Abstract
Alphaherpesvirus reactivation from thoracic sympathetic ganglia (TSG) and transaxonal spread to target organs cause human visceral disease. Yet alphaherpesvirus latency in TSG has not been well characterized. In this study, quantitative PCR detected varicella-zoster virus (VZV), herpes simplex virus 1 (HSV-1), and HSV-2 DNA in 117 fresh TSG obtained postmortem from 15 subjects. VZV DNA was found in 76 (65%) ganglia from all subjects, HSV-1 DNA was found in 5 (4%) ganglia from 3 subjects, and no HSV-2 was found.
TEXT
After primary infection, varicella-zoster virus (VZV) becomes latent in cranial nerve ganglia, dorsal root ganglia, and autonomic ganglia along the entire neuraxis (1–7), herpes simplex virus 1 (HSV-1) DNA becomes latent in cranial nerve, dorsal root, and autonomic ganglia (2, 5–10), and HSV-2 DNA becomes latent primarily in sacral ganglia as well as in cranial nerve and other dorsal root ganglia (5, 9). Alphaherpesvirus reactivation from thoracic sympathetic ganglia (TSG) and transaxonal spread to target organs cause human visceral disease. However, latency in TSG has not been well characterized because earlier studies used formalin-fixed paraffin-embedded (FFPE) tissue. To assess autonomic ganglionic infection, quantitative PCR analyzed VZV, HSV-1, and HSV-2 DNA in 117 fresh unfixed TSG.
Briefly, 117 TSG from 15 deidentified autopsied subjects were deemed exempt by the Colorado Institutional Review Board (IRB). Table 1 provides age, sex, cause of death, and postmortem interval of the subjects. Of the 15 subjects, 5 (33%) were women and 10 (67%) were men. Ages ranged from 42 to 84 years (mean, 61 ± 12 years; median, 62 years). None of the subjects died of causes related to herpesvirus infection.
TABLE 1.
Clinical features of subjects from whom TSG were analyzed
| Subject | Age (yr), gendera | Cause(s) of deathb | Postmortem interval (h) |
|---|---|---|---|
| 1 | 70, M | Interstitial lung disease | 17 |
| 2 | 47, M | Bowel ischemia | 16 |
| 3 | 64, M | CAD | 14 |
| 4 | 62, F | Liver disease | 9 |
| 5 | 50, M | CAD | 24 |
| 6 | 70, F | Breast cancer | 8 |
| 7 | 65, M | CAD | 24 |
| 8 | 72, F | Respiratory failure and CAD | 11 |
| 9 | 68, F | Cancer | 17 |
| 10 | 44, M | Cancer | 5 |
| 11 | 84, F | CAD | 16 |
| 12 | 59, M | Leukemia | 24 |
| 13 | 61, M | Cancer | >24 |
| 14 | 52, M | Pneumonia | 23 |
| 15 | 42, M | Brain cancer | 13 |
M, male; F, female.
CAD, coronary artery disease.
Ganglia were obtained <24 h postmortem, except for subject 13, whose ganglia were obtained >24 h postmortem; ganglia were rinsed in phosphate-buffered saline, and extraneous tissue was removed. DNA was extracted using the DNeasy blood and tissue kit (Qiagen, Valencia, CA). DNA concentration and purity were determined with a Thermo Scientific Nanodrop ND-1000 spectrophotometer. DNA was quantitated with a TaqMan real-time PCR on a model 7500 real-time PCR system (Applied Biosystems, Grand Island, NY) as described previously (11) using glyceraldehyde-3-phosphate dehydrogenase (GAPDH), VZV, and HSV-1 DNA-specific primers and probe (12) and HSV-2 DNA-specific primers (forward, 5′-TACCACGCGTCGCTTTTG-3′; reverse, 5′-TAAACGTGCGGCCCGTAAT-3′ and probe/56-FAM/TTGCTCCCCAGAGCCTG/3IABkFQ_3′/). Primer efficiencies for VZV, HSV-1, and HSV-2 were similar (104, 104, and 102, respectively). Amplification of serial dilutions of known concentrations of GAPDH, VZV, HSV-1, and HSV-2 DNA provided positive controls. DNA was omitted from reaction wells as a negative control. Ganglia were considered positive for viral DNA if (i) no virus amplification was detected in wells with water, (ii) GAPDH was detected in wells with ganglionic DNA, and (iii) at least two of four PCR replicates amplified target DNA.
