Abstract
Objective
To describe auxologic, physical, and behavioral features in a large cohort of males with 47,XYY (XYY), ages newborn to young adult.
Study design
This is a cross-sectional descriptive study of male subjects with XYY who were evaluated at 1 of 2 specialized academic sites. Subjects underwent a history, physical examination, laboratory testing, and cognitive/behavioral evaluation.
Results
In 90 males with XYY (mean age 9.6 ± 5.3 years [range 0.5-36.5]), mean height SD was above average (1.0 ± 1.2 SD). Macrocephaly (head circumference >2 SD) was noted in 28/84 (33%), hypotonia in 57/90 (63%), clinodactyly in 47/90 (52%), and hypertelorism in 53/90 (59%). There was testicular enlargement for age (>2 SD) in 41/82 (50%), but no increase in genital anomalies. No physical phenotypic differences were seen in boys diagnosed prenatally vs postnatally. Testosterone, luteinizing hormone, and follicle stimulating hormone levels were in the normal range in most boys. There was an increased incidence of asthma, seizures, tremor, and autistic spectrum disorder (ASD) compared with the general population rates. Prenatally diagnosed boys scored significantly better on cognitive testing and were less likely to be diagnosed with ASD (P < .01).
Conclusions
The XYY phenotype commonly includes tall stature, macrocephaly, macroorchidism, hypotonia, hypertelorism, and tremor. Physical phenotypic features were similar in boys diagnosed prenatally vs postnatally. Prenatal diagnosis was associated with higher cognitive function and less likelihood of an ASD diagnosis.
Despite the fact that 1 in 1000 boys1-6 have the karyotype 47,XYY (XYY), there is a paucity of information about the phenotype, and approximately 85% or more of males with XYY are never diagnosed.1,4,7-13 In addition to the lack of large-scale studies, another barrier to understanding the breadth of the phenotype in XYY is ascertainment bias. Historically, studies of adults with XYY ascertained subjects from samples of men who were tall,9,14 or had psychiatric diagnoses or behavior problems.5,15 A few studies describe cohorts diagnosed by newborn screening,1,6,7,10,16,17 which are likely to best reflect the true diversity of the phenotype. However, because these studies are rare and most newborn screening programs do not include analysis of sex chromosome or karyotype, recent studies typically describe boys diagnosed for clinical reasons. Most boys with XYY are diagnosed in the first decade of life because of developmental delays, behavioral issues,2 and tall stature.6 Studies describing those diagnosed postnatally are inherently biased toward describing patients with more severe clinical involvement, given the atypical development, behavior or other clinical concerns that led to their diagnosis.2,4,9
Previously described physical features include birth weight and length in the normal range, with tall stature starting in childhood and exceeding the midparental target height.1,6,18 There are reports of genitourinary anomalies, including microphallus, hypoplastic scrotum, cryptorchidism, and hypospadias in XYY.11,19
Aspects of the phenotype including pubertal development, testosterone levels, and testicular function have been topics of debate because males with XYY were first described in the 1960s.9 An initial report described men institutionalized for antisocial behavior who were found to have an increased frequency of the XYY karyotype,9 and males in prison with XYY had higher testosterone than controls with healthy age-matched male controls.9 There are few studies investigating testicular function in XYY during childhood and adolescence. Ratcliffe described 19 boys with XYY as having normal or delayed puberty.1
A large cohort study with the aim of comparing mortality and cancer incidence in 667 males identified with XYY at a cytogenetics center found increased risks for seizures, respiratory diseases, genitourinary problems, and congenital anomalies.20 Another study reported increased risk of asthma in boys with XYY.8 Language delay has been recognized in some boys with XYY. Motor development either is normal or mildly delayed. Studies of cognitive functioning have revealed a normal IQ or slightly low IQ and/or educational difficulties. There are also reports of increased risk of behavioral problems, attention deficit hyperactivity disorder (ADHD), and autistism spectrum disorder (ASD).4,13,21-23
The aim of this study was to describe a large group of boys diagnosed with XYY and evaluated at 2 centers in the Eastern and Western US, in an attempt to expand knowledge of physical, hormonal, and cognitive/behavioral characteristics, and to answer the following questions: Are there physical/behavioral features present during childhood that will guide the pediatrician to earlier diagnosis during childhood?; What are the differences between boys who are diagnosed prenatally vs postnatally?; Do boys with XYY have pubertal or hormonal differences?; and What medical conditions occur with increased frequency in XYY? Novel aspects of this study include the large cohort size, the contrast between subjects diagnosed prenatally vs postna-tally, the summary of medical and psychiatric diagnoses, and the inclusion of physical, behavior, and autism behavioral aspects of the phenotype.
Methods
Subjects were recruited to the Philadelphia XYY study from a broad geographic and socioeconomic distribution through the support of the national XYY advocacy organization, by direct referral through an established referral network of university- and community-based pediatricians, and through the genetics clinic at A.I. duPont Hospital for Children. Patients also were recruited from the eXtraordinarY Kids clinic, a specialized multidisciplinary clinic for children with sex chromosome disorders at Children's Hospital Colorado, which serves as a clinical referral center for patients with XYY in Colorado and nationally. All boys with XYY had postnatal karyotypes confirming the diagnosis of XYY. None was mosaic. The study was approved by the Human Studies Committees at Thomas Jefferson University and the University of Colorado. Informed consent was obtained in all cases. The clinical/cognitive evaluation was performed at both sites. The hormone measurements and behavioral questionnaires were completed in a subset of the patients at both sites.
Parents were interviewed by providers to obtain information regarding previous diagnoses, medications, and interventions. Medical and psychiatric diagnoses were documented prior to the study visit.
The clinical assessment included conversion of measurements to SD scores using age- and sex-specific norms for height (by stadiometer), head circumference (HC), weight, waist circumference (WC), and body mass index (BMI).24 Testicular size was measured using standard Prader orchidometer beads and converted to SD scores. Pubic hair was assessed according to Tanner staging.25 Muscle tone was evaluated clinically as increased, normal, mildly decreased, or severely decreased by assessing the degree of resistance to passive movement at the elbow and knee, and the degree of pes planus and pronation of the foot. Fifth finger clinodactyly was assessed by visual inspection of the angle of the fifth finger distal interphalangeal joint. Hypertelorism was defined as inner canthal distance (cm) ≥2 SD.25
Cognitive Functioning
Subjects were individually administered neuropsychological testing, including the Differential Ability Scales-Second Edition26 at the Thomas Jefferson University site, or by the Wechsler Abbreviated Scale of Intelligence, the Wechsler Intelligence Scale for Children–Fourth Edition,27 or the Mullen Scales of Early Learning 28 (3-5 years of age), at the Denver site, to assess cognitive abilities. Raw scores were converted to standard scores (mean of 100, SD 15), based on the test-specific norms. Index scores examined were general conceptual ability (full scale IQ [FSIQ]), verbal cluster (verbal IQ), and nonverbal cluster (performance IQ, nonverbal spatial ability). The general conceptual ability score presents an overall indication of an individual's ability to perform complex mental processing of information.
