Abstract
Previously institutionalized older patients with schizophrenia show changes in cognitive and functional capacity over time. This study examined changes in real-world functioning in a sample of people with schizophrenia who varied in their history of long-term institutionalization and related changes in real world functioning to changes in cognition and functional capacity over the follow-up period.
Older patients with schizophrenia (n=111) were examined with assessments of cognitive functioning, functional capacity, clinical symptoms, and everyday functioning. They were then followed up to 45 months and examined up to two times. Mixed-model regression was used to examine changes in real-world functioning in social, everyday living, and vocational domains over the follow-up period and identify potential predictors of change.
Everyday functioning worsened over time in all three domains. Although length of longest hospitalization predicted worsening, this influence was eliminated when the course of functional capacity was used to predict the course of everyday functioning. For both vocational and everyday living domains, as well as the composite score on functional status, worsening in performance based measures of everyday functioning and social competence predicted worsening in real world functioning. Changes in negative symptoms further predicted worsening in the everyday living domain.
Worsening in everyday functioning is found in people with schizophrenia and those with a history of greater chronicity and severity of illness seem more affected. These influences seem to be expressed through worsening in the ability to perform everyday functional skills. Potential causes of these changes and implications for reducing these impairments are discussed.
Introduction
Schizophrenia is a highly debilitating illness and a chronic condition that leads to disruptions in daily life functioning. Schizophrenia patients demonstrate impairment in their everyday functioning, with deficiencies in social, cognitive and real world activities that are detectable at the time of the first episode of illness. In recent years interest in the relationship between cognitive and functional impairments in patients with schizophrenia has grown (Green et al., 2000). Abundant evidence suggests that cognitive deficits are important determinants of impairments in most functional domains and are targeted for treatment in schizophrenia (Buchanan et al., 2005). Cognitive impairments have been shown to exert a general influence on functioning (Evans et al., 2003), with minimal evidence of differential correlations with different functional domains. Cognitive impairments are evident at the onset of illness (Speidman et al., 2010), are stable over the lifetime for the majority of patients (Heaton et al., 2001), appear to worsen only in the “oldest-old” patients (Loewenstein et al., in press) and can be affected by treatments (Kurtz et al., 2009).
Real world disabilities may be directly influenced by deficits in functional capacity (Patterson et al., 2001a), measured by the ability to perform everyday living skills, social activities and vocational abilities (Harvey et al., 2007). Leifker et al (Liefker et al., 2009) suggested that performance-based measures of everyday functioning are more directly related to everyday functioning than NP test performance, although other studies have found slightly different results (Heinrichs et al., 2010). Severity of symptoms negatively impacts on everyday functioning in the absence of major correlations with either NP test performance or functional capacity (Bowie et al., 2008, 2010).
A large body of literature supports the notion that cognitive functioning has a generally “normal” course of age-related decline, and that the decline among most schizophrenia patients is similar to healthy populations. However, older patients with an extensive history of illness and protracted institutionalization have shown a greater progressive decline (Fucetola et al., 2000, Harvey et al., 1999). These declines have also been observed among patients who are no longer institutionalized (Harvey et al., 2010). Specifically, our recent work indicated that formerly institutionalized patients demonstrated decline in functional capacity over time compared to healthy controls and patients who had never experienced a lengthy institutional stay. We found that longer duration of lifetime hospitalization was associated with worsening on performance-based measures of social and everyday living skills, suggesting the potential for worsening in this subgroup of patients even if they are no longer institutionalized. Further, risk factors previously identified as predictors of functional decline, including educational attainment, exerted an adverse impact on the course of functional capacity in our previous study.
In the present study, we examine the course of real world functioning in the same sample of older patients with schizophrenia who were examined in our previous report. The present analysis is unique for several reasons. First, there has never been a short-term longitudinal study of older patients that was oriented toward decline in everyday functioning. This study examines the same sample of patients wherein a subset worsened in their functional capacity in the previous follow-up analyses. Second, we are able to examine the longitudinal influence of previously identified cross-sectional predictors of impairments in everyday functioning in three functional domains: social, community activities, and vocational functioning (Bowie et al., 2008, 2010). These predictors include cognitive functioning, functional capacity, and social skills. In addition, we use the previously identified longitudinal predictors of decline in functional capacity (length of institutional stay, education) to predict the longitudinal course of real-world outcomes in these same three domains. Finally, we prospectively examine the influence of symptoms that had previously been reported to influence everyday functioning (i.e., depression and negative symptoms) on a cross-sectional basis. We examine the course of the three domains of everyday functioning, as well as a composite score based on all three domains, and used the variables described above to predict the course of everyday functioning in these three domains.
