Abstract
Many opportunities to explain ADHD-related risk of substance use/disorder (SUD) remain available for study. We detail these opportunities by considering characteristics of children with ADHD and factors affecting their outcomes side-by-side with overlapping variables in the developmental literature on SUD etiology. Although serious conduct problems are a known contributor to ADHD-related risk of SUD, few studies have considered their emergence developmentally and in relation to other candidate mediators and moderators that could also explain risk and be intervention targets. Common ADHD-related impairments, such as school difficulties, are in need of research. Heterogeneous social impairments have the potential for predisposing, and buffering, influences. Research on neurocognitive domains should move beyond standard executive function batteries to measure deficits in the interface between cognitive control, reward, and motivation. Ultimately, maximizing prediction will depend, as it has in the SUD literature, on simultaneous consideration of multiple risk factors.
Keywords: attention deficit disorder, adolescent, substance-related disorders, alcoholism, young adult
INTRODUCTION
In the 1960s, substance use among youth in the United States was on the rise and headed toward its zenith in the late 1970s (Johnston et al. 2013). In an effort to understand this trend, longitudinal studies of children and adolescents were launched to uncover the possibility of early dispositional vulnerabilities to later drug abuse. These studies improved upon the methodologic limitations of their less-compelling cross-sectional predecessors. Theories of drug addiction readily emerged to emphasize the role of personality and temperament traits as well as the contribution of other individual difference variables such as differential sensitivity to alcohol, cognitive factors, and attitudes (e.g., Tarter et al. 1985). This class of variables, emerging from research that predated DSM-III-driven (American Psychiatric Association 1980) research on categorical diagnoses, was thought to partially reflect inherited liabilities that interacted with socio-environmental conditions to produce addiction vulnerability (Glantz & Pickens 1992). As studies of these contributing factors rapidly multiplied, findings revealed that drug abuse vulnerability is multifaceted, with individual factors accounting for additive and interactive influences on substance use initiation, escalation, and course (including progression to addiction and sometimes recovery). One of these findings, replicated across many studies, is the prediction of substance use from a constellation of temperament and personality traits that overlap almost entirely with the defining symptoms of Attention-Deficit/Hyperactivity Disorder (ADHD).
ADHD: HISTORY, DEFINITION, AND COURSE
ADHD has been variably named and defined over the past 60 years in which it has been studied. In the 1960’s the terms “minimal brain damage or dysfunction” were commonly used to describe the children, who would ultimately be labeled in Diagnostic and Statistical Manual of Mental Disorders (DSM IV; American Psychiatric Association 1994) as having ADHD, though labeled Attention Deficit Disorder with and without Hyperactivity in DSM III (American Psychiatric Association 1980). However, through the decades of research on diagnosis/identification and in all versions of the DSM, the core characteristics of the disorder have been the same--levels of inattention, impulsivity, and hyperactivity that were substantially different from typically developing children and that are accompanied by problems in daily living (impairment). Prior to the publication of DSM III and beginning in the early 1960’s, ADHD was defined with child behavior rating scales that included items reflecting the core characteristics of ADHD, conduct problems and aggression, peer relationships, and internalizing problems. These scales included the early Behavior Problem Checklist, the Conners Teacher Rating Scale (CTRS), and the Child Behavior Checklist (Achenbach et al. 1989), and were typically completed by parents or teachers or both. The developers of these scales had started in part with item pools that were derived from clinic-based records of children with behavior or emotional problems (e.g., Robins 1966), were typically factor analyzed to produce scales, and had response formats based on frequency or severity of behaviors. With respect to ADHD, the one that became most commonly employed was the CTRS—particularly the short form or the Abbreviated Conners (ACTRS; Conners 1969). For more than a decade, a score of 15 on the ACTRS was the most common way of diagnosing ADHD (Pelham et al. 2005).
When DSM III was developed, researchers took the DSM items that defined ADHD and put them in a format in which they could be rated by parents, teachers, and clinicians (see Atkins et al. 1985). Implicit in the original rating scale definitions and in the DSM III was that children had to be impaired in their functioning in order to be diagnosed with ADHD. Impairment was later codified as a part of the diagnostic schema in the fourth edition of the DSM (APA 1994). In DSM III and continuing into DSM 5 (APA 2013), ADHD is defined according to the number of symptoms endorsed by parents or teachers or clinicians in two lists—one reflecting inattention and the other reflecting impulsivity/hyperactivity. If six symptoms—5 for older adolescents and adults--are endorsed by parent or teacher, with at least some symptoms and related impairment occurring across settings, and with an onset prior to age 12, ADHD can be diagnosed. A child can meet criteria on the inattention list, the hyperactive/impulsive list, or both, and be diagnosed with an inattentive (or impulsive) subtype or with a combined subtype. There is debate regarding whether structured interviews with parents are needed to buttress parent and teacher ratings, but the procedures for symptom counting are similar for interviews and rating scales. It turns out that DSM symptom scales and the empirically derived scales that predated DSM III are very highly correlated and identify the same children – particularly the combined subtype, the most common diagnosis (Pelham et al. 2005).
The broad spectrum rating scales that predated the DSM III were developed with large item pools that reflected a broad spectrum of problem behaviors. Factors reflecting aggression/conduct problems and ADHD symptoms were always obtained, but they were also highly correlated; most children high on one scale were high on the other. The same is true for DSM diagnoses. As we discuss below, conduct disorder (CD) and oppositional defiant disorder (ODD) are highly comorbid with ADHD and complicate the picture of ADHD risk for later substance use disorder (SUD).
It is now well established that ADHD is a chronic condition that begins in early childhood and continues through life. Indeed, the vast majority of children diagnosed with ADHD continue to have symptoms of ADHD and related impairments into adulthood (Barkley et al. 2008). There has been debate about this point for some time, but recent longitudinal studies have made it clear that if symptoms are assessed appropriately (i.e., using other rather than self report), the majority of children with ADHD become teenagers with ADHD (Sibley et al. 2012a) and the majority of teenagers with ADHD become adults with ADHD (Sibley et al. 2012b). Further, even when symptoms of ADHD necessarily decline (attention and impulsivity are constructs that improve with age in all children), the problems of daily living that characterize the disorder do not decline and often worsen (Barkley et al. 2006). We review in sections below a number of domains in which impairment continues to be present for individuals with ADHD as they grow up.
ADHD AS A RISK FACTOR FOR SUBSTANCE USE DISORDER
Childhood Traits that Predict Substance Use in NonADHD Studies
Longitudinal studies of young children, not diagnosed with ADHD but followed into adolescence and adulthood, provide compelling evidence that early occurring behaviors, of the type that overlap with symptoms of ADHD, are signals of substance disorder risk. In one of the first demonstrations of adolescent substance use predicted from nursery school-age behavior, Block and colleagues predicted adolescent substance use from nursery school teacher ratings of behavior that clearly included difficulties with self-regulation in multiple behavioral, emotional, and social respects (Block et al. 1988). From a large sample of kindergarten boys in Montreal, Masse and Tremblay (1997) predicted early adolescent substance use from “novelty-seeking” measured as teacher-ratings of restlessness, not keeping still, fidgety, etc. In New Zealand, the well-known large Dunedin Health and Development study found increased adult alcohol dependence by age 21 from observations of “behavioral undercontrol” at age 3 (e.g., impulsivity, impersistence, difficulty sitting still) (Caspi et al. 1996) and increased substance dependence by age 32 from a composite measure of “self-control” through childhood (aggregated across childhood observations and ratings of impulsive aggression, hyperactivity, lack of persistence, inattention, and impulsivity) (Moffit et al. 2011). Other prospective longitudinal studies have reported similar findings (Niemela et al. 2006, Reinherz et al. 2000, Tarter et al. 2004). Collectively, these studies identify a constellation of traits variously identified in the addiction literature, such as behavioral undercontrol (Chassin et al. in press) or behavioral disinhibition (Iacono et al. 2008). These temperament traits are strongly correlated with ADHD symptoms (Martel et al. 2009a, White 1999) and in fact might be argued to be the same behaviors – just expressed in different language. Their occurrence in early childhood predicts later ADHD diagnosis and also substance use (Martel et al. 2009b). Thus, theoretical models of ADHD that discuss the disorder for some children as a reflection of extreme temperament traits (e.g., Carey 2002), dovetails nicely with theoretical models of substance disorder that posit these aspects of temperament vulnerability as contributors to addiction.
Longitudinal Studies of Children Diagnosed with ADHD
There has been interest in an ADHD-substance disorder connection for many decades—based primarily on the temperament-symptom link and early reports of co-occurrence in families (Cantwell 1972, Pelham & Lang 1993, Tarter et al. 1985). Longitudinal studies have accumulated since the earliest papers were published and substance use was a nascent interest among ADHD researchers (e.g., Barkley et al. 1990, Gittelman et al. 1985, Hartsough & Lambert 1987, Hechtman & Weiss 1986). Two recent meta-analyses found evidence of increased risk of abuse/dependence on nicotine, alcohol, marijuana, cocaine, and other unspecified substances (Charach 2011, Lee et al. 2011). However, puzzling results emerged, such as a non-significant association between childhood ADHD and lifetime alcohol use (as opposed to disorder). Moreover, effect sizes were modest and in some cases statistically variable (marijuana disorder). (The association of nicotine dependence, however, has consistently been shown across multiple studies to be strongly associated with childhood ADHD, with 40%-50% of children with ADHD expected to be daily smokers by adulthood, Molina 2011.) Virtually absent from these studies, most of which were initiated in the 1970s and 1980s, were assessments of substance use initiation and quantity/frequency escalation that are standard fare in developmental studies of substance use. Thus, risk for substance disorder by adulthood for individuals with ADHD is apparent, but the bulk of the literature is relatively uninformative regarding the unfolding of substance use at younger ages. This unfolding has important implications for long-term course in adulthood (Chassin et al. in press, Derefinko & Pelham in press, Masten et al. 2009, Molina 2011).
The more recent longitudinal studies of childhood ADHD—including the first to focus primarily on substance use and disorder (Pittsburgh ADHD Longitudinal Study; PALS)--have incorporated comprehensive substance use and SUD assessments to enable detection of onset, escalation, and course into adulthood (e.g., PALS; the Berkeley Girls ADHD Longitudinal Study; the longitudinal follow-up of the Multimodal Treatment of ADHD Study, MTA). For example, Molina and Pelham (2003) reported more frequent drunkenness and marijuana use, daily smoking, non-marijuana illicit drug use, and alcohol problems for adolescents with childhood ADHD compared to a demographically similar nonADHD comparison group. An absence of group differences and high prevalence of any lifetime alcohol use were illustrative of the need to measure substance use using developmentally appropriate methods to detect age-atypical levels of use. Taken together the literature suggests moderate, at times inconsistent, risk for some substance-related outcomes in individuals with ADHD (e.g., Babinski et al. 2011 and Hinshaw et al. 2006, found no increased risk of substance use or SUD for girls with ADHD). Studies of onset, escalation, and course are needed, but perhaps more importantly, integration of more sophisticated substance use measurement with consideration of factors that explain heterogeneity of ADHD-related addiction risk.
Other Types of Studies Demonstrating ADHD-Substance Use Links
There is ample indirect evidence of an association between ADHD and substance disorder vulnerability from other types of studies. Alcohol and other substance disorders occur at greater rates among the parents of children with ADHD (Biederman et al. 2008). Children of parents with substance disorders are more likely to have ADHD or ADHD symptoms (Carbonneau et al. 1998, Martel et al. 2009) or to score higher on temperament or personality traits that include dimensional expressions of ADHD symptomatology such as impulsivity (Handley et al. 2011, King et al. 2009, Martel et al. 2009, Molina et al. 2010, Tarter et al. 2004). Approximately two-thirds of adolescents in treatment for substance disorders are diagnosable with ADHD (Chan et al. 2008). Further, genetic and neurobiological models of addiction and ADHD share strikingly similar overlap in targeted regions of interest (e.g., Arcos-Burgos et al. 2012, Goldstein & Volkow, 2002). Finally, studies of parent-child interactions have validated the possibility that ADHD behaviors in children cause stress-mediated increases in parent alcohol use (Pelham et al. 1997) and that parent alcohol use causes parenting styles that are ineffective with ADHD-affected children (Lang et al. 1999). Thus, a transactional, dynamic relationship between problematic child and parent behavior may involve alcohol in families affected by ADHD.
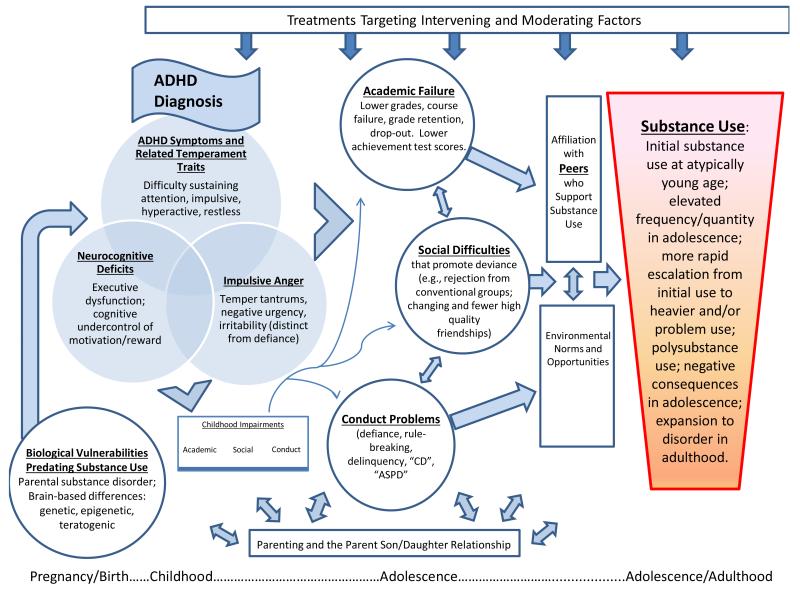
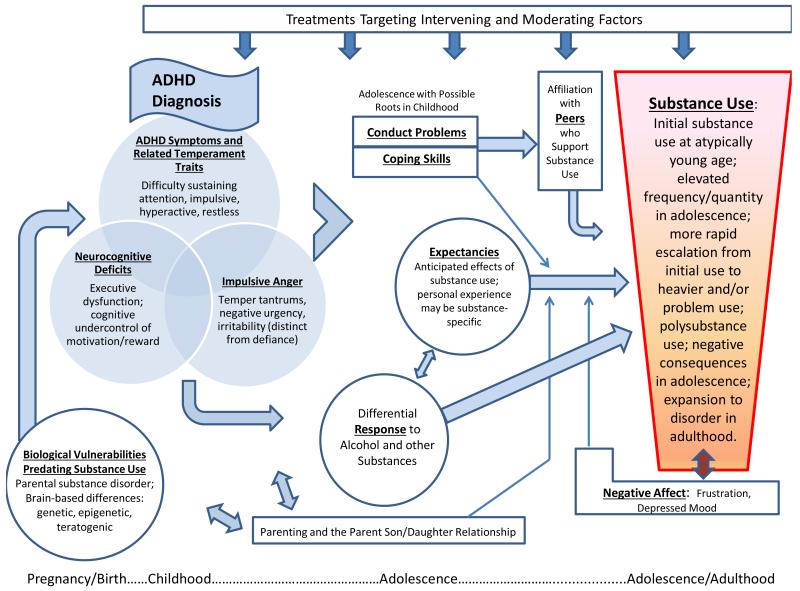
Multiple reviews of SUD liability have emerged in the last twenty plus years to produce theoretical models identifying additional research needs (Chassin et al. in press; Iacono et al. 2008, Sher 1991, Sher et al. 2005, Tarter 2002, Zucker, 2006). When juxtaposed with the empirical literature characterizing ADHD and its associated impairments (e.g., Barkley et al. 2008), opportunities to clarify ADHD risk of addiction, both magnitude and mechanisms, become apparent. These opportunities may be loosely organized around the recent distinction offered by Chassin and colleagues (in press) between pathways of generalized versus substance-specific risk. The former include risk pathways that are non-specific to substance disorder outcome and incorporate externalizing and internalizing disorder tendencies and their attendant mediators and moderators. ADHD and its downstream consequences, including multiple impairments and substance use/SUD, figure prominently in the externalizing (or deviance-proneness) pathway (e.g., Iacono et al. 2008, Sher 1991). Additional avenues for hypothesis-testing emerge from consideration of other substance-specific pathways to addiction as well the need to consider developmentally-specific mechanisms (e.g., differential response to alcohol in ADHD, Weafer et al. 2009). These pathways to substance disorder provide a backdrop against which new hypotheses about ADHD-related risk of SUD may be generated. We caution that these hypotheses, depicted in Figures 1 and 2, are not mutually exclusive, nor exhaustive; rather, they are meant collectively to provide a heuristic framework from which future research on ADHD and SUD risk may build.
