Abstract
The accuracy of saccades, as maintained by saccade adaptation, has been shown to be context dependent: able to have different amplitude movements to the same retinal displacement dependent on motor contexts such as orbital starting location. There is conflicting evidence as to whether purely visual cues also effect contextual saccade adaptation and, if so, what function this might serve. We tested what visual cues might evoke contextual adaptation. Over 5 experiments, 78 naive subjects made saccades to circularly moving targets, which stepped outward or inward during the saccade depending on target movement direction, speed, or color and shape. To test if the movement or context postsaccade were critical, we stopped the postsaccade target motion (experiment 4) or neutralized the contexts by equating postsaccade target speed to an intermediate value (experiment 5). We found contextual adaptation in all conditions except those defined by color and shape. We conclude that some, but not all, visual cues before the saccade are sufficient for contextual adaptation. We conjecture that this visual contextuality functions to allow for different motor states for different coordinated movement patterns, such as coordinated saccade and pursuit motor planning.
Keywords: saccade adaptation, context learning, motor learning, visual cues
movement adaptation gradually remaps the relationship between the retinal vector of the target and the movement executed to reduce endpoint errors. Adaptation of our rapid, gaze reorienting, saccadic eye movements shares many similarities with adaptation of rapid arm movements, and concepts and data from one modality are often translatable to the other (Herman et al. 2013a). Examples of these overlaps include the use of sensory prediction error signals (Bahcall and Kowler 2000; Wolpert et al. 1998), multiple timescales of motor memory (Kording et al. 2007; Joiner and Smith 2008), and adaptation that is context specific (Pelisson et al. 2010; Wolpert et al. 1998).
Visual cues are routinely used by arm movement systems to adjust motor parameters. Whether one judges one's glass half full or half empty, different sets of grip and arm muscle forces are predictively engaged when picking it up compared with a full or empty glass. In addition to this motor prediction, it is hardly surprising, therefore, that perturbations to movements in a given context can induce motor adaptation that is also highly context dependent (Vetter and Wolpert 2000), including the use of visual cues that help guide the action (Wing and Lederman 1998; Mon-Williams and Murray 2000; Howard et al. 2013). The fixed muscle load of the eye means there is less obvious need for such rich contextuality, and yet saccade adaptation can be highly context specific.
Indeed, saccade adaptation is context specific to various motor states. The simple act of looking upward vs. downward involves different sets of muscle commands, whose independent motor states provide sufficient contexts to allow independent adaptation. For example, shifting a target onward during 10° rightward saccades can induce lengthening of saccades in the upper visual field, while on interleaved trials, backward shifts in the lower visual field can simultaneously shorten the same vector saccades in the lower visual field (Alahyane and Pelisson 2004; Shelhamer and Clendaniel 2002; Tian and Zee 2010; Zimmermann and Lappe 2011; Havermann et al. 2011; Wulff et al. 2012). Similarly, saccade gain (saccade amplitude/initial target amplitude) can be adapted separately depending on orbital eccentricity or vergence command contexts (Chaturvedi and van Gisbergen 1997). All these context-specific adaptations are entirely consistent with the dominant view that saccade adaptation functions as a relatively simple motor recalibration process (for review see Hopp and Fuchs 2004).
Visual cues alone have been shown to cause contextual saccade adaptation, but there are conflicting reports. We found that intrasaccadic steps of either flickering or nonflickering targets led to different adaptations to the two different target types, providing both visual contexts were frequently intermixed (Herman et al. 2009). The only other studies reporting visual contexts found that color and shape were largely ineffective cues, as adaptation was broadly similar for red circles and green crosses with different intrasaccadic perturbations (Deubel 1995) or for diamonds vs. squares (Bahcall and Kowler 2000). The methods were so different that it is difficult to compare the three studies. A major aim of the current work was to test a wider variety of visual cues under the same experimental paradigm to establish the limits of visually induced contextual saccade adaptation and thereby clarify its possible function. The broader importance of this issue is that if visual cues can reliably produce context-specific saccade adaptation, it would question the simple recalibration model of conventional saccade adaptation: why would a calibration system care about visual target properties? Instead, visual contextuality might imply that saccade adaptation can access a form of associative learning in which perhaps any informative cue could be used to reduce endpoint errors.
We chose to test visual cues in contextual adaptation of saccades to moving targets. The saccade system extrapolates target motion when planning saccade amplitudes to moving targets (Gellman and Carl 1991). We conjectured that a context with a strong existing predictive element based on visual properties of the target might lend itself relatively strongly to contextual adaptation (somewhat analogously to the arm reaching for a glass example above). Noncontextual saccade adaptation of the first saccade to moving targets has recently been demonstrated via intrasaccadic target position step (Schutz and Souto 2011), and postsaccadic onsets of pursuit have also been shown to adapt the preceding saccade amplitude (Havermann et al. 2012). There have been no studies of contextual saccade adaptation to moving targets of which we are aware, although adaptation of pursuit (changing target velocity after pursuit onset) has shown context specificity to orbital position (Takagi et al. 2000). An additional novelty was our use of circular pursuit trajectories around the central fixation point. This has the methodological advantage of the required saccade amplitude being independent of latency. Moreover, recent work has shown that saccade gains decrease systematically as circular pursuit velocity increases (Azadi et al. 2012), revealing a surprising inherent flexibility in saccade gains, which might further encourage contextual adaptation.
We present five experiments in which alternate visual cues were defined by target properties of motion direction (experiments 1 and 4), speed (experiments 2 and 5), or color and shape (experiment 3), with each contextual cue (e.g., clockwise vs. anticlockwise motion) resulting in either inward or outward position steps of the pursuit trajectory. Furthermore, we tested the importance of postsaccadic contexts by removing the contextual differences upon saccade, by either stopping the target (experiment 4), or by equating target speeds after the saccade to an intermediate velocity (experiment 5). In addition to testing the importance of context contiguity across saccades for the first time, experiments 4 and 5 aimed to remove the smooth pursuit movement as a possible motor state context. That is, in experiments 1 and 2 the different pursuit velocities after the saccade might have provided a context for differential adaptation instead of the visual properties before the saccade (although one can always argue that different pursuit movements were still prepared, just not executed in experiments 4 and 5, and that that motor planning provided a context). Visual cues were effective in eliciting contextual adaptation in all experiments except for the color and shape cues of experiment 3.
METHODS
Subjects
We tested 78 subjects (n = 16, 24, 18, 10, and 10 in experiments 1–5; 47 female, 31 male). All subjects were undergraduate students, with no experience in psychophysical or eye-movement experiments and naive to the goals of the study. They had normal or corrected to normal vision. Written informed consent was obtained from all subjects, and all protocols were approved by the City College of New York Institutional Review Board.
Apparatus
Subjects sat in a darkened room with their heads stabilized via chin and forehead rests, 57 cm from a 19-in. wide CRT display. Stimuli were presented using the Psychophysics Toolbox (Brainard 1997; Pelli 1997) in Matlab (The Mathworks, Natick, MA) at a 120-Hz refresh rate on an 800 × 600 pixel resolution monitor. The luminance of the gray background was 12.5 cd/m2. Eye level was aligned with the middle of the monitor horizontally but 100 pixel (4.9°) below the center vertically. The Eyelink 1000 (SR Research, Osgoode, Ontario, Canada) sampled right eye position at 1,000 Hz.