PCR detected cellular GAPDH DNA in all 117 ganglia (data not shown), VZV DNA in 76 ganglia (65%), and HSV-1 DNA in 5 ganglia (4%); HSV-2 DNA was not found in any ganglia (Table 2). VZV DNA was detected in at least one TSG from all 15 subjects, and HSV-1 DNA was found in at least one TSG from 3 (20%) subjects. The VZV DNA load, which varied among subjects (Fig. 1 and 2), was <1,000 copies/100 ng of total DNA in all subjects except subject 14, in whom 3 TSG contained >1,000 copies of VZV DNA per 100 ng total DNA (Table 2). In TSG from subjects 1, 5, and 14 that contained HSV-1 DNA, the average load was ≤302 HSV-1 DNA copies/100 ng total DNA (Table 2).
TABLE 2.
Presence of VZV, HSV-1, and HSV-2 DNA in TSGa
| Subject | TSG | Copy number of alphaherpesvirus/100 ng DNA (avg ± SD)b |
||
|---|---|---|---|---|
| VZV | HSV-1 | HSV-2 | ||
| 1 | 1 | <100c | 302 ± 8 | 0 |
| 2 | 0 | 0 | 0 | |
| 3 | <100c | 0 | 0 | |
| 4 | 0 | 0 | 0 | |
| 5 | <100c | 0 | 0 | |
| 6 | <100c | 0 | 0 | |
| 7 | 0 | 0 | 0 | |
| 8 | <100c | 0 | 0 | |
| 9 | <100c | 0 | 0 | |
| 2 | 1 | 0 | 0 | 0 |
| 2 | 0 | 0 | 0 | |
| 3 | 0 | 0 | 0 | |
| 4 | 28 ± 11 | 0 | 0 | |
| 5 | 0 | 0 | 0 | |
| 6 | 0 | 0 | 0 | |
| 7 | 0 | 0 | 0 | |
| 8 | 0 | 0 | 0 | |
| 9 | 0 | 0 | 0 | |
| 10 | 0 | 0 | 0 | |
| 3 | 1 | <10c | 0 | 0 |
| 2 | 18 ± 2 | 0 | 0 | |
| 3 | 33 ± 15 | 0 | 0 | |
| 4 | 7 ± 1 | 0 | 0 | |
| 5 | 4 ± 1 | 0 | 0 | |
| 6 | 21 ± 8 | 0 | 0 | |
| 7 | 24 ± 2 | 0 | 0 | |
| 4 | 1 | <10c | 0 | 0 |
| 2 | <10c | 0 | 0 | |
| 3 | 0 | 0 | 0 | |
| 5 | 1 | <10c | 0 | 0 |
| 2 | <10c | 0 | 0 | |
| 3 | <10c | 0 | 0 | |
| 4 | <10c | 0 | 0 | |
| 5 | 31 ± 11 | 0 | 0 | |
| 6 | 18 ± 11 | 0 | 0 | |
| 7 | <10c | 0 | 0 | |
| 8 | 22 ± 3 | 0 | 0 | |
| 9 | 12 ± 2 | 0 | 0 | |
| 10 | <10c | 0 | 0 | |
| 11 | <10c | 0 | 0 | |
| 12 | <10c | 0 | 0 | |
| 13 | <10c | <100c | 0 | |
| 14 | <100c | <100c | 0 | |
| 15 | 0 | 0 | 0 | |
| 6 | 1 | 99 ± 25 | 0 | 0 |
| 2 | 316 ± 5 | 0 | 0 | |
| 3 | 185 ± 17 | 0 | 0 | |
| 4 | 92 ± 7 | 0 | 0 | |
| 5 | 0 | 0 | 0 | |
| 6 | 278 ± 125 | 0 | 0 | |
| 7 | 0 | 0 | 0 | |
| 7 | 1 | 265 ± 3 | 0 | 0 |
| 2 | 0 | 0 | 0 | |
| 3 | 52 ± 24 | 0 | 0 | |
| 8 | 1 | 0 | 0 | 0 |
| 2 | 28 ± 18 | 0 | 0 | |
| 3 | 54 ± 31 | 0 | 0 | |
| 4 | 32 ± 6 | 0 | 0 | |
| 5 | 36 ± 37 | 0 | 0 | |
| 6 | <10c | 0 | 0 | |
