Abstract
Purpose of Review
While a large number of novel broadly neutralizing antibodies has been recently described, the induction of such antibodies via vaccination has proven difficult. By contrast, non-neutralizing antibodies arise early during infection and have been repeatedly associated with both protection from infection and disease progression.
Recent findings
We are beginning to gain new insights into the broader landscape of antiviral mechanisms that non-neutralizing antibodies may harness to fight HIV, providing an unprecedented breadth of approaches by which HIV can be blocked and contained.
Summary
In this review we summarize the characteristics of non-neutralizing antibodies, their role in HIV infection, and new paradigm-shifting functions that may be exploited by next generation vaccine approaches aimed at blocking HIV infection.
Keywords: ADCC, HIV, antibodies
Introduction
Recent results from the RV144 vaccine trial [1] and Ad26 vaccination in non-human primates [2] argue that some degree of protection from infection can be achieved in the absence of neutralizing antibodies or cytotoxic T cell responses. In RV144, qualitative differences in the vaccine-induced humoral response, associated with low-levels of IgA and high levels of antibody-dependent cellular cytotoxicity (ADCC), may have contributed to the limited protection observed [3]. This protection, observed in only 30% of vaccinees, waned rapidly after the final boost. Interestingly, compared with other vaccine trials, RV144 induced lower levels of HIV-specific antibody titers, suggesting that (a) increased titers do not necessarily correlate with better protection, (b) qualitative features of non-neutralizing antibodies may be a more critical measure of protective efficacy, and (c) vaccine strategies able to increase both the titer and functional potency of antibodies may provide enhanced protection from infection. In this review we will summarize the characteristics of non-neutralizing antibodies, their role in HIV infection, and new paradigm-shifting functions that may be exploited by next generation vaccine approaches aimed at blocking HIV infection.
Two ends of an antibody
Antibodies consist of two antigen-binding domains (variable domains, Fv) and a constant domain (constant domain, Fc). The double antigen-binding domain structure provides high-avidity tethering of the target antigen. Conversely, the Fc provides instructions to the immune system on how the target antigen should be destroyed. While the Fv and Fc domains have largely separable functions, changes in both these ends of the antibody are tightly regulated by a single enzyme, activation-induced cytidine deaminase (AID) [4]. While AID drives somatic hypermutation of the Fv domain, allowing for affinity maturation and diversification of the antibody, AID also drives class switch recombination (CSR) of the Fc domain. Despite its name, the Fc domain varies significantly both in protein sequence, depending on the Ab isotype, and glycosylation, both of which are involved in determining the functional activity of the antibody.
Isotype selection
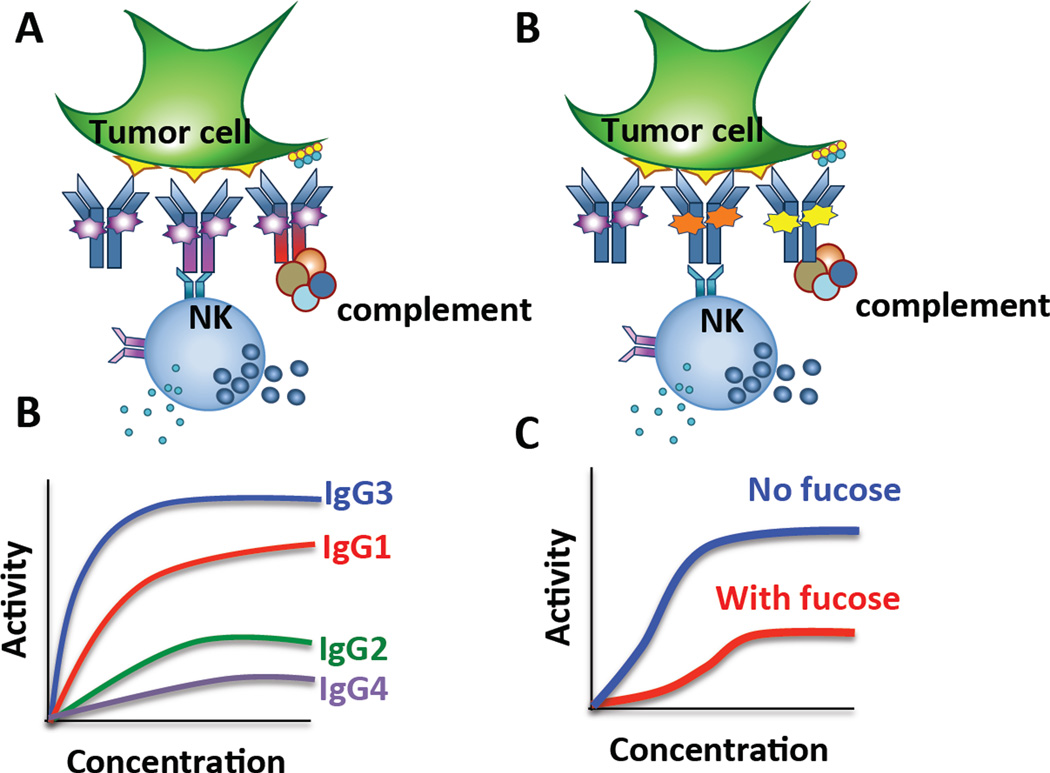
Upon B cell activation, a naïve B cell can functionally tune its Ab response from the production of IgM to the production of IgG, IgE, or IgA through CSR [5]. As Ab isotypes and subclasses are specialized to have different functional activities and to respond to and eliminate distinct types of pathogens, isotype switching is a highly regulated process largely controlled by cytokines and T helper–provided signals [6] received during B cell priming. For example, IL-4 selectively induces IgG4 and IgE [7, 8], whereas IL-10 and IL-21 induce IgG1 and IgG3 [9]. Furthermore, IgG subclass selection varies by infection and is related both to the inflammatory state induced by the pathogen and the location of the pathogen (intra- vs. extracellular) [10]. Ultimately, differences in functionality are often linked to the Fc affinity for Fcγ receptors (FcγRs) and complement [11, 12] (Figure 1). In general with IgG3 has the highest affinity for FcγRs followed by IgG1 and then IgG2 and IgG4 (Figure 2a and b). Importantly, specific Ab isotypes have been associated with protective humoral immune responses in various diseases, such as malaria where IgG3 levels predict parasite control [10].
