, Pulendran B, Brasel K, Teepe M, Pettit D, Lynch DH, Vremec D, Robb L, Shortman K, McKenna HJ, Maliszewski CR, Maraskovsky E. 2000. Polyethylene glycol-modified GM-CSF expands CD11b(high)CD11c(high) but notCD11b(low)CD11c(high) murine dendritic cells in vivo: a comparative analysis with Flt3 ligand. J. Immunol. 165:49–58. 10.4049/jimmunol.165.1.49 [DOI] [PubMed] [Google Scholar]
- 63.Pulendran B, Smith JL, Caspary G, Brasel K, Pettit D, Maraskovsky E, Maliszewski CR. 1999. Distinct dendritic cell subsets differentially regulate the class of immune response in vivo. Proc. Natl. Acad. Sci. U. S. A. 96:1036–1041. 10.1073/pnas.96.3.1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Maraskovsky E, Brasel K, Teepe M, Roux ER, Lyman SD, Shortman K, McKenna HJ. 1996. Dramatic increase in the numbers of functionally mature dendritic cells in Flt3 ligand-treated mice: multiple dendritic cell subpopulations identified. J. Exp. Med. 184:1953–1962. 10.1084/jem.184.5.1953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shurin MR, Pandharipande PP, Zorina TD, Haluszczak C, Subbotin VM, Hunter O, Brumfield A, Storkus WJ, Maraskovsky E, Lotze MT. 1997. FLT3 ligand induces the generation of functionally active dendritic cells in mice. Cell. Immunol. 179:174–184. 10.1006/cimm.1997.1152 [DOI] [PubMed] [Google Scholar]
- 66.Pulendran B, Lingappa J, Kennedy MK, Smith J, Teepe M, Rudensky A, Maliszewski CR, Maraskovsky E. 1997. Developmental pathways of dendritic cells in vivo: distinct function, phenotype, and localization of dendritic cell subsets in FLT3 ligand-treated mice. J. Immunol. 159:2222–2231 [PubMed] [Google Scholar]
- 67.Mott KR, Allen SJ, Zandian M, Akbari O, Hamrah P, Maazi H, Wechsler SL, Sharpe AH, Freeman GJ, Ghiasi H. 2014. Inclusion of CD80 in HSV targets the recombinant virus to PD-L1 on DCs and allows productive infection and robust immune responses. PLoS One 9:e87617. 10.1371/journal.pone.0087617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Maraskovsky E, Daro E, Roux E, Teepe M, Maliszewski CR, Hoek J, Caron D, Lebsack ME, McKenna HJ. 2000. In vivo generation of human dendritic cell subsets by Flt3 ligand. Blood 96:878–884 [PubMed] [Google Scholar]
- 69.Pulendran B, Banchereau J, Burkeholder S, Kraus E, Guinet E, Chalouni C, Caron D, Maliszewski C, Davoust J, Fay J, Palucka K. 2000. Flt3-ligand and granulocyte colony-stimulating factor mobilize distinct human dendritic cell subsets in vivo. J. Immunol. 165:566–572. 10.4049/jimmunol.165.1.566 [DOI] [PubMed] [Google Scholar]
- 70.Lyman SD, James L, Vanden Bos T, de Vries P, Brasel K, Gliniak B, Hollingsworth LT, Picha KS, McKenna HJ, Splett RR, Fletcher FA, Maraskovsky E, Farrah T, Foxworthe D, Williams DE, Beckmann MP. 1993. Molecular cloning of a ligand for the flt3/flk-2 tyrosine kinase receptor: a proliferative factor for primitive hematopoietic cells. Cell 75:1157–1167. 10.1016/0092-8674(93)90325-K [DOI] [PubMed] [Google Scholar]
- 71.Sitnicka E, Bryder D, Theilgaard-Monch K, Buza-Vidas N, Adolfsson J, Jacobsen SE. 2002. Key role of flt3 ligand in regulation of the common lymphoid progenitor but not in maintenance of the hematopoietic stem cell pool. Immunity 17:463–472. 10.1016/S1074-7613(02)00419-3 [DOI] [PubMed] [Google Scholar]
- 72.Lyman SD, Jacobsen SE. 1998. c-kit ligand and Flt3 ligand: stem/progenitor cell factors with overlapping yet distinct activities. Blood 91:1101–1134 [PubMed] [Google Scholar]
- 73.Shurin MR, Esche C, Lotze MT. 1998. FLT3: receptor and ligand. Biology and potential clinical application. Cytokine Growth Factor Rev. 9:37–48. 10.1016/S1359-6101(97)00035-X [DOI] [PubMed] [Google Scholar]