Methods
Participants
We previously reported on the collection and assessment of our schizophrenia sample in several cross-sectional reports and one longitudinal analysis (Bowie et al., 2008, Harvey et al., 2010, Liefker et al., 2009). Older community dwelling outpatients who met DSM-IV criteria for schizophrenia or schizoaffective disorder were enrolled in this longitudinal study investigating the course of cognitive and functional status. Exclusion criteria consisted of a primary DSM-IV Axis I diagnosis other than schizophrenia or schizoaffective disorder, a lifetime history of past substance dependence or current substance abuse disorders, Mini-Mental State Examination (MMSE) (Folstein et al., 1975) score below 18, Wide Range Achievement Test 3rd Edition (WRAT-3) (Jastak and Wilkinson, 1994) reading grade-equivalent of grade 6 or less, or any medical illnesses that might interfere with the assessment of cognitive functioning. The Comprehensive Assessment of Symptoms and History (CASH) (Andreasen et al., 1992) was completed by trained research assistants and diagnosis was confirmed by a senior clinician. Subjects were also required to demonstrate continued illness at the time of recruitment, as evidenced by meeting at least one of three criteria: 1) an inpatient admission for psychosis in the past two years; 2) an emergency room visit for psychosis in the past two years; or 3) a score on the PANSS positive symptoms items delusions, hallucinations, or conceptual disorganization of 4 (moderate) or more at the time of their baseline assessment. The reason for this criterion was to ensure that patients had not fully recovered from their illness. Outpatient status was defined as living outside of any institutional setting, including a nursing home while not excluding a group home setting.
In this patient group, all subjects were receiving treatment with second-generationantipsychotic medications at the time of the assessment. Recruitment was conducted at a VA or State Hospital or academic medical center and each subject received $50 for their participation at each assessment. After the testing procedures were fully explained, all subjects signed a written informed consent form approved by the institutional review board at each research site, where ethical approval was obtained. Healthy controls were included in the overall project, but are not included in these analyses because they did not have case managers to rate their everyday functioning.
Measures
All subjects completed the test battery in a fixed order. All interviewers received extensive training in performing all assessments and every three months their performance was evaluated through re-rating of training tapes, dual-ratings of the functional status measures with a senior clinical psychologist, and quality assurance assessments of all testing.
Performance-based Measures of Functional Capacity
The UCSD Performance-Based Skills Assessment Battery (UPSA) (Patterson et al., 2001a) was designed to directly evaluate the ability to perform everyday tasks that are considered necessary for independent functioning in the community. This test uses standardized role-playing situations to evaluate skills in different functional domains. In this study, four derived domains of the UPSA were used: Comprehension/Planning (e.g., organizing outings to the beach or the zoon), Finance (e.g., counting change, and paying bills), Transportation (e.g., using public transportation), and Communication (e.g., using the telephone, rescheduling medication appointments). We excluded the household chores subtest because the analogue kitchen required was not portable enough to be used at field sites. We then re-standardized the scores to a 100-point scale, like the original 5-subtest UPSA, thus allowing comparisons to previous results. This modified version was used in our previous reports with the UPSA (Bowie et al., 2008, Harvey et al., 2010).
The Social Skills Performance Assessment (SSPA) (Patterson et al., 2001b) is a social role-play task in which the subject initiates and maintains a conversation in two 3-minute role-play tasks: greeting a new neighbor and calling a landlord to request a repair for a leak that has gone unfixed. These sessions were audiotaped and scored by a trained rater who was unaware of diagnosis (patient or HC as part of a larger study) and all other data. Dimensions of social skills scored include fluency, clarity, focus, negotiation ability, persistence, and social appropriateness. These raters were trained to the gold standard ratings of the instrument developers, with an Intraclass Correlation Coefficient (ICC) of .86 and high inter-rater reliability was maintained at three months (ICC=.87). The mean of the ratings on these variables across the two measures was used in this study.
Cognitive Assessment
Neuropsychological tests were selected to represent diverse cognitive domains that were previously shown to be the most consistently correlated with functional skills (Green et al., 2000, Harvey et al., 2004). These tests included the Trail Making Test Parts A and B (Reitan and Wolfson, 1993), learning trials 1-5, from the Rey Auditory Learning Test (RAVLT) (Spreen and Strauss, 1998), FAS verbal fluency (Spreen and Strauss, 1998), animal naming (Spreen and Strauss, 1998), The Wisconsin Card Sorting Test-64 card version (Heaton et al., 1993), and the Digit Span, Letter-Number-Sequencing, and Digit Symbol Coding subtests of the Wechsler Adult Intelligence Scale, 3rd edition (WAIS-III) (Wechsler, 1997) were also included.This battery was developed prior to the finalization of the MATRICS consensus cognitive battery (Neuchterlein et al., 2008), but covers similar cognitive domains. Normatively corrected t-scores were created from published norms as we previously described.
