Abstract
Aims
The nuclear hormone receptor estrogen-related receptor α (ERRα) regulates the activation of mitochondrial genes in various human tissues, but its role in the adrenal gland and its disorders has not been defined. Therefore, we examined ERRα expression in both normal adrenal cortex (NAC) and adrenocortical tumors (ACT) in order to study the possible correlation of ERRα with adrenal development and tumor development.
Methods
Human adrenal specimens (non-pathological fetal n=7; non-pathological post-birth n=40; aldosterone producing adenoma (APA) n=11; cortisol producing adenoma (CPA) n=11; adrenocortical carcinoma (ACC) n=8) were immunohistochemically examined in this study. NAC (n=13) and ACT (n=14) frozen tissue specimens were also available for studying ERRα mRNA levels.
Key findings
In fetal NAC tissues, ERRα labeling index (LI) in fetal zone (FC) was significantly higher that that in neocortex (NC), and the difference among age groups for overall mean LI was statistically significant when analyzed by cortical layer. ERRα LI was also significantly higher in adrenocortical carcinomas (ACCs) than in other types of ACTs. ACC tended to have the highest mRNA levels for ERRα compared to other adrenocortical tumors.
Significance
Results of our present study suggest a possible role of ERRα in adrenal development and ACC.
Keywords: estrogen-related receptor α (ERRα), adrenal gland, development, immunohistochemistry
1. INTRODUCTION
Nuclear receptors (NRs) play pivotal roles during the development of many vertebrates and also in regulation of physiological functions in adults (Chambon 2005; Evans 2005). The estrogen related receptor α (ERRα) belongs to the NR superfamily, a group of 48 structurally related, ligand-activated transcription factors (Alaynick 2008; Giguere 2008; Tremblay and Giguere 2007). ERRα was originally cloned by DNA sequence homology to the estrogen receptor α (ERα, NR3A1) (Giguere et al. 1988), but subsequent ligand binding and transient transfection experiments with reporter genes could not demonstrate natural estrogens as its ligands, as could not identify any natural ligands for this receptor (Deblois and Giguere 2011). Therefore, ERRα is currently considered an orphan nuclear receptor (Deblois and Giguere 2011; Giguere et al. 1988).
ERRα is expressed in several tissues requiring high energy demand, including the heart, skeletal muscle and the brain (Heard et al. 2000). Results of both in vitro and in vivo studies suggest that ERRα is required for the activation of mitochondrial genes as well as increased mitochondrial biogenesis (Schreiber et al. 2004; Villena et al. 2007). It has been reported that ERRα can activate reporters containing steroidogenesis factor 1 (SF-1) response elements as a result of transient transfection assays (Bonnelye et al. 1997). A possible role of ERRα in steroidogenesis with relation to SF-1 was subsequently demonstrated in vitro by Seely et al., who also demonstrated ERRα immunoreactivity in the three layers of the adult adrenal (Seely et al., 2005). However, ERRα expression in the adrenal gland, in which activation of mitochondrial genes play important roles, has not been examined in detail.
ERRα has been studied at the RNA or protein level in various cancer types, and high expression levels were reported to be associated with a poor prognosis in various human malignancies including colon, endometrium, ovary, prostate and breast cancers (Ariazi et al. 2002; Bianco et al. 2011; Cavallini et al. 2005; Fujimoto and Sato 2009; Fujimura et al. 2007; Sun et al. 2005; Suzuki et al. 2004). ERRα has been previously reported to be present in H295R adrenocortical carcinoma cells (Seely et al., 2005) but it has not been studied at all in human adrenal gland disorders.
Therefore, in this study, we evaluated ERRα expression in both normal and neoplastic adrenocortical tissues in order to clarify its roles in adrenocorical development and tumorigenesis.
2. MATERIALS AND METHODS
2.1 Human adrenal samples
Research protocols were approved by the ethics committee at Tohoku University Graduate School of Medicine (Sendai, Japan) and the Fukushima Medical University (Fukushima, Japan).