Figure 1.
ADHD-related impairment pathways to substance use disorder.
Figure 2.
Negative affect, expectancies, and coping pathways to substance use disorder.
DEVIANCE-PRONENESS AND IMPAIRMENT PATHWAYS TO SUD IN ADHD
The Role of Conduct Disorder in ADHD Risk of SUD
The best known correlate of ADHD that is omnipresent in discussions of substance use/disorder risk for ADHD is Conduct Disorder (CD). Children with ADHD have a well-established increased likelihood of having, or of developing, behavior problems such as defiance and rule-breaking (Barkley et al. 2008) that are known to strongly correlate with substance use in adolescence and to predict adult substance disorder in nonADHD samples (Zucker 2006). For youth, serious delinquency may result in a diagnosis of CD in the clinical setting and this comorbidity occupies a prominent role in studies of ADHD risk for substance disorder (Flory & Lynam 2003). Rates of diagnosable CD generally range from 25% to 30% of boys with ADHD diagnosed in childhood and slightly lower, 17% to 23%, of girls with this same history (Molina 2011). Samples that reach more broadly into the community to include primary care populations may have somewhat lower rates (Brown et al. 2001).
In adolescence, co-occurrence of heavier substance use and serious conduct problems was established years ago and continues, to date, to be strongly supported by many studies. Jessor and colleagues’ hypothesis that adolescent substance use and conduct problems are reflections of a general tendency toward deviant behavior (Jessor & Jessor 1977) has been supported in many studies with replicated findings of shared predictors that lead to both outcomes. Moreover, emergence of conduct problems prior to substance use by children with ADHD has been found in multiple studies (Molina 2011). As a result, a knee-jerk reaction to the need to “control for” CD in analyses of ADHD substance use risk has promulgated. However, such an approach may remove key components of ADHD, such as impulsivity, that are associated with ADHD and CD and that are important for the prediction of atypical levels of substance use (Derefinko & Pelham in press). This approach also tends to miss the potential for detecting the cascading pattern of vulnerability that begins with difficult temperament, leads in childhood to escalation of behavior problems such as defiance and rule-breaking (in interaction with environmental factors such as parenting), and culminates in adolescence with expanded behavior problems that include early/heavier/escalating/problematic substance use. Martel and colleagues found this cascading pattern in a high risk sample of children of parents with alcoholism and antisocial personality (Martel et al. 2009).
Published studies of children with ADHD are beginning to incorporate these strategies. Brook and colleagues (2010) found support for CD as a mediator of the association between ADHD and adult substance disorder, although both ADHD and CD were measured in adolescence. Sibley and colleagues (under review) found that when modeling behavior longitudinally from age five, that ADHD and not CD symptoms predicted adolescent substance use. Findings such as these, and others that find limited or inconsistent prediction of substance use from childhood, as opposed to adolescent, CD (Molina et al. 2007), suggest that although later CD may be a crucial comorbidity, childhood ADHD may set the stage for later onset and escalation of conduct problems including delinquency that creates substance disorder risk. As such, we include conduct problems both in childhood, and downstream in adolescence (including delinquency), in our illustrated models of ADHD risk for SUD (Figs. 1, 2). The “developmental roots” of delinquency remain a matter of debate, but there is support from multiple studies for prospective prediction of deviant behavior from temperamental traits that map onto aspects of ADHD (Eisenberg et al. 2005).
ADHD-Related Impairment Pathways to SUD: Academic and Vocational problems
One of the most common precipitants to professional evaluation for childhood ADHD is academic underachievement (Loe & Feldman 2007). The primary referral basis for ADHD is problems in school settings that include both academic deficits and disruptive classroom behavior. Children with ADHD have impairing academic problems and disruptive behavior in elementary school (e.g., Atkins et al. 1985, Pelham & Bender 1982), middle school (Langberg et al. 2011) and high school (Kent et al. 2011). These problems persist into college and vocational years (Kuriyan et al. 2013) which, not surprisingly, are predicted by problems at earlier ages. In elementary school, children with ADHD are off task more than peers, fail to finish seatwork and homework, and fall behind in standardized achievement. They violate classroom rules, disobey the teacher, and disturb peers at very high rates. In middle school, they take poor notes, fail to study during assigned time periods, and fail to turn in homework assignments. The same difficulties continue into high school, resulting in lower class placements, higher rates of course failure, and higher rates of retention and dropout. In one very large study of predictors of later achievement in school, the only variable measured at school entry other than baseline achievement that predicted later achievement was problems in attention (Duncan et al. 2007)—neither IQ nor social/emotional behaviors predicted beyond baseline achievement and attentional skills.
With respect to substance use, academic underachievement in childhood and in adolescence predicts later substance use in prospective longitudinal studies (Brook et al. 1995, Wills et al. 2004) even after controlling for other variables such as delinquency and associating with drug-using peers (Ellickson et al. 2004). For example, repetition of a term or grade by age 14 and cheating each prospectively predicted drug use by age 22 (Brook et al. 1995). Although strong concurrent relations between substance use and academic performance indicators are frequently found (Verweig et al. 2013), prospective prediction from childhood academic difficulties (before substance use has begun) has also been found (Brook et al. 1995) although there are exceptions (Ensminger et al. 2002).
Achievement problems are thought to be an important reflection of failure to successfully engage in activities that promote future goal attainment and healthy adjustment (Brook & Brook 1990, Jessor & Jessor 1977). As such, although underachievement often increases risk of substance use, the presumed explanation is that multiple correlated and contributing factors are involved. Early warning signs of school disengagement, such as excessive absences and course failure, predict both drop-out in adolescence and, in adulthood, criminal behavior and problematic substance use (Henry et al. 2012). This may explain why shared environmental factors were found to underlie early school leaving and adolescent marijuana use in a large twin sample of adults’ recollections (Verweig et al. 2013). In addition to factors such as parenting and neighborhood, individual difference variables that convey shared liability probably contribute to both. In the Dunedin Multidisciplinary Health and Development Study (Moffit et al. 2011), low self-control in childhood predicted early school leaving which in turn partially accounted for prediction of age 32 substance dependence. Combined with the high rates of academic problems experienced by children with ADHD, this latter finding implicates an academic performance pathway to substance dependence for children with ADHD.
Academic difficulties have been little studied for their role in substance disorder vulnerability in research on ADHD. In a study specifically targeting academic performance as a mediator of growth in ADHD-related alcohol use, grades earned in secondary school partially mediated the relation between childhood ADHD diagnosis and frequency of alcohol use at age 17 (Molina et al. 2012). Delinquency was part of this “academic deviance pathway” to drinking, supporting the applicability of general “problem-behavior proneness” rather than academic difficulties per se as substance use risk (see the correlated academic problems and delinquency in Fig. 1). However, much work remains to appreciate whether deflecting achievement trajectories, prior to and during vulnerable periods, would have beneficial effects on substance use outcomes. Academic difficulties may only create substance risk under specific conditions or in certain developmental windows not yet investigated in research on ADHD (e.g., adulthood).
Another fruitful area for research is in the disentangling of ADHD symptoms and cognitive impairments versus social/emotional behaviors that lead to academic difficulties (Duncan et al. 2007) and may have differential implications for substance disorder risk. For example, inattention symptoms and their neurocognitive underpinnings are thought to primarily underlie the academic difficulties of ADHD (Hinshaw 1992, Lee & Hinshaw 2006, Massetti et al. 2008, Pingault et al. 2011), but compared to impulsivity, this dimension of ADHD symptoms is less often specifically hypothesized to be causal in substance abuse vulnerability. There is inconsistency across studies that attempt to isolate which dimension of ADHD symptoms matters for substance use/disorder risk, presumably because of the high intercorrelation between the two dimensions. An important question for future research is whether early emerging school difficulties set the stage for subsequent disengagement and deviance that propel substance use involvement, and whether particular symptom profiles over time coupled with academic problems and deviance, are important (as opposed to inattention symptoms and academic problems alone). Whether early and/or continuing cognitive-academic interventions (Sibley et al. in press) may avert these costly outcomes is also crucial to understand.
ADHD-Related Impairment Pathways to SUD: Heterogeneous Social Difficulties in ADHD with Mixed Implications for SUD
It has long been known that many children with ADHD have difficulties in their relationships with peers that lead to their being disliked and/or ignored more often than children without ADHD. Compared to typical children, children with ADHD have fewer friends and they are less popular and more often rejected by their peers (Hoza 2007, McQuade & Hoza 2008, Milich & Landau 1982, Pelham & Bender 1982). For example, after collecting gold standard measurement of social standing among peers using sociometric methods (classmate ratings of likeability), Hoza and colleagues found that 52% of children with ADHD were identified by their peers as children they did not want to befriend (Hoza et al. 2005). Children with ADHD also had fewer reciprocally confirmed friendships. Children with ADHD act in ways that engender these reactions with astonishing rapidity (Erhardt & Hinshaw 1994, Pelham & Bender 1982) and stability (Hinshaw 2002). Their social difficulties continue well into adolescence and beyond (Bagwell et al. 2001, Molina et al. 2009). The causes of these underlying social difficulties remain a subject of investigation, but chief contenders are the symptoms themselves that are annoying to peers (Pelham & Bender 1982), social cognitive deficits that prevent accrual and correct utilization of skills (Hoza et al. 2000), and intrafamilial transmission that may include parental skill deficits (Mikami et al. 2010). Although executive functions and in particular inhibitory control have been proposed as responsible for multiple deficits experienced in ADHD (Barkley 1997), evidence of their ability to explain social behaviors that lead to rejection is mixed (Diamantopoulou et al. 2007, Huang-Pollock et al. 2009).
The heterogeneity of interpersonal difficulties experienced by children with ADHD is noteworthy and may be partly connected to variable phenotypic expression of symptoms. Children with the full complement of ADHD symptoms, who are typically diagnosed with combined subtype, have the highest rates of social rejection and aggression (Hinshaw 2002, Hodgens et al. 2000, Mikami et al. 2007, Pelham & Bender 1982). For example, among preadolescent girls with ADHD, teacher ratings of aggression including relational forms (e.g., excluding peers or spreading rumors), staff observed aggression, and sociometric ratings of dislike, were highest for those with combined versus inattentive subtype (Hinshaw 2002). The social problems experienced by children with ADHD also include social inhibition and appear to be more connected to the inattentive than to the combined subtype. The social problems of children with predominantly inattentive symptoms tend to be of the shy, isolated, and passive nature (Carlson et al. 1987, McQuade & Hoza 2008, Willcutt et al. 2012). For example, ADHD inattentive type was associated with social withdrawal and peer ratings of shyness in a sample of boys with ADHD (Hodgens et al. 2000). These different types of social profiles have potentially important implications for understanding ADHD-related substance abuse risk.
Adolescent substance use primarily occurs in social contexts (Chassin et al. in press), and affiliation with peers who support substance use is among the strongest predictors of adolescent substance use (Barnes et al. 2006, Hawkins et al. 1992, Reifman et al. 1998). Longitudinal studies support both selection and influence effects such that friendship with “users” predicts increased use, and substance use predicts gravitation to peers who support use (e.g., Curran et al. 1997). Moreover, analysis of sibling and best friend substance use indicates that vulnerability to best friend substance use is greatest for adolescents with the highest (behavioral) genetic vulnerability (Harden et al. 2008). Also pertinent to ADHD, longitudinal studies of general community children have found that social deficits in childhood of the type that commonly characterize children with ADHD, such as mismanagement of conflict and lower peer acceptance (Hops et al. 1999), aggression, and “shyness” (which included having few friends) (Kellam et al. 1980) predicted adolescent drug use. Social skill difficulties such as these are thought to result in exclusion from conventional peer groups. Together these findings illustrate the crucial importance of peer-mediated socialization to adolescent substance use in models of addiction vulnerability for high risk individuals as defined by early traits and behaviors common to children with ADHD. We emphasize the proximal importance, and potential selection and influence effects, of affiliation with substance-supportive peer groups by its placement with double-headed arrows near Substance Use in Figures 1 and 2.
Limited attempts have been made to relate these aspects of social functioning in ADHD to substance use. Marshal and colleagues (2003) found that adolescents with childhood ADHD were more likely to report friendships with substance-using and -tolerant peers than adolescents without childhood ADHD (Marshal et al. 2003). The association between peer use/tolerance and the adolescent’s self-reported substance use was also stronger in the ADHD versus nonADHD group which suggested greater vulnerability to substance use within the peer context. However, the cross-sectional nature of these associations has yet to be tested with longitudinal data that can distinguish selection from influence effects, and research with adolescents has relied on subject perceptions which may be biased. The expected finding, to parallel the literature outside of ADHD interests, is that both types of effects will be operating (see bidirectional associations in Fig. 1 and 2). More importantly, however, will be the determination of factors that moderate this risk. For example, effective parenting practices that are known to decrease substance disorder risk have yet to be studied as moderator of peer-mediated substance use socialization processes. Whether parents of adolescents with ADHD can have similar effects (indicated by upward pointing arrows from Parenting in Fig. 1) is unknown but important for understanding the mutability of this important risk pathway.
The heterogeneity of social difficulties in ADHD has been largely ignored with respect to substance disorder risk and may explain sometimes modest and inconsistent associations between childhood ADHD and substance use. In particular, the implications of social neglect, passivity, shyness, and withdrawal, experienced by some children with ADHD, may have unexpected implications. These particular social characteristics, or variants such as social phobia or anxiety, have been shown in some studies to predict decreased risk of adolescent substance use in samples not selected for ADHD (Frojd et al. 2011, Kaplow et al. 2001), presumably due to decreased social opportunities related to substance use. Parental alcohol problems has also been found to predict more sociability and less shyness in early childhood (Kendler et al. 2013). In a longitudinal study of childhood ADHD and adolescent alcohol use (Molina et al. 2012), parent ratings of relationship problems with same-aged peers mediated the association between childhood ADHD and adolescent alcohol use, but two pathways with opposite effects on drinking suggested that suppression effects may be operating. In addition to an expected social impairment pathway that included increased delinquency and drinking, another pathway from childhood ADHD to increased social impairment predicted lower drinking frequency. These findings suggested that future research on adolescent substance use initiation and escalation may need to distinguish between these types of social tendencies. In addition, it is unclear whether these social difficulties may engender similar effects in adulthood when access to substances broadens and motives for continued use and addictive tendencies evolves.
NEUROCOGNITIVE UNDERPINNINGS OF ADHD WITH RESPECT TO SUD
Neuropsychological Deficits in ADHD
The neurocognitive aspects of ADHD that have been extensively researched bear striking resemblance to parallel domains of inquiry with respect to substance disorder vulnerability. Studies of neuropsychological task performance in children with ADHD have been conducted for over 50 years (Douglas 1972, Nigg 2001); they have principally focused on deficits of executive function (EF). There are multiple overlapping definitions of EF (Barkley 2012) that roughly emphasize flexible control over one’s own thought and actions in pursuit of goal-directed behavior. Commonly included in measurement batteries of EF are assessments of inhibitory control, resistance to interference, working memory, cognitive flexibility, planning, and other related variables (Miyake et al. 2000, Welsh & Pennington 1988).
In addition to group differences in general measures of intelligence, robust ADHD group differences are found for processing speed, vigilance, motor, set-shifting, short-term memory, planning, and especially response inhibition, response variability, and working memory (Willcutt et al. 2012). Importantly, and especially when considering implications for substance disorder vulnerability, the inattention symptom domain appears to be most strongly related to these cognitive deficits. Willcutt and colleagues’ recent meta-analytic review of research informing the distinctiveness of the DSM-IV ADHD symptom dimensions and subtypes found no appreciable differences between performance on these tasks across ADHD combined and inattentive subtype groups and significantly stronger correlations between task performance and inattention symptoms than with impulsivity-hyperactivity symptoms (Willcutt et al. 2012). Given the prominence of behavioral disinhibition in models of addiction vulnerability (Iacono et al. 2008), (with the possible exception of tobacco dependence, Burke et al. 2001), the implications of these findings are somewhat puzzling in terms of understanding substance disorder risk in ADHD.
