Abstract
Exercise-induced hyperemia is often normalized for muscle mass, and this value is sometimes evaluated at relative exercise intensities to take muscle recruitment into account. Therefore, this study sought to better understand the impact of muscle mass on leg blood flow (LBF) during exercise. LBF was assessed by Doppler ultrasound in 27 young healthy male subjects performing knee-extensor (KE) exercise at three absolute (5, 15, and 25 W) and three relative [20, 40, and 60% of maximum KE (KEmax)] workloads. Thigh muscle mass (5.2–8.1 kg) and LBF were significantly correlated at rest (r = 0.54; P = 0.004). Exercise-induced hyperemia was linearly related to absolute workload, but revealed substantial between-subject variability, documented by the coefficient of variation (5 W: 17%; 15 W: 16%; 25 W: 16%). Quadriceps muscle mass (1.5–2.7 kg) and LBF were not correlated at 5, 15, or 25 W (r = 0.09–0.01; P = 0.7–0.9). Normalizing blood flow for quadriceps muscle mass did not improve the coefficient of variation at each absolute workload (5 W: 21%; 15 W: 21%; 25 W: 22%), while the additional evaluation at relative exercise intensities resulted in even greater variance (20% KEmax: 29%; 40% KEmax: 29%; 60% KEmax: 27%). Similar findings were documented when subjects were parsed into high and low aerobic capacity. Thus, in contrast to rest, blood flow during exercise is unrelated to muscle mass, and simply normalizing for muscle mass or comparing normalized blood flow at a given relative exercise intensity has no effect on the inherent blood flow variability. Therefore, during exercise, muscle mass does not appear to be a determinant of the hyperemic response.
Keywords: blood flow, muscle mass, knee extension, normalization
due to basic cellular requirements, skeletal muscle oxygen delivery and metabolism are tightly linked. Although skeletal muscle blood flow has the potential to be modulated by factors such as oxygen carrying capacity and tissue oxygen extraction, it is tightly related to muscle metabolism. Indeed, at rest, Dinenno et al. (7) identified a significant positive correlation between blood flow in the femoral artery and leg oxygen consumption (V̇o2). Therefore, under resting conditions, there is also likely a link between muscle mass and blood flow with a larger volume of muscle consuming more oxygen, although this may be influenced by muscle fiber type (4, 20). Thus, at rest, it seems reasonable to expect skeletal muscle blood flow to be dependent on muscle metabolic demand, which is, in turn, related to muscle mass.
The blood flow response to exercise appears to be dictated by the metabolic requirement to perform external work. This relationship was highlighted in the seminal study by Andersen and Saltin (2), which not only documented a tight relationship between leg blood flow (LBF) and oxygen demand, but also identified inherent intersubject variability in the blood flow response. The technique of normalizing blood flow to muscle mass during exercise is commonly utilized in an attempt to account for this variability, especially in studies where subject populations typically have a significantly different muscle mass (i.e., age or sex differences). For example, Donato et al. (8) reported that a potential reduction in exercising LBF, postulated to be due to aging, was abolished when blood flow was normalized for muscle mass. Another study, by Parker et al. (23), observed that a significant sex difference in exercising LBF was further augmented by muscle mass normalization. Thus some would argue that the between-subject variability in exercise-induced hyperemia can be explained, at least to some extent, by muscle mass. However, currently it is unknown whether normalizing blood flow to total muscle mass is an appropriate method to reduce this variability and perhaps allow a better comparison between subjects.
Studies examining quadriceps muscle recruitment during both cycling (28) and single-leg knee-extensor (KE) exercise (27) suggest that muscle recruitment is dependent on exercise intensity relative to peak workload. The potential impact of this recruitment pattern on blood flow was first postulated by Ray and Dudley (27), who suggested that muscle recruited during exercise is maximally perfused, and thus taking active muscle mass at a given absolute work rate into account would reduce the between-subject variability in exercise-induced hyperemia. Shortly thereafter, Radegran et al. (26) utilized this approach to examine the hyperemic response to submaximal and maximal exercise in healthy young subjects. If blood flow is, indeed, dictated by muscle recruitment, normalizing for muscle mass combined with exercise performed at a similar relative intensity may be the correct way to report exercise-induced skeletal muscle blood flow. Donato and colleagues (8) utilized this concept and reported that LBF per kilogram of muscle was reduced with age when examined at the same given relative intensity in both old and young subjects. Although a link between relative effort, muscle recruitment, and blood flow appears reasonable, this tenet has never been validated.
Consequently, there are uncertainties about the relationship between metabolic demand, muscle recruitment, blood flow, and the potential role of muscle mass as a modulator of exercise-induced hyperemia. Therefore, in a group of subjects uncomplicated by differences in sex and age, this study sought to better understand the impact of quadriceps muscle mass (QMM) on femoral artery blood flow during one-legged KE exercise at absolute and relative submaximal workloads. We hypothesized that, at rest, muscle mass would be related to blood flow, but muscle mass would have no effect on exercise-induced blood flow examined at either absolute or relative work rates. Also, the wide spectrum of physical activity exhibited by the subjects afforded the opportunity to examine the effect of muscle mass on blood flow in subjects with high and low aerobic capacity.
MATERIALS AND METHODS
Subjects.
Twenty-seven young, healthy men between the ages of 18–35 yr and free of overt cardiovascular or metabolic disease took part in the study. All procedures were approved by the Institutional Review Boards of the University of Utah and the Salt Lake City Veteran Affairs Medical Center, and written, informed consent was attained according to their guidelines.