Symptom Assessment
Severity of schizophrenia symptoms was assessed using the Positive and Negative Syndrome Scale (PANSS) (Kay, 1991). This is a 30-item scale with seven items measuring positive symptoms, seven items measuring negative symptoms, and sixteen items measuring general aspects of psychopathology and is completed after a structured interview. Each item is scored on a scale ranging from 1 (absent) to 7 (extreme), with item ratings incorporating the behavioral effects of symptoms as well as their severity. Raters were trained to adequate reliability with ICC's from .86 to .92. We used the White et al. (White et al., 1997a) factor analysis derived scores for negative symptoms on the PANSS, in line with our previous studies. Patients reported their depression severity on the Beck Depression Inventory-Second edition (BDI-II) (Beck et al., 1996).
Assessment of everyday functioning
In order to examine real-world functional performance, the Specific Levels of Function Scale (SLOF) (Schneider and Steuening, 1983) was employed. This scale is a 43-item caretaker report of a patient's behavior and functioning across the following domains: Interpersonal Relationships (e.g., initiating, accepting and maintaining social contacts; effectively communicating), Activities (e.g., shopping, using telephone, paying bills, use of leisure time, use of public transportation), and Work Skills (e.g., employable skills, level of supervision, punctuality). In order to obtain these ratings, a corroborated informant report method was used. With the patient's permission, a caseworker for the patient was directly interviewed in order to obtain information on real-world performance. All informants indicated that they knew the patient “very well” on the SLOF's 5-point Likert scale. The scale has excellent interrater reliability, factorial validity, and internal consistency (Leifker et al., 2011). There are three other subscales on the SLOF that were not administered because they either assessed aspects of function that are not associated with chronic mental illness (physical functioning and Personal care) or because they measure disruptive behaviors (Social acceptability). We used the three subscales and a composite generated from total scores on these three scales as our everyday functioning measures.
Procedures
Assessment and Follow-up
This project was approved by all applicable local IRBs and all participants provided signed informed consent for all initial and subsequent follow-up assessments, which were scheduled at 18 month intervals after initial entry into the study. Patients were examined with the same assessments in the same sequence at each subsequent follow-up visit (with the exception of the MMSE and WRAT Recognition). Alternate test forms were not available for most of the tests, so the same forms were used for all assessments for all participants. Telephone calls and mail contact were used to stay in touch with participants between assessments.
Determination of Institutionalization Status
Length of longest single hospital stay in months was a critical variable in the analyses of institutionalization status. This information was collected from medical records only, because of concerns with the accuracy of self report on the part of patients, using the same procedure as our previous report. Some data were not available because of missing information as described before (Harvey et al., 2010).
Data Analyses
A mixed-effects model repeated-measures (MMRM) (Gibbons, 2000) was used for the main set of analyses which examined the effect of individual differences variables: longest hospitalization, sex, age, and education on the course of everyday functioning. We then repeated these analyses including the dynamic performance-based covariates, including composite NP test performance, the UPSA, and the SSPA. In final set of analyses, we added the symptom variables that had been shown to have a cross-sectional relationship with everyday functioning, including PANSS negative symptoms and self-reported depression, also entered as dynamic covariates.
To complement the main analysis and to determine if changes in everyday functioning are evident across ages, or restricted to aging population, a Generalized Linear Mixed-Model (GLMM) regression was used. GLMM technique maximizes the use of the longitudinal nature of the data as it permits the inclusion of single as well as multiple measurements per person, and irregular intervals between measurements. The effect of interest was age (time). Splines were used to model age effects (i.e., time) on SLOF score. A spline model estimates the response relation without assuming that the data follow a particular form, such as linear or cubic. This allows for an evaluation of the functional form of associations. Analyses were carried using SAS software version 9.3 (Cary, NC, USA).
Results
111 patients were assessed at baseline. 102 were assessed twice, and 57 were assessed three times. The average time interval between first to second assessment was 1.5 years (SD=0.57). Average time to third assessment was 1.75 years (SD=0.58). Table 1 presents the baseline scores on the demographic variables and the performance-based predictor variables. Table 2 presents the observed case scores on the performance-based predictors.
Table 1.