Social/Emotional/Behavioral
Parents were asked to complete the Child Behavior Checklist (CBCL) (ages 4-18).29 The CBCL includes 118 items that describe the child's behavioral, emotional, and social problems over the past 6 months. It includes 3 behavior summary scales, 9 problem behavior scales, and 3 social competence scales.
Participants were asked to complete 2 self-report questionnaires to assess symptoms of anxiety and depression. These measures included the Revised Children's Manifest Anxiety Scale–Second Edition (ages 6-19)30 and the Children's Depression Inventory–Second Edition (CDI-2) (ages 6-17).31 If the children were unable to read the questionnaires, they were read to them.
ASD Measures
To assess symptoms of ASD, 2 parent-report questionnaires were completed. The Social Communicative Questionnaire (SCQ)32 is a screening tool for an ASD diagnosis and includes the current version for children 4-5 years of age, and the lifetime version for children 6 years and above. Scores at or exceeding 15 are generally suggestive of a need for more definitive evaluation for autism. The Social Responsiveness Scale (SRS)33 is a rating scale that assesses socialization, communication, and repetitive behaviors associated with ASD.
Additionally, in a subset of the Philadelphia sample, parents were interviewed by research-reliable personnel (B.W.) using the Autism Diagnostic Interview–Revised (ADI-R),34 a semistructured standardized parent interview developed to assess the presence and severity of autism behaviors throughout childhood across the 3 main symptom domains of reciprocal social interaction, qualitative abnormalities in communication, and restricted, repetitive, and stereotyped patterns of behavior.
Socioeconomic Status
Socioeconomic status (SES) estimate was calculated for children using the Hollingshead 2-Factor Index of Social Status based on education and occupation of parents.35 Higher numbers are associated with university education and professional degrees.
Laboratory Test
Hormone levels (testosterone, estradiol, luteinizing hormone [LH], follicle stimulating hormone [FSH], inhibin B, anti-mullerian hormone [AMH]) were measured.36 Reference values for age and pubic hair Tanner staging are derived from the same laboratory used to analyze the hormone levels in this study. Serum testosterone levels were measured by means of coated tube radioimmunoassays (CIS Biointernational, Gif-sur-Yvette, France). Testosterone sensitivity was 0.01 ng/mL (0.05 nmol/L). Estradiol levels were measured by means of a double-antibody RIA (Diasorin, Anthony, France), and FSH and LH by means of fluoroimmometric assays (AutoDelfia reagents; Perkin-Elmer, Courtaboeuf, France). AMH and inhibin-B were measured by means of enzyme-linked immunosorbent assays (DSL-France, Cergy-Pontoise, France), with respective sensitivities of 6 pg/mL and 0.35 ng/mL (2.5 pmol/L).
Statistical Analyses
All results are presented as mean SD scores, standard scores, and t scores ±SD. Statistical comparisons included t tests, the Pearson correlations, and Fisher exact test comparisons. Results were considered statistically significant at P values of ≤.05.
Results
Patients were evaluated at 2 sites: 43 patients in Philadelphia and 47 in Denver. Overall, the mean age ± SD (range) was 9.6 ± 5.3 years (0.5-36.5 years). Seventy-seven participants were Caucasian, 6 Hispanic, 2 African American, and 5 were of mixed race. Thirty-five boys were diagnosed prenatally (33 for advanced maternal age and 2 for abnormal triple screens), and 55 boys were diagnosed postnatally (5 for hypotonia, 7 for language delay, 17 for behavior issues, and 26 for miscellaneous reasons [eg, other developmental delay, dysmorphic features, mother's request]). The mean age at evaluation was younger in the prenatal vs postnatal groups (8.2 ± 5.4 vs 10.5 ± 5.1 years; P = .05). The patients seen in Philadelphia vs Denver were of similar age (P = .91). Mean SES was slightly lower in the Denver cohort (46 ± 12 vs 51 ± 10; P = .03). Physical, cognitive, and behavioral results of a subset of these XYY subjects was previously reported.22,23
Auxologic Physical Features
Height
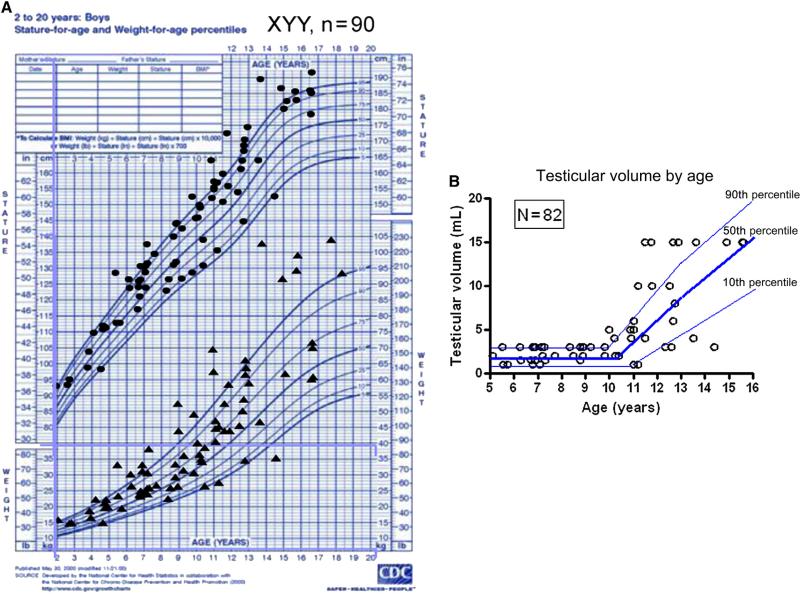
Analysis of cross-sectional height SD demonstrated that mean height SD was generally above average (1.0 ± 1.2 SD; Figure 1 and Table I). Most boys (67/86, 78%) were taller than the mean and 13 boys (15%) were ≥2 SD above the mean. Height SD increased with age (r = 0.29, P = .006). Height SD for boys <6 years (n = 21) was average (0.4 ± 1.3 SD), but was above average in those (n = 65) >6 years (1.1 ± 1.2 SD).
Figure 1.
