Abstract
Background
Sphingolipids take part in immune response and can initiate and/or sustain inflammation. Various inflammatory diseases have been associated with increased ceramide content, and pharmacological reduction of ceramide diminishes inflammation damage in vivo. Inflammation and susceptibility to microbial infection are two elements in a vicious circle. Recently, sphingolipid metabolism inhibitors were used to reduce infection. Cystic Fibrosis (CF) is characterized by a hyper-inflammation and an excessive innate immune response, which fails to evolve into adaptive immunity and to eradicate acute infection. This results in chronic infections, lung damage, and patient morbidity. Indeed, ceramide content in mucosa airways is higher in CF mouse models and in patients than in control mice or healthy subjects.
Methods
The potential of the de novo ceramide synthesis inhibitor myriocin in CF therapy was investigated in cells and mice models.
Results
We treated CF human respiratory epithelial cells with myriocin, an inhibitor of the rate limiting enzyme involved in de novo ceramide synthesis Serine Palmitoyl Transferase (SPT). This treatment resulted in reduced basal, as well as TNFα -stimulated, inflammation. In turn, TNFα induced an increase in SPT in these cells, linking de novo synthesis of ceramide and inflammation in a noxious loop. Furthermore, myriocin-loaded nanocarrier, injected intratrachea prior to P.aeruginosa challenge, allowed a significant reduction of lung infection and reduced inflammation.
Conclusion
The presented data suggest that de novo sphingolipid synthesis is constitutively enhanced in CF mucosa and that it can be envisaged as pharmacological target for modulating inflammation and restoring effective innate immunity against acute infection.
General significance
Myriocin stands as a powerful immunomodulatory agent for inflammatory and infectious diseases.
Introduction
Sphingolipids (SPL) are a broad class of membrane components and signaling mediators involved in cell survival and function. It is becoming increasingly apparent that SPL take part to inflammation and to host innate response upon infection (1-3). The sphingolipid ceramide is highly effective in the activation of inflammation related transcription factors, such as NF-kB (4) and AP1(5) and in receptors clustering /signaling upon inflammatory stimula (6). Patients suffering from chronic inflammation such as irritable bowel syndrome (7), emphysema lung injury and chronic obstructive pulmonary disease (8-10), exhibit increased levels of mucosal ceramide as compared to controls. A vicious loop relates the excessive inflammation to the susceptibility to microbial infection. Hyper-inflammation associates with the inability to clear infections at their early stage, as well as to mature the adaptive immune response, thus allowing the chronic establishment of pathogens communities. Ceramide accumulation may derive from increased synthesis de novo or from altered metabolism of complex sphingolipids such as sphingomyelin or glycosphingolipids. The role of sphingomyelinases, neutral and acidic, in inflammation has been extensively investigated (11; 12). The hydrolysis of plasma-membrane sphingomyelin is responsible for ceramide-rich membrane platforms formation and required for signal transduction of inflammatory stimula, such as TNFα, ILβ, INFγ (6; 8; 13; 14), increased vascular permeability (15; 16) as well as for the internalization of microorganisms (13; 17). On the other side, only a few reports underscore the involvement of de novo synthesis of ceramide in the inflammatory responses (10; 18-20). Thus, emphysema lung damage (10) and immune-reactivity in murine dorsal horn of the lumbar spinal cord were reduced by Fumonisin B1, an inhibitor of ceramide synthase (18). Furthermore, mice feeding with Myriocin (Myr), an inhibitor of Serine Palmitoyl Transferase (SPT), decreased radiation- induced inflammation and fibrosis (21). A recent finding demonstrated that reduced de novo ceramide synthesis by fenretinide, associated with the increase of its precursor dihydroceramide, impairs bacterial infection in macrophages (19).
Cystic Fibrosis (CF) is an inherited autosomal recessive disease caused by CF transmembrane conductance regulator (CFTR) mutations. CF patients develop mucus viscosity, impaired mucociliary transport, hyper- inflammation and severe alteration of all mucosal functions with lung disease (22; 23) and chronic opportunistic infections (mainly Pseudomonas aeruginosa, Bhurkolderia cepacia complex, and Staphylococcus aureus) remaining the main cause of morbidity and mortality (24; 25). Inflammation is an independent risk factor for CF disease progression. Even the uninfected CF lungs of foetuses or two years old infants, develop a pathological inflammatory condition (26-29), confirming the severe immune alteration of mucosa in the respiratory tract.
