Abstract
Purpose
To expand our predictive models for progression to advanced stages of age-related macular degeneration (AMD) based on demographic, environmental, genetic, and ocular factors, using longer follow-up, time varying analyses, calculation of absolute risks, adjustment for competing risks, and detailed baseline AMD and drusen status.
Design
Prospective, longitudinal study.
Participants
We included 2937 individuals in the Age-Related Eye Disease Study, of which 819 subjects progressed to advanced AMD during 12 years of follow-up.
Methods
Cox proportional hazards regression analyses were performed to calculate hazard ratios for progression. Covariates included demographic and environmental factors, 6 variants in 5 genes, baseline macular drusen size, and presence and type of advanced AMD in 1 eye at baseline. To assess the ability of risk scores based on all covariates to discriminate between progressors and nonprogressors, an algorithm was developed and the area under the receiver operating characteristic curve (AUC) was calculated. To validate the overall model, the total sample was randomly subdivided into derivation and test samples. Another model was built based on the derivation sample and assessed for calibration and discrimination in the test sample. Sample sizes needed for testing new treatments in clinical trials were estimated based on models with and without genetic variables.
Main Outcome Measures
Progression to advanced AMD, including geographic atrophy and neovascular disease.
Results
In multivariate models, age, smoking, body mass index, single nucleotide polymorphisms in the CFH, ARMS2/HTRA1, C3, C2, and CFB genes, as well as presence of advanced AMD in 1 eye and drusen size in both eyes were all independently associated with progression. The AUC for progression at 10 years in the model with genetic factors, drusen size, and environmental covariates was 0.915 in the total sample. In the test sample, based on a model estimated from the derivation sample, the AUC was 0.908. The sample sizes needed for clinical trials were estimated to be lower when genetic susceptibility was considered.
Conclusions
Factors reflective of nature and nurture were incorporated into an expanded algorithm for risk prediction, which performed very well in both derivation and test samples. Risk scores and predicted progression rates will be useful for AMD surveillance and for designing clinical trials.
Financial Disclosure(s)
Proprietary or commercial disclosure may be found after the references.
Age-related macular degeneration (AMD) is the leading cause of visual loss among the elderly. The 2 advanced forms, geographic atrophy (GA) and neovascular disease (NV) can cause irreversible blindness.1,2 Several genetic variants as well as modifiable factors including smoking, lower intake of dietary antioxidants and omega-3 fatty acids, and higher body mass index (BMI) are known to be associated with higher rates of progression from the early to advanced stages of AMD.1–8 Characteristics of macular drusen, which are deposits under the retinal pigment epithelium and the clinical hallmark of the early stages of AMD, are also associated with progression to advanced AMD.5,9 We previously created risk prediction models based on demographic, environmental, and genetic factors that can predict the occurrence of AMD and its progression from early and intermediate stages to the advanced forms.10–13 Herein, we report new information and expand upon our previous models in the following ways: (1) considering progression in both eyes, (2) incorporating the baseline macular phenotypes of drusen size and presence of advanced AMD in 1 eye and status of the fellow eye, (3) accounting for time varying rates of progression by using AMD grades at all follow-up visits, (4) extending the follow-up period to 12 years, and (5) including a greater number of participants. We also calculate absolute risk for individuals given a specific set of demographic, ocular, and genetic risk factors, adjusting for competing risks according to age and gender of the subject, and validate our model in a test sample that differed from the sample used to derive the model. Furthermore, we estimate sample sizes needed for clinical trials based on use of these models. Results of our expanded risk score models could be useful for targeting high risk individuals for lifestyle changes that reduce risk of AMD progression. We also show that these risk models could help to identify subjects for participation in clinical trials involving new treatments.
Methods
Phenotype and Progression Data
The Age-Related Eye Disease Study (AREDS) included a randomized, clinical trial to assess the effect of antioxidant and mineral supplements on risk of AMD and cataract, and a longitudinal study of AMD that ended in December 2005. Study procedures have been previously reported.9 Based on ocular examination and photographic grading of fundus photographs, participants were defined at baseline as AREDS category 1 in both eyes (essentially free of age-related macular abnormalities), category 2 in the worse eye (mild changes including multiple small drusen, nonextensive intermediate drusen, and/or pigment abnormalities), category 3 in the worse eye (≥1 large drusen of ≥125 micron in diameter, extensive intermediate drusen, and/or non-central GA), category 4 in 1 eye (advanced AMD, either neovascular or central GA, or visual loss owing to AMD regardless of phenotype), or category 4 in both eyes. Non-Caucasians were excluded from these analyses because the distribution of advanced AMD in that population differs considerably compared with Caucasians.1 Because group 3 patients in the original AREDS classification included non-central GA and group 4 included both advanced forms of AMD as well as visual loss regardless of phenotype,9 we reclassified these groups independent of visual acuity level into grades 4 and 5, with grade 4 including both non-central and central GA, and grade 5 including NV, using the Clinical Age-Related Maculopathy Grading System.14
Maximum drusen size within the grid (a 3000-micron [μm] radius centered on the fovea) at baseline was used to assess drusen phenotypes for eyes without advanced AMD. Drusen size was based on standard circles with diameters corresponding to 63, 125, and 250 μm.15 Drusen size was divided into the following categories: <63, 63 to 124, 125 to 249, and ≥250 μm.
Progression was defined as either eye progressing from a grade 1, 2, or 3 to either a 4 or a 5 at any follow-up visit to the end of the study within each individual. Time to progression was recorded for the first eye to progress if both eyes were at risk, and for the fellow eye if 1 eye was at risk. Individuals were considered progressors if there was no advanced AMD in either eye at baseline and they developed AMD in ≥1 eye during follow-up (group A), or they had advanced AMD in 1 eye at baseline and progressed to AMD in the fellow eye during follow-up (group B). For subjects in group A, we controlled for drusen size in each eye at baseline and evaluated the time to progression in each eye and used the earlier of the 2 progression times if both eyes progressed at different times. For subjects in group B, we controlled for AMD category in the affected eye at baseline (i.e., GA or NV), drusen size in the unaffected eye at baseline, and evaluated the time to progression in the fellow eye.
Demographic and risk factor data, including education, smoking history, and BMI, were obtained at the baseline visit from questionnaires and height and weight measurements. Antioxidant status was defined as taking antioxidants (antioxidants alone or antioxidants and zinc) or no antioxidants (placebo or zinc alone) in the clinical trial. The clinical trial treatment groups included placebo, antioxidants alone, zinc, and antioxidants plus zinc. The research protocol was approved by institutional review boards and all participants signed the AREDS general consent statements. Research adhered to the tenets of the Declaration of Helsinki.
Genotype Data
The following 6 common single nucleotide polymorphisms (SNPs) associated with AMD were evaluated: (1) Complement factor H (CFH) Y402H (rs1061170) in exon 9 of the CFH gene on chromosome 1q31, a change 1277T<C, resulting in a substitution of histidine for tyrosine at codon 402 of the CFH protein,16–19 (2)CFH rs1410996, an independently associated SNP variant within intron 14 of CFH,10 (3) ARMS2/HTRA1 (rs10490924) in the LOC387715/HTRA1 region of chromosome 10, a nonsynonymous coding SNP variant in exon 1 of LOC387715, resulting in a substitution of the amino acid serine for alanine at codon 69,20–24 (4) Complement component 2 or C2 E318D (rs9332739), the nonsynonymous coding SNP variant in exon 7 of C2 resulting in the amino acid glutamic acid changing to aspartic acid at codon 318,10,25 (5) Complement Factor B or CFB R32Q (rs641153), the nonsynonymous coding SNP variant in exon 2 of CFB resulting in the amino acid glutamine changing to arginine at codon 32,10,25 (6) Complement component 3 or C3 R102G (rs2230199), the nonsynonymous coding SNP variant in exon 3 of C3 resulting in the amino acid glycine to arginine at codon 102.26,27 For the genetic variant on chromosome 10, ARMS2, it remains a subject of debate whether the gene HTRA1 adjacent to it may in fact be the AMD-susceptibility gene on 10q2622–24; however, the relevant SNPs in these 2 genes have been reported to be nearly perfectly correlated. Thus, although the other SNP is a promising candidate variant, rs10490924 used in this study can be considered a surrogate for the causal variant that resides in this region. For the C2/CFB genes, there are 2 independent associations to the C2/CFB locus, but because of linkage disequilibrium we do not know which of the 2 genes or whether both are functionally affected.10,25 Genotyping was performed using primer mass extension and MALDI-TOF MS analysis (MassARRAY iPLEX platform of Sequenom, San Diego, CA) at the Broad Institute Center for Geno-typing and Analysis (Cambridge, MA).
Statistical Analyses
Analyses were performed using the Cox proportional hazards model to evaluate relationships between progression of AMD and the following variables: genotypes, age (<65, 65–74, ≥75 years), gender, education (high school or less, more than high school), cigarette smoking (never, past, current), and BMI, which was calculated as the weight in kilograms divided by the square of the height in meters (<25, 25–29.9, ≥30). The treatment assignment in the randomized clinical trial was also added to the multivariate model (taking a supplement containing antioxidants or taking study supplements containing no antioxidants).