A, Height and weight plotted on growth chart of 90 males with XYY. B, Testicular volume (mL) in boys with XYY in relationship to age (years).
Table I.
Physical features, services received, and medical and psychiatric diagnoses in XYY males, by prenatal vs postnatal ascertainment
| Prenatal | Postnatal | Combined | P value* | Prevalence in childhood | |
|---|---|---|---|---|---|
| N | 35 | 55 | 90 | ||
| Age, y | 8.2 ± 5.4 | 10.5 ± 5.1 | 9.6 ± 5.3 | .05 | |
| (mean ± SD, range) | 0.5-21.5 | 2.8-36.5 | 0.5-36.5 | ||
| SES | 52 ± 10 | 47 ± 11 | 49 ± 11 | .05 | |
| Measurements | |||||
| Height SDS (mean ± SDS) | 0.9 ± 1.3 | 1.0 ± 1.2 | 1.0 ± 1.2 | .72 | |
| Weight SDS | 0.6 ± 1.4 | 0.9 ± 1.2 | 0.8 ± 1.3 | .40 | |
| BMISDS | 0.5 ± 1.1 | 0.5 ± 1.2 | 0.5 ± 1.2 | .95 | |
| HC SDS | 1.3 ± 1.6 | 1.3 ± 1.7 | 1.3 ± 1.6 | .97 | |
| WC >90 percentile for age | 19% | 19% | 19% | 10% | |
| Testicular volume SDS (right) | 2.0 ± 2.5 | 2.1 ± 2.2 | 2.0 ± 2.3 | .88 | |
| Penile length SDS | —0.6 ± 1.4 | —0.5 ± 1.4 | —0.5 ± 1.4 | .76 | |
| Features | |||||
| Hypotonia | 51% | 71% | 63% | .08 | |
| Low musculature | 20% | 20% | 20% | .99 | |
| Clinodactyly | 46% | 57% | 52% | .39 | <10% |
| Hypertelorism | 53% | 64% | 59% | .38 | |
| Tremor | 41% | 45% | 43% | .83 | 0.1%-22% |
| Dental problems | 29% | 18% | 22% | .30 | |
| Flat feet | 44% | 59% | 53% | .19 | |
| Scoliosis | 9% | 16% | 13% | .36 | 4% |
| Services | |||||
| OT or PT | 59% | 70% | 66% | .36 | |
| Other special education | 23% | 52% | 43% | .01 | |
| Speech therapy | 64% | 94% | 83% | .01 | |
| Medical diagnoses | |||||
| Motor delay/dyspraxia | 23% | 18% | 20% | .60 | <5% |
| Asthma | 44% | 36% | 39% | .51 | 9.6% |
| Seizures | 3% | 20% | 13% | .03 | 1% |
| Psychiatric diagnoses | |||||
| ADD or ADHD | 40% | 60% | 52% | .08 | 2%-16% |
| Verbal or motor tic | 18% | 18% | 18% | .99 | 5%-10% |
| Oppositional defiant | 6% | 5% | 6% | .99 | 1%-16% |
| Depression | 11% | 15% | 13% | .76 | <1% |
| Anxiety | 17% | 31% | 26% | .22 | 15%-20% |
| ASD/pervasive Developmental disorder | 11% | 40% | 29% | .004 | 1% |
| Bipolar | 0% | 13% | 8% | .04 | 0.4%-6.3% |
OT,occupational therapy; PT,physical therapy; ADD, attention deficit disorder; N, number. Bolded values are P < .05.
Pvalue, prenatal vs postnatal.
Weight and BMI
Mean weight and BMI SD were also increased at 0.8 ± 1.3 and 0.5 ± 1.2 but to a lesser degree than height. Fifteen of 86 boys (17%) were ≥2 SD above the mean for weight. Eighteen percent of boys who had WC measurements had WC >90 percentile for age. Therefore, most boys were of relatively normal weight, but there was a trend toward central adiposity (Figure 1 and Table I).
HC
Mean HC SD was increased (1.3 ± 1.6) and macrocephaly (HC SD >2) was observed in 28/84 (33%). There was a trend toward increasing HC SD with age (P = .054) (Table I).
Other Physical Features
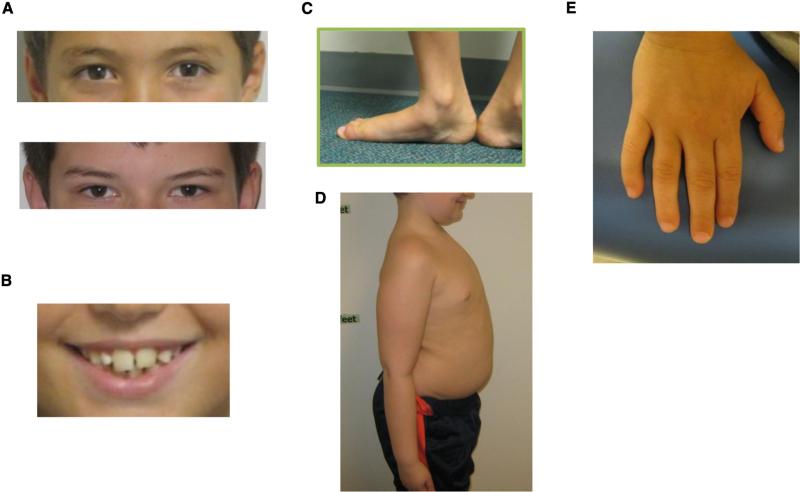
Clinodactyly was noted in 47/90 (52%) patients and hypertelorism was common in XYY (59%) (Figure 2). Mild resting and/or intention tremors were found in 39/90 (43%) patients. Dental problems were reported in 22%, including prognathic jaw with underbite and macrodontia. Hypotonia was noted in 57/90 (63%) patients, and flat feet were common (52%).
Figure 2.
Physical features in boys with XYY. A, Hypertelorism. B, Big teeth. C, Pes planus (decreased tone). D, Central adiposity. E, Clinodactyly.
Genitalia
All boys younger than 10 years had testicular volume of ≤3 mL and Tanner I pubic hair, indicating no increased incidence of precocious puberty or precocious adrenarche (Figure 1, B). Hypospadias was reported in 1/ 90, cryptorchidism in 2/90, and inguinal hernia in 5/90. Of these, only the prevalence of inguinal hernias was increased relative to reported population results.37
Testicular enlargement for age (macroorchidism) was common. The frequency of testicular volume ≥10 mL in the early pubertal age range of 11-13 years was 8/19 (42%) (Figure 1, B). One patient had delayed puberty, which was defined as testicular volume <4 mL at age ≥14 years. Most of the boys had testicular volume that was greater than average for age (>0 SDS) in 64/82 (78%), and significantly above average (>2 SD) in 41/82 (50%). There was no significant correlation between testicular volume SDS and age (P = .18).