For immunohistochemical analysis, specimens of non-pathological adrenal glands were obtained from autopsy files (fetal, n=7; post-birth 4 to 80 years of age, n=40) from Tohoku University Hospital (Sendai, Japan). Thirty cases of adrenocortical tumors (11 aldosterone producing adenomas (APAs), 11 cortisol producing adenomas (CPAs), and 8 adrenocortical carcinomas (ACCs)) were retrieved from the surgical pathology files of Tohoku University Hospital.
For quantitative RT-PCR analysis, twenty-eight cases of adrenocortical neoplasms (5 APAs, 6 CPAs, and 3 ACCs obtained from surgical procedures performed at Tohoku University Hospital and 6 APAs, 6 CPAs, and 2 ACCs obtained from Fukushima Medical University) as well as 13 samples of normal adrenal cortex (NAC), obtained from autopsies at Tohoku University Hospital, were available for RT-PCR analysis.
2.2 Immunohistochemistry
The specimens were fixed in 10% formalin for 24–48 h at room temperature and embedded in paraffin wax. Mouse monoclonal antibody for ERRα (2ZH5844H) was purchased from Perseus Proteomics Inc. (Tokyo, Japan). Antigen retrieval for immunostaining of ERRα was performed by heating the slides in an autoclave at 121°C for 5 min in citric acid buffer (2 mM citric acid and 9 mM trisodium citrate dehydrate, pH 6.0). The dilution of the primary antibody was 1:100. Immunostaining was performed employing the streptavidin-biotin amplification method using a Histofine Kit (Nichirei, Tokyo, Japan). The antigen-antibody complex was visualized with 3.3′diaminobenzidine (DAB) solution (1 mM DAB, 50 mM Tris-HCl buffer (pH 7.6), and 0.006% H2O2), and counterstained with hematoxylin. For negative controls 0.01M PBS instead of the primary antibody was incubated and no specific immunoreactivity was detected in these tissue sections, and for positive control we used a human heart sample (data not shown).
2.3 Evaluation of immunoreactivity
After completely reviewing the slides, relative immunoreactivity for ERRα in each zone of the adrenocortex as well as in tumor specimens was evaluated by labeling index (LI), carried out by examining high-power fields and counting 1000 cells. The LIs were independently and blindly evaluated by two of the authors (S.J.A.F. and X.G.H.) and the mean of these two values was used for analysis. The normal adrenals were further subclassified into the following age groups: fetal (n=7), 4–10 years of age (y.o.) (n=6), 11–20 y.o. (n=10), 21–40 y.o. (n=16), and 41–80 y.o. (n=15).
2.4 RNA isolation and quantitative RT-PCR (qPCR)
Specimens were snap-frozen for RNA isolation and stored at -80°C until use. Total RNA was carefully extracted from the 28 specimens of adrenocortical neoplasms and 13 specimens of normal adrenocortex by using the RNeasy Mini Kit (QIAGEN, Hilden, Germany) after cryostate disruption at -20°C.
cDNA was produced with Quantitec reverse transcription kit (QIAGEN) and RT-PCR was performed with LightCycler FastStart DNA Master SYBR Green I kit (Roche, Basel, Switzerland) in a LightCycler equipment (Roche). The primer sequences used in our study were: ERRα forward: 5′-CAC CAT CAG CTG GGC CAA GAG-3′ and reverse: 5′-GGT CAG ACA GCG ACA GCG ATG-3′ (Seely et al. 2005); RPL13A forward: 5′-CCT GGA GGA GAA GAG GAA AG-’3 and reverse 5′-TTG AGG ACC TCT GTG TAT TT-’3. The samples previously submitted to total RNA extraction and cDNA production were analyzed. The melting curve analysis was performed in order to verify amplification of the expected sequence. Negative control experiments did not contain cDNA substrate to check for the possibility of exogenous contaminant DNA, and no amplified products were detected under these conditions.