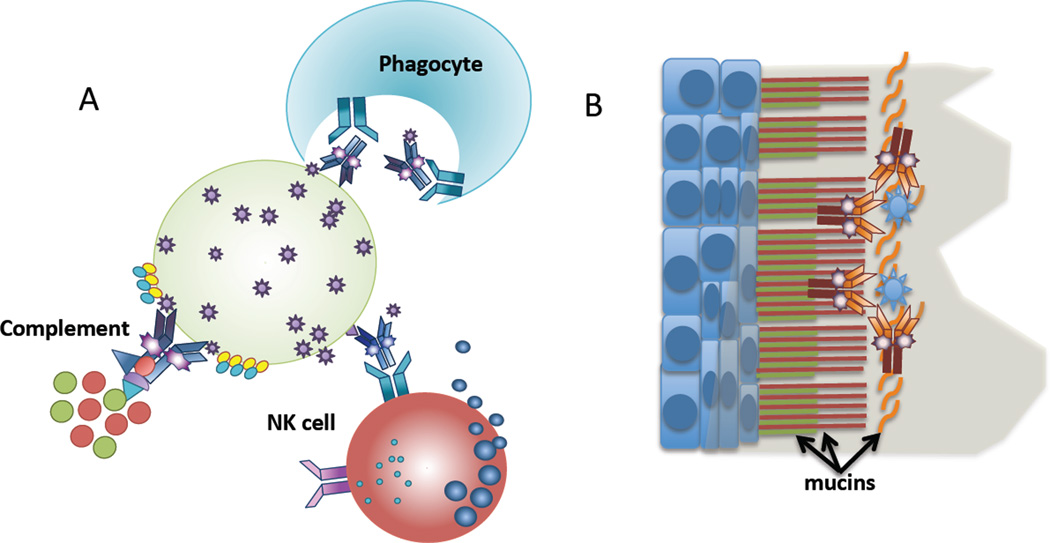
Figure 1. Antiviral properties of non-neutralizing HIV-specific antibodies.
(A) HIV-specific antibodies act as beacons aimed at attracting innate immune cells or complement to kill infected cells through various different mechanisms including, but not limited to, ADCC, ADCP, and complement activation. (B) Newer data suggest that HIV-specific antibodies may also act to trap the virus above mucosal membranes, through the interaction with mucus components, preventing infection, and therefore potentially offering a novel mechanism by which vaccine induced antibodies may prevent infection.
Figure 2. Antibody functionality is tuned through isotype selection and altered glycosylation.
(A) The generation of monoclonal therapeutics on difference constant domain isotypes can drive significantly different functions, and (B) induce hierarchically different levels of tumor mediated killing in the presence of NK cells. (C) Similarly, simply altering the glycan structure attached to the same antibody heavy chain car lead to different antibody effector functions, (D) where the removal of a single sugar, fucose, has been shown to improve NK cell mediated tumor clearance dramatically.
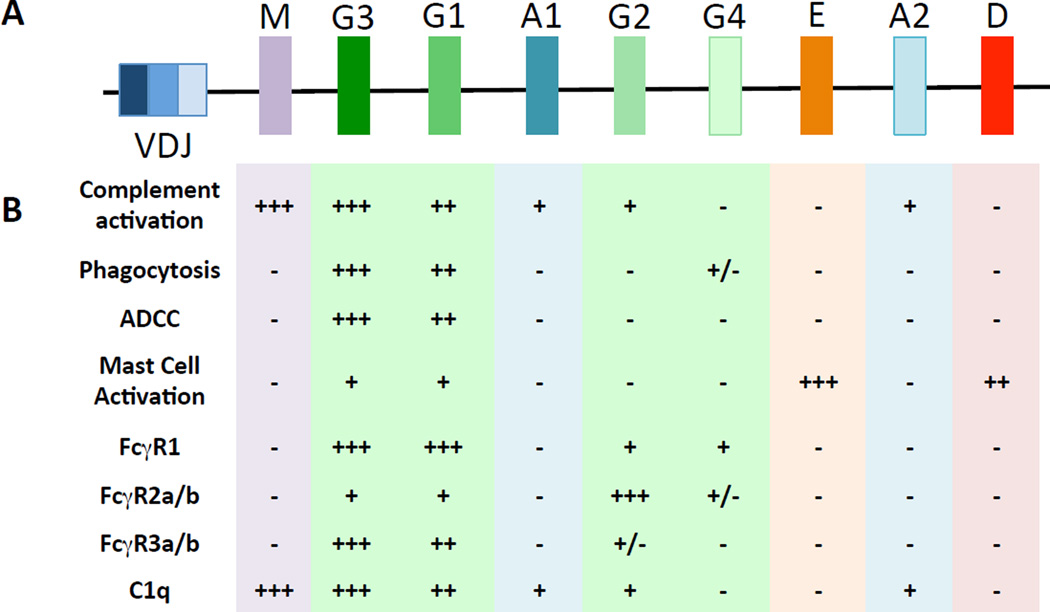
Within the human heavy chain locus (IGH@), the IgG3 constant region is the first subtype [13]( (Figure 1), suggesting that it is the first IgG subclass that may be selected during an acute immune response. Moreover, the IGH subclass sequence follows the FcγR binding affinity, and functional potency, of the subclasses (IgG3>IgG1>IgG2>IgG4), suggesting that IgG subclass selection is evolutionarily programmed to allow for the production of the most functional antibodies early in the immune response, followed by less inflammatory Ab subclasses in subsequent waves. Along these lines, HIV-specific IgG3 antibodies have been proposed as markers of early HIV infection due to their enrichment during the first few weeks of infection [14]. However, IgG3 antibody levels decline rapidly following acute infection and are replaced by a strong IgG1 response in the chronic phase of the disease [15]. Additionally, spontaneous control of HIV infection (Controllers) in the absence of antiretroviral therapy is associated with the induction of high levels of p24- and gp120-specific IgG1 and the maintenance of gp120-specific IgG3 antibodies [16], whereas progression to AIDS has been associated with increasing levels HIV-specific IgG4 antibodies [17]. Together, these data suggest that spontaneous control is associated with the induction of functionally enhanced antibody isotypes (IgG1 and IgG3), whereas progression to disease is associated with the selection of poorly functional antibodies [16].
