Abstract
Midthoracic spinal cord injury (SCI) is associated with enhanced cardiac sympathetic activity and reduced cardiac parasympathetic activity. The enhanced cardiac sympathetic activity is associated with sympathetic structural plasticity within the stellate ganglia, spinal cord segments T1–T4, and heart. However, changes to cardiac parasympathetic centers rostral to an experimental SCI are relatively unknown. Importantly, reduced vagal activity is a predictor of high mortality. Furthermore, this autonomic dysregulation promotes progressive left ventricular (LV) structural remodeling. Accordingly, we hypothesized that midthoracic spinal cord injury is associated with structural plasticity in premotor (preganglionic parasympathetic neurons) cardioinhibitory vagal neurons located within the nucleus ambiguus as well as LV structural remodeling. To test this hypothesis, dendritic arborization and morphology (cholera toxin B immunohistochemistry and Sholl analysis) of cardiac projecting premotor cardioinhibitory vagal neurons located within the nucleus ambiguus were determined in intact (sham transected) and thoracic level 5 transected (T5X) rats. In addition, LV chamber size, wall thickness, and collagen content (Masson trichrome stain and structural analysis) were determined. Midthoracic SCI was associated with structural changes within the nucleus ambiguus and heart. Specifically, following T5 spinal cord transection, there was a significant increase in cardiac parasympathetic preganglionic neuron dendritic arborization, soma area, maximum dendritic length, and number of intersections/animal. This parasympathetic structural remodeling was associated with a profound LV structural remodeling. Specifically, T5 spinal cord transection increased LV chamber area, reduced LV wall thickness, and increased collagen content. Accordingly, results document a dynamic interaction between the heart and its parasympathetic innervation.
Keywords: autonomic nervous system, cardiac, paraplegia
midthoracic spinal cord injury [SCI; T5 spinal cord transection (T5X)] reduced cardiac parasympathetic tonus (40), increased cardiac sympathetic tonus (14, 40, 41, 61), and increased the susceptibility to life-threatening, sustained ventricular tachyarrhythmias (14, 40, 41, 63). These functional changes were associated with structural sympathetic hyperinnervation of the heart (45). For example, using injection of cholera toxin B into the left and right stellate ganglia (which provides >90% of sympathetic supply to the heart), or pericardial sac (43), we documented a significant increase in cardiac-sympathetic preganglionic (45) and postganglionic neuron (43) dendritic arborization following T5X. Furthermore, there was a significant increase in left ventricular sympathetic innervation density, as measured through tyrosine hydroxylase immunohistochemistry following T5X (40, 45). Thus, midthoracic spinal cord injury results in cardiac sympathetic hyperinnervation and increased the susceptibility to life-threatening arrhythmias.
The origin of parasympathetic innervation to the heart (preganglionic parasympathetic neurons) is premotor cardioinhibitory vagal neurons located within the nucleus ambiguus (47, 48). These premotor nucleus ambiguus neurons synapse with the postganglionic neurons within the heart (6, 21) and dominate the control of heart rate (48). Acetylcholine is released from the preganglionic nerve terminal and binds to nicotinic acetylcholine receptors located on the postganglionic neuron. In response, the postganglionic neuron depolarizes and releases acetylcholine at the sinoatrial and atrioventricular nodes, activating M2 muscarinic receptors and slowing heart rate.
Although T5X reduces cardiac parasympathetic drive (40), almost nothing is known about structural alterations in premotor cardioinhibitory vagal neurons located within the nucleus ambiguus. Furthermore, reduced vagal activity is a predictor of high mortality (31, 33) and promotes progressive left ventricular (LV) structural remodeling (64, 65). For example, pharmacological agents that reduce heart rate improve survival and prevent or attenuate progressive LV remodeling in animals with heart failure (10, 72). Furthermore, electrical vagus nerve stimulation prevents sudden cardiac death in dogs with myocardial infarction and improves survival in rats with chronic heart failure (35, 73).
A dynamic interaction between a target tissue and its innervation is required for optimal functioning (60, 74). Importantly, cardiac function is lower in rats with midthoracic SCI compared with sham-operated intact rats following blockade of the sympathetic nervous system, documenting LV dysfunction (43). Accordingly, T5X-induced changes in cardiac function may be associated with changes in premotor cardioinhibitory vagal neuron morphology as well as progressive LV remodeling (12, 13, 39–41, 43, 45, 61–63). Therefore, the hypotheses that T5X is associated with decreased premotor cardioinhibitory vagal neuron dendritic arborization and LV remodeling was tested. To test these hypotheses, cholera toxin B subunit (CTB) immunohistochemistry procedures and Sholl analysis were used to examine the dendritic branching pattern of cardiac-projecting premotor cardioinhibitory vagal neurons located within the nucleus ambiguus. Specifically, CTB was injected into the pericardial space to retrogradely label premotor cardioinhibitory vagal neurons projecting to the heart. This is an important procedure because only cardiac-projecting premotor cardioinhibitory vagal neurons were examined. In addition, the hearts were harvested, sectioned, and processed with Masson trichrome stain for morphological analysis (chamber size, wall thickness, septal area, and collagen content). Results document structural plasticity within the nucleus ambiguus and heart following midthoracic spinal cord transection.
MATERIALS AND METHODS
Surgical Procedures
Experimental procedures and protocols were reviewed and approved by the Animal Care and Use Committee of Wayne State University and complied with the American Physiological Society's Guiding Principles in the Care and Use of Animals.
Twelve adult Sprague-Dawley male rats [n = 6, midthoracic spinal cord transected (T5X); and n = 6, sham transected (intact)] were studied to determine structural remodeling of the heart and its premotor cardioinhibitory vagal neurons. Specifically, rats were studied to determine cardiac morphology (Masson trichrome staining) and nucleus ambiguus neuron dendritic arborization and morphology (CTB immunohistochemistry and Sholl analysis) 28 days post-spinal cord transection or sham transection. Intact and T5X rats were studied 28 days after the intervention because we were interested in the chronic state after adaptive responses reached a steady state. For example, we reported recently that sympathetic support of heart rate and ventricular function increased nerve growth factor as early as 1–3 days after a T5 spinal cord injury, but nerve growth factor content within the stellate ganglia did not increase until 28 days posttransection (or sometime between days 7 and 28 postinjury) (43).
All surgical procedures were performed using aseptic surgical techniques. Rats were anesthetized with pentobarbital sodium (50 mg/kg ip), atropinized (0.05 mg/kg ip), intubated, and prepared for aseptic surgery by removing the fur over the surgical site and cleansing the skin with a povidone-iodine solution. Subsequently, the rats were mechanically ventilated and placed on a feedback-based temperature control system (model no. 40-90-8; FHC, Bowdoin, ME) for monitoring and maintaining body temperature within the physiological range. Supplemental doses of pentobarbital sodium (10–20 mg/kg ip) were administered if the rats regained the blink reflex or responded during the surgical procedures.