Ascertainment
There was no significant difference in auxologic measurements including height, weight, BMI, or HC or in phenotypic features in the prenatal vs the postnatal groups (Table I).
Hormone Measurements
Testosterone
Thirty-nine of 42 boys with XYY (93%) had testosterone levels in the normal range for age and pubic hair Tanner staging. One boy had testosterone levels that were elevated for age and Tanner stage. He was 16.4 years old, had testicular volume of 25 mL, Tanner 5 pubic hair, and a testosterone level of 1152 ng/dL. There was 1 boy with delayed puberty and low testosterone for age; he was 14.4 years old, Tanner 1 pubic hair, testicular volume of 3 mL, and had a testosterone of 18.8 ng/dL. Testosterone values correlated with testicular size (r = 0.8, P < .01). The boy with an elevated testosterone level did not have any significant behavioral concerns.
Estradiol
Estradiol levels were in the normal range (defined as undetectable in prepuberty-36 pg/mL at Tanner V) in 32/34 boys (94%). Two boys had estradiol levels above normal for age and pubic hair stage, both of whom also had elevated testosterone levels.
Gonadotropins
FSH levels were normal in 28/37 (77%) and were slightly elevated for pubic hair Tanner stage and age in 9/37 (24%). None were in the castrate range, and there was no correlation with inhibin B. Similarly, LH levels were normal in 32/38 and were slightly elevated in 6/38, but none were in the castrate range.
Inhibin B
Twenty-nine of 35 boys had normal inhibin B levels, 2 were slightly high for age and stage of puberty, and 4 were low. All boys <9 years had normal inhibin B levels. Four of 26 boys ages 9-16 had a low inhibin B level, in contrast with the normal physiologic increase seen starting at age 9 years and peaking in puberty. Three of the 4 boys with low inhibin B levels had slightly elevated FSH levels and normal testosterone levels.
Antimullerian Hormone
AMH levels, another marker of Sertoli cell number and function, were low in 2 boys (ages 11.5 and 12.7 years, 4.7 and 5.3 ng/mL, one of whom had high testosterone for age), high in 6 boys (ages 4-10 years, normal testosterone levels), and normal in the remaining 27 boys. There was an inverse correlation with age (r = –.5, P < .01), FSH (r = –0.6, P < .01), LH (r = –0.5, P < .01), and testosterone (r = –0.4, P < .01) and a positive correlation with inhibin B (r = 0.4, P = .03). There was no significant correlation with testicular volume SDS.
Comorbid Diagnoses
Asthma was diagnosed in 35/89 (39%) of XYY patients, which is significantly greater than the prevalence in the general population (9.6%). Seizure activity had occurred in 12/89 (13%), all but 1 diagnosed postnatally (prenatal vs postnatal, P = .03). This represents a significant increase when compared with 1% of the general childhood population.38
Cognition and Behavior Results
Behavior problems or a psychiatric diagnosis were reported in 52/90 (58%) boys. Comorbid psychiatric diagnoses were reported in 27/90 (35%) of the XYY boys, of which 7 were diagnosed prenatally with XYY. A total of 50/90 (56%) of the group had received treatment with a stimulant or psycho-tropic medication. The diagnosis of ADHD was most common: 47/90 (52%) of the combined group (40% in prenatal vs 60% in postnatal, P = .08). Mild verbal and/or motor tics were noted in 16/89 (18%) boys. Other diagnoses included ASDs (26/90 [29%] combined, 11% prenatal vs 40% postnatal, P < .004), anxiety (23/90 [26%]), depression (13%), bipolar disorder (8%), and oppositional defiant disorder (6%). There was no significant correlation between psychiatric or behavioral diagnoses and testicular volume SD. Both ASD and bipolar diagnoses were significantly more common in the postnatal group.
Neuropsychological testing was performed on 80 patients. Combined mean verbal IQ was 88 ± 16, combined mean performance IQ was 95 ± 21, and combined mean full scale was 91 ± 16 (Table II). Prenatal diagnosis was associated with a mean IQ similar to the general population and was significantly higher than in the group with postnatal diagnosis (P < .001). There was no significant correlation between FSIQ and SES (P = .73), or between FSIQ and psychiatric diagnosis (P = .71).
Table II.
Cognitive ability and behavior results (mean standard/t score ± SD)
| N | Prenatal diagnosis | N | Postnatal diagnosis | Combined | P value* | |
|---|---|---|---|---|---|---|
| IQ (DAS-2, WISC-IV, Mullen) | ||||||
| Verbal IQ | 36 | 101 ± 11 | 44 | 82 ± 15 | 88 ± 16 | .001 |
| Performance IQ | 36 | 107 ± 15 | 44 | 90 ± 19 | 95 ± 21 | .001 |
| FSIQ | 36 | 102 ± 14 | 44 | 85 ± 15 | 91 ± 16 | .001 |
| CBCL (parent questionnaire, t scores) Behavior total | 15 | 63.0 ± 12.3 | 25 | 66.8 ± 11.7 | 65.4 ± 11.9 | .33 |
| Internalizing total | 15 | 61.5 ± 13.7 | 25 | 62.8 ± 12.5 | 62.3 ± 12.8 | .79 |
| Externalizing total | 15 | 57.2 ± 11.6 | 25 | 60.7 ± 13.3 | 59.4 ± 12.7 | .41 |
| CBCL problem behaviors scales | ||||||
| Withdrawn | 15 | 61.9 ± 12.6 | 25 | 63.7±11.1 | 63.1 ± 11.5 | .64 |
| Somatic complaints | 15 | 60.7 ± 9.7 | 25 | 61.2 ± 11.0 | 61.1 ± 10.4 | .88 |
| Anxious/depressed | 15 | 61.4 ± 11.4 | 25 | 61.8 ± 12.0 | 61.6 ± 11.7 | .92 |
| Social problems | 15 | 64.5 ± 15.4 | 25 | 70.8 ± 11.8 | 68.3 ± 13.4 | .15 |
| Thought problems | 15 | 62.7 ± 9.8 | 25 | 67.6 ± 10.5 | 65.8 ± 10.4 | .16 |
| Attention problems | 15 | 65.9 ± 13.2 | 25 | 70.7 ± 11.6 | 68.9 ± 12.3 | .23 |
| Delinquent behavior | 15 | 60.9 ± 8.2 | 25 | 61.4 ± 11.4 | 61.2 ± 10.2 | .90 |
| Aggressive behavior | 15 | 58.1 ± 8.7 | 25 | 62.9 ± 10.7 | 61.1 ± 10.2 | .16 |
| CBCL social competence scales | ||||||
| Activities total | 12 | 54.2 ± 7.0 | 25 | 58.1 ± 9.6 | 56.8 ± 8.9 | .23 |
| Social total | 12 | 59.1 ± 11.5 | 25 | 64.5 ± 10.0 | 62.6 ± 10.7 | .16 |