Functional tuning by glycosylation
In addition to isotype-associated differences in activity, antibody glycosylation profoundly modulates antibody function. For example, IgG antibodies lacking fucose exhibit increased cytotoxic activity [18–23] as the result of increased affinity to the activating FcγR3A [18] (Figure 2c and d). Therapeutic antibodies optimized to contain low levels of fucose residues are more effective in clearing tumor cells than their fucosylated counterparts [24, 25]. Conversely, increased terminal sialylation imbues antibodies with anti-inflammatory properties [26], and the bioactive fraction of IVIG that is used to treat many inflammatory and autoimmune conditions is thought to be mediated by a fraction of sialylated antibodies within the larger polyclonal pool [27].
In the context of HIV infection, recent data suggest that significant changes occur in both bulk and HIV-specific antibody glycosylation [28, 29]. Specifically, HIV infection is associated with a significant increase in agalactosylated antibodies [29], which are highly inflammatory and typically enriched in patients with inflammatory conditions such as rheumatoid arthritis [27]. Interestingly, the most dramatic enrichment of agalactosylated antibodies was observed among Controllers, suggesting that while these individuals exhibit less overall immune activation [28], B cells in Controllers continue to receive inflammatory signals that drive the secretion of antibodies with glycan structures associated with chronic inflammation. Intriguingly, the glycosylation of HIV-specific antibodies was further skewed towards a more highly inflammatory glycoform, containing less galactose, fucose and sialic acid [28]. The most profound enrichment of afucosylated, agalactosylated antibodies was observed among HIV-specific antibodies in Controllers, who also exhibit enhanced ADCC activity, suggesting that the B cells in these individuals were specifically programmed to make highly functional antibodies that may contribute to persistent viral control. Yet, to date, little is known about the mechanism by which B cells program antibody glycosylation, even though it may offer new alternatives by which antibody effector functions may be actively recruited through vaccination.
Recruitment of innate immune cells through FcγRs
Beyond neutralization, antibodies mediate a broad array of functions including ADCC, antibody-mediated cellular phagocytosis (ADCP), complement-mediated killing (Figure 3A), and antibody-dependent cell-mediated virus inhibition (ADCVI). On a cellular level, the biological functions of IgG are mediated by interactions between Fc and FcγRs, which are found on all innate immune cells.
Figure 3. The IGH locus and associated function.
(A) depicts a rearranged variable domain (VDJ) (left) followed by the intact sequence of the different constant domain isotypes. (B) summarizes the breadth of functions and protein interaction potencies for each of the antibody constant domain isotypes.
Six major FcγRs have been identified in humans: FcγRI, FcγRIIa, FcγRIIb, FcγRIIc, FcγRIIIa, and FcγRIIIb [30] (Figure 1). With the exception of FcγRIIb, which inhibits activation, all other FcγRs are activating receptors, signaling either directly through immune tyrosine activating motifs (ITAMs) in their cytoplasmic tail or through interactions with separate ITAM-containing proteins [30–32]. The genes encoding FcγRIIa and FcγRIIIa contain single nucleotide polymorphisms that result in receptors that differ in their binding affinities for different IgG subclasses [33]. NK cells generally express FcγRIIIa and, in some individuals, FcγRIIc. Other cell types likely to be involved in preventing or controlling HIV infection, such as monocytes and macrophages may express all of the FcγRs with the exception of FcγRIIIb, which is primarily expressed on neutrophils and eosinophils. It should be emphasized that expression of FcγRs on any given cell type may differ according to activation state, cytokine milieu, and location.
Over the past three decades, significant work has pointed toward a role for Fc-FcγR interactions in both infection and vaccine-mediated control of HIV/SIV in vitro and in vivo. While several studies have explored the potential role of FcγR-mediated antibody functions in preventing HIV infection [34–37], only one study directly implicated these functions in prevention [38]. In this study, infusion of IgG1 b12, a monoclonal antibody (mAb) that engages FcγRs, protected macaques from vaginal exposure to SHIV162p3, and this protection was partially lost when the mAb was mutated to abrogate FcγR binding. Although this study directly demonstrated the importance of Fc-FcγR interactions in augmenting protection, the precise antibody function(s) responsible remain unknown. Thus, while Fc-FcγR interactions have been directly identified as a mechanism of protection in a rhesus macaque model of vaginal SHIV infection, the contribution of any single FcγR-mediated antibody function to protective immunity remains unknown.
Function depends on multimerization
While passive transfer strategies using non-neutralizing, functional antibodies alone have failed to definitively demonstrate protection from HIV/SIV infection [35–37, 39], the biology underlying the protective activity of neutralizing and non-neutralizing antibodies is likely different. Therefore, comparisons of passively administered single mAbs may not be informative. Unlike neutralizing antibodies that block a limited number of viral epitopes on the surface of a virus [40], non-neutralizing antibodies must form avid immune complexes that are able to recruit the low-affinity receptors or innate immune proteins necessary for their function [12]. Due to the limited amount of envelope on the surface of HIV [41], non-neutralizing antibodies may have a limited impact on the virus itself; however, because HIV assembles in lipid rafts [42], the concentration of HIV antigens on the surface of cells may allow non-neutralizing antibodies to bind and cluster Fc receptors on effector cells. However, polyclonal non-neutralizing antibodies simultaneously targeting several epitopes on a single envelope may have a better opportunity to form large avid immune complexes than mAbs. Therefore, while previous passive transfer efforts of single, non-neutralizing antibodies exhibited limited success [34], it is likely that cocktails of high-affinity, Fc-enhanced non-neutralizing antibodies that could simultaneously decorate the surface of HIV envelope could mediate sterilizing protection from infection.