Dual Process Models of ADHD and SUD
Historically, the EF functions were thought to be controlled by dorsal system regions within the brain but mixed findings along these lines has resulted in an expansion of theory (Cubillo et al.2012) that helps to relate cognitive control and motivational-affective deficits of ADHD to substance use vulnerability. Dual- and multi-pathway models of the disorder have emerged that take into account the heterogeneity of ADHD with regard to symptom presentation, comorbidity, and course (Castellanos et al. 2006, Nigg 2001, Sonuga-Barke 2003). These models acknowledge the potentially crucial role of subcortical regions in motivation and reward, and their under-developed connections with cortical regions, that have implications for both ADHD and addiction. For example, the development of “top down” neural systems with age has been hypothesized to account for the post-childhood reduction of ADHD symptoms that occurs for some (Halperin and Schulz 2006) and, if under-developed, are implicated in ADHD symptom persistence in relation to substance use (Barkley et al. 2008, Molina et al. 2012, Wilens et al. 2011). These expanded models, such as the distinction between hot and cool EF (Zelazo & Carlson 2012), allow incorporation of co-occurring features such as anger control problems (see Fig. 1) inherent to oppositional defiant disorder that relate more strongly to symptoms of impulsivity than to inattention (Lahey et al. 2004, Pillow et al. 1998), and poor delay of gratification, a construct important in addiction vulnerability and recovery (Stanger et al. 2012, Yi et al. 2010). Aspects of cortical-subcortical connectivity, including delayed development when social opportunities to use drugs and alcohol are at their peak (adolescence and young adulthood), may underlie vulnerability to substance disorder in ADHD. These expanded neurobiological models of ADHD also converge with dual process approaches to understanding addiction (Chassin et al. in press), in which automatic, impulsive approach tendencies to seek immediate reward are (or, in the case of addictive tendencies, are not) regulated by a higher order, rationale, effortful cognitive control system (e.g., Bechara 2005).
Executive Functioning in ADHD and SUD
Research attempting to isolate the neuropsychological vulnerabilities within ADHD that portend initial substance use and later disorder is nascent. As a measure of executive inhibitory control, Nigg and colleagues (2006) measured speed of response inhibition using the tracking version of the Stopping Task. Stop signal reaction time (SSRT) was significantly correlated with alcohol-related problems and illicit drug use in late adolescence, with associations ranging from .09 to .18, and with elementary school aged ratings of ADHD (.17) and CD (.15). ADHD and CD were correlated with the later substance use outcomes (.20 to .45). However, after all variables were entered in regression equations, prediction from ADHD fell to non-significance, and SSRT and CD continued to predict substance use, suggesting a unique independent additive effect of a specific aspect of executive functioning in substance abuse vulnerability. However, it’s unclear whether ADHD symptoms failed to predict due to the usual high correlation with CD, or whether mediational pathways were operating such that this specific form of EF may be partly explaining which children with ADHD have increased substance disorder risk. In addition, heavy drinking by some adolescents, known to worsen performance on many EF tasks (Tapert et al. 2002), may have predated and contributed to the findings. Replication is needed.
Several other studies have failed to successfully link ADHD and substance use outcomes via EF deficits. Wilens and colleagues measured EF as abnormal performance on 2/6 standard neuropsychological tests (including response inhibition and working memory among others). EF, which characterized 41% of the ADHD group, failed to predict late adolescent and early adult substance use disorder or cigarette use in ADHD and in nonADHD groups (Wilens et al. 2011). Handley and colleagues also found mixed results in a multigenerational family alcoholism study of adolescent offspring that included measures of ADHD and CD symptoms and substance use (Handley et al. 2011). Only one of three EF measures (response inhibition – an amalgam of working memory and response inhibition) related to ADHD symptoms. Response inhibition also related to CD symptoms, which was promising given the importance of conduct problems in the pathway to substance use, but none of the three EF measures were associated with familial alcohol or drug disorders or with adolescent substance use.
A recent study of EF in young adults with/without childhood ADHD and frequent past year marijuana use (2x2 design) found expected robust ADHD group differences on multiple tests of EF including errors of commission, poorer working memory, verbal memory, decision making, and cognitive interference (Tamm et al. 2013). However, only one of these variables was also associated with past year marijuana use (decision-making based on the IOWA Gambling Task). Although largely speculative due to the isolated finding, and complicated by the possibility of neurocognitive effects from drug use (Hanson et al. 2011), an inability to balance rewards and punishments may reflect cognitive-motivational circuitry deficiencies in ADHD that partially explain addiction vulnerability (Tamm et al. 2013). To date, such tasks have not been well-tapped in neurocognitive batteries designed to prospectively predict substance use in ADHD. These deficiencies, which only partly overlap with the clinical symptoms of ADHD, are depicted in Figures 1 and 2 as part of the Venn diagram.
EF deficits are measured by a large number of neuropsychological tasks, each of which assesses narrowly defined aspects of cognition and behavior, by design. However, their individual explanatory power with respect to conditions in which children with ADHD have elevated risk for SUD remains weak especially relative to traditional questionnaire measures of temperament and personality (e.g., Handley et al. 2011, White et al. 1994). An important test would be aggregated or longitudinally modeled measurement across repeated assessments of EF; such repeated assessments of behavior should have the capacity to better estimate true individual trait-level differences in functioning especially when including trajectories of change with age (Ludtke et al. 2008). Such analyses, if analyzed in interaction with traditional measures of behavior modeled similarly, may be better able to identify the subgroup of youth with the greatest cognitive vulnerability (for example, the intersecting area between ADHD symptoms and neurocognitive deficits in the Venn diagrams of Figures 1 and 2). Utilization of tasks specifically designed to tap the effective functioning of neural systems invoked in decisional balance regarding rewards and punishments, and including recent innovations that incorporate personalized reward value (Geier & Luna 2012), may also more effectively reflect cognitive-motivational imbalance within ADHD that creates addiction vulnerability. Identification of these brain-behavior relationships, and plasticity of the imbalance between cold and hot EF systems particularly in response to environmental shaping (e.g., effects of parenting shown throughout development in Figures 1 and 2), may help to better explain the brain-based reasons that some children with ADHD have elevated risk for substance disorder. An interesting question will be whether such discoveries can lead to interventions that shift the vulnerability trajectory as well as, or in addition to, interventions that target their outward behavioral expressions (principally, ADHD-related impairments that presumably increase substance disorder risk).
NEGATIVE AFFECT, EXPECTANCIES, AND COPING PATHWAYS
A widely held belief is that children with ADHD, due to their frequent performance failures in multiple domains of functioning, suffer low self-esteem and increased likelihood of internalizing disorders (specifically, depression). These consequences, in turn, are expected to produce substance disorder as a means of coping with the ensuing negative affect. Self-medication for the management of negative affect (Khantzian 1985) was hypothesized some time ago, outside of ADHD research, and enjoys popular clinical support, but mixed and complicated empirical support. If applicable to ADHD-related substance use, we would expect higher rates of internalizing disorder in ADHD, increased beliefs that drugs and alcohol alleviate negative affect and improve mood, strong connections between these states and substance use, and in general statistically significant associations in stress-negative affect pathways to substance use sometimes identified in other non-referred and high-risk populations (Chassin et al. 1993).
Depression, Other Negative Affect (Impulsive Anger), and SUD
Although prevalence varies widely across studies, with some reporting negative results (Bagwell et al. 2006, Claude & Firestone 1995, Mannuzza et al. 1998), childhood ADHD sometimes predicts increased rates of depression (Daviss 2008). Modestly but significantly higher rates of major depressive disorder (MDD) were found for ADHD combined and inattentive subtypes in the Willcutt et al (2012) meta-analytic review. A recent young adult follow-up study of girls with ADHD found higher internalizing composite scores on the adult form of the Achenbach behavior checklist, but not higher rates of depression symptoms or disorder per se (Hinshaw et al. 2012). However, prior to Bonferroni correction, the group differences in depression/dysthymia were statistically significant, with 7% of the nonADHD comparison group versus 19.5% and 21.2% of the ADHD inattentive and combined type groups, respectively, meeting diagnostic criteria. Chronis-Tuscano and colleagues (2010) found an increased rate of recurrent depressive episodes by age 18 in a longitudinally followed sample of young children with and without ADHD (18.4% vs 1.6%), even after controlling for maternal depression and other potentially confounding variables. In adulthood, rates of depression have been higher, particularly in the case of persisting ADHD symptoms and treatment-seeking populations (Barkley et al. 2008).
Increased risk of depression among individuals with ADHD, however, conflicts to some extent with the frequently documented tendency for children, adolescents, and young adults with ADHD to perceive their symptoms and impairment as better than others report. This bias in self-perceived competence, found across multiple domains of functioning, has been shown in a number of studies to be larger than in nonADHD comparison groups and tends to be largest for domains of greatest impairment (Hoza et al. 2002, 2004). Two studies of children with ADHD followed into adulthood showed dramatically lower rates of ADHD diagnosis at follow-up for self- as opposed to parent report of symptoms (Barkley et al. 2002, Sibley et al. 2012b). In the Barkley study, employer ratings and other data helped to validate parent ratings and supported the observation of positive bias in self-reports, at least with regard to ADHD symptoms. What remains puzzling, however, is that this positive lens should theoretically reduce risk of depression. As such, positive bias that characterizes only a portion of children with ADHD (estimated at 30-57% of children with ADHD combined subtype; Linnea et al. 2012), should theoretically decrease risk of depression. There is some support for an inverse association between positive bias and depression (Hoza et al. 2002, 2004) although prospective longitudinal data did not support this result (Hoza et al. 2010). Instead, positive bias appears to be most compellingly associated with externalizing behavior, social, and academic impairment, which are strong correlates and predictors of substance use. However, no studies have simultaneously examined self- and other-reports of symptoms and impairment in relation to substance use onset and escalation.
Translating ADHD-related risk of depression into hypotheses regarding substance use and disorder risk requires consideration of the complex relationship between negative affect and drug addiction. Drug use as a means of reducing aversive emotional states, and/or promoting positive emotions, has been of interest to researchers for many years (Kassel & Veilleux 2010). However, consistent findings regarding the prospective predictive utility of internalizing disorders for later substance use and disorder has been elusive. For example, Lansford and colleagues found cross-sectional associations between internalizing disorders at age 18 and substance use, but when analyzed longitudinally, only behavior disorders or comorbid behavior-internalizing disorders predicted change (i.e., slope) in illicit substance use from childhood to age 22 (Lansford et al. 2008). Other studies have also failed to find prospective prediction after controlling for externalizing covariates (e.g., Fergusson et al. 2009, Reinherz et al. 2000). These variable results, combined with robust cross-sectional associations of substance use/disorder with depression and anxiety in multiple studies at different developmental periods and for different populations (e.g., Chan et al. 2008) have prompted a more refined consideration of the processes that may underpin the association. This includes consideration of internalizing symptoms that onset early and persist into adulthood, disaggregation of internalizing symptoms to distinguish identification of states that may inhibit behavior and therefore decrease substance use risk, consideration of moderators such as externalizing symptoms, deviant peers, and opportunities for use, and utilization of daily assessment designs that better match theory and method (Hussong et al. 2011).
Relatively little research has been conducted to consider the contribution of mood or anxiety disorders to substance disorder vulnerability in ADHD, and none of this research has yet to incorporate recommendations such as those suggested above by Hussong and colleagues (2011). Wilens and colleagues have systematically examined internalizing disorder comorbidity within ADHD as a predictor of later substance disorder. Despite relatively high rates of major depressive (42%-52%) and anxiety disorders (23%-24%) at baseline (ages 6-17), neither of these comorbidities predicted the overall category of SUD at 10 year follow-up (Wilens et al. 2011). (Although prior work from this group has shown strong associations with bipolar disorder, e.g., Biederman et al. 1997). Usually, rates of psychiatric comorbidity are quite high for teens with substance use disorders (Chan et al. 2008, Rohde et al. 1996), but only 12.5% of adolescents met DSM-IV criteria for major depressive disorder (MDD) in a multi-site treatment trial of ADHD and SUD (Warden et al. 2012). Those with MDD reported more drug use days, before and throughout the trial, which is consistent with prior study findings that MDD comorbidity is associated with increased substance disorder severity. Outside of treatment seeking populations, there is some evidence that depression and ADHD symptoms mediate the intergenerational transmission of smoking from parent to offspring (Zoloto et al. 2012), although there appeared to be some sex-specificity in these associations (mediation by ADHD for girls and by depression for boys). Clearly, much work remains to be done to determine the circumstances under which various types of internalizing disorders increase substance disorder risk, to include the ever-important need to covary externalizing comorbidity but within a developmental design (i.e., dynamic and reciprocal effects over time with age), and to test associations using methods (i.e., daily assessments, see Hussong et al. 2011) that match hypotheses about affect and associated substance use.
An associated affective feature of ADHD, that has yet to be considered within research on ADHD-related substance disorder risk, is difficulty with impulsive anger or irritability. Low frustration tolerance, susceptibility to temper tantrums/hot temper, and quick anger/annoyance have long been recognized clinically as “associated features” of ADHD; these traits are depicted in the Venn diagrams of Figures 1 and 2. They are directly reflected in the emotional symptoms of ODD which is highly comorbid in ADHD and predictive of SUD (e.g., Wilens et al. 2011). They are also subsumed within CD comorbidity when children with reactive aggression are included. Multiple studies now identify an anger-irritability subset of ODD symptoms (loses temper, touchy or easily annoyed, angry or resentful) as distinct from the remaining ODD items measuring defiance and vindictiveness (Drabick et al. 2012, Mick et al. 2005, Stringaris et al. 2009). This construct has been strongly associated with multiple impairments for adults with ADHD. It also relates to persistence of ADHD symptoms into adulthood and explains variance in impairment above and beyond symptom persistence. It overlaps conceptually with “negative urgency,” a facet of impulsive behavior in response to negative affect (Whiteside and Lynam 2001), and may have relevance for problem drinking (Dick et al. 2010). Research on this aspect of ADHD, which characterizes only a portion of individuals with ADHD, but which may also signify a specific endophenotype vulnerable to addiction (as opposed to social binge drinking typical of college students, Cyders et al. 2009), is recommended.
Perceived Effects of Alcohol and Other Drugs (Expectancies)
Initially developed within the field of alcoholism research, and initiated with the pioneering work of Brown and Goldman (Brown et al. 1980), expectations of the effects of alcohol are now widely recognized to play a significant role in the initiation and development of alcohol use and disorder. Later expanded to include cognitions regarding the negative, as well as the positive, consequences of drinking (Fromme et al. 1993), expectancies include cognitions regarding positive reinforcement (positive outcomes of drinking), negative reinforcement (reduction in negative affect or other states), and punishment (negative outcomes occurring as a function of drinking) from alcohol use (Wiers et al. 2007). Expectancies begin to form at a young age long before alcohol consumption begins (Zucker et al. 1995) and they prospectively predict drinking in adolescence (e.g., Christiansen et al. 1989). They also correlate strongly with alcohol use and this association changes with age such that social and sexual enhancement expectancies relate most strongly for younger, versus older, adults (Pabst et al. 2010). Social learning experiences are generally believed to drive the early development of expectancies, while more sophisticated models incorporating the development of cognitive-affective-motivational processes in relation to expectancy formation and utilization (Wiers et al. 2007) have been proposed to explain the role of expectancies in the development of alcohol and other drug addiction.
Very little research has been conducted to directly examine expectancies and their relation to any type of substance use for individuals with ADHD. However, research on individual difference variables pertaining to ADHD-like traits and risk profiles pertinent to ADHD (e.g., familial alcohol and drug abuse) suggest interesting hypotheses about expectancies in this population. Substance disorders occur at higher rates than chance in families affected by ADHD (Biederman et al. 2008), and a positive family history of alcoholism is correlated with expectancies. For example, Wiers and colleagues found stronger expectancies of positive arousal after the onset of drinking for adolescent children of alcoholics (COAs; Wiers et al. 1998). Evidence that COAs experience more positive arousing and less negative and sedating effects of alcohol is thought to partly underlie these differences (Newlin & Thompson 1990). Schuckit and colleagues’ work suggests that a high tolerance to the negative effects of alcohol may be part of a pathway from familial alcoholism to adult alcohol problems that includes externalizing behaviors and positive alcohol expectancies (Schuckit & Smith 2006). This would suggest that in vulnerable individuals, presumably those genetically predisposed to a differential response, that negative expectancies would be lower and less strongly related to alcohol use in COAs (Wiers et al. 2007). Positive expectancies, given their reflection of appetitive processes that may be under-regulated in adolescence, and particularly so for individuals with ADHD, should relate more strongly to alcohol use in ADHD.