| 7 | <10c | 0 | 0 | |
| 8 | 92 ± 47 | 0 | 0 | |
| 9 | 126 ± 19 | 0 | 0 | |
| 10 | <10c | 0 | 0 | |
| 11 | <10c | 0 | 0 | |
| 9 | 1 | 46 ± 9 | 0 | 0 |
| 2 | 23 ± 0 | 0 | 0 | |
| 3 | 0 | 0 | 0 | |
| 4 | 0 | 0 | 0 | |
| 5 | 62 ± 22 | 0 | 0 | |
| 6 | 0 | 0 | 0 | |
| 7 | 30 ± 9 | 0 | 0 | |
| 8 | 0 | 0 | 0 | |
| 9 | 17 ± 0 | 0 | 0 | |
| 10 | <100c | 0 | 0 | |
| 10 | 1 | 10 ± 7 | 0 | 0 |
| 2 | 2 ± 0 | 0 | 0 | |
| 3 | 2 ± 0 | 0 | 0 | |
| 4 | 9 ± 0 | 0 | 0 | |
| 5 | 9 ± 1 | 0 | 0 | |
| 6 | <100c | 0 | 0 | |
| 7 | <100c | 0 | 0 | |
| 8 | 322 ± 73 | 0 | 0 | |
| 9 | <100c | 0 | 0 | |
| 10 | 2 ± 1 | 0 | 0 | |
| 11 | <100c | 0 | 0 | |
| 12 | 0 | 0 | 0 | |
| 11 | 1 | 0 | 0 | 0 |
| 2 | 0 | 0 | 0 | |
| 3 | 0 | 0 | 0 | |
| 4 | 0 | 0 | 0 | |
| 5 | 0 | 0 | 0 | |
| 6 | 0 | 0 | 0 | |
| 7 | <10c | 0 | 0 | |
| 8 | 0 | 0 | 0 | |
| 9 | 0 | 0 | 0 | |
| 10 | 0 | 0 | 0 | |
| 12 | 1 | 0 | 0 | 0 |
| 2 | 0 | 0 | 0 | |
| 3 | 0 | 0 | 0 | |
| 4 | 0 | 0 | 0 | |
| 5 | 0 | 0 | 0 | |
| 6 | 0 | 0 | 0 | |
| 7 | <100c | 0 | 0 | |
| 13 | 1 | <100c | 0 | 0 |
| 2 | 127 ± 5 | 0 | 0 | |
| 3 | 0 | 0 | 0 | |
| 4 | 225 ± 22 | 0 | 0 | |
| 5 | 0 | 0 | 0 | |
| 6 | 0 | 0 | 0 | |
| 7 | 574 ± 261 | 0 | 0 | |
| 14 | 1 | 1,645 ± 781 | <100c | 0 |
| 2 | 1,246 ± 479 | 106 ± 4 | 0 | |
| 3 | 1,251 ± 425 | 0 | 0 | |
| 15 | 1 | 0 | 0 | 0 |
| 2 | <100c | 0 | 0 | |
| 3 | 4 ± 1 | 0 | 0 | |
| 4 | 120 ± 34 | 0 | 0 | |
Of a total of 117 TSG, 76 were VZV positive, 5 were HSV-1 positive, and none were HSV-2 positive.
SD, standard deviation.
Copy number was detectable but not quantifiable based on amplification of the standard curve.
FIG 1.
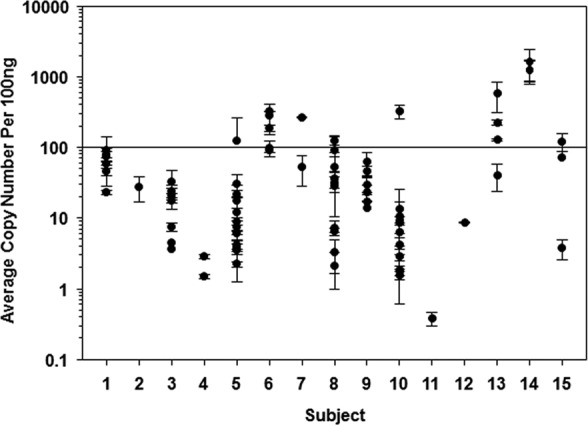
VZV DNA burden in human thoracic sympathetic ganglia (TSG). A total of 117 TSG from 15 subjects were obtained. DNA was extracted from each ganglion and quantitative PCR (qPCR) was performed using VZV-specific primers and probe to determine viral DNA copy numbers per 100 ng of DNA. Each point represents one ganglion. Ganglia that did not contain VZV DNA are not shown. DNA copy numbers were determined using a standard curve for known viral DNA concentrations. Based on the standard curve, some samples had copy numbers of <100 (detectable but not quantifiable). VZV DNA was found in all 15 subjects and in 76 (65%) of the 117 ganglia.