In all experiments, the relative mRNA expression was calculated as the ratio between the quantity of ERRα and house-keeping gene RPL13A cDNA transcripts, as previously described (Nakamura et al. 2006; Shibahara et al. 2012).
2.5 Statistical analysis
The labeling index (LI) obtained by evaluation of immunoreactivity as the percentages of stained cell nuclei, and the ERRα/RPL13A relative mRNA values obtained from qPCR data, were submitted to statistical evaluation using Mann-Whitney or Kruskal-Wallis non-parametric tests. P<0.05 was considered significant.
3. RESULTS
3.1 Immunohistochemical analysis
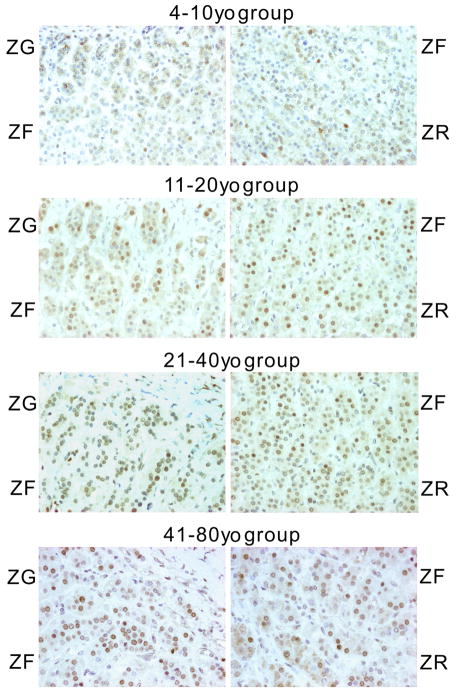
Results for ERRα expression in fetal and post-birth NAC were summarized in Figures 1 and 2, respectively. In post-birth NAC, ERRα immunoreactivity was detected in the nuclei of the zona glomerulosa (ZG), the zona fasciculata (ZF) and the zona reticularis (ZR) cells. In fetal adrenal gland, immunoreactivity was detected in neocortex (NC), with fewer nuclei positive in the fetal zone (FZ). In post-birth NAC samples, the lowest LI was observed in the 4–10 y.o., and the highest in the 41–80 y.o. age group (Figure 3A) in each zone. Statistical difference of LI among the three cortical layers was observed within the 11–20 y.o. age group (P=0.021). The difference among age groups for overall mean LI was statistically significant as well as when analyzed by cortical layer (P=0.022 for ZG, P=0.008 for ZF and P<0.001 for ZR, respectively). In fetal adrenal glands, ERRα LI in the NC was significantly higher in that in the FZ (P=0.0017) (Figure 3B). In adrenal tumors, ERRα LI was significantly higher in ACC when compared to both APA and CPA (P<0.005) (Figure 4A).
FIG 1. Post-birth adrenal cortex immunostaining.
ERRα immunoreactivity was detected in the nuclei of the Zona Glomerulosa (ZG), the Zona Fasciculata (ZF) and the Zona Reticularis (ZR). The 4–10y.o. age group showed the lowest labeling index (LI), and the 41–80y.o. group, the highest LI.
FIG 2. Fetal adrenal immunostaining.
ERRα immunoreactivity was virtually detected in the neocortex (NC) nuclei, in contrast to the fetal zone (FZ), which showed a low labeling index (LI).
FIG 3.
3A. Post-birth labeling indexExcept for the 11–20y.o. group (*P=0.021), no other significant difference was detected among the three cortical layers in post-birth adrenal cortex within the same age groups, but there were differences between age groups within the same layer (** P<0.001) - ZG: zona glomerulosa, ZF: zona fasciculate, ZR: zona reticularis.
3B. Fetal labeling index: A higher labeling index (LI) was detected in the neocortex (NC) of the fetal adrenal, when compared to the fetal zone (FZ) (*** P=0.0017).