The dual process model of expectancies proposed by Stacy and Wiers (2010), however, asserts that for individuals with poor EF, expectancies of the type measured in traditional questionnaires may be less strongly tied to behavior than expectancies measured using tasks that indirectly assess attentional bias or memory associations around alcohol (e.g., associative memory tasks; Stacy 1997) and which are presumed to tap automatic cognitive processes regarding alcohol. Indeed, Thrush and colleagues (2008) found that adolescent alcohol use was more related to implicit, than to explicit, alcohol expectancies for adolescents with low working memory. Similarly, positive implicit associations about alcohol were more strongly related to young adult alcohol use for individuals high in impulsivity as indicated by high positive or negative urgency (Burton et al. 2012).
These findings suggest that expectancies might be different (in absolute value), and operate differently, for individuals with versus without ADHD. Only two studies have directly tested expectancies in ADHD and these are limited to cognitions about alcohol. In a study of college students, Datillo and colleagues found that positive expectancies were higher and more strongly associated with alcohol use for students with higher ADHD symptom scores (Datillo et al. 2013). In contrast, children with ADHD followed into adolescence reported fewer positive (sociability and liquid courage) and fewer negative (cognitive and behavioral impairment) expectancies than comparison adolescents without ADHD histories. Additionally, negative expectancies were less predictive of drinking one year later (Pedersen et al. in press). These findings suggest the possibility, although untested, that substance use (in this case, alcohol use) may be more strongly affected in ADHD by automatic appetitive processes rather than by those governed by EF capacities, and that studies of implicit expectancies are needed for this population (Pedersen et al. in press). Moreover, the findings suggest the intriguing possibility that reliable assessment of the subpopulation of ADHD that is persistently weak in higher order cognitive control, yet strong in emotional urges, may be at greatest risk (see above, regarding impulsive anger and negative urgency). Lack of self-awareness may also be a contributing factor in these ADHD-specific findings for explicitly measured alcohol expectancies.
Coping Skills
As mentioned earlier, substance use to cope with negative affect has been a prominent concept in the addiction literature (Khantzian 1985). However, rather than inciting initial use and escalation in substance use, negative-affect motivated use has been more clearly connected to cycles of repeated intoxication and withdrawal that result in recurring aversive emotions and motivation to consume drugs and alcohol for relief (Chassin et al. in press, Koob & Volkow 2010). This recursive process, illustrated in Figure 2 with a bidirectional arrow, is more likely to be relevant in advanced stages of addiction of the type seen in adulthood (although there certainly are teenagers with advanced stages of addiction). In fact, relatively few youth report drinking to copy with negative affect (Kuntsche et al. 2005). Negative affect-motivated substance use, therefore, may be most likely to occur for individuals with ADHD in adulthood, when rates of depression appear to rise a bit, rather than in adolescence. This hypothesis remains untested.
Coping skills should have some importance with regard to this potential pathway, with regard to the ability to refrain from socially mediated temptations in adolescence and in early adulthood, and for effective recovery once addiction occurs. Effective coping skills (adaptive behavioral strategies) by teens have been found to predict less growth in substance use than strategies that are typically less effective (anger coping, helpless coping, and hangout coping) (Wills et al. 2001). However, as might be expected for teenagers with cognitive and behavioral control problems, teenagers with ADHD histories have less effective coping skills, at least as reported by their mothers. These deficiencies were associated with cigarette smoking (Molina et al. 2005), similar to Wills’ findings. We depict this pathway in Figure 2. An important question for future research is whether individuals with cognitive-behavioral deficits of the sort that characterize individuals with ADHD, can change their coping skill repertoire, particularly in tempting situations that invoke automatic reward-seeking responses. For example, effective coping skills may dampen utilization of drugs and alcohol in response to expectations of social enhancement (e.g., alcohol) or anxiolytic effects (e.g., marijuana). This possible moderating effect of coping on the association between expectancies and substance use is denoted in Figure 2.
PARENTING, THE PARENT-CHILD RELATIONSHIP, AND OTHER “EXTERNAL” INFLUENCES
The parenting environment and other social, cultural, and structural environmental factors extrinsic to the vulnerable child are often conceptualized as potential targets for intervention to mitigate substance disorder risk and to treat ongoing substance disorder. Although it is widely recognized that risk variables for substance use outcomes tend to aggregate across individual, familial, and even neighborhood levels (Zucker 2006) that may reciprocally exacerbate with age and time (CPPRG 2011), and therefore parenting is not independent of child risk profiles (in this case, ADHD), parenting strategies and the parent-child relationship are among the most widely researched variables for their capacity to affect pre-existing addiction liability.
Parenting, the Parent-Child Relationship, ADHD, and Substance Use
It has long been known that parents of children with disruptive behavior disorders, including ADHD, often employ inappropriate parenting strategies (e.g., inconsistent discipline, reduced positive interactions) that reinforce their children’s negative behaviors and inadvertently contribute to the children’s poor outcomes (Johnston & Mash 2001, Patterson 1982, Patterson & Fisher 2002). It has also long been known that a dynamic, transactional model best explains the relationship between ADHD/externalizing child behaviors and parenting (Johnston & Chronis-Toscano in press). Thus, we include bidirectional arrows in our figures with respect to the associations between parenting and the parent-child relationship with child and adolescent behavior. Although parenting is not believed to initially cause ADHD, the impulsive behavior of children with ADHD and, when it occurs, inappropriate parenting strategies each contribute to the development of the other in a reciprocal, transactional manner. Longitudinal studies (Patterson & Fisher 2002) have documented these effects, as have controlled laboratory studies. In one illustrative set of studies, child actors were employed to exhibit ADHD and noncompliant behaviors to examine their impact on parenting and subsequent parental alcohol consumption, and parental alcohol consumption was manipulated to examine the impact on parenting (Lang et al. 1999, Pelham et al. 1997). The results showed that parents respond to ADHD/oppositional behavior in children with high levels of stress and negative affect and increased alcohol consumption when given the opportunity to drink when anticipating another interaction with the same child. When alcohol was manipulated, the strategies that parents employed to control the confederates’ behavior became inappropriate in ways that the literature would predict. Together these studies demonstrate the reciprocal relationship that exists between externalizing child behavior and parenting. These patterns are exacerbated by the fact that the parents of children with ADHD themselves have high rates of ADHD and other forms of psychopathology (including substance disorders) that interfere with their parenting (Johnston et al. 2012).
Parenting strategies such as developmentally appropriate limit-setting with enforcement, including positive and negative consequences, and good parent-child relationship quality (e.g., empathy, warmth, instrumental and emotional support) have been shown in many studies to be prospectively related to adolescent substance use (e.g., Barnes et al. 2006, Ellickson et al. 2004) and even to predict substance use into early adulthood (Guo et al. 2001, Raudino et al. 2013). For example, quality of the parent-child relationship in adolescence (bonding and attachment) predicted multiple outcomes by age 30, including reduced illicit drug abuse and dependence, even after controlling for multiple confounding variables (Raudino et al. 2013). Parenting behavior moderates the impact of genetic vulnerability that may characterize a subgroup within ADHD. Otten and colleagues found that carriers of the DRD4-7 repeat allele were prospectively more likely to report marijuana use in the presence of low parental monitoring (Otten et al. 2012). Interventions that include enhancement of parenting skill also begin to shape the substance use risk trajectory well before substance use onset (e.g., CPPRG 2011) and have been shown to delay alcohol onset (Koning et al. 2013) and decrease days of illicit drug use following brief strategic family therapy (Robbins et al. 2011). Thus, interventions that improve parenting have the potential to reap benefits for multiple emotional and behavioral health outcomes that include substance use (although the maintenance of these effects beyond their acute and short-term follow-up windows remains stubbornly unimpressive, Lochman et al. 2010).
Only two studies of independent samples from the same research group have examined the contribution of parenting variables to adolescent substance use in relation to ADHD. In one study, parental knowledge of the adolescent’s activities and whereabouts moderated the association between childhood ADHD and growth in alcohol use frequency through adolescence (Molina et al. 2012). In this case, parental knowledge was based on the adolescent’s report that parents “really knew” about their friendships, location at night, how free time was spent, and so on. When knowledge was low (i.e., parents only knew these things “some of the time” or less often), childhood ADHD predicted a higher frequency of alcohol use by age 17. Mediating pathways through secondary school GPA, ADHD symptom persistence, social impairment, and delinquency indicated that these impairments contributed importantly to the overall risk profile defined by lower parental awareness and childhood ADHD. Another study of an independent sample, covering approximately the same age range but assessed several years earlier, revealed that parental knowledge (also adolescent-reported) was more strongly associated with alcohol use in the ADHD than in the nonADHD group and after controlling for the effects of other parenting variables, namely discipline consistency, support, and conflict, all of which were significantly worse in the ADHD group (Walther et al. 2012). Variance shared by the parenting variables, as indicated by a latent factor in a “general-specific” model of parenting, also predicted delinquency more strongly for the ADHD versus nonADHD group.
These findings suggest that intervention to increase the effectiveness of parental monitoring, as well as other developmentally appropriate parenting techniques, should dampen addiction vulnerability for children with ADHD. However, there are only a handful of studies of parent training or family therapy for adolescents with ADHD (cf Barkley et al. 1992, 2001), and those have shown only moderately beneficial results. In contrast, more than 90% of the psychosocial treatment studies for ADHD are with younger children, and the results are quite robust (Pelham & Fabiano 2008). In several trials in which intensive behavioral treatment has been used successfully at an early age in ADHD samples, benefits from the behavioral interventions on a variety of outcomes, including very early substance use, have been found at 2-3 year follow-up (August et al. 2002, Molina et al. 2007) but lost in later follow up (e.g., Molina et al. 2013). In neither of these trials was the intensive intervention in young childhood followed by ongoing work with parents/families into adolescence. It is possible that the impact of early intervention will maintain if treatment is reinstated during the offsprings’ adolescence when the skills that parents need are substantively different from those they implement during childhood. Thus, more research is needed both to identify the parenting behaviors that are thought to impact substance use and substance disorder vulnerability amongst teens with ADHD and to develop and evaluate interventions to improve these parenting skills during the adolescent years. Research is also needed to determine the best strategies for parents to use during emerging adulthood when substance use reaches its peak but individuals with ADHD histories are ill-equipped to handle the autonomy they desire.
Two avenues for further investigation are suggested from related research. First, the extent to which parenting affects other processes known to be areas of vulnerability in ADHD should be examined. For example, Giannotta and colleagues (2011) found that parental knowledge of child daily activities decreased the effect of peer influence on cigarette smoking. This potential moderating effect has not been tested in ADHD. It is also unlikely that successful monitoring alone would be sufficient, particularly when parenting an adolescent whose interpersonal skills preclude easy establishment of healthy friendships (such as might occur for adolescents with ADHD). Second, a nascent but growing body of research is uncovering the potential added value of substance-specific parenting. Alcohol-specific rules have been shown to predict less alcohol use in adolescence (Mares et al. 2012). However, parents may also need to be careful about their disclosures of their own personal negative experiences with alcohol which have been shown to increase teen drinking at least for mothers (Handley & Chassin 2013). Thus, added traction may be gained in parenting intervention development by considering not only universally beneficial parenting practices but also communications and actions specific to potential substances of abuse. However, parental history of alcoholism, other substance disorder, or cigarette smoking, known to be higher in families affected by ADHD, may require special separate attention (Chassin et al. 2005).
CNS Stimulant Medication and Substance Use
One reason for continued consideration of psychosocial interventions that target parenting, parent-child relationships, and other therapeutic targets discussed above, such as academic functioning, peer relations, and coping skills, is that stimulant medication, the most commonly used and widely available treatment for ADHD (American Academy of Pediatrics 2011), has not been shown to protect against the development of substance use and disorder (Humphreys et al. 2013, Molina et al. 2013). A very large literature documents that stimulants are acutely effective for the reduction of ADHD symptoms and related measures of functional impairment in the majority of treated children. The improvements in treated children occur in many of the domains we have discussed above, including core symptoms of inattention and impulsivity, daily academic performance and classroom deportment, peer interactions, and parent ratings of children’s behavior (e.g., Pelham et al. 2001). These acute benefits continue for at least two years if medication is continued (Abikoff et al. 2004; Multimodal Treatment of ADHD (MTA) Cooperative Group, 2004), and the acute benefits in adolescents have been similar (e.g., Evans et al. 2001, Smith et al. 1998). These medication effects in domains predictive of substance use and also demonstrated in treated adolescents suggest that pharmacotherapy should lead to reduced risk for substance use in individuals with ADHD. However, this hypothesis has not been consistently supported.
These negative findings may be the result of several factors. First, although most children with ADHD are treated at some point with stimulants, few continue beyond a year (Marcus et al. 2005). The rate at which children with ADHD continue to take psychoactive medication drops consistently with each increasing year-age after approximately fourth or fifth grade, and the vast majority of treated children have discontinued medication by the time they reach the years at which teens typically begin substance use (Barkley et al. 2003, Molina et al. 2009). Second, the impact of stimulant medication on the impairments of ADHD that are also thought to increase risk of substance dependence, as reviewed herein, is not robust. For example, although stimulants improve daily academic seatwork performance in children and teens in controlled classroom studies, long-term substantial effects on school grades, retention, dropout and the other domains that are so problematic in middle and high school for children with ADHD (e.g., Kent et al. 2011, Langberg et al. 2011) and central to risk for drug use in these years, are typically not found (Molina et al. 2009). A few uncontrolled studies have shown beneficial effects on standardized tests using large national samples of convenience and examining records of medication and achievement (e.g., Scheffler et al. 2009). However, the results, though statistically significant with the large sample, were not meaningful--an average of .19 and .26 school-years of additional achievement in math and reading throughout the entirety of elementary school. Finally, parental reliance on pharmacotherapy for their children with ADHD may undermine parental motivation to seek, learn, and implement the psychosocial interventions (e.g., parent training, family therapies) that are explicitly targeted at the key mediators of substance use risk (e.g., Pelham et al. 2007).
SUMMARY AND CONCLUSIONS
Two mostly independent literatures – one on ADHD and the other on substance disorder risk – have meaningful points of convergence that produce ample opportunities for research on ADHD-related risk of substance disorder. Longitudinal studies of ADHD have revealed significantly increased risk of alcohol, nicotine, and other drug disorder by adulthood (Lee et al. 2011), but with the exception of nicotine dependence, findings are somewhat variable, age specific, and effect sizes are modest. We reviewed the relevant literature to determine what methodological considerations might be contributing to these findings and to ascertain opportunities for future research on factors that might contribute to heterogeneity of substance disorder risk within ADHD. We summarize our conclusions in a series of bullets below.
Longitudinal studies of non-referred children show that temperament and personality traits measured from a very young age, and that are the same behaviors captured in more extreme form in the ADHD symptom list, predict later substance use and substance disorder into adulthood.
A statistically significant association between childhood ADHD and substance disorder by adulthood has been demonstrated, but there is some heterogeneity in findings, and more sophisticated substance use measurement is needed. Continued study of the association needs to employ developmentally appropriate assessments that produce age-specific variables for consideration throughout multiple developmental periods.
Consideration of CD comorbidity should move beyond analysis of its contemporaneous co-occurrence, especially in adolescence, to study its development over time in relation to development of ADHD symptoms and other putative mediators and moderators of substance disorder risk in ADHD.
Common ADHD-related impairments, such as poor performance in school and social rejection, as well as growth in serious conduct problems, should be studied for their contribution to substance disorder. Studies need to be done with measurement both in childhood and in adolescence. Impairments such as these provide immediate targets for intervention development.
Interpersonal difficulties are heterogeneous for individuals with ADHD histories. Some sequelae may increase substance disorder risk (e.g., social rejection that leads to affiliation with substance-using peers) while others may suppress vulnerability (e.g., social passivity and isolation). These associations, and candidate moderators (e.g., parenting strategies), need longitudinal study in adolescence and in adulthood.