FIG 2.
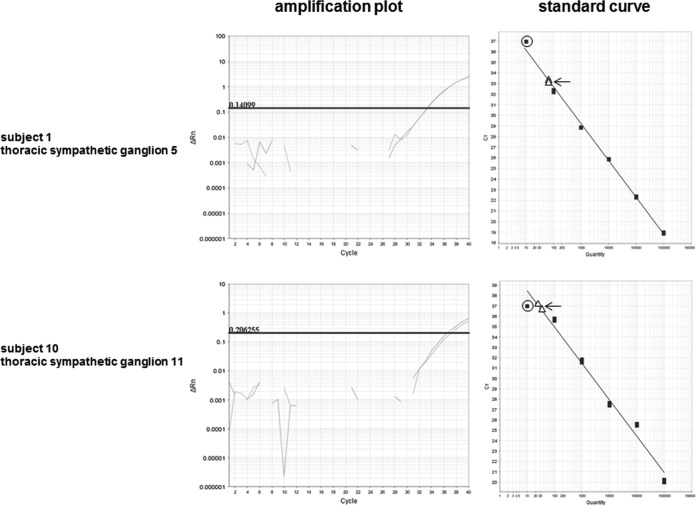
Amplification plot derived from two human thoracic sympathetic ganglia that contained low-abundance VZV DNA with corresponding standard curves using known concentrations of VZV DNA. Black squares (≥100 copies of VZV DNA) reflect duplicate data points; circles around black squares on the standard curves indicate single data point (10 copies of VZV DNA). Arrows indicate duplicate thoracic sympathetic ganglionic samples containing 10 to 100 copies of VZV DNA that are denoted as <100 copies in Table 2.
Both fresh and FFPE autonomic ganglia have been examined by PCR for alphaherpesvirus DNA. In one study (3), VZV DNA was detected in FFPE celiac (sympathetic) ganglia in 5/12 (42%) subjects and nodose ganglia from 1/11 (9%) subjects, while HSV-1 DNA was found in nodose ganglia of 1/11 (9%) subjects but not in celiac ganglia. Subsequent PCR analysis of fresh postmortem sympathetic ganglia did not detect VZV DNA in three subjects but did reveal HSV-1 DNA in two subjects; unfortunately, the type of sympathetic ganglia obtained was not identified (10). More recent analyses of multiple fixed human autonomic ganglia revealed VZV and HSV-1 DNA, respectively, in 11/58 and 23/58 pterygopalatine ganglia, in 14/60 and 25/60 ciliary ganglia, in 8/50 and 15/50 otic ganglia, in 4/47 and 14/47 submandibular ganglia, in 10/58 and 18/58 superior cervical ganglia, and in 1/36 and 12/36 nodose ganglia (7). Our detection of VZV DNA in greater numbers of freshly isolated TSG clearly indicates that the VZV DNA is from VZV viremia in childhood; the presence of HSV-1 and HSV-2 DNA is rare to nonexistent, with HSV-1 latency mostly limited to cranial nerve ganglia and HSV-2 mostly limited to sacral ganglia, presumably after retrograde transaxonal transport of virus from the face to cranial nerve ganglia.
Our current detection of VZV DNA in TSG from 100% of the subjects most likely reflects enhanced PCR amplification of DNA from fresh rather than FFPE tissue. Recently, VZV DNA was found in resected segments of gastrointestinal tract from 6/6 children with a history of varicella and in 6/7 children who received varicella vaccine but not in 7 children with no history of varicella or varicella vaccination (13). Overall, VZV DNA is present not only in cranial nerve ganglia and dorsal root ganglia but also in sympathetic and parasympathetic ganglia of most humans as well as in the enteric nervous system.