T5X
Twelve rats underwent complete T5X or sham transection (6 T5X and 6 sham T5X). Following anesthesia the rats were positioned prone over a thoracic roll that slightly flexed the trunk. The fourth thoracic vertebra was exposed via a midline dorsal incision, and the spinous process and laminae were removed. Because the spinal cord is shorter than the vertebral column, spinal cord segment T5 lies at the level of the T4 vertebral body. Two ligatures (6.0 silk) were tightened around the underlying spinal cord between the fifth and sixth thoracic segments, and the spinal cord was completely transected by cutting between the ligatures with scissors (40, 41). In this way, there was minimal bleeding. Identical procedures were followed for the 6 sham T5X rats, except the spinal cord was not tied or transected. Sympathetic innervation to the heart is derived from preganglionic fibers that exit the spinal cord at the first through fourth thoracic levels (71). Transection between the fifth and sixth thoracic levels of the spinal cord preserves supraspinal control of cardiac sympathetic activity. Importantly, because cardiac vagal fibers do not pass through the spinal cord, spinal cord transection does not interrupt cardiac parasympathetic activity. Specifically, the origin of parasympathetic innervation to the heart is premotor (preganglionic) cardioinhibitory vagal neurons located within the nucleus ambiguus (47, 48) and travel in the vagus nerve to the heart. The completeness of the transection was confirmed by visual inspection of the lesion site. The diets of all rats were supplemented postsurgery with palatable, nutritious treats (Bio-Serv, Frenchtown, NJ). No other dietary interventions were necessary.
Intrapericardial Sac Injections
Twenty-one to 25 days after the T5X or sham transection, the neuronal tracer CTB was injected into the pericardial space, as described recently (43). Briefly, the animals were anesthetized as described above, and the heart was approached via a thoracotomy through the second or third intercostal space. Ten microliters of 1% CTB mixed with 1 μl of 3% Evans blue dye was injected into the pericardial space by inserting a pipette tip through the thymus gland (43). The Evans blue dye was used to visualize the injectate because CTB is colorless. This assured localization within the pericardial sac. All injections were confined within the pericardial sac. Five to 7 days were allowed for CTB to be picked up at synaptic endings and transported in a retrograde fashion back to the cell bodies of neurons located within the nucleus ambiguus. Subsequently, the animals were deeply anesthetized and perfused transcardially, and the heart and brain stems were preserved as described previously (45).
Tissue Processing, Analysis, and Immunohistochemistry
Nucleus ambiguus.
Investigators have reported independent and differential control of sinoatrial and atrioventricular nodal function extending from the central nervous system to the level of the heart (2, 20). In this context, heart rate is modulated predominantly by neurons in the right nucleus ambiguus, whereas atrioventricular conduction is controlled predominantly by neurons in the left nucleus ambiguus. Accordingly, the right nucleus ambiguus was selected to include neurons controlling cardiac rate.
To achieve this goal, the right brain stem from six T5X and six intact (sham T5X) rats was sectioned sagittally at 30-μm intervals. Tissue sections were washed in 10 mM Tris, 0.9% NaCl, and 0.05% thimerosal in 10 mM phosphate buffer, pH 7.4 (TPBS), containing 0.3% Triton X-100 for 3 × 10 min then incubated in 10% heat-inactivated normal horse serum (NHS; Invitrogen) in TPBS-Triton for ≥1 h. The sections were then incubated in goat anti-CTB antiserum (1:25,000; List Biologicals) in TPBS-Triton containing 10% NHS for 3 days at room temperature. After rinsing (TPBS, 3 × 10 min each), sections were incubated with biotinylated donkey anti-goat immunoglobulin (1:500; Jackson Laboratories) in TPBS-Triton with 1% NHS overnight at room temperature. Sections were rinsed again (TPBS, 3 × 10 min each) and incubated for 4–6 h in 1:1,500 ExtrAvidin-HRP (catalog no. E-2886; Sigma) in TPBS-Triton. Immunoreactive neurons were revealed with the nickel-intensified diaminobenzidine reaction (37).
Structural analysis.
CTB-labeled nucleus ambiguus neurons were examined with an Olympus BH-2 microscope outfitted with a motorized stage, Neurolucida imaging software, and a high-resolution digital camera. Multiplanar photomicrographs were taken and the images stacked using MicroBrightfield Neurolucida software. Selection criteria were similar to previous studies examining dendritic arborization in other regions (51, 75). Cell bodies and dendrites were reconstructed using the neuron-tracing feature on the Neurolucida system, and dendritic branching was assessed in the NeuroExplorer 3D visualization and morphometric analysis program included with the Neurolucida system.
Morphological features of cardiac-projecting nucleus ambiguus neurons were analyzed as described by Nelson and colleagues (50–52). Specifically, each neuron was analyzed using the Sholl analysis of dendritic branching (45, 67), which assumes that dendritic arborization is an indirect measurement of available postsynaptic space. A series of concentric rings calibrated at 10-μm intervals was superimposed on each neuron and centered on the cell body. Intersections between dendrites and each concentric ring were then counted. The location and number of intersections were plotted (45) and used for statistical comparisons. In each animal, nine to 10 sections were examined and nine to 10 neurons per section measured using Neurolucida software. Each cell's morphometric features were measured within one 30-μm-thick section. Only cells with clearly distinguishable perikarya and dendritic trees were assessed. Specifically, to be selected for analysis, CTB-labeled neurons satisfied the following criteria: 1) dark and consistent staining in the entire dendritic tree, 2) lack of truncated dendrites, and 3) relative separation from nearby stained neurons to avoid overlapping dendrites. The examined neurons were chosen randomly. Three additional morphological features were recorded: area of soma, overall length of all visible processes (maximum dendritic length), and number of intersections per animal. Finally, every labeled neuron from each section was counted. The morphological features and number of neurons were compared between T5X and sham-operated intact rats.
Preparation of heart sections.
The left and right atria and large vessels were removed. The heart was washed in TPBS (3 × 10 min) then cryoprotected overnight in 30% sucrose (prepared in half-strength TPBS). Subsequently, the heart was embedded in optimal cutting temperature compound and sliced transversely from the apex to the base at 10-μm thickness with the use of a cryostat (42, 44). An interval of 300 μm was maintained between each section. All sections were thaw-mounted on Superfrost Plus slides and stained with Masson trichrome for quantitative analysis of LV chamber area, septal area, wall thickness, and collagen content (42, 44). Specifically, all histological sections were examined with an Olympus BH-2 microscope using a 1× objective for LV chamber area, septal area, and wall thickness and 100× objective for collagen content. Photomicrographs were obtained with a high-resolution digital camera and merged using Adobe PhotoShop CS2 (Adobe Systems, San Jose, CA). Our primary end points were LV chamber area, septal area, and wall thickness. To achieve this end point, every section was quantified from the digital photomicrographs using image analysis software (Image J 1.45s). Finally, the collagen volume fraction (area of the collagen/area of field of vision × 100%) was measured. Twelve separate areas of high-power fields (×100 in each section) were visualized under light microscope. The twelve sections from each rat were averaged and compared between intact and T5X rats (80). Adobe PhotoShop CS3 (Adobe Systems) was used to size and sharpen digital images, construct montages, adjust contrast and brightness, equalize illumination, and prepare figures.
