Abstract
Medial olivocochlear (MOC) neurons are efferent neurons that project axons from the brain to the cochlea. Their action on outer hair cells reduces the gain of the “cochlear amplifier,” which shifts the dynamic range of hearing and reduces the effects of noise masking. The MOC effects in one ear can be elicited by sound in that ipsilateral ear or by sound in the contralateral ear. To study how MOC neurons project onto the cochlea to mediate these effects, single-unit labeling in guinea pigs was used to study the mapping of MOC neurons for neurons responsive to ipsilateral sound vs. those responsive to contralateral sound. MOC neurons were sharply tuned to sound frequency with a well-defined characteristic frequency (CF). However, their labeled termination spans in the organ of Corti ranged from narrow to broad, innervating between 14 and 69 outer hair cells per axon in a “patchy” pattern. For units responsive to ipsilateral sound, the midpoint of innervation was mapped according to CF in a relationship generally similar to, but with more variability than, that of auditory-nerve fibers. Thus, based on CF mappings, most of the MOC terminations miss outer hair cells involved in the cochlear amplifier for their CF, which are located more basally. Compared with ipsilaterally responsive neurons, contralaterally responsive neurons had an apical offset in termination and a larger span of innervation (an average of 10.41% cochlear distance), suggesting that when contralateral sound activates the MOC reflex, the actions are different than those for ipsilateral sound.
Keywords: cochlear amplifier, outer hair cell, masking, acoustic protection, descending system
hair cells of auditory and vestibular sense organs receive a prominent efferent innervation from the brainstem (reviewed by Ryugo et al. 2011). One group of efferent neurons, the medial olivocochlear (MOC) neurons, terminates on outer hair cells (OHCs) of the cochlea. Activation of these neurons hyperpolarizes the OHCs (Fuchs 2002) and reduces the vibration of the basilar membrane (Murugasu and Russell 1996; Cooper and Guinan 2006), the responses of inner hair cells (Brown and Nuttall 1984), and the firing of auditory-nerve fibers (Gifford and Guinan 1987). MOC neurons are thought to act by reducing the gain of the “cochlear amplifier,” the process by which the OHC electromotility enhances motion of the receptor organ, the organ of Corti (Dallos 1992). This process, mediated by the OHC protein prestin (Dallos et al. 2008), generates the high sensitivity and sharp tuning of the cochlea. MOC action has several beneficial effects. It shifts the dynamic range of auditory-nerve fibers to higher levels (Wiederhold and Kiang 1970), and it decreases the masking effects of steady background noise (Winslow and Sachs 1988; Kawase et al. 1993; Jennings et al. 2011). Finally, MOC action protects the inner ear from damage due to high-level sound (Reiter and Liberman 1995; Maison and Liberman 2000).
To perform these functions, MOC neurons respond to sound (Fex 1962) as part of the MOC reflex (Liberman and Guinan 1998). Individual MOC neurons are sharply tuned to sound frequency, with selectivity measures equal or almost equal to those of the auditory-nerve fibers (Robertson and Gummer 1985; Liberman and Brown 1986; Brown 1989). This high selectivity suggests that the MOC system feeds back onto the cochlea with a frequency-specific mapping. However, the mapping of MOC neurons, including its relationship to that of auditory-nerve fibers, is not known. Only a handful of MOC axons have been reconstructed to all their terminations (Liberman and Brown 1986; Brown 1989). Some of the published axonal terminations represent only portions of axons (Robertson and Gummer 1985). Thus available data do not rule out an MOC termination just basal to the CF position of auditory-nerve fibers, the location of the OHCs involved in the cochlear amplifier (Neely and Kim 1986; Cody 1992; Patuzzi 1996; Pang and Guinan 1997; Shera 2007; Fisher et al. 2012).
MOC neurons consist of three response types (Robertson and Gummer 1985; Liberman and Brown 1986; Brown 1989). About two-thirds of the neurons, Ipsi units, respond only to monaural sound presented to the ipsilateral ear (that ear which receives the MOC terminations). About one-third, Contra units, respond to sound in the contralateral ear, and a small percentage of units, Either Ear units, respond to sound in either ear. A comparison of terminations of the types of neurons has not been made, an important issue since many studies have used contralateral sound to elicit MOC effects with the implicit assumption that they are similar to the effects elicited by ipsilateral or bilateral sound (Veuillet et al. 1991; Chery-Croze et al. 1993; Abdala et al. 2009; Henin et al. 2011). To address these issues, the present study constructs the cochlear frequency mapping for MOC axons and compares the terminations of Ipsi, Contra, and Either-Ear units.
MATERIALS AND METHODS
All experimental procedures on animals were in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and were performed under approved protocols at the Massachusetts Eye and Ear Infirmary. Experiments were carried out within a sound-attenuating and electrically shielded chamber in which the air temperature was heated. Guinea pigs were of the Hartley strain and were of either sex; those used for biocytin injections were anesthetized with urethane (1,100 mg/kg) plus fentanyl (0.2 mg/kg) and droperidol (10 mg/kg). Guinea pigs used for horseradish peroxidase (HRP) injections were anesthetized with pentobarbital sodium (Nembutal; 15 mg/kg) plus the above dose of fentanyl and droperidol. Supplemental doses of anesthesia (∼1/3 of the original dose) were administered as needed. After anesthesia, a tracheal cannula was inserted, both pinnae were removed, and the animal's head was placed in a headholder. A rectal thermometer was used in conjunction with the heated air temperature in the chamber and a heating pad to control the rectal temperature to 38°C. The bulla on the left side was opened posterior to the ear canal and a silver wire was placed on the round window of the cochlea to record the compound action potential, with a reference electrode in the neck muscles. Sound stimuli were produced by half-inch condenser microphones driven as a sound sources and led into the right and left ear canals using couplers. Cochlear sensitivity was tested using tone pips (duration: 5 ms, 0.5-ms rise/fall time, repeated 10/s in alternate polarity pairs, frequency: 4–32 kHz). Responses were amplified (×10,000) and averaged with artifact rejection, and a 15-μV criterion was used for threshold. The thresholds were checked periodically during the experiment, and only preparations with minimal threshold shifts (<10 dB) relative to the beginning of the experiment were used. Surgical methods to access the cochlear structures via the scala tympani were as described previously (Sellick and Russell 1979; Robertson and Gummer 1985; Brown 1989). A fenestration of the bone over scala tympani in the basal turn was made, and the spiral ganglion was visualized. Pinpoint access to the ganglion was made by piercing the overlying bone with a Minutien insect pin, usually at the peripheral edge of the ganglion where the MOC axons run in the intraganglionic spiral bundle. The pin was coated with pencil graphite to mark the circumference of the opening for aiming electrodes and for postexperiment histological recovery of the recording site. In some preparations, to gain access to the modiolus to record from auditory-nerve fibers of lower CF, the cochlear opening was enlarged and an opening into the modiolus was made (Alder and Johnstone 1978).
Sharp electrodes for recording single units were glass micropipettes usually filled with 4% biocytin in 0.45 M KCl in 0.05 M Tris buffer or sometimes with 5% HRP in 0.15 M KCl in 0.05 M Tris buffer. The electrodes were advanced into the spiral ganglion pinpoint opening while binaural noise bursts at 80 dB sound pressure level (SPL) were presented as search stimuli. All sound stimuli for single unit responses were of 50-ms duration and repeated 10/s. After a unit was obtained, it was first classified (Fex 1962; Robertson and Gummer 1985; Liberman and Brown 1986; Brown 1989) as either auditory-nerve fiber (irregular spike intervals) or MOC neuron (regular interspike intervals). MOC neurons were further classified as Ipsi units (responsive to 65 dB SPL noise bursts presented to the ipsilateral ear but not to noise bursts presented to the contralateral ear; the ipsilateral ear is where the recordings are being made), Contra units (responsive to noise bursts presented to the contralateral ear but not to noise bursts presented to the ipsilateral ear), or Either Ear units (responsive to noise bursts presented to either ear). An automated tuning curve algorithm (Kiang et al. 1970; Liberman 1978) was used to obtain the tuning curve (for MOC neurons the response window was delayed 15 ms to compensate for the longer latency relative to auditory-nerve fibers). The CF was obtained from the tuning curve. For Either-Ear units, the CF used was for the ear with the lower threshold (one Either-Ear unit had a lower threshold for its ipsilateral ear and the other had a lower threshold for its contralateral ear; the CFs of the other ears were within 0.1 octave). To impale the unit, the electrode was moved back and forth in small increments to obtain a negative DC resting membrane potential. To inject the unit, positive iontophoretic current (4–7 nA, 50 ms on, 50 ms off) was applied for a total of 0.5–6 min.
