Abstract
The prefrontal cortex (PFC) maintains information about relevant sensory stimuli, in a process thought to rely on dopamine release. In a recent paper, Jacob et al. (J Neurosci 33: 13724–13734, 2013) demonstrated one way in which dopamine might facilitate this process. The authors recorded from PFC neurons in monkeys during local application of dopamine. They found that dopamine increases the gain of sensory-evoked responses in putative pyramidal neurons in PFC, potentially by inhibiting local interneurons.
Keywords: dopamine, prefrontal cortex, gating, working memory
almost 20 years ago Mirenowicz and Schultz (1994) discovered a peculiar feature of dopamine neurons' responses to reward. When an animal is given an unexpected reward or reward-predicting cue, dopamine neurons fire a burst of action potentials. If an expected reward is delivered at the expected time, dopamine neurons do not respond. Finally, if an expected reward is not delivered at the expected time, dopamine neurons dip below their baseline firing rate. Together, these findings suggest that dopamine neurons encode reward prediction error, or the difference between the reward an animal expects and the reward it actually receives. Such a signal dovetails beautifully with models for how animals learn from their environment, prompting intense interest in how dopamine can promote learning and motivated behavior. Although many studies have suggested that dopamine guides learning through long-term changes in synaptic strength (Reynolds et al. 2001), accumulating evidence suggests that dopamine can also act rapidly and reversibly to affect information processing as it happens (Arnsten et al. 2012). In their elegant recent study, Jacob and colleagues (2013) contribute to this field by showing how dopamine modulates the activity of neurons in one of the principal targets of dopamine release: the prefrontal cortex (PFC; see Fig. 1).
Fig. 1.
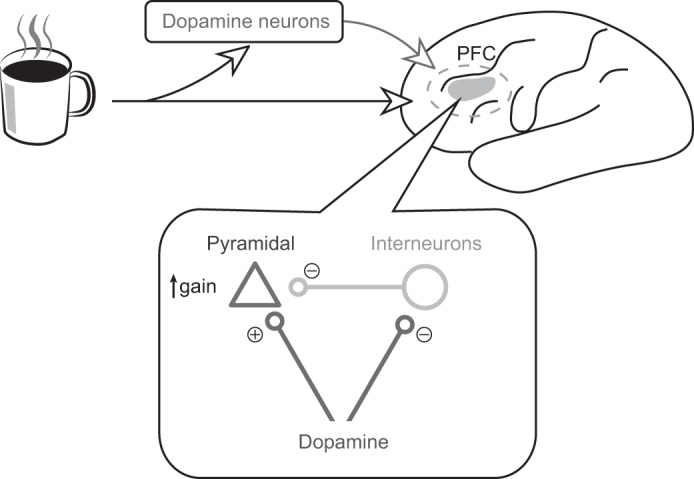
How does the brain represent salient stimuli (e.g., a coffee mug in the morning) to elicit the appropriate response (e.g., drinking the coffee)? Neurons in the prefrontal cortex (PFC) maintain relevant information in working memory, in a process thought to rely on dopamine release. But what is the mechanism of dopamine's action in PFC? In a recent paper, Jacob et al. (2013) recorded from PFC neurons while monkeys performed a simple working-memory task. During the recording, the authors locally applied either dopamine or saline and measured how these manipulations affected PFC responses. They found that dopamine decreased stimulus-evoked activity in putative interneurons (circle), while increasing the gain of activity in putative pyramidal neurons (triangle). Such a gain increase is consistent with the popular “gating” model of dopamine function, which posits that dopamine enhances PFC responses to relevant input, allowing PFC neurons to stably represent important information without distraction from irrelevant stimuli.
Previously, most work on dopamine and the PFC studied the neuromodulator's potential role in working memory. In a series of seminal reports, Sawaguchi and Goldman-Rakic (1991) found that neurons in dorsolateral PFC show sustained activity during the delay period of a working memory task, and that this activity is disrupted by either blocking or overdosing D1 receptors in this region (Sawaguchi and Goldman-Rakic 1991; Vijayraghavan et al. 2007). Thus, optimal tonic levels of D1 stimulation may contribute to the stability of working memory representations in PFC. However, in physiological conditions, dopamine acts on both the D1 and D2 families of receptors. It is worth testing, therefore, how dopamine itself modulates PFC neurons, rather than drugs targeting particular receptors. In addition, we know that dopamine neurons respond to unexpected or salient events (Mirenowicz and Schultz 1994), and that they fire more when working memory demand is high (Matsumoto and Takada 2013). In tasks such as these, how does dopamine affect stimulus representations in PFC?