Procedure
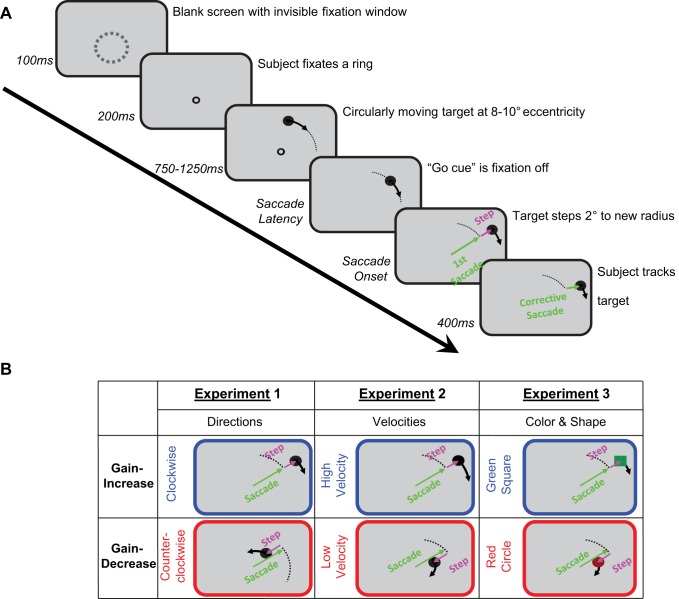
The procedure was similar for all five experiments and is illustrated in Fig. 1. After subjects looked inside an invisible circular boundary (6° diameter, centered on subject's eye level) for 100 ms, a 0.6° red fixation circle appeared. After allowing subjects 200 ms to precisely locate the fixation circle, the target, a 1° diameter white circle, appeared and started moving through a circular pathway around the fixation circle at a random eccentricity between 8 and 10°. After a random time between 750 and 1,250 ms, the fixation circle disappeared, which served as a go cue for subjects to saccade to the moving target. In adapting phases, the detection of the saccade triggered either an inward or outward step of 2° of the moving target, depending on one of two possible contexts in a given experiment (see below). The target was visible for 400 ms after the saccade detection and was smoothly pursued by subjects except in experiment 4 in which the target was stopped following its intrasaccadic position step. The trial was terminated and excluded from further analysis, if subjects blinked or made a saccade during the fixation period. The online saccade detection corresponded to eye position crossing the invisible boundary 3° from fixation center.
Fig. 1.
Experimental design. A: schematic of a trial in adapting phases. The ring with dashed line in the first schematic screen shows the invisible boundary that subjects needed to fixate inside of for 100 ms for the black fixation ring to appear. After 200 ms further fixation the moving target appears; the black arrows show its movement direction. Fixation off, used as “Go cue,” at a random time between 750 and 1,250 ms. The target stepped 2° on saccade onset (pre- and postadapting phases did not contain this target step). Subjects usually made corrective saccades and finally pursued the target for 400 ms until it disappeared. B: examples of the 3 presaccadic contexts and their target steps on saccade onset. Experiment 1: direction was used as context; targets stepped outward in clockwise and inward in counterclockwise moving directions. Experiment 2: velocity was used as context; high velocity targets stepped outward and low velocity targets stepped backward. Experiment 3: shape and color were used as context; moving green squares stepped outward and moving red circles stepped inward. In all 3 experiments, the reverse conditions (the other combinations of step direction and contexts) were counterbalanced across subjects.
The fixation-off go-signal was timed to elicit saccades in a sector between 15 and 75° in the right upper quadrant. From pilot data, we found an average latency of 210 ms and used this timing and a random range of starting angles to try to approximately cover the 15–75 degree sector. We wanted to avoid the cardinal meridians in case these had special contextual value.
All experiments had four phases: baseline, miniblock adapting, random-switch adapting, and postadapting. The second and third phases contained intrasaccadic steps of the target (adapting phases). The first two phases had trials blocked into short runs of each context (random “miniblock” lengths between 3–9 trials), as used previously (Herman et al. 2009); the last two phases had each context randomly interleaved. The rationale for these trial structures was that before context-specific learning is established, if context switched every trial any learning of one context would be detrimental to performance on the next trial. Conversely, if context switched only very rarely its utility would also be reduced (nonspecific saccade adaptation would be effective in reducing error within these long blocks). These miniblocks may therefore facilitate development of contextual adaptation, which can then become robust enough to be maintained during random context switching.
Because rest intervals have been reported to facilitate contextual adaptation (Shelhamer et al. 2005), we gave subjects regular breaks: every 1 min after finishing the running miniblock (approximately every 22 trials depending on subject reaction time), we gave subjects a 5 s-long break, followed by an Eyelink drift correction before the experiment continued.
The five experiments used pairs of contexts defined by the motion direction, speed, or color and shape of the target.
Experiment 1: direction.
Clockwise moving targets stepped inward and counterclockwise moving targets stepped outward for eight subjects, with the reverse contingencies for the other eight subjects. Target speed was always 1/16 Hz (22.5°/s).
Experiment 2: speed of movement.
Low velocity moving targets (1/16 Hz) stepped inward, and high velocity moving targets (1/4 Hz) stepped outward for 16 subjects, with reverse contingencies for the other eight subjects. Target direction was always clockwise.
Experiment 3: color and shape.
The color and shape were used as a context in this experiment, for nine subjects, a 1° diameter red circle (luminance 14.1 cd/m2), stepped inward and a 1° side green square (luminance 18.1 cd/m2) stepped outward, and it was reversed for the other nine subjects. The target always moved clockwise, with speed of 1/16 Hz.
Experiment 4: direction and stop-on saccade.
As in experiment 1, but the target motion stopped on detection of saccade, eliminating postsaccadic contextual information. The intrasaccadic inward or outward step still occurred, with the target remaining visible for 400-ms postsaccade. Removing the target motion postsaccade removed the possibility that different pursuit movements could serve as motor contexts for differential adaptation, but left the possibility that different, unfulfilled pursuit planning before the saccade formed a context.
Experiment 5: high and low speeds become intermediate-on saccade.
As in experiment 2, but the target changed speed to 1/8 Hz upon saccade, as well as making its intrasaccadic position steps inward or outward. Similar to experiment 4, by equating all target velocities after the saccade, there were no pursuit movement differences that could form contexts for differential adaptation. However, different pursuit planning before the saccade might still have provided a context signal, in addition to the visual differences in target speed before the saccade.
Data Analysis
We present data with each trial normalized to their average baseline gain. This is because we are interested in gain changes relative to baseline and because faster target speeds lead to lower gain saccades (Azadi et al. 2012); thus, when context is defined by speed, we need to normalize for these differences, and we chose to normalize each context in case of other idiosyncratic baseline differences in individual subjects.
Saccades were detected offline with minimum eye velocity and acceleration criteria of 75°/s and 2,500°/s2. Start and end points were marked when velocity fell below 15°/s.
The first saccade amplitude, after the go signal, was used to calculate saccadic gain, based on the target eccentricity. Gains <0.4 were excluded from future analyzes (<2% of trials).
Statistics
We used bootstrapping methods (resampling with replacement 100,000 times) to estimate all the statistical parameters such as mean, SE, and 95% confidence intervals (CIs; Efron 1979), as well as for performing hypothesis testing.
Benjamini-Hochberg procedure.
For multiple comparisons, Benjamini-Hochberg is a statistical method to control false discovery rate, the proportion of incorrectly rejected null hypothesis (type I error), by adjusting p-values (Benjamini and Hochberg 1995). Moreover, this method is useful to adjust false covering range, the average of false coverage in selected intervals (Benjamini and Yekutieli 2005).
We used the following false covering range algorithm to adjust P values and CIs in multiple comparisons:
1) Sort the P values used for testing the m hypotheses regarding the parameters, P(1) ≤ P(2) ≤…≤ P(m), where m is the number of P values. For example in experiment1, we had 4 phases and 16 subjects, making m = 64 P values for comparing saccadic gain in each phase in each subject.
2) Calculate R = max{i: P(i) ≤ i·q/m}: find R, which is the rank of the highest P value that satisfies the above inequality; i is the rank of the P value (from 1 to m) in the sorted set of P values; q is a fixed value between 0 and 1, and m is the total number of P values. Various values of q have been used in the literature with q = 0.1 a frequent choice. For a more conservative outcome, we used q = α = 0.05.
3) H0(i) is rejected when P(i) ≤ R· q/m.
4) Construct new marginal CIs with confidence level of [1 − R·q/m], instead of [1 − α] for each parameter selected.