Executive functioning deficits as measured by standard neuropsychological test batteries describe only a subset of children with ADHD and, to date, do not help distinguish those with substance dependence vulnerability. Expansion of batteries that tap motivation-reward processes and the interface with higher order cognitive control may help uncover brain-based vulnerabilities that better relate to substance disorder outcomes.
Longitudinal studies that utilize trajectory-based methods to distinguish individuals with specific patterns of functioning over time should improve detection of risk beyond single point-in-time assessments. This suggestion may apply to neurocognitive assessments as well as to ADHD symptom/personality-type measures.
Stress-negative affect models, including the self-medication hypothesis, although clinically appealing, have received mixed support in the nonADHD literature and little research in the ADHD-substance disorder literature. Substance use as a function of negative affect may be more relevant in adulthood when rates of depression appear to increase in ADHD and when coping with withdrawal-related negative is more likely.
Trait impulsive anger, that characterizes a portion of individuals with ADHD and predicts worse impairment long-term, may increase risk of problems from substance use. Beyond including children with reactive aggression in subgroups of children with CD diagnosis, no research has specifically addressed this more refined temperament question in the association between ADHD and substance disorder vulnerability.
Beliefs about the effects of alcohol and other drugs (expectancies) have received minimal research attention in the ADHD literature despite their well-documented contribution to alcohol disorder. This is a fruitful area for future research, for both alcohol and other drugs of abuse, that should consider current models of expectancy formation and utilization as well as limitations of interpretation resulting from the cognitive and self-awareness deficits of ADHD. 11. Effective use of developmentally appropriate parenting strategies and a supportive parent-child/adolescent relationship, known to be important in substance disorder vulnerability for all children, may protect against substance use/disorder in ADHD, if implemented during periods of risk in addition to childhood. However, additional research is needed, including the impact of moderators (e.g., impact of parental mental health and substance use problems), and age (e.g., effect on emerging adult substance use when day-to-day parental involvement typically decreases).
Stimulant medication treatment, despite its well-documented acute effects on ADHD symptoms and impairment, has failed to demonstrate protective effects against substance use and disorder, as it has failed to demonstrate beneficial long-terms effects in all of the key domains that mediate the development of SUD.
Simultaneous consideration of multiple risk factors, as has been used in substance use research on normative samples that predict up to 50% of the variance in substance use (Donovan 1996), is likely to be crucial for understanding and maximizing prediction of substance use and disorder for children with ADHD.
Research on the development and empirical evaluation of interventions that simultaneously target ADHD-related impairments and substance-specific vulnerabilities, for the adolescent and young adult age ranges, is needed. Given the difficulty experienced with demonstration of long-term effects of any treatment for ADHD and SUDs, these interventions should acknowledge and potentially incorporate the need for life-long management of ADHD-related SUD vulnerability.
Acknowledgments
This research was principally supported by AA011873, DA-8-5553, and DA12414. Additional support was provided by AA12342, AA00202, MH50467, MH12010, ESO5015, DA016631, MH065899, KAI-118-S1, DA85553, MH077676, MH069614, MH62946, MH065899, MH53554, MH069434, IES LO3000665A, IESR324B060045, & NS39087.
Footnotes
DISCLOSURE STATEMENT
The author is not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
Contributor Information
Brooke S.G. Molina, Departments of Psychiatry and Psychology, University of Pittsburgh, Pittsburgh, PA 15213
William E. Pelham, Jr., Departments of Psychology and Psychiatry, Florida International University, Miami, FL 33199, wpelham@fiu.edu
LITERATURE CITED
- Abikoff H, Hechtman L, Klein RG, Weiss G, Fleiss K, et al. Symptomatic improvement in children with ADHD treated with long-term methylphenidate and multimodal psychosocial treatment. Journal of the American Academy of Child and Adolescent Psychiatry. 2004;43:802–11. doi: 10.1097/01.chi.0000128791.10014.ac. [DOI] [PubMed] [Google Scholar]
- Achenbach TM, Conners CK, Quay HC, Verhulst FC, Howell CT. Replication of empirically derived syndromes as a basis for taxonomy of child/adolescent psychopathology. J Abnorm Child Psychol. 1989;17:299–323. doi: 10.1007/BF00917401. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 3rd Edition. American Psychiatric Association; Washington, DC: 1980. [Google Scholar]
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th Edition. American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 5th Edition. American Psychiatric Association; Washington, DC: 2013. [Google Scholar]
- Arcos-Burgos M, Velez JI, Solomon BD, Muenke M. A common genetic network underlies substance use disorders and disruptive or externalizing disorders. Hum Genet. 2012;131:917–29. doi: 10.1007/s00439-012-1164-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins MS, Pelham WE, Licht MH. A comparison of objective classroom measures and teacher ratings of Attention Deficit Disorder. J Abnorm Child Psychol. 1985;13:155–67. doi: 10.1007/BF00918379. [DOI] [PubMed] [Google Scholar]
- Atkins MS, Pelham WE, Licht MH. The differential validity of teacher ratings of inattention/overactivity and aggression. J Abnorm Child Psychol. 1989;17:423–35. doi: 10.1007/BF00915036. [DOI] [PubMed] [Google Scholar]
- August GJ, Hektner JM, Egan EA, Realmuto GM, Bloomquist ML. The early risers longitudinal prevention trial: Examination of 3-year outcomes in aggressive children with intent-to-treat and as-intended analyses. Psychology of Addictive Behaviors. 2002;16:S27–S39. doi: 10.1037/0893-164x.16.4s.s27. [DOI] [PubMed] [Google Scholar]
- Babinski DE, Pelham WE, Molina BSG, Gnagy EM, Waschbusch DA, et al. Late adolescent and young adult outcomes of girls diagnosed with ADHD in childhood: An exploratory investigation. Journal of Attention Disorders. 2011;15:204–14. doi: 10.1177/1087054710361586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagwell CL, Molina BSG, Kashdan TB, Pelham WE, Hoza B. Anxiety and mood disorders in adolescents with childhood attention-deficit hyperactivity disorder. Journal of Emotional and Behavioral Disorders. 2006;14:178–87. [Google Scholar]
- Bagwell CL, Molina BS, Pelham WE, Jr., Hoza B. Attention-deficit hyperactivity disorder and problems in peer relations: predictions from childhood to adolescence. J Am Acad Child Adolesc Psychiatry. 2001;40:1285–92. doi: 10.1097/00004583-200111000-00008. [DOI] [PubMed] [Google Scholar]
- Barkley R. Executive Functions: What They Are, How They Work, and Why They Evolved. Guilford Press; New York, NY: 2012. [Google Scholar]
- Barkley RA, Edwards G, Laneri M, Fletcher K, Metevia L. The efficacy of problem-solving communication training alone, behavior management training alone, and their combination for parent-adolescent conflict in teenagers with ADHD and ODD. Journal of Consulting and Clinical Psychology. 2001;69:926–41. [PubMed] [Google Scholar]
- Barkley RA, Fischer M, Edelbrock CS, Smallish L. The adolescent outcome of hyperactive children diagnosed by research criteria: I. An 8-year prospective follow-up study. Journal of the American Academy of Child and Adolescent Psychiatry. 1990;29:546–57. doi: 10.1097/00004583-199007000-00007. [DOI] [PubMed] [Google Scholar]
- Barkley RA, Fischer M, Smallish L, Fletcher KE. Does the treatment of Attention-Deficit/Hyperactivity Disorder with stimulants contribute to drug use/abuse? A 13-year prospective study. Pediatrics. 2003;111:97–109. doi: 10.1542/peds.111.1.97. [DOI] [PubMed] [Google Scholar]
- Barkley RA, Fischer M, Smallish L, Fletcher KE. Young adult outcome of hyperactive children: Adaptive functioning in major life activities. Journal of the American Academy of Adolescent Psychiatry. 2006;45:192–202. doi: 10.1097/01.chi.0000189134.97436.e2. [DOI] [PubMed] [Google Scholar]
- Barkley RA, Guevremont DC, Anastopoulos AD, Fletcher KE. A comparison of three family therapy programs for treating family conflicts in adolescents with attention-deficit hyperactivity disorder. Journal of Consulting and Clinical Psychology. 1992;60:450–62. doi: 10.1037/0022-006X.60.3.450. [DOI] [PubMed] [Google Scholar]
- Barkley RA, Murphy KR, Fischer M. ADHD in Adults: What the Science Says. The Guilford Press; New York: 2008. p. 500. [Google Scholar]
- Barnes GM, Hoffman JH, Welte JW, Farrell MP, Dintcheff BA. Effects of Parental Monitoring and Peer Deviance on Substance Use and Delinquency. Journal of Marriage and Family. 2006;68:1084–104. [Google Scholar]
- Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nat Neurosci. 2005;8:1458–63. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- Becker SP, McBurnett K, Hinshaw SP, Pfiffner LJ. Negative Social Preference in Relation to Internalizing Symptoms Among Children with ADHD Predominantly Inattentive Type: Girls Fare Worse Than Boys. Journal of Clinical Child & Adolescent Psychology. 2013:1–12. doi: 10.1080/15374416.2013.828298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederman J, Petty CR, Wilens TE, Fraire MG, Purcell CA, et al. Familial risk analyses of attention deficit hyperactivity disorder and substance use disorders. Am J Psychiatry. 2008;165:107–15. doi: 10.1176/appi.ajp.2007.07030419. [DOI] [PubMed] [Google Scholar]
- Biederman J, Wilens T, Mick E, Faraone SV, Weber W, et al. Is ADHD a risk factor for psychoactive substance use disorders? Findings from a four-year prospective follow-up study. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:21–29. doi: 10.1097/00004583-199701000-00013. [DOI] [PubMed] [Google Scholar]
- Blachman DR, Hinshaw SP. Patterns of friendship among girls with and without attention-deficit/hyperactivity disorder. J Abnorm Child Psychol. 2002;30:625–40. doi: 10.1023/a:1020815814973. [DOI] [PubMed] [Google Scholar]
- Block J, Block JH, Keyes S. Longitudinally foretelling drug usage in adolescence: Early childhood personality and environmental precursors. Child Development. 1988;59:336–55. doi: 10.1111/j.1467-8624.1988.tb01470.x. [DOI] [PubMed] [Google Scholar]
- Brook DW, Brook JS, Zhang CS, Koppel J. Association Between Attention-Deficit/Hyperactivity Disorder in Adolescence and Substance Use Disorders in Adulthood. Archives of Pediatrics & Adolescent Medicine. 2010;164:930–34. doi: 10.1001/archpediatrics.2010.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook JS, Brook DW. The psychosocial etiology of adolescent drug use: A family interactional approach. Genetic, Social & General Psychology Monographs. 1990;116:111. [PubMed] [Google Scholar]
- Brook JS, Rubenstone E, Zhang C, Finch SJ, Brook DW. The Intergenerational Transmission of Smoking in Adulthood: A 25-Year Study of Maternal and Offspring Maladaptive Attributes. Addictive behaviors. 2013 doi: 10.1016/j.addbeh.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook JS, Whiteman M, Cohen P, Shapiro J, Balka E. Longitudinally predicting late adolescent and young adult drug use: Childhood and adolescent precursors. Journal of the American Academy of Child and Adolescent Psychiatry. 1995;34:1230–38. doi: 10.1097/00004583-199509000-00022. [DOI] [PubMed] [Google Scholar]
- Brown RT, Freeman WS, Perrin JM, Stein MT, Amler RW, et al. Prevalence and assessment of attention-deficit/hyperactivity disorder in primary care settings. Pediatrics. 2001;107 doi: 10.1542/peds.107.3.e43. [DOI] [PubMed] [Google Scholar]
- Brown SA, Goldman MS, Inn A, Anderson LR. Expectations of reinforcement from alcohol: their domain and relation to drinking patterns. Journal of Consulting and Clinical Psychology. 1980;48:419–26. doi: 10.1037//0022-006x.48.4.419. [DOI] [PubMed] [Google Scholar]
- Burke JD, Loeber R, Lahey BB. Which aspects of ADHD are associated with tobacco use in early adolescence? Journal of Child Psychology and Psychiatry and Allied Disciplines. 2001;42:493–502. [PubMed] [Google Scholar]
- Burton CM, Pedersen SL, McCarthy DM. Impulsivity moderates the relationship between implicit associations about alcohol and alcohol use. Psychol Addict Behav. 2012;26:766–72. doi: 10.1037/a0028742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantwell DP. Psychiatric illness in the families of hyperactive children. Arch Gen Psychiatry. 1972;27:414–7. doi: 10.1001/archpsyc.1972.01750270114018. [DOI] [PubMed] [Google Scholar]
- Carbonneau R, Tremblay RE, Vitaro F, Dobkin PL, Saucier JF, Pihl RO. Paternal alcoholism, paternal absence and the development of problem behaviors in boys from age six to twelve years. J Stud Alcohol. 1998;59:387–98. doi: 10.15288/jsa.1998.59.387. [DOI] [PubMed] [Google Scholar]
- Carlson CL, Lahey BB, Frame CL, Walker J, Hynd GW. Sociometric status of clinic-referred children with Attention Deficit Disorders with and without Hyperactivity. Journal of Abnormal Child Psychology. 1987;15:537–47. doi: 10.1007/BF00917239. [DOI] [PubMed] [Google Scholar]
- Carey W. Is ADHD a valid disorder? In Attention Deficit Hyperactivity Disorder: State of the Science. Best Practices. Civic Research Institute; Kingston NJ: 2002. [Google Scholar]
- Caspi A, Moffitt TE, Newman DL, Silva PA. Behavioral Observations at Age 3 Years Predict Adult Psychiatric Disorders: Longitudinal Evidence From a Birth Cohort. Arch Gen Psychiatry. 1996;53:1033–39. doi: 10.1001/archpsyc.1996.01830110071009. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Sonuga-Barke EJ, Milham MP, Tannock R. Characterizing cognition in ADHD: beyond executive dysfunction. Trends Cogn Sci. 2006;10:117–23. doi: 10.1016/j.tics.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Chan YF, Dennis ML, Funk RR. Prevalence and comorbidity of major internalizing and externalizing problems among adolescents and adults presenting to substance abuse treatment. J Subst Abuse Treat. 2008;34:14–24. doi: 10.1016/j.jsat.2006.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charach A, Yeung E, Climans T, Lillie E. Childhood Attention-Deficit/Hyperactivity Disorder and Future Substance Use Disorders: Comparative Meta-Analyses. Journal of the American Academy of Child and Adolescent Psychiatry. 2011;50:9–21. doi: 10.1016/j.jaac.2010.09.019. [DOI] [PubMed] [Google Scholar]
- Chassin L, Colder C, Hussong A, Sher KJ. Substance use and substance use disorders. In: Cicchetti D, editor. Developmental Psychopathology. In press. [Google Scholar]
- L Chassin, DR Pillow, PJ Curran, BSG Molina, M Barrera. Relation of parental alcoholism to early adolescent substance use: A test of three mediating mechanisms. Journal of Abnormal Psychology. 1993;102:3–19. doi: 10.1037//0021-843x.102.1.3. [DOI] [PubMed] [Google Scholar]
- Chassin L, Presson CC, Rose J, Sherman SJ, Davis MJ, Gonzalez JL. Parenting style and smoking-specific parenting practices as predictors of adolescent smoking onset. J Pediatr Psychol. 2005;30:333–44. doi: 10.1093/jpepsy/jsi028. [DOI] [PubMed] [Google Scholar]
- Christiansen BA, Smith GT, Roehling PV, Goldman MS. Using alcohol expectancies to predict adolescent drinking after one year. Journal of Consulting and Clinical Psychology. 1989;57:93–99. doi: 10.1037//0022-006x.57.1.93. [DOI] [PubMed] [Google Scholar]