FIG 4.
4A. Adrenocortical tumors labeling index
A higher labeling index (LI) was detected in adrenocortical carcinoma (ACC) when compared to aldosterone producing adenoma (APA) and cortisol producing adenoma (CPA) samples.
4B. qPCR analysis: Adrenocortical carcinoma (ACC) has the highest mRNA levels for ERRα when compared to aldosterone producing adenoma (APA), cortisol producing adenoma (CPA) and normal adrenal cortex (NAC), although only a tendency was detected on statistical difference (P=0.25).
3.2 qPCR analysis
The qPCR results of ERRα mRNA levels were summarized in Figure 4B. ERRα mRNA was detected in the NAC examined. ACC tended to have the highest mRNA levels for ERRα compared to other adrenocortical tumors although it did not reach a statistical significance (P=0.25).
4. DISCUSSION
In our present study, we detected high levels of ERRα expression in fetal adrenal NC, and low levels in post-birth NAC, with a steady rise following the period corresponding to adrenarche. In addition, we also demonstrated that ACC had higher ERRα expression levels than benign ACT. A high ERRα LI was detected in the NC of fetal adrenal gland. This layer is well known to differentiate into the three layers of NAC following birth and ERRα is considered to be involved in this process of adrenocortical differentiation. The 4–10 y.o. group had the lowest LI among post-birth NAC, and relative increment of overall ERRα LI of that age group corresponds to the post-adrenarche period - usually girls over 8 y.o. and boys over 9 y.o. (Idkowiak et al. 2011; Williams et al. 2012).
At adrenarche, the ZR of the adrenal cortex is well known to start to produce increasing amounts of the androgen precursor dehydroepiandrosterone (DHEA) and its sulfate ester DHEA-sulphate (DHEAS) (Auchus and Rainey 2004; Idkowiak et al. 2011; Rainey and Nakamura 2008). The actual physiological mechanisms of adrenarche still remains unknown at this juncture, for instance the mechanisms of the ZR development have not been clarified (Auchus and Rainey 2004; Idkowiak et al. 2011; Williams et al. 2012).
The transcriptional activation of CYP17A1 and SULT2A1 by ERRα has been proposed as the mechanism of action behind the rise in DHEAS serum levels (Seely et al. 2005). Recent studies using H295R cell line suggest that an increment of intra-adrenal cortisol probably results in higher DHEA production and initiation of adrenarche (Topor et al. 2011). However, whether a rise in ERRα levels is a cause or consequence of those events remains unknown. In our present study, a rise in ERRα LI among after-birth age groups was most clearly detected after 21 y.o. for the three cortical layers, when compared to below 20 y.o. (ZR) or below 10 y.o. subjects (ZG and ZF). Results of these findings are also consistent with those of human adrenal development including the abrupt decrease of DHEAS a few months after birth, with levels increasing gradually after 10 y.o. with its peak between 20 and 30 y.o. (Rainey et al. 2002; Rainey and Nakamura 2008). A circulating DHEAS level is high in the immediate neonatal period but quickly decreases below the limit of detection during the first few months of life subsequent to the involution of the FZ of the adrenal cortex (de Peretti and Forest 1976; de Peretti and Forest 1978; Idkowiak et al. 2011). However, in contrast to the DHEAS levels during human development as reported by our groups (Rainey and Nakamura 2008), the ERRα expression in adrenal did not necessarily decrease after 30 y.o. Instead, the ERRα levels increased with age in human adrenal, in our present study, suggesting other functions to this receptor besides the regulation of DHEAS production. It is true that DHEAS production decreases with age, but the regulation of mitochondrial genes related and required for the production of other steroids may increase with time.