Data Analysis
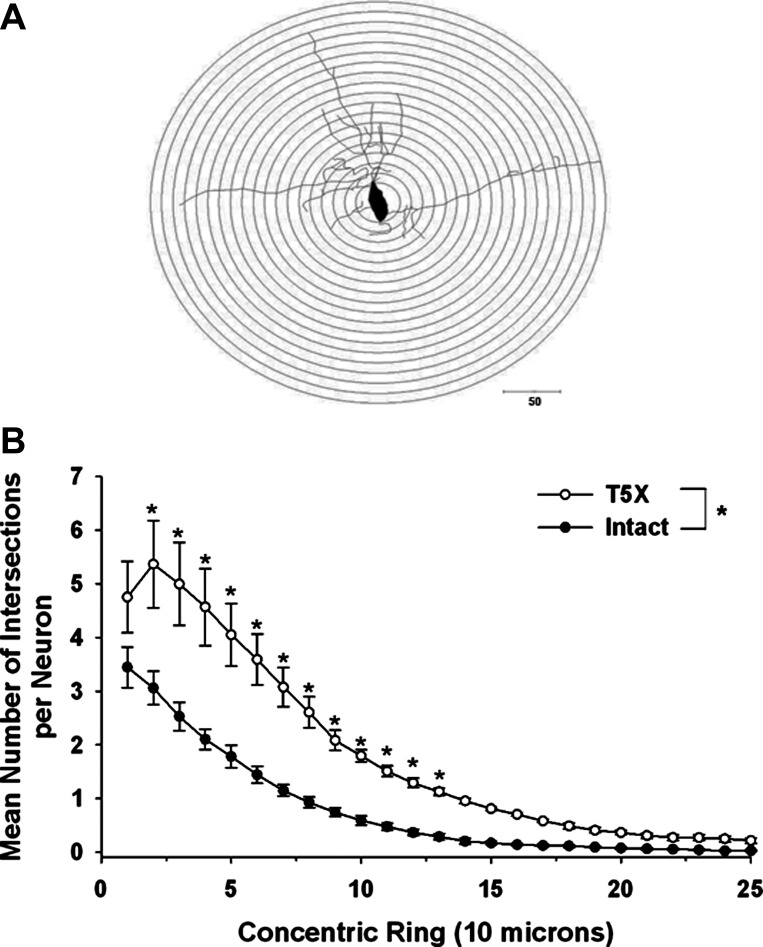
The Sholl analysis was evaluated using a two-way ANOVA with repeated measurements on one factor (branching order) on the dendritic intersections found at each concentric ring. Specifically, a two-way repeated measures ANOVA [group (sham T5X or T5X) × branching order] was applied to the numbers of dendrites according to their order of branching (Fig. 2). Post hoc Holm-Sidak analysis was used to document a significant difference between the two groups for every point.
Fig. 2.
Dendritic branching pattern of premotor cardioinhibitory vagal neurons located within the nucleus ambiguus from 6 intact (sham-operated) and 6 T5X rats was analyzed 28 days after T5X or sham transection, using the Sholl analysis of dendritic branching. A: a series of concentric rings calibrated at 10-μm intervals were superimposed on each neuron and centered on the cell body. B: no. of dendritic intersections within each concentric ring was counted, and the dendritic length was measured. The mean no. of intersections per neuron at each point (with the exception of the tails) was significantly increased in rats with midthoracic T5X. *P ≤ 0.05, intact vs. T5X.
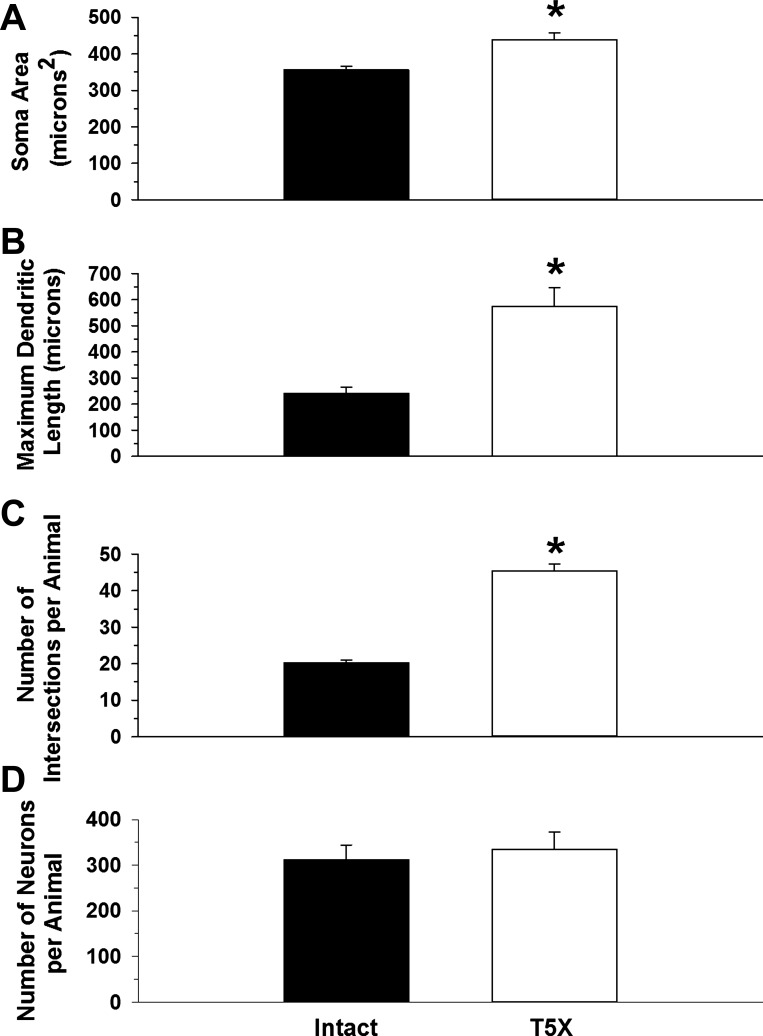
A Student unpaired t-test was used to compare premotor cardioinhibitory vagal neuron soma size, maximum dendritic length, number of intersections/animal (which represents the total dendritic field), and neuron number between sham-operated intact and T5 spinal cord-transected rats (Fig. 3).
Fig. 3.
Morphological features of premotor cardioinhibitory vagal neurons located within the nucleus ambiguus from 6 intact (sham-operated) and 6 T5X rats were analyzed using the Neurolucida software. Soma area (A), maximum dendritic length (B), no. of intersections/animal (C), and total no. of neurons (D) of premotor cardioinhibitory vagal neurons located within the nucleus ambiguus projecting to heart are presented. Soma area, maximum dendritic, length, and total dendritic field were increased in T5X rats. *P ≤ 0.05, intact vs T5X.
Finally, a Student unpaired t-test was used to compare LV chamber size, septal area, wall thickness, and collagen content between sham-operated intact and T5 spinal cord-transected rats (Fig. 4). For all comparisons, significance was set at P ≤ 0.05.
Fig. 4.