Hazard ratios (HRs) and 95% confidence intervals were calculated for demographic, behavioral, ocular, and genetic factors. Tests for trend for the number of risk alleles (0, 1, or 2) for each genetic variant were calculated.28 The method for calculation of the AMD progression risk score based on regression coefficients of all demographic, environmental, genetic, and ocular factors is shown in Table 1 (available online at http://aaojournal.org), which gives examples of sets of variables for 1 individual who progressed and 1 individual who did not. Table 1 also shows the total risk score and total genetic load for the progressor and the nonprogressor, and the regression coefficients associated with each variable that can be used to calculate a risk score. In addition, survival analysis was used to determine 5- and 10-year cumulative incidence rates of AMD for individual subjects with various risk factor levels at baseline, adjusting for competing mortality risks according to age and gender. The total risk score can be used to estimate HRs for specific subjects relative to a subject with no risk factors, and thereby estimate the AMD survival curve for individual subjects based on specific levels of risk factors using the Baseline option of PROC PHREG of SAS (version 9.1; SAS, Inc., Cary, NC). One can then estimate AMD progression over different periods of time for individual subjects.
Table 1.
Calculation of Age-Related Macular Degeneration (AMD) Progression Risk Score in Individuals with Advanced AMD in One Eye at Baseline, Based on Demographic, Environmental, Genetic and Ocular Characteristics
| Regression Coefficient | Code | Non-Progressor-Subject A | Progressor-Subject B | |||
|---|---|---|---|---|---|---|
| Variables | ||||||
| Demographic | ||||||
| Age | ||||||
| 55-64 | −0.60967 | 1 | −0.60967 | 1 | −0.60967 | |
| 65-74 | −0.30308 | 0 | 0 | 0 | 0 | |
| Gender | 0.02907 | 1=m /0=f | 0 | 0 | 1 | 0.02907 |
| Education | −0.12266 | 1=some college/0=high school or less | 1 | −0.12266 | 1 | −0.12266 |
| Environmental | ||||||
| Smoking | ||||||
| Current | 0.5691 | 1=yes/0=no | 0 | 0 | 0 | 0 |
| Past | 0.12149 | 1=yes/0=no | 0 | 0 | 1 | 0.12149 |
| BMI | ||||||
| 25-29 | 0.06915 | 1=yes/0=no | 0 | 0 | 1 | 0.06915 |
| 30+ | 0.25687 | 1=yes/0=no | 0 | 0 | 0 | 0 |
| Antioxidant | −0.09171 | 1=yes/0=no | 0 | 0 | 1 | −0.09171 |
| Genetic | ||||||
| CFH :rs1061170 (Y402H) | ||||||
| CT | 0.05449 | 1=yes/0=no | 0 | 0 | 0 | 0 |
| CC | 0.16544 | 1=yes/0=no | 0 | 0 | 1 | 0.16544 |
| CFH :rs1410996 | ||||||
| CT | 0.65396 | 1=yes/0=no | 0 | 0 | 0 | 0 |
| CC | 0.8162 | 1=yes/0=no | 0 | 0 | 1 | 0.8162 |
| ARMS2/HTRA1 :rs10490924(A69s) | ||||||
| GT | 0.30063 | 1=yes/0=no | 1 | 0.30063 | 1 | 0.30063 |
| TT | 0.60353 | 1=yes/0=no | 0 | 0 | 0 | 0 |
| CFB :rs641153(R32Q) | ||||||
| CT/TT | −0.38387 | 1=yes/0=no | 0 | 0 | 0 | 0 |
| C2 :rs9332739(E318D) | ||||||
| CG/CC | −0.44619 | 1=yes/0=no | 0 | 0 | 0 | 0 |
| C3 :rs2230199(R102G) | ||||||
| CG | 0.16624 | 1=yes/0=no | 0 | 0 | 0 | 0 |
| GG | 0.37935 | 1=yes/0=no | 0 | 0 | 0 | 0 |
| Ocular | ||||||
| Advanced Age-Related Macular Degeneration in One Eye At Baseline | ||||||
| Geographic Atrophy | 1.9822 | 1=yes/0=no | 0 | 0 | 0 | 0 |
| Neovascular Disease | 1.62015 | 1=yes/0=no | 1 | 1.62015 | 1 | 1.62015 |
| Individuals with Advanced Age-Related Macular Degeneration in One Eye at Baseline: Largest Drusen size in non-Advanced eye (microns) | ||||||
| ≥250 | 2.4629 | 1=yes/0=no | 0 | 0 | 0 | 0 |
| 125-249 | 1.98696 | 1=yes/0=no | 1 | 1.98696 | 1 | 1.98696 |
| 63-124 | 1.4201 | 1=yes/0=no | 0 | 0 | 0 | 0 |
| Individuals Without Advanced Age-Related Macular Degeneration at Baseline: Size of Drusen (microns) in each eye | ||||||
| ≥250, ≥250 | 3.98295 | 1=yes/0=no | 0 | 0 | 0 | 0 |
| ≥250, 125-249 | 3.78268 | 1=yes/0=no | 0 | 0 | 0 | 0 |
| ≥250, ≤124 | 3.33299 | 1=yes/0=no | 0 | 0 | 0 | 0 |
| 125-249, 125-249 | 3.2573 | 1=yes/0=no | 0 | 0 | 0 | 0 |
| 125-249, 63-124 | 2.71209 | 1=yes/0=no | 0 | 0 | 0 | 0 |
| 125-249, <63 | 2.05322 | 1=yes/0=no | 0 | 0 | 0 | 0 |
| 63-124, 63-124 | 2.02435 | 1=yes/0=no | 0 | 0 | 0 | 0 |
| 63-124, <63 | 1.24127 | 1=yes/0=no | 0 | 0 | 0 | 0 |
| Total Risk Score* | 3.17541 | 4.28505 | ||||
| Genetic Load† | 0.30063 | 1.28227 | ||||
Calculated by adding all regression coefficients
Calculated by adding all genotype regression coefficients
The area under the receiver operating characteristic curve (AUC) was obtained for progression within 5 years and progression within 10 years. In addition, an age-adjusted concordant or “C” statistic based on the curve was calculated for different combinations of these factors to assess the probability that the risk score based on the group of risk factors in that model from a random progressor was higher than the corresponding risk score from a random nonprogressor within the same 10-year age group.13,29 We obtained standard errors of estimated C statistics and compared C statistics from alternative risk prediction models using correlated receiver operating characteristic curve methods.30 Attributable risks were calculated based on combinations of environmental, genetic, and supplement variables, which were conditional on demographic factors and drusen size.
In addition, the study population was subdivided into 2 sub-samples: a derivation sample consisting of 1505 individuals and a test sample of 1432 subjects by using a SAS uniform random number generator (RANUNI) with allocation probabilities of 0.5 in each group. To validate the model, 3 multivariate models were estimated from the derivation sample and then applied separately to both the derivation and the test samples. To assess discrimination, the model obtained from the derivation sample was applied to the test sample and the AUC was determined for both derivation and test samples. To assess the calibration of the model, the subjects in the test sample were subdivided into age-specific deciles of predicted 5-year AMD risk as obtained from the model including genes, environment, drusen, and baseline AMD status based on the derivation sample. The expected number of subjects who progressed to AMD over 5 years adjusted for competing mortality risks (as obtained from 2006 life tables) was then compared with the observed number of subjects who progressed over 5 years, estimated from Kaplan–Meier curves obtained for individual subjects within each age-specific risk decile in the test sample. A Hosmer–Lemeshow test statistic was then computed to compare the observed and predicted counts by risk decile summed over all age groups. Counts for deciles 1 to 4 were combined due to small numbers. These predicted survival curves were a function of the specific demographic, environmental, genetic, and ocular risk factors of the individual subject, and were also adjusted for competing mortality risks over a 10-year period according to age and gender of subjects at baseline.
Results
There were 819 individuals who progressed to either GA or NV in ≥1 eye and 2118 who did not progress during the course of the study. There were 265 progressors to GA (but not NV), and 379 progressors to NV in ≥1 eye (but not GA), and 175 individuals who progressed to GA in 1 eye and NV in the fellow eye. The mean ages at baseline (± standard deviation) of progressors and nonprogressors were 70.2 (±5.2) and 68.1 (±4.7), respectively. The average follow-up time was 9.2 years (range, 0.5–13) for individuals without advanced AMD in either eye at baseline (n = 2519), and was 6.7 years (range, 0.5–12) for subjects who had 1 eye with advanced AMD at baseline (n = 418).
Overall, there were 341 people who were not followed for 5 years and did not progress within 5 years (12%), and 423 people who were not followed for 10 years and did not progress within 10 years (14%). Persons lost to follow-up over 10 years were slightly older (mean age of 69.9 vs 68.5 years), and tended to have better macular status at baseline than subjects who were followed for ≥10 years. There were no differences according to gender or smoking status.
Overall rates of progression over time for these 2 groups (1 vs 2 eyes at risk of progression to advanced AMD) are shown in Fig 1 (available online at http://aaojournal.org), and the corresponding overall probabilities of progression at 2, 5, and 10 years for individuals in these 2 ocular categories at baseline are shown in Table 2 (available online at http://aaojournal.org). The progression rate over 10 years for individuals with drusen size of 125 to 249 microns in both eyes at baseline was 21%, and for individuals with advanced AMD at baseline in 1 eye and the same drusen size in the other eye, the progression rate at 10 years was 61%.