ERRα was reported to act as a transcriptional activator of CYP11B1 and CYP11B2, which indicates that this nuclear receptor is required for the production of both cortisol and aldosterone in the adrenal gland (Cheng et al. 2012). Dufour et al. demonstrated that ERRα is required for the maintenance of diurnal cholesterol, glucose, insulin, bile acid, and trygliceride levels as well as locomotor rhythms in mice (Dufour et al. 2011). In addition, ERRα was recently identified as an important regulator of the mammalian circadian clock, and its output pathways at both transcriptional and physiological levels regulated the expression of transcription factors involved in metabolic homeostasis (Dufour et al. 2011).
ERRα is related to mithocondrial function but studies involving ERRα-null mice suggested that this receptor, while dispensable for basal cellular function, is definitely necessary to provide the levels of energy necessary to respond to physiological and pathological insults in diverse tissues (Deblois and Giguere, 2011). It is well-known that various physiopathological insults increase in an organism with the passage of time (Barzilai et al. 2012; Constantini et al. 2011; Mora et al. 2012). Therefore, we suggest that ERRα is required for basal adrenal metabolism in maintaining homeostatic levels of adrenocorticoids, which awaits further investigations for clarification.
Results of our present study also demonstrated higher expressions of ERRα mRNA as well as its immunoreactivity in ACC when compared to APA and CPA, which suggest that this nuclear receptor might play a role also in malignant behaviour of adrenocortical tumors. Similar finding have been reported in many human malignancies (Ariazi et al. 2002; Bianco et al. 2011; Cavallini et al. 2005; Fujimoto and Sato 2009; Fujimura et al. 2007; Sun et al. 2005; Suzuki et al. 2004), which suggests higher metabolic rate and mitochondrial usage in cancer cells when compared to benign ACT. ERRα is also considered to be required for cancer cells growth and stability.
In breast cancer MDA-MB-231 cells, depletion of ERRα results in a reduction of the migratory potential of the cells and decreases the tumor growth rate, and ERRα antagonists inhibit tumor growth of both estrogen receptor α (ERα) positive MCF-7 and ERα-negative T47D cells in mouse xenografts (Chisamore et al. 2009; Stein et al. 2008), as well as MCF-7 in vitro cultures (Bianco et al. 2009). In other primary tumors, ERRα was reported to be associated with reduced survival in ovarian cancer (Sun et al. 2005), with a higher tumor grade in colon cancer (Cavallini et al. 2005), with high grade and invasion in endometrial cancer (Fujimoto and Sato 2009) and with clinical advanced stage in prostate cancer (Fujimura et al. 2007). These results are all consistent with the results in ACT, and the role of this nuclear receptor in adrenocortical malignancy may well be related to tumoral metabolism and survival, which require further investigations to confirm..
5. CONCLUSION
In NAC tissues, ERRα expression is possibly related to adrenal development, playing a role not only in fetal adrenal function, in DHEAS production in adrenarche, and also in steroid production of post-adrenarche/adult life. ERRα may also play a role in ACC cell proliferation and survival.
Acknowledgments
We would like to thank Ms. Kazue Ise for the technical support of immunohistochemical analysis.
This work was supported by The National Institutes of Health, Prime Award DK069950. This work was also partly supported by the Takeda Science Foundation.
The first author received scholarship support from the Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT).