Familiarization and KE maximum determination.
Initial visits to the laboratory involved familiarization with study equipment and the performance of a graded KE test on a single-leg KE ergometer (2) to determine maximum work rate (KEmax). Subjects were given a 2- to 3-min warm-up at a low submaximal workload, which was then followed by 5-W increases in the workload every minute until the subjects could no longer maintain 60 revolutions/min (17).
Experimental protocol.
Subjects reported to the laboratory for the experimental trial in a fasted state and having abstained from exercise for at least 24 h. The subjects performed three consecutive absolute workloads (5, 15, and 25 W), followed by a 5-min break, and then three consecutive relative workloads (20, 40, and 60% of the subject's KEmax). Each workload was performed for 3 min at 60 revolutions/min. Blood flow and mean arterial pressure were assessed before exercise and during the final minute of each 3-min workload when blood flow and pressure are in steady-state conditions (2, 34). Mean arterial pressure was determined with finger photoplethysmography (Finometer, Finapres Medical Systems, Amsterdam, The Netherlands).
LBF.
Measurements of common femoral artery (CFA) blood velocity and vessel diameter were performed on the exercising leg using a Logic 7 ultrasound Doppler system (General Electric Medical Systems, Milwaukee, WI). The Logic 7 was equipped with linear array transducers operating at an imaging frequency of 14 MHz. CFA diameter was determined at a perpendicular angle along the central axis of the scanned area, and blood velocity was measured using the same transducer with a frequency of 5 MHz. All blood velocity measurements were obtained with the probe appropriately positioned to maintain an insonation angle of 60° or less. The sample volume was maximized according to vessel size and was centered within the vessel based on real-time ultrasound visualization. Using CFA diameter and mean velocity (Vmean) (angle corrected and intensity weighted), blood flow was automatically calculated by commercially available software (Logic 7) as Vmeanπ (vessel diameter × 0.5)2 × 60, where blood flow is in ml/min.
Body composition.
Height and weight were measured with a standing scale (Seca, Birmingham, UK). Body mass index was calculated using these height and weight data. Whole body fat percentage and muscle mass were determined with a dual-energy X-ray absorptiometry (DEXA) scanner (Lunar Prodigy, General Electic Medical Systems, Milwaukee, WI). Additionally, utilizing the DEXA scan, thigh muscle mass was determined using the ischial tuberosity and the knee joint as the upper and lower margins. As the quadriceps are the primary muscle group used to perform KE exercise (29) and DEXA cannot differentiate between quadriceps and hamstring muscle mass, anthropometric measures were utilized to determine solely QMM. These anthropometric measures involved three thigh circumference measures, one length measure, and one thigh skinfold measure and utilized the prediction equation compiled by Jones and Pearson (11), as well as the correction equation reported by Radegran et al. (26) to more accurately predict muscle mass, based on computer tomography scans. Anthropometric measures of thigh volume, utilizing these equations, have been previously reported to be strongly correlated to the results attained both by DEXA (22) and magnetic resonance imaging (18). As anticipated, anthropometric measures utilized in this study were strongly correlated to DEXA measures (r = 0.9).
Maximal V̇o2.
A maximal oxygen uptake (V̇o2max) test was performed on an electronically braked cycle ergometer (Lode, The Netherlands), and pulmonary V̇o2 was measured continuously throughout the test (Parvomedics, Sandy, UT). The V̇o2max protocol consisted of a 1-min warm-up at 25 W and then an increase in 25 W each minute until exhaustion (1). Criteria for the attainment of V̇o2max were a respiratory exchange ratio of >1.1 and a maximal heart rate within 10 beats/min of the predicted value (220 − age), with the highest average 30-s V̇o2 reading before cessation of exercise, after the achievement of these criteria, considered to be V̇o2max.
Physical activity measures.
Subjects wore an accelerometer (GT1M; Actigraph, Pensacola, FL) for 7 days to monitor physical activity level. Daily physical activity was totaled, averaged, and expressed in total counts and total step counts per day. Further analysis was performed to classify average minutes per day in sedentary, light, moderate, vigorous, and very vigorous activity levels.
Blood analyses.
A prestudy blood draw was taken from the antecubital vein. Blood was analyzed for lipids, hemoglobin, glucose, and a complete blood cell count.
Aerobic capacity and physical activity assessment.
To evaluate aerobic capacity and physical activity as modulators of blood flow, subjects were parsed into groups of either high (n = 9) or low (n = 10) aerobic capacity. Relative V̇o2max values of ≤45 ml·kg−1·min−1 combined with KEmax values of ≤55 W were used to place individuals in the low aerobic capacity group, while relative V̇o2max values of ≥50 ml·kg−1·min−1 in combination with KEmax values of ≥60 W were used to categorize the high aerobic capacity group. Both accelerometer and self-report physical activity data were also utilized to confirm the appropriate placement of subjects in the high and low aerobic capacity groups. Subjects that did not meet these combined criteria, and therefore fell in between high and low aerobic capacity, were excluded from the analysis (n = 8).
Statistical analysis.