- Chronis-Tuscano A, Molina BS, Pelham WE, Applegate B, Dahlke A, et al. Very early predictors of adolescent depression and suicide attempts in children with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2010;67:1044–51. doi: 10.1001/archgenpsychiatry.2010.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claude D, Firestone P. The development of ADHD boys: A 12-year follow-up. Canadian Journal of Behavioral Science. 1995;27:226–49. [Google Scholar]
- Conners CK. A Teacher Rating Scale for Use in Drug Studies with Children. American Journal of Psychiatry. 1969;126:884–888. doi: 10.1176/ajp.126.6.884. [DOI] [PubMed] [Google Scholar]
- Cubillo A, Halari R, Smith A, Taylor E, Rubia K. A review of fronto-striatal and fronto-cortical brain abnormalities in children and adults with Attention Deficit Hyperactivity Disorder (ADHD) and new evidence for dysfunction in adults with ADHD during motivation and attention. Cortex. 2012;48:194–215. doi: 10.1016/j.cortex.2011.04.007. [DOI] [PubMed] [Google Scholar]
- Curran PJ, Stice E, Chassin L. The relation between adolescent alcohol use and peer alcohol use: A longitudinal random coefficients model. Journal of Consulting and Clinical Psychology. 1997;65:130–40. doi: 10.1037//0022-006x.65.1.130. [DOI] [PubMed] [Google Scholar]
- Cyders MA, Flory K, Rainer S, Smith GT. The role of personality dispositions to risky behavior in predicting first-year college drinking. Addiction. 2009;104:193–202. doi: 10.1111/j.1360-0443.2008.02434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dattilo L, Murphy KG, Van Eck K, Flory K. Do ADHD symptoms moderate the relation between positive alcohol expectancies and alcohol-related outcomes? Atten Defic Hyperact Disord. 2013;5:93–104. doi: 10.1007/s12402-012-0098-y. [DOI] [PubMed] [Google Scholar]
- Daviss WB. A review of co-morbid depression in pediatric ADHD: etiology, phenomenology, and treatment. J Child Adolesc Psychopharmacol. 2008;18:565–71. doi: 10.1089/cap.2008.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derefinko KD, Pelham WE. ADHD and substance use. In: Sher K, editor. Oxford handbook of substance use and substance dependence. Oxford; New York: In press. [Google Scholar]
- Diamantopoulou S, Rydell AM, Thorell LB, Bohlin G. Impact of executive functioning and symptoms of attention deficit hyperactivity disorder on children’s peer relations and school performance. Dev Neuropsychol. 2007;32:521–42. doi: 10.1080/87565640701360981. [DOI] [PubMed] [Google Scholar]
- Dick DM, Smith G, Olausson P, Mitchell SH, Leeman RF, et al. Understanding the construct of impulsivity and its relationship to alcohol use disorders. Addiction Biology. 2010;15:217–26. doi: 10.1111/j.1369-1600.2009.00190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan JE. Problem-behavior theory and the explanation of adolescent marijuana use. Journal of Drug Issues. 1996;26:379–404. [Google Scholar]
- Douglas VI. Stop, look, and listen: the problem of sustained attention and impulse control in hyperactive and normal children. Can J Behav Sci. 1972:4-259–282. [Google Scholar]
- Drabick DA, Gadow KD. Deconstructing oppositional defiant disorder: clinic-based evidence for an anger/irritability phenotype. J Am Acad Child Adolesc Psychiatry. 2012;51:384–93. doi: 10.1016/j.jaac.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan GJ, Dowsett CJ, Claessens A, Magnuson K, Huston AC, et al. School readiness and later achievement. Dev Psychol. 2007;43:1428–46. doi: 10.1037/0012-1649.43.6.1428. [DOI] [PubMed] [Google Scholar]
- Earls F, Reich W, Jung KG, Cloninger CR. Psychopathology in children of alcoholic and antisocial parents. Alcohol Clin Exp Res. 1988;12:481–7. doi: 10.1111/j.1530-0277.1988.tb00230.x. [DOI] [PubMed] [Google Scholar]
- Eiden RD, Edwards EP, Leonard KE. A conceptual model for the development of externalizing behavior problems among kindergarten children of alcoholic families: role of parenting and children’s self-regulation. Dev Psychol. 2007;43:1187–201. doi: 10.1037/0012-1649.43.5.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg N, Zhou Q, Spinrad TL, Valiente C, Fabes RA, Liew J. Relations among positive parenting, children’s effortful control, and externalizing problems: A three-wave longitudinal study. Child Development. 2005;76:1055–71. doi: 10.1111/j.1467-8624.2005.00897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellickson PL, Tucker JS, Klein DJ, Saner H. Antecedents and outcomes of marijuana use initiation during adolescence. Prev Med. 2004;39:976–84. doi: 10.1016/j.ypmed.2004.04.013. [DOI] [PubMed] [Google Scholar]
- Ensminger ME, Juon HS, Fothergill KE. Childhood and adolescent antecedents of substance use in adulthood. Addiction. 2002;97:833–44. doi: 10.1046/j.1360-0443.2002.00138.x. [DOI] [PubMed] [Google Scholar]
- Erhardt D, Hinshaw SP. Initial sociometric impressions of attention-deficit hyperactivity disorder and comparison boys: predictions from social behaviors and from nonbehavioral variables. J Consult Clin Psychol. 1994;62:833–42. doi: 10.1037/0022-006X.62.4.833. [DOI] [PubMed] [Google Scholar]
- Evans SW, Pelham WE, Smith BH, Bukstein OG, Gnagy EM, et al. Dose-response effects of methylphenidate on ecologically valid measures of academic performance and classroom behavior in adolescents with ADHD. Experimental and Clinical Psychopharmacology. 2001;9:163–75. doi: 10.1037//1064-1297.9.2.163. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Boden JM, Horwood LJ. Tests of Causal Links Between Alcohol Abuse or Dependence and Major Depression. Archives of General Psychiatry. 2009;66:260–66. doi: 10.1001/archgenpsychiatry.2008.543. [DOI] [PubMed] [Google Scholar]
- Flory K, Lynam DR. The relation between Attention Deficit Hyperactivity Disorder and substance abuse: What role does conduct disorder play? Clinical Child and Family Psychology Review. 2003;6:1–16. doi: 10.1023/a:1022260221570. [DOI] [PubMed] [Google Scholar]
- Frazier TW, Youngstrom EA, Glutting JJ, Watkins MW. ADHD and achievement: meta-analysis of the child, adolescent, and adult literatures and a concomitant study with college students. J Learn Disabil. 2007;40:49–65. doi: 10.1177/00222194070400010401. [DOI] [PubMed] [Google Scholar]
- Frojd S, Ranta K, Kaltiala-Heino R, Marttunen M. Associations of social phobia and general anxiety with alcohol and drug use in a community sample of adolescents. Alcohol Alcohol. 2011;46:192–9. doi: 10.1093/alcalc/agq096. [DOI] [PubMed] [Google Scholar]
- Fromme K, Stroot E, Kaplan D. Comprehensive effects of alcohol: Development and psychometric assessment of a new expectancy questionnaire. Psychological Assessment. 1993;5:19–26. [Google Scholar]
- Galera C, Melchior M, Chastang JF, Bouvard MP, Fombonne E. Childhood and adolescent hyperactivity-inattention symptoms and academic achievement 8 years later: the GAZEL Youth study. Psychol Med. 2009;39:1895–906. doi: 10.1017/S0033291709005510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier CF, Luna B. Developmental effects of incentives on response inhibition. Child Dev. 2012;83:1262–74. doi: 10.1111/j.1467-8624.2012.01771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannotta F, Ortega E, Ciairano S. A two-year follow-up investigation of parenting and peer influences on tobacco use onset among Italian early adolescents. European Journal of Developmental Psychology. 2011;8:573–86. [Google Scholar]
- Gittelman R, Mannuzza S, Shenker R, Bonagura N. Hyperactive boys almost grown up. I. Psychiatric status. Arch Gen Psychiatry. 1985;42:937–47. doi: 10.1001/archpsyc.1985.01790330017002. [DOI] [PubMed] [Google Scholar]
- Glantz M, Pickens RW. Vulnerability to drug abuse. American Psychological Association; Washington, DC: 1992. [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–52. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottman JM, Katz LF, Hooven C. Meta-emotion: How families communicate emotionally. Psychology Press; Hillsdale, NJ: 1997. [Google Scholar]
- Group. CPPRG. The effects of the fast track preventive intervention on the development of conduct disorder across childhood. Child Dev. 2011;82:331–45. doi: 10.1111/j.1467-8624.2010.01558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, Hawkins JD, Hill KG, Abbott RD. Childhood and adolescent predictors of alcohol abuse and dependence in young adulthood. J Stud Alcohol. 2001;62:754–62. doi: 10.15288/jsa.2001.62.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halperin JM, Schulz KP. Revisiting the role of the prefrontal cortex in the pathophysiology of attention-deficit/hyperactivity disorder. Psychol Bull. 2006;132:560–81. doi: 10.1037/0033-2909.132.4.560. [DOI] [PubMed] [Google Scholar]
- Handley ED, Chassin L. Alcohol-Specific Parenting as a Mechanism of Parental Drinking and Alcohol Use Disorder Risk on Adolescent Alcohol Use Onset. Journal of Studies on Alcohol and Drugs. 2013;74:684–93. doi: 10.15288/jsad.2013.74.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handley ED, Chassin L, Haller MM, Bountress KE, Dandreaux D, Beltran I. Do executive and reactive disinhibition mediate the effects of familial substance use disorders on adolescent externalizing outcomes. Journal of Abnormal Psychology. 2011;120:528–542. doi: 10.1037/a0024162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson KL, Medina KL, Padula CB, Tapert SF, Brown SA. Impact of Adolescent Alcohol and Drug Use on Neuropsychological Functioning in Young Adulthood: 10-Year Outcomes. J Child Adolesc Subst Abuse. 2011;20:135–54. doi: 10.1080/1067828X.2011.555272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harden KP, Hill JE, Turkheimer E, Emery RE. Gene-environment correlation and interaction in peer effects on adolescent alcohol and tobacco use. Behavior Genetics. 2008;38:339–47. doi: 10.1007/s10519-008-9202-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartsough CS, Lambert NM. Pattern and progression of drug use among hyperactives and controls: A prospective short-term longitudinal study. Journal of Child Psychology and Psychiatry. 1987;28:543–53. doi: 10.1111/j.1469-7610.1987.tb00222.x. [DOI] [PubMed] [Google Scholar]
- Hawkins JD, Catalano RF, Miller JY. Risk and protective factors for alcohol and other drug problems in adolescence and early adulthood: implications for substance abuse prevention. Psychol Bull. 1992;112:64–105. doi: 10.1037/0033-2909.112.1.64. [DOI] [PubMed] [Google Scholar]
- Hechtman L, Weiss G. Controlled prospective fifteen year follow-up of hyperactives as adults: Non-medical drug and alcohol use and anti-social behavior. Canadian Journal of Psychiatry. 1986;31:557–67. doi: 10.1177/070674378603100614. [DOI] [PubMed] [Google Scholar]
- Henry KL, Knight KE, Thornberry TP. School disengagement as a predictor of dropout, delinquency, and problem substance use during adolescence and early adulthood. J Youth Adolesc. 2012;41:156–66. doi: 10.1007/s10964-011-9665-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinshaw SP. Externalizing behavior problems and academic underachievement in childhood and adolescence: causal relationships and underlying mechanisms. Psychol Bull. 1992;111:127–55. doi: 10.1037/0033-2909.111.1.127. [DOI] [PubMed] [Google Scholar]
- Hinshaw SP. Preadolescent girls with attention-deficit/hyperactivity disorder: I. Background characteristics, comorbidity, cognitive and social functioning, and parenting practices. J Consult Clin Psychol. 2002;70:1086–98. doi: 10.1037//0022-006x.70.5.1086. [DOI] [PubMed] [Google Scholar]
- Hinshaw SP, Owens EB, Sami N, Fargeon S. Prospective follow-up of girls with attention-deficit/hyperactivity disorder into adolescence: Evidence for continuing cross-domain impairment. Journal of Consulting and Clinical Psychology. 2006;74:489–9. doi: 10.1037/0022-006X.74.3.489. [DOI] [PubMed] [Google Scholar]
- Hinshaw SP, Owens EB, Zalecki C, Huggins SP, Montenegro-Nevado AJ, et al. Prospective Follow-Up of Girls With Attention-Deficit/Hyperactivity Disorder Into Early Adulthood: Continuing Impairment Includes Elevated Risk for Suicide Attempts and Self-Injury. J Consult Clin Psychol. 2012 doi: 10.1037/a0029451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgens JB, Cole J, Boldizar J. Peer-based differences among boys with ADHD. J Clin Child Psychol. 2000;29:443–52. doi: 10.1207/S15374424JCCP2903_15. [DOI] [PubMed] [Google Scholar]
- Hops H, Davis B, Lewin LM. The development of alcohol and other substance use: a gender study of family and peer context. J of Studies on Alcohol. 1999;13:22–31. doi: 10.15288/jsas.1999.s13.22. [DOI] [PubMed] [Google Scholar]
- Hoza B. Peer functioning in children with ADHD. Ambul Pediatr. 2007;7:101–6. doi: 10.1016/j.ambp.2006.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoza B, Gerdes AC, Hinshaw SP, Arnold LE, Pelham WE, Jr., et al. Self-perceptions of competence in children with ADHD and comparison children. J Consult Clin Psychol. 2004;72:382–91. doi: 10.1037/0022-006X.72.3.382. [DOI] [PubMed] [Google Scholar]
- Hoza B, Mrug S, Gerdes AC, Hinshaw SP, Bukowski WM, et al. What aspects of peer relationships are impaired in children with attention-deficit/hyperactivity disorder. J Consult Clin Psychol. 2005;73:411–23. doi: 10.1037/0022-006X.73.3.411. [DOI] [PubMed] [Google Scholar]
- Hoza B, Murray-Close D, Arnold LE, Hinshaw SP, Hechtman L, Group MTAC. Time-dependent changes in positively biased self-perceptions of children with attention-deficit/hyperactivity disorder: a developmental psychopathology perspective. Dev Psychopathol. 2010;22:375–90. doi: 10.1017/S095457941000012X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoza B, Pelham WE, Dobbs J, Owens JS, Pillow DR. Do boys with attention-deficit/hyperactivity disorder have positive illusory self-concepts. Journal of Abnormal Psychology. 2002;111:268–78. doi: 10.1037//0021-843x.111.2.268. [DOI] [PubMed] [Google Scholar]
- Hoza B, Waschbusch DA, Pelham WE, Molina BS, Milich R. Attention-deficit/hyperactivity disordered and control boys’ responses to social success and failure. Child Dev. 2000;71:432–46. doi: 10.1111/1467-8624.00155. [DOI] [PubMed] [Google Scholar]
- Huang-Pollock CL, Mikami AY, Pfiffner L, McBurnett K. Can executive functions explain the relationship between Attention Deficit Hyperactivity Disorder and social adjustment. J Abnorm Child Psychol. 2009;37:679–91. doi: 10.1007/s10802-009-9302-8. [DOI] [PubMed] [Google Scholar]
- Humphreys KL, Eng T, Lee SS. Stimulant Medication and Substance Use Outcomes A Meta-analysis. Jama Psychiatry. 2013;70:740–49. doi: 10.1001/jamapsychiatry.2013.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussong AM, Jones DJ, Stein GL, Baucom DH, Boeding S. An internalizing pathway to alcohol use and disorder. Psychol Addict Behav. 2011;25:390–404. doi: 10.1037/a0024519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacono WG, Malone SM, McGue M. Behavioral disinhibition and the development of early-onset addiction: common and specific influences. Annu Rev Clin Psychol. 2008;4:325–48. doi: 10.1146/annurev.clinpsy.4.022007.141157. [DOI] [PubMed] [Google Scholar]
- Jessor R, Jessor SL. Problem behavior and psychosocial development: A longitudinal study of youth. Academic Press; New York, New York: 1977. [Google Scholar]
- Johnston C, Chronis-Tuscano AM. Families of children with ADHD. In: Barkley RA, editor. Attention-Deficit/Hyperactivity Disorder: A handbook for diagnosis and treatment. 4th Ed. Guilford; New York: In press. [Google Scholar]
- Johnston C, Mash EJ. Families of children with Attention-Deficit/Hyperactivity Disorder: Review and recommendations. Clinical Child and Family Psychology Review. 2001;4:183–207. doi: 10.1023/a:1017592030434. [DOI] [PubMed] [Google Scholar]