RESULTS
All five experiments involved adaptation of the initial saccades towards circularly moving targets, with the moving target stepping to a nearer (gain-decreasing) or further (gain-increasing) eccentricity depending on contextual cues present before the saccade. The greater potency of gain-decreasing adaptation, in general, led to downward adaptation, but, with the exception of experiment 3 (color and shape cues), all experiments showed a strongly significant, gradual buildup in differential adaptation states between the pair of interleaved contexts.
Experiment 1: Direction Context
Different target motion directions can serve as contexts to elicit saccades of different gains for the same saccade vector. The Cartesian landing positions of the two contexts from the first adapting phase (“miniblock” adapting phase) are shown for a representative subject (Fig. 2A). Despite having the same initial target vector (black dashed line), gain-decreasing contexts (red points) had lower gains than their interleaved increasing contexts (blue points; P < 0.001). In this example the red points were from clockwise moving targets, and the blue from anticlockwise ones. The direction was unimportant, per se, and the opposite pairings of motion direction and intrasaccadic step perturbations were counterbalanced across subjects. There were no significant effects of the choice of pairings across experiments, and so all the data are pooled together throughout (i.e., we present gain-decreasing vs. gain-increasing contexts defined by, for example, direction but with the directions in each gain context pooled).
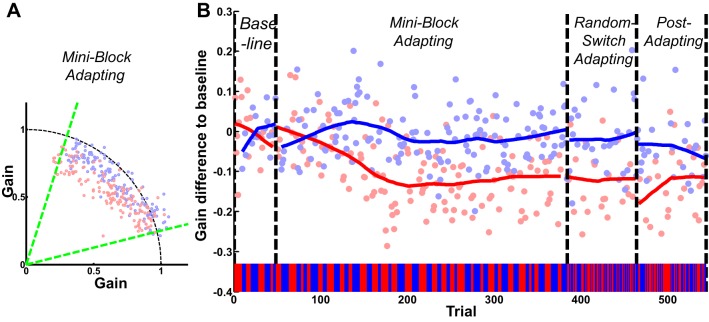
Fig. 2.
Representative subject from the motion direction context (experiment 1). Blue objects belong to gain increasing context and red objects belong to gain decreasing context. A: Cartesian saccade landing position in the miniblock adapting phase; circles indicate saccade landing position. Black dashed line indicates the target trajectory and the ideal landing position. Green dashed line indicates the overlapping data for both contexts. The nonoverlapping data were excluded from the further analysis. B: gain differences in each trial: x-axis indicates trial number; the color bar indicates type of context in each trial. In the 1st 2 phases, baseline and miniblock adapting phase, blocks of 3–9 trials in each context were used; therefore, context was not changed randomly. In contrast, context was changed randomly in the 2 last phases: random switch adapting and postadapting phase. Each circle indicates the gain difference to the baseline in a trial, which is the saccadic gain of the trial subtracted from the average gain for that context in the baseline phase. Solid lines are the moving average of gain differences to the baseline.
Orbital position effects, which can induce contextual adaptation, or direction-specific effects cannot explain this contextual gain dependence. Our saccade go-cue was timed to elicit saccades within a specific vector range (15–75°), and we excluded saccades beyond the overlapping range of each context vector range (green lines in Fig. 2A). Moreover, we summarize the lack of orbital effect in all the five experiments at the end of the results. Because of this lack of orbital or direction effect, we present the rest of our data in non-Cartesian form, using saccade amplitude/target eccentricity gains. We computed gain differences to the baseline phase (no intrasaccadic steps) in each context and gain differences between contexts. We also analyzed the correlation between target eccentricity, which was chosen randomly between 8 and 10°, and the saccadic gain, to rule out any possibility of target eccentricity providing an alternative context for adaptation. There were no significant correlations in any phase for any subject (P > 0.1 for all 312 Pearson correlation tests; 78 subjects × 4 phases).
The gain differences between contexts emerged gradually across the miniblock adapting phase, and the significant differences were maintained in the random switching adapt, and postadapt, phases. This can be seen in the representative subject (Fig. 2B; P < 0.001 in each phase), with the color bar along the bottom axis indicating the alternating contexts from the three- to nine-trial long miniblocks to completely random switching phase.
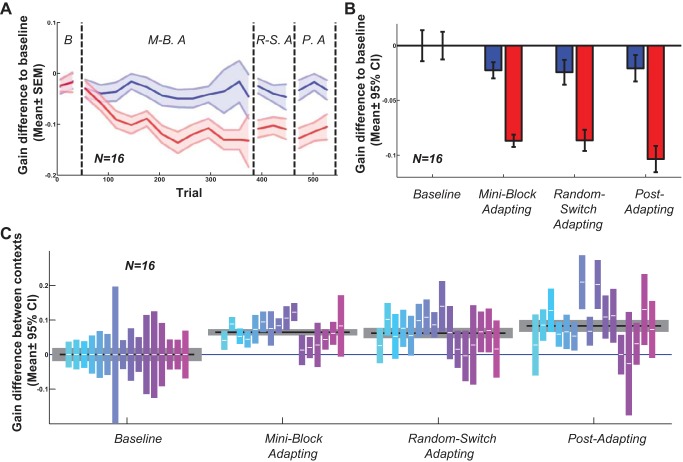
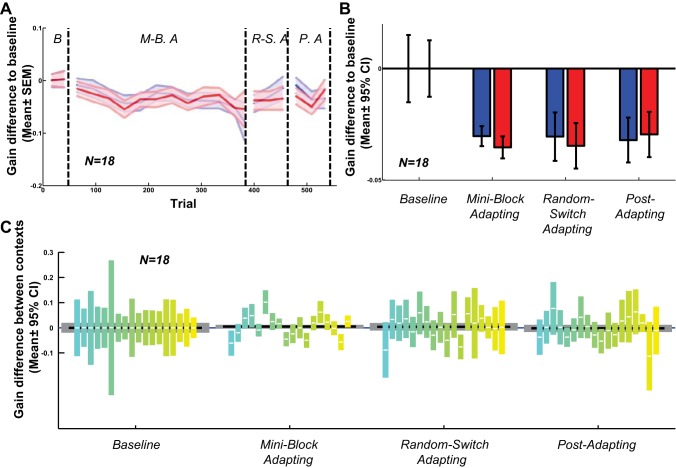
The group data from all 16 naive subjects showed the same gradual emergence of context specificity (Fig. 3). Gain decreased significantly in each phase on the interleaved step-back trials (red bars in Fig. 3B, P values: miniblock adapting phase: <0.001; random-switch adapting phase, <0.001; and postadapting phase, <0.001), but the alternate gain-increasing context did not change the saccadic gain statistically (blue bars; P values: miniblock adapting phase, 0.166; random-switch adapting phase, 0.203; and postadapting phase, 0.458). (Because we computed gain differences from the baseline phase for each subject, the baseline values average to 0 with 95% CIs as shown.) Next, we computed gain differences between each context in each subject and phase (Fig. 3C). Each color indicates the data from one subject. We used bootstrapping and the Benjamini-Hochberg multiple comparison procedure to construct 95% CIs (see methods). CIs not crossing the y = 0 line for individual subjects, or the group (gray boxes), indicate significant gain differences between contexts. For example, 12 out of 16 subjects had significant contextual differences in the miniblock adapting phase. At the group level, the motion-defined context was significant in all test phases (P values: miniblock adapting phase, <0.001; random-switch adapting phase, <0.001; and postadapting phase, <0.001).
Fig. 3.