Pearson correlations were utilized to identify the effect of QMM on LBF at each absolute workload. To examine the variability of the blood flow responses, coefficients of variation (CV) were calculated at each absolute and relative workload with and without muscle mass normalization. To assess the effect of aerobic capacity on LBF responses, a 2 (aerobic capacity) × 3 (absolute workload) repeated-measures ANOVA was utilized. Additional Pearson correlations were performed on QMM and blood flow measures following the separation of subjects into high and low aerobic capacity. All data are presented as either individual data or means ± SD, and the level of significance for all tests was set a priori at α = 0.05.
RESULTS
Subjects.
Subject characteristics, including anthropometric data, body composition, blood analysis profile, aerobic capacity, and average daily physical activity levels for all subjects and subjects separated into low and high aerobic capacity groups, are described in Table 1.
Table 1.
Subject characteristics for all subjects and two subsets of these subjects parsed into low and high aerobic capacity
| All Subjects | Low Aerobic Capacity Group | High Aerobic Capacity Group | |
|---|---|---|---|
| n | 27 | 10 | 9 |
| Age, yr | 27 ± 4 | 26 ± 4 | 27 ± 4 |
| Height, cm | 178 ± 6 | 179 ± 6 | 177 ± 5 |
| Weight, kg | 77 ± 14 | 81 ± 16 | 72 ± 12 |
| Body mass index, kg/m2 | 24 ± 3 | 25 ± 3 | 23 ± 2 |
| Body fat, % | 19 ± 8 | 23 ± 9 | 14 ± 3 |
| Thigh volume, liters | 7 ± 1.1 | 7.1 ± 1.2 | 6.9 ± 0.7 |
| Quadriceps muscle mass, kg | 2.0 ± 0.3 | 2.1 ± 0.4 | 1.9 ± 0.2 |
| Thigh mass, kg | 6 ± 0.9 | 6 ± 0.9 | 6 ± 0.9 |
| Total leg mass, kg | 10 ± 1.2 | 10 ± 1.1 | 10 ± 1.3 |
| Mean arterial pressure, mmHg | 92 ± 1 | 101 ± 3 | 94 ± 3 |
| Hemoglobin, g/dl | 16 ± 1 | 16 ± 1 | 16 ± 1 |
| Cholesterol, mg/dl | 180 ± 41 | 196 ± 47 | 180 ± 38 |
| Triglycerides, mg/dl | 121 ± 80 | 124 ± 69 | 122 ± 105 |
| HDL, mg/dl | 46 ± 11 | 46 ± 12 | 47 ± 9 |
| LDL, mg/dl | 116 ± 35 | 130 ± 40 | 117 ± 36 |
| V̇o2max, ml·kg−1·min−1 | 47 ± 10 | 39 ± 7 | 57 ± 7* |