Individual antibody functions and their role in HIV infection
Antibodies are able to recruit a spectrum of different antiviral functions including:
ADCC. ADCC occurs when an antibody forms a bridge between antigen-expressing target cells and FcγR-expressing effector cells; the cross-linking of FcγRs on the effector cells results in target cell death. While several studies have explored the role of vaccine-elicited ADCC-inducing antibodies in preventing HIV, SIV, or SHIV infection, there has been no direct demonstration of a protective effect. However, as mentioned above, in secondary analyses of the RV144 data, the combination of low vaccine-elicited plasma anti-gp120 IgA levels and high ADCC antibody activity was associated with a lower infection rate [3]. Furthermore, in macaques, vaccine-induced ADCC activity has been correlated with infection outcomes [37, 39, 43]. For example, a very strong trend towards protection from infection was observed following heterologous prime-boost vaccinated macaques exposed to repetitive SIVmac251 intrarectal challenge[2]. Likewise, ADCC-inducing responses elicited by live, attenuated SIVΔnef correlated with protection from intravenous or vaginal challenge with SIVmac251 [43]. Additionally, ADCC-mediating IgG in cervico-vaginal secretions has been elicited by vaccination of macaques, and it is possible that such ADCC activity plays a role in protection against SHIV162p3 vaginal challenge [44]. Other studies have found either an inverse correlation between ADCC activity prior to challenge and acute or chronic viremia levels or a direct correlation between ADCC activity and the number of challenges required for infection [35, 37, 45], suggesting that these responses may have post-acquisition antiviral effects.
However, no study to date has directly demonstrated that an ADCC-mediating antibody is responsible for preventing HIV/SIV infection. Moreover, ADCC-inducing responses following MVA vaccination were not associated with the risk of SIVsmE660 infection after rectal challenge [46]. Furthermore, a recent mAb passive infusion study has now called into question whether NK cell–mediated ADCC activity is involved in protection against mucosal SHIV challenge [47]. In this study, animals were infused with either IgG1 b12 or a non-fucosylated version of the mAb, designed to increase FcγRIIIa binding and NK cell-mediated ADCC activity. Although a small number of animals were studied, there was no difference in protection against repeated low-dose vaginal SHIV challenge for the two forms of the mAb, suggesting that other FcγR interactions could be more critical in driving antiviral control and clearance, particularly at mucosal surfaces.
Phagocytosis. Phagocytes, such as monocytes, macrophages, and dendritic cells, can internalize antibody-coated virus or antibody-coated infected cells. Like ADCC-inducing antibodies, it is likely that phagocytosis of HIV-1 immune complexes augments the anti-viral activity of neutralizing or non-neutralizing antibodies. However, interestingly, depending on the inflammatory state, phagocytes, and not CD16+ NK cells, are more abundant at mucosal membranes where the large majority of infections occur [48, 49]. Therefore, it is possible that antibodies able to mediate the rapid clearance of the virus or virally infected cells could have a beneficial role in preventing HIV infection [50–53]. Recently, antibodies elicited in healthy human volunteers by an Ad26 vaccine expressing clade A HIV-1 Env were shown to mediate phagocytosis of gp120-coated beads [54].
As with other FcγR-mediated antibody functions, IgG subclass is a determinant of phagocytosis, and it is likely that FcγRIIa or FcγRIIIa genotypes and differential binding to different FcγRs impact phagocytosis in vivo [55, 56]. Subjects possessing the FcγRIIa RR genotype demonstrated reduced internalization of HIV-1 immune complexes than subjects with the RH or HH genotypes, and this reduced phagocytic activity was linked to more a rapid decline in CD4 cells, suggesting that phagocytic clearance of virus and/or virally infected cells plays a role in post-acquisition disease control [57]. Similarly, quantitative differences in the capacity of antibodies to drive phagocytosis were recently associated with qualitative differences in FcγR binding profiles. Specifically, antibodies from Controllers exhibited increased phagocytic activity that was associated with the enhanced capacity of both bulk plasma and HIV-specific IgG to engage FcγRIIa and not FcγR2IIb [56], this altered FcγR binding profile was associated with changes in antibody glycosylation, potentially pointing to new antibody glycoforms that may allow antibodies to prevent infection more robustly at mucosal membranes, where phagocytic cells are more abundant. Moreover, as mentioned above, although the non-fucosylated b12 did not exhibit enhanced protection from infection, it is plausible that modifications, such as those elicited by Controllers that enhance phagocytosis, may offer an alternate mechanism by which passively transferred mAbs may mediate enhanced protection from infection via the utilization of the phagocytic cells that abundantly line mucosal membranes.
ADCVI. ADCVI is not an antibody function itself; rather, it is a measure of net antiviral activity provided by antibody in combination with FcγR-bearing effector cells. The components contributing to the antiviral effect measured in ADCVI assays include ADCC, the production of β chemokines, and phagocytosis [58]. ADCVI antibody activity has been shown to rise early and to correlate with the fall in viremia observed during acute infection [58]. Moreover, ADCVI activity is enriched in Controllers and is associated with the preferential accumulation of agalactosylated antibodies [28]. As with ADCC, ADCVI-inducing antibodies elicited by vaccination or present in passively infused reagents correlate with protection or reductions in viral load in monkeys after various types of challenge with SIV or SHIV [35–38, 59, 60]. In humans, increased ADCVI activity elicited by a recombinant gp120 vaccine in the Vax004 trial was associated with lower infection rates [61]. However, the ADCVI response to primary infection with HIV-1 did not correlate with the risk of superinfection in a cohort of women in Kenya [62].