Motion direction context (experiment 1), all subjects. A: moving average of gain difference to baseline in each trial for all 16 subjects. Blue lines indicate gain increasing context and red lines indicate gain decreasing context, shading indicates means ± SE. There is no difference between contexts in the 1st, baseline phase. The gains gradually separate between the 2 contexts from the beginning of the 2nd phase, which contains inward/outward target steps on saccade onset. The separation remains during the random-switching adapt (R.-S.A.) and even in the postadapt phase (P.A.). M.-B.A., miniblock adapting. B: difference between saccadic gains in each phase and the baseline phase; blue bars belong to the gain increasing context and red bars to the gain decreasing context. Error bars indicate 95% confidence interval. The gain increasing context did not change saccadic gains significantly, but the gain decreasing context induced gain decrease saccade adaptation. C: gain difference between 2 contexts in each phase (gain increase context minus gain decrease context); the gray bars indicate the difference across subjects with 95% confidence interval. The color bars indicate the difference between 2 contexts for each subject individually with 95% confidence interval, note that each bar, crossing 0 (blue line), is not significantly different from 0 with 95% confidence interval.
Simple differences in gains between contexts could arise without being strongly tied to the presaccadic visual cue. Because the contextual cue (clockwise or anticlockwise motion) was paired with an intrasaccadic step (onward or backward step), experience of a change in intrasaccadic step (or a change in predicted error) might itself act as a contextual cue (rather than the visual cue itself). Moreover, regardless of any contextuality, the reversed perturbation direction will change gain itself on trials after the transition. Both of these confounds would predict changes in gain on the second or later trial after context switch. Hence, as a stronger test of contextuality, we compared gains before and after context switches.
Gain differences occurred on the first trial of a switch between contexts. Our Miniblock adapting phase had runs of three to nine consecutive trials of the same context. Therefore, there were at least three trials before and after each switching point with the same context. Figure 4A shows saccadic gains around switching points for a typical subject in which “trial −1” is the last trial in one context and “trial +1” is the first trial in the other context. Solid lines show the switch from gain-decreasing to gain-increasing context, and dashed lines show the reverse switching. The gain changed immediately in the first trial after switching the context before any experience of the consequences of the switched context. Figure 4B plots the saccadic gain around the switching point in all 16 subjects in experiment 1, and the differences between gains before and after the context switch were statistically significant (P values: miniblock adapting phase, <0.001; random-switch adapting phase, <0.001; and postadapting phase, <0.001). Hence, the contextual adaptation was strongly tied to the presaccadic context.
Fig. 4.
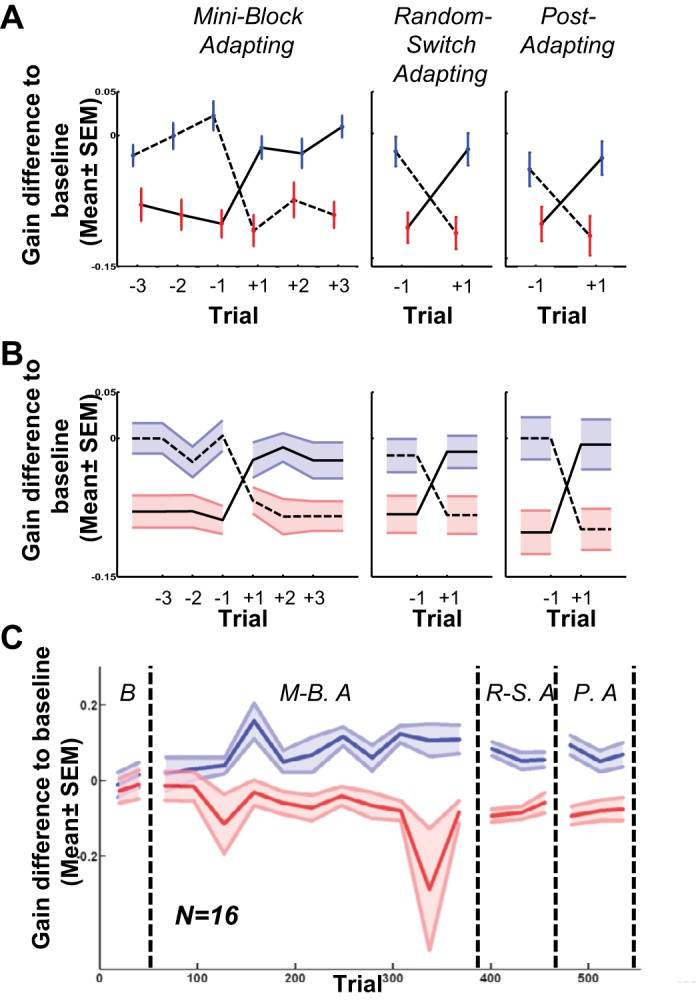
Analysis of the contextual switch transitions in the motion context, experiment 1. A: results from a typical subject. Solid lines indicate saccadic gains in context switching from gain decreasing to gain increasing context, dashed lines indicate context switching from gain increasing to gain decreasing context. Error bars indicate means ± SE, whereas blue indicates gain increasing context and red indicates gain decreasing context. Trial −1 indicates the last trial before context switching and +1 indicates the very 1st trial after context switching. In miniblock adapting phase, we used 3–9 consecutive trials in the same context; therefore, there are at least 3 trials before and after context switching point in each context. The saccadic gain in the very 1st trial after context switching (trial +1), is different from saccadic gains in the previous trials (trial −1, −2 and −3). B: results from all 16 subjects. Symbols as in A, with shading representing means ± SE. Similar to the typical subject, the saccadic gains in the 1st trial after context switching (trial +1) are different from the last trial in the other context (trial −1). P value in all phases across subjects <0.001. C: gain differences at the transition switches (+1 to −1 and vice versa) accumulate gradually over the experiment, and remain in random and postadapt phases.
This strong switch contextuality, like the overall contextual differences in Fig. 3, developed gradually (Fig. 4C) across the miniblock adapt phase and persisted into the random-adapt, and even postadapt, phases.
Experiment 2: Speed Context
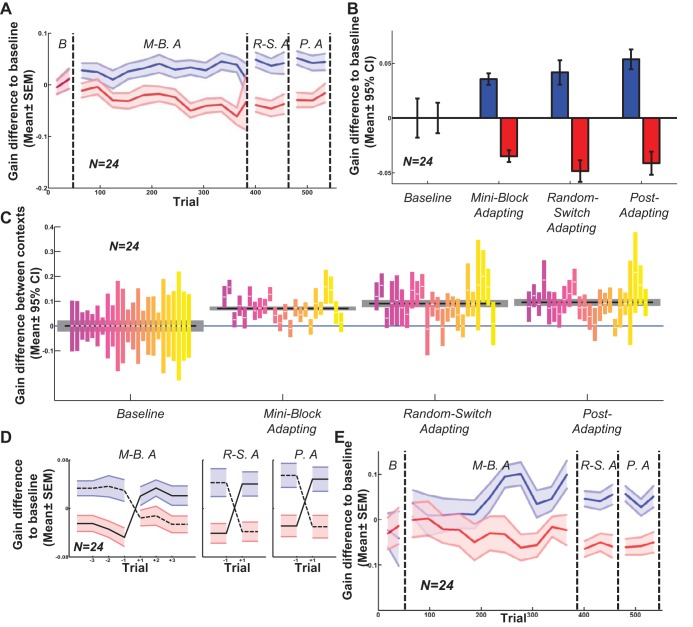
Different speeds of moving targets induce contextual saccade adaptation. This experiment was identical to the previous one, except that targets always moved clockwise and the second context was defined by a faster speed of rotation (1/4 Hz). A new group of 24 naive subjects participated. Similar to experiment 1, there was a gradual separation between saccadic gains in different contexts from the beginning of the miniblock adapting phase, which persisted in the random adapting and postadapt phases (Fig. 5A). However, unlike experiment 1, the contextual differences resulted from an approximately symmetrical change in gain in gain-decreasing vs. gain-increasing contexts (Fig. 5B). Each context was different from baseline in each phase at the P < 0.001 significance level. The gain differences between the two contexts were significantly different in 18 out of 24 subjects in the miniblock adapting phase (Fig. 5C). Again at the group level each phase had differences between contexts significant at the P < 0.001 level. Similar to experiment 1, the stronger contextual test of differences between gains before and after switching points were significant across subjects (Fig. 5D; P values: miniblock adapting phase, <0.001; random-switch adapting phase, <0.001; and postadapting phase: <0.001). Moreover, this contextual switch effect built up gradually over the experiment (Fig. 5E).