| Maximum knee extensor, W | 58 ± 16 | 46 ± 7 | 71 ± 12* |
| Steps/day | 8,197 ± 3,790 | 6,598 ± 2,276 | 10,208 ± 5,240 |
| Sedentary, min/day | 1,234 ± 71 | 1,264 ± 82 | 1,205 ± 56 |
| Light activity, min/day | 123 ± 46 | 123 ± 62 | 127 ± 41 |
| Moderate activity, min/day | 43 ± 16 | 34 ± 16 | 47 ± 10* |
| Vigorous activity, min/day | 6 ± 6 | 5 ± 5 | 10 ± 8 |
| Very vigorous activity, min/day | 5 ± 16 | 1 ± 2 | 13 ± 26 |
Values are means ± SD; n, no. of subjects. V̇o2max, maximal oxygen uptake.
Significantly different than low aerobic capacity group, P < 0.05.
LBF.
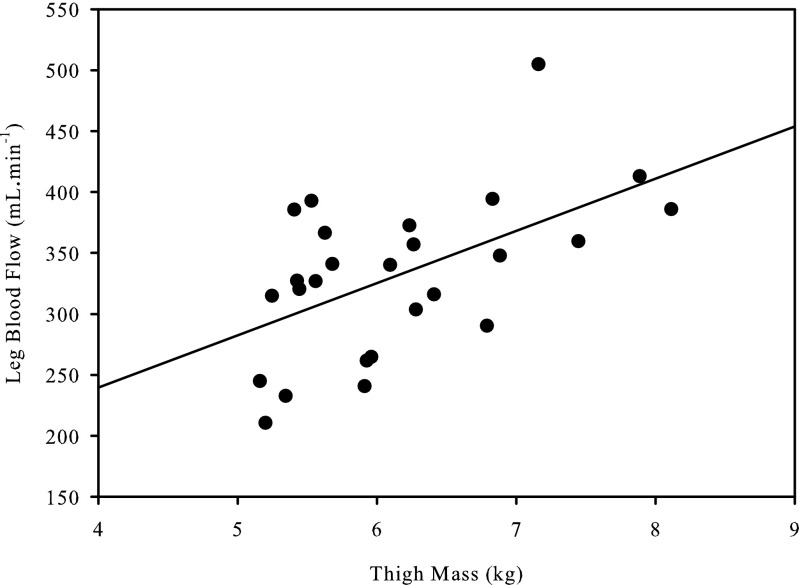
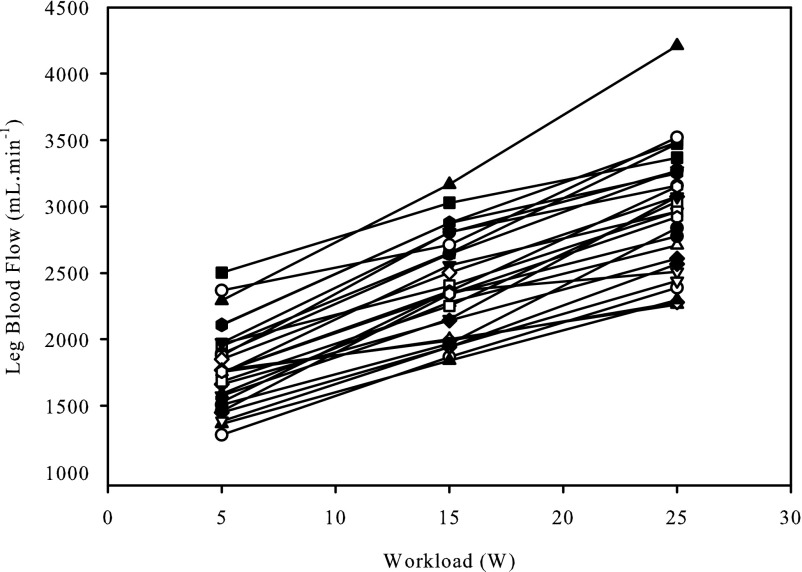
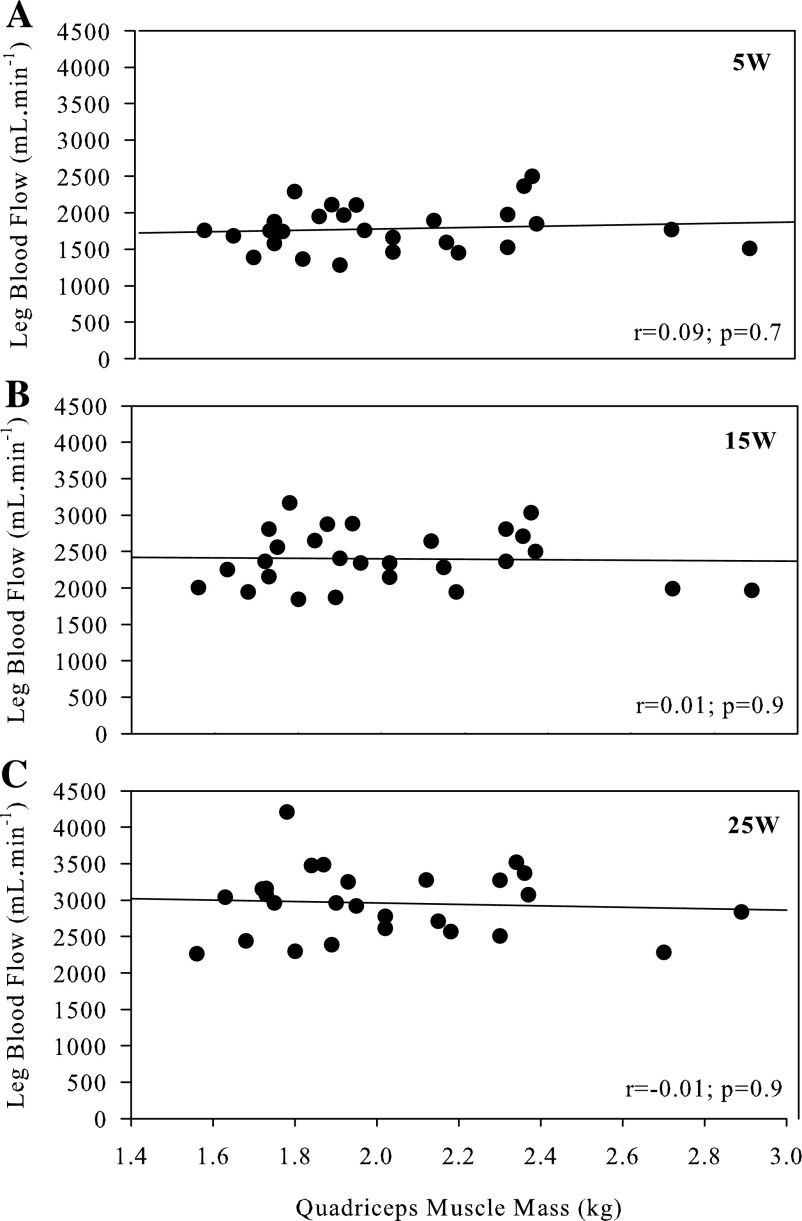
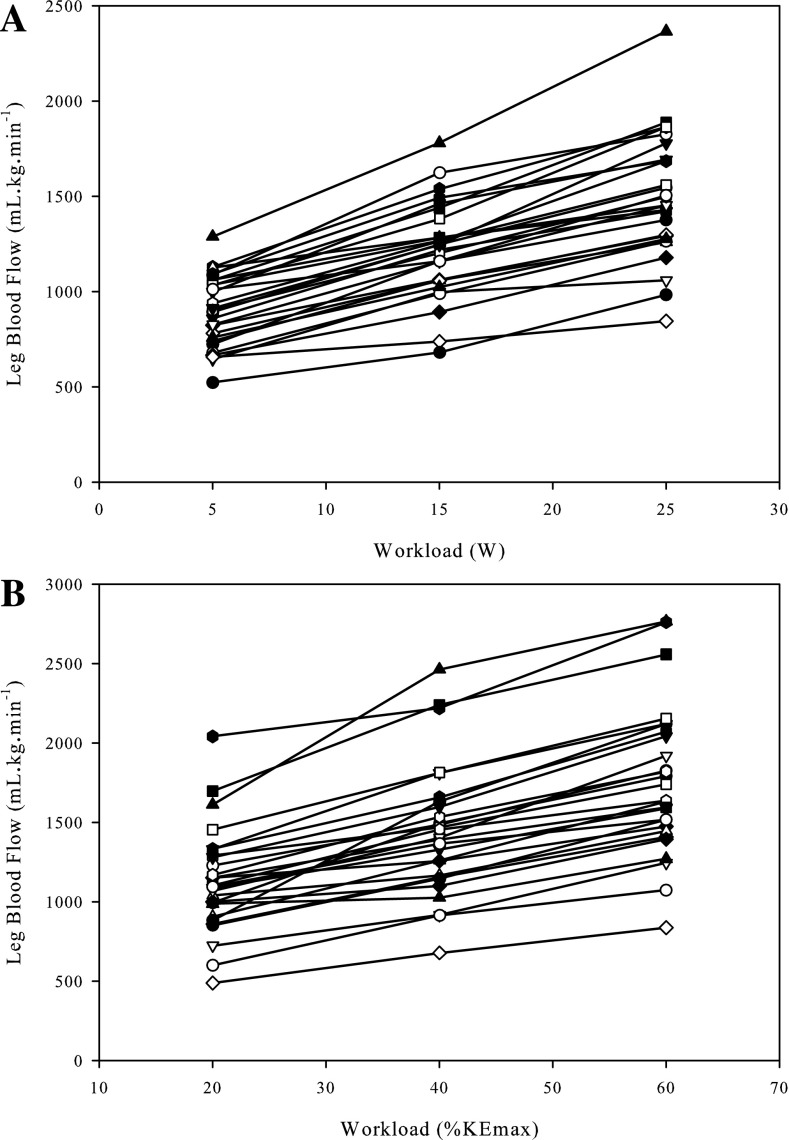
Thigh mass and LBF were significantly correlated at rest (r = 0.54; P < 0.01), (Fig. 1). There was a strong relationship between LBF and absolute KE workload (y = 58x − 1,500; r = 0.99 ± 0.02; SD of slope = 15; SD of intercept = 308); however, there was substantial variability between subjects at each absolute workload as assessed by the CV (5 W: 17%; 15 W: 16%; 25 W: 16%) (Fig. 2). During exercise, QMM and LBF were not significantly correlated (Fig. 3) at 5, 15, and 25 W. Following normalization for QMM, the CV at each absolute workload (5 W: 21%; 15 W: 21%; 25 W: 22%) were unchanged (Fig. 4A). The CV of LBF normalized for muscle mass assessed during the relative workloads were all significantly greater than the previous assessments of blood flow variability (20% KEmax: 33%; 40% KEmax: 30%; 60% KEmax: 30%) (Fig. 4B).
Fig. 1.
Correlation between thigh muscle mass measured by dual-energy x-ray absorptiometry and resting leg blood flow.
Fig. 2.
Individual exercise-induced leg blood flow responses across multiple absolute workloads.
Fig. 3.
Correlation between exercise-induced leg blood flow and quadriceps muscle mass at three absolute workloads: 5 W (A), 15 W (B), and 25 W (C).
Fig. 4.
Individual exercise-induced leg blood flow responses normalized to quadriceps muscle mass across absolute and relative work rates. A: blood flow per unit of muscle across absolute knee extensor work rates. B: blood flow per unit of muscle across work rate relative to knee extensor maximum work rate.