| School total | 12 | 64.2 ± 12.2 | 25 | 68.7 ± 6.6 | 67.1 ± 8.9 | .16 |
| Child questionnaires (standard scores) | ||||||
| CDI-2 total | 8 | 100.2 ± 19.4 | 18 | 102.7 ± 14.9 | 101.9 ± 16.0 | .72 |
| RCMAS-2 total | 9 | 90.5 ± 19.1 | 18 | 99.9 ± 15.9 | 96.8 ± 17.2 | .19 |
| Autism evaluation | ||||||
| ADI-R (raw scores) | ||||||
| Reciprocal social interaction | 12 | 6.9 ± 6.9 | 19 | 13.4 ± 8.5 | 10.9 ± 8.4 | .04 |
| % ≥cutoff = 10 | 3/12(25%) | 12/19 (63%) | 15/31 (48%) | .07 | ||
| Communication | 12 | 6.9 ± 4.7 | 19 | 10.7 ± 4.9 | 9.2 ± 5.1 | .04 |
| % ≥cutoff = 8 | 6/12(50%) | 12/19 (63%) | 18/31 (58%) | .71 | ||
| Restrictive/repetitive behavior | 12 | 2.3 ± 2.0 | 19 | 3.3 ± 3.1 | 2.9 ± 2.7 | .32 |
| % ≥cutoff = 3 | 5/12(42%) | 11/19 (58%) | 16/31 (52%) | .47 | ||
| SRS (t scores) | ||||||
| Total | 17 | 66.5 ± 19.1 | 25 | 72.5 ± 15.3 | 70.1 ± 17.0 | .31 |
| Social awareness | 17 | 61.8 ± 14.4 | 25 | 66.4 ± 14.0 | 64.5 ± 14.2 | .25 |
| Social cognition | 17 | 64.1 ± 18.2 | 25 | 73.3 ± 15.8 | 69.6 ± 17.2 | .07 |
| Social communication | 17 | 65.7 ± 18.2 | 25 | 70.5 ± 16.8 | 68.6 ± 17.3 | .54 |
| Social motivation | 17 | 63.7 ± 18.1 | 25 | 64.3 ± 14.7 | 64.0 ± 15.9 | .64 |
| Autistic mannerisms | 17 | 65.2 ± 17.8 | 25 | 73.5 ± 16.8 | 70.2 ± 17.2 | .23 |
| SCQ (raw scores) | 21 | 9.1 ± 7.9 | 34 | 15.1 ± 9.1 | 12.8 ± 9.1 | .02 |
| % ≥cutoff = 15 | 5/21 (24%) | 15/34 (44%) | 20/55 (36%) | .16 | ||
DAS-2, Differential Ability Scales-Second Edition; RCMAS-2, Revised Children′s Manifest Anxiety Scale-Second Edition; WISC-IV, Wechsler Intelligence Scale for Children-Fourth Edition. Bolded values are P < .05.
Pvalue, prenatal vs postnatal.
Parental Reporting of Behavioral Problems and Social Competence
Parents of prenatally and postnatally diagnosed boys with XYY assessed their children's CBCL measures of behavior and social competence (Table II). Although scores were somewhat higher (more severe) in the postnatal group, the results did not differ between the groups. The most severe behavioral issues for both cohorts, as indicated by a mean t score >1 SD (60) on the CBCL scales, were in areas of behavior total, internalizing total, withdrawn behavior, somatic complaints, anxiety/depressed complaints, social problems, thought problems, attention problems, and delinquent behavior. Mean CBCL measures of social competence were within 1 SD on parental scoring in areas of overall social ability and school sociability, with ratings in the typical range on activity sociability.
Child Self-Report Anxiety and Depression Behavioral Questionnaires Results
There were no significant differences between the 2 groups on responses from self-report questionnaires of anxiety (Revised Children's Manifest Anxiety Scale–Second Edition) and depression (CDI-2 ) (Table II). The groups reported themselves as in the normal range in categories of total CDI-2 score, negative mood, ineffectiveness, and negative self-esteem, thereby indicating an overall lack of anxiety or depressive characteristics on these instruments. Boys with XYY did not report increased sentiments of anxiety or depression, compared with the general population.
Autistic Features
Previous diagnosis of ASD was less common in the prenatal cohort. A total of 11% of the prenatal vs 40% of the postnatal XYY group had received the diagnosis of ASD/pervasive developmental delay (Table I, P < .004). Mean scores on the SCQ were significantly lower in the prenatal group (P = .02), but the proportion of boys with scores exceeding the cutoff score of 15 (24% of prenatally diagnosed boys and 44% of postnatally diagnosed boys) did not differ between the groups (P = .16). For the SRS, t scores for both groups fell in the mild to moderate range of autistic features and did not differ between groups. For the ADI-R, mean scores were significantly lower for the prenatal group for reciprocal social interaction and communication (P < .04), but the proportion whose score exceeded the cutoff were similar in both groups, suggesting both groups were at risk for ASD features. A total of 3/12 (25%) of the prenatal cohort met ADI-R criteria for ASD in the 3 domains vs 9/19 (47%) of the postnatal group. Thus, a significant proportion of both the prenatal and postnatal cohorts met ASD criteria compared with the 1% incidence of ASD in the population. Age, SES, and verbal scores were not correlated with SCQ, ADI-R, or SRS scores.
Discussion
This cross-sectional study describes 90 males diagnosed both pre- and postnatally with XYY, ages 0.5-36.5 years. This is a large cohort for phenotypic description. Tall stature was evident in most boys, starting at approximately age 6 years. Weight generally was increased, with a trend toward central adiposity. HC was above average size in most boys. Pubertal onset was generally normal, with a trend toward enlarged testicular size, starting in early puberty. Muscular tone was decreased in over one-half the boys, and common physical features included clinodactyly, hypertelorism, macroorchidism, and a mild tremor. Pubertal hormone values in most boys were within the normal range, although 3 boys had abnormally low inhibin B and mildly elevated FSH, indicating possible risk of decreased fertility. Seizures, asthma, and dental problems were more prevalent than in the general pediatric population. General intelligence, on average, was similar between the prenatally diagnosed cohort and the general population, but was lower in the postnatally diagnosed cohort. Similarly, autistic spectrum metrics measured in this study were more severe in the post-natally ascertained boys, although there was a significantly increased ASD risk in both XYY cohorts. There was no increase in reported symptoms of anxiety or depression reported by boys in either group.