MOC neurons (and usually auditory-nerve fibers as well) were labeled in 30 guinea pigs (26 with biocytin and 4 with HRP). These latter four guinea pigs were part of a previous report (Brown 1989). In five other guinea pigs, only auditory-nerve fibers were labeled. In 17 guinea pigs, 1–3 MOC neurons were injected and the same numbers of labeled MOC axons were recovered. Most of these units had resting membrane potentials more negative than −10 mV at the time of injection. In other cases, more units were injected (1–4) than axons recovered (0–3), probably because of the poor quality of some injection parameters (membrane potentials less negative than −10 mV or electrodes passing less injection current). In cases with multiple MOC neurons labeled or attempted to label, the injection parameters as well as the CF and point of innervation along the cochlea (Brown 1989) were used to match a given arbor to the recorded neuron. Postinjection survival times averaged 5 h, 5 min (range 0.5 to 10.5 h.). Guinea pigs were then killed by perfusion with 3.5% paraformaldehyde mixed with 0.5% glutaraldehyde in 0.1 M phosphate buffer pH 7.3. After dissection from the head, cochleas were postfixed for 1 h and then transferred to phosphate buffer overnight.
Cochleas were decalcified in refrigerated 0.1 M EDTA for ∼5 days. For the biocytin injections, they were then frozen for a few minutes (to increase membrane permeability to allow reagents to penetrate) and then bisected and microdissected into hemi-turns containing the organ of Corti, the osseous spiral lamina, and the spiral ganglion. The tectorial membrane was removed from the organ of Corti. Pieces were incubated in 0.5% hydrogen peroxide, washed in 0.1 M phosphate buffer, and then incubated overnight in an ABC kit at room temperature. Then, they were incubated in diaminobenzidine alone for 15 min, followed by diaminobenzidine containing 0.5% hydrogen peroxide for ∼7 min and then washed again in buffer. After processing, the pieces were dehydrated in an ethanol series, then infiltrated in propylene oxide, and finally embedded in epon in a holder of the size and shape of a glass microscope “slide.” The slide was left overnight at room temperature to allow the propylene oxide to evaporate, and then it was cured in a 60° oven overnight. The slide was removed from the holder and examined with a compound microscope. Important pieces were sometimes removed, individually thinned, and glued to a glass slide, so that a high-power, oil-immersion lens could be focused on the specimen. For the HRP cases, cochleas were embedded in a polymerized gelatin/albumin mixture and sectioned as described previously (Brown 1989). Brainstems were processed similarly to the cochlear sections and some auditory-nerve fibers were recovered in the cochlear nucleus, but the central axon of only one MOC neuron was recovered and only as far as the vestibular nerve root (not to its cell body of origin).
The database consists of 35 axons that were filled well enough to determine the basal-most and apical-most positions of their innervations. In 32 of these axons, the OHCs innervated and counts of endings were determined, but in the other three axons (2 Ipsi and 1 Contra units) the number of OHCs could not be determined because of missing tissue (1 axon) or fading of the reaction product with increasing distance from the injection site in the apical direction (2 axons). Points of innervation along the organ of Corti were drawn using a compound microscope with a camera lucida attachment. From the drawings, the span of the MOC axon innervation from the most basal to the most apical hair cell innervated was obtained. The midpoint position of this span along the cochlear spiral, divided by the organ of Corti's total length (measured from drawings done at low magnification), was defined as the “innervation midpoint.” Within the axon span, the center of gravity was determined by calculating a weighted sum of all endings on all three rows of OHCs numbered from the basal-most to the apical-most position and dividing by the hair cell number. This measure is expressed as a fraction of the distance from the basal-most to apical-most innervated OHC (e.g., see Fig. 8A) or as the position of the fraction along the cochlea divided total cochlear distance (e.g., see Fig. 7C). Statistical tests were computed using Kaleidagraph software using the 0.05 significance level.
Fig. 8.
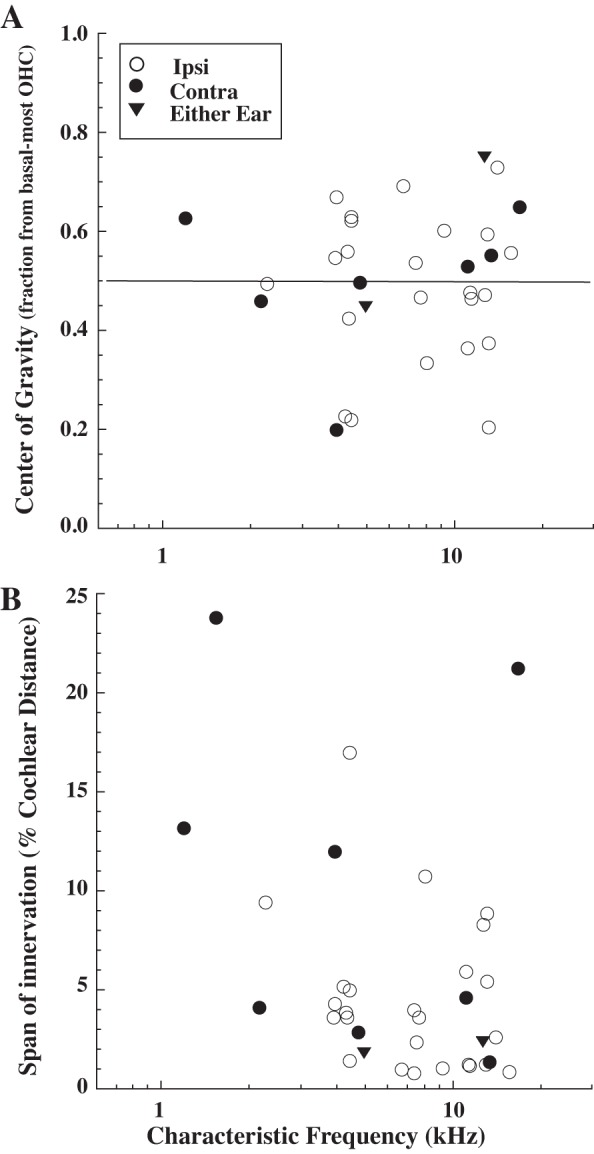
A: fractional center of gravity of MOC terminal arbors (see Fig. 3 and materials and methods) as a function of CF. B: innervation span for MOC axons as a function of CF. Span (in %cochlear distance) is measured from the most basal to the most apically innervated OHC (see Fig. 3).
Fig. 7.
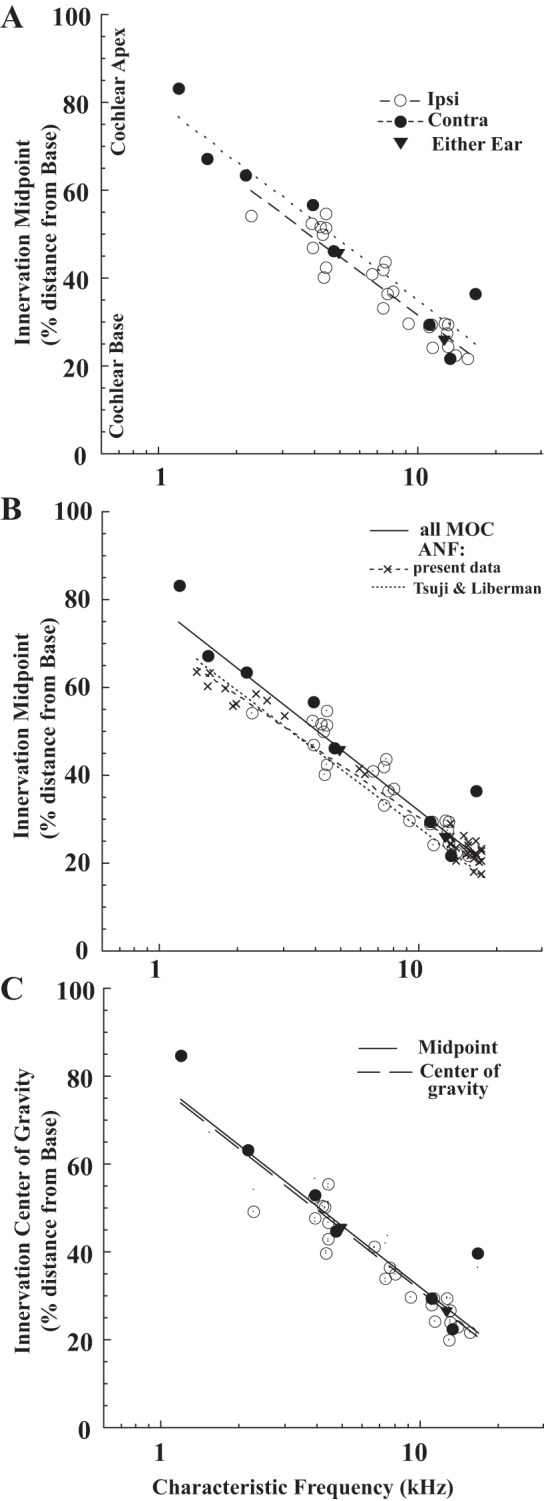
Mappings for labeled MOC axons and auditory-nerve fibers onto the organ of Corti as a function of CF. Innervation midpoint or center of gravity (e.g., Fig. 3) are organ of Corti distance from basal end as a percent of total distance from base to apex. A: innervation midpoint for axons of MOC neurons (see key for response type), with best-fit lines separately for Ipsi units [dashed line, %Dis = 75.53 − 43.85 × log(CF), R = 0.93] and Contra units [dotted line, %Dis =80.50 − 44.92 × log(CF), R = 0.95]. B: innervation midpoint for MOC axons compared with innervation point for auditory-nerve fibers, with best-fit regression for all MOC axons [solid line, %Dis = 78.2 − 46.1 × log(CF), R = 0.95] and for nerve fibers [dashed line, %Dis = 70.2 − 40.0 × log(CF), R = 0.99]. Dotted line shows the mapping for auditory-nerve fibers from Tsuji and Liberman (1997). An adjusted version of the MOC mapping (see results), using the positions of nerve fibers of similar CFs in individual cochleas (Fig. 4), was %Dis = 78.73 − 45.96 × log(CF), R = 0.93 (not plotted), which differed little from the unadjusted best-fit line above. C: innervation center of gravity position mapping for MOC units [points, dashed line: %Dis =77.5 − 46.3 × log(CF), R = 0.95], which is similar to the innervation midpoint line (solid line from B).