Table 2.
Overall Probability of Progression to Advanced Age-Related Macular Degeneration According to Baseline Macular Characterstics
| Macular Drusen (125- 249 μm) Both Eyes | Advanced AMD in 1 Eye, Drusen (125-249 μm) in Fellow Eye | |
|---|---|---|
| Years | ||
| 2 | 0.052 | 0.20 |
| 5 | 0.12 | 0.404 |
| 10 | 0.21 | 0.61 |
Table 3 (available online at http://aaojournal.org) shows the univariate associations between AMD progression and demographic, ocular, environmental, and genetic factors at baseline. Progressors were older, had less education, were more likely to be past or current smokers, and had somewhat higher BMI compared with nonprogressors. All 6 genetic variants were significantly related to progression from nonadvanced to advanced stages of AMD.
Table 3.
Univariate Associations Between Baseline Demographic, Environmental, Genetic and Ocular Characteristics and Incident Advanced Age-Related Macular Degeneration
| Progressors N (%) | Non-progressors N (%) | Incidence Rates (%) | Hazard Ratio (95% Confidence Interval) | P-value | |
|---|---|---|---|---|---|
| Total patients | 819 | 2118 | |||
| Variables | |||||
| Demographic | |||||
| Age (years) | |||||
| <65 | 110 (13) | 455 (21) | 19 | 1.0 | |
| 65-74 | 504 (62) | 1413 (67) | 26 | 1.4 (1.2 - 1.8) | 0.0005 |
| 75+ | 205 (25) | 250 (12) | 45 | 3.0 (2.4 - 3.8) | <0.0001 |
| Sex | |||||
| Female | 460 (56) | 1201 (57) | 28 | 1.0 | |
| Male | 359 (44) | 917 (43) | 28 | 1.0(0.9-1.2) | 0.758 |
| Education | |||||
| ≤ High School | 321 (39) | 656 (31) | 33 | 1.0 | |
| > High School | 498 (61) | 1462 (69) | 25 | 0.7(0.6-0.8) | <0.0001 |
| Environmental | |||||
| Smoking | |||||
| Never | 319(39) | 1068 (50) | 23 | 1.0 | |
| Past | 429(52) | 944 (45) | 31 | 1.4(1.2-1.6) | <0.0001 |
| Current | 71(9) | 106 (5) | 40 | 2.1(1.6-2.7) | <0.0001 |
| Body Mass Index | |||||
| <25 | 245 (30) | 716 (34) | 25 | 1.0 | |
| 25-29 | 334 (41) | 902 (43) | 27 | 1.1(0.9-1.3) | 0.319 |
| 30+ | 240 (29) | 500 (24) | 32 | 1.4(1.1-1.6) | 0.001 |
| Antioxidants | |||||
| No | 420 (51) | 1044 (49) | 29 | 1.0 | |
| Yes | 399 (49) | 1074 (51) | 27 | 0.9(0.8-1.1) | 0.294 |
| Genetic | |||||
| CFH :rs1061170 (Y402H) | |||||
| TT | 131 (16) | 767 (36) | 15 | 1.0 | |
| CT | 359 (44) | 979 (46) | 27 | 2.0(1.6-2.4) | <0.0001 |
| CC | 329 (40) | 372 (18) | 47 | 3.8(3.1-4.7) | <0.0001 |
| CFH :rs1410996 | |||||
| TT | 31 (4) | 349 (16) | 8 | 1.0 | |
| CT | 247 (30) | 974 (46) | 20 | 2.6(1.8-3.8) | <0.0001 |
| CC | 541 (66) | 795 (38) | 40 | 6.6 (4.1-8.5) | <0.0001 |
| ARMS2/HTRA1 :rs10490924 (A69S) | |||||
| GG | 255 (31) | 1237 (58) | 17 | 1.0 | |
| GT | 396 (48) | 749 (35) | 35 | 2.2(1.9-2.6) | <0.0001 |
| TT | 168 (21) | 132 (6) | 56 | 4.4(3.6-5.4) | <0.0001 |
| C2 :rs9332739(E318D) | |||||
| GG | 792 (97) | 1949 (92) | 29 | 1.0 | |
| CG/CC | 27 (3) | 169 (8) | 14 | 0.4(0.3-0.6) | <0.0001 |
| CFB :rs641153(R32Q) | |||||
| CC | 762 (93) | 1770(84) | 30 | 1.0 | |
| CT/TT | 57 (7) | 348(16) | 14 | 0.4(0.3-0.6) | <0.0001 |
| C3 :rs2230199(R102G) | |||||
| CC | 388 (47) | 1299 (61) | 23 | 1.0 | |
| CG | 357 (44) | 732 (35) | 33 | 1.5(1.3-1.8) | <0.0001 |
| GG | 74 (9) | 87 (4) | 46 | 2.3(1.8-2.9) | <0.0001 |
| Ocular | |||||
| Individuals with Advanced Age-Related Macular Degeneration in One Eye at Baseline | |||||
| Neither Eye | 553 (68) | 1966 (93) | 22 | 1.0 | |
| 1 Eye with Geographic Atrophy | 52 (6) | 8 (0) | 87 | 10.0 (3.9-25.2) | <0.0001 |
| 1 Eye with Neovascular Disease | 214 (26) | 144 (7) | 60 | 7.6 (3.2-18.5) | <0.0001 |
| Largest Drusen Size (microns) in Non-Advanced Eye for Individuals with Advanced Age-Related Macular Degeneration in One Eye At Baseline | |||||
| <63 | 7 (3) | 45 (30) | 13 | 1.0 | |
| 63-124 | 48 (18) | 54 (36) | 47 | 4.3 (1.9-9.5) | 0.0003 |
| 125-249 | 85 (32) | 38 (25) | 69 | 7.8 (3.6-16.9) | <0.0001 |
| ≥ 250 | 126 (47) | 15 (10) | 89 | 13.9 (6.5-29.9) | <0.0001 |
| Individuals Without Advanced Age-Related Macular Degeneration at Baseline Size of Drusen (microns) in each eye | |||||
| <63, <63 | 17 (3) | 872 (44) | 2 | 1.0 | |
| 63-124, <63 | 30 (5) | 422 (21) | 7 | 3.7 (2.0-6.5) | <0.0001 |
| 63-124, 63-124 | 35 (6) | 180 (9) | 16 | 9.1 (5.1-16.2) | <0.0001 |
| 125-249, <63 | 22 (4) | 135 (7) | 14 | 7.9 (4.2-14.9) | <0.0001 |
| 125-249, 63-124 | 68 (12) | 160 (8) | 30 | 18.1 (10.6-30.8) | <0.0001 |
| 125-249, 125-249 | 94 (17) | 102 (5) | 48 | 33.4 (19.9-56.1) | <0.0001 |
| ≥ 250, ≤124 | 27 (5) | 27 (1) | 50 | 36.7 (20.0-67.4) | <0.0001 |
| ≥ 250, 125-249 | 102 (18) | 42 (2) | 71 | 60.0 (36.0-100.6) | <0.0001 |
| ≥ 250, ≥250 | 158 (29) | 26 (1) | 86 | 88.4 (53.7-146.4) | <0.0001 |
Table 3 also shows the influence of macular status at baseline on progression. Individuals with advanced AMD in 1 eye at baseline had a significantly higher hazard of progression to advanced AMD in the fellow eye (HR, 10.0 [95% CI, 3.9–25.2] for GA, and HR, 7.6 [95% CI, 3.2–18.5] for NV) compared with those without advanced AMD at baseline. Hazard of progression increased as drusen size increased for all categories. For example, the HR was 4.3 for a drusen size of 63 to 124 μm in one eye and advanced AMD in the other eye, and the HR was 13.9 for a drusen size of ≥250 μm in one eye, and advanced AMD in the other eye. Compared with individuals in which both eyes had no drusen or very small drusen (<63 μm), individuals with a drusen size of 63 to 124 μm in both eyes had a HR of 9.1 (95% CI, 5.1–16.2). Individuals with a drusen size of 63 to 124 μm in 1 eye and larger drusen (125–249 μm) in the other eye had a HR of 18.1 (95% CI, 10.6–30.8).
Table 4 (as well as Table 5 available online at http://aaojournal.org) displays the multivariate associations between demographic, ocular, environmental, and genetic characteristics and AMD progression in the 3 models: Model A controls for demographic, environmental characteristics, genetic factors, and AMD status (whether 1 eye had advanced AMD at baseline, and if so the type of advanced AMD); model B controls for demographic, environmental factors, drusen phenotypes, and AMD status; and model C (Table 4) includes all variables in models A and B. Baseline drusen pheno-types were strongly related to progression. Smoking, higher BMI, and all genetic variants were also associated with worsening of macular disease over time. Environmental characteristics had similar effects on progression in models A, B, and C. Genetic factors were all significantly associated with AMD progression in model A, and these effects were weakened, but remained significant (except for CFH rs1061170), with the addition of drusen pheno-types to the model C. Larger drusen size was very strongly associated with progression to advanced AMD as seen in model B, and these effects were reduced slightly with the addition of genetic factors to the model (model C, Table 4).
Table 4.