Fig. 5.
Context defined by target speed in clockwise direction (experiment 2). A: moving average of gain difference to baseline in each trial for all 24 subjects. Formatting schemes as in Figs. 3–4. Similar to experiment 1, there is no difference between contexts in the 1st, baseline phase, but contextual separation gradually emerges in the 2nd phase, remaining into the postadapting phase. B: difference between saccadic gains in each phase and baseline phase. Unlike experiment 1, both gain decreasing and gain increasing contexts changed significantly. C: gain difference between 2 contexts in each phase. D: saccadic gains in context switching points.; similar to experiment 1, the saccadic gain before and after context switching are significantly different (P value in all phases across subjects <0.001). E: context switch gains increase gradually over the 1st 200 trials.
The gain-increasing context might have been more apparent in this experiment due to lower baseline gains. Faster rotating targets elicit initial saccades of lower gains (Azadi et al 2012). We replicated that previous finding: baseline gains for the slow target were 0.91 compared with 0.86 for the faster target (P < 0.05). These were lower than the gains in the baseline for experiment 1 (0.93), which was also the slower target speed. These differences in baseline gains are masked by plotting gain changes from baseline in Fig. 5. Perhaps this lower baseline level allowed greater scope for increasing gain or the lower baseline level inhibited the gain decrease here compared with experiment 1 (i.e., floor effects). Possibly consistent with this, in experiment 5 below, which had identical conditions to experiment 2 before the saccade, the baseline gains were 0.93 for the fast speed and 0.98 for the slow speed, and the adaptation decreased in both contexts.
Experiment 3: Color and Shape
Different color and shape of moving targets did not induce contextual saccade adaptation. Contexts defined by clockwise, slow-moving targets (1/16 Hz) of different shapes and colors led to downward adaptation (Fig. 6, A and B; P < 0.001 for both contexts in all test phases), which was not different in the different contexts (P > 0.05 for all test phases). The downward adaptation is unsurprising given the predominance of gain-decreasing over gain-increasing perturbations (Pelisson et al. 2010). The lack of context specificity can be seen in the individual subject data, with only 1 out of 18 subjects showing a significant context effect in the random-switch adapting phase (Fig. 6C) and in the wrong direction.
Fig. 6.
Shape and color combination defined context (experiment 3). A: moving average of gain difference to baseline in each trial for all 18 subjects. Unlike experiment 1 and experiment 2, there is no difference between contexts in any phase and after starting saccade adaptation in miniblock phase; saccadic gains gradually decrease in both contexts similarly. B: difference between saccadic gains in each phase and baseline phase; unlike experiment 1 and experiment 2, saccadic gains in both contexts decrease in adapting phases and postadapting phase. C: gain difference between 2 contexts in each phase were neither significant at the group level nor for most of the individual subjects. Negative contextual gain differences are opposite to the intrasaccadic step contexts.
Experiment 4: No Postsaccadic Motion
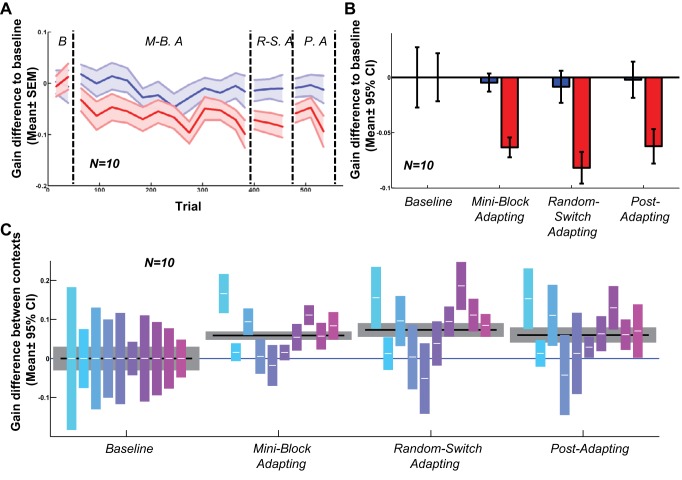
Contexts defined by different motion direction only before the saccade, with target motion stopped upon saccade, induced robust contextual adaptation. The results were entirely compatible with those from the direction context in experiment 1: gains gradually separated over the course of the experiment (Fig. 7A); the gain-decreasing direction adapted downwards significantly (Fig. 7B; P < 0.001 in all test phases), while the gain-increasing direction did not (P > 0.05 in all test phases); the contextual differences were highly significant (P < 0.001) in all test phases (Fig. 7C).
Fig. 7.
Motion direction context defined only before the saccade with target stopped on saccade (experiment 4). A: moving average of gain difference to baseline in each trial for all 10 subjects. B: difference between saccadic gains in each phase and baseline phase; eliminating postsaccadic contextual information did not stop robust contextual adaptation in all phases. C: gain difference between 2 contexts in each.
Experiment 5: Intermediate Postsaccadic Motion
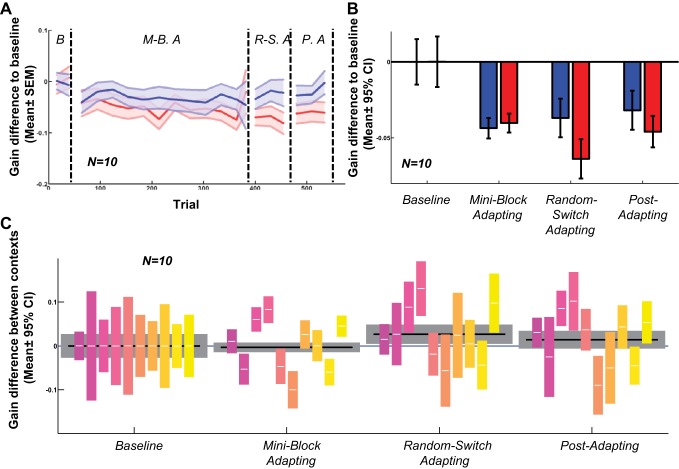
Contexts defined by different clockwise motion speeds only before the saccade, with an intermediate speed of target motion upon saccade, induced modest contextual adaptation. The conditions before the saccade were identical to those of experiment 2, albeit in a new, smaller group of subjects. Unlike experiment 2, gains in both contexts gradually decreased over the course of the experiment (Fig. 8, A and B; P < 0.001 in all test phases), with slowly emerging and weaker contextual differences. There was considerable intersubject variability throughout, with overall no significant contextual effect during the miniblock adapting and postadapt phases (Fig. 8C). However, the gradual accumulation of contextual difference at the group level did reach significance in the final adaptation phase (random-switch adapt, Fig. 8C; P < 0.05).
Fig. 8.
Motion velocity context defined only before the saccade with target speed changing to intermediate value upon saccade (experiment 5). A: moving average of gain difference to baseline in each trial for all 10 subjects. B: difference between saccadic gains in each phase and baseline phase. C: gain difference between 2 contexts in each phase. All formatting schemes are as in Figs. 3–7.
Comparison Across Experiments
Our consistent design allowed us to compare the efficacy of contextual cues across the five experiments (Fig. 9). We chose to compare the random-adapting switch phases, since these were at the end of the adaptation session and random-switching requires inherently more context specificity than blocked-switching. Speed and direction were similarly strong context cues, but speed was significantly more effective (P < 0.05). On the one hand, postsaccade contexts were not necessary, since removing the context after the saccade did not reduce the adaptation in experiment 4 compared with experiment 1 (P = 0.47). On the other hand, providing intermediate postsaccadic context that conflicted with the presaccadic contexts greatly reduced the contextual adaptation in experiment 5 compared with experiment 2 (P < 0.001).
Fig. 9.