New mechanisms of protection by non-neutralizing antibodies
Antibodies are present at the majority of the mucosal surfaces exposed to HIV during sexual transmission. In addition to the presence of antibodies within the fluids associated with these barriers, the antibodies may also be able to interact with the mucosal and/or mucus components themselves (Figure 3b). The first barrier encountered by HIV at mucosal epithelial barriers is composed of secreted mucins, which coat the mucosal surfaces of the colorectal tract and the female reproductive tract. Because most HIV infections are caused by a single transmitted founder virus [63], the majority of viruses must get stuck or cleared at the mucosal surface. Therefore, recent efforts have been focused on dissecting the potential properties of antibodies that promote more effective interactions with mucins to trap the virus, a mechanism that would effectively neutralize the virus by preventing if from reaching potential target cells.
Although the interaction of antibodies and mucus has not been well defined, there have been a number of recent paper that suggest that antibodies and mucus can function together to reduce the pathogenic consequences of microbes. For example, mucus in combination with secretory IgA provided optimal protection from toxin-induced injury in a tissue culture model [64]. These results echo studies published a decade ago suggesting that the secretory component of IgA mediates the protective effects of mucus-bound IgA [65]. Similarly, the deletion of MyD88 in mice decreased mucin 2 and secretory IgA production, leading to compromised antibacterial immunity [66]. IgG may also interact comparably with mucus. For example, the presence of IgG antibodies to Helicobacter pylori in cervical mucus has been associated with infertility [67].
Antibodies may also interact with the mucosal surfaces, potentially retaining HIV at the interface of the mucosal epithelium and the lumen. Trapped at the surface, HIV would not be able to penetrate the mucosal epithelial barrier. These interactions could take place between cell-associated mucins (e.g., MUC1, MUC4, and MUC16) and the Fc portions of antibodies. Antibodies have also been observed to accumulate in the superficial aspects of the squamous epithelium of the ectocervix [68, 69]. However, while these observations suggest another level of potential protection provided by non-neutralizing antibodies at the mucosal epithelium, these interactions need to be better defined.
Summary
While a large number of novel broadly neutralizing antibodies has been recently described [70–72] the induction of such antibodies via vaccination has proven difficult [73]. By contrast, non-neutralizing antibodies arise early during infection and have been repeatedly associated with both protection from infection and disease progression. Moreover, we are beginning to gain new insights into the broader landscape of antiviral mechanisms that non-neutralizing antibodies may harness to fight HIV, providing an unprecedented breadth of approaches by which HIV can be blocked and contained. Together with new insights into the biophysical properties that allow antibodies to recruit specific innate effector functions, significant progress is being made in our understanding of the humoral parameters that might be manipulated via vaccination to improve antiviral control of HIV.
Key Bullet Points.
-
-
Beyond neutralization, antibodies are able to recruit a wide array of antiviral activities against HIV through the innate immune system.
-
-
Antiviral activity of non-neutralizing antibodies is programmed through selective antibody subclass selection and antibody glycosylation.
-
-
Beyond traditional antiviral effector mechanisms, antibodies may also trap virus at mucosal membranes thereby preventing HIV infection.
Footnotes
The authors do not have any conflicts of interest.
References
- 1.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361:2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 2.Barouch DH, Liu J, Li H, et al. Vaccine protection against acquisition of neutralization-resistant SIV challenges in rhesus monkeys. Nature. 2012;482:89–93. doi: 10.1038/nature10766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haynes BF, Gilbert PB, McElrath MJ, et al. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med. 2012;366:1275–1286. doi: 10.1056/NEJMoa1113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keim C, Kazadi D, Rothschild G, et al. Regulation of AID, the B-cell genome mutator. Genes Dev. 2013;27:1–17. doi: 10.1101/gad.200014.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kinoshita K, Lee CG, Tashiro J, et al. Molecular mechanism of immunoglobulin class switch recombination. Cold Spring Harb Symp Quant Biol. 1999;64:217–226. doi: 10.1101/sqb.1999.64.217. [DOI] [PubMed] [Google Scholar]
- 6.Banchereau J, Blanchard D, Briere F, et al. Role of cytokines in human B lymphocyte growth and differentiation. Nouv Rev Fr Hematol. 1993;35:61–66. [PubMed] [Google Scholar]
- 7.Lundgren M, Persson U, Larsson P, et al. Interleukin 4 induces synthesis of IgE and IgG4 in human B cells. Eur J Immunol. 1989;19:1311–1315. doi: 10.1002/eji.1830190724. [DOI] [PubMed] [Google Scholar]
- 8.Pene J, Rousset F, Briere F, et al. IgE production by normal human lymphocytes is induced by interleukin 4 and suppressed by interferons gamma and alpha and prostaglandin E2. Proc Natl Acad Sci U S A. 1988;85:6880–6884. doi: 10.1073/pnas.85.18.6880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pene J, Gauchat JF, Lecart S, et al. Cutting edge: IL-21 is a switch factor for the production of IgG1 and IgG3 by human B cells. J Immunol. 2004;172:5154–5157. doi: 10.4049/jimmunol.172.9.5154. [DOI] [PubMed] [Google Scholar]
- 10.Garraud O, Perraut R, Riveau G, et al. Class and subclass selection in parasite-specific antibody responses. Trends Parasitol. 2003;19:300–304. doi: 10.1016/s1471-4922(03)00139-9. [DOI] [PubMed] [Google Scholar]
- 11.Jefferis R. Glycosylation of antibody therapeutics: optimisation for purpose. Methods Mol Biol. 2009;483:223–238. doi: 10.1007/978-1-59745-407-0_13. [DOI] [PubMed] [Google Scholar]
- 12.Nimmerjahn F, Ravetch JV. FcgammaRs in health and disease. Curr Top Microbiol Immunol. 2011;350:105–125. doi: 10.1007/82_2010_86. [DOI] [PubMed] [Google Scholar]
- 13.Murphy K, Travers P, Walport M, et al. Janeway's immunobiology. 8th ed. xix. New York: Garland Science; 2012. p. 868. [Google Scholar]
- 14. Yates NL, Lucas JT, Nolen TL, et al. Multiple HIV-1-specific IgG3 responses decline during acute HIV-1: implications for detection of incident HIV infection. AIDS. 2011;25:2089–2097. doi: 10.1097/QAD.0b013e32834b348e. This manuscript points to an early selection of the IgG3 subclass following acute HIV infection, that precipitously declines following infection, potentially useful as a marker of early disease.