Multivariate Association Between Demographic, Environmental, Genetic and Macular Characteristics and Progression to Advanced Age-Related Macular Degeneration
| Variables | Hazard Ratio (95% CI) | P Value |
|---|---|---|
| Progressors/nonprogressors | 819/2118 | |
| Demographic | ||
| Age (y) | ||
| <65 | 1.0 | |
| 65–74 | 1.4 (1.1–1.7) | 0.004 |
| ≥75 | 1.8 (1.5–2.3) | <0.0001 |
| Gender | ||
| Female | 1.0 | |
| Male | 1.0 (0.9–1.2) | 0.704 |
| Education | ||
| ≤High school | 1.0 | |
| >High school | 0.9 (0.8–1.0) | 0.098 |
| Environmental | ||
| Smoking | ||
| Never | 1.0 | |
| Past | 1.1 (1.0–1.3) | 0.121 |
| Current | 1.8 (1.4–2.3) | <0.0001 |
| Body mass index (kg/m2) | ||
| <25 | 1.0 | |
| 25–29 | 1.1 (0.9–1.3) | 0.425 |
| ≥30 | 1.3 (1.1–1.6) | 0.006 |
| Antioxidants | ||
| No | 1.0 | |
| Yes | 0.9 (0.8–1.0) | 0.196 |
| Genetic | ||
| CFH:rs1061170 (Y402H) | ||
| TT | 1.0 | |
| CT | 1.1 (0.8–1.3) | 0.639 |
| CC | 1.2 (0.9–1.5) | 0.214 |
| CFH:rs1410996* | ||
| TT | 1.0 | |
| CT | 1.9 (1.3–2.9) | 0.002 |
| CC | 2.3 (1.5–3.5) | 0.0002 |
| ARMS2/HTRA1: rs10490924 (A69S) | ||
| GG | 1.0 | |
| GT | 1.4 (1.1–1.6) | 0.0003 |
| TT | 1.8 (1.5–2.2) | <0.0001 |
| C2:rs9332739(E318D) | ||
| GG | 1.0 | |
| CG/CC | 0.6 (0.4–0.9) | 0.024 |
| CFB:rs641153(R32Q)* | ||
| CC | 1.0 | |
| CT/TT | 0.7 (0.5–0.9) | 0.006 |
| C3:rs2230199(R102G)* | ||
| CC | 1.0 | |
| CG | 1.2 (1.0–1.4) | 0.025 |
| GG | 1.5 (1.1–1.9) | 0.004 |
| Ocular | ||
| Advanced AMD in 1 eye at baseline | ||
| Neither eye | 1.0 | |
| 1 eye with geographic atrophy | 7.3 (2.9–18.4) | <0.0001 |
| 1 eye with neovascular disease | 5.1 (2.1–12.2) | 0.0003 |
| Largest drusen size (microns) in non-advanced fellow eye | ||
| <63 | 1.0 | |
| 63–124 | 4.1 (1.9–9.2) | 0.001 |
| 125–249 | 7.3 (3.4–15.8) | <0.0001 |
| ≥250 | 11.7 (5.4–25.3) | <0.0001 |
| No advanced AMD at baseline: size of drusen (microns) OU | ||
| <63, <63 | 1.0 | |
| 63–124, <63 | 3.5 (1.9–6.3) | <0.0001 |
| 63–124, 63–124 | 7.6 (4.2–13.5) | <0.0001 |
| 125–249,<63 | 7.8 (4.1–14.7) | <0.0001 |
| 125–249, 63–124 | 15.1 (8.8–25.7) | <0.0001 |
| 125–249, 125–249 | 26.0 (15.4–43.7) | <0.0001 |
| ≥ 250, <124 | 28.0 (15.2–51.6) | <0.0001 |
| ≥ 250, 125–249 | 43.9 (26.1–73.9) | <0.0001 |
| ≥ 250, ≥250 | 53.7 (32.2–89.4) | <0.0001 |
SNP is coded by minus DNA strand.
AMD = age-related macular degeneration; CI = confidence interval.
Table 5.
Multivariate Association Between Demographic, Environmental, Genetic and Drusen Characteristics and Progression to Advanced Age-Related Macular Degeneration
| Model A* (Genes) Hazard Ratio (95% Confidence Interval) | P-value | Model B† (Drusen) Hazard Ratio (95% Confidence Interval) | P-value | Model C‡ (Genes & Drusen) Hazard Ratio (95% Confidence Interval) | P-value | |
|---|---|---|---|---|---|---|
| Progressors/Non-Progressors | 819/2118 | 819/2118 | 819/2118 | |||
| Variables | ||||||
| Demographic | ||||||
| Age (years) | ||||||
| <65 | 1.0 | 1.0 | 1.0 | |||
| 65-74 | 1.5 (1.2 - 1.9) | <0.0001 | 1.3 (1.1 - 1.6) | 0.008 | 1.4 (1.1 - 1.7) | 0.004 |
| 75+ | 2.6 (2.1 - 3.3) | <0.0001 | 1.8 (1.4 - 2.2) | <0.0001 | 1.8 (1.5 - 2.3) | <0.0001 |
| Sex | ||||||
| Female | 1.0 | 1.0 | 1.0 | |||
| Male | 0.9 (0.8-1.1) | 0.281 | 1.0 (0.9-1.2) | 0.659 | 1.0 (0.9-1.2) | 0.704 |
| Education | ||||||
| ≤ High School | 1.0 | 1.0 | 1.0 | |||
| > High School | 0.8 (0.7-1.0) | 0.02 | 0.9 (0.8-1.0) | 0.149 | 0.9 (0.8-1.0) | 0.098 |
| Environmental | ||||||
| Smoking | ||||||
| Never | 1.0 | 1.0 | 1.0 | |||
| Past | 1.4 (1.2-1.6) | <0.0001 | 1.1 (1.0-1.3) | 0.150 | 1.1 (1.0-1.3) | 0.121 |
| Current | 1.8 (1.4-2.4) | <0.0001 | 1.8 (1.4-2.4) | <0.0001 | 1.8 (1.4-2.3) | <0.0001 |
| Body Mass Index | ||||||
| <25 | 1.0 | 1.0 | 1.0 | |||
| 25-29 | 1.1 (0.9-1.3) | 0.422 | 1.1 (0.9-1.3) | 0.526 | 1.1 (0.9-1.3) | 0.425 |
| 30+ | 1.3 (1.0-1.5) | 0.015 | 1.2 (1.0-1.5) | 0.026 | 1.3 (1.1-1.6) | 0.006 |
| Antioxidants | ||||||
| No | 1.0 | 1.0 | 1.0 | |||
| Yes | 0.9 (0.8-1.1) | 0.451 | 0.9 (0.8-1.1) | 0.217 | 0.9 (0.8-1.0) | 0.196 |
| Genetic | ||||||
| CFH :rs1061170 (Y402H) | ||||||
| TT | 1.0 | 1.0 | ||||
| CT | 1.3 (1.0-1.6) | 0.045 | 1.1 (0.8-1.3) | 0.639 | ||
| CC | 1.7 (1.3-2.1) | 0.0001 | 1.2 (0.9-1.5) | 0.214 | ||
| CFH :rs1410996 | ||||||
| TT | 1.0 | 1.0 | ||||
| CT | 2.0 (1.3-3.0) | 0.001 | 1.9 (1.3-2.9) | 0.002 | ||
| CC | 3.1 (2.0-4.7) | <0.0001 | 2.3 (1.5-3.5) | 0.0002 | ||
| ARMS2/HTRA1 :rs10490924 (A69S) | ||||||
| GG | 1.0 | 1.0 | ||||
| GT | 1.9 (1.6-2.2) | <0.0001 | 1.4 (1.1-1.6) | 0.0003 | ||
| TT | 3.2 (2.6-3.9) | <0.0001 | 1.8 (1.5-2.2) | <0.0001 | ||
| C2 :rs9332739(E318D) | ||||||
| GG | 1.0 | 1.0 | ||||
| CG/CC | 0.5 (0.3-0.7) | <0.0001 | 0.6 (0.4-0.9) | 0.024 | ||
| CFB :rs641153(R32Q) | ||||||
| CC | 1.0 | 1.0 | ||||
| CT/TT | 0.5 (0.4-0.7) | <0.0001 | 0.7 (0.5-0.9) | 0.006 | ||
| C3 :rs2230199(R102G) | ||||||
| CC | 1.0 | 1.0 | ||||
| CG | 1.4 (1.2-1.6) | <0.0001 | 1.2 (1.0-1.4) | 0.025 | ||
| GG | 1.8 (1.4-2.3) | <0.0001 | 1.5 (1.1-1.9) | 0.004 | ||
| Ocular | ||||||
| Individuals with Advanced Age-Related Macular Degeneration in One Eye at Baseline | ||||||
| Neither Eye | 1.0 | 1.0 | 1.0 | |||
| 1 Eye with Geographic Atrophy | 3.7(2.7-4.9) | 8.7 (3.4-22.1) | <0.0001 | 7.3 (2.9-18.4) | <0.0001 | |
| 1 Eye with Neovascular Disease | 2.0(1.1-2.4) | 6.4 (2.6-15.4) | <0.0001 | 5.1 (2.1-12.2) | 0.0003 | |
| Largest Drusen Size (microns) in Non-Advanced Eye for Individuals with Advanced Age-Related Macular Degeneration in One Eye At Baseline | ||||||
| <63 | 1.0 | 1.0 | ||||
| 63 -124 | 4.4 (2.0-9.6) | 0.0003 | 4.1 (1.9-9.2) | 0.001 | ||
| 125-249 | 8.0 (3.7-17.2) | <0.0001 | 7.3 (3.4-15.8) | <0.0001 | ||
| ≥250 | 14.5 (6.7-31.2) | <0.0001 | 11.7 (5.4-25.3) | <0.0001 | ||
| Individuals Without Advanced Age-Related Macular Degeneration at Baseline Size of Drusen (microns) in each eye | ||||||
| x<63, <63 | 1.0 | 1.0 | ||||
| 63-124, <63 | 3.6 (2.0-6.5) | <0.0001 | 3.5 (1.9-6.3) | <0.0001 | ||
| 63-124, 63-124 | 8.7 (4.9-15.5) | <0.0001 | 7.6 (4.2-13.5) | <0.0001 | ||
| 125-249,<63 | 7.8 (4.1-14.6) | <0.0001 | 7.8 (4.1-14.7) | <0.0001 | ||
| 125-249, 63-124 | 17.3 (10.2-29.4) | <0.0001 | 15.1 (8.8-25.7) | <0.0001 | ||
| 125-249,125-249 | 33.0 (19.7-55.3) | <0.0001 | 26.0 (15.4-43.7) | <0.0001 | ||
| ≥ 250, <124 | 35.2 (19.1-64.7) | <0.0001 | 28.0 (15.2-51.6) | <0.0001 | ||
| ≥ 250, 125-249 | 58.2 (34.7-97.3) | <0.0001 | 43.9 (26.1-73.9) | <0.0001 | ||
| ≥ 250, ≥ 250 | 80.9 (48.9-133.8) | <0.0001 | 53.7 (32.2-89.4) | <0.0001 | ||
Model A includes age, sex, education, smoking, body mass index (BMI), all genes listed in the table and age-related macular degeneration (AMD) status.