Importantly, TSG supply postganglionic fibers to blood vessels, skin, heart, lung, pancreas, gastrointestinal tract, liver, spleen, adrenal glands, kidneys, ureters, and bladder and also connect to dorsal root ganglia via gray communicating rami (14, 15). Thus, virus reactivation from TSG and dorsal root ganglia followed by virus spread to target organs may provide a potential pathway for virus-induced visceral disease. This is supported by VZV-induced pancreatitis and the presence of VZV antigen, large basophilic inclusions, and Cowdry type A inclusion bodies in associated sympathetic ganglia of a patient with fatal VZV meningoradiculitis without skin involvement (16), as well as additional reports of zoster-associated pancreatitis and hepatitis, and the presence of VZV DNA and VZV antigen in biopsy specimens of patients with zoster-associated gastritis (17–23).
VZV is well known to reactivate in the absence of rash (24). Two reports describe VZV-verified hepatitis and gastritis without rash in bone marrow transplant recipients. The first report was of a 74-year-old patient with common variable immunodeficiency of uncertain origin who died of fulminant hepatic failure, and VZV DNA was found in the blood and liver (25); serum of the second patient contained anti-VZV IgM antibody, and the patient was successfully treated with intravenous acyclovir (19). Finally, there is a remarkable report of VZV reactivation from multiple ganglia in a patient who developed right thoracic zoster sine herpete, followed 6 days later by left thoracic-distribution zoster associated with pancreatitis, cholecystitis, and gastric ulcerations that contained VZV DNA (21).
ACKNOWLEDGMENTS
This work was supported by Public Health Service grants AG006127 (D.G.), AG032958 (D.G.), and NS 067070 (M.A.N.) from the National Institutes of Health.
We thank Marina Hoffman for editorial review and Lori DePriest for manuscript preparation.
We report no conflicts of interest.
Footnotes
Published ahead of print 30 April 2014
REFERENCES
- 1.Gilden DH, Vafai A, Shtram Y, Becker Y, Devlin M, Wellish M. 1983. Varicella-zoster virus DNA in human sensory ganglia. Nature 306:478–480. 10.1038/306478a0 [DOI] [PubMed] [Google Scholar]
- 2.Mahalingam R, Wellish MC, Dueland AN, Cohrs RJ, Gilden DH. 1992. Localization of herpes simplex virus and varicella zoster virus DNA in human ganglia. Ann. Neurol. 31:444–448. 10.1002/ana.410310417 [DOI] [PubMed] [Google Scholar]
- 3.Kennedy PG, Grinfeld E, Gow JW. 1998. Latent varicella-zoster virus is located predominantly in neurons in human trigeminal ganglia. Proc. Natl. Acad. Sci. U. S. A. 95:4658–4662. 10.1073/pnas.95.8.4658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kennedy PG, Grinfeld E, Gow JW. 1999. Latent varicella-zoster virus in human dorsal root ganglia. Virology 258:451–454. 10.1006/viro.1999.9745 [DOI] [PubMed] [Google Scholar]
- 5.Pevenstein SR, Williams RK, McChesney D, Mont EK, Smialek JE, Straus SE. 1999. Quantitation of latent varicella-zoster virus and herpes simplex virus genomes in human trigeminal ganglia. J. Virol. 73:10514–10518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gilden DH, Gesser R, Smith J, Wellish M, Laguardia JJ, Cohrs RJ, Mahalingam R. 2001. Presence of VZV and HSV-1 DNA in human nodose and celiac ganglia. Virus Genes 23:145–147. 10.1023/A:1011883919058 [DOI] [PubMed] [Google Scholar]
- 7.Richter ER, Dias JK, Gilbert JE, II, Atherton SS. 2009. Distribution of herpes simplex virus type 1 and varicella zoster virus in ganglia of the human head and neck. J. Infect. Dis. 200:1901–1906. 10.1086/648474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Croen KD, Ostrove JM, Dragovic LJ, Smialek JE, Straus SE. 1987. Latent herpes simplex virus in human trigeminal ganglia. Detection of an immediate early gene “anti-sense” transcript by in situ hybridization. N. Engl. J. Med. 317:1427–1432 [DOI] [PubMed] [Google Scholar]