- 15.Tomaras GD, Yates NL, Liu P, et al. Initial B-cell responses to transmitted human immunodeficiency virus type 1: virion-binding immunoglobulin M (IgM) and IgG antibodies followed by plasma anti-gp41 antibodies with ineffective control of initial viremia. J Virol. 2008;82:12449–12463. doi: 10.1128/JVI.01708-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Banerjee K, Klasse PJ, Sanders RW, et al. IgG subclass profiles in infected HIV type 1 controllers and chronic progressors and in uninfected recipients of Env vaccines. AIDS Res Hum Retroviruses. 2010;26:445–458. doi: 10.1089/aid.2009.0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ljunggren K, Broliden PA, Morfeldt-Manson L, et al. IgG subclass response to HIV in relation to antibody-dependent cellular cytotoxicity at different clinical stages. Clin Exp Immunol. 1988;73:343–347. [PMC free article] [PubMed] [Google Scholar]
- 18.Shields RL, Lai J, Keck R, et al. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human Fcgamma RIII and antibody-dependent cellular toxicity. J Biol Chem. 2002;277:26733–26740. doi: 10.1074/jbc.M202069200. [DOI] [PubMed] [Google Scholar]
- 19.Shinkawa T, Nakamura K, Yamane N, et al. The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity. J Biol Chem. 2003;278:3466–3473. doi: 10.1074/jbc.M210665200. [DOI] [PubMed] [Google Scholar]
- 20.Yamane-Ohnuki N, Kinoshita S, Inoue-Urakubo M, et al. Establishment of FUT8 knockout Chinese hamster ovary cells: an ideal host cell line for producing completely defucosylated antibodies with enhanced antibody-dependent cellular cytotoxicity. Biotechnol Bioeng. 2004;87:614–622. doi: 10.1002/bit.20151. [DOI] [PubMed] [Google Scholar]
- 21.Mori K, Iida S, Yamane-Ohnuki N, et al. Non-fucosylated therapeutic antibodies: the next generation of therapeutic antibodies. Cytotechnology. 2007;55:109–114. doi: 10.1007/s10616-007-9103-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsumiya S, Yamaguchi Y, Saito J, et al. Structural comparison of fucosylated and nonfucosylated Fc fragments of human immunoglobulin G1. J Mol Biol. 2007;368:767–779. doi: 10.1016/j.jmb.2007.02.034. [DOI] [PubMed] [Google Scholar]
- 23.Peipp M, Lammerts van Bueren JJ, Schneider-Merck T, et al. Antibody fucosylation differentially impacts cytotoxicity mediated by NK and PMN effector cells. Blood. 2008;112:2390–2399. doi: 10.1182/blood-2008-03-144600. [DOI] [PubMed] [Google Scholar]
- 24.Junttila TT, Parsons K, Olsson C, et al. Superior in vivo efficacy of afucosylated trastuzumab in the treatment of HER2-amplified breast cancer. Cancer Res. 2010;70:4481–4489. doi: 10.1158/0008-5472.CAN-09-3704. [DOI] [PubMed] [Google Scholar]
- 25.Niwa R, Natsume A, Uehara A, et al. IgG subclass-independent improvement of antibody-dependent cellular cytotoxicity by fucose removal from Asn297-linked oligosaccharides. J Immunol Methods. 2005;306:151–160. doi: 10.1016/j.jim.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 26.Anthony RM, Nimmerjahn F, Ashline DJ, et al. Recapitulation of IVIG anti-inflammatory activity with a recombinant IgG Fc. Science. 2008;320:373–376. doi: 10.1126/science.1154315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaneko Y, Nimmerjahn F, Ravetch JV. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science. 2006;313:670–673. doi: 10.1126/science.1129594. [DOI] [PubMed] [Google Scholar]
- 28. Ackerman ME, Crispin M, Yu X, et al. Natural variation in Fc glycosylation of HIV-specific antibodies impacts antiviral activity. J Clin Invest. 2013;123:2183–2192. doi: 10.1172/JCI65708. This study demonstrates the unusual accumulation of inflammatory antibody glycans among HIV infected patients, that are agalactosylated and asialated. However, beyond these changes, spontaneous HIV controllers elicited more inflammatory glycoforms associated with higher ADCVI functionality, that were additionally afucosylated.