In mouse models of CF, an age-related accumulation of ceramide in respiratory epithelium was associated to the pathological inflammatory state and infection susceptibility. Sphingomyelinase (9; 30; 31), or ceramide synthase (9) inhibition, or fenretinide, an inhibitor of ceramide formation from its precursor dihydro-ceramide (32), reduced inflammation and infection in CF mice.
Similarly, in CF patients, increased ceramide content was found in nasal respiratory epithelium and lungs (30; 33; 34). Amitriptyline, an inhibitor of acid sphingomyelinase used in a phase II study, reduced ceramide levels in the respiratory epithelial cells of treated patients and this was accompanied by a significant increase in lung function (30; 31).
In spite of the increased ceramide mass in CF mucosa, there is no evidence on what is the rate of ceramide synthesis versus its release from membrane sphingomyelin. We hypothesized that preventing de novo sphingolipid synthesis with Myr could reduce excessive lung inflammation in CF and allow an efficient innate response to acute infection. In this article we demonstrated that Myr reduces IL-8 and IL6 release in human CF respiratory epithelium. Given the hydrophobicity of the compound, we sought to deliver Myr in vivo, in murine airways, by means of Solid Lipid Nanoparticles (SLN) (35). We demonstrated that Myr- loaded SLN are able to reduce inflammation and infection in CF mice lung.
Materials and Methods
Reagents and Antibodies
Myr was purchased from Fermentek LTD (Israel), MTT and bovine serum albumin (BSA) were from Sigma-Aldrich (US). LHC Basal, LHC-8 w/o gentamicin culture media (Gibco, US), Penicillin and streptomycin (Invitrogen) were purchased from Life Technologies Italia (Italy). Fetal bovine serum (FBS) and the chemiluminescence system LiteAbLot were purchased from EuroClone Life Science Division (Italy). Human Fibronectin and Bovine Collagen were from Becton-Dickinson Italia (Italy). Human and mouse IL-8, IL-6 mini EDK and Human TNF-α were from Peprotech (UK). The synthetic oligonucleotides used in this study were purchased from M-Medical (Italy). All reagents were of the maximal available purity degree.
Myriocin stock solution preparation
Myr powder was weighted and dissolved in DMSO by warming up at 37°C, to a final concentration of 2 mM. Solution was sterile filtered (0.22 μm pore diameter, Nalgene) and stored at 4°C until used. This stock solution was diluted in medium for cell treatment (final treatment concentration: 10 μM) and in sterile saline for animal treatments (final treatment concentration: 420 μM, equal to 11,95 μg of Myr per mouse).
Myriocin loaded-Solid Lipid Nanoparticles (SLN) used for mice treatment
Treatment of mice with Myr was achieved by using Myr-loaded SLNs (Nanovector srl, Italy) prepared as previously described (35). SLN loaded with drug were measured for Myr content and a 1 mM Myr-SLN stock solution was prepared. This solution was diluted 1:12 in sterile saline and 75 μl (1.7 μg of Myr and 8% SLN) were used for each mouse administration in the airways.
Cell lines and treatments
IB3-1 cells, an adeno-associated virus-transformed human bronchial epithelial cell line derived from a CF patient (ΔF508/W1282X) and its isogenic C38 cells, corrected by insertion of CFTR, have been both obtained from LGC Promochem (US) and kindly provided by the Cystic Fibrosis animal Core Facility (CFaCore, San Raffaele Hospital, Milan, Italy). Cells were grown in LHC-8 media supplemented with 5% FBS. Both culture flasks and plates were coated with a solution of LHC-basal medium containing 35 μg/ml bovine collagen, 1 μg/ml bovine serum albumin and 10 μg/ml human fibronectin as described (36). For experiments, cells were seeded in 6 multi-wells plate or 100 mm petri dishes at 3×105 and 2×106 cells/plate respectively. Twenty four h after seeding, when cells reached about 60% confluence, medium was replaced with fresh one, containing either Myr (10 μM) or vehicle (DMSO). Eight h after Myr treatment, human TNFα (20ng/ml) was added to both treated and untreated cells. Incubation proceeded for further 18 h and then samples were collected for analyses.