Impact of aerobic fitness and activity level.
Eight subjects whose aerobic capacity and activity level were determined to be neither high nor low were excluded from this analysis. By experimental design, those selected for the high aerobic capacity group (n = 9) had significantly greater KEmax (P = 0.001), V̇o2max (P = 0.001), and minutes per day of moderate intensity physical activity (P = 0.03) compared with the low aerobic capacity group (n = 10) (Table 1). Further analysis revealed no significant differences between groups in terms of stature, body composition, blood profile, or average daily physical activity data. LBF (F = 2.17; P = 0.16) compared at absolute KE work rates were not different between the subjects with high and low aerobic capacity. CV for LBF in the high aerobic capacity (5 W: 18%; 15 W: 14%; 25 W: 14%) and low aerobic capacity groups (5 W: 18%; 15 W: 14%; 25 W: 14%) were unchanged following normalization for QMM (high aerobic capacity: 5 W: 21%; 15 W: 20%; 25 W: 19%; low aerobic capacity: 5 W: 21%; 15 W: 22%; 25 W: 22%). The greatest between-subject variability was evident when the data were presented as blood flow normalized for muscle mass at relative exercise intensities (high aerobic capacity: 20% KEmax: 31%; 40% KEmax: 22%; 60% KEmax: 20%; low aerobic capacity: 20% KEmax: 25%; 40% KEmax: 24%; 60% KEmax: 23%). Again, no significant relationships between QMM and LBF in either the high (5 W: r = 0.2; P = 0.7; 15 W: r = 0.1; P = 0.9; 25 W: r = 0.3; P = 0.4) or low aerobic capacity groups (5 W: r = 0.2; P = 0.9; 15 W: r = 0.1; P = 0.7; 25 W: r = 0.3; P = 0.9) were revealed. The eight subjects excluded from this analysis were also examined as a moderate aerobic capacity group and revealed no significant differences in LBF compared at absolute KE work rates. Similar to the high and low aerobic capacity groups, QMM and LBF were not significantly correlated in the moderate aerobic capacity.