- 29.Moore JS, Wu X, Kulhavy R, et al. Increased levels of galactose-deficient IgG in sera of HIV-1-infected individuals. AIDS. 2005;19:381–389. doi: 10.1097/01.aids.0000161767.21405.68. [DOI] [PubMed] [Google Scholar]
- 30.Takai T. Roles of Fc receptors in autoimmunity. Nat Rev Immunol. 2002;2:580–592. doi: 10.1038/nri856. [DOI] [PubMed] [Google Scholar]
- 31.Dijstelbloem HM, van de Winkel JG, Kallenberg CG. Inflammation in autoimmunity: receptors for IgG revisited. Trends Immunol. 2001;22:510–516. doi: 10.1016/s1471-4906(01)02014-2. [DOI] [PubMed] [Google Scholar]
- 32.Budde P, Bewarder N, Weinrich V, et al. Tyrosine-containing sequence motifs of the human immunoglobulin G receptors FcRIIb1 and FcRIIb2 essential for endocytosis and regulation of calcium flux in B cells. J Biol Chem. 1994;269:30636–30644. [PubMed] [Google Scholar]
- 33.Bruhns P, Iannascoli B, England P, et al. Specificity and affinity of human Fcgamma receptors and their polymorphic variants for human IgG subclasses. Blood. 2009;113:3716–3725. doi: 10.1182/blood-2008-09-179754. [DOI] [PubMed] [Google Scholar]
- 34.Burton DR, Hessell AJ, Keele BF, et al. Limited or no protection by weakly or nonneutralizing antibodies against vaginal SHIV challenge of macaques compared with a strongly neutralizing antibody. Proc Natl Acad Sci U S A. 2011;108:11181–11186. doi: 10.1073/pnas.1103012108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xiao P, Zhao J, Patterson LJ, et al. Multiple vaccine-elicited nonneutralizing antienvelope antibody activities contribute to protective efficacy by reducing both acute and chronic viremia following simian/human immunodeficiency virus SHIV89.6P challenge in rhesus macaques. J Virol. 2010;84:7161–7173. doi: 10.1128/JVI.00410-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Florese RH, Demberg T, Xiao P, et al. Contribution of nonneutralizing vaccine-elicited antibody activities to improved protective efficacy in rhesus macaques immunized with Tat/Env compared with multigenic vaccines. J Immunol. 2009;182:3718–3727. doi: 10.4049/jimmunol.0803115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hidajat R, Xiao P, Zhou Q, et al. Correlation of vaccine-elicited systemic and mucosal nonneutralizing antibody activities with reduced acute viremia following intrarectal simian immunodeficiency virus SIVmac251 challenge of rhesus macaques. J Virol. 2009;83:791–801. doi: 10.1128/JVI.01672-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hessell AJ, Hangartner L, Hunter M, et al. Fc receptor but not complement binding is important in antibody protection against HIV. Nature. 2007;449:101–104. doi: 10.1038/nature06106. [DOI] [PubMed] [Google Scholar]
- 39.Gomez-Roman VR, Patterson LJ, Venzon D, et al. Vaccine-elicited antibodies mediate antibody-dependent cellular cytotoxicity correlated with significantly reduced acute viremia in rhesus macaques challenged with SIVmac251. J Immunol. 2005;174:2185–2189. doi: 10.4049/jimmunol.174.4.2185. [DOI] [PubMed] [Google Scholar]
- 40.Koff WC. HIV vaccine development: challenges and opportunities towards solving the HIV vaccine-neutralizing antibody problem. Vaccine. 2012;30:4310–4315. doi: 10.1016/j.vaccine.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 41.Klasse PJ. Modeling how many envelope glycoprotein trimers per virion participate in human immunodeficiency virus infectivity and its neutralization by antibody. Virology. 2007;369:245–262. doi: 10.1016/j.virol.2007.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Campbell SM, Crowe SM, Mak J. Lipid rafts and HIV-1: from viral entry to assembly of progeny virions. J Clin Virol. 2001;22:217–227. doi: 10.1016/s1386-6532(01)00193-7. [DOI] [PubMed] [Google Scholar]
- 43. Alpert MD, Harvey JD, Lauer WA, et al. ADCC develops over time during persistent infection with live-attenuated SIV and is associated with complete protection against SIV(mac)251 challenge. PLoS Pathog. 2012;8:e1002890. doi: 10.1371/journal.ppat.1002890. This study points to a potential protective role for ADCC inducing antibodies, induced by vaccination with the live-attenuated SIV, against infection, pointing to a prophylactic
- 44.Bomsel M, Tudor D, Drillet AS, et al. Immunization with HIV-1 gp41 subunit virosomes induces mucosal antibodies protecting nonhuman primates against vaginal SHIV challenges. Immunity. 2011;34:269–280. doi: 10.1016/j.immuni.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 45.Xiao P, Patterson LJ, Kuate S, et al. Replicating adenovirus-simian immunodeficiency virus (SIV) recombinant priming and envelope protein boosting elicits localized, mucosal IgA immunity in rhesus macaques correlated with delayed acquisition following a repeated low-dose rectal SIV(mac251) challenge. J Virol. 2012;86:4644–4657. doi: 10.1128/JVI.06812-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lai L, Kwa SF, Kozlowski PA, et al. SIVmac239 MVA vaccine with and without a DNA prime, similar prevention of infection by a repeated dose SIVsmE660 challenge despite different immune responses. Vaccine. 2012;30:1737–1745. doi: 10.1016/j.vaccine.2011.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Moldt B, Shibata-Koyama M, Rakasz EG, et al. A nonfucosylated variant of the anti-HIV-1 monoclonal antibody b12 has enhanced FcgammaRIIIa-mediated antiviral activity in vitro but does not improve protection against mucosal SHIV challenge in macaques. J Virol. 2012;86:6189–6196. doi: 10.1128/JVI.00491-12. This manuscript shows a lack of an enhanced protective effect against HIV acquisition following administration of an ADCC-enhanced (afucosylated) variant of B12, suggesting that the Fc-mediated protective role of B12 may be mediated through other effector mechanisms than ADCC.