Physical findings and medical morbidity in boys diagnosed prenatally do not differ in this study in major ways from those diagnosed postnatally, which is important for genetic counseling in agreement and is consistent with previous reports.39 The increased prevalence of seizures, dental problems, tremor, and asthma in this cohort is likely related to the presence of an extra Y chromosome. For example, tall stature is related to the extra copy of the short stature homeobox gene40 in the pseudoautosomal region of the extra Y chromosome. It is possible that the extra Y chromosome may be amplifying the risk of atopy, as there is a greater prevalence of atopy/asthma and a reduced relative airway size in boys compared with girls.41 Hypertelorism also has been found in association with XXYY, XXXY, and XXXXY syndromes,23 suggesting that the distance between the eyes may be related to dosage of sex chromosomes genes. Both the increased HC and the increased risk for seizure activity may be related to increased cerebral grey matter and white matter described in XYY.42 Increased grey matter may be the result of reduced synaptic pruning, leading to altered synaptic function and perhaps increased seizure risk.
Normal total testosterone levels were detected in nearly all boys in this XYY cohort. In the early literature describing males with XYY, there were reports of increased testosterone in institutionalized men with XYY,43 which led to the belief that elevated testosterone in men with XYY leads to aggressive behavior. Follow-up studies of males with XYY have indeed found an increase in some criminal convictions, although not related to aggression.1 Twelve men with XYY identified in a birth cohort of tall men in Denmark, were found to have high normal total testosterone levels compared with matched peers, but the investigators did not find an association with aggressive behavior.9 Studies have revealed normal adult testosterone levels compared with controls.44 A single boy in our study had significantly elevated testosterone, but he did not have any behavior problems.
Markers of testicular function including AMH and inhibin B were normal in most, but not all, boys with XYY. In puberty, inhibin B levels are thought to reflect Sertoli cell number and/or spermatogenesis. Four boys had low inhibin B levels, 3 of whom had elevated FSH. A study by Aksglaede describing 9 boys, ages 9.2-20 years, also reported 1 boy with an extremely high FSH and an immeasurably low inhibin B.18 This constellation can be seen in Sertoli-cell-only syndrome, which has been reported in adults with XYY,11,45-47 and is associated with decreased fertility. Gonadal histology in a study of XYY fetuses showed fewer spermatogonia than in XY controls.48 Infertility is associated with microdeletions in the long arm of the Y chromosome, specifically a gene in the Yq11 region, the azoospermic factor.49 It is also possible that extra copies of genes in this region could contribute to decreased fertility. The natural history of testicular development in males with XYY is not well known. The significance of the enlarged testes in boys with XYY is a previously unreported finding and is also of unknown significance; enlargement may be due to Sertoli, interstitial cell, or Leydig cell proliferation.
This study extends previous findings by presenting an in-depth cognitive analysis of boys diagnosed with XYY prenatally and postnatally. Prenatally diagnosed boys had a higher mean IQ and were less likely to have received special education or speech therapy services.
Psychiatric diagnoses were more common in boys diagnosed postnatally and were often the reason these boys had karyotype evaluation. The mean age of the prenatally diag nosed group was 2 years younger, which likely also contributed to the difference in psychiatric diagnoses. One-half of the boys with XYY had the diagnosis of ADHD or attention deficit disorder, which is significantly more than the 16% prevalence of the general school aged-population. In general, ADHD is more common in boys than girls (4:1), and also may be related to genes on the Y chromosome. Nearly one-third of the boys in this cohort had received a diagnosis of ASD compared with a 1% prevalence in the general population, similar to results of Bishop et al.13 We and others 13 hypothesize that increased Y chromosome gene copy number in XYY leads to overexpression of 1 or more Y-linked genes involved in brain development or function, thereby increasing risk for manifestations of ASD. One Y gene in particular, neuroligin 4Y (NLGN4Y) is involved in synaptic function and is therefore a compelling ASD candidate for attributability for XYY.
Behavioral questionnaires revealed that children self-report much better scores of anxiety and depression than their parents. This speaks positively to the self-esteem of XYY children and suggests that behavioral dysregulation does not impact their feelings of self-worth.
Despite the large number of boys described, there are limitations of this study. There is a bias toward inclusion of boys with delays or behavior problems as participating families may have been seeking more information about XYY or treatment for behavioral issues. We attempted to shed light on this bias by comparing the groups diagnosed pre- and postnatally; however, there are likely to be many boys not diagnosed or studied who have not required intervention in school or for behavior modification. The cross-sectional nature of the study only gives a snapshot of different boys, and we were not able to determine the course of growth and development, testicular growth, or hormone levels in individuals.
Children may be identified earlier in childhood if the practitioner is aware of the physical, medical, and neurodevelopmental features. Clues to the diagnosis of XYY include tall stature, macrocephaly, macroorchidism, hypotonia, hypertelorism, and tremor in boys. Once the diagnosis is made, children should be screened carefully and treated for asthma and dental problems. Most seizures are clinically apparent by convulsive symptoms and should be treated with standard antiepileptic medications, however, neurologic evaluation and possible electroencephalogram should be considered for atypical staring spells, significant sleep disturbance, or significant unexplained behavioral dysregulation. Tremor is usually mild, however, if it impairs handwriting, occupational or self-care skills (ie, eating, dressing), then occupational therapy evaluation, educational/workplace supports, and medication treatments can be considered. Neurodevelopmental screenings also should be performed given the increased risk for developmental delays, language disorders, ADHD, and ASDs.22 The increased incidence in XYY may help to shed light on the genetics of asthma and disorders of attention, autism, socialization, and communication. Males with XYY have increased testicular volume in childhood, and a minority may be at risk for decreased fertility.
The relatively normal cognitive ability and behavioral phenotype of the prenatally diagnosed cohort can provide evidence to reassure parents whose children are karyotyped as XYY in utero and will prove useful in genetic counseling. Future studies might investigate the hormonal profile during puberty, and the genetic link between XYY and asthma, ADHD, and autism.
Acknowledgments
J.R. received support for this study from the Delaware Health Science Alliance Pilot Award. N.T. receives support from National Institute of Neurological Disorders and Stroke (1K23NS070337), National Center for Advancing Translational Sciences (Colorado CTSI UL1 TR000154), and Children's Hospital Colorado Research Institute. Contents are the authors' sole responsibility and do not necessarily represent official views of the National Institutes of Health. The authors declare no conflicts of interest.