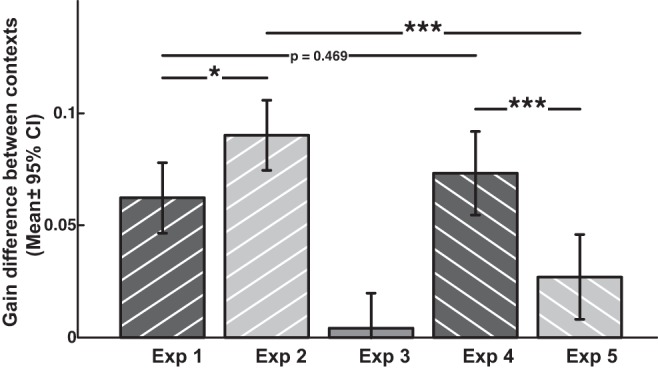
Comparing contextual differences between the 5 experiments. Note there are no significant differences between experiments 1 and 4 (direction contexts), but there is significantly less adaptation in experiments 5 vs. 2 (speed contexts), despite the same presaccadic conditions in both these pairs (NB: different subjects used in each experiment). *P < 0.05, ***P < 0.001.
Saccade Angles
There was no relationship between the contextual differences that we found in saccade gain and small differences in saccade angles between contexts. We designed the timing of our go-signal to try to produce overlapping saccade vectors between contexts and restricted our analyses to overlapping saccade vectors (see above). However, if there had been consistent differences in saccade angular vectors leftover between contexts (e.g., clockwise motion producing saccades clustered more clockwise than anticlockwise moving targets), these might have allowed for direction-specific adaptation (Frens and van Opstal 1997) or even contextual adaptation based on postsaccadic orbital position, as opposed to the visual contexts we were testing. As can be seen in Fig. 10, the magnitudes of angular differences in the two contexts were typically <5°, and there was no relationship between any angular differences and gain differences between contexts in all five experiments (correlation coefficients, r = −0.27, 0.17, −0.12. 0.20, and −0.47 with P values of 0.31, 0.42, 0 .65, 0.58, and 0.17, respectively).
Fig. 10.
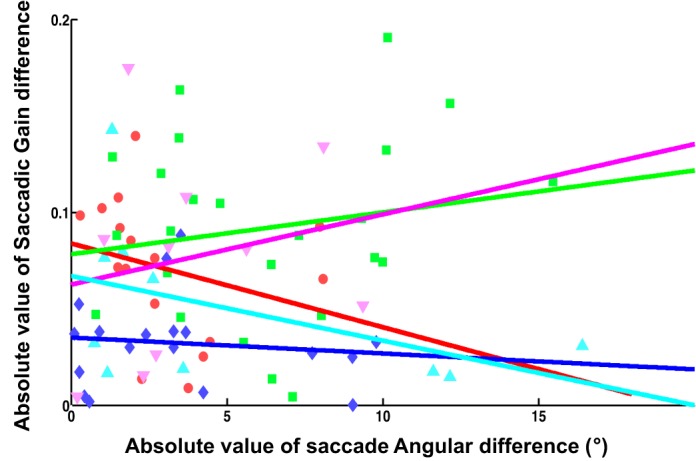
Lack of relationship between saccadic gain difference and saccadic angular difference in random-switching adapting phase in all 5 experiments. Each symbol indicates data from a specific subject in phase 3. Blue, green, red, magenta, and cyan indicate experiments 1, 2, 3, 4, and 5. Angular difference is the difference in the mean vectorial angle in each context.
DISCUSSION
Our results show that some, but not all, visual cues before a saccade are sufficient to induce context-specific saccade adaptation. We had subjects make saccades to targets moving smoothly on a circular trajectory around the fixation point, and upon saccade we increased or decreased the eccentricity of the circular motion on any given trial depending on one of two interleaved visual contexts. Visual contexts tested were motion direction (clockwise vs. counter-clockwise), motion speed (22.5 vs. 90°/s), or target color and shape (red circle vs. green square). Saccade amplitudes adapted gradually to separate gain values for the alternative contexts when direction (Fig. 3) or speed were the visual cues (Fig. 5) but not for the color and shape cues (Fig. 6).
We use the term “visual cue” to emphasize the distinction between contexts based on motor states (as tested in most earlier context-specific saccade adaptation reports, see below) and those based on visual contexts. Here, because the motor state was the same (fixation at the same location) when the contexts were defined, and the saccade vector was equated in all conditions, and without orbital position biases (Fig. 10), the different target properties provided visual contexts. To further underscore the visual nature of the context, we showed that a postsaccade movement context was unnecessary to generate contextual adaptation by stopping the targets moving upon saccade (Fig. 7). Similarly, removing the contextual differences postsaccade by having a neutral, intermediate speed after the movement, again resulted in distinct adaptations (Fig. 8), also showing that the visual cue before the saccade was the key to establishing the contextual adaptation. This work resolves previous conflicts in the literature, giving support to saccade adaptation being susceptible to visual cues, while placing limits on the types of those cues.
Comparison to Previous Studies
Most context specificities explored in saccade adaptation have consisted of different motor states. Arguably the strongest motor context is saccade direction, although this type of vector specificity is usually not labeled as “contextual.” However, clearly to compensate for damage to individual muscles or groups of motoneurons and so on, saccade adaptation has to be highly contextual to direction and amplitude vectors, as is well established (Pelisson et al. 2010). There seems little difference, in principle, between vector specificity and motor contexts defined by orbital position. The orbital dependency of eye muscle strength (Collins et al. 1975) requires different sets of saccadic commands for different orbital starting positions, making strong orbital-specific adaptation readily explicable (Alahyane and Pelisson 2004; Shelhamer and Clendaniel 2002; Tian and Zee 2010; Zimmermann and Lappe 2011; Havermann et al. 2011; Wulff et al. 2012). Similarly, we redirect our gaze in combined eye and head movements, and hence motor states incorporating head position signals such as head tilt (Shelhamer and Clendaniel 2002) would also be expected to show context specificity.
Understanding contextual adaptation to visual cues is more challenging. The presumed function of saccade adaptation is typically as a pure recalibration mechanism, and why might this be interested in visual properties of targets? Previous studies testing visual properties such as shape and color (Deubel 1995) or shape alone (Bahcall and Kowler 2000) have not found significant evidence of contextual saccade adaptation. Our lack of contextuality from color and shape cues (experiment 3) are, thus, entirely consistent with these previous reports, as well as the impotence of color and shape in contextual adaptation of smooth pursuit (Takagi et al. 2000), and arm movement (Howard et al. 2013).
In contrast, our findings that visual cues can evoke contextual adaptation (experiments 1, 2, 4, and 5) are consistent with our previous report in which we found context effects to flickering vs. nonflickering targets (Herman et al. 2009), as well as recent evidence from arm movement adaptation (Howard et al. 2013). Howard et al. tested the ability of subjects to simultaneously learn two oppositely directed force field perturbations for the same movement vectors. Two visual contexts led to distinct adaptations: displacement offsets of the projected hand cursor position to the left or right, and, similarly, a disc oriented to the left or right of the hand cursor (to simulate a hand-held object). Arbitrary motion cues that were spatially disconnected from the task, and background or cursor colors had no contextual effect. The implication of our current work, and that of Howard et al., is that spatially relevant visual cues (e.g., motion direction and speed of our pursuit targets, or the projected spatial location of arm movement) are much more effective in eliciting contextual adaptation than feature-based cues disconnected to the movement plan.
In our case the movement plan was a coordinated saccade and pursuit plan, which may be important in at least three ways. First, to an isolated saccade system our cues were purely visual (see above), but if we assume a representation of the motion signals in the motor response of the saccade, then the contextual adaptation could be thought of as motor or visuomotor specific. Similarly, different planned pursuit states might have provided a smooth movement context for differential adaptation, regardless of whether they were enacted or cancelled as in experiment 4. Second, a natural use of contextual adaptation might be in incorporating visual motion information to coordinate saccadic and smooth eye movements without necessarily impairing saccades to stationary objects. This conjecture may be plausible given that reflexive saccades can be adapted without affecting voluntary saccades to stationary targets (Pelisson et al. 2010). Future work could test the transfer between our contextual adaptation and voluntary saccades. Third, a similar but more specific proposal is that our contextual adaptation compensates for the recently discovered effect of rotation speed on saccade gain (Azadi et al. 2012). In that work using an essentially identical, but nonadaptation, task, saccade gain decreased systematically with increasing rotation velocity (e.g., to 0.66 at 1 HZ). We found the same trend with the two speeds used here in experiments 2 and 5. If our contextual adaptation helped to compensate for speed-related gain differences, it might explain why speed was a more effective context than direction (Fig. 9, experiment 2 > experiment 1). Baseline gain differences related to rotation speed might also explain some differences in adaptation symmetry between gain-increasing and gain-decreasing contexts, due to floor or ceiling effects (see results).