DISCUSSION
The main goal of this study was to determine the role of muscle mass as a modulator of exercise-induced blood flow. The major finding of this work was that, in contrast to rest, exercise-induced blood flow was not correlated with muscle mass at any given absolute work rate. Therefore, normalizing the blood flow response for muscle mass did not reduce the considerable between-subject variability of blood flow. Additionally, the approach of normalizing blood flow to muscle mass across relative work rates did not reduce the between-subject variation in exercise-induce hyperemia. Finally, aerobic capacity did not alter the blood flow-to-work rate relationship, and, as there was still no relationship between muscle mass and absolute work rate, neither approach to correct blood flow for muscle mass had any effect on the variance in exercise-induced hyperemia in the subjects with high or low aerobic capacities. Thus, despite the dogma that normalizing blood flow for muscle mass is an important analysis tool to better interpret exercise-induced hyperemia, these data reveal that this is not only an unnecessary procedure, but may actually add additional variance.
Muscle mass and resting blood flow.
Our current data support the notion that, under resting conditions, due to basic cellular requirements, blood flow is linked to muscle mass, with larger volume of muscle consuming more oxygen and, therefore, requiring a greater oxygen delivery achieved by a greater blood flow. However, it should be noted that, although the relationship between thigh muscle mass and resting blood flow was significant, thigh muscle mass only accounted for 30% of the resting blood flow variability. Based on the Fick equation, the strength of this relationship can be modulated by oxygen carrying capacity (9) and tissue oxygen extraction (6), but may also be influenced by muscle fiber type (4, 20). Thus, although clearly not the only factor, skeletal muscle mass does play a significant role in determining muscle blood flow at rest.
Muscle mass and exercise-induced blood flow.
The normalization of blood flow to muscle mass during exercise is common, especially in studies where subject populations typically have a significantly different muscle mass (e.g., due to age or sex). However, as blood flow may actually differ purely as a consequence of sex differences (23, 24) and age (5, 17, 24, 25, 33), this study focused on the relationship between muscle mass and exercise-induced blood flow only in young men, avoiding these other, potentially confounding, factors.
The simple normalization for total limb muscle mass may not be appropriate, as exercise-induced hyperemia is predominantly dependent on the metabolic demand required to sustain the external work and potentially influenced by the level of muscle recruitment. Indeed, studies examining quadriceps muscle recruitment during both cycling (28) and single-leg KE exercise (27) suggest that muscle recruitment and, therefore, blood flow (26, 27) may be dependent on exercise intensity relative to peak workload. Nevertheless, it is presently unclear whether normalizing exercise-induced blood flow for the entire muscle mass or taking into account the percentage of muscle mass recruited is most appropriate.
Although, in the present study, the relationship between muscle mass and resting blood flow proved to be well related, this relationship did not persist during exercise. While the individual LBF response to increasing absolute workloads resulted in a strong relationship between LBF and workload, in agreement with prior studies (12, 30) there was considerable between-subject variability in the hyperemic response at each given workload, which was not reduced by normalizing for muscle mass. Additionally, there was no relationship between LBF and QMM at any of the absolute work rates employed in the present study, questioning the link between these two variables. As already acknowledged, others have suggested a more complex relationship in that muscle recruitment is dependent on exercise intensity relative to peak workload, and thus exercise-induced hyperemia should be assessed as normalized blood flow at a given relative intensity of exercise (26, 27). However, the present data refute the concept utilized by Ray and Dudley (27), which takes into account maximally perfused active muscle mass, because, instead of reducing the difference between subjects, this approach actually yielded the largest intersubject variability. Interestingly, the previously purported relationship between muscle recruitment and perfusion has been questioned by disparities between electromyographic activity and muscle perfusion of the different muscles of the quadriceps (15). Additionally, studies performed in rats (16) and swine (3) report significant blood flow heterogeneity, both across and within exercising muscles, with perfusion being highly dependent on muscle fiber type. As the intramuscular fiber type of human muscle is typically heterogeneous, especially in the muscles of the thigh studied here, it is presently unclear if the lack of an effect of muscle mass on blood flow during exercise can be translated to animal models. However, these data indicate that muscle mass, per se, does not play a role in the exercise-induced blood flow response in humans.
In actuality, the blood flow response to exercise is predominantly dictated by the metabolic requirement to perform external work. This straightforward concept was recognized by Limberg et al. (19) in a study that examined muscle blood flow in obese and lean individuals. Accordingly, the authors did not normalize for muscle mass in groups with very different proportions of lean and fat mass, revealing preserved blood flow regulation in the obese. Of note, if the authors had opted to normalize the recorded blood flows to muscle mass, the results would have incorrectly pointed to a marked decrease in LBF in the obese subjects compared with the lean controls, yielding the incorrect conclusion that blood flow is compromised in this population. In the present study, muscle mass did not explain the between-subject variability in the exercise-induced muscle blood flow, leaving a considerable amount of between-subject variability unaccounted for. A similar inherent LBF variability during KE exercise across absolute workloads was previously reported by Andersen and Saltin (2), who utilized thermodilution to measure LBF, a measure that is strongly correlated to data collected by Doppler ultrasound (32).