Glossary
- ADHD
Attention deficit hyperactivity disorder
- ADI-R
Autism Diagnostic Interview–Revised
- AMH
Anti-mullerian hormone
- ASD
Autistism spectrum disorder
- BMI
Body mass index
- CBCL
Child Behavior Checklist
- CDI-2
Children's Depression Inventory–Second Edition
- FSH
Follicle stimulating hormone
- FSIQ
Full scale IQ
- HC
Head circumference
- SCQ
Social Communicative Questionnaire
- LH
Luteinzing hormone
- SES
Socioeconomic status
- SRS
Social Responsiveness Scale
- WC
Waist circumference
- XYY
47,XYY
Footnotes
Portions of this study were presented during the Pediatric Academic Societies' Meeting, Boston, MA, Apr 28-May 1, 2012.
References
- 1.Ratcliffe S. Long-term outcome in children of sex chromosome abnormalities. Arch Dis Child. 1999;80:192–5. doi: 10.1136/adc.80.2.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abramsky L, Chapple J. 47,XXY (Klinefelter syndrome) and 47,XYY: estimated rates of and indication for postnatal diagnosis with implications for prenatal counselling. Prenat Diagn. 1997;17:363–8. doi: 10.1002/(sici)1097-0223(199704)17:4<363::aid-pd79>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 3.Hook EB. Behavioral implications of the human XYY genotype. Science. 1973;179:139–50. doi: 10.1126/science.179.4069.139. [DOI] [PubMed] [Google Scholar]
- 4.Geerts M, Steyaert J, Fryns JP. The XYY syndrome: a follow-up study on 38 boys. Genet Couns. 2003;14:267–79. [PubMed] [Google Scholar]
- 5.Ruud A, Arnesen P, Stray LL, Vildalen S, Vesterhus P. Stimulant medication in 47,XYY syndrome: a report of two cases. Dev Med Child Neurol. 2005;47:559–62. doi: 10.1017/s001216220500109x. [DOI] [PubMed] [Google Scholar]
- 6.Robinson A, Bender BG, Puck MH, Salbenblatt JA. Growth and development of children with a 47,XYY karyotype. Alan R. Liss, Inc; New York: 1985. [Google Scholar]
- 7.Nielsen J, Wohlert M. Chromosome abnormalities found among 34,910 newborn children: results from a 13-year incidence study in Arhus, Denmark. Hum Genet. 1991;87:81–3. doi: 10.1007/BF01213097. [DOI] [PubMed] [Google Scholar]
- 8.Linden MG, Bender BG. Fifty-one prenatally diagnosed children and adolescents with sex chromosome abnormalities. Am J Med Genet. 2002;110:11–8. doi: 10.1002/ajmg.10394. [DOI] [PubMed] [Google Scholar]
- 9.Schiavi RC, Theilgaard A, Owen DR, White D. Sex chromosome anomalies, hormones, and aggressivity. Arch Gen Psychiatry. 1984;41:93–9. doi: 10.1001/archpsyc.1984.01790120097012. [DOI] [PubMed] [Google Scholar]
- 10.Walzer S, Bashir AS, Silbert AR. Cognitive and behavioral factors in the learning disabilities of 47,XXY and 47,XYY boys. Birth Defects Orig Artic Ser. 1990;26:45–58. [PubMed] [Google Scholar]
- 11.Fryns JP, Kleczkowska A, Kubien E, Van den Berghe H. XYY syndrome and other Y chromosome polysomies. Mental status and psychosocial functioning. Genet Couns. 1995;6:197–206. [PubMed] [Google Scholar]
- 12.Netley CT. Summary overview of behavioural development in individuals with neonatally identified X and Y aneuploidy. Birth Defects Orig Artic Ser. 1986;22:293–306. [PubMed] [Google Scholar]
- 13.Bishop DV, Jacobs PA, Lachlan K, Wellesley D, Barnicoat A, Boyd PA, et al. Autism, language and communication in children with sex chromosome trisomies. Arch Dis Child. 2010;96:954–9. doi: 10.1136/adc.2009.179747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Philip J, Lundsteen C, Owen D, Hirschhorn K. The frequency of chromosome aberrations in tall men with special reference to 47, XYY and 47, XXY. Am J Hum Genet. 1976;28:404–11. [PMC free article] [PubMed] [Google Scholar]
- 15.Christensen AL, Nielsen J. Psychological studies of ten patients with the XYY syndrome. Br J Psychiatry. 1973;123:219–21. doi: 10.1192/bjp.123.2.219. [DOI] [PubMed] [Google Scholar]
- 16.Bender BG, Puck MH, Salbenblatt JA, Robinson A. The development of four unselected 47,XYY boys. Clin Genet. 1984;25:435–45. doi: 10.1111/j.1399-0004.1984.tb02013.x. [DOI] [PubMed] [Google Scholar]
- 17.Salbenblatt JA, Meyers DC, Bender BG, Linden MG, Robinson A. Gross and fine motor development in 47,XXY and 47,XYY males. Pediatrics. 1987;80:240–4. [PubMed] [Google Scholar]
- 18.Aksglaede L, Jensen RB, Carlsen E, Kok P, Keenan DM, Veldhuis J, et al. Increased basal and pulsatile secretion of FSH and LH in young men with 47,XXY or 46,XX karyotypes. Eur J Endocrinol. 2008;158:803–10. doi: 10.1530/EJE-07-0709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carakushansky G, Neu RL, Gardner LI. XYY with abnormal genitalia. Lancet. 1968;2:1144. doi: 10.1016/s0140-6736(68)91622-x. [DOI] [PubMed] [Google Scholar]
- 20.Higgins CD, Swerdlow AJ, Schoemaker MJ, Wright AF, Jacobs PA. Mortality and cancer incidence in males with Y polysomy in Britain: a cohort study. Hum Genet. 2007;121:691–6. doi: 10.1007/s00439-007-0365-8. [DOI] [PubMed] [Google Scholar]
- 21.Nicolson R, Bhalerao S, Sloman L. 47,XYY karyotypes and pervasive developmental disorders. Can J Psychiatry. 1998;43:619–22. doi: 10.1177/070674379804300611. [DOI] [PubMed] [Google Scholar]
- 22.Cordeiro L, Tartaglia N, Roeltgen D, Ross J. Social deficits in male children and adolescents with sex chromosome aneuploidy: a comparison of XXY, XYY, and XXYY syndromes. Res Dev Disabil. 2012;33:1254–63. doi: 10.1016/j.ridd.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ross JL, Roeltgen DP, Kushner H, Zinn AR, Reiss A, Bardsley MZ, et al. Behavioral and social phenotypes in boys with 47,XYY syndrome or 47,XXY Klinefelter syndrome. Pediatrics. 2012;129:769–78. doi: 10.1542/peds.2011-0719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamill PV, Drizd TA, Johnson CL, Reed RB, Roche AF, Moore WM. Physical growth: National Center for Health Statistics percentiles. Am J Clin Nutr. 1979;32:607–29. doi: 10.1093/ajcn/32.3.607. [DOI] [PubMed] [Google Scholar]