Bayesian Switching and Integration of Sensory Predictions
There is increasing evidence that saccade adaptation uses sensory prediction errors as its key error signal (Bahcall and Kowler 2000; Chen-Harris et al. 2008; Ethier et al. 2008; Wong and Shelhamer 2011; Collins and Wallman 2012; Herman et al. 2013b), rather than simple sensory (retinal) error (Wallman and Fuchs 1998; Noto and Robinson 2001). In the parlance of internal model theories, a “forward model” estimates the predicted sensory error of a movement, while an “inverse model” translates the desired movement goal into the necessary motor commands to control the dynamics of the eye and muscle plant. Pairs of forward and inverse models are thought to integrate sensory prediction errors into updating one's motor state in a particular context. Switching between contexts is seen as switching between pairs of models based on estimates of the best context pair to optimize task performance (Wolpert et al. 1998; Vetter and Wolpert 2000; Körding and Wolpert 2006). The updating of context and motor models are believed to be based on Bayesian inference about the environmental or motor state, respectively.
Within this general theoretical framework, our observed failure of color and shape as contexts for saccade adaptation can be seen as the system having a very low, or nonexistent, prior for these features; conversely, making saccades to moving targets would have a strong existing prior for using motion direction and speed information in nonadaptation situations (Gellman and Carl 1991; de Brouwer et al. 2002) and thus may be able to incorporate this information more readily into context-specific adaptations. Indeed, this was a rationale for our choice of task (see Introduction). Importantly, our data are not simple extrapolations of motion information, because our perturbation was orthogonal to the motion path and the development of this contextual prediction gradually accumulated in time (Figs. 4C and 5E). This gradual learning of the contextual switch from zero-change to a strong immediate adaptation on the first trial in a new context is what would be expected from the Bayesian learning of environmental state argument above. Note that most previous contextual saccade adaptation studies have used longer blocks of one context and not analyzed the contextual transitions themselves.
Finally, from the viewpoint of a trial-by-trial Bayesian updating of contextual predictions (learning about the environment state not the motor state, per se), one would expect that changing the context across the movement would interfere with the accumulation of contextual learning. We found a strong reduction in the contextual adaptation in experiment 5, in which the speed-defined context before the saccade was replaced with a neutral, intermediate speed context after the saccade (Fig. 9). When there was no conflict, because the motion context was removed upon saccade (experiment 4), there was no reduction in contextual adaptation. Note that the change in speed across the saccade might also cause noncontextual adaptation (Havermann et al. 2012), which could interfere with or add variability to the contextual adaptation.
Can Feature-Based Contextual Cues Be Trained?
Although spatially relevant visual cues for movement planning are more effective for contextual adaptation, extensive training of feature-based cues has led to contextual learning in arm movement adaptation (Krouchev and Kalaska 2003; Wada et al. 2003). Low prior probabilities of a context being utilized may simply require more exposure to evoke significant contextual adaptation. For example, the arbitrary motion cue used by Howard et al. (2013) began to induce some contextuality towards the end of their experiment, even though it was not significant over the experiment as a whole. In humans, the only repeat-exposure data presented in contextual saccade adaptation showed the single subject increase their contextual difference in gains to different shaped targets, but it did not reach significance (Bahcall and Kowler 2000). In monkeys, the only repeat-exposure data in contextual saccade adaptation showed a gradual significant increase over three sessions in the one monkey tested (Tian and Zee 2010).
The subject of repeat exposure and retention during conventional saccade adaptation has been explored (Alahyane and Pelisson 2005; Robinson et al. 2006; Mueller et al. 2012), and a systematic study in contextual saccade adaptation would be interesting. Anecdotal evidence from our laboratory suggests that there may be considerable buildup in one's contextual adaptability over several months. Our previous finding that purely visual cues (flickering vs. nonflickering targets) induced robust contextual adaptation (Herman et al. 2009) has proven difficult to replicate more recently. Regardless of their naiveté of the experimental details, many of the experienced subjects used in the earlier work had participated in multiple pilot versions of the contextual paradigm. Novelties introduced in that work included training “miniblocks” before random-switching phases, and different lengths of miniblock have been explored over different sessions. In motor adaptation it has been shown that frequent and random switching between contexts increases the learning (Osu et al. 2004; Wada et al. 2003), as perhaps does reduced movement dimensionality (Addou et al. 2011). This repeat exposure to random switching of contextual adaptation may have increased our previous subjects' prior likelihood of responding to purely visual cues. In the current set of experiments, we chose to use a large number of complete novices to oculomotor experiments (78 undergraduates), and, although there is natural intersubject variability in the adaptation, our contextual effects cannot be due to training.
Possible Pathways
The inefficacy of our color cues to elicit differential adaptation states implies that contexts in our paradigm are mediated by the magnocellular system. However, our targets contained luminance as well as chromatic contrast (luminance contrasts >12%). If these suprathreshold luminance contrasts were effectively “acontextual” in not providing cues as to the different contexts, one might argue that the achromatic and acontextual information dominated weaker parvocellular contextual information because motion processing is more effective for luminance contrast (e.g., Braun et al. 2008). If we had used isoluminant color stimuli to define the alternate contexts in experiment 3, it might have unmasked a weak parvocellular contribution to contextual saccade adaptation.
Conclusions
In summary, we have found strong evidence of contextual adaptation in saccades to moving targets. Visual cues that are spatially relevant to coordinated movement planning between saccades and pursuit movements are much more effective in eliciting differential adaptation than feature-based cues such as color and shape. While contextual cues before movement are sufficient for adaptation, the present study leaves open whether these cues are purely visual or include cancelled pursuit planning. We have shown for the first time that the postsaccade contextual information can interfere with this adaptation.
GRANTS
This research was funded by National Science Foundation Grant BCS-0842464 and, in part, by National Institutes of Health Grants 1R01-EY-019508, 2G12-RR-03060-26A1, and 8G12-MD-007603-27.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: R.A. and M.R.H. conception and design of research; R.A. performed experiments; R.A. analyzed data; R.A. and M.R.H. interpreted results of experiments; R.A. prepared figures; R.A. and M.R.H. drafted manuscript; R.A. and M.R.H. edited and revised manuscript; R.A. and M.R.H. approved final version of manuscript.