Increased blood flow to the skin (14) and inactive leg muscle (i.e., hamstrings) (10) is an inevitable consequence of exercise and most likely contributed to the inherent blood flow variability reported in this study. Previous research by Richardson and colleagues (29) reported the quadriceps as the primary muscle group recruited during KE exercise and, therefore, the principal target for increased LBF and oxygen delivery, but these other factors may have played a role in the observed between-subject variability. In an attempt to control for differences in skin blood flow, subjects were evaluated in a thermoneutral environment. Subjects also performed significant KE exercise practice to ensure the utilization of only the quadriceps during this exercise modality. In summary, the between-subject LBF variability was likely explained by blood flow to inactive muscles and skin, as well as the factors that can modulate resting blood flow, including arterial blood oxygen content, oxygen extraction, and muscle fiber type.
Aerobic capacity, blood flow, and muscle mass.
The wide spectrum of physical activity levels exhibited by the subjects in this study afforded us the opportunity to examine the effect of muscle mass on blood flow in subjects with high and low aerobic capacity, as well as the impact of aerobic capacity on skeletal muscle blood flow during exercise. The impact of endurance training, which raises aerobic capacity, on blood flow in the exercising muscle has been equivocal, with previous studies reporting an increase in blood flow (21, 31), a decrease in blood flow (17), and more subtle changes, such as reduced blood flow heterogeneity (13). These endurance training-induced effects have been attributed to changes in oxygen delivery and extraction (13, 17, 21, 31), as well as an enhanced matching of blood flow to oxygen demand (12). In the present study, although blood flow tended to be higher in the high aerobic capacity group, this difference did not achieve statistical significance when assessed across absolute workloads. It was anticipated that, if the high aerobic capacity group did indeed have better matching of blood flow to oxygen demand, this would reduce the intrinsic blood flow variability, independent of muscle mass, and reveal a clearer picture of the relationship between exercise-induced blood flow and muscle mass. Thus, in keeping with the main goal of this study, an assessment of muscle mass and blood flow in both the high and low aerobic capacity groups revealed no relationship, solidifying the original hypothesis that muscle mass would have no effect on blood flow during exercise, and extending this finding to people with differing aerobic capacities.
Conclusion.
This study documented that, although blood flow is linked to muscle mass at rest, due to the proportional cellular requirements, exercise-induced hyperemia is independent of muscle mass and is instead dependent on the level of external work. Although sizeable between-subject blood flow variability was reported, this variability was not related to muscle mass and is most likely due to a combination of other factors. Thus, despite the dogma that normalizing blood flow for muscle mass is an important analysis tool to better interpret exercise-induced hyperemia, this study has revealed that this is not only an unnecessary procedure, but may actually add variance.
GRANTS
This study received financial support from Veterans Affairs Merit Grant E6910R (R. S. Richardson) and National Heart, Lung, and Blood Institute Grant P01 HL-091830 (R. S. Richardson). R. S. Garten was supported by an Advanced Fellowship in Geriatrics from the Veterans Affairs Medical Center.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: R.S.G. and R.S.R. conception and design of research; R.S.G., H.J.G., M.J.R., and J.R.G. performed experiments; R.S.G. analyzed data; R.S.G. prepared figures; R.S.G. drafted manuscript; R.S.G., H.J.G., M.J.R., J.R.G., and R.S.R. edited and revised manuscript; R.S.G., H.J.G., M.J.R., J.R.G., and R.S.R. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank all of the study participants.
REFERENCES
- 1.Amann M, Subudhi A, Foster C. Influence of testing protocol on ventilatory thresholds and cycling performance. Med Sci Sports Exerc 36: 613–622, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Andersen P, Saltin B. Maximal perfusion of skeletal muscle in man. J Physiol 366: 233–249, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armstrong RB, Delp MD, Goljan EF, Laughlin MH. Distribution of blood flow in muscles of miniature swine during exercise. J Appl Physiol (1985) 62: 1285–1298, 1987 [DOI] [PubMed] [Google Scholar]
- 4.Behnke BJ, McDonough P, Padilla DJ, Musch TI, Poole DC. Oxygen exchange profile in rat muscles of contrasting fibre types. J Physiol 549: 597–605, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carlson RE, Kirby BS, Voyles WF, Dinenno FA. Evidence for impaired skeletal muscle contraction-induced rapid vasodilation in aging humans. Am J Physiol Heart Circ Physiol 294: H1963–H1970, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Clark MG, Rattigan S, Clerk LH, Vincent MA, Clark AD, Youd JM, Newman JM. Nutritive and non-nutritive blood flow: rest and exercise. Acta Physiol Scand 168: 519–530, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Dinenno FA, Jones PP, Seals DR, Tanaka H. Limb blood flow and vascular conductance are reduced with age in healthy humans: relation to elevations in sympathetic nerve activity and declines in oxygen demand. Circulation 100: 164–170, 1999 [DOI] [PubMed] [Google Scholar]