ABBEVIATIONS
- NAC
normal adrenal cortex
- ACT
adrenocortical tumors
- APA
aldosterone producing adenoma
- CPA
cortisol producing adenoma
- ACC
adrenocortical carcinoma
- NC
neocortex
- FZ
fetal zone
- ZG
zona glomerulosa
- ZF
zona fasciculate
- ZR
zona reticularis
- LI
labeling index
- y.o
years of age
References
- Alaynick WA. Nuclear receptors, mithocondria and lipid metabolism. Mitochondrion. 2008;8:329–37. doi: 10.1016/j.mito.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariazi EA, Clark GM, Mertz JE. Estrogen-related receptor α and estrogen-related receptor γ associate with unfavorable and favorable biomarkers, respectively, in human breast cancer. Cancer Res. 2002;62:6510–6518. [PubMed] [Google Scholar]
- Auchus R, Rainey WE. Adrenarche – physiology, biochemistry and human disease. Clinical Endocrinol. 2004;60:288–296. doi: 10.1046/j.1365-2265.2003.01858.x. [DOI] [PubMed] [Google Scholar]
- Barzilai N, Huffman DM, Muzumdar RH, Bartke A. The critical role of metabolic pathways in aging. Diabetes. 2012;61:1315–22. doi: 10.2337/db11-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco S, Lanvin O, Tribollet V, Macari C, North S, Vanacker JM. Modulating estrogen receptor-related receptor-α activity inhibits cell prolifereation. J Biol Chem. 2009;284:23286–23292. doi: 10.1074/jbc.M109.028191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco S, Sailland J, Vanacker JM. ERRs and cancers: effects on metabolism and on proliferation and migration capacities. J Steroid Biochem Mol Biol. 2012;130:180–5. doi: 10.1016/j.jsbmb.2011.03.014. [DOI] [PubMed] [Google Scholar]
- Bird IM, Mason JI, Rainey WE. Hormonal regulation of angiotensin II type 1 receptor expression and AT1-R mRNA levels in human adrenocortical cells. Endocr Res. 1995;21:169–82. doi: 10.3109/07435809509030432. [DOI] [PubMed] [Google Scholar]
- Bonnelye E, Vanacker JM, Dittmar T, Begue A, Desbiens X, Denhardt DT, Aubin JE, Laudet V, Fournier B. The ERR-1 orphan receptor is a transcriptional activator expressed during bone development. Mol Endocrinol. 1997;11:905–916. doi: 10.1210/mend.11.7.9948. [DOI] [PubMed] [Google Scholar]
- Cavallini A, Notarnicola M, Giannini R, Montemurro S, Lorusso D, Visconti A, Minervini F, Caruso MG. Oestrogen receptor-related receptor alpha (ERRα) and oestrogen receptors (ERα and β) exhibit different gene expression in human colorectal tumor progression. Eur J Cancer. 2005;41:1487–1494. doi: 10.1016/j.ejca.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Chambon P. The nuclear receptor superfamily: a personal retrospect on the first two decades. Mol Endocrinol. 2005;19:1418–1428. doi: 10.1210/me.2005-0125. [DOI] [PubMed] [Google Scholar]
- Cheng LC, Pai TW, Li LA. Regulation of human CYP11B1 and CYP11B2 promoters by transposable elements and conserved cis elements. Steroids. 2012;77:100–109. doi: 10.1016/j.steroids.2011.10.010. [DOI] [PubMed] [Google Scholar]
- Chisamore MJ, Wilkinson HA, Flores O, Chen JD. Estrogen-related receptor-alpha antagonist inhibits both estrogen receptor-positive and estrogen receptor-negative breast tumor growth in mouse xenografts. Mol Cancer Ther. 2009;8:672–681. doi: 10.1158/1535-7163.MCT-08-1028. [DOI] [PubMed] [Google Scholar]
- Costantini D, Marasco V, Møller AP. A meta-analysis of glucocorticoids as modulators of oxidative stress in vertebrates. J Comp Physiol B. 2011;181:447–56. doi: 10.1007/s00360-011-0566-2. [DOI] [PubMed] [Google Scholar]