Model B includes age, sex, education, smoking, BMI, drusen phenotypes and AMD status.
Model C includes age, sex, education, smoking, BMI, all genes listed in the table, drusen phenotypes and AMD status
An example of the use of Tables 4 and Table 5 to derive a total risk score and also a risk score based only on the genetic components (genetic load) is shown in Table 1 (available online at http://aaojournal.org) for 2 individuals with advanced AMD in 1 eye at baseline: one who progressed and one who did not progress. The nonprogressor had a total risk score of 3.17 and a genetic load of 0.30, whereas the progressor had a total risk score of 4.28 and a genetic load of 1.28. The information in Table 1 can be used to define the HR for a subject versus a reference person with a 0 value for all covariates. If this HR is combined with the survival curve of the reference subject obtained using the baseline option in SAS PROC PHREG, one can obtain survival rates or curves for each person.
Table 6 shows risk profiles for progression to advanced AMD according to demographic, environmental, genetic, and ocular factors for representative individuals aged 55 to 64 years with a drusen size of 125 to 249 μm at baseline, and for individuals with advanced NV at baseline in the same age group and same size drusen in the fellow eye. Individuals are classified as being in the low, medium, or high risk groups based on combinations of demographic, environmental, and genetic factors. The low, medium, and high risk individuals correspond to subjects with overall risk scores in the 10th, 50th, and 90th percentiles, respectively, among individuals in the same age groups and drusen size characteristics mentioned.
Table 6.
Risk Profiles and Probabilities for Progression to Advanced Age-Related Macular Degeneration According to Demographic, Environmental, and Genetic Factors for Different Baseline Ocular Characteristics
| Risk Groups Among Subjects with Macular Drusen (125–249 μm) in Both Eyes† |
Risk Groups Among Subjects with Macular Drusen (125–249 μm) in One Eye and Neovascular Disease in Fellow Eye‡ |
|||||
|---|---|---|---|---|---|---|
| Variables* | Low | Medium | High | Low | Medium | High |
| Gender | 0 | 1 | 0 | 0 | 1 | 1 |
| Education | 1 | 1 | 1 | 1 | 1 | 0 |
| Smoking | ||||||
| Past | 0 | 0 | 1 | 0 | 1 | 0 |
| Current | 0 | 1 | 0 | 0 | 0 | 1 |
| Body mass index (kg/m2) | ||||||
| 25–29 | 1 | 0 | 0 | 0 | 1 | 1 |
| ≥30 | 0 | 0 | 1 | 0 | 0 | 0 |
| Antioxidant use | 1 | 1 | 0 | 0 | 1 | 1 |
| CFH:rs1061170 | ||||||
| CT | 0 | 0 | 1 | 0 | 0 | 1 |
| CC | 0 | 1 | 0 | 0 | 1 | 0 |
| CFH:rs1410996 | ||||||
| CT | 0 | 0 | 1 | 0 | 0 | 0 |
| CC | 0 | 1 | 0 | 0 | 1 | 1 |
| ARMS2/HTRA1:rs10490924 | ||||||
| GT | 1 | 1 | 1 | 1 | 1 | 0 |
| TT | 0 | 0 | 0 | 0 | 0 | 1 |
| CFB:rs641153 | ||||||
| TT | 0 | 0 | 0 | 0 | 0 | 0 |
| C2:rs9332739 | ||||||
| CC | 0 | 0 | 0 | 0 | 0 | 0 |
| C3:rs2230199 | ||||||
| CG | 1 | 0 | 0 | 0 | 0 | 1 |
| GG | 0 | 0 | 0 | 0 | 0 | 0 |
| Probability of progression to advanced AMD at 2, 5, and 10 years according to risk groups§ | ||||||
| 2 Years | 0.037 | 0.088 | 0.14 | 0.045 | 0.131 | 0.298 |
| 5 Years | 0.095 | 0.217 | 0.331 | 0.116 | 0.311 | 0.61 |
| 10 Years | 0.198 | 0.418 | 0.588 | 0.238 | 0.561 | 0.876 |
Gender: 0 = female, 1 = male; education: 0 = ≤high school, 1 = >high school; all other variables: 0 = no, 1 = yes.
Progression risk groups defined for representative individuals at the low (10th), medium (50th), and high (90th) percentile for subjects age 55–64 and drusen size 125–249 μm in each eye based on combinations of the listed variables as shown in the table.
Progression risk groups defined for representative individuals at the low (10th), medium (50th), and high (90th) percentile for subjects age 55–64 and drusen size 125–249 μm in the nonadvanced eye.
Controlling for competing risks.
The individual in the high risk group with drusen in both eyes at baseline had a history of smoking, BMI ≥30, and ≥1 risk allele for both CFH variants and ARMS2, whereas the individual in the low risk group with drusen in both eyes had no smoking history, had lower BMI, and no risk alleles for either of the CFH variants. The individual in the high risk group with NV disease in 1 eye at baseline was a current smoker, had a BMI of 25 to 29.9 kg/m2, and ≥1 risk allele for both CFH variants, ARMS2/HTRA1, and C3, whereas the individual in the low risk group with NV in 1 eye at baseline had no environmental risk factors and was heterozygous for ARMS2/HTRA1, with no other risk genotypes.
The probabilities of progression to advanced AMD shown in Table 6 are displayed in Fig 2 representing different risk groups. The individual in the high risk group with bilateral drusen size of 125 to 249 μm at baseline had a 59% risk of progressing to advanced AMD at 10 years, whereas the individual in the low risk group with bilateral drusen had a 20% risk of progression to advanced AMD over this same time period (Fig 2A). The individual in the high risk group with advanced AMD at baseline and a high risk score had an 88% probability of progression at 10 years, compared with a 24% probability of progression for the individual with advanced AMD at baseline in the low risk group (Fig 2B). This demonstrates the influence of genetic load and other risk factors on risk of progression within the same macular phenotype.
Figure 2.
Rates of progression over 12 years for selected individuals in the low (10th percentile), medium (50th percentile), and high (90th percentile) risk groups, based on a combination of genetic, demographic, environmental, and ocular characteristics. A, Rates of progression for individuals with a drusen size of 125 to 249 μm in both eyes at baseline. B, Rates of progression for individuals with neovascular AMD in 1 eye and a drusen size of 125 to 249 μm in the nonadvanced eye at baseline.
In Table 7, we present the AUC or C statistics, predicting progression to advanced AMD for the models shown in Table 4 and Table 5 (available online at http://aaojournal.org) with different combinations of demographic, environmental, genetic, and ocular variables. For the overall sample (Table 4 and Table 5 [available online at http://aaojournal.org]), model A (which includes genetic and environmental factors), the 5-year and 10- year C statistics (± standard error) were 0.790±0.011 and 0.810±0.009, respectively. In Table 5 (available online at http://aaojournal.org), model B (ocular and environmental covariates), the C statistics increased to 0.873±0.009 and 0.901±0.007 for 5- and 10-year progression, respectively. Model C as shown in Table 4 (all variables together from models A and B), there was an increase in the C statistic to 0.885±0.008 for 5-year progression, and 0.915±0.006 for the 10-year progression model. The AUCs for models A versus C and B versus C were different from one another (P<0.001 for 10-year progression). Table 7 also displays the statistics for the derivation sample and the test sample. There is a slight decrease in C statistic from the derivation to test sample. However, the overall C statistic in the test sample for model C is >90%. In addition, the attributable risks were 18.1% based on environmental variables, 83.6% based on genetic variables, 87% based on environmental and genetic variables, and 87.2% based on genetic, environmental and treatment variables. This means that 18.1% of progression could be prevented if all subjects changed from current or past smoking to nonsmoking, and/or changed from overweight or obese to normal weight. The other attributable risks are interpreted similarly.