Demographic and baseline performance and clinical characteristics
| Demographic characteristics | ||||||
| N | 111 | |||||
| % Male | 73 | |||||
| Ethnicity (%) | ||||||
| Caucasian | 58 | |||||
| African-American | 33 | |||||
| Other | 9 | |||||
| M | SD | |||||
| Age | 56.99 | 9.00 | ||||
| Years of Education | 11.86 | 4.42 | ||||
| UPSA Scaled Score | 72.19 | 17.38 | ||||
| SSPA Mean Score | 3.89 | 0.74 | ||||
| Cognitive Composite Score | −1.47 | 0.95 | ||||
|
Illness History Variables | ||||||
| M | SD | Min | Max | Median | ||
| Longest Hospital Stay (months) | 16.12 | 28.61 | 1 | 360 | 6 | |
| Number of Hospitalizations | 7.72 | 6.95 | 1 | 36 | 5 | |
| Total Lifetime Months in Hospital | 43.62 | 61.40 | 1 | 366 | 20 | |
| Age At First Admission | 23.65 | 10.26 | 16 | 45 | 23 | |
|
Baseline Illness Severity Variables | ||||||
| PANSS Negative Symptoms | 2.05 | 0.82 Reported as mean item score | ||||
| PANSS Positive Symptoms | 1.86 | 0.83 Reported as mean item score | ||||
| BDI Depression | 10.69 | 9.91 | ||||
Table 2.
Functional Capacity, Neuropsychological Performance, and Everyday functioning over the follow-up period.
| First Followup (N=104) | ||
|---|---|---|
| M | SD | |
| UPSA | 74.60 | 18.25 |
| SSPA | 3.92 | 0.74 |
| NP Performance | −1.52 | 0.90 |
|
Second Follow up (N=57) | ||
| M | SD | |
| UPSA | 73.18 | 18.27 |
| SSPA | 3.82 | 0.75 |
| NP Performance | −1.60 | 0.92 |
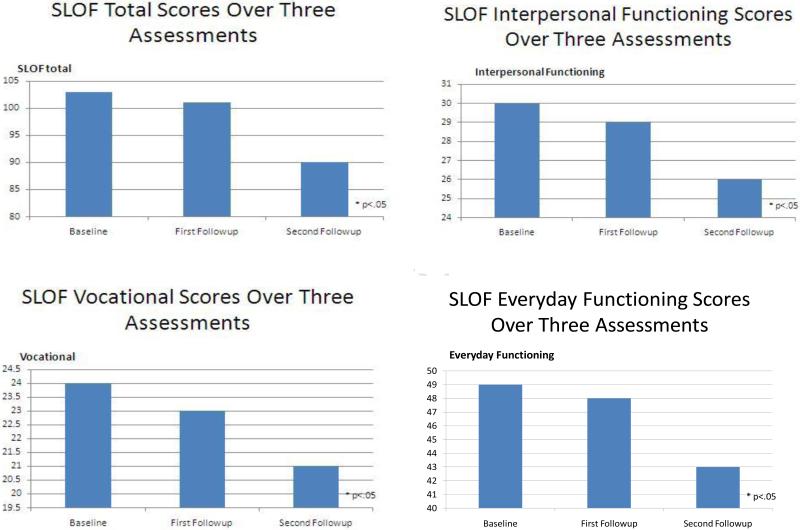
Figure 1 presents everyday function for the SLOF composite measure and three SLOF subscales. Our first analysis focused on demographic predictors of change over time in SLOF total score, evaluating the time effects and also predicting the change in SLOF scores using the unchanging covariates age, education, and longest psychiatric hospitalization. In the first model, we examined the effects of time, age, sex, education, and longest hospital stay on SLOF total score to determine if there were significant overall effects. For SLOF total score, the overall model was statistically significant [X2(3)=60.31, p<.001]. There were statistically significant effects of time [X2(1)=8.01, p<.003], age, [X2(1)=59.41, p<.001], and longest institutional stay [X2(1)= 5.64, p<.05]. Sex and education were not statistically significantly associated with SLOF scores.
Figure 1.
Everyday functioning over the follow-up period
Next, performance-based measures (UPSA, SSPA, NP performance) were added to the model. The overall model was statistically significant [X2(7)=105.74, p<.001]. There were statistically significant effects of time [X2(1)=7.67, p<.005], and age[X2(1)=20.16, p<.001]. There were also statistically significant effect of change in UPSA scores [X2(1)=12.85, p<0.001], and SSPA scores [X2(1)= 5.26, p<0.05]. For both worsening in performance on the functional capacity measures was associated with worsening in total estimates of real-world functioning. NP performance was not associated with SLOF scores, and in the extended model the effect of longest institutional stay was no longer statistically significant [X2(1)=2.35, p=.125].