- Johnston C, Mash EJ, Miller N, Ninowski JE. Parenting in adults with attention- deficit/hyperactivity disorder (ADHD) Clin Psychol Rev. 2012;32:215–28. doi: 10.1016/j.cpr.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future national survey results on drug use, 1975-2012. Volume II: College students and adults ages 19-50. Institute for Social Research, The University of Michigan; Ann Arbor: 2013. p. 400. [Google Scholar]
- Kaplow JB, Curran PJ, Angold A, Costello EJ. The prospective relation between dimensions of anxiety and the initiation of adolescent alcohol use. J Clin Child Psychol. 2001;30:316–26. doi: 10.1207/S15374424JCCP3003_4. [DOI] [PubMed] [Google Scholar]
- Kaplow JB, Curran PJ, Dodge KA, Gr CPPR. Child, parent, and peer predictors of early-onset substance use: A multisite longitudinal study. Journal of Abnormal Child Psychology. 2002;30:199–216. doi: 10.1023/a:1015183927979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassel JD, Veilleux JC. The complex interplay between substance abuse and emotion. American Psychological Association; Washingon, DC: 2010. [Google Scholar]
- Kellam SG, Ensminger ME, Simon MB. Mental health in first grade and teenager drug, alcohol, and cigarette use. Drug and Alcohol Dependence. 1980;5:273–304. doi: 10.1016/0376-8716(80)90003-4. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Gardner CO, Edwards A, Hickman M, Heron J, et al. Dimensions of Parental Alcohol Use/Problems and Offspring Temperament, Externalizing Behaviors, and Alcohol Use/Problems. Alcohol Clin Exp Res. 2013 doi: 10.1111/acer.12196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent KM, Pelham WE, Jr., Molina BS, Sibley MH, Waschbusch DA, et al. The academic experience of male high school students with ADHD. J Abnorm Child Psychol. 2011;39 doi: 10.1007/s10802-010-9472-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khantzian EJ. The self-medication hypothesis of addictive disorders: focus on heroin and cocaine dependence. Am J Psychiatry. 1985;142:1259–64. doi: 10.1176/ajp.142.11.1259. [DOI] [PubMed] [Google Scholar]
- King SM, Keyes M, Malone SM, Elkins I, Legrand LN, et al. Parental alcohol dependence and the transmission of adolescent behavioral disinhibition: a study of adoptive and non-adoptive families. Addiction. 2009;104:578–86. doi: 10.1111/j.1360-0443.2008.02469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollins SH, McClernon FJ, Fuemmeler BF. Association between smoking and attention-deficit/hyperactivity disorder symptoms in a population-based sample of young adults. Arch Gen Psychiatry. 2005;62:1142–7. doi: 10.1001/archpsyc.62.10.1142. [DOI] [PubMed] [Google Scholar]
- Koning IM, van den Eijnden RJ, Verdurmen JE, Engels RC, Vollebergh WA. A cluster randomized trial on the effects of a parent and student intervention on alcohol use in adolescents four years after baseline; no evidence of catching-up behavior. Addict Behav. 2013;38:2032–9. doi: 10.1016/j.addbeh.2012.12.013. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–38. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuntsche E, Knibbe R, Gmel G, Engels R. Why do young people drink? A review of drinking motives. Clin Psychol Rev. 2005;25:841–61. doi: 10.1016/j.cpr.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Kuriyan AB, Pelham WE, Jr., Molina BS, Waschbusch DA, Gnagy EM, et al. Young adult educational and vocational outcomes of children diagnosed with ADHD. Journal of Abnormal Child Psychology. 2013;41 doi: 10.1007/s10802-012-9658-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahey BB, Applegate B, Waldman ID, Loft JD, Hankin BL, Rick J. The structure of child and adolescent psychopathology: generating new hypotheses. J Abnorm Psychol. 2004;113:358–85. doi: 10.1037/0021-843X.113.3.358. [DOI] [PubMed] [Google Scholar]
- Lang AR, Pelham WE, Atkeson BM, Murphy DA. Effects of Alcohol Intoxication on Parenting Behavior in Interactions With Child Confederates Exhibiting Normal or Deviant Behaviors. Journal of Abnormal Child Psychology. 1999;27:177–89. doi: 10.1023/a:1021996122095. [DOI] [PubMed] [Google Scholar]
- Langberg JM, Molina BS, Arnold LE, Epstein JN, Altaye M, et al. Patterns and predictors of adolescent academic achievement and performance in a sample of children with attention-deficit/hyperactivity disorder. J Clin Child Adolesc Psychol. 2011;40:519–31. doi: 10.1080/15374416.2011.581620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansford JE, Erath S, Yu T, Pettit GS, Dodge KA, Bates JE. The developmental course of illicit substance use from age 12 to 22: links with depressive, anxiety, and behavior disorders at age 18. J Child Psychol Psychiatry. 2008;49:877–85. doi: 10.1111/j.1469-7610.2008.01915.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latimer WWAG, Newcomb MD, Realmuto GM, Hektner JM, Mathy RM. Child and familial pathways to academic achievement and behavioral adjustment: a prospective six-year study of children with and without ADHD. Journal of Attention Disorders. 2003;7:101–16. doi: 10.1177/108705470300700204. [DOI] [PubMed] [Google Scholar]
- Lee SS, Hinshaw SP. Predictors of adolescent functioning in girls with attention deficit hyperactivity disorder (ADHD): the role of childhood ADHD, conduct problems, and peer status. J Clin Child Adolesc Psychol. 2006;35:356–68. doi: 10.1207/s15374424jccp3503_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SS, Humphreys KL, Flory K, Liu R, Glass K. Prospective association of childhood attention-deficit/hyperactivity disorder (ADHD) and substance use and abuse/dependence: a meta-analytic review. Clin Psychol Rev. 2011;31:328–41. doi: 10.1016/j.cpr.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnea K, Hoza B, Tomb M, Kaiser N. Does a positive bias relate to social behavior in children with ADHD. Behav Ther. 2012;43:862–75. doi: 10.1016/j.beth.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochman JE, Bierman KL, Coie JD, Dodge KA, Greenberg MT, McMahon RJ, Pinderhughes EE. The difficulty of maintaining positive intervention effects: A look at disruptive behavior, deviant peer relations, and social skills during the middle school years. Journal of Early Adolescence. 2010;30 doi: 10.1177/0272431609340513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loe IM, Feldman HR. Academic and Educational Outcomes of Children With ADHD. Journal of Pediatric Psychology. 2007;32:643–54. doi: 10.1093/jpepsy/jsl054. [DOI] [PubMed] [Google Scholar]
- Ludtke O, Marsh HW, Robitzsch A, Trautwein U, Asparouhov T, Muthen B. The multilevel latent covariate model: a new, more reliable approach to group-level effects in contextual studies. Psychol Methods. 2008;13:203–29. doi: 10.1037/a0012869. [DOI] [PubMed] [Google Scholar]
- Mannuzza S, Klein RG, Bessler A, Malloy P, LaPadula M. Adult psychiatric status of hyperactive boys grown up. Am J Psychiatry. 1998;155:493–8. doi: 10.1176/ajp.155.4.493. [DOI] [PubMed] [Google Scholar]
- Marcus SC, Wan GJ, Kemner JE, Olfson M. Continuity of methylphenidate treatment for attention-deficit/hyperactivity disorder. Arch Pediatr Adolesc Med. 2005;159:572–8. doi: 10.1001/archpedi.159.6.572. [DOI] [PubMed] [Google Scholar]
- Mares SHW, Lichtwarck-Aschoff A, Burk WJ, van der Vorst H, Engels RCME. Parental alcohol-specific rules and alcohol use from early adolescence to young adulthood. Journal of Child Psychology and Psychiatry. 2012;53:798–805. doi: 10.1111/j.1469-7610.2012.02533.x. [DOI] [PubMed] [Google Scholar]
- Marshal MP, Molina BSG, Pelham WE. Childhood ADHD and adolescent substance use: An examination of deviant peer group affiliation as a risk factor. Psychology of Addictive Behaviors. 2003;17:293–302. doi: 10.1037/0893-164X.17.4.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel MM, Nigg JT, von Eye A. How do trait dimensions map onto ADHD symptom domains. J Abnorm Child Psychol. 2009a;37:337–48. doi: 10.1007/s10802-008-9255-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel MM, Pierce L, Nigg JT, Jester JM, Adams K, et al. Temperament Pathways to Childhood Disruptive Behavior and Adolescent Substance Abuse: Testing a Cascade Model. Journal of Abnormal Child Psychology. 2009b;37:363–73. doi: 10.1007/s10802-008-9269-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masse LC, Tremblay RE. Behavior of boys in kindergarten and the onset of substance use during adolescence. Archives of General Psychiatry. 1997;54:62–68. doi: 10.1001/archpsyc.1997.01830130068014. [DOI] [PubMed] [Google Scholar]
- Massetti GMLB, Pelham WE, Loney J, Ehrhardt A, Lee SS, Kipp H. Academic achievement over 8 years among children who met modified criteria for attention-deficit/hyperactivity disorder at 4-6 years of age. J Abnorm Child Psychol. 2008;36:399–410. doi: 10.1007/s10802-007-9186-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten AS, Faden VB, Zucker RA, Spear LP. A Developmental Perspective on Underage Alcohol Use. Alcohol Research & Health. 2009;32:3–15. [PMC free article] [PubMed] [Google Scholar]
- McQuade JD, Hoza B. Peer problems in Attention Deficit Hyperactivity Disorder: current status and future directions. Dev Disabil Res Rev. 2008;14:320–4. doi: 10.1002/ddrr.35. [DOI] [PubMed] [Google Scholar]
- Mick E, Spencer T, Wozniak J, Biederman J. Heterogeneity of irritability in attention-deficit/hyperactivity disorder subjects with and without mood disorders. Biol Psychiatry. 2005;58:576–82. doi: 10.1016/j.biopsych.2005.05.037. [DOI] [PubMed] [Google Scholar]
- Mikami AY, Huang-Pollock CL, Pfiffner LJ, McBurnett K, Hangai D. Social skills differences among attention-deficit/hyperactivity disorder types in a chat room assessment task. Journal of Abnormal Child Psychology. 2007;35:509–21. doi: 10.1007/s10802-007-9108-5. [DOI] [PubMed] [Google Scholar]
- Mikami AY, Jack A, Emeh CC, Stephens HF. Parental influence on children with attention-deficit/hyperactivity disorder: I. Relationships between parent behaviors and child peer status. Journal of abnormal child psychology. 2010;38:721–36. doi: 10.1007/s10802-010-9393-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milich R, Landau S. Socialization and peer relations in hyperactive children. Advances in Learning & Behavioral Disabilities. 1982 [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex “Frontal Lobe” tasks: a latent variable analysis. Cogn Psychol. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Moffitt TE, Arseneault L, Belsky D, Dickson N, Hancox RJ, et al. A gradient of childhood self-control predicts health, wealth, and public safety. Proc Natl Acad Sci USA. 2011;108:2693–8. doi: 10.1073/pnas.1010076108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina BSG. Delinquency and Substance Use in ADHD: Adolescent and Young Adult Outcomes in Developmental Context. In: Evans SW, Hoza B, editors. Treating Attention Deficit Hyperactivity Disorder: Assessment and Intervention in Developmental Context. Vol. 19. Civic Research Institute, Inc.; Kingston, NJ: 2011. pp. 1–52. [Google Scholar]
- Molina BS, Donovan JE, Belendiuk KA. Familial loading for alcoholism and offspring behavior: mediating and moderating influences. Alcohol Clin Exp Res. 2010;34:1972–84. doi: 10.1111/j.1530-0277.2010.01287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina BSG, Hinshaw SP, Arnold LE, Swanson JM, Swanson JM, et al. Adolescent Substance Use in the Multimodal Treatment Study of Attention-Deficit/Hyperactivity Disorder (ADHD) (MTA) as a Function of Childhood ADHD, Random Assignment to Childhood Treatments, and Subsequent Medication. Journal of the American Academy of Child and Adolescent Psychiatry. 2013;52:250–63. doi: 10.1016/j.jaac.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina BSG, Hinshaw SP, Swanson JM, Arnold LE, Vitiello B, et al. The MTA at 8 Years: Prospective follow-Up of children treated for combined type ADHD in a multisite study. J of the American Academy of Child and Adolescent Psychiatry. 2009;48:484–500. doi: 10.1097/CHI.0b013e31819c23d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina BSG, Marshal MP, Pelham WE, Wirth RJ. Coping skills and parent support mediate the association between childhood ADHD and adolescent cigarette use. Journal of Pediatric Psychology. 2005;30:345–57. doi: 10.1093/jpepsy/jsi029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina BSG, Pelham WE. Childhood predictors of adolescent substance use in a longitudinal study of children with ADHD. Journal of Abnormal Psychology. 2003;112:497–507. doi: 10.1037/0021-843x.112.3.497. [DOI] [PubMed] [Google Scholar]
- Molina BS, Pelham WE, Cheong J, Marshal MP, Gnagy EM, Curran PJ. Childhood attention-deficit/hyperactivity disorder (ADHD) and growth in adolescent alcohol use: the roles of functional impairments, ADHD symptom persistence, and parental knowledge. J Abnorm Psychol. 2012;121:922–35. doi: 10.1037/a0028260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina BSG, Pelham WE, Gnagy EM, Thompson AL, Marshal MP. ADHD risk for heavy drinking and alcohol use disorder is age-specific. Alcoholism: Clinical and Experimental Research. 2007;31:643–54. doi: 10.1111/j.1530-0277.2007.00349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MTA Cooperative Group. National Institute of Mental Health multimodal treatment study of ADHD follow-up: 24-month outcomes of treatment strategies for attention-deficit/hyperactivity disorder (ADHD) Pediatrics. 113:754–761. doi: 10.1542/peds.113.4.754. [DOI] [PubMed] [Google Scholar]
- Newlin DB, Thomson JB. Alcohol challenge with sons of alcoholics: a critical review and analysis. Psychol Bull. 1990;108:383–402. doi: 10.1037/0033-2909.108.3.383. [DOI] [PubMed] [Google Scholar]
- Niemela S, Sourander A, Poikolainen K, Helenius H, Sillanmaki L, et al. Childhood predictors of drunkenness in late adolescence among males: a 10-year population-based follow-up study. Addiction. 2006;101:512–21. doi: 10.1111/j.1360-0443.2006.01381.x. [DOI] [PubMed] [Google Scholar]
- Nigg J. Is ADHD a disinhibitory disorder. Psychological Bulletin. 2001;127:571–98. doi: 10.1037/0033-2909.127.5.571. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Wong MM, Martel MM, Jester JM, Puttler LI, et al. Poor response inhibition as a predictor of problem drinking and illicit drug use in adolescents at risk for alcoholism and other substance use disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 2006;45:468–75. doi: 10.1097/01.chi.0000199028.76452.a9. [DOI] [PubMed] [Google Scholar]
- Normand S, Schneider BH, Lee MD, Maisonneuve MF, Chupetlovska-Anastasova A, et al. Continuities and Changes in the Friendships of Children with and Without ADHD: A Longitudinal, Observational Study. J Abnorm Child Psychol. 2013;41:1161–75. doi: 10.1007/s10802-013-9753-9. [DOI] [PubMed] [Google Scholar]
- Otten R, Barker ED, Huizink AC, Engels RC. The Interplay between Parental Monitoring and the Dopamine D4 Receptor Gene in Adolescent Cannabis Use. PloS one. 2012;7:e49432. doi: 10.1371/journal.pone.0049432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabst A, Baumeister SE, Kraus L. Alcohol-expectancy dimensions and alcohol consumption at different ages in the general population. J Stud Alcohol Drugs. 2010;71:46–53. doi: 10.15288/jsad.2010.71.46. [DOI] [PubMed] [Google Scholar]
- Patterson GR. Coercive family process. Castalia; Eugene, OR: 1982. [Google Scholar]
- Patterson GR, Fisher PA. Handbook of Parenting. Lawrence Erlbaum; New Jersey: 2002. Recent developments in our understanding of parenting: Bidirectional effects, causal models, and the search for parsimony; pp. 59–88. [Google Scholar]
- Pedersen SL, Harty S, Pelham WE, Gnagy EM, Molina BSG. Differential associations between alcohol expectancies and adolescent alcohol use as a function of childhood ADHD. Journal of Studies on Alcohol and Drugs. doi: 10.15288/jsad.2014.75.145. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham WE, Bender ME. Peer relationships in hyperactive children: Description and treatment. Advances in Learning & Behavioral Disabilities. 1982 [Google Scholar]