- 9.Obara Y, Furuta Y, Takasu T, Suzuki S, Suzuki H, Matsukawa S, Fujioka Y, Takahashi H, Kurata T, Nagashima K. 1997. Distribution of herpes simplex virus types 1 and 2 genomes in human spinal ganglia studied by PCR and in situ hybridization. J. Med. Virol. 52:136–142. [DOI] [PubMed] [Google Scholar]
- 10.Chen T, Hudnall SD. 2006. Anatomical mapping of human herpesvirus reservoirs of infection. Mod. Pathol. 19:726–737. 10.1038/modpathol.3800584 [DOI] [PubMed] [Google Scholar]
- 11.Cohrs RJ, Gilden DH. 2007. Prevalence and abundance of latently transcribed varicella-zoster virus genes in human ganglia. J. Virol. 81:2950–2956. 10.1128/JVI.02745-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nagel MA, Choe A, Traktinskiy I, Cordery-Cotter R, Gilden D, Cohrs RJ. 2011. Varicella-zoster virus transcriptome in latently infected human ganglia. J. Virol. 85:2276–2287. 10.1128/JVI.01862-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen JJ, Gershon AA, Li Z, Cowles RA, Gershon MD. 2011. Varicella zoster virus (VZV) infects and establishes latency in enteric neurons. J. Neurovirol. 17:578–589. 10.1007/s13365-011-0070-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Standring S. 2008. Gray's anatomy, 40th ed. Churchill Livingstone, Elsevier, London, England [Google Scholar]
- 15.Marieb EN, Wilhelm PB, Mallatt J. 2011. The autonomic nervous system and visceral sensory neurons. In Human anatomy, 6th ed. Pearson Education Inc., San Francisco, CA [Google Scholar]
- 16.Dueland AN, Devlin M, Martin JR, Mahalingam R, Cohrs R, Manz H, Trombley I, Gilden D. 1991. Fatal varicella-zoster virus meningoradiculitis without skin involvement. Ann. Neurol. 29:569–572. 10.1002/ana.410290520 [DOI] [PubMed] [Google Scholar]
- 17.Schiller GJ, Nimer SD, Gajewski JL, Golde DW. 1991. Abdominal presentation of varicella-zoster infection in recipients of allogeneic bone marrow transplantation. Bone Marrow Transplant. 7:489–491 [PubMed] [Google Scholar]
- 18.Pulik M, Teillet F, Teillet-Thiebaud F, Lionnet F, Genet Petitdidier PC. 1995. Varicella-zoster virus pancreatitis in hematologic diseases. Ann. Med. Interne (Paris) 146:292–294 (In French.) [PubMed] [Google Scholar]
- 19.Yagi T, Karasuno T, Hasegawa T, Yasumi M, Kawamoto S, Murakami M, Uosima N, Nakamura H, Hiraoka A, Masaoka T. 2000. Acute abdomen without cutaneous signs of varicella zoster virus infection as a late complication of allogeneic bone marrow transplantation: importance of empiric therapy with acyclovir. Bone Marrow Transplant. 25:1003–1005. 10.1038/sj.bmt.1702340 [DOI] [PubMed] [Google Scholar]
- 20.Stratman E. 2002. Visceral zoster as the presenting feature of disseminated herpes zoster. J. Am. Acad. Dermatol. 46:771–774. 10.1067/mjd.2002.119091 [DOI] [PubMed] [Google Scholar]
- 21.Kurtovic J, Webster GJ, Singh-Grewal I, Bullpitt P, Haindl W, Wakefield D, Riordan SM. 2005. Acalculous cholecystitis, multifocal gastrointestinal infarction and pancreatitis resulting from varicella-zoster virus. Intern. Med. J. 35:69–70. 10.1111/j.1445-5994.2004.00724.x [DOI] [PubMed] [Google Scholar]
- 22.Milligan KL, Jain AK, Garrett JS, Knutsen AP. 2012. Gastric ulcers due to varicella-zoster reactivation. Pediatrics 130:e1377–81. 10.1542/peds.2011-3491 [DOI] [PubMed] [Google Scholar]
- 23.Remmerswaal RG, de Vries AC, Ramsoekh D, van Buuren HR. 2012. Varicella zoster-associated gastric ulcers, hepatitis and pancreatitis in an immunocompromised patient. Endoscopy 44(Suppl 2 UCTN(:E140. 10.1055/s-0030-1256934 [DOI] [PubMed] [Google Scholar]
- 24.Gilden D, Cohrs RJ, Mahalingam R, Nagel MA. 2010. Neurological disease produced by varicella zoster virus reactivation without rash. Curr. Top. Microbiol. Immunol. 342:243–253. 10.1007/82_2009_3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rogers SY, Irving W, Harris A, Russell NH. 1995. Visceral varicella zoster infection after bone marrow transplantation without skin involvement and the use of PCR for diagnosis. Bone Marrow Transplant. 15:805–807 [PubMed] [Google Scholar]