In their study, Jacob and colleagues (2013) used electrophysiology in behaving primates to address how local application of dopamine modulates lateral PFC neurons' responses to relevant sensory cues. The authors presented rhesus monkeys with brief flashes of weak visual stimuli. Following a delay, the monkeys used one of two actions to report whether they detected the stimulus. Importantly, the precise action the monkeys needed to take was signaled by a second cue at the end of the delay. Thus, the authors neatly separated working memory (“Did I see the stimulus?”) from motor planning (“I must press the lever”). In this way, the neurons' activity after stimulus onset was a pure representation of the stimulus, divorced from the monkeys' future action. During recording, either dopamine or saline was applied to the vicinity of recorded cells, allowing the authors to document how dopamine affected stimulus representations in PFC.
Jacob et al. (2013) found that neurons in lateral PFC showed two classes of responses to dopamine iontophoresis: some increased their spontaneous firing rate (dopamine-excited) while others decreased it (dopamine-inhibited). In both cases, the effect of dopamine was not due to long-lasting changes in synaptic strength, since it was rapidly reversed after washing out the drug. This is consistent with the idea that in the PFC, dopamine can act in a transient fashion, rapidly and reversibly regulating specific ion channels on dendritic spines (Arnsten et al. 2012).
When the monkeys were presented with visual stimuli, both classes of PFC neurons were excited, but the effect of dopamine on these responses was quite different. The dopamine-inhibited neurons underwent subtraction, meaning they decreased both their baseline activity and their response to stimuli by the same number of spikes. On the other hand, the dopamine-excited neurons underwent an increase in gain: dopamine enhanced stimulus-evoked activity more than it enhanced baseline activity, keeping the ratio between the two responses the same. In addition, dopamine-excited neurons showed decreased response variability, resulting in a better signal-to-noise ratio.
Do these dopamine-excited and dopamine-inhibited neurons belong to different cell types? To find out, the authors clustered the recorded units' spike waveforms into two groups. Based on previous literature, neurons with longer spike waveforms are likely to be pyramidal, while neurons with shorter waveforms are putative interneurons. Strikingly, all of the dopamine-excited neurons fit into the pyramidal cell group, while putative interneurons were all dopamine-inhibited. Thus, dopamine might preferentially increase the gain of pyramidal neurons while inhibiting interneuron firing.
These results have deep implications for how dopamine might contribute to cognitive control. In particular, the observation that dopamine-excited neurons increased their signal-to-noise ratio after dopamine application directly supports the so-called “gating” model of dopamine function (Cohen et al. 1996). In this model, dopamine released after reward-predicting or salient events transiently enhances the gain of afferents to PFC, allowing the PFC to update its representations to include the newly relevant inputs. In support of this hypothesis, a recent paper by D'Ardenne and colleagues (2012) used brain imaging in human volunteers to show correlated signals in midbrain dopamine regions and PFC when subjects updated their working memory to reflect task demands. Furthermore, disrupting this updating signal in PFC using transcranial magnetic stimulation interrupted the subjects' ability to perform the task. Jacob et al. (2013) nicely complement these findings by demonstrating dopamine-evoked gain increases at a cellular level. Moreover, their results suggest a possible mechanism for these gain increases in pyramidal neurons, i.e., the inhibition of local interneurons when dopamine is released in response to relevant stimuli.
Although this is the closest study yet to examine how dopamine might affect phasic responses in PFC during behavior, there are important caveats. First, the authors used 10-min infusions of dopamine, far from the 100-ms signals that occur to salient stimuli in natural situations (Matsumoto and Takada 2013). Future experiments taking advantage of optogenetic stimulation of dopamine terminals in PFC could help resolve this issue, either in a mouse model or when these tools become available in primates. Because they more closely mimic physiological situations, such studies could tease apart the role of glutamate corelease from dopamine terminals, which is known to cause phasic excitatory responses in PFC neurons (Lavin et al. 2005). Second, this study does not assess whether dopamine or the PFC is actually required for performance on this task. In fact, it has been shown that monkeys with PFC lesions show deficits only when asked to remember the position of objects in space, which the current task did not require (Goldman-Rakic 1987). Thus, to demonstrate that the authors' findings are relevant for normal behavior, it would be necessary to disrupt PFC activity or block dopamine release in this area. Third, using waveforms to identify cells as either pyramidal neurons or interneurons is not reliable (Nowak et al. 2003; Vigneswaran et al. 2011). More robust clustering would be useful, either through anatomical methods or a combination of other electrophysiological characteristics. Last, it would be interesting to determine how dopamine's effect varies between cortical layers. It has been hypothesized that neurons with sustained activity during the delay period belong primarily to layers III or IV, while neurons with phasic responses to the cue belong to layer II (Arnsten et al. 2012). Jacob et al. (2013) recorded many neurons with phasic cue responses but few with sustained activity, leaving it unclear whether the lack of sustained responses is due to a quirk of their task or a bias in recording location.