MTT assay
Cell proliferation was examined in triplicate samples by the MTT assay as previously described (37). Proliferation index represents fold increase proliferation over vehicle-treated cells at time zero.
ELISA
Released IL-8 and IL-6 were determined in supernatants collected from the cell cultures using ELISA kits according to the manufacturer's instructions (Vinci Biochem, Italy). Values were normalized to 106 cells. KC and IL-6 concentration were determined in lung homogenates by ELISA, according to manufacturer's instructions.
LC–MS analysis
Sphingolipid extracts from both mice lungs and treated cells, fortified with internal standards (N-dodecanoylsphingosine, N-dodecanoylglucosylsphingosine, N-dodecanoylsphingosyl phosphorylcholine, C17-sphinganine (0.2 nmol each) and C17-sphinganine-1-phosphate (0.1 nmol), were prepared and analysed as reported (38).
Western blotting
Cells were scraped in ice-cold phosphate buffered saline (PBS) containing proteases inhibitors (Roche Italia, Milan, Italy) and spun at 1,200 × g for 5 min at 4°C. An aliquot was used for protein quantification; the remaining cells were resuspended in Laemmli buffer, boiled for 8 min and stored at -20°C. Cytosolic and nuclear extracts were obtained by NE-PER Nuclear and Cytoplasmic Extraction Reagents (Pierce, US), according to manufacturer protocols. Equal amount of proteins (20 μg for SPT and 15μg for NF-kB 40μg for IK-Bα) were separated on 10% acrylamide gels by SDS-electrophoresis and transferred onto nitrocellulose membranes. After blocking unspecific binding sites with 5% dry skimmed milk in PBS-Tween 0.1% (PBST), the membranes were incubated (47°C/overnight) with primary antibodies (Anti-Serine Palmitoyltransferase, anti-SPTLC 1 and 2, antibodies were kindly provided by Dr. T. Hornemann University Hospital Zürich, Switzerland; anti IK-Bα and anti NF-kB p65 were from Calbiochem, US) diluted 1:1,000 in PBST-3% BSA, followed by incubation (room temperature/2 h) with the appropriate HRP-secondary antibodies (Jackson Laboratories US) diluted 1:10,000 in PBST-3% BSA. The same membranes were immunoblotted against β-actin (dilution 1:5,000) for data normalization. Proteins were detected by chemiluminescence and bands intensity was quantified by Gel Doc 2000, using Quantity One Software (BioRad Life Science, US).
RNA extraction and quantitative RT-PCR
RNA extraction and quantitative RT-PCR for murine KC and IL-6 and human IL-8 and IL-6 were performed as previously reported (39). Human SPTLC 1 and 2 transcripts (mRNA) were evaluated by RT-PCR as previously reported (40).
Mice treatment and infection
Fourteen weeks old gut-corrected CFTR deficient mice B6.129P2- Cftrtm1UNCTgN(FABPCFTR) (group KO) and congenic wild-type (Case Western Reserve University, Cleveland, Ohio, USA) (group WT) male and female mice were used (41; 42).