RESULTS
Tuning curves and morphology of labeled axons.
Tuning curves from the 35 labeled MOC neurons were sharply tuned to sound frequency (Fig. 1) and encompassed a wide range of CFs (1.19–16. 7 kHz). Thresholds at CF averaged 32.7 dB (SD 16.8, range 9.5–81.1) and were not correlated with CF or response type (data not shown). This large range has been previously reported (Liberman and Brown 1986; Brown 1989).
Fig. 1.
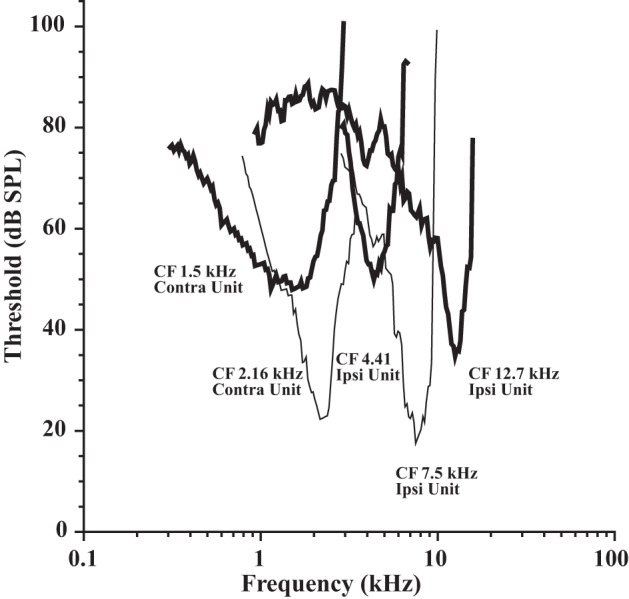
Tuning curves from labeled medial olivocochlear (MOC) neurons. Response type and characteristic frequency (CF) are indicated next to each tuning curve. Each neuron was from a different preparation. SPL, sound pressure level; Ipsi, ipsilateral.
The OHCs act as the cochlear amplifier (Dallos 1992; Dallos et al. 2008), and they were the target of the labeled axons of the database (25 Ipsi units, 8 Contra units, and 2 Either-Ear units). A photomicrograph of a portion of a labeled axon is shown in Fig. 2. The high density of labeling makes it possible to resolve innervation of individual OHCs (see counts in Fig. 2 legend). Conversely, the low background in the immediately adjacent areas of the organ of Corti makes it clear that there is no additional labeling (this axon produced 2 other patches of innervation that were located apically outside the field of view). For another axon, the complete reconstruction of its termination is shown in the drawing of Fig. 3. This axon had 2 patches of innervated OHCs (dots), a small basal patch of 5 and a larger apical patch of 25. Other than OHCs, the database contained only two examples of other terminations: a single terminal branch from one axon (from a neuron with a low CF of 1.53 kHz) that ended in nearby Hensen's cells and a single terminal branch from another axon that ended in the inner hair cell region.
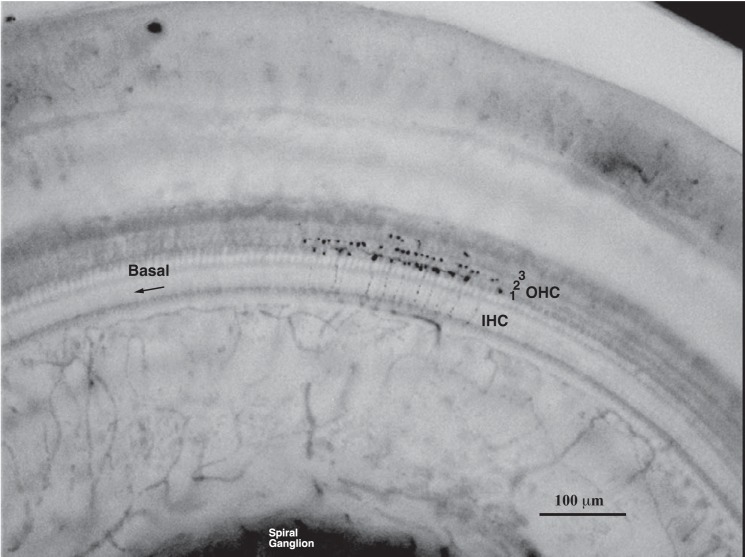
Fig. 2.
Photomicrograph of the termination of a labeled MOC branch in the organ of Corti, from the second turn of the cochlea. A total of 44 outer hair cells (OHCs) were innervated by this branch (15 in row 1, 23 in row 2, and 6 in row 3), and it formed a total of 64 endings (26 in row 1, 30 in row 2, and 8 in row 3). Two other branches of this axon terminated out of the field of view in smaller patches apically. This labeled axon was from an MOC Ipsi unit with a CF of 4.21 kHz.
Fig. 3.
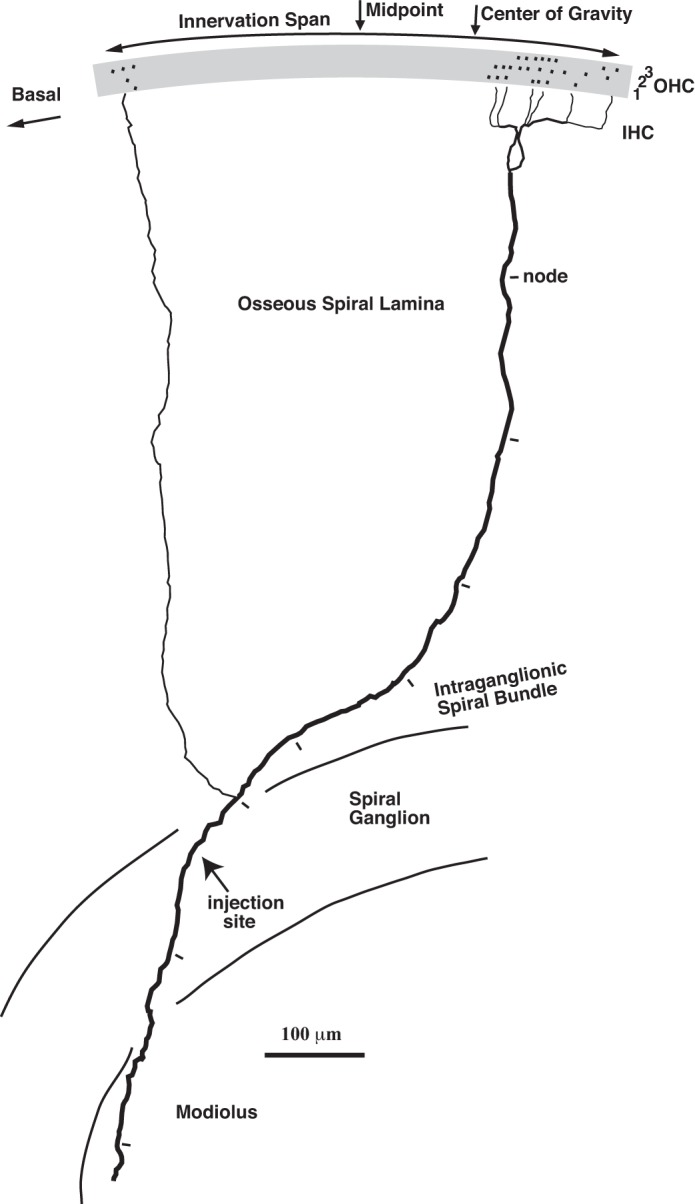
Camera lucida drawing of the labeled axon of a single MOC neuron (Ipsi unit, CF 14.0 kHz) that terminates in the lower first (basal) turn of the cochlea. The drawing is a surface view of the organ of Corti, osseous spiral lamina, and intraganglionic spiral bundle. From the bottom of the drawing, the axon emerged from the modiolus and crossed the spiral ganglion. This crossing position for all axons of the database (avg. 15.13% distance from basal end) was independent of CF and did not differ significantly between Ipsi and contralateral (Contr)a axons (avg. 13.95 vs. 17.34%, t-test, P = 0.21). For the illustrated axon, the injection site (indicated by the axon swelling, nearby red blood cells, and graphite from the pin used to access the ganglion) was found in the spiral ganglion. At the peripheral edge of the ganglion, the axon ran briefly in the intraganglionic spiral bundle, where it gave off 2 branches that crossed the osseous spiral lamina (in our database the average number was 2.0 and the range was 1–4). The thicker branch had periodic constrictions (indicated by tick marks). These constrictions were interpreted as nodes of Ranvier (Liberman and Oliver 1984). In contrast, the thinner branch (in this case and in others) lacked nodes and was apparently unmyelinated or lightly myelinated. This thin branch formed one tunnel-crossing branch to innervate a small patch of OHCs (shading) basally, whereas the thicker branch formed 6 tunnel-crossing branches to innervate a larger patch of OHCs apically (in the database, the average number of tunnel-crossing branches per axon was 6.5 and the range was 2–14). The fine terminal branches are not indicated on the drawing, but they formed endings on OHCs. Each innervated OHC is indicated by a dot, and the hair cell rows are indicated by the numerals. A total of 30 OHCs were innervated (8 in row 1, 11 in row 2, and 11 in row 3) by a total of 35 endings (9 in row 1, 12 in row 2, and 14 in row 3). The arc indicates the span of innervation (497 μm, which is 2.6% of the total cochlear distance), and arrows indicate the innervation midpoint (located at a position that was 22.40% of total cochlear distance from the base to the apex) and center of gravity (a fraction 0.729 of the distance from the basal-most to apical-most innervated OHC, which is at a position 22.99% of the total cochlear distance, see materials and methods). This axon was labeled with horseradish peroxidase but the appearance of MOC axons labeled with biocytin was qualitatively similar. This was the only MOC neuron injected in this cochlea and this was the only axon that was recovered. An auditory-nerve fiber was recorded at the same site and its CF was 17.6 kHz, but it was not injected.
Rather than a stereoptypical number of OHCs innervated by single MOC axons, there was large variability. The total number of OHCs innervated per axon ranged from 14 to 69 (avg. 38.6, SD 14.0, n = 32 axons). High-CF axons (Figs. 3 and 4, A and B) tended to innervate fewer hair cells than the low-CF axons (Figs. 4, C and D). The dependence on CF is plotted for all the axons in Fig. 5A. There was little difference between Ipsi units (avg. 38.6 OHCs innervated per axon, SD 14.0) and Contra units (avg. 39.4 OHC/axon, SD 13.6) although the greater number of low-CF Contra units may make this a biased comparison. The two Either-Ear units innervated somewhat fewer hair cells (avg. 33.5 OHC/axon). The MOC endings were large (Fig. 2) and were usually formed at the bases of the hair cells. Sometimes multiple endings were formed on individual OHCs (see counts in legends of Figs. 2 and 3). On average, the number of endings per axon was 46.8 (SD 17.2, range 16–87). The number of endings per axon is also a decreasing function of CF (Fig. 5B).
Fig. 4.
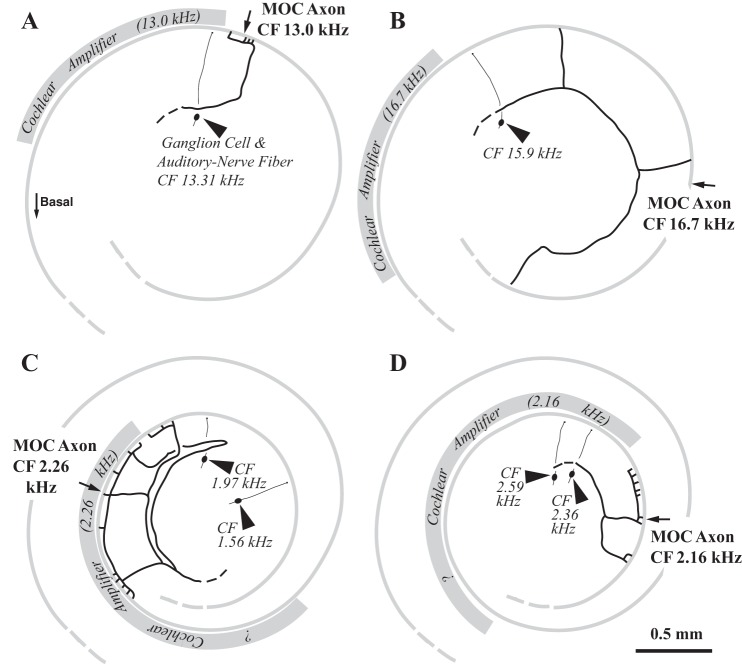
Drawings of labeled axons of 4 other MOC neurons, positioned onto templates of a portion of the cochlear spiral (thin gray shading indicates position of OHCs). The templates are aligned such that the 13.18-kHz point is uppermost on the drawing (an orientation similar to Fig. 3). The MOC unit CFs are given next to the drawings and the midpoints of innervation are indicated by the arrows. The unit ID numbers, response types, numbers of innervated hair cells, and spans are as follows: A: MCB 460.6, Ipsi unit, 24 OHCs, 230 μm (1.28% cochlear distance); B: MCB 432.5b, Contra unit, 14 OHCs, 3,334 μm (21.24%); C: MCB 454.2, Ipsi unit, 69 OHCs, 1,567 μm (9.47%); D: MCB 521.7, Contra unit, 56 OHCs, 816 μm (4.25%). Labeled auditory-nerve fibers and ganglion cells (arrowheads, with their CFs given in italicized numbers) in the same cochleas are shown. The positions of these nerve fibers, along with the formula for nerve-fiber mappings (see legend of Fig. 7B), were used to calculate the location of the cochlear amplifier for the CF of the MOC axons (gray shading, also see Fig. 9). The cochlear amplifier location (for a nerve fiber) begins at the CF position and extends basally one-fourth octave in the cochlear base (A and B). The extent of the amplifier may be larger apically (C and D), since there is an increase in 2-tone suppression (Prijs 1989) and calculated gain functions (Shera 2007). A doubling in extent of the amplifier is assumed but the “?” symbols on the figure indicate the amount of increase is unknown.
Fig. 5.
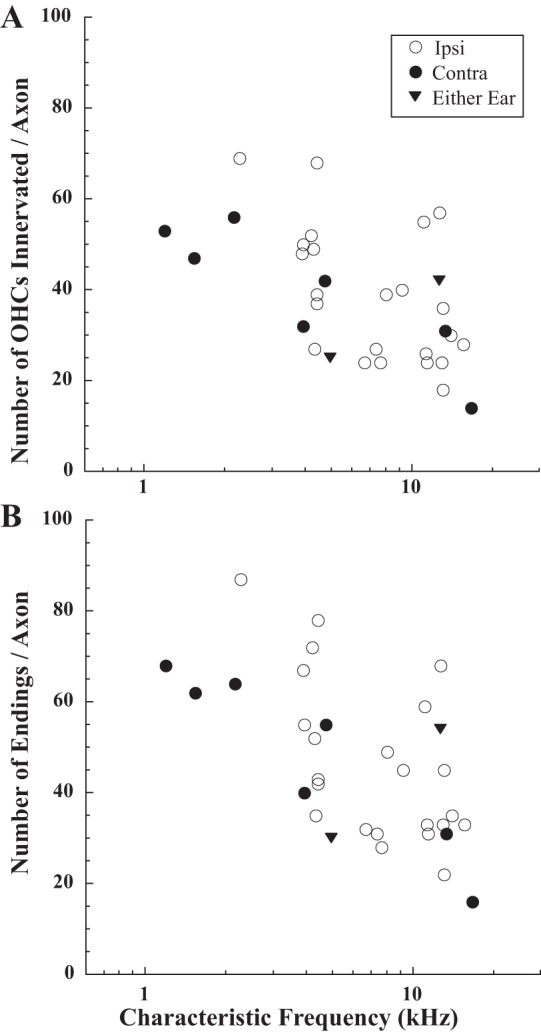
Number of outer hair cells innervated (A) and endings formed (B) for MOC axons, plotted as a function of CF. Symbols code for response type (see key).
Row 1 OHCs play an important role in the cochlear amplifier (Liberman and Dodds 1984), and MOC axons preferentially innervated this row (Fig. 6). For the total of 1,273 innervated hair cells in the database, 47.8% were in row 1. On a per axon basis, the average number of innervated OHCs in row 1 was significantly larger than the average number in row 2 (18.5 vs. 12.9 per axon, t-test, P = 0.01), and the average number in row 2 was significantly larger than the average number in row 3 (12.9 vs. 7.2, t-test, P = 0.0001). The highest numbers of row 1 OHCs were innervated by axons with CFs <5 kHz (30–50 per axon, Fig. 6A), whereas these axons only innervated 0–15 in row 3 (Fig. 6C). The small number of OHCs innervated in row 3 is observed in the example axon terminating in the second cochlear turn (Fig. 2) compared with the more even coverage of the hair cell rows by the example axon terminating in the lower first (basal) turn (Fig. 3). Although Ipsi units innervated fewer row 1 OHCs per axon than Contra units, this difference was not significant (avg. 16.3 vs. 24.5, t-test, P = 0.06).
Fig. 6.
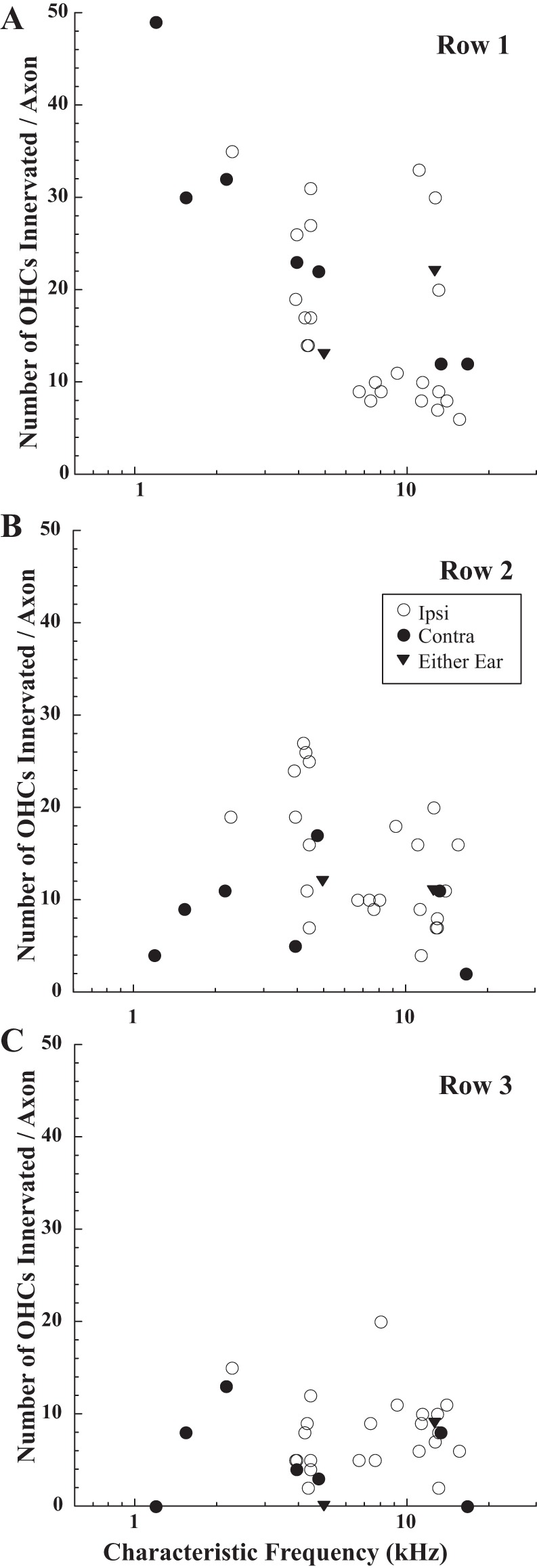
Number of OHCs innervated by MOC axons in row 1 (A), row 2 (B), and row 3 (C), plotted as a function of CF. Symbols code for response type (see key).
Mappings.
The mapping of CF to region of cochlear termination for MOC neurons was constructed from physiological and anatomical data like that contained in Figs. 1, 3, and 4. For the axon of Fig. 3, the midpoint of innervation (middle arrow), normalized by the total cochlear distance, was located at a position 22.40% from the basal end of the cochlea. Its CF was 14.0 kHz and it was an Ipsi unit. The midpoints for the axons of Fig. 4 are indicated (arrows), and the physiological data are adjacent to the drawings. The mapping for all axons of the database (Fig. 7A) shows a tonotopic relationship, with low-CF axons terminating apically and high-CF axons terminating more basally. Contra units terminated slightly more apically than Ipsi units for a given CF. The best-fit lines for the two unit types (see Fig. 7A legend) predict that, at a CF equal to 4 kHz, there is a difference of 4.32% distance (∼1/3 octave in guinea pigs: Tsuji and Liberman 1997). The two Either-Ear units in the database followed the mapping for Ipsi units. Both Ipsi and Contra units showed considerable spread of data from the lines. One cluster of data points from units with CFs ∼4 kHz, all Ipsi units, illustrates this variability: the most basally terminating axon had a midpoint of 40.16% and the most apically terminating axon had a midpoint of 54.68%.
To gain insight into the relationship of MOC terminations to the site of the cochlear amplifier, auditory-nerve fibers were labeled (Fig. 4, arrowheads). Their terminals contact a single inner hair cell, which is a “pinpoint” mapping compared with the broader swath of MOC axon terminations. The overall data set for nerve fibers consisted of 39 fibers, which were labeled either via the spiral-ganglion recording site in the basal turn (n = 27, CFs 13.3–17.8 kHz, e.g., Fig. 4, A and B) or at a modiolar recording site (n = 12, CFs of 1.4 - 6.2 kHz, e.g., Fig. 4, C and D). The nerve-fiber data had less spread from the best-fit line (Fig. 7B, dashed line, R = 0.99) compared with the MOC mapping. In this characteristic, as well as in slope and intercept, the nerve fiber mapping is similar to that of an earlier study in guinea pig (Tsuji and Liberman 1997; Fig. 7B, dotted line). With the nerve-fiber line as a reference, the best-fit line for all types of MOC neurons (Fig. 7B, solid line) is just apical (0.8% cochlear distance) at 15 kHz and further apical (8.0% cochlear distance) at 1 kHz. The best-fit lines for the nerve-fiber data (from the present study) and MOC data can be compared using statistical tests (Kleinbaum et al. 2008). The null hypotheses of parallel lines and equal intercepts are rejected (P = 0.016, and P < 0.001, respectively), that is, the two lines are significantly different. However, another test that used only data from Ipsi units (Fig. 7A, dashed line) indicates parallel lines (P = 0.646) and equal intercepts (although just at the edge of being significantly different, P = 0.053). Thus Ipsi MOC units and nerve fibers have similar mappings, but Contra units have a different mapping.
The relationship between nerve fibers and MOC axons of similar CFs was examined in individual cochleas where both types of neurons were labeled (Fig. 4). Twenty-two cases had pairs with similar CFs (within 1 octave) using labeled nerve fibers (e.g., Fig. 4) or a visible recording site (e.g., Fig. 3) from which a radial line was drawn (9 other cochleas did not have pairs or labeled MOC neurons close in CF to the recording site and were not adjusted). It was assumed that the labeled nerve fiber position anchored at that point the nerve-fiber CF mapping for that cochlea. Then, the difference between this anchor point and the CF mapping for nerve fibers (see Fig. 7B legend) was used to adjust the MOC innervation position. The average adjustment was 0.65% (range −3.49 to 6.34%) and using this adjusted position did not significantly affect the MOC mapping (see Fig. 7B legend). The nerve-fiber terminals in the organ of Corti also indicate the position of OHCs involved in the cochlear amplifier for their CF, which starts at this position and extends basally (Neely and Kim 1986; Cody 1992; Patuzzi 1996; Pang and Guinan 1997; Shera 2007; Fisher et al. 2012). Where there were labeled pairs, the cochlear amplifier position can be calculated accurately for the CF of the MOC neurons (gray shading on Fig. 4). In Fig. 4, only one MOC neuron terminations had extensive overlap with the predicted position of the cochlear amplifier (Fig. 4C), but the other three neuron terminations had no overlap because the amplifier was located basally (Fig. 4, A, B, and D).
The mappings of MOC innervation presented so far were based on midpoint, and such a measure may give a misleading view of the effects of MOC action if there is a disproportionate number of endings toward one edge of the MOC axon termination. For example, the axon shown in Fig. 3 had most of its endings in the large patch at the apical edge of its span (toward the right in Fig. 3). To quantify this asymmetry, the center of gravity (see materials and methods) was computed for each axon; for the axon of Fig. 3 the center of gravity (Fig. 3, right arrow) was the fraction 0.729 of the distance from the basal end of its innervation span. The cochlear mapping for this measure, expressed as a percent distance along the cochlear spiral, is plotted in Fig. 7C. The best-fit lines (dashed) vs. the earlier-described midpoint of innervation (solid) are almost the same. The center of gravity (expressed as a fraction) also had no obvious pattern across CF (Fig. 8A). The center of gravity did not differ significantly (t-test, P = 0.85) between Ipsi units (avg. 0.490, SD 0.148) and Contra units (avg. 0.503, SD 0.150).
Innervation spans.
The labeled MOC axons had large variability in their spans of termination, encompassing spans that were small (Fig. 4A), intermediate (Figs. 3 and Fig. 4D), and large (Fig. 4, B and C). For all the labeled axons (Fig. 8B), spans ranged from 150 to 4910 μm, which corresponds to between 0.79 and 23.80% of the total cochlear distance. Axons with small spans were not due to fading of the reaction product along their course. Also, axons with small spans were not potentially confused with other axons, since 8 of 10 axons with spans of <2% cochlear distance were the only axons labeled in those particular cochleas. There was a trend for the lower CF units to have larger spans (Figs. 8B and 9). For example, units with CFs <6 kHz had spans that averaged 7.43% (SD 6.43, n = 15), whereas units with CFs >6 kHz have spans that averaged 4.45% (SD 4.93, n = 20). This difference was not statistically significant (t-test, P = 0.13), and there is a low R value to a linear fit to the points (data not shown) because of the large variability. The spans for Ipsi units were significantly smaller than those for Contra units (t-test, P = 0.01). For Ipsi units, the average span was 4.52% cochlear distance (SD 3.88, range 0.79 to 17.03) and for Contra units, the average span was 10.41% (SD 8.59, range 1.41 to 23.80). For the two Either-Ear units, the spans were 1.84 and 2.40%, more like Ipsi units than Contra units.
Fig. 9.
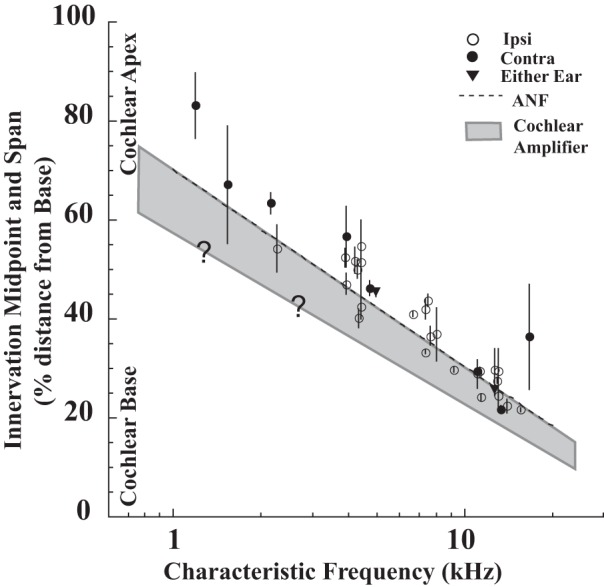
Comparison of MOC axon midpoints (symbols) and spans (bars) with the extent of the cochlear amplifier. Innervation midpoints are plotted as a function of CF, with bars showing the innervation span for each axon. Gray shading shows the approximate extent of the OHCs involved in the cochlear amplifier, which occupies a one-quarter octave extent basal to the auditory-nerve fiber CF position in the cochlear base. The graphed extent increases apically, since there is an increase in two-tone suppression (Prijs 1989) and calculated gain functions (Shera, 2007). On the graph, the amount of increase is a doubling in the apex, but the “?” signifies that the extent of this increase is unknown.
DISCUSSION
Anatomical terminations of MOC neurons.
The sample of labeled MOC axon terminations documented here is large enough to compare the cochlear frequency mapping to auditory-nerve fibers and show that it is similar in many ways. While the mapping of individual nerve fibers is pinpoint and precise (Liberman 1982; Tsuji and Liberman 1997), the mapping of MOC axons consists of broader swaths that have higher variability. Contra MOC units had apical offsets and broader innervation spans relative to Ipsi units even though they innervated about the same number of hair cells. Either-Ear units have not previously been labeled; although only two of these uncommon units were labeled here, both had anatomical characteristics like those of Ipsi units. The relatively broad pattern of innervation contrasts with the sharply tuned responses of MOC neurons (Fig. 1).
Single-unit recordings tend to sample large neurons (Stone 1973), and the following calculation suggests that the axons sampled in the present study have above-average numbers of endings. The average number of MOC endings observed in the present study (avg. 46.8/axon), multiplied by the number of MOC neurons projecting to one cochlea (n = 615: Robertson et al. 1987; Brown et al. 2013, although larger n's were found by Aschoff and Ostwald 1987), yields a total of 28,782 MOC endings per cochlea. Dividing by the number of OHC in the guinea pig cochlea (2,400; Coleman 1976) yields ∼12 endings per OHC, which is higher than the number of efferent endings per OHC (8–10) reported previously in the guinea pig (Engstrom 1960). Thus the present data appear to have oversampled axons that form the largest numbers of endings, which may be those with the largest diameters.
MOC terminations vs. the site of the cochlear amplifier.
The present results, showing preferential MOC termination on OHCs in row 1, are consistent with the importance of this row in the function of the cochlear amplifier. The importance of row 1 was originally shown in noise-exposed preparations in which lesions of stereocilia on row 1 hair cells, with virtually no other damage to OHCs, were accompanied by large losses in auditory-nerve sensitivity near CF (Liberman and Dodds 1984). Present data indicate a trend in innervation between the OHC rows (1 > 2 > 3) and suggest a similar trend for contribution to the amplifier function. MOC innervation trends between the rows and along the cochlear length have been reported previously (Ishii and Balough 1968; Guinan et al. 1984; Brown 1987; Liberman et al. 1990).
The CF-to-position mapping of MOC neurons described in the present study is one of many such tonotopic mappings in the auditory pathway (Clopton et al. 1974; Ryan et al. 1982; Friauf 1992; Muniak et al. 2013). Present results suggest that the MOC mapping is similar to the auditory-nerve fiber mapping, either indistinguishable from it (for MOC Ipsi units) or just apical to it (for MOC Contra units, although these data are more limited). Previous studies of MOC labeling in cat and guinea pig, most of which are Ipsi units, are consistent with present results (Robertson and Gummer 1985; Liberman and Brown 1986). Thus the Ipsi units form a feedback system that is in frequency alignment with their CFs and those of auditory-nerve fibers. The more apical offset of Contra units is reminiscent of earlier projection data from injections made into the superior olivary complex (Guinan et al. 1984). Without any other information, such data suggest that MOC neuron effects would be largest at places tuned to their CFs or slightly below. With the use of contralateral sound to activate MOC neurons, largest effects on auditory-nerve fibers are indeed found for frequencies around CF (Warren and Liberman 1989). Similarly, contralateral sound is most effective for frequencies close to the frequency of an otoacoustic emission in the ipsilateral ear (Veuillet et al. 1991; Chery-Croze et al. 1993; Lilaonitkul and Guinan 2012), although caution is needed here because the spatial extent of the emission generators is not clearly known and almost certainly depends on sound level and the type of emission. In the present study, in contrast, there is the direct measurement of the position of MOC innervation compared with the CF place for auditory-nerve fibers.
The region of OHCs that is most active in the amplifier, however, is ∼650 μm (¼ octave) basal to the CF place for nerve fibers of the basal turn (gray shading on Fig. 9), as shown by lesion and perturbation studies (Cody 1992; Fisher et al. 2012). Although those studies are limited to the cochlear basal turn, a basal offset in all cochlear regions is indicated by modeling studies (Neely and Kim 1986; de Boer and Nuttall 2000), by calculated gain functions from basilar membrane measurements (Shera 2007), and by extensive studies of two-tone suppression in which the nerve-fiber response to a probe at CF is suppressed by a second tone that presumably “jams” the cochlear amplifier (Sachs and Kiang 1968; Schmiedt 1982; Prijs 1989; Geisler et al. 1990; Kanis and de Boer 1994; Nobili and Mammano 1996; reviewed by Patuzzi 1996). However, there is much less known about extent of the cochlear amplifier in the apical cochlear regions (and MOC labeling data there are also lacking). Given that the MOC and nerve-fiber mappings are close, and that the cochlear amplifier extends basally, the MOC terminations of a given CF do not generally reach basally to the site of the cochlear amplifier for that CF (Fig. 9). Only about half (17 of 34) of the MOC axon spans in the present study infringe on this region, and almost all of the terminations extend apical to this region. Similar conclusions are seen for individual cochleas, where auditory nerve fibers were used to compute the location of the cochlear amplifier and where the location of the amplifier for the CF of the labeled MOC axons was usually basal to the MOC termination (Fig. 4, A, B, and D). It seems unlikely that terminations of MOC axons were missed in the present study, because of the excellent signal-to-noise of the labeling (Fig. 2), because all branches and endings were recovered without evidence of fading for almost all axons, and because the important basal part of the terminations is nearest the injection site (Fig. 3) where the reaction product is the darkest.
How can these data explain the biggest effects at CF with few projections to the position of the amplifier? This question does not appear to be fully explained by existing knowledge, but part of the answer requires consideration of sound level. Cochlear amplifier measurements are usually performed at low sound levels where the contribution of the amplifier is maximal (Patuzzi 1996). In contrast, the MOC system acts mostly at moderate and high sound levels. MOC firing rates do not become significant until high sound levels (Liberman 1988a, 1988b; Brown et al. 1998), and high rates of stimulation of the OC bundle are needed for peripheral effects (Wiederhold and Kiang 1970; Brown and Nuttall 1984). At these high sound levels, the pattern of MOC firing shifts such that maximal rates are in response to frequencies just below MOC neuron CF (Liberman 1988b), and this offset, while variable, averages about a one-quarter octave (Fig. 12C in Warren and Liberman 1989). Thus, if frequency for maximal MOC firing rate was plotted instead of CF, the points of Fig. 9 would shift toward the left so that a larger portion would have involvement with the cochlear amplifier. With this consideration, the MOC firing at high sound levels is a better match for the position of the cochlear amplifier. An untested idea is that those MOC terminations that have the most deviation from the region of the amplifier (such as Contra units) might have the most downward shift in frequency from CF to frequency of maximal firing. These shifts might also reduce some of the variability in termination observed for MOC axons. However, another part of the answer to the alignment problem may be that MOC neurons have actions that use mechanisms other than altering the gain of the cochlear amplifier (Guinan and Stankovic 1996). These mechanisms might be important in the MOC function to protect the cochlea from overstimulation, which occurs at the highest sound levels where the cochlear amplifier contribution is minimal.
Functional effects of large MOC spans.
The present study demonstrates that some MOC axons terminate in wide spans along the organ of Corti. The largest span of the present study, a Contra unit, was almost 24% of the total cochlear length, which would correspond to approximately two octaves of sound frequency. Like those studied here in the guinea pig, MOC terminations in other species can also be broad. In the cat, 23–84 OHCs were innervated per MOC axon and the spans were up to 2.8 mm, just under an octave span (Liberman and Brown 1986). In mice, axonal spans are at least as broad, 8.8–35% of the total cochlear length (Wilson et al. 1991) and 35% of the cochlear length (Brown et al. 1991).
Significant termination span is the anatomical substrate for a single point along the cochlea to be affected by MOC neurons tuned to a variety of CFs. In fact, in human experiments, an otoacoustic emission can be affected by a range of frequencies presented to the contralateral ear (Veuillet et al. 1991; Chery-Croze et al. 1993; Lilaonitkul and Guinan 2009a), and the range extends over several octaves (Lilaonitkul and Guinan 2012). Although the spatial extent of the emission generators is not known, these data suggest wide effects of Contra MOC neurons. A second mechanism explaining wide effects of these reflex elicitors is the variability in MOC projection, so that innervation midpoints or centers of gravity do not fall right on the best-fit line (Fig. 7). This variability would tend to broaden the functional effects of the overall MOC system. In the present study, Contra units had innervation spans that were on average about twice as great as those of Ipsi units. These large spans offer a mechanism for the greater spatial summation of effects from contralateral elicitors vs. ipsilateral elicitors as bandwidth increases (Lilaonitkul and Guinan 2009b). This increase recruits additional, off-CF MOC neurons, and these Contra neurons have spans wide enough to encompass the CF position (whereas many Ipsi neurons do not). Overall, such ipsilateral/contralateral differences in the MOC reflex suggest differences in the functional roles of the two types of MOC neurons leading to the OHCs, even in the way the cochlear amplifier is controlled depending on whether sound is presented to the ipsilateral vs. the contralateral ear.
GRANTS
This work was supported by National Institute of Child Health and Human Development Grant DC-01089.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.C.B. conception and design of research; M.C.B. performed experiments; M.C.B. analyzed data; M.C.B. interpreted results of experiments; M.C.B. prepared figures; M.C.B. drafted manuscript; M.C.B. edited and revised manuscript; M.C.B. approved final version of manuscript.
ACKNOWLEDGMENTS
I thank Dr. M. Charles Liberman for generous help throughout the project and Drs. John J. Guinan, Jr., Chris Shera, and the many reviewers for helpful comments on earlier versions of the manuscript.
REFERENCES
- Abdala C, Mishra SK, Williams TL. Considering distortion product otoacoustic emission fine structure in measurements of the medial olivcochlear reflex. J Acoust Soc Am 125: 1584–1594, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alder VA, Johnstone BM. A new approach to the guinea pig auditory nerve. J Acoust Soc Am 64: 684–687, 1978 [DOI] [PubMed] [Google Scholar]
- Aschoff A, Ostwald J. Different origins of cochlear efferents in some bat species, rats, and guinea pigs. J Comp Neurol 264: 56–72, 1987 [DOI] [PubMed] [Google Scholar]
- Brown MC. Morphology of labeled efferent fibers in the guinea pig cochlea. J Comp Neurol 260: 605–618, 1987 [DOI] [PubMed] [Google Scholar]
- Brown MC. Morphology and response properties of single olivocochlear fibers in the guinea pig. Hear Res 40: 93–110, 1989 [DOI] [PubMed] [Google Scholar]
- Brown MC, Nuttall AL. Efferent control of cochlear inner hair cell responses in the guinea-pig. J Physiol 354: 625–646, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MC, Pierce S, Berglund AM. Cochlear-nucleus branches of thick (medial) olivocochlear fibers in the mouse: A cochleotopic projection. J Comp Neurol 303: 300–315, 1991 [DOI] [PubMed] [Google Scholar]
- Brown MC, Kujawa SG, Duca ML. Single olivocochlear neurons in the guinea pig: I. Binaural facilitation of responses to high-level noise. J Neurophysiol 79: 3077–3087, 1998 [DOI] [PubMed] [Google Scholar]
- Brown MC, Mukerji S, Drottar M, Windsor A, Lee DJ. Identification of inputs to olivocochlear neurons using transneuronal labeling with pseudorabies virus (PRV). J Assoc Res Otolaryngol 14: 703–717, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chery-Croze A, Moulin A, Collet L. Effect of contralateral sound stimulation on the distortion product 2f1-f2 in humans: Evidence of a frequency specificity. Hear Res 68: 53–58, 1993 [DOI] [PubMed] [Google Scholar]
- Clopton BM, Winfield JA, Flammino FJ. Tonotopic organization: review and analysis. Brain Res 76: 1–20, 1974 [DOI] [PubMed] [Google Scholar]
- Cody AR. Acoustic lesions in the mammalian cochlea: Implications for the spatial distribution of the “active process”. Hear Res 62: 166–171, 1992 [DOI] [PubMed] [Google Scholar]
- Coleman JW. Hair cell loss as a function of age in the normal cochlea of the guinea pig. Acta Otolaryngol (Stockh) 82: 33–40, 1976 [DOI] [PubMed] [Google Scholar]
- Cooper NP, Guinan JJ., Jr Efferent-mediated control of basilar membrane motion. J Physiol 576: 49–54, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallos P. The active cochlea. J Neurosci 12: 4575–4585, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallos P, Cheatham MA, Gao J, Zheng J, Anderson CT, Jia S, Wang X, Cheng WH, Sengupta S, He DZ, Zuo J. Prestin-based outer hair cell motility is necessary for mammalian cochlear amplification. Neuron 58: 333–339, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer E, Nuttall AL. The mechanical waveform of the basilar membrane. III. Intensity effects. J Acoust Soc Am 107: 1497–1507, 2000 [DOI] [PubMed] [Google Scholar]
- Engstrom H. Electron microscopic studies of the receptor cells of the organ of Corti. In: Neural Mechanisms of the Auditory and Vestibular Systems, edited by Rasmussen GS, Windle SF. Springfield, IL: C. C. Thomas 1960, p. 48–64 [Google Scholar]
- Fex J. Auditory activity in centrifugal and centripetal cochlear fibers in cat. Acta Physiol Scand 189: 1–68, 1962 [PubMed] [Google Scholar]
- Fisher JAN, Nin F, Reichenbach T, Uthaiah RC, Hudspeth AJ. The spatial pattern of cochlear amplification. Neuron 76: 989–997, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friauf E. Tonotopic order in the adult and developing auditory system of the rat as shown by c-fos immunocytochemistry. Eur J Neurosci 4: 798–812, 1992 [DOI] [PubMed] [Google Scholar]
- Fuchs PA. The synaptic physiology of cochlear hair cells. Audiol Neurotol 7: 40–44, 2002 [DOI] [PubMed] [Google Scholar]
- Geisler CD, Yates GK, Patuzzi RB, Johnstone BM. Saturation of outer hair cell receptor currents causes two-tone suppression. Hear Res 44: 241–256, 1990 [DOI] [PubMed] [Google Scholar]
- Gifford ML, Guinan JJ., Jr Effects of electrical stimulation of medial olivocochlear neurons on ipsilateral and contralateral cochlear responses. Hear Res 29: 179–194, 1987 [DOI] [PubMed] [Google Scholar]
- Guinan JJ, Jr, Stankovic TK. Medial efferent inhibition produces the largest equivalent attenuations at moderate to high sound levels in cat auditory-nerve fibers. J Acoust Soc Am 100: 1680–1690, 1996 [DOI] [PubMed] [Google Scholar]
- Guinan JJ, Jr, Warr WB, Norris BE. Topographic organization of the olivocochlear projections from the lateral and medial zones of the superior olivary complex. J Comp Neurol 226: 21–27, 1984 [DOI] [PubMed] [Google Scholar]
- Henin S, Thompson S, Abdelrazeq S, Long GR. Changes in amplitude and phase of distortion-product otoacoustic emission fine-structure and separated components during efferent activation. J Acoust Soc Am 129: 2068–2079, 2011 [DOI] [PubMed] [Google Scholar]
- Ishii D, Balough K., Jr Distribution of efferent endings in the organ of Corti. Acta Otolaryngol (Stockh) 66: 282–288, 1968 [DOI] [PubMed] [Google Scholar]
- Jennings SG, Heinz MG, Strickland EA. Evaluating adaptation and olivocochlear efferent feedback as potential explanations of psychophysical overshoot. J Assoc Res Otolaryngol 12: 345–360, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanis LJ, de Boer E. Two-tone suppression in a locally active nonlinear model of the cochlea. J Acoust Soc Am 96: 2156–2165, 1994 [DOI] [PubMed] [Google Scholar]
- Kawase T, Delgutte B, Liberman MC. Antimasking effects of the olivocochlear reflex. II. Enhancement of auditory-nerve response to masked tones. J Neurophysiol 70: 2533–2549, 1993 [DOI] [PubMed] [Google Scholar]
- Kiang NY, Moxon EC, Levine RA. Auditory-nerve activity in cats with normal, and abnormal cochleas. In: Ciba Foundation Symposium on Sensorineural Hearing Loss, edited by Wolstenholme GE, Knight J.London, UK: J&A Churchill, 1970, p. 241–273 [DOI] [PubMed] [Google Scholar]
- Kleinbaum DG, Kupper LL, Nizam A, Muller KE. Applied Regression Analysis and Other Multivariate Methods. Belmont, CA: Duxbury, 2008 [Google Scholar]
- Liberman MC. Auditory-nerve responses from cats raised in a low-noise chamber. J Acoust Soc Am 63: 442–455, 1978 [DOI] [PubMed] [Google Scholar]
- Liberman MC. The cochlear frequency map for the cat: labeling auditory-nerve fibers of known characteristic frequency. J Acoust Soc Am 72: 1441–1449, 1982 [DOI] [PubMed] [Google Scholar]
- Liberman MC. Physiology of cochlear efferent and afferent neurons: direct comparisons in the same animal. Hear Res 34: 179–192, 1988a [DOI] [PubMed] [Google Scholar]
- Liberman MC. Response properties of cochlear efferent neurons: monaural vs. binaural stimulation and the effects of noise. J Neurophysiol 60: 1779–1798, 1988b [DOI] [PubMed] [Google Scholar]
- Liberman MC, Guinan JJ., Jr Feedback control of the auditory periphery: anti-masking effects of middle ear muscles vs. olivocochlear efferents. J Commun Disord 31: 471–483, 1998 [DOI] [PubMed] [Google Scholar]
- Liberman MC, Oliver ME. Morphometry of intracellularly labeled neurons of the auditory nerve: correlations with functional properties. J Comp Neurol 223: 163–176, 1984 [DOI] [PubMed] [Google Scholar]
- Liberman MC, Dodds LW. Single neuron labeling and chronic cochlear pathology. III. Stereocilia damage and alterations of threshold tuning curves. Hear Res 16: 55–74, 1984 [DOI] [PubMed] [Google Scholar]
- Liberman MC, Brown MC. Physiology and anatomy of single olivocochlear neurons in the cat. Hear Res 24: 17–36, 1986 [DOI] [PubMed] [Google Scholar]
- Liberman MC, Dodds LW, Pierce S. Afferent and efferent innervation of the cat cochlea: quantitative analysis with light and electron microscopy. J Comp Neurol 301: 443–460, 1990 [DOI] [PubMed] [Google Scholar]
- Lilaonitkul W, Guinan JJ., Jr Reflex control of the human inner ear: a half-octave offset in medial efferent feedback that is consistent with an efferent role in the control of masking. J Neurophysiol 101: 1394–1406, 2009a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilaonitkul W, Guinan JJ., Jr Human medial olivcochlear reflex: effects as functions of contralateral, ipsilateral, and bilateral elicitor bandwidths. J Assoc Res Otolaryngol 10: 459–470, 2009b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilaonitkul W, Guinan JJ., Jr Frequency tuning of medial-olivocochlear-efferent acoustic reflexes in humans as functions of probe frequency. J Neurophysiol 107: 1598–1611, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maison SF, Liberman MC. Predicting vulnerability to acoustic injury with a noninvasive assay of olivocochlear reflex strength. J Neurosci 20: 4701–4707, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muniak MA, Rivas A, Montey KL, May BJ, Francis HW, Ryugo DK. 3D model of frequency representation in the cochlear nucleus of the CBA/J mouse. J Comp Neurol 521: 1510–1532, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murugasu E, Russell IJ. The effect of efferent stimulation on basilar membrane displacement in the basal turn of the guinea pig cochlea. J Neurosci 16: 325–332, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neely ST, Kim DO. A model for active elements in cochlear biomechanics. J Acoust Soc Am 79: 1472–1480, 1986 [DOI] [PubMed] [Google Scholar]
- Nobili R, Mammano F. Biophysics of the cochlea. II. Stationary nonlinear phenomenology. J Acoust Soc Am 99: 2244–2255, 1996 [DOI] [PubMed] [Google Scholar]
- Pang XD, Guinan JJ., Jr Growth rate of simultaneous masking in cat auditory-nerve fibers: relationship to the growth of basilar-membrane motion and the origin of two-tone suppression. J Acoust Soc Am 102: 3564–3575, 1997 [DOI] [PubMed] [Google Scholar]
- Patuzzi R. Cochlear micromechanics and macromechanics. In: The Cochlea, edited by Dallos P, Popper AN, Fay RR. New York: Springer-Verlag, 1996, p. 186–257 [Google Scholar]
- Prijs VF. Lower boundaries of two-tone suppression in the guinea pig. Hear Res 42: 73–82, 1989 [DOI] [PubMed] [Google Scholar]
- Reiter ER, Liberman MC. Efferent mediated protection from acoustic overexposure: relation to “slow” effects of olivocochlear stimulation. J Neurophysiol 73: 506–514, 1995 [DOI] [PubMed] [Google Scholar]
- Robertson D, Gummer M. Physiological and morphological characterization of efferent neurons in the guinea pig cochlea. Hear Res 20: 63–77, 1985 [DOI] [PubMed] [Google Scholar]
- Robertson D, Cole KS, Corbett K. Quantitative estimate of bilaterally projecting medial olivocochlear neurons in the guinea pig brainstem. Hear Res 27: 177–181, 1987 [DOI] [PubMed] [Google Scholar]
- Ryan AF, Woolf NK, Sharp FR. Tonotopic organization in the central auditory pathway of the mongolian gerbil: a 2-deoxyglucose study. J Comp Neurol 207: 369–380, 1982 [DOI] [PubMed] [Google Scholar]
- Ryugo DK, Fay RR, Popper AN. Auditory and Vestibular Efferents. New York: Springer Science & Business Media, 2011 [Google Scholar]
- Sachs MB, Kiang NY. Two-tone inhibition in auditory-nerve fibers. J Acoust Soc Am 43: 1120–1128, 1968 [DOI] [PubMed] [Google Scholar]
- Schmiedt RA. Boundaries of two-tone rate suppression of cochlear-nerve activity. Hear Res 7: 335–351, 1982 [DOI] [PubMed] [Google Scholar]
- Sellick PM, Russell IJ. Intracellular studies of the receptor potentials of inner hair cells of the guinea pig cochlea: techniques. In: Auditory Investigation: The Scientific and Technological Basis, edited by Beagley HA. Oxford, UK: Oxford Univ Press, 1979, p. 368–381 [Google Scholar]
- Shera CA. Laser amplification with a twist: traveling-wave propagation and gain functions from throughout the cochlea. J Acoust Soc Am 122: 2738–2758, 2007 [DOI] [PubMed] [Google Scholar]
- Stone J. Sampling properties of microelectrodes assessed in the cat's retina. J Neurophysiol 36: 1071–1079, 1973 [DOI] [PubMed] [Google Scholar]
- Tsuji J, Liberman MC. Intracellular labeling of the auditory nerve fibers in guinea pig: central and peripheral projections. J Comp Neurol 381: 188–202, 1997 [PubMed] [Google Scholar]
- Veuillet E, Collet L, Duclaux R. Effect of contralateral acoustic stimulation on active cochlear micromechanical properties in human subjects: dependence on stimulus variables. J Neurophysiol 65: 724–735, 1991 [DOI] [PubMed] [Google Scholar]
- Warren EH, 3rd, Liberman MC. Effects of contralateral sound on auditory-nerve responses II. Dependence on stimulus variables. Hear Res 37: 105–122, 1989 [DOI] [PubMed] [Google Scholar]
- Wiederhold ML, Kiang NYS. Effects of electric stimulation of the crossed olivocochlear bundle on single auditory-nerve fibers in the cat. J Acoust Soc Am 48: 950–965, 1970 [DOI] [PubMed] [Google Scholar]
- Wilson JL, Henson MM, Henson OW., Jr Course and distribution of efferent fibers in the cochlea of the mouse. Hear Res 55: 98–108, 1991 [DOI] [PubMed] [Google Scholar]
- Winslow R, Sachs MB. Single-tone intensity discrimination based on auditory-nerve rate responses in backgrounds of quiet, noise, and with stimulation of crossed olivocochlear bundle. Hear Res 35: 165–190, 1988 [DOI] [PubMed] [Google Scholar]