- 25.Hall JG, Froster-Iskenius Ursula G, Allanson JE. Handbook of normal physical measurements. Oxford University Press; Oxford: 1995. [Google Scholar]
- 26.Elliott CD. Differential ability scales-introductory and technical handbook. Harcourt, Brace, Jovanovich; San Diego, CA: 1983. [Google Scholar]
- 27.Wechsler DA. Wechsler Intelligence Scale for Children–Fourth edition. Administration and scoring manual. Psychological Corporation; San Antonio: 2003. [Google Scholar]
- 28.Mullen E. Mullen scales of early learning. Total Child, Inc; Cranston, RI: 1989. [Google Scholar]
- 29.Achenbach T, Edlebrock C. The child behavior profile: II boys age 12-16 and girls ages 6-11 and 12-16. J Consulting Clin Psychol. 1979;47:223–33. doi: 10.1037//0022-006x.47.2.223. [DOI] [PubMed] [Google Scholar]
- 30.Reynolds CR, Richmond BO. What I think and feel: a revised measure of children's manifest anxiety. J Abn Child Psychol. 1978;6:271–80. doi: 10.1007/BF00919131. [DOI] [PubMed] [Google Scholar]
- 31.Kovacs MaMS. CDI Children's depression inventory technical manual update. MHS; North Tonawanda, NY: 2003. [Google Scholar]
- 32.Rutter M, Bailey A, Lord C. The Social Communication Questionnaire manual. WPS. Los Angeles, CA: 2003. [Google Scholar]
- 33.Constantino J. Western Psychological Services; Los Angeles: 2005. Social Responsiveness Scale (SRS). [Google Scholar]
- 34.Rutter M, Le Couteur A, Lord C. ADI-R Autism Diagnostic Interview Revised WPS Manual. Western Psychological Services; Los Angeles, CA: 2008. [Google Scholar]
- 35.Hollingshead AB, Redlich F. Social class and mental illness. John Wiley; New York: 1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Soriano-Guillen L, Mitchell V, Carel JC, Barbet P, Roger M, Lahlou N. Activating mutations in the luteinizing hormone receptor gene: a human model of non-follicle-stimulating hormone-dependent inhibin production and germ cell maturation. J Clin Endocrinol Metab. 2006;91:3041–7. doi: 10.1210/jc.2005-2564. [DOI] [PubMed] [Google Scholar]
- 37.Grosfeld JL. Current concepts in inguinal hernia in infants and children. World J Surg. 1989;13:506–15. doi: 10.1007/BF01658863. [DOI] [PubMed] [Google Scholar]
- 38.Centers for Disease Control and Prevention Vital signs: asthma prevalence, disease characteristics, and self-management education: United States, 2001–2009. MMWR. 2011;60:547–52. [PubMed] [Google Scholar]
- 39.Lalatta F, Folliero E, Cavallari U, Di Segni M, Gentilin B, Fogliani R, et al. Early manifestations in a cohort of children prenatally diagnosed with 47,XYY. Role of multidisciplinary counseling for parental guidance and prevention of aggressive behavior. Ital J Pediatr. 2012;38:52. doi: 10.1186/1824-7288-38-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ottesen AM, Aksglaede L, Garn I, Tartaglia N, Tassone F, Gravholt CH, et al. Increased number of sex chromosomes affects height in a nonlinear fashion: a study of 305 patients with sex chromosome aneuploidy. Am J Med Genet A. 2010;152A:1206–12. doi: 10.1002/ajmg.a.33334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weiss ST. Bronchial asthma mechanisms and therapeutics. 3rd ed. Little, Brown; Boston: 1993. Epidemiology and natural history. [Google Scholar]
- 42.Bryant DM, Hoeft F, Lai S, Lackey J, Roeltgen D, Ross J, et al. Sex chromosomes and the brain: a study of neuroanatomy in XYY syndrome. Dev Med Child Neurol. 2010;54:1149–56. doi: 10.1111/j.1469-8749.2012.04418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rudd BT, Galal OM, Casey MD. Testosterone excretion rates in normal males and males with an XYY complement. J Med Genet. 1968;5:286–8. doi: 10.1136/jmg.5.4.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Benezech M, Noel B. Hormonal and Biochemical Abnormalities in XYY Males. The Y chromosome, part B: clinical aspects of Y chromosome abnormalities. 1985:301–22. [Google Scholar]
- 45.Martini E, Geraedts JP, Liebaers I, Land JA, Capitanio GL, Ramaekers FC, et al. Constitution of semen samples from XYY and XXY males as analysed by in situ hybridization. Hum Reprod. 1996;11:1638–43. doi: 10.1093/oxfordjournals.humrep.a019461. [DOI] [PubMed] [Google Scholar]
- 46.Milazzo JP, Rives N, Mousset-Simeon N, Mace B. Chromosome constitution and apoptosis of immature germ cells present in sperm of two 47,XYY infertile males. Hum Reprod. 2006;21:1749–58. doi: 10.1093/humrep/del051. [DOI] [PubMed] [Google Scholar]
- 47.Moretti E, Anichini C, Sartini B, Collodel G. Sperm ultrastructure and meiotic segregation in an infertile 47, XYY man. Andrologia. 2007;39:229–34. doi: 10.1111/j.1439-0272.2007.00791.x. [DOI] [PubMed] [Google Scholar]
- 48.Autio-Harmainen H, Rapola J, Aula P. Fetal gonadal histology in XXXXY, XYY and XXX syndromes. Clin Genet. 1980;18:1–5. doi: 10.1111/j.1399-0004.1980.tb01356.x. [DOI] [PubMed] [Google Scholar]
- 49.Ferlin A, Arredi B, Speltra E, Cazzadore C, Selice R, Garolla A, et al. Molecular and clinical characterization of Y chromosome microdeletions in infertile men: a 10-year experience in Italy. J Clin Endocrinol Metab. 2007;92:762–70. doi: 10.1210/jc.2006-1981. [DOI] [PubMed] [Google Scholar]