Mice were housed in filtered cages under specific-pathogen conditions and permitted unlimited access to food and water. Once a deep stage of anesthesia with 2,2,2,-tribromoethanol (Avertin, Sigma-Aldrich, US) was reached, Myr either dissolved in DMSO or uploaded in SLN, was intra-trachea instilled by means of MicroSprayer® Aerosolizer – Model IA1C, attached to “FMJ-250 High Pressure Syringe” (Penn-Century Inc., US). Control animals were treated with the corresponding empty vehicle. The total volume introduced in lungs was 75 μl, in mice of approximately 30 gr each. This volume contained either 11.95 μg of compound/mouse in 10%DMSO-saline solution or 1.75 μg of compound/mouse in 8%SLN-saline solution. 24 h after treatment, animals (both KO and WT) were infected with 3-10 × 105 CFU of P. aeruginosa strain PAO1 planktonic cells, as previously described (43). After 18 h post-infection, mice were euthanized and lungs were perfused with PBS and homogenized in 1 ml of PBS containing protease inhibitors (Roche Italia, Italy). Part of the lung homogenates was centrifuged at 13,000 rpm for 30 minutes at 4°C and the supernatants were stored at −20°C for cytokine analysis. The remaining lung homogenate was divided in two aliquots: one part was lyophilized and lipids were extracted for LC–MS analysis; the other aliquot was serially diluted 1:10 in PBS and plated onto TSA plates to determine lung bacterial load. All experiments were performed with a minimum of five animals per group.
Ethics Statement
Animal studies were conducted according to protocols approved by the San Raffaele Scientific Institute (Milan, Italy) Institutional Animal Care and Use Committee (IACUC) and adhered strictly to the Italian Ministry of Health guidelines for the use and care of experimental animals.
Statistical analysis
Data significance was evaluated by unpaired two-tailed Student t-test (P < 0.05) or by one-way ANOVA followed by the Bonferroni multiple comparisons test when significant (P < 0.05). Data are expressed as mean ±SEM.
Results
1. Myriocin differently affects proliferation of IB3 vs C38 cells
To assess whether IB3 cells (a cystic fibrosis cell line, Δ508/W128X) and C38 cells (a cell line derived from IB3, but stably expressing the wild-type CFTR) respond to sphingolipid de novo synthesis inhibitor, we evaluated the proliferation rate of cells treated with Myr vs untreated control cells (DMSO vehicle only) for different times. Myr inhibitory effect on proliferation was significantly lower (50%) on IB3 with respect to C38 cells, at 48 and at 72 h (Figure 1A and B). In order to exclude that sphingolipid synthesis inhibition was affecting cell survival, we performed a Trypan blue exclusion test in IB3 and C38 cells treated with Myr. As shown in Figure 1C, percentage of alive cells was not affected by Myr treatment at any time point (24, 48, 72 h).
Figure 1. Myriocin differently affects proliferation of C38 vs IB3 cells.
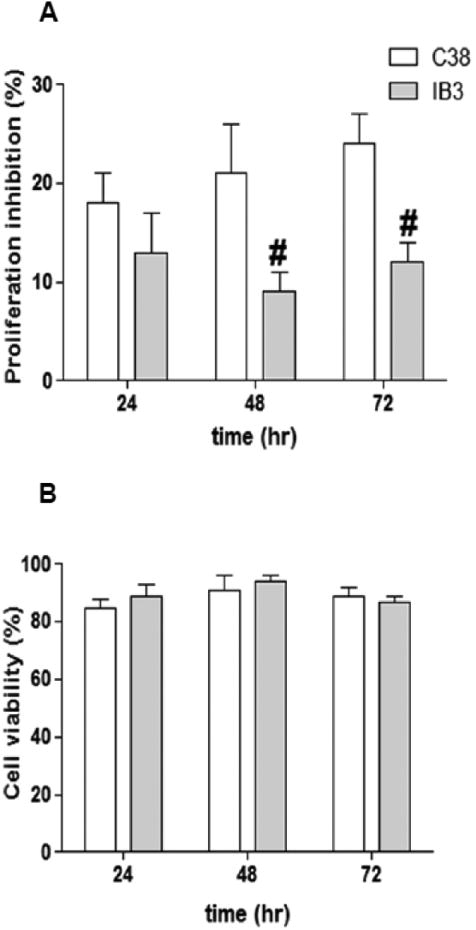
Inhibitory effect of Myr (10 μM) on IB3 and C38 cells proliferation at 24, 48, 72 h, expressed as percentage of proliferation inhibition in treated vs control (A). Significance was evaluated by one-way ANOVA as reported in Table 1. #, vs C38 cells. Cell viability, calculated as percentage of alive vs total cells in IB3 and C38 cells treated