Next we repeated the analysis for each of the three SLOF everyday functioning subscales (i.e. social, everyday activities, and vocational). For social functioning, the overall model was statistically significant [X2(3)=19.18, p<0.01]. However, only time had a significant association [X2(1)=7.28, p<.007]. For everyday activities, the overall model was significant[X2(6)=157.96, p<.001]. There were significant effects of time [X2(1)=4.64, p<.05], and age [X2(1)=43.05, p<.001], but education, sex, and longest hospitalization were not significant predictors. Declines in UPSA scores [X2(1)=24.01, p<.001], and SSPA scores[X2(1)=4.83, p=.03], were significant predictors of worsening of SLOF everyday functioning, but changes in NP test performance did not. Finally, for vocational functioning, the overall model was significant [X2(6)=83.06, p<.001]. The effect of time of assessment was significant [X2(1)=8.11, p=.004], as was the effect of age [X2(1)=16.80, p<.001]. Worsening UPSA scores [X2(1)=6.51, p<.025], and SSPA scores [X2(1)=4.60, p=.04] were significant predictors of worsening in vocational functioning, but NP test performance again did not predict changes over time.
We next examined if symptoms were significant predictors of course of everyday functioning. Results remained unchanged after adding symptom severity to the models described above. Change in negative symptoms predicted the course of everyday activities [X2 (1)=5.35, p<.05]. No other associations between symptoms and everyday functioning were statistically significant.
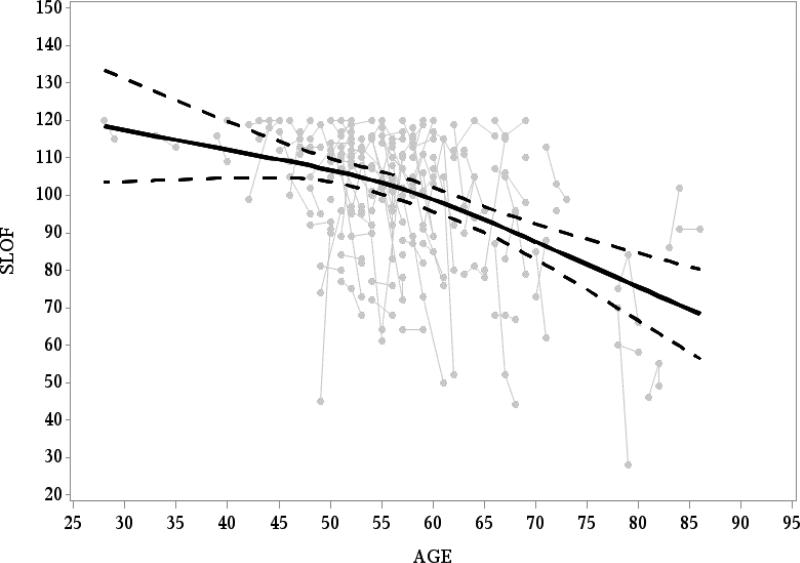
Figure 2 presents the SLOF composite measure as a function of the age of the participants. As the figure demonstrates, everyday functioning continuously deteriorates across adult life [F(2,139)=22.15, p<0.0001]. Although visual inspection of Figure 2 suggested that the functional deterioration may accelerate after age 55, this was not supported by a formal statistical test [F test for non-linear trend(1,139)=1.61, p=0.20], but sample size in the older ages may limit statistical power.
Figure 2. Age-associated changes in everyday functioning.
Gray dots represent Individual participants. Black line represents the average SLOF score per age as modelled using spline; Dotted black line represents the 95% Confidence Intervals.
Discussion
There are several findings of potential interest in this study. First, older people with schizophrenia show modest worsening in their everyday functioning as a group and these changes are predicted by several of the variables that we measured. Importantly, we expand on our previous findings that history of longer institutional stay predicts worsening in performance-based measures of functional abilities by showing that history of institutionalization predicts worsening in real-world functioning as well. The results also include a demonstration that worsening in performance on measures of social skills and functional capacity predicts worsening of everyday functioning in two of the three everyday functional domains that we measured. Further, these data also suggest that longer institutionalization influences worsening in real-world functioning by leading to deterioration in functional skills. Worsening in negative symptoms was also associated with worsening in everyday activities, while the previous observed cross-sectional influence of depression on everyday functioning did not hold up in a longitudinal study.
Limitations of this study should be acknowledged. Follow up assessments did not occur for every patient, but we previously (Harvey et al., 2010) found no difference in any of the current predictor variables across patients who were and were not followed up. Other factors, including medical illness, were not measured sufficiently to be used as predictor variables. This will be an important topic for future research. Patients’ symptom severity was not substantial leading to reduced ability to predict changes based on symptomatic worsening. Some of our previous cross-sectional findings including the correlation of depression with everyday activities and social amotivation as a predictor of social dysfunction, were challenging to evaluate in a sample with only minimal symptom dynamics. We argue that this does not suggest that these factors are reduced in their potential importance, but rather that we could not prove that they produce functional decline.