Table 7.
C Statistics for Progression to Advanced Age-Related Macular Degeneration Based on Models with Different Combinations of Genetic, Environmental, and Macular Characteristics in Overall, Derivation, and Test Samples
|
Overall Sample
|
Derivation Sample*
|
Test Sample*
|
||||
|---|---|---|---|---|---|---|
| Model | 5-Year Progression Area Under the Curve ± Standard Error | 10-Year Progression Area Under the Curve ± Standard Error | 5-Year Progression Area Under the Curve ± Standard Error | 10-Year Progression Area Under the Curve ± Standard Error | 5-Year Progression Area Under the Curve ± Standard Error | 10-Year Progression Area Under the Curve ± Standard Error |
| A | 0.790±0.011 | 0.810±0.009 | 0.805±0.015 | 0.814±0.013 | 0.774±0.017 | 0.798±0.013 |
| B | 0.873±0.009 | 0.901±0.007 | 0.887±0.011 | 0.907±0.009 | 0.869±0.013 | 0.900±0.009 |
| C | 0.885±0.008 | 0.915±0.006 | 0.899±0.010 | 0.919±0.008 | 0.876± 0.012 | 0.908±0.009 |
| Model A vs C (P value) | <0.001 | <0.001 | ||||
| Model B vs C (P value) | 0.010 | <0.001 | ||||
Model A: Genetic and environmental variables: Smoking (never, past, current), body mass index (<25, 25–29.9, 30+), education (≤high school, >high school), antioxidant treatment (yes, no), AMD status at baseline, CFH Y402H (TT, CT, CC), CFHrs1410966 (TT, CT, CC), ARMS2/HTRA1 (GG, GT, TT), C2 (GG, CG/CC), CFB (CC, CT/TT), C3 (CC, CG, GG).
Model B: Macular drusen and environmental variables: Smoking (never, past, current), body mass index (<25, 25–29.9, 30+), education (≤high school, >high school), antioxidant treatment (yes, no), AMD status at baseline and drusen size in each eye (μm) as shown in Table 4.
Model C: All variables in models A and B.
Derivation sample, n = 1505; test sample, n = 1432.
For comparative purposes, we also considered AUCs for simpler models for the overall 12-year follow-up time. Specifically, we considered a model without genetic variables but with age, gender, and education (denoted model A1) and a model with the same variables as this model A1 plus cigarette smoking and BMI (denoted model A2) and compared these models with model A in Table 4, which also includes the genetic variables. The AUCs for models A1, A2, and A over the 12-year period were 0.540, 0.601, and 0.803, respectively, with highly significant differences for each of models A1 and A2, versus model A (P<0.001). This suggests the added value of the genetic variables in addition to the demographic and environmental variables.
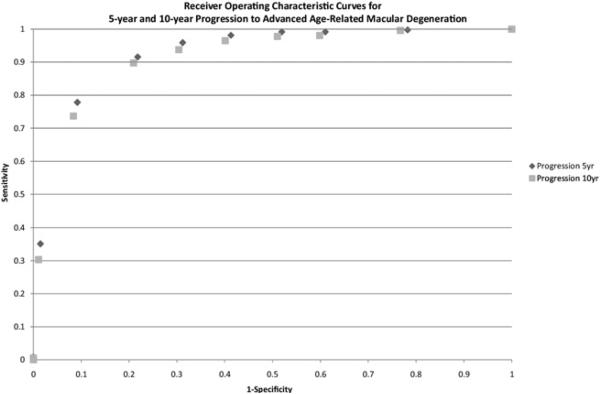
The ROC curves for progressors and nonprogressors at 5 and 10 years for the model shown in Table 4 are displayed in Fig 3. Based on this curve, if a test positive subject is defined as having a risk score ≥3.0, then the sensitivity and specificity are 92% and 78% for 5-year progression, and 90% and 79% for 10-year progression, respectively.
Figure 3.
Receiver operating characteristic curves for 5-year and 10-year progression to advanced age-related macular degeneration based on model in Table 4 (age, gender, education, smoking, BMI, CFH:rs1410996, CFH: rs1061170, ARMS2/HTRA1:rs10490924, C2:rs9332739, CFB:rs641153, C3:rs2230199, drusen phenotypes and AMD status at baseline).
The calibration of the risk prediction model was assessed in the test sample based on a model fit to the derivation sample. The results are shown in Table 8 (available online at http://aaojournal.org). The observed and expected counts within age-specific risk deciles in the test sample were not significantly different (Hosmer-Lemeshow chi-square = 8.16; P = 0.23), indicating adequate calibration of the model in a separate sample. Results based on discrimination and calibration in the test sample indicate that the risk model would likely perform well in other populations of individuals at risk of progression to advanced AMD.
Table 8.
Calibration of Risk Prediction Model
| Risk Decile | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1-4 | 5 | 6 | 7 | 8 | 9 | 10 | ||||||||||
| Age group | Observed | Expected | Observed | Expected | Observed | Expected | Observed | Expected | Observed | Expected | Observed | Expected | Observed | Expected | Chi square | p-value |
| 55-64 | 0 | 0.384 | 0 | 0.332 | 0 | 0.623 | 2 | 1.666 | 3 | 3.984 | 6 | 7.573 | 12 | 14.252 | ||
| 65-74 | 7 | 2.452 | 3 | 2.77 | 8 | 6.798 | 14 | 12.421 | 30 | 22.068 | 41 | 34.934 | 45 | 54.941 | ||
| 75-86 | 1 | 2.612 | 6 | 3.015 | 4 | 4.849 | 11 | 6.868 | 11 | 9.681 | 12 | 12.012 | 16 | 15.341 | ||
| Total | 8 | 5.448 | 9 | 6.117 | 12 | 12.27 | 27 | 20.955 | 44 | 35.733 | 59 | 54.519 | 73 | 84.534 | 8.158624 | 0.226711 |
An assessment of the effect of interactions between genotype and antioxidant/mineral supplement groups on progression to advanced AMD controlling for all demographic, environmental, genetic, and ocular factors among individuals with a baseline grade of ≥2 is shown in Table 9 (available online at http://aaojournal.org). There was an interaction between treatment group and the CFH rs1061170 Y402H variant indicating that the effect of supplementation on risk of progression was significantly different among the 3 CFH genotypes when considered both as a categorical variable (P = 0.038) as well as in a codominant model according to the number of risk alleles (P = 0.013). Specifically, there was a protective effect of the combination antioxidant/zinc group compared with placebo for subjects with the TT non-risk genotype (HR, 0.5; 95% CI, 0.3–0.6), but there did not seem to be a significant beneficial effect for the CC (homozygous risk) geno-type. There were no other interactions between antioxidant/mineral supplements and the other genetic variants.
Table 9.