- 74.Gilliet M, Boonstra A, Paturel C, Antonenko S, Xu XL, Trinchieri G, O'Garra A, Liu YJ. 2002. The development of murine plasmacytoid dendritic cell precursors is differentially regulated by FLT3-ligand and granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 195:953–958. 10.1084/jem.20020045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mott KR, Underhill D, Wechsler SL, Town T, Ghiasi H. 2009. A role for the JAK-STAT1 pathway in blocking replication of HSV-1 in dendritic cells and macrophages. Virol. J. 6:56. 10.1186/1743-422X-6-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Engelmayer J, Larsson M, Subklewe M, Chahroudi A, Cox WI, Steinman RM, Bhardwaj N. 1999. Vaccinia virus inhibits the maturation of human dendritic cells: a novel mechanism of immune evasion. J. Immunol. 163:6762–6768 [PubMed] [Google Scholar]
- 77.Granelli-Piperno A, Delgado E, Finkel V, Paxton W, Steinman RM. 1998. Immature dendritic cells selectively replicate macrophagetropic (M-tropic) human immunodeficiency virus type 1, while mature cells efficiently transmit both M- and T-tropic virus to T cells. J. Virol. 72:2733–2737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Geijtenbeek TB, Kwon DS, Torensma R, van Vliet SJ, van Duijnhoven GC, Middel J, Cornelissen IL, Nottet HS, KewalRamani VN, Littman DR, Figdor CG, van Kooyk Y. 2000. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell 100:587–597. 10.1016/S0092-8674(00)80694-7 [DOI] [PubMed] [Google Scholar]
- 79.Wu SJ, Grouard-Vogel G, Sun W, Mascola JR, Brachtel E, Putvatana R, Louder MK, Filgueira L, Marovich MA, Wong HK, Blauvelt A, Murphy GS, Robb ML, Innes BL, Birx DL, Hayes CG, Frankel SS. 2000. Human skin Langerhans cells are targets of dengue virus infection. Nat. Med. 6:816–820. 10.1038/77553 [DOI] [PubMed] [Google Scholar]
- 80.Mott KR, Allen SJ, Zandian M, Ghiasi H. 26 March 2014. Coregulatory interactions between CD8α DCs, LAT, and PD-1 contribute to higher HSV-1 latency. J. Virol. 10.1128/JVI.00590-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kabashima K, Banks TA, Ansel KM, Lu TT, Ware CF, Cyster JG. 2005. Intrinsic lymphotoxin-beta receptor requirement for homeostasis of lymphoid tissue dendritic cells. Immunity 22:439–450. 10.1016/j.immuni.2005.02.007 [DOI] [PubMed] [Google Scholar]
- 82.De Trez C, Schneider K, Potter K, Droin N, Fulton J, Norris PS, Ha SW, Fu YX, Murphy T, Murphy KM, Pfeffer K, Benedict CA, Ware CF. 2008. The inhibitory HVEM-BTLA pathway counter regulates lymphotoxin receptor signaling to achieve homeostasis of dendritic cells. J. Immunol. 180:238–248. 10.4049/jimmunol.180.1.238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stark GR, Kerr IM, Williams BR, Silverman RH, Schreiber RD. 1998. How cells respond to interferons. Annu. Rev. Biochem. 67:227–264. 10.1146/annurev.biochem.67.1.227 [DOI] [PubMed] [Google Scholar]
- 84.Choubey D, Moudgil KD. 2011. Interferons in autoimmune and inflammatory diseases: regulation and roles. J. Interferon Cytokine Res. 31:857–865. 10.1089/jir.2011.0101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Moudgil KD, Choubey D. 2011. Cytokines in autoimmunity: role in induction, regulation, and treatment. J. Interferon Cytokine Res. 31:695–703. 10.1089/jir.2011.0065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hall JC, Rosen A. 2010. Type I interferons: crucial participants in disease amplification in autoimmunity. Nat. Rev. Rheumatol. 6:40–49. 10.1038/nrrheum.2009.237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Robbins SH, Walzer T, Dembele D, Thibault C, Defays A, Bessou G, Xu H, Vivier E, Sellars M, Pierre P, Sharp FR, Chan S, Kastner P, Dalod M. 2008. Novel insights into the relationships between dendritic cell subsets in human and mouse revealed by genome-wide expression profiling. Genome Biol. 9:R17. 10.1186/gb-2008-9-1-r17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shortman K, Lahoud MH, Caminschi I. 2009. Improving vaccines by targeting antigens to dendritic cells. Exp. Mol. Med. 41:61–66. 10.3858/emm.2009.41.2.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lauterbach H, Bathke B, Gilles S, Traidl-Hoffmann C, Luber CA, Fejer G, Freudenberg MA, Davey GM, Vremec D, Kallies A, Wu L, Shortman K, Chaplin P, Suter M, O'Keeffe M, Hochrein H. 2010. Mouse CD8alpha+ DCs and human BDCA3+ DCs are major producers of IFN-lambda in response to poly IC. J. Exp. Med. 207:2703–2717. 10.1084/jem.20092720 [DOI] [PMC free article] [PubMed] [Google Scholar]