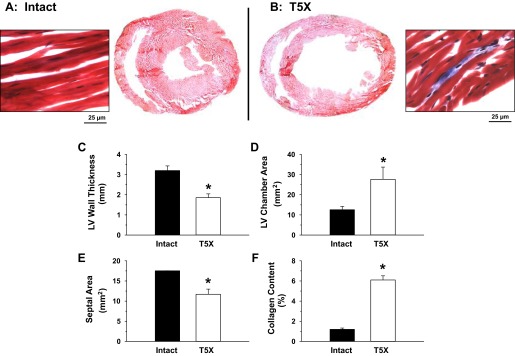
Morphological features of the heart from 6 intact (sham-operated) and 6 T5X rats were analyzed from the digital photomicrographs (A and B) using image analysis software (Image J 1.45s). Higher magnifications (×100, boxed areas) were analyzed to determine collagen content. Left ventricular (LV) wall thickness (C), chamber area (D), septal area (E), and collagen content (F) from the 6 transected rat hearts as well as the 6 hearts from the sham-transected rats are presented. LV chamber area and collagen content were significantly larger in spinal cord-transected rats. In contrast, LV wall thickness and septal area were smaller after spinal cord transection. *P ≤ 0.05, intact vs. T5X.
RESULTS
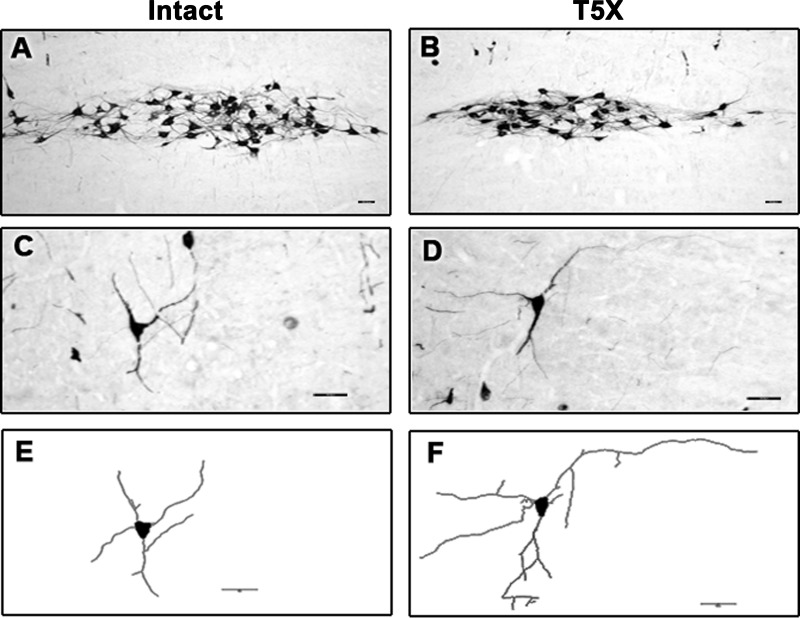
Photomicrographs of a 30-μm sagittal section through the right brainstem processed for CTB immunoreactivity from one sham-operated intact and one T5 spinal cord-transected rat are presented in Fig. 1, A and B, respectively. Higher-power photomicrographs (Fig. 1, C and D) from Fig. 1, A and B, show details of CTB-labeled neurons and dendritic branching. Note the extensive dendritic branching in the T5X rat compared with the intact rat. Finally, cell bodies and dendrites from Fig. 1, C and D, were reconstructed, and dendritic branching was assessed (Fig. 1, E and F).
Fig. 1.
Photomicrographs of a 30-μm sagittal section through the right brainstem processed for cholera toxin B subunit immunoreactivity (CTB-IR; black) from 1 sham-operated intact (A) and one T5 spinal cord transected rat (T5X; B) are presented. CTB was injected into the pericardial sac to retrogradely label premotor cardioinhibitory vagal neurons located within the nucleus ambiguus projecting to the heart. Higher-power photomicrographs (C and D) from A and B show details of CTB-labeled neurons and dendritic branching. Note the extensive dendritic branching in the T5X rat compared with the intact rat. Cell bodies and dendrites from C and D were reconstructed using the neuron-tracing feature on the Neurolucida system, and dendritic branching was assessed in the NeuroExplorer 3D visualization and morphometric analysis program included with the Neurolucida system (E and F, respectively). Scale bar, 50 μm.
Each neuron was analyzed using the Sholl analysis of dendritic branching, which assumes that dendritic arborization is an indirect measurement of available postsynaptic space. A series of concentric rings calibrated at 10-μm intervals were superimposed on each neuron and centered on the cell body (Fig. 2A). Intersections between dendrites and each concentric ring were then counted. For example, Fig. 2B presents the dendritic branching pattern of cardiac-projecting premotor cardioinhibitory vagal neurons located within the nucleus ambiguus from six intact and six T5X rats 28 days after T5X or sham transection. To identify premotor cardioinhibitory vagal neurons, CTB was injected into the pericardial space 21–25 days after T5X or sham transection to retrogradely label premotor cardioinhibitory vagal neurons projecting to the heart. Five to 7 days later the brainstems were harvested. This is an important procedure because only cardiac-projecting premotor cardioinhibitory vagal neurons were examined. Specifically, Fig. 2B presents the mean number of intersections per CTB-labeled, cardiac-projecting, premotor cardioinhibitory vagal neurons between each ring in a series of concentric rings. Premotor cardioinhibitory vagal neuron dendritic arborization was increased in T5X rats compared with sham-operated intact rats (Fig. 2B). Specifically, the mean number of intersections per neuron at each point (with the exception of the tails) was significantly increased in T5X rats.
Premotor cardioinhibitory vagal neuron soma area (Fig. 3A), maximum dendritic length (Fig. 3B), number of intersections/animal (Fig. 3C), and total number of neurons (Fig. 3D) are presented in Fig. 3. Premotor cardioinhibitory vagal neuron soma area (Fig. 3A), maximum dendritic length (Fig. 3B), and the total dendritic field (Fig. 3C) were increased in spinal cord-transected rats. There was no difference in the total number of labeled neurons (Fig. 3D).
Twenty-eight days (4 wk) after the T5X or sham transection, the hearts from six intact and six T5X rats were harvested, sectioned, and stained with Masson trichrome stain. Photomicrographs of heart sections from one spinal cord-transected rat and one sham-transected rat are presented in Fig. 4, A and B. Higher magnifications (×100) are shown in the boxed areas of this figure. Sections were processed with Masson trichrome stain. Note the small LV chamber area, the thick LV wall, and the absence of collagen (no blue stain) in the heart from the sham-transected rat. Figure 4B presents the heart from the spinal cord-transected rat. Note the large LV chamber area, the thin LV wall, and the presence of collagen (blue stain).
Figure 4 also presents the LV wall thickness (Fig. 4C), LV chamber area (Fig. 4D), septal area (Fig. 4E), and collagen content (Fig. 4F) from six intact and six spinal cord-transected rats 28 days after T5X or sham transection. LV chamber area was significantly larger in spinal cord-transected rats. In contrast, LV wall thickness and septal area were smaller after T5X. Finally collagen content was higher in spinal cord-transected rats.