- Deblois G, Giguere V. Functional and physiological genomics of estrogen-related receptors (ERRs) in health and disease. Biochemica et Biophysica Acta. 2011;1812:1032–1040. doi: 10.1016/j.bbadis.2010.12.009. [DOI] [PubMed] [Google Scholar]
- De Peretti E, Forest MG. Unconjugated dehydroepiandrosterone plasma levels in normal subjects from birth to adolescence in human: the use a sensitive radioimmunoassay. J Clinical Endocrinol and Metabol. 1976;43:982–991. doi: 10.1210/jcem-43-5-982. [DOI] [PubMed] [Google Scholar]
- De Peretti E, Forest MG. Pattern of plasma dehydroepiandrosterone sulfate levels in humans from birth to adulthood: evidence for testicular production. J Clinical Endocrinol and Metabol. 1978;47:572–577. doi: 10.1210/jcem-47-3-572. [DOI] [PubMed] [Google Scholar]
- Dufour CR, Levasseur MP, Pham NHH, Eichner LJ, Wilson BJ, Charest-Marcotte A, Duguay D, Poirier-Heon JF, Cermakian N, Giguere V. Genomic convergence among ERRα, PROX1, and BMAL1 in the control of metabolic clock outputs. PLoS Genet. 2011;7:e1002143. doi: 10.1371/journal.pgen.1002143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans RM. The nuclear receptor superfamily: a rosetta stone for physiology. Mol Endocrinol. 2005;19:1429–1438. doi: 10.1210/me.2005-0046. [DOI] [PubMed] [Google Scholar]
- Fujimoto J, Sato E. Clinical implication of estrogen-related receptor (ERR) in uterine endometrial cancers. J Steroid Biochem Mol Biol. 2009;116:71–75. doi: 10.1016/j.jsbmb.2009.04.012. [DOI] [PubMed] [Google Scholar]
- Fujimura T, Takahashi S, Urano T, Kumagai J, Ogushi T, Horie-Inoue K, Ouchi Y, Kitamura T, Muramatsu M, Inoue S. Increased expression of estrogen-related receptor alpha (ERRalpha) is a negative prognostic predictor in human prostate cancer. Int J Cancer. 2007;120:2325–2330. doi: 10.1002/ijc.22363. [DOI] [PubMed] [Google Scholar]
- Giguere V. Transcriptional control of energy homeostasis by the estrogen related receptors. Endocrine Reviews. 2008;29:677–96. doi: 10.1210/er.2008-0017. [DOI] [PubMed] [Google Scholar]
- Giguere V, Yang N, Segui P, Evans RM. Identification of a new class of steroid hormone receptors. Nature. 1988;331:91–94. doi: 10.1038/331091a0. [DOI] [PubMed] [Google Scholar]
- Heard DJ, Norby PL, Holloway J, Vissing H. Human ERR gamma, a third member of the estrogen receptor-related receptor (ERR) subfamily of orphan nuclear receptors: Tissue-specific isoforms are expressed during development and in the adult. Mol Endocrinol. 2000;14:382–392. doi: 10.1210/mend.14.3.0431. [DOI] [PubMed] [Google Scholar]
- Idkowiak J, Lavery GG, Dhir V, Barrett TG, Stewart PM, Krone N, Arlt W. Premature adrenarche: novel lessons from early onset androgen excess. Eur J Endocrinol. 2011;165:189–207. doi: 10.1530/EJE-11-0223. [DOI] [PubMed] [Google Scholar]
- Mora F, Segovia G, Del Arco A, de Blas M, Garrido P. Stress, neurotransmitters, corticosterone and body-brain integration. Brain Res. 2012 doi: 10.1016/j.brainres.2011.12.049. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Suzuki S, Suzuki T, Ono K, Miura I, Satoh F, Moriya T, Saito H, Yamada S, Ito S, Sasano H. MDM2: a novel mineralocorticoid-responsive gene involved in aldosterone-induced human vascular structural remodeling. Am J Pathol. 2006;169:362–371. doi: 10.2353/ajpath.2006.051351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainey WE. Adrenal zonation: clues from 11β-hydroxilase and aldosterone synthase. Mol Cell Endocrinol. 1999;151:151–160. doi: 10.1016/s0303-7207(99)00051-9. [DOI] [PubMed] [Google Scholar]