- Pelham WE, Fabiano GA. Evidence-Based Psychosocial Treatments for Attention-Deficit/Hyperactivity Disorder. Journal of Clinical Child & Adolescent Psychology. 2008;37:184–214. doi: 10.1080/15374410701818681. [DOI] [PubMed] [Google Scholar]
- Pelham WE, Fabiano GA, Massetti GM. Evidence-Based Assessment of Attention Deficit Hyperactivity Disorder in Children and Adolescents. Journal of Clinical Child and Adolescent Psychology. 2005;34:449–76. doi: 10.1207/s15374424jccp3403_5. [DOI] [PubMed] [Google Scholar]
- Pelham WE, Fabiano G, Waxmonsky J, Greiner A, Burrows-MacLean L, Massetti G, Washbusch D, Hoffman M, Murphy S, Carter R, Gnagy E, Bhatia I, Verley J, Mitchell C. Adaptive Pharmacological and Behavioral Treatments for Children with ADHD: Sequencing, Combining, and Escalating Doses; Poster presented at the Institute of Education Sciences Research Conference; Washington, DC. 2007. [Google Scholar]
- Pelham WE, Gnagy EM, Burrows-Maclean L, Williams A, Fabiano GA, et al. Once-a-day Concerta methylphenidate versus three-times-daily methylphenidate in laboratory and natural settings. Pediatrics. 2001;107:e105–e05. doi: 10.1542/peds.107.6.e105. [DOI] [PubMed] [Google Scholar]
- Pelham WE, Lang AR. Parental alcohol consumption and deviant child behavior: Laboratory studies of reciprocal effects. Clinical Psychology Review. 1993;13:1–22. [Google Scholar]
- Pelham WE, Lang AR, Atkeson B, Murphy DA, Gnagy EM, et al. Effects of Deviant Child Behavior on Parental Distress and Alcohol Consumption in Laboratory Interactions. Journal of Abnormal Child Psychology. 1997;25:413–24. doi: 10.1023/a:1025789108958. [DOI] [PubMed] [Google Scholar]
- Pillow DR, Pelham WE, Jr., Hoza B, Molina BS, Stultz CH. Confirmatory factor analyses examining attention deficit hyperactivity disorder symptoms and other childhood disruptive behaviors. J Abnorm Child Psychol. 1998;26:293–309. doi: 10.1023/a:1022658618368. [DOI] [PubMed] [Google Scholar]
- Pingault JB, Tremblay RE, Vitaro F, Carbonneau R, Genolini C, et al. Childhood trajectories of inattention and hyperactivity and prediction of educational attainment in early adulthood: a 16-year longitudinal population-based study. Am J Psychiatry. 2011;168:1164–70. doi: 10.1176/appi.ajp.2011.10121732. [DOI] [PubMed] [Google Scholar]
- Polderman TJCBD, Bartels M, Verhulst FC, Huizink AC. A systematic review of prospective tudies on attention problems and academic achievement. Acta Psychiatr Scand. 2010;122:271–84. doi: 10.1111/j.1600-0447.2010.01568.x. [DOI] [PubMed] [Google Scholar]
- Rapport MD, Scanlan SW, Denney CB. Attention-deficit/hyperactivity disorder and scholastic achievement: a model of dual developmental pathways. J Child Psychol Psychiatry. 1999;40:1169–83. [PubMed] [Google Scholar]
- Raudino A, Fergusson DM, Horwood LJ. The quality of parent/child relationships in adolescence is associated with poor adult psychosocial adjustment. J Adolesc. 2013;36:331–40. doi: 10.1016/j.adolescence.2012.12.002. [DOI] [PubMed] [Google Scholar]
- Reifman A, Barnes GM, Dintcheff BA, Farrell MP, Uhteg L. Parental and peer influences on the onset of heavier drinking among adolescents. J Stud Alcohol. 1998;59:311–7. doi: 10.15288/jsa.1998.59.311. [DOI] [PubMed] [Google Scholar]
- Reinherz HZ, Giaconia RM, Hauf AM, Wasserman MS, Paradis AD. General and specific childhood risk factors for depression and drug disorders by early adulthood. J Am Acad Child Adolesc Psychiatry. 2000;39:223–31. doi: 10.1097/00004583-200002000-00023. [DOI] [PubMed] [Google Scholar]
- Robins LN. Deviant children grown up; a sociological and psychiatric study of sociopathic personality. xiv. Williams & Wilkins; Baltimore, MD: 1966. p. 351. [Google Scholar]
- Robbins MS, Feaster DJ, Horigian VE, Rohrbaugh M, Shoham V, et al. Brief strategic family therapy versus treatment as usual: results of a multisite randomized trial for substance using adolescents. J Consult Clin Psychol. 2011;79:713–27. doi: 10.1037/a0025477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohde P, Lewinsohn PM, Seeley JR. Psychiatric comorbidity with problematic alcohol use in high school students. Journal of the American Academy of Child and Adolescent Psychiatry. 1996;35:101–09. doi: 10.1097/00004583-199601000-00018. [DOI] [PubMed] [Google Scholar]
- Scheffler RM, Brown TT, Fulton BD, Hinshaw SP, Levine P, Stone S. Positive association between attention-deficit/hyperactivity disorder medication use and academic achievement during elementary school. Pediatrics. 2009;123:1273–9. doi: 10.1542/peds.2008-1597. [DOI] [PubMed] [Google Scholar]
- Schoechlin C, Engel RR. Neuropsychological performance in adult attention-deficit hyperactivity disorder: meta-analysis of empirical data. Arch Clin Neuropsychol. 2005;20:727–44. doi: 10.1016/j.acn.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Scholtens S, Rydell AM, Yang-Wallentin F. ADHD symptoms, academic achievement, self-perception of academic competence and future orientation: a longitudinal study. Scand J Psychol. 2013;54:205–12. doi: 10.1111/sjop.12042. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL. An evaluation of the level of response to alcohol, externalizing symptoms, and depressive symptoms as predictors of alcoholism. Journal of Studies on Alcohol. 2006;67:215–27. doi: 10.15288/jsa.2006.67.215. [DOI] [PubMed] [Google Scholar]
- Sher K. Children of alcoholics: A critical appraisal of theory and research. University of Chicago Press; Chicago, IL: 1991. [Google Scholar]
- Sher KJ, Grekin ER, Williams NA. The development of alcohol use disorders. Annual Review of Clinical Psychology. 2005;1:493–523. doi: 10.1146/annurev.clinpsy.1.102803.144107. [DOI] [PubMed] [Google Scholar]
- Sibley MH, Pelham WE, Jr, Derefinko KJ, Kuriyan AB, Sanchez F, Graziano PA. A Pilot Trial of Supporting teens’ Academic Needs Daily (STAND): A Parent-Adolescent Collaborative Intervention for ADHD. Journal of Psychopathology and Behavioral Assessment. 2013:1–14. [Google Scholar]
- Sibley MH, Pelham WE, Jr., Molina BS, Gnagy EM, Waschbusch DA, et al. Diagnosing ADHD in adolescence. J Consult Clin Psychol. 2012a;80:139–50. doi: 10.1037/a0026577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley MH, Pelham WE, Molina BS, Gnagy EM, Waxmonsky JG, et al. When diagnosing ADHD in young adults emphasize informant reports, DSM items, and impairment. J Consult Clin Psychol. 2012b;80:1052–61. doi: 10.1037/a0029098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley MH, Pelham WE, Molina BSG, Kipp H, Gnagy EM, Meinzer M, Ross JM, Lahey BB. The role of early childhood ADHD and subsequent CD in the initiation and escalation of adolescent cigarette, alcohol, and marijuana use. doi: 10.1037/a0036585. Under Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith BH, Pelham WE, Evans S, Gnagy E, Molina B, et al. Dosage effects of methylphenidate on the social behavior of adolescents diagnosed with attention-deficit hyperactivity disorder. Exp Clin Psychopharmacol. 1998;6:187–204. doi: 10.1037/1064-1297.6.2.187. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJ. The dual pathway model of AD/HD: an elaboration of neurodevelopZelazomental characteristics. Neurosci Biobehav Rev. 2003;27:593–604. doi: 10.1016/j.neubiorev.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Stacy AW. Memory activation and expectancy as prospective predictors of alcohol and marijuana use. J Abnorm Psychol. 1997;106:61–73. doi: 10.1037//0021-843x.106.1.61. [DOI] [PubMed] [Google Scholar]
- Stacy AW, Wiers RW. Implicit cognition and addiction: a tool for explaining paradoxical behavior. Annu Rev Clin Psychol. 2010;6:551–75. doi: 10.1146/annurev.clinpsy.121208.131444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanger C, Ryan SR, Fu H, Landes RD, Jones BA, et al. Delay discounting predicts adolescent substance abuse treatment outcome. Exp Clin Psychopharmacol. 2012;20:205–12. doi: 10.1037/a0026543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L. A Social Neuroscience Perspective on Adolescent Risk-Taking. Dev Rev. 2008;28:78–106. doi: 10.1016/j.dr.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringaris A, Goodman R. Longitudinal outcome of youth oppositionality: irritable, headstrong, and hurtful behaviors have distinctive predictions. J Am Acad Child Adolesc Psychiatry. 2009;48:404–12. doi: 10.1097/CHI.0b013e3181984f30. [DOI] [PubMed] [Google Scholar]
- Subcommittee on Attention-Deficit/Hyperactivity D, Steering Committee on Quality I, Management. Wolraich M, Brown L, et al. ADHD: clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics. 2011;128:1007–22. doi: 10.1542/peds.2011-2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamm L, Epstein JN, Lisdahl KM, Molina B, Tapert S, et al. Impact of ADHD and cannabis use on executive functioning in young adults. Drug Alcohol Depend. 2013 doi: 10.1016/j.drugalcdep.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapert SF, Granholm E, Leedy NG, Brown SA. Substance use and withdrawal: neuropsychological functioning over 8 years in youth. J Int Neuropsychol Soc. 2002;8:873–83. doi: 10.1017/s1355617702870011. [DOI] [PubMed] [Google Scholar]
- Tarter RE. Etiology of Adolescent Substance Abuse: A Developmental Perspective. The American Journal on Addictions. 2002;11:171–91. doi: 10.1080/10550490290087965. [DOI] [PubMed] [Google Scholar]
- Tarter RE, Alterman AI, Edwards KL. Vulnerability to alcoholism in men: a behavior-genetic perspective. J Stud Alcohol. 1985;46:329–56. doi: 10.15288/jsa.1985.46.329. [DOI] [PubMed] [Google Scholar]
- Tarter RE, Kirisci L, Habeych M, Reynolds M, Vanyukov M. Neurobehavior disinhibition in childhood predisposes boys to substance use disorder by young adulthood: direct and mediated etiologic pathways. Drug and Alcohol Dependence. 2004;73:121–32. doi: 10.1016/j.drugalcdep.2003.07.004. [DOI] [PubMed] [Google Scholar]
- Thrush C, Wiers RW, Ames SL, Grenard JL, Sussman S, Stacy AW. Interactions between implicit and explicit cognition and working memory capacity in the prediction of alcohol use in at-risk adolescents. Drug Alcohol Depend. 2008;94:116–24. doi: 10.1016/j.drugalcdep.2007.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verweig KJHHA, Agrawal A, Martin NG, Lynskey M. Is the relationship between early-onset cannabis use and educational attainment causal or due to common liability? Drug and Alcohol Dependence. 2013 doi: 10.1016/j.drugalcdep.2013.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther CA, Cheong J, Molina BS, Pelham WE, Jr., Wymbs BT, et al. Substance use and delinquency among adolescents with childhood ADHD: the protective role of parenting. Psychol Addict Behav. 2012;26:585–98. doi: 10.1037/a0026818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warden D, Riggs PD, Min SJ, Mikulich-Gilbertson SK, Tamm L, et al. Major depression and treatment response in adolescents with ADHD and substance use disorder. Drug Alcohol Depend. 2012;120:214–9. doi: 10.1016/j.drugalcdep.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weafer J, Fillmore MT, Milich R. Increased Sensitivity to the Disinhibiting Effects of Alcohol in Adults With ADHD. Experimental and Clinical Psychopharmacology. 2009;17:113–21. doi: 10.1037/a0015418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiland BJ, Nigg JT, Welsh RC, Yau WY, Zubieta JK, et al. Resiliency in adolescents at high risk for substance abuse: flexible adaptation via subthalamic nucleus and linkage to drinking and drug use in early adulthood. Alcohol Clin Exp Res. 2012;36:1355–64. doi: 10.1111/j.1530-0277.2012.01741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh MC, Pennington BF. Assessing frontal lobe functioning in children: Views from developmental psychology. Developmental neuropsychology. 1988;4:199–230. [Google Scholar]
- White JD. Personality, temperament and ADHD: a review of the literature. Personality and Individual Differences. 1999;27:589–98. [Google Scholar]
- White JL, Moffitt TE, Caspi A, Bartusch J, Needles DJ, Stouthamer-Loeber M. Measuring impulsivity and examining its relationship to delinquency. Journal of Abnormal Psychology. 1994;103:192–205. doi: 10.1037//0021-843x.103.2.192. [DOI] [PubMed] [Google Scholar]
- Whiteside SP, Lynam D. The Five Factor Model and impulsivity: Using a structural model of personality to understand impulsivity. Personality and Individual Differences. 2001;30:669–89. [Google Scholar]
- Wiers RW, Bartholow BD, van den Wildenberg E, Thush C, Engels RC, et al. Automatic and controlled processes and the development of addictive behaviors in adolescents: a review and a model. Pharmacology Biochemistry and Behavior. 2007;86:263–83. doi: 10.1016/j.pbb.2006.09.021. [DOI] [PubMed] [Google Scholar]
- Wiers RW, Gunning WB, Sergeant JA. Do young children of alcoholics hold more positive or negative alcohol-related expectancies than controls. Alcohol Clin Exp Res. 1998;22:1855–63. [PubMed] [Google Scholar]
- Wilens TE, Martelon M, Fried R, Petty C, Bateman C, Biederman J. Do executive function deficits predict later substance use disorders among adolescents and young adults. J Am Acad Child Adolesc Psychiatry. 2011;50:141–9. doi: 10.1016/j.jaac.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilens TE, Martelon M, Joshi G, Bateman C, Fried R, et al. Does ADHD predict substance-use disorders? A 10-year follow-up study of young adults with ADHD. J Am Acad Child Adolesc Psychiatry. 2011;50:543–53. doi: 10.1016/j.jaac.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcutt EG, Nigg JT, Pennington BF, Solanto MV, Rohde LA, et al. Validity of DSM-IV attention deficit/hyperactivity disorder symptom dimensions and subtypes. J Abnorm Psychol. 2012;121:991–1010. doi: 10.1037/a0027347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills TA, Resko JA, Ainette MG, Mendoza D. Role of parent support and peer support in adolescent substance use: a test of mediated effects. Psychology of Addictive Behaviors. 2004;18:122–34. doi: 10.1037/0893-164X.18.2.122. [DOI] [PubMed] [Google Scholar]
- Wills TA, Sandy JM, Yaeger AM, Cleary SD. Coping dimensions, life stress, and adolescent substance use: A latent growth analysis. J of Abnormal Psychology. 2001;110:309–323. doi: 10.1037//0021-843x.110.2.309. [DOI] [PubMed] [Google Scholar]
- Yi RMS, Bickel WK. Delay discounting and substance abuse-dependence. In: Madden GJ, Bickel WK, editors. Impulsivity: The behavioral and neurological science of discounting. American Psychological Association; Washington DC: 2010. pp. 191–211. [Google Scholar]
- Zelazo PD, Carlson SM. Hot and Cool Executive Function in Childhood and Adolescence: Development and Plasticity. Child Development Perspectives. 2012;6:354–60. [Google Scholar]
- Zoloto A, Nagoshi CT, Presson C, Chassin L. Attention deficit/hyperactivity disorder symptoms and depression symptoms as mediators in the intergenerational transmission of smoking. Drug Alcohol Depend. 2012;126:147–55. doi: 10.1016/j.drugalcdep.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker RA. Alcohol use and the alcohol use disorders: A developmental-biopsychosocial systems formulation covering the life course. In: Cicchetti D, Cohen DJ, editors. Developmental psychopathology, Vol 3: Risk, disorder, and adaptation (2nd ed.) John Wiley & Sons Inc; Hoboken, NJ US: 2006. pp. 620–56. [Google Scholar]
- Zucker RA, Kincaid SB, Fitzgerald HE, Bingham CR. Alcohol schema acquisition in preschoolers: differences between children of alcoholics and children of nonalcoholics. Alcohol Clin Exp Res. 1995;19:1011–7. doi: 10.1111/j.1530-0277.1995.tb00982.x. [DOI] [PubMed] [Google Scholar]