DISCUSSION
In this study, the effects of midthoracic spinal cord injury on cardiac parasympathetic preganglionic neuron dendritic arborization and morphology as well as LV structural remodeling were determined. For the evaluation of cardiac parasympathetic preganglionic neuron dendritic arborization, sham-operated intact rats were compared with T5X rats (4 wk post-T5 spinal cord transection or sham transection). Following T5 spinal cord transection, there was a significant increase in cardiac parasympathetic preganglionic neuron dendritic arborization (Fig. 2) and morphology (Fig. 3). This parasympathetic structural remodeling is similar to that reported for cardiac sympathetic innervation following T5X. Specifically, after T5X, sympathetic hyperinnervation consisting of a significant increase in cardiac sympathetic preganglionic (45) and postganglionic neuron (43) dendritic arborization and increased LV sympathetic innervation density has been reported.
For the evaluation of LV structural remodeling, the same sham-operated intact rats were compared with T5X rats (4 wk post-T5 spinal cord transection or sham transection). Following T5 spinal cord transection, there was profound LV structural remodeling. Specifically, T5 spinal cord transection increased LV chamber area and collagen content as well as reduced LV wall thickness and septal area (Fig. 4). This cardiac structural remodeling is similar to that reported following a myocardial infarction (42, 44). Specifically, after a chronic myocardial infarction, cardiac structural remodeling consisting of chamber dilatation and wall thinning in the infarcted region has been reported (46, 55, 58).
The increased cardiac parasympathetic preganglionic neuron dendritic arborization (Fig. 2) and morphology (Fig. 3) was an unexpected finding because in sympathetic circuits the size of the soma and the complexity of the dendrites correlate with the extent of the terminal fields and the number of synaptic inputs, respectively, (59). However, T5X is associated with reduced cardiac parasympathetic drive (40). Thus, we were expecting shrunken parasympathetic preganglionic neurons. Consequently, our observations may indicate a difference between sympathetic and parasympathetic neurons. Alternatively, and in contrast, neurons with larger surface areas are less excitable due to a decrease in input resistance, an increased membrane capacitance, and a shorter length constant (the size principle) (4, 23, 28, 68, 70). Importantly, the vast majority of neuronal surface area is dendritic arborization (78). Accordingly, the larger soma and dendritic arborization may have reduced excitability and contribute in part to the reduced cardiac parasympathetic drive in animals with T5X (40).
Furthermore, the reduced cardiac parasympathetic drive, following T5X, may be due to changes at several sites along the innervation pathway. Specifically, the reduced cardiac parasympathetic drive may be mediated in part by reduced generation of vagal impulses within the nucleus ambiguus, reduced release of acetylcholine by preganglionic fibers, altered signaling at the level of nicotinic acetylcholine receptor, reduced release of acetylcholine by postganglionic fibers, enhanced acetylcholinesterase activity, reduced density of and/or binding to M2 muscarinic receptors, or altered intracellular signaling pathways (5). In addition, hypertrophy of vagal postganglionic neurons in cardiac ganglia could account for the parasympathetic withdrawal that accompanies T5X (68). In fact, reduced cardiac parasympathetic drive in individuals with heart failure is reportedly mediated by reduced signaling at the level of the nicotinic acetylcholine receptor (5) as well as hypertrophy of vagal postganglionic neurons in cardiac ganglia (68). Taken together, the results from this study suggest that the increase in cardiac parasympathetic preganglionic neuron dendritic arborization (Fig. 2) and morphology (Fig. 3), following T5 spinal cord transection, is a compensatory response to, rather than a cause for, the reduced cardiac parasympathetic drive (40), although this suggestion requires further investigation.
It is also important to consider that individuals with spinal cord injury above the sixth thoracic level experience episodic bouts of severe hypertension as part of a condition termed autonomic dysreflexia (AD) (22, 36). Many of these same individuals also experience episodes of low blood pressure due to orthostatic hypotension (OH) (11). Episodes of AD and OH frequently occur numerous times per day, such that blood pressure fluctuates dramatically (1). The effects of such pronounced changes in blood pressure on autonomic structural and functional remodeling in individuals with spinal cord injury are unknown and may account in part for the findings reported in this study. Finally, it is well documented that similar changes in cardiac structure are reported in inactive humans following bed rest (34, 57), space flight (56), and spinal cord injury (16, 18, 26, 27, 49). However, the influence of inactivity on the reported results was not investigated and remains unknown but merits future investigation.
As discussed above, the mechanisms mediating the observed changes are not established in this work. However, the observations are important, and several interesting hypotheses and future experiments to address this critical issue are discussed. In this context, midthoracic spinal cord injury is associated with enhanced cardiac sympathetic function and reduced cardiac parasympathetic function. This altered cardiac autonomic function may be due in part to a baroreflex-mediated response following the hypotension that occurs due to loss of sympathetic vasoconstrictor tone below the site of the lesion. The reduced parasympathetic drive and the enhanced sympathetic drive may subsequently mediate the structural changes in the autonomic nervous system, which in turn alters the structure and function of the heart. This activity-dependent neuroplasticity hypothesis, mediated by hypotension-induced baroreflex unloading, merits further investigation.
Importantly, the results from this study document a dynamic interaction between the heart and its parasympathetic innervation. This interaction between the heart and its parasympathetic innervation was associated with profound LV remodeling. However, it is unknown whether the neuroplastic changes in cardiac parasympathetic innervation are a cause or a consequence of the profound LV remodeling. However, reduced vagal activity has been documented to promote progressive left ventricular structural remodeling (64, 65). Furthermore, reducing heart rate improves survival and attenuates the progression of LV remodeling in animals with heart failure (10, 72). Finally, vagal nerve stimulation prevents sudden cardiac death in dogs with myocardial infarction and improves survival in rats with chronic heart failure (35, 73). Thus, although additional work is required before it can be concluded whether the parasympathetic structural neuroplasticity is a cause or a consequence of the profound LV remodeling, it is likely that the autonomic dysregulation mediated the target tissue changes. Nevertheless, a similar dynamic interaction between the heart and its sympathetic innervation following T5X has been documented. Specifically, T5X increased cardiac sympathetic tonus (40), altered cardiac electrophysiology (14, 63), and increased cardiac sympathetic preganglionic and postganglionic neuron dendritic arborization (40, 45).
Perspective
Using a combination of techniques and lines of evidence, we documented that midthoracic spinal cord injury results in cardiac parasympathetic and cardiac structural remodeling. These results have important implications for understanding the mechanisms responsible for the high mortality rates and incidence of cardiovascular disease in individuals with spinal cord injury (9, 17, 32, 76, 77) as well as other pathophysiological conditions that are associated with low cardiac parasympathetic drive (64, 65).
Results from this study, as well as from a number of other studies (15, 69, 81), document a dynamic interaction between the target tissue and its innervation. In this context, pathological plasticity following spinal cord injury is associated with many lingering complications, such as increased susceptibility to life-threatening, sustained, ventricular arrhythmias, chronic pain, spasticity, neurogenic bladder, and autonomic dysreflexia (3, 7, 8, 19, 24, 25, 29, 30, 38, 40, 41, 45, 53, 54, 66, 79).