- Rainey WE, Carr BR, Sasano H, Suzuki T, Mason JI. Dissecting human adrenal androgen production. Trends Endocrinol Metab. 2002;13:234–239. doi: 10.1016/s1043-2760(02)00609-4. [DOI] [PubMed] [Google Scholar]
- Rainey WE, Nakamura Y. Regulation of the adrenal androgen biosynthesis. Journal of Steroid Biochem Mol Biol. 2008;108:281–286. doi: 10.1016/j.jsbmb.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero LM. Physiological stress in ecology: lessons from biomedical research. Trends Ecol Evol. 2004;19:249–255. doi: 10.1016/j.tree.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Krey LC, McEwen BS. The neuroendocrinology of stress and aging: the glucocorticoid cascade hypothesis. Endocr Rev. 1986;7:284–301. doi: 10.1210/edrv-7-3-284. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Schreiber SN, Emter R, Hock MB, Knutti D, Cardenas J, Podvinec M, Oakeley EJ, Kralli A. The estrogen related receptor alpha (ERR alpha) functions in PPAR gamma coactivator 1 alpha (PGC-1alpha)-induced mitochondrial biogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:6472–6477. doi: 10.1073/pnas.0308686101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seely J, Amigh KS, Suzuki T, Mayhew B, Sasano H, Giguere V, Laganière J, Carr BR, Rainey WE. Transcriptional regulation of dehydroepiandrosterone sulfotransferase (SULT2A1) by estrogen-related receptor alpha. Endocrinology. 2005;146:3605–3613. doi: 10.1210/en.2004-1619. [DOI] [PubMed] [Google Scholar]
- Shibahara Y, Miki Y, Onodera Y, Hata S, Chan MS, Yiu CC, Loo TY, Nakamura Y, Akahira J-i, Ishida T, Abe K, Hirakawa H, Chow LW, Suzuki T, Ouchi N, Sasano H. Aromatase inhibitor treatment of breast cancer cells increases the expression of let-7f, a microRNA targeting CYP19A1. J Pathol. 2012;227:357–366. doi: 10.1002/path.4019. [DOI] [PubMed] [Google Scholar]
- Stein RA, Chang CY, Kazmin DA, Way J, Schroeder T, Wergin M, Dewhirst MW, McDonnel DP. Estrogen-related receptor α is critical for the growth of estrogen receptor-negative breast cancer. Cancer Res. 2008;68:8805–8812. doi: 10.1158/0008-5472.CAN-08-1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun P, Sehouli J, Denkert C, Mustea A, Konsgen D, Koch I, Oskay-Ozcelik G, Wei L, Lichtenegger W. Expression of estrogen receptor-related receptors, a subfamily of orphan nuclear receptors, as new tumor biomarkers in ovarian cancer cells. J Mol Med. 2005;83:457–467. doi: 10.1007/s00109-005-0639-3. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Miki Y, Moriya T, Shimada N, Ishida T, Hirakawa H, Ohuchi N, Sasano H. Estrogen-related receptor α in human breast carcinoma as a potent prognosis factor. Cancer Res. 2004;64:4670–4676. doi: 10.1158/0008-5472.CAN-04-0250. [DOI] [PubMed] [Google Scholar]
- Tremblay AM, Giguere V. The NR3B subgroup: an ovERRview. Nuclear Receptor Signaling. 2007;5:e009. doi: 10.1621/nrs.05009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topor LS, Asai M, Dunn J, Majzoub JA. Cortisol stimulates secretion of dehydroepiandrosterone in human adrenocortical cells through inhibition of 3betaHSD2. J Clin Endocrinol Metab. 2011;96:E31–E39. doi: 10.1210/jc.2010-0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villena JA, Hock MB, Chang WY, Barcas JE, Giguere V, Kralli A. Orphan nuclear receptor estrogen-related receptor alpha is essential for adaptative thermogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:1418–1423. doi: 10.1073/pnas.0607696104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RM, Ward CE, Hughes IA. Premature adrenarche. Arch Dis Child. 2012;97:250–4. doi: 10.1136/archdischild-2011-300011. [DOI] [PubMed] [Google Scholar]