- 48.Sips M, Sciaranghella G, Diefenbach T, et al. Altered distribution of mucosal NK cells during HIV infection. Mucosal Immunol. 2012;5:30–40. doi: 10.1038/mi.2011.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smith PD, Meng G, Salazar-Gonzalez JF, et al. Macrophage HIV-1 infection and the gastrointestinal tract reservoir. J Leukoc Biol. 2003;74:642–649. doi: 10.1189/jlb.0503219. [DOI] [PubMed] [Google Scholar]
- 50.Holl V, Hemmerter S, Burrer R, et al. Involvement of Fc gamma RI (CD64) in the mechanism of HIV-1 inhibition by polyclonal IgG purified from infected patients in cultured monocyte-derived macrophages. J Immunol. 2004;173:6274–6283. doi: 10.4049/jimmunol.173.10.6274. [DOI] [PubMed] [Google Scholar]
- 51.Holl V, Peressin M, Decoville T, et al. Nonneutralizing antibodies are able to inhibit human immunodeficiency virus type 1 replication in macrophages and immature dendritic cells. J Virol. 2006;80:6177–6181. doi: 10.1128/JVI.02625-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Holl V, Peressin M, Schmidt S, et al. Efficient inhibition of HIV-1 replication in human immature monocyte-derived dendritic cells by purified anti-HIV-1 IgG without induction of maturation. Blood. 2006;107:4466–4474. doi: 10.1182/blood-2005-08-3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Forthal DN, Moog C. Fc receptor-mediated antiviral antibodies. Curr Opin HIV AIDS. 2009;4:388–393. doi: 10.1097/COH.0b013e32832f0a89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barouch DH, Liu J, Peter L, et al. Characterization of humoral and cellular immune responses elicited by a recombinant adenovirus serotype 26 HIV-1 Env vaccine in healthy adults (IPCAVD 001) J Infect Dis. 2013;207:248–256. doi: 10.1093/infdis/jis671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Forthal DN, Landucci G, Ding H, et al. IgG2 inhibits HIV-1 internalization by monocytes, and IgG subclass binding is affected by gp120 glycosylation. AIDS. 2011;25:2099–2104. doi: 10.1097/QAD.0b013e32834b64bd. [DOI] [PubMed] [Google Scholar]
- 56.Ackerman ME, Dugast AS, McAndrew EG, et al. Enhanced Phagocytic Activity of HIV-Specific Antibodies Correlates with Natural Production of Immunoglobulins with Skewed Affinity for FcgammaR2a and FcgammaR2b. J Virol. 2013;87:5468–5476. doi: 10.1128/JVI.03403-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Forthal DN, Landucci G, Bream J, et al. FcgammaRIIa genotype predicts progression of HIV infection. J Immunol. 2007;179:7916–7923. doi: 10.4049/jimmunol.179.11.7916. [DOI] [PubMed] [Google Scholar]
- 58.Forthal DN, Landucci G, Daar ES. Antibody from patients with acute human immunodeficiency virus (HIV) infection inhibits primary strains of HIV type 1 in the presence of natural-killer effector cells. J Virol. 2001;75:6953–6961. doi: 10.1128/JVI.75.15.6953-6961.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Forthal DN, Landucci G, Cole KS, et al. Rhesus macaque polyclonal and monoclonal antibodies inhibit simian immunodeficiency virus in the presence of human or autologous rhesus effector cells. J Virol. 2006;80:9217–9225. doi: 10.1128/JVI.02746-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bialuk I, Whitney S, Andresen V, et al. Vaccine induced antibodies to the first variable loop of human immunodeficiency virus type 1 gp120, mediate antibody-dependent virus inhibition in macaques. Vaccine. 2011;30:78–94. doi: 10.1016/j.vaccine.2011.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Forthal DN, Gilbert PB, Landucci G, et al. Recombinant gp120 vaccine-induced antibodies inhibit clinical strains of HIV-1 in the presence of Fc receptor-bearing effector cells and correlate inversely with HIV infection rate. J Immunol. 2007;178:6596–6603. doi: 10.4049/jimmunol.178.10.6596. [DOI] [PubMed] [Google Scholar]
- 62.Forthal DN, Landucci G, Chohan B, et al. Antibody-dependent cell-mediated virus inhibition (ADCVI) antibody activity does not correlate with risk of HIV-1 superinfection. J Acquir Immune Defic Syndr. 2013 doi: 10.1097/QAI.0b013e3182874d41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Boeras DI, Hraber PT, Hurlston M, et al. Role of donor genital tract HIV-1 diversity in the transmission bottleneck. Proc Natl Acad Sci U S A. 2011;108:E1156–E1163. doi: 10.1073/pnas.1103764108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Olson A, Diebel LN, Liberati DM. Effect of host defenses on Clostridium difficile toxin-induced intestinal barrier injury. J Trauma Acute Care Surg. 2013;74:983–989. doi: 10.1097/TA.0b013e3182858477. discussion 989-90. [DOI] [PubMed] [Google Scholar]
- 65.Phalipon A, Cardona A, Kraehenbuhl JP, et al. Secretory component: a new role in secretory IgA-mediated immune exclusion in vivo. Immunity. 2002;17:107–115. doi: 10.1016/s1074-7613(02)00341-2. [DOI] [PubMed] [Google Scholar]
- 66.Frantz AL, Rogier EW, Weber CR, et al. Targeted deletion of MyD88 in intestinal epithelial cells results in compromised antibacterial immunity associated with downregulation of polymeric immunoglobulin receptor, mucin-2, and antibacterial peptides. Mucosal Immunol. 2012;5:501–512. doi: 10.1038/mi.2012.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ambrosini G, Andrisani A, Fiore C, et al. Anti-Helicobacter pylori antibodies in cervical mucus: a new cause of infertility. Eur J Obstet Gynecol Reprod Biol. 2011;155:157–160. doi: 10.1016/j.ejogrb.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 68.Blaskewicz CD, Pudney J, Anderson DJ. Structure and function of intercellular junctions in human cervical and vaginal mucosal epithelia. Biol Reprod. 2011;85:97–104. doi: 10.1095/biolreprod.110.090423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fahrbach KM, Barry SM, Anderson MR, et al. Enhanced cellular responses and environmental sampling within inner foreskin explants: implications for the foreskin's role in HIV transmission. Mucosal Immunol. 2010;3:410–418. doi: 10.1038/mi.2010.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Huang J, Ofek G, Laub L, et al. Broad and potent neutralization of HIV-1 by a gp41-specific human antibody. Nature. 2012;491:406–412. doi: 10.1038/nature11544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Walker LM, Huber M, Doores KJ, et al. Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature. 2011;477:466–470. doi: 10.1038/nature10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wu X, Yang ZY, Li Y, et al. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science. 2010;329:856–861. doi: 10.1126/science.1187659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mascola JR, Montefiori DC. The role of antibodies in HIV vaccines. Annu Rev Immunol. 2010;28:413–444. doi: 10.1146/annurev-immunol-030409-101256. [DOI] [PubMed] [Google Scholar]