Although novel insights have been made, critical questions remain, and a number of potential mechanisms must be identified. For example, further examination of parasympathetic neuroplasticity within distinct and diverse regions of the myocardium as well as potential changes along the innervation pathway are expected to provide important insights. In addition, determining whether the changes in cardiac parasympathetic innervation are a cause or a consequence of the profound LV remodeling merits further investigation. However, although clinically relevant and physiologically interesting questions remain, important new insights into the dynamic interaction between the heart and its parasympathetic innervation following midthoracic spinal cord injury are reported.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grant HL-088615.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
H.L.L., H.J., and S.E.D. conception and design of research; H.L.L., H.J., and S.E.D. performed experiments; H.L.L., H.J., and S.E.D. analyzed data; H.L.L., H.J., and S.E.D. interpreted results of experiments; H.L.L., H.J., and S.E.D. prepared figures; H.L.L. and S.E.D. drafted manuscript; H.L.L. and S.E.D. edited and revised manuscript; H.L.L., H.J., and S.E.D. approved final version of manuscript.
REFERENCES
- 1.Alan N, Ramer LM, Inskip JA, Golbidi S, Ramer MS, Laher I, Krassioukov AV. Recurrent autonomic dysreflexia exacerbates vascular dysfunction after spinal cord injury. Spine J 10: 1108–1117, 2010 [DOI] [PubMed] [Google Scholar]
- 2.Ardell JL, Randall WC. Selective vagal innervation of sinoatrial and atrioventricular nodes in canine heart. Am J Physiol Heart Circ Physiol 251: H764–H773, 1986 [DOI] [PubMed] [Google Scholar]
- 3.Arnold JM, Feng QP, Delaney GA, Teasell RW. Autonomic dysreflexia in tetraplegic patients: evidence for alpha-adrenoceptor hyper-responsiveness. Clin Auton Res 5: 267–270, 1995 [DOI] [PubMed] [Google Scholar]
- 4.Arora RC, Cardinal R, Smith FM, Ardell JL, Dell'Italia LJ, Armour JA. Intrinsic cardiac nervous system in tachycardia induced heart failure. Am J Physiol Regul Integr Comp Physiol 285: R1212–R1223, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Bibevski S, Dunlap ME. Evidence for impaired vagus nerve activity in heart failure. Heart Fail Rev 16: 129–135, 2011 [DOI] [PubMed] [Google Scholar]
- 6.Bluemel KM, Wurster RD, Randall WC, Duff MJ, O'Toole MF. Parasympathetic postganglionic pathways to the sinoatrial node. Am J Physiol Heart Circ Physiol 259: H1504–H1510, 1990 [DOI] [PubMed] [Google Scholar]
- 7.Brock JA, Yeoh M, McLachlan EM. Enhanced neurally evoked responses and inhibition of norepinephrine reuptake in rat mesenteric arteries after spinal transection. Am J Physiol Heart Circ Physiol 290: H398–H405, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Brown A, Weaver LC. The dark side of neuroplasticity. Exp Neurol 235: 133–141, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Disease Control. Cardiovascular-cardiopulmonary secondary disabilities. First Colloquim on Preventing Secondary Disabilities Among People With Spinal Cord Injuries. Atlanta, GA: Centers for Disease Control, 1991, p. 47–54 [Google Scholar]
- 10.Cheng Y, George I, Yi GH, Reiken S, Gu A, Tao YK, Muraskin J, Qin S, He KL, Hay I, Yu K, Oz MC, Burkhoff D, Holmes J, Wang J. Bradycardic therapy improves left ventricular function and remodeling in dogs with coronary embolization-induced chronic heart failure. J Pharmacol Exp Ther 321: 469–476, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Claydon VE, Krassioukov AV. Orthostatic hypotension and autonomic pathways after spinal cord injury. J Neurotrauma 23: 1713–1725, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Collins HL, DiCarlo SE. Acute exercise reduces the response to colon distension in T5 spinal rats. Am J Physiol Heart Circ Physiol 282: H1566–H1570, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Collins HL, DiCarlo SE. TENS attenuates response to colon distension in paraplegic and quadriplegic rats. Am J Physiol Heart Circ Physiol 283: H1734–H1739, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Collins HL, Rodenbaugh DW, DiCarlo SE. Spinal cord injury alters cardiac electrophysiology and increases the susceptibility to ventricular arrhythmias. Prog Brain Res 152: 275–288, 2006 [DOI] [PubMed] [Google Scholar]
- 15.de Groat WC, Kawatani M, Hisamitsu T, Cheng CL, Ma CP, Thor K, Steers W, Roppolo JR. Mechanisms underlying the recovery of urinary bladder function following spinal cord injury. J Auton Nerv Syst 30 Suppl: S71–S77, 1990 [DOI] [PubMed] [Google Scholar]
- 16.de Groot PC, van Dijk A, Dijk E, Hopman MT. Preserved cardiac function after chronic spinal cord injury. Arch Phys Med Rehabil 87: 1195–1200, 2006 [DOI] [PubMed] [Google Scholar]
- 17.DeVivo MJ, Black KJ, Stover SL. Causes of death during the first 12 years after spinal cord injury. Arch Phys Med Rehabil 74: 248–254, 1993 [PubMed] [Google Scholar]
- 18.Eysmann SB, Douglas PS, Katz SE, Sarkarati M, Wei JY. Left ventricular mass and diastolic filling patterns in quadriplegia and implications for effects of normal aging on the heart. Am J Cardiol 75: 201–203, 1995 [DOI] [PubMed] [Google Scholar]
- 19.Fouad K, Pedersen V, Schwab ME, Brosamle C. Cervical sprouting of corticospinal fibers after thoracic spinal cord injury accompanies shifts in evoked motor responses. Curr Biol 11: 1766–1770, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Gatti PJ, Johnson TA, Massari VJ. Can neurons in the nucleus ambiguus selectively regulate cardiac rate and atrio-ventricular conduction? J Auton Nerv Syst 57: 123–127, 1996 [DOI] [PubMed] [Google Scholar]
- 21.Geis WP, Kaye MP, Randall WC. Major autonomic pathways to the atria and S-A and A-V nodes of the canine heart. Am J Physiol 224: 202–208, 1973 [DOI] [PubMed] [Google Scholar]
- 22.Helkowski WM, Ditunno JF, Jr, Boninger M. Autonomic dysreflexia: incidence in persons with neurologically complete and incomplete tetraplegia. J Spinal Cord Med 26: 244–247, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Henneman E, Somjen G, Carpenter DO. Functional significance of cell size in spinal motoneurons. J Neurophysiol 28: 560–580, 1965 [DOI] [PubMed] [Google Scholar]