The present sample is a unique one and there are few current patients who will experience extended institutional stays like some of the members of the current sample experienced. However, the historical reasons for extended institutional stays, including treatment resistance and aggressive behavior (White et al., 1997b), have not been resolved as clinical issues. Later research should determine whether treatment resistance, which is associated with longitudinal ventricular enlargement (Davis et al., 1998) is the operative factor in the functional declines seen and whether patients with persistently severe psychotic symptoms evidence functional decline as previous data has suggested (Harvey et al., 2003).
When functional worsening is detected, it would be important to at least speculate about the potential causes. This is a unique and rarely studied population, with minimal longitudinal data available on truly older patients. As a result, we have three hypotheses based on our own research with patients who are similar to the current population. The first is that chronic psychosis associated with long-term institutional stay exerts a neurotoxic effect. Multiple relapses early in the illness have been shown to lead to cortical gray matter loss (Cahn et al., 2006), consistent with the findings that persistent psychosis leads to ventricular enlargement in chronic patients (Davis et al., 1998). There are neurodegenerative possibilities as well. We (Rapp et al., 2011), reported that in postmortem brain specimens from people with schizophrenia, evaluated during life, the numbers of amyloid plaques and neurofibrillary tangles predicted the level of cognitive impairments at the last assessment during life. These patients were selected for not meeting neuropathological criteria for Alzheimer's disease, suggesting that normal range neurodegenerative changes may induce greater cognitive deficit in people with lifelong cortical challenge.
A third possibility arises from the domain of alterations in brain structure. We (Friedman et al., 2008) have shown that chronic patients show evidence of reduced organization of white matter as indexed by reduced levels of regional fractional anisoptropy in chronic patients compared to first episode patients. While these data are cross-sectional, they suggest possible longitudinal changes in brain structure. Thus, progressive changes in both the volume and structure of the brain, involving white and gray matter, have been detected and might be a possible mechanism that underlies the current findings.
Conclusions
Worsening in everyday functioning is found in people with schizophrenia and those with a history of greater chronicity and severity of illness seem more affected. Direct interventions on functional capacity and underlying cognitive impairments are plausible treatment targets. Cognitive remediation interventions improve cognition and functioning (Wykes et al., 2011) and work even in very poor outcome patients 0). Reduction of risk factors for cortical deterioration, might provide a complementary approach.
Acknowledgement
This study was funded by the NIMH (Grants numbered MH 63116 and 78775) to Dr. Harvey.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
Dr Bowie has served as a consultant to Abbott Laboratories and has received a grant from Johnson and Johnson. Dr. Harvey has received consulting fees from Abbott Labs, Boehringer-Ingleheim, Bristol Myers Squibb, En Vivo, Genentech, Johnson and Johnson, Pharma Neuro Boost, Sunovion Pharma, and Takeda Pharma during the past year.
References
- Andreasen NC, Flaum M, Arndt S. The comprehensive assessment of symptoms and history (CASH): An instrument for assessing diagnosis and psychopathology. Archives of General Psychiatry. 1992;49:615–623. doi: 10.1001/archpsyc.1992.01820080023004. [DOI] [PubMed] [Google Scholar]
- Beck A, Steer R, Brown G. BDI-II, The Beck Depression Inventory Second Edition. The Psychological Corporation; San Antonio, TX.: 1996. [Google Scholar]