Despite several decades of work, the effects of dopamine release on information processing in the rest of the brain remain quite mysterious. Rather than a unified role in every downstream area, dopamine acts through diverse mechanisms over multiple timescales. By combining pharmacology with careful in vivo recording, this study takes a significant step towards understanding dopamine's function in PFC.
GRANTS
This work was supported by National Institutes of Health Grants T32-GM-007753, T32-MH-020017-12, and F30-MH-100729-01A1, as well as the Sackler Fellowship in Psychobiology.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
N.E. and J.T. drafted manuscript; N.E. and J.T. edited and revised manuscript; N.E. and J.T. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Jeremiah Cohen and Naoshige Uchida for helpful discussions.
REFERENCES
- Arnsten AFT, Wang MJ, Paspalas CD. Neuromodulation of thought: flexibilities and vulnerabilities in prefrontal cortical network synapses. Neuron 76: 223–239, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JD, Braver TS, O'Reilly RC. A computational approach to prefrontal cortex, cognitive control and schizophrenia: recent developments and current challenges. Philos Trans R Soc Lond B Biol Sci 351: 1515–1527, 1996 [DOI] [PubMed] [Google Scholar]
- D'Ardenne K, Eshel N, Luka J, Lenartowicz A, Nystrom LE, Cohen JD. Role of prefrontal cortex and the midbrain dopamine system in working memory updating. Proc Natl Acad Sci USA 109: 19900–19909, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Circuitry of primate prefrontal cortex and regulation of behavior by representational memory. In: Handbook of Physiology: The Nervous System, edited by Plum F. Bethesda, MD: Am. Physiol. Soc., 1987, p. 373–417 [Google Scholar]
- Jacob SN, Ott T, Nieder A. Dopamine regulates two classes of primate prefrontal neurons that represent sensory signals. J Neurosci 33: 13724–13734, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavin A, Nogueira L, Lapish CC, Wightman RM, Phillips PE, Seamans JK. Mesocortical dopamine neurons operate in distinct temporal domains using multimodal signaling. J Neurosci 25: 5013–5023, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Takada M. Distinct representations of cognitive and motivational signals in midbrain dopamine neurons. Neuron 79: 1011–1024, 2013 [DOI] [PubMed] [Google Scholar]
- Mirenowicz J, Schultz W. Importance of unpredictability for reward responses in primate dopamine neurons. J Neurophysiol 72: 1024–1027, 1994 [DOI] [PubMed] [Google Scholar]
- Nowak LG, Azouz R, Sanchez-Vives MV, Gray CM, McCormick DA. Electrophysiological classes of cat primary visual cortical neurons in vivo as revealed by quantitative analyses. J Neurophysiol 89: 1541–1566, 2003 [DOI] [PubMed] [Google Scholar]
- Reynolds JN, Hyland BI, Wickens JR. A cellular mechanism of reward-related learning. Nature 413: 67–70, 2001 [DOI] [PubMed] [Google Scholar]
- Sawaguchi T, Goldman-Rakic PS. D1 dopamine receptors in prefrontal cortex: involvement in working memory. Science 251: 947–950, 1991 [DOI] [PubMed] [Google Scholar]
- Vigneswaran G, Kraskov A, Lemon RN. Large identified pyramidal cells in macaque motor and premotor cortex exhibit “thin spikes”: implications for cell type classification. J Neurosci 31: 14235–14242, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayraghavan S, Wang M, Birnbaum SG, Williams GV, Arnsten AFT. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat Neurosci 10: 376–384, 2007 [DOI] [PubMed] [Google Scholar]