Assessment of Effect of Genotype-Supplement Interactions on Risk of Progression to Advanced Age-Related Macular Degeneration
| Placebo Hazard Ratio (95% Confidence Interval) | Antioxidant Hazard Ratio (95% Confidence Interval) | Zinc Hazard Ratio (95% Confidence Interval) | Antioxidant-Zinc Hazard Ratio (95% Confidence Interval) | P-interaction (6 df)† | P-interaction (3 df)‡ | |
|---|---|---|---|---|---|---|
| Progressors/Non-Progressors | 191/318 | 183/365 | 218/339 | 203/358 | ||
| CFH rs:1061170(Y402H) | ||||||
| TT | 1.0 | 0.7 (0.4 - 0.9) | 0.7 (0.4 - 1.1) | 0.5 (0.3 - 0.6) | 0.038 | 0.013 |
| CT | 0.8(0.5 - 1.3) | 0.6 (0.4 - 0.8) | 0.7 (0.4 - 1.0) | 0.7 (0.5 - 0.9) | ||
| CC | 0.6 (0.4 - 1.0) | 0.7 (0.5 - 0.9) | 0.9 (0.6 - 1.3) | 0.8 (0.5 - 1.0) | ||
| CFH:1410996 | ||||||
| TT | 1.0 | 0.6 (0.2 - 1.0) | 0.6 (0.2 - 1.5) | 0.5 (0.2 - 0.9) | 0.678 | 0.265 |
| CT | 1.5 (0.7 - 3.0) | 1.1 (0.6 - 1.6) | 1.2 (0.6 - 2.4) | 1.1 (0.6 - 1.6) | ||
| CC | 1.4 (0.7 - 2.8) | 1.3 (0.7 - 1.9) | 1.5 (0.8 - 3.0) | 1.5 (0.7 - 2.1) | ||
| ARMS2/HTRA1 rs:10490924(A69S) | ||||||
| GG | 1.0 | 1.0 (0.7 -1.2) | 1.0 (0.7 - 1.5) | 1.1 (0.7 - 1.3) | 0.852 | 0.518 |
| GT | 1.5 (1.1 - 2.1) | 1.2 (0.9 - 1.5) | 1.4 (1.0 - 2.0) | 1.5 (1.1 - 1.8) | ||
| TT | 2.3 (1.5 - 3.5) | 1.8 (1.2 - 2.2) | 1.8 (1.2 - 2.6) | 1.6 (1.1 - 2.0) | ||
| C2 :rs9332739(E318D) | ||||||
| GG | 1.0 | 0.5 (0.4 - 0.5) | 0.9 (0.8 - 1.1) | 0.9 (0.7 - 1.0) | 0.697 | |
| CG/CC | 0.3 (0.1 - 1.1) | 0.5 (0.2 - 0.8) | 0.7 (0.4 - 1.4) | 0.5 (0.2 - 0.8) | ||
| CFB :rs641153(R32Q) | ||||||
| CC | 1.0 | 0.8 (0.7 - 0.9) | 1.0 )0.8 - 1.2) | 0.9 (0.8 - 1.0) | 0.530 | |
| CT/TT | 0.8 (0.4 - 1.3) | 0.8 (0.5 - 1.0) | 0.6 (0.3 - 1.0) | 0.5 (0.3 - 0.7) | ||
| C3 :rs2230199(R102G) | ||||||
| CC | 1.0 | 0.9 (0.7 -1.0) | 1.0 (0.7 - 1.3) | 0.8 (0.6 - 1.0) | 0.120 | 0.618 |
| CG | 1.3 (1.0 - 1.8) | 0.9 (0.6 - 1.0) | 1.1 (0.8 - 1.4) | 1.1 (0.8 - 1.3) | ||
| GG | 0.9 (0.6 - 1.5) | 2.0 (1.1 - 2.6) | 1.5 (0.9 - 2.4) | 1.3 (0.7 - 1.8) |
*Hazard ratios adjusted for age (<70, ≥70), sex, education (≤ high school, > high school), smoking (never, past, current), body mass index (<25, 25 - 29, 30+), genotypes, supplement groups (placebo, antioxidants, zinc, and antioxidants plus zinc), baseline AMD and drusen status.
Interaction of supplement group by genotype group.
Interaction of supplement group by number of risk/protective alleles.
Discussion
To our knowledge, this is the first model of AMD progression that includes time varying rates of progression up to 12 years, AMD status at baseline, macular drusen size in both eyes at baseline, 6 genetic variants, and demographic and environmental factors. We also calculated absolute risk for individuals based on different combinations of risk factors adjusting for competing mortality risks. Presence of drusen and increasing drusen size in 1 or both eyes are strong risk factors for progression to advanced AMD adjusting for environmental and genetic factors. Individuals with advanced AMD in 1 eye at baseline had a 7- to 10-fold greater hazard of progression in the fellow eye compared with those without advanced AMD at baseline, controlling for genetic and other factors (Table 3, available at http://aaojournal.org). Among individuals with the same baseline drusen phenotype and AMD status, the addition of demographic, lifestyle, and genetic factors was able to differentiate those who were at low, medium, and high risk of progression. Models with varying combinations of these variables have excellent probability of predicting progression to advanced AMD.
Since 2006, we have designed several predictive models for AMD, including a polygenic score in an association study,10 a model in a case-control study with 1 genetic variant (CFH) along with demographic and behavioral factors,11 a model predicting progression over time including the CFH and ARMS2/HTRA1 genes along with the same set of nongenetic factors,12 and an expanded model with 6 genetic variants, together with ocular, demographic, and environmental factors with excellent ability to predict worsening of the disease.13 The C statistic (± standard error) based on an algorithm used in that model, which combined 6 genetic variants and demographic and environmental variables was 0.831±0.013 with an average follow-up time of 6.3 years. Our current analyses expand upon these models further and add the presence and size of macular drusen and severity of AMD in each eye at baseline, grade of AMD at each follow-up visit, longer follow-up time, and a larger sample size. The C statistic improved to 0.885 and 0.915 for 5- and 10-year progression, respectively. Results have practical applications in clinical settings and for designing clinical trials.
The Muenster Aging and Retina Study group performed a cross-sectional study with a population of 730 individuals with combined early and late AMD and 183 controls.31 In a model that included 2 genes, age, gender, and smoking, the C statistic for advanced AMD was 0.81.31 McKay et al32 in another cross-sectional study of 437 cases and 436 controls in a Northern Irish population calculated a C statistic of 0.86 for advanced AMD including genetic factors, smoking, and age. To our knowledge, other than our previous report on predictive models for prevalence and progression,13 there is only 1 other predictive model for AMD progression that involves GA only.33 We also assessed a predictive model with the incorporation of the biomarkers in plasma, com plement components and activation fragments, along with the environmental and genetic variables, which improved prediction,34 although assessment of such biomarkers may not be cost effective or easily obtained in a clinical setting. None of these studies used time-varying rates of progression over 12 years or all of the covariates included in this report.
The advantages of this study include the evaluation of the predictive power of demographic, environmental, genetic, and ocular variables based on a large, well-characterized population of Caucasian patients from various geographic regions around the United States. Additional strengths include the standardized collection of risk factor information, direct measurements of height and weight, measurements of drusen size, and classification of maculopathy by standardized ophthalmic examinations and grading of fundus photographs. Misclassification was unlikely, because grades were assigned without knowledge of risk factors or geno-type. There may be some other unmeasured and, therefore, uncontrolled factors that confound these relationships, but, to explain these results, they would have to be highly related to genotype, smoking, BMI, and treatment assignment and a strong risk factor for AMD. Although this is a selected population, the subjects probably represent the typical patient at risk for progression to advanced AMD, and the overall population is similar to other clinic populations in this age range in terms of smoking and prevalence of obesity as well as the distribution of the genotypes. Furthermore, the biologic effects of the genetic variants do not seem to differ in major ways among various Caucasian populations with AMD. Therefore, results are likely applicable to other Caucasian populations but may not apply to other ethnic groups.
Interpretation of Results as Applied to Clinical Trials
To provide some practical insight into how this information could be useful for planning future clinical trials of various treatments for advanced AMD, we calculated sample sizes needed for (a) high risk individuals, age 55 to 64 years, with advanced AMD in 1 eye and a drusen size of 125 to 249 μm in the fellow eye, and (b) medium risk individuals with bilateral drusen of that size in both eyes but no advanced AMD (Fig 4).
Figure 4.
Estimated sample sizes needed for a clinical trial of progession to advanced age-related macular degeneration (AMD) with 80% power to detect a 50% treatment effect based on presence or absence of genetic screening.
In the presence of genetic testing (left side of Fig 4), we assume that we can identify individuals in the top quintile of risk based on a combination of demographic, environmental, genetic and ocular variables as shown in Table 4. We also assume that the subject at the 90th percentile (Table 6) would represent the average risk of the top quintile, which is 30% incidence over 2 years for the unilateral advanced cases, and 14% for the bilateral drusen category. If we project that incidence rates for progression would be reduced by 50% in the treatment group, then to achieve 80% power would require 120 subjects per treatment arm (total n = 240) in the unilateral advanced AMD case group, and 300 (total n = 600) per group in the bilateral drusen subjects. To identify those with the highest risk of developing advanced AMD (top quintile), 1200 individuals with uni-lateral advanced disease would need to undergo genetic testing to identify 240 subjects (120 per treatment arm) for a proposed clinical trial. Similarly, 3000 people with bilateral drusen would need to undergo genetic testing to identify approximately 600 people (300 per treatment arm) in the top quintile of risk.
In the absence of genetic testing, assuming the medium risk group (Table 6) represents the median risk overall, the 2-year risk in an average subject would be 13.1% and 8.8% with unilateral advanced AMD and bilateral drusen, respectively. If we assume progression rates would be reduced by 50% with a new treatment, to achieve 80% power we would need to enroll 600 individuals in the unilateral advanced AMD group and 1000 in the bilateral drusen group. On average, 400 additional subjects would need to be enrolled for the bilateral drusen group, and 360 additional subjects would need to be enrolled for the unilateral advanced AMD group compared with selecting only high risk individuals based on genetic testing. The cost of genotyping a small number of genetic variants would likely be less than the cost of enrolling an additional 400 people and following them for 2 years in a clinical trial.
In summary, there is increasing interest in personalized medicine and the use of data as presented herein for individual risk prediction for AMD and its progression. The algorithms could be very useful for identifying high risk individuals for future clinical trials designed to evaluate new treatments. Fewer patients would need to be enrolled in such a study if both genetic and nongenetic parameters were part of the screening procedures before enrollment. On an individual basis, the models could someday be used to generate scores for genetic risk, or genetic load, and overall risk for developing the advanced forms of AMD with visual loss over a specific period of time. However, it may be premature to recommend such testing now except for certain high risk families. The overall benefit of these models to improve prediction of individual progression rates may be realized only if genetic testing becomes part of routine clinical practice.
Acknowledgments
The authors thank Marion McPhee, BEd, from the Harvard University Channing Laboratory, Boston, Massachusetts for her programming assistance, and the AREDS Research Group.
Financial Disclosure(s):
The authors have made the following disclosures.