- 24.Hill CE, Beattie MS, Bresnahan JC. Degeneration and sprouting of identified descending supraspinal axons after contusive spinal cord injury in the rat. Exp Neurol 171: 153–169, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Hou S, Duale H, Cameron AA, Abshire SM, Lyttle TS, Rabchevsky AG. Plasticity of lumbosacral propriospinal neurons is associated with the development of autonomic dysreflexia after thoracic spinal cord transection. J Comp Neurol 509: 382–399, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huonker M, Schmid A, Sorichter S, Schmidt-Trucksab A, Mrosek P, Keul J. Cardiovascular differences between sedentary and wheelchair-trained subjects with paraplegia. Med Sci Sports Exerc 30: 609–613, 1998 [DOI] [PubMed] [Google Scholar]
- 27.Kessler KM, Pina I, Green B, Burnett B, Laighold M, Bilsker M, Palomo AR, Myerburg RJ. Cardiovascular findings in quadriplegic and paraplegic patients and in normal subjects. Am J Cardiol 58: 525–530, 1986 [DOI] [PubMed] [Google Scholar]
- 28.Koester J. Local signaling: passive electrical properties of the neuron. In: Principles of Neural Science, edited by Kandel ER, Schwartz JH, Jessell TM. New York: McGraw-Hill, 2000, p. 136–143 [Google Scholar]
- 29.Krassioukov AV, Weaver LC. Reflex and morphological changes in spinal preganglionic neurons after cord injury in rats. Clin Exp Hypertens 17: 361–373, 1995 [DOI] [PubMed] [Google Scholar]
- 30.Krenz NR, Meakin SO, Krassioukov AV, Weaver LC. Neutralizing intraspinal nerve growth factor blocks autonomic dysreflexia caused by spinal cord injury. J Neurosci 19: 7405–7414, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.La Rovere MT, Bigger JT, Jr, Marcus FI, Mortara A, Schwartz PJ. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) Investigators. Lancet 351: 478–84, 1998 [DOI] [PubMed] [Google Scholar]
- 32.Le CT, Price M. Survival from spinal cord injury. J Chronic Dis 35: 487–492, 1982 [DOI] [PubMed] [Google Scholar]
- 33.Lechat P, Hulot JS, Escolano S, Mallet A, Leizorovicz A, Werhlen-Grandjean M, Pochmalicki G, Dargie H. Heart rate and cardiac rhythm relationships with bisoprolol benefit in chronic heart failure in CIBIS II Trial. Circulation 103: 1428–1433, 2001 [DOI] [PubMed] [Google Scholar]
- 34.Levine BD, Zuckerman JH, Pawelczyk JA. Cardiac atrophy after bed-rest deconditioning: a nonneural mechanism for orthostatic intolerance. Circulation 96: 517–525, 1997 [DOI] [PubMed] [Google Scholar]
- 35.Li M, Zheng C, Sato T, Kawada T, Sugimachi M, Sunagawa K. Vagal nerve stimulation markedly improves long-term survival after chronic heart failure in rats. Circulation 109: 120–124, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Lindan R, Joiner E, Freehafer AA, Hazel C. Incidence and clinical features of autonomic dysreflexia in patients with spinal cord injury. Paraplegia 18: 285–292, 1980 [DOI] [PubMed] [Google Scholar]
- 37.Llewellyn-Smith IJ, DiCarlo SE, Collins HL, Keast JR. Enkephalin-immunoreactive interneurons extensively innervate sympathetic preganglionic neurons regulating the pelvic viscera. J Comp Neurol 488: 278–289, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Llewellyn-Smith IJ, Weaver LC. Changes in synaptic inputs to sympathetic preganglionic neurons after spinal cord injury. J Comp Neurol 435: 226–240, 2001 [DOI] [PubMed] [Google Scholar]
- 39.Llewellyn-Smith I, Martin C, Fenwick N, DiCarlo S, Lujan H, Schreihofer A. VGLUT1 and VGLUT2 innvervation in autonomic regions of intact and transected rat spinal cord. J Comp Neurol 503: 741–767, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Lujan HL, Chen Y, DiCarlo SE. Paraplegia increased cardiac NGF content, sympathetic tonus, and the susceptibility to ischemia-induced ventricular tachycardia in conscious rats. Am J Physiol Heart Circ Physiol 296: H1364–H1372, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lujan HL, DiCarlo SE. T5 spinal cord transection increases susceptibility to reperfusion-induced ventricular tachycardia by enhancing sympathetic activity in conscious rats. Am J Physiol Heart Circ Physiol 293: H3333–H3339, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Lujan HL, DiCarlo SE. Cardiac output, at rest and during exercise, before and during myocardial ischemia, reperfusion, and infarction in conscious mice. Am J Physiol Regul Integr Comp Physiol 304: R286–R295, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lujan HL, Janbaih H, DiCarlo SE. Dynamic interaction between the heart and its sympathetic innervation following T5 spinal cord transection. J Appl Physiol 113: 1332–1341, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lujan HL, Janbaih H, Feng HZ, Jin JP, DiCarlo SE. Myocardial ischemia, reperfusion, and infarction in chronically instrumented, intact, conscious, and unrestrained mice. Am J Physiol Regul Integr Comp Physiol 302: R1384–R1400, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lujan HL, Palani G, DiCarlo SE. Structural neuroplasticity following T5 spinal cord transection: increased cardiac sympathetic innervation density and SPN arborization. Am J Physiol Regul Integr Comp Physiol 299: R985–R995, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lutgens E, Daemen MJ, de Muinck ED, Debets J, Leenders P, Smits JF. Chronic myocardial infarction in the mouse: cardiac structural and functional changes. Cardiovasc Res 41: 586–593, 1999 [DOI] [PubMed] [Google Scholar]
- 47.Mendelowitz D. Advances in Parasympathetic Control of Heart Rate and Cardiac Function. News Physiol Sci 14: 155–161, 1999 [DOI] [PubMed] [Google Scholar]
- 48.Mendelowitz D, Kunze DL. Identification and dissociation of cardiovascular neurons from the medulla for patch clamp analysis. Neurosci Lett 132: 217–221, 1991 [DOI] [PubMed] [Google Scholar]
- 49.Nash MS, Bilsker S, Marcillo AE, Isaac SM, Botelho LA, Klose KJ, Green BA, Rountree MT, Shea JD. Reversal of adaptive left ventricular atrophy following electrically-stimulated exercise training in human tetraplegics. Paraplegia 29: 590–599, 1991 [DOI] [PubMed] [Google Scholar]
- 50.Nelson AJ, Iwamoto GA. Reversibility of exercise-induced dendritic attenuation in brain cardiorespiratory and locomotor areas following exercise detraining. J Appl Physiol 101: 1243–1251, 2006 [DOI] [PubMed] [Google Scholar]
- 51.Nelson AJ, Juraska JM, Musch TI, Iwamoto GA. Neuroplastic adaptations to exercise: neuronal remodeling in cardiorespiratory and locomotor areas. J Appl Physiol 99: 2312–2322, 2005 [DOI] [PubMed] [Google Scholar]
- 52.Nelson AJ, Juraska JM, Ragan BG, Iwamoto GA. Effects of exercise training on dendritic morphology in the cardiorespiratory and locomotor centers of the mature rat brain. J Appl Physiol 108: 1582–1590, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ondarza AB, Ye Z, Hulsebosch CE. Direct evidence of primary afferent sprouting in distant segments following spinal cord injury in the rat: colocalization of GAP-43 and CGRP. Exp Neurol 184: 373–380, 2003 [DOI] [PubMed] [Google Scholar]