- Bowie CR, Leung WW, Reichenberg A, McClure MM, Patterson TL, Heaton RK, Harvey PD. Predicting schizophrenia patients’ real world behavior with specific neuropsychological and functional capacity measures. Biol Psychiatry. 2008;63:505–511. doi: 10.1016/j.biopsych.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie CR, Depp C, McGrath JA, Wolyniec P, Mausbach BT, Thornquist MH, et al. Prediction of real-world functional disability in chronic mental disorders: a comparison of schizophrenia and bipolar disorder. Am J Psychiatry. 2010;167:1116–1124. doi: 10.1176/appi.ajp.2010.09101406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan RW, Davis M, Goff D, Green MF, Keefe RSE, Leon AC, et al. A summary of the FDA-NIMH-MATRICS workshop on clinical trial design for neurocognitive drugs for schizophrenia. Schizophr Bull. 2005;31:5–19. doi: 10.1093/schbul/sbi020. [DOI] [PubMed] [Google Scholar]
- Cahn W, van Haren NE, Hulshoff Pol HE., Schnack HG, Caspers E, Laponder DA, Kahn RS. Brain volume changes in the first year of illness and 5-year outcome of schizophrenia. Br J Psychiatry. 2006;189:381–382. doi: 10.1192/bjp.bp.105.015701. [DOI] [PubMed] [Google Scholar]
- Davis KL, Buchsbaum MS, Shihabuddin L, Spiegel-Cohen J, Metzger M, Frecska E, et al. Ventricular enlargement in poor-outcome schizophrenia. Biol Psychiatry. 1998;43(11):783–793. doi: 10.1016/s0006-3223(97)00553-2. [DOI] [PubMed] [Google Scholar]
- Evans JD, Heaton RK, Paulsen JS, Palmer BW, Patterson T, Jeste DV. The relationship of neuropsychological abilities to specific domains of functional capacity in older schizophrenia patients. Biol Psychiatry. 2003;53(5):422–430. doi: 10.1016/s0006-3223(02)01476-2. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Friedman JI, Tang C, Carpenter D, Buchsbaum MS, Schmeidler J, Flanagan L, et al. Diffusion Tensor Imaging Findings in First-Episode and Chronic Schizophrenia Patients. Am J Psychiatry. 2008;165:1024–32. doi: 10.1176/appi.ajp.2008.07101640. [DOI] [PubMed] [Google Scholar]
- Fucetola R, Seidman LJ, Kremen WS, Faraone SV, Goldstein JM, Tsuang MT. Age and neuropsychologic function in schizophrenia: A decline in executive abilities beyond that observed in healthy volunteers. Biol Psychiatry. 2000;48:137–146. doi: 10.1016/s0006-3223(00)00240-7. [DOI] [PubMed] [Google Scholar]
- Gibbons RD. Mixed-effects models for mental health services research. Health Serv Outcomes Res Methodol. 2000;1:91–129. [Google Scholar]
- Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: Are we measuring the “right stuff”? Schizophr Bull. 2000;26:119–136. doi: 10.1093/oxfordjournals.schbul.a033430. [DOI] [PubMed] [Google Scholar]
- Harvey PD, Silverman JM, Mohs RC, Parrella M, White L, Powchick P, et al. Cognitive decline in late-life schizophrenia: A longitudinal study of geriatric chronically hospitalized patients. Biol Psychiatry. 1999; 45:32–40. doi: 10.1016/s0006-3223(98)00273-x. [DOI] [PubMed] [Google Scholar]
- Harvey PD, Bertisch H, Friedman JI, Marcus S, Parrella M, White L, Davis KL. The course of functional decline in geriatric patients with schizophrenia: Cognitive-functional and clinical symptoms as determinants of change. . Am J Geriatr Psychiatry. 2003;11:610–619. doi: 10.1176/appi.ajgp.11.6.610. [DOI] [PubMed] [Google Scholar]
- Harvey PD, Green MF, Keefe RSE, Velligan D. Cognitive function in schizophrenia: Its role in the definition and evaluation of effective treatments for the illness. J Clin Psychiatry. 2004;2004;65:361–372. [PubMed] [Google Scholar]
- Harvey PD, Velligan DI, Bellack AS. Performance-based measures of functional skills: Usefulness in clinical treatment studies. Schizophrenia Bull. 2007;33:1138–1148. doi: 10.1093/schbul/sbm040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PD, Reichenberg A, Bowie CR, Patterson TL, Heaton RK. The course of neuropsychological performance and functional capacity in older patients with schizophrenia: Influences of previous history of long-term institutional stay. Biol Psychiatry. 2010;67:933–939. doi: 10.1016/j.biopsych.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Chelune GJ, Talley JL, Kay GG, Curtiss G. Wisconsin Card Sorting Test manual – Revised and expanded. Psychological Assessment Resources, Inc.; Florida: 1993. [Google Scholar]
- Heaton RK, Gladsjo JA, Palmer BW, Kuck J, Marcotte TD, Jeste DV. Stability and course of neuropsychological deficits in schizophrenia. Arch Gen Psychiatry. 2001;58:24–32. doi: 10.1001/archpsyc.58.1.24. [DOI] [PubMed] [Google Scholar]
- Heinrichs RW, Ammari N, Miles AA, McDermid Vaz S. Cognitive performance and functional competence as predictors of community independence in schizophrenia. Schizophr Bull. 2010;36:381–387. doi: 10.1093/schbul/sbn095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jastak S, Wilkinson G. The Wide Range Achievement Test. 3rd ed. The Psychological Corporation; San Antonio: 1994. [Google Scholar]