Tufts Medical Center and Massachusetts General Hospital (JMS and MJD) have filed patent applications for materials related to this work.
Supported by grant RO1-EY11309 from the National Institutes of Health; the Massachusetts Lions Eye Research Fund Inc., New Bedford, MA; unrestricted grants from Research to Prevent Blindness Inc., New York, NY; the American Macular Degeneration Foundation, Northampton, MA; Virginia B Smith Fund, Tufts Medical Center; and the Age-Related Macular Degeneration Research Fund, Ophthalmic Epidemiology and Genetics Service, Tufts Medical Center, Tufts University School of Medicine.
Footnotes
Presented at: Association for Research in Vision and Ophthalmology, Ft. Lauderdale, Florida, May 4, 2011.
References
- 1.Seddon JM, Sobrin L. Epidemiology of age-related macular degeneration. In: Albert D, Miller J, Azar D, Blodi B, editors. Albert and Jakobiec's Principles and Practice of Ophthalmology. 3rd ed. Vol. 1. Saunders; Philadelphia, PA: 2008. pp. 413–22. [Google Scholar]
- 2.de Jong PT. Age-related macular degeneration. N Engl J Med. 2006;355:1474–85. doi: 10.1056/NEJMra062326. [DOI] [PubMed] [Google Scholar]
- 3.Seddon JM, Ajani UA, Sperduto RD, et al. Eye Disease Case Control Study Group. Dietary carotenoids, vitamins A, C, and E and advanced age-related macular degeneration. JAMA. 1994;272:1413–20. [PubMed] [Google Scholar]
- 4.Seddon JM, Willett WC, Speizer FE, Hankinson SE. A prospective study of cigarette smoking and age-related macular degeneration in women. JAMA. 1996;276:1141–6. [PubMed] [Google Scholar]
- 5.Seddon JM, Cote J, Davis N, Rosner B. Progression of age-related macular degeneration: association with body mass index, waist circumference, and waist-hip ratio. Arch Ophthalmol. 2003;121:785–92. doi: 10.1001/archopht.121.6.785. [DOI] [PubMed] [Google Scholar]
- 6.Seddon JM, Cote J, Rosner B. Progression of age-related macular degeneration: association with dietary fat, transun-saturated fat, nuts, and fish intake. Arch Ophthalmol. 2003;121:1728–37. doi: 10.1001/archopht.121.12.1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seddon JM, Rosner B, Sperduto RD, et al. Dietary fat and risk for advanced age-related macular degeneration. Arch Ophthalmol. 2001;119:1191–9. doi: 10.1001/archopht.119.8.1191. [DOI] [PubMed] [Google Scholar]
- 8.Age-Related Eye Disease Study Research Group. The relationship of dietary lipid intake and age-related macular degeneration in a case-control study: AREDS report no. 20. Arch Ophthalmol. 2007;125:671–9. doi: 10.1001/archopht.125.5.671. [DOI] [PubMed] [Google Scholar]
- 9.Age-Related Eye Disease Study Research Group A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and beta carotene, and zinc for age-related macular degeneration and vision loss. AREDS report no. 8. Arch Ophthalmology. 2001;119:1417–36. doi: 10.1001/archopht.119.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maller J, George S, Purcell S, et al. Common variation in three genes, including a noncoding variant in CFH, strongly influences risk of age-related macular degeneration. Nat Genet. 2006;38:1055–9. doi: 10.1038/ng1873. [DOI] [PubMed] [Google Scholar]
- 11.Seddon J, George S, Rosner B, Klein M. CFH gene variant Y402H, and smoking, body mass index, environmental associations with advanced age-related macular degeneration. Hum Hered. 2006;61:157–65. doi: 10.1159/000094141. [DOI] [PubMed] [Google Scholar]
- 12.Seddon JM, Francis PJ, George S, et al. Association of CFH Y402H and LOC387715 A69S with progression of age related macular degeneration. JAMA. 2007;297:1793–800. doi: 10.1001/jama.297.16.1793. [DOI] [PubMed] [Google Scholar]
- 13.Seddon JM, Reynolds R, Maller J, et al. Prediction model for prevalence and incidence of advanced age-related macular degeneration based on genetic, demographic, and environmental variables. Invest Ophthalmol Vis Sci. 2009;50:2044–53. doi: 10.1167/iovs.08-3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seddon JM, Sharma S, Adelman RA. Evaluation of the Clinical Age-Related Maculopathy Staging system. Ophthalmology. 2006;113:260–6. doi: 10.1016/j.ophtha.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 15.Klein R, Davis MD, Magli YL, et al. The Wisconsin Age-Related Maculopathy Grading System. Ophthalmology. 1991;98:1128–34. doi: 10.1016/s0161-6420(91)32186-9. [DOI] [PubMed] [Google Scholar]
- 16.Klein RJ, Zeiss C, Chew EY, et al. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308:385–9. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edwards AO, Ritter R, III, Abel KJ, et al. Complement factor H polymorphism and age-related macular degeneration. Science. 2005;308:421–4. doi: 10.1126/science.1110189. [DOI] [PubMed] [Google Scholar]
- 18.Haines JL, Hauser MA, Schmidt S, et al. Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005;308:419–21. doi: 10.1126/science.1110359. [DOI] [PubMed] [Google Scholar]
- 19.Hageman GS, Anderson DH, Johnson LV, et al. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proc Natl Acad Sci U S A. 2005;102:7227–32. doi: 10.1073/pnas.0501536102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jakobsdottir J, Conley YP, Weeks DE, et al. Susceptibility genes for age-related maculopathy on chromosome 10q26. Am J Hum Gen. 2005;77:389–407. doi: 10.1086/444437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rivera A, Fisher SA, Fritsche LG, et al. Hypothetical LOC387715 is a second major susceptibility gene for age related macular degeneration, contributing independently of complement factor H to disease risk. Hum Mol Genet. 2005;14:3227–36. doi: 10.1093/hmg/ddi353. [DOI] [PubMed] [Google Scholar]
- 22.Dewan A, Liu M, Hartman S, et al. HTRA1 promoter polymorphism in wet age-related macular degeneration. Science. 2006;314:989–92. doi: 10.1126/science.1133807. [DOI] [PubMed] [Google Scholar]
- 23.Yang Z, Camp NJ, Sun H, et al. A variant of the HTRA1 gene increases susceptibility to age-related macular degeneration. Science. 2006;314:992–3. doi: 10.1126/science.1133811. [DOI] [PubMed] [Google Scholar]
- 24.Kanda A, Chen W, Othman M, et al. A variant of mitochondrial protein LOC387715/ARMS2, not HTRA1, is strongly associated with age-related macular degeneration. Proc Natl Acad Sci U S A. 2007;104:16227–32. doi: 10.1073/pnas.0703933104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gold B, Merriam JE, Zernant J, et al. Variation in factor B (BF) and complement component 2 (C2) genes is associated with age-related macular degeneration. Nat Genet. 2006;38:458–62. doi: 10.1038/ng1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maller JB, Fagerness JA, Reynolds RC, et al. Variation in complement factor 3 is associated with risk of age related macular degeneration. Nat Genet. 2007;39:1200–1. doi: 10.1038/ng2131. [DOI] [PubMed] [Google Scholar]
- 27.Yates JR, Sepp T, Matharu BK, et al. Genetic Factors in AMD Study Group Complement C3 variant and the risk of age related macular degeneration. N Engl J Med. 2007;357:553–61. doi: 10.1056/NEJMoa072618. [DOI] [PubMed] [Google Scholar]
- 28.Rosner B. Fundamentals of Biostatistics. 7th ed. Brooks/Cole; Boston, MA: 2011. pp. 774–5. [Google Scholar]
- 29.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143:29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- 30.Rosner B, Glynn RJ. Power and sample size estimation for the Wilcoxon rank sum test with application to comparisons of C statistics from alternative prediction models. Biometrics. 2009;65:188–97. doi: 10.1111/j.1541-0420.2008.01062.x. [DOI] [PubMed] [Google Scholar]
- 31.Farwick A, Dasch B, Weber BH, et al. Variations in five genes and the severity of age-related macular degeneration: results from the Muenster Aging and Retina Study. Eye (Lond) 2009;23:2238–44. doi: 10.1038/eye.2008.426. [DOI] [PubMed] [Google Scholar]
- 32.McKay GJ, Dasari S, Patterson CC, et al. Complement component 3: an assessment of association with AMD and analysis of gene-gene and gene-environment interactions in a Northern Irish cohort. [April 18, 2011];Mol Vis [serial online] 2010 16:194–9. Available at: http://www.molvis.org/molvis/v16/a24/. [PMC free article] [PubMed] [Google Scholar]
- 33.Ying GS, Maguire MG. Complications of Age-related Macular Degeneration Prevention Trial Research Group. Development of a risk score for GA in Complications of the Age-related Macular Degeneration Prevention Trial. Ophthalmology. 2011;118:332–8. doi: 10.1016/j.ophtha.2010.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reynolds R, Hartnett ME, Atkinson JP, et al. Plasma complement components and activation fragments: associations with age-related macular degeneration genotypes and phenotypes. Invest Ophthalmol Vis Sci. 2009;50:5818–27. doi: 10.1167/iovs.09-3928. [DOI] [PMC free article] [PubMed] [Google Scholar]