- 54.Pan B, Kim EJ, Schramm LP. Increased close appositions between corticospinal tract axons and spinal sympathetic neurons after spinal cord injury in rats. J Neurotrauma 22: 1399–1410, 2005 [DOI] [PubMed] [Google Scholar]
- 55.Patten RD, Aronovitz MJ, Deras-Mejia L, Pandian NG, Hanak GG, Smith JJ, Mendelsohn ME, Konstam MA. Ventricular remodeling in a mouse model of myocardial infarction. Am J Physiol Heart Circ Physiol 274: H1812–H1820, 1998 [DOI] [PubMed] [Google Scholar]
- 56.Perhonen MA, Franco F, Lane LD, Buckey JC, Blomqvist CG, Zerwekh JE, Peshock RM, Weatherall PT, Levine BD. Cardiac atrophy after bed rest and spaceflight. J Appl Physiol 91: 645–653, 2001 [DOI] [PubMed] [Google Scholar]
- 57.Perhonen MA, Zuckerman JH, Levine BD. Deterioration of left ventricular chamber performance after bed rest: “cardiovascular deconditioning” or hypovolemia? Circulation 103: 1851–1857, 2001 [DOI] [PubMed] [Google Scholar]
- 58.Pfeffer JM, Pfeffer MA, Fletcher PJ, Braunwald E. Progressive ventricular remodeling in rat with myocardial infarction. Am J Physiol Heart Circ Physiol 260: H1406–H1414, 1991 [DOI] [PubMed] [Google Scholar]
- 59.Purves D, Rubin E, Snider WD, Lichtman J. Relation of animal size to convergence, divergence, and neuronal number in peripheral sympathetic pathways. J Neurosci 6: 158–163, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Purves D, Snider WD, Voyvodic JT. Trophic regulation of nerve cell morphology and innervation in the autonomic nervous system. Nature 336: 123–128, 1988 [DOI] [PubMed] [Google Scholar]
- 61.Rodenbaugh DW, Collins HL, DiCarlo SE. Increased susceptibility to ventricular arrhythmias in hypertensive paraplegic rats. Clin Exp Hypertens 25: 349–358, 2003 [DOI] [PubMed] [Google Scholar]
- 62.Rodenbaugh DW, Collins HL, DiCarlo SE. Paraplegia differentially increases arterial blood pressure related cardiovascular disease risk factors in normotensive and hypertensive rats. Brain Res 980: 242–248, 2003 [DOI] [PubMed] [Google Scholar]
- 63.Rodenbaugh DW, Collins HL, Nowacek DG, DiCarlo SE. Increased susceptibility to ventricular arrhythmias is associated with changes in Ca2+ regulatory proteins in paraplegic rats. Am J Physiol Heart Circ Physiol 285: H2605–H2613, 2003 [DOI] [PubMed] [Google Scholar]
- 64.Sabbah HN, Shimoyama H, Kono T, Gupta RC, Sharov VG, Scicli G, Levine TB, Goldstein S. Effects of long-term monotherapy with enalapril, metoprolol, and digoxin on the progression of left ventricular dysfunction and dilation in dogs with reduced ejection fraction. Circulation 89: 2852–2859, 1994 [DOI] [PubMed] [Google Scholar]
- 65.Sabbah HN, Stanley WC, Sharov VG, Mishima T, Tanimura M, Benedict CR, Hegde S, Goldstein S. Effects of dopamine beta-hydroxylase inhibition with nepicastat on the progression of left ventricular dysfunction and remodeling in dogs with chronic heart failure. Circulation 102: 1990–1995, 2000 [DOI] [PubMed] [Google Scholar]
- 66.Seki S, Sasaki K, Fraser MO, Igawa Y, Nishizawa O, Chancellor MB, de Groat WC, Yoshimura N. Immunoneutralization of nerve growth factor in lumbosacral spinal cord reduces bladder hyperreflexia in spinal cord injured rats. J Urol 168: 2269–2274, 2002 [DOI] [PubMed] [Google Scholar]
- 67.Sholl DA. Dendritic organization in the neurons of the visual and motor cortices of the cat. J Anat 87: 387–406, 1953 [PMC free article] [PubMed] [Google Scholar]
- 68.Singh S, Sayers S, Walter JS, Thomas D, Dieter RS, Nee LM, Wurster RD. Hypertrophy of neurons within cardiac Ganglia in human, canine, and rat heart failure: the potential role of nerve growth factor. J Am Heart Assoc 2: e000210, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Steers WD, Ciambotti J, Erdman S, de Groat WC. Morphological plasticity in efferent pathways to the urinary bladder of the rat following urethral obstruction. J Neurosci 10: 1943–1951, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Steinback CD, Salmanpour A, Breskovic T, Dujic Z, Shoemaker JK. Sympathetic neural activation: an ordered affair. J Physiol 588: 4825–4836, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Strack AM, Sawyer WB, Marubio LM, Loewy AD. Spinal origin of sympathetic preganglionic neurons in the rat. Brain Res 455: 187–191, 1988 [DOI] [PubMed] [Google Scholar]
- 72.Swedberg K, Komajda M, Böhm M, Borer JS, Ford I, Dubost-Brama A, Lerebours G, Tavazzi L; SHIFT Investigators. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet 376: 875–885, 2010 [DOI] [PubMed] [Google Scholar]
- 73.Vanoli E, De Ferrari GM, Stramba-Badiale M, Hull SS, Jr, Foreman RD, Schwartz PJ. Vagal stimulation and prevention of sudden death in conscious dogs with a healed myocardial infarction. Circ Res 68: 1471–1481, 1991 [DOI] [PubMed] [Google Scholar]
- 74.Voyvodic JT. Peripheral target regulation of dendritic geometry in the rat superior cervical ganglion. J Neurosci 9: 1997–2010, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vyas A, Jadhav S, Chattarji S. Prolonged behavioral stress enhances synaptic connectivity in the basolateral amygdala. Neuroscience 143: 387–393, 2006 [DOI] [PubMed] [Google Scholar]
- 76.Whiteneck GG, Charlifue SW, Frankel HL, Fraser MH, Gardner BP, Gerhart KA, Krishnan KR, Menter RR, Nuseibeh I, Short DJ, Silver JR. Mortality, morbidity, and psychosocial outcomes of persons spinal cord injured more than 20 years ago. Paraplegia 30: 617–630, 1992 [DOI] [PubMed] [Google Scholar]
- 77.Wicks JR, Oldridge NB, Cameron NB, Jones NL. Arm cranking and wheelchair ergometry in elite spinal cord injured athletes. Med Sci Sports Excerc 15: 224–231, 1983 [PubMed] [Google Scholar]
- 78.Xi X, Randall WC, Wurster RD. Morphology of intracellularly labeled canine intracardiac ganglion cells. J Comp Neurol 314: 396–402, 1991 [DOI] [PubMed] [Google Scholar]
- 79.Yeoh M, McLachlan EM, Brock JA. Chronic decentralization potentiates neurovascular transmission in the isolated rat tail artery, mimicking the effects of spinal transection. J Physiol 561: 583–596, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yu Q, Li Q, Na R, Li X, Liu B, Meng L, Liutong H, Fang W, Zhu N, Zheng X. Impact of repeated intravenous bone marrow mesenchymal stem cells infusion on myocardial collagen network remodeling in a rat model of doxorubicin-induced dilated cardiomyopathy. Mol Cell Biochem 387: 279–285, 2014 [DOI] [PubMed] [Google Scholar]
- 81.Zucker IH, Patel KP, Schultz HD. Neurohumoral stimulation. Heart Fail Clin 8: 87–99, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]