- Kay SR. Positive and negative syndromes in schizophrenia. Brunner/Mazel; New York: 1991. [Google Scholar]
- Kurtz MM, Seltzer JC, Fujimoto M, Shagan DS, Wexler BE. Predictors of change in life skills in schizophrenia after cognitive remediation. Schizophr Res. 2009;107(2-3):267–274. doi: 10.1016/j.schres.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leifker FR, Bowie CR, Harvey PD. Determinants of everyday outcomes in schizophrenia: The influences of cognitive impairment, functional capacity, and symptoms. Schizophrenia Research. 2009;115:82–87. doi: 10.1016/j.schres.2009.09.004. [DOI] [PubMed] [Google Scholar]
- Leifker FR, Patterson TL, Heaton RK, Harvey PD. Validating measures of real-world outcome: The results of the VALERO Expert Survey and RAND Appropriateness Panel. Schizophr Bull. 2011; 37:334–343. doi: 10.1093/schbul/sbp044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewenstein DA, Czaja SJ, Bowie CR, Harvey PD. Age associated differences in cognitive performance in older patients with schizophrenia: A comparison with healthy older adults. Am J Geriatr Psychiatry. doi: 10.1097/JGP.0b013e31823bc08c. in press. [DOI] [PubMed] [Google Scholar]
- Neuchterlein KH, Green MF, Kern RS, Baade LE, Barch D, Cohen J, et al. The MATRICS consensus cognitive battery: Part 1, test selection, reliability, and validity. Am J Psychiatry. 2008;165:203–213. doi: 10.1176/appi.ajp.2007.07010042. [DOI] [PubMed] [Google Scholar]
- Patterson TL, Goldman S, McKibbin CL, Hughs T, Jeste DV. UCSD performance-based skills assessment: Development of a new measure of everyday functioning for severely mentally ill adults. Schizophr Bull. 2001a;27:235–245. doi: 10.1093/oxfordjournals.schbul.a006870. [DOI] [PubMed] [Google Scholar]
- Patterson TL, Moscona S, McKibbin CL, Davidson K, Jeste DV. Social skills performance assessment among older patients with schizophrenia. Schizophr Res. 2001b;48:351–60. doi: 10.1016/s0920-9964(00)00109-2. [DOI] [PubMed] [Google Scholar]
- Rapp MA, Schnaider-Beeri M, Purohit DP, Reichenberg A, McGurk SR, Haroutunian V, Harvey PD. Cortical neuritic plaques and hippocampal neurofibrillary tangles are related to dementia severity in elderly schizophrenia patients. Schizophr Res. 2011;116:90–96. doi: 10.1016/j.schres.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan M, Wolfson D. The Halstead–Reitan Neuropsychological Test Battery: Theory and clinical interpretation. 2nd ed. Neuropsychology Press; Tucson: 1993. [Google Scholar]
- Schneider LC, Streuening EL. SLOF: A behavioral rating scale for assessing the mentally ill. Soc Work Res and Abstr. 1983;6:9–21. doi: 10.1093/swra/19.3.9. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Giuliano AJ, Meyer EC, Addington J, Cadenhead KS, Cannon TD, et al. Neuropsychology of the prodrome to psychosis in the NAPLS consortium: relationship to family history and conversion to psychosis. Arch Gen Psychiatry. 2010;67:578–88. doi: 10.1001/archgenpsychiatry.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreen O, Strauss E. A Compendium of neuropsychological tests: Administration, norms, and commentary. 2nd ed. Oxford University Press; New York: 1998. [Google Scholar]
- Wechsler D. WAIS-III administration and scoring manual. The Psychological Corporation; San Antonio: 1997. [Google Scholar]
- White L, Harvey PD, Opler L, Lindenmayer JP. Empirical assessment of the factorial structure of clinical symptoms in schizophrenia. A multisite, multimodel evaluation of the factorial structure of the Positive and Negative Syndrome Scale. The PANSS Study Group. Psychopathology. 1997a;30(5):263–74. doi: 10.1159/000285058. [DOI] [PubMed] [Google Scholar]
- White L, Parrella M, McCrystal-Simon J, Harvey PD, Masiar S, Davidson M. Characteristics of elderly psychiatric patients retained in a state hospital during downsizing: A prospective study with replication. Int J Geriatr Psychiatry. 1997b;12:474–480. doi: 10.1002/(sici)1099-1166(199704)12:4<474::aid-gps530>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Wykes T, Huddy V, Cellard C, McGurk SR, Czobor P. A meta-analysis of cognitive remediation for schizophrenia: Methodology and effect sizes. Am J Psychiatry. 2011;168:472–85. doi: 10.1176/appi.ajp.2010.10060855. [DOI] [PubMed] [Google Scholar]