- 8.Donato AJ, Uberoi A, Wray DW, Nishiyama S, Lawrenson L, Richardson RS. Differential effects of aging on limb blood flow in humans. Am J Physiol Heart Circ Physiol 290: H272–H278, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez-Alonso J, Richardson RS, Saltin B. Exercising skeletal muscle blood flow in humans responds to reduction in arterial oxyhaemoglobin, but not to altered free oxygen. J Physiol 530: 331–341, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heinonen I, Duncker DJ, Knuuti J, Kalliokoski KK. The effect of acute exercise with increasing workloads on inactive muscle blood flow and its heterogeneity in humans. Eur J Appl Physiol 112: 3503–3509, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Jones PR, Pearson J. Anthropometric determination of leg fat and muscle plus bone volumes in young male and female adults. J Physiol 204: 63P–66P, 1969 [PubMed] [Google Scholar]
- 12.Kalliokoski KK, Knuuti J, Nuutila P. Relationship between muscle blood flow and oxygen uptake during exercise in endurance-trained and untrained men. J Appl Physiol (1985) 98: 380–383, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Kalliokoski KK, Oikonen V, Takala TO, Sipila H, Knuuti J, Nuutila P. Enhanced oxygen extraction and reduced flow heterogeneity in exercising muscle in endurance-trained men. Am J Physiol Endocrinol Metab 280: E1015–E1021, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Kenney WL, Johnson JM. Control of skin blood flow during exercise. Med Sci Sports Exerc 24: 303–312, 1992 [PubMed] [Google Scholar]
- 15.Laaksonen MS, Kyrolainen H, Kalliokoski KK, Nuutila P, Knuuti J. The association between muscle EMG and perfusion in knee extensor muscles. Clin Physiol Funct Imaging 26: 99–105, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Laughlin MH, Armstrong RB. Muscular blood flow distribution patterns as a function of running speed in rats. Am J Physiol Heart Circ Physiol 243: H296–H306, 1982 [DOI] [PubMed] [Google Scholar]
- 17.Lawrenson L, Poole JG, Kim J, Brown C, Patel P, Richardson RS. Vascular and metabolic response to isolated small muscle mass exercise: effect of age. Am J Physiol Heart Circ Physiol 285: H1023–H1031, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Layec G, Venturelli M, Jeong EK, Richardson RS. The validity of anthropometric leg muscle volume estimation across a wide spectrum: From able bodied adults to individuals with a spinal cord injury. J Appl Physiol (1985); 10.1152/japplphysiol.01120.2013, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Limberg JK, De Vita MD, Blain GM, Schrage WG. Muscle blood flow responses to dynamic exercise in young obese humans. J Appl Physiol 108: 349–355, 2010 [DOI] [PubMed] [Google Scholar]
- 20.McDonough P, Behnke BJ, Padilla DJ, Musch TI, Poole DC. Control of microvascular oxygen pressures in rat muscles comprised of different fibre types. J Physiol 563: 903–913, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mourtzakis M, Gonzalez-Alonso J, Graham TE, Saltin B. Hemodynamics and O2 uptake during maximal knee extensor exercise in untrained and trained human quadriceps muscle: effects of hyperoxia. J Appl Physiol 97: 1796–1802, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Nishiyama SK, Wray DW, Richardson RS. Aging affects vascular structure and function in a limb-specific manner. J Appl Physiol (1985) 105: 1661–1670, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Parker BA, Smithmyer SL, Pelberg JA, Mishkin AD, Herr MD, Proctor DN. Sex differences in leg vasodilation during graded knee extensor exercise in young adults. J Appl Physiol 103: 1583–1591, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Parker BA, Smithmyer SL, Pelberg JA, Mishkin AD, Proctor DN. Sex-specific influence of aging on exercising leg blood flow. J Appl Physiol (1985) 104: 655–664, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Proctor DN, Koch DW, Newcomer SC, Le KU, Leuenberger UA. Impaired leg vasodilation during dynamic exercise in healthy older women. J Appl Physiol (1985) 95: 1963–1970, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Radegran G, Blomstrand E, Saltin B. Peak muscle perfusion and oxygen uptake in humans: importance of precise estimates of muscle mass. J Appl Physiol 87: 2375–2380, 1999 [DOI] [PubMed] [Google Scholar]
- 27.Ray CA, Dudley GA. Muscle use during dynamic knee extension: implication for perfusion and metabolism. J Appl Physiol 85: 1194–1197, 1998 [DOI] [PubMed] [Google Scholar]
- 28.Reid RW, Foley JM, Jayaraman RC, Prior BM, Meyer RA. Effect of aerobic capacity on the T(2) increase in exercised skeletal muscle. J Appl Physiol 90: 897–902, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Richardson RS, Frank LR, Haseler LJ. Dynamic knee-extensor and cycle exercise: functional MRI of muscular activity. Int J Sports Med 19: 182–187, 1998 [DOI] [PubMed] [Google Scholar]
- 30.Richardson RS, Haseler LJ, Nygren AT, Bluml S, Frank LR. Local perfusion and metabolic demand during exercise: a noninvasive MRI method of assessment. J Appl Physiol 91: 1845–1853, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Rud B, Foss O, Krustrup P, Secher NH, Hallen J. One-legged endurance training: leg blood flow and oxygen extraction during cycling exercise. Acta Physiol (Oxf) 205: 177–185, 2012 [DOI] [PubMed] [Google Scholar]
- 32.Wray DW, Fadel PJ, Smith ML, Raven P, Sander M. Inhibition of alpha-adrenergic vasoconstriction in exercising human thigh muscles. J Physiol 555: 545–563, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wray DW, Nishiyama SK, Monnet A, Wary C, Duteil S, Carlier PG, Richardson RS. Multiparametric NMR-based assessment of skeletal muscle perfusion and metabolism during exercise in elderly persons: preliminary findings. J Gerontol A Biol Sci Med Sci 64: 968–974, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wray DW, Nishiyama SK, Richardson RS. Role of α1-adrenergic vasoconstriction in the regulation of skeletal muscle blood flow with advancing age. Am J Physiol Heart Circ Physiol 296: H497–H504, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]