REFERENCES
- Addou T, Krouchev N, Kalaska JF. Colored context cues can facilitate the ability to learn and to switch between multiple dynamical force fields. J Neurophysiol 106: 163–183, 2011 [DOI] [PubMed] [Google Scholar]
- Alahyane N, Pélisson D. Eye position specificity of saccadic adaptation. Invest Ophthalmol Vis Sci 45: 123–130, 2004 [DOI] [PubMed] [Google Scholar]
- Alahyane N, Pélisson D. Long-lasting modifications of saccadic eye movements following adaptation induced in the double-step target paradigm. Learn Mem 12: 43–443, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azadi R, Holcombe A, Edelman J. Dysmetric saccades to targets moving in predictable but nonlinear trajectories. J Vis 12: 409, 2012 [Google Scholar]
- Bahcall DO, Kowler E. The control of saccadic adaptation: implications for the scanning of natural visual scenes. Vision Res 40: 2779–2796, 2000 [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Royal Stat Soc Series B 57: 289–300, 1995 [Google Scholar]
- Benjamini Y, Yekutieli D. False discovery rate-adjusted multiple confidence intervals for selected parameters. J Am Stat Assoc 100: 71–81, 2005 [Google Scholar]
- Brainard DH. The Psychophysics Toolbox. Spat Vis 10: 433–436, 1997 [PubMed] [Google Scholar]
- Braun DI, Mennie N, Rasche C, Schutz AC, Hawken MJ, Gegenfurtner KR. Smooth pursuit eye movements to isoluminant targets. J Neurophysiol 100: 1287–1300, 2008 [DOI] [PubMed] [Google Scholar]
- Chaturvedi V, van Gisbergen JA. Specificity of saccadic adaptation in three-dimensional space. Vision Res 37: 1367–1382, 1997 [DOI] [PubMed] [Google Scholar]
- Chen-Harris H, Joiner WM, Ethier V, Zee DS, Shadmehr R. Adaptive control of saccades via internal feedback. J Neurosci 28: 2804–2813, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins CC, O'Meara D, Scott AB. Muscle tension during unrestrained human eye movements. J Physiol 245: 351–369, 1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins T, Wallman J. The relative importance of retinal error and prediction in saccadic adaptation. J Neurophysiol 107: 3342–3348, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Brouwer S, Missal M, Barnes G, Lefèvre P. Quantitative analysis of catch-up saccades during sustained pursuit. J Neurol 87: 1772–1780, 2002 [DOI] [PubMed] [Google Scholar]
- Deubel H. Separate adaptive mechanisms for the control of reactive and volitional saccadic eye movements. Vision Res 35: 3529–3540, 1995 [DOI] [PubMed] [Google Scholar]
- Efron B. Bootstrap methods: another look at the jackknife. Ann Stat 7: 1–26, 1979 [Google Scholar]
- Ethier V, Zee DS, Shadmehr R. Changes in control of saccades during gain adaptation. J Neurosci 28: 13929–13937, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frens MA, Van Opstal AJ. Monkey superior colliculus activity during short-term saccadic adaptation. Brain Res Bull 43: 473–483, 1997 [DOI] [PubMed] [Google Scholar]
- Gellman R, Carl J. Motion processing for saccadic eye movements in humans. Exp Brain Res 84: 660–667, 1991 [DOI] [PubMed] [Google Scholar]
- Havermann K, Volcic R, Lappe M. Saccadic adaptation to moving targets. PLoS One 7: e39708, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havermann K, Zimmermann E, Lappe M. Eye position effects in saccadic adaptation. J Neurophysiol 106: 2536–2545, 2011 [DOI] [PubMed] [Google Scholar]
- Herman JP, Blangero A, Madelain L, Khan A, Harwood MR. Saccade adaptation as a model of flexible and general motor learning. Exp Eye Res 114: 6–15, 2013a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP, Cloud CP, Wallman J. End-point variability is not noise in saccade adaptation. PLoS One 8: e59731, 2013b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP, Harwood MR, Wallman J. Saccade adaptation specific to visual context. J Neurophysiol 101: 1713–1721, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopp JJ, Fuchs AF. The characteristics and neuronal substrate of saccadic eye movement plasticity. Progr Neurobiol 72: 27–53, 2004 [DOI] [PubMed] [Google Scholar]
- Howard IS, Wolpert DM, Franklin DW. The effect of contextual cues on the encoding of motor memories. J Neurophysiol 109: 2632–2644, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner WM, Smith MA. Long-term retention explained by a model of short-term learning in the adaptive control of reaching. J Neurophysiol 100: 2948–2955, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kording KP, Tenenbaum JB, Shadmehr R. The dynamics of memory as a consequence of optimal adaptation to a changing body. Nat Neurosci 10: 779–786, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Körding KP, Wolpert DM. Bayesian decision theory in sensorimotor control. Trends Cogn Sci 10: 319–326, 2006 [DOI] [PubMed] [Google Scholar]
- Krouchev NI, Kalaska JF. Context-dependent anticipation of different task dynamics: rapid recall of appropriate motor skills using visual cues. J Neurophysiol 89: 1165–1175, 2003 [DOI] [PubMed] [Google Scholar]
- Mon-Williams M, Murray AH. The size of the visual size cue used for programming manipulative forces during precision grip. Exp Brain Res 135: 405–410, 2000 [DOI] [PubMed] [Google Scholar]
- Mueller AL, Davis AJ, Robinson FR. Long term size-increasing adaptation of saccades in macaques. Neuroscience 224: 38–47, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noto CT, Robinson FR. Visual error is the stimulus for saccade gain adaptation. Brain Res Cogn Brain Res 12: 301–305, 2001 [DOI] [PubMed] [Google Scholar]
- Osu R, Hirai S, Yoshioka T, Kawato M. Random presentation enables subjects to adapt to two opposing forces on the hand. Nat Neurosci 7: 111–112, 2004 [DOI] [PubMed] [Google Scholar]
- Pelisson D, Alahyane N, Panouilleres M, Tilikete C. Sensorimotor adaptation of saccadic eye movements. Neurosci Biobehav Rev 34: 1103–1120, 2010 [DOI] [PubMed] [Google Scholar]
- Pelli DG. The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spat Vis 10: 437–442, 1997 [PubMed] [Google Scholar]
- Robinson FR, Soetedjo R, Noto C. Distinct short-term and long-term adaptation to reduce saccade size in monkey. J Neurophysiol 96: 1030–1041, 2006 [DOI] [PubMed] [Google Scholar]
- Schutz AC, Souto D. Adaptation of catch-up saccades during the initiation of smooth pursuit eye movements. Exp Brain Res 209: 537–549, 2011 [DOI] [PubMed] [Google Scholar]
- Shelhamer M, Aboukhalil A, Clendaniel R. Context-specific adaptation of saccade gain is enhanced with rest intervals between changes in context state. Ann NY Acad Sci 1039: 166–175, 2005 [DOI] [PubMed] [Google Scholar]
- Shelhamer M, Clendaniel RA. Context-specific adaptation of saccade gain. Exp Brain Res 146: 441–450, 2002 [DOI] [PubMed] [Google Scholar]
- Takagi M, Abe H, Hasegawa S, Usui T, Hasebe H, Miki A, Zee DS. Context-specific adaptation of pursuit initiation in humans. Invest Ophthalmol Vis Sci 41: 3763–3769, 2000 [PubMed] [Google Scholar]
- Tian J, Zee DS. Context-specific saccadic adaptation in monkeys. Vision Res 50: 2403–2410, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter P, Wolpert DM. Context estimation for sensorimotor control. J Neurophysiol 84: 1026–1034, 2000 [DOI] [PubMed] [Google Scholar]
- Wada Y, Kawabata Y, Kotosaka S, Yamamoto K, Kitazawa S, Kawato M. Acquisition and contextual switching of multiple internal models for different viscous force fields. Neurosci Res 46: 319–331, 2003 [DOI] [PubMed] [Google Scholar]
- Wallman J, Fuchs AF. Saccadic gain modification: visual error drives motor adaptation. J Neurophysiol 80: 2405–2416, 1998 [DOI] [PubMed] [Google Scholar]
- Wing AM, Lederman SJ. Anticipating load torques produced by voluntary movements. J Exp Psychol 24: 1571–1581, 1998 [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Miall RC, Kawato M. Internal models in the cerebellum. Trends Cogn Sci 2: 338–347, 1998 [DOI] [PubMed] [Google Scholar]
- Wong AL, Shelhamer M. Sensorimotor adaptation error signals are derived from realistic predictions of movement outcomes. J Neurophysiol 105: 1130–1140, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulff S, Bosco A, Havermann K, Placenti G, Fattori P, Lappe M. Eye position effects in saccadic adaptation in macaque monkeys. J Neurophysiol 108: 2819–2826, 2012 [DOI] [PubMed] [Google Scholar]
- Zimmermann E, Lappe M. Eye position effects in oculomotor plasticity and visual localization. J Neurosci 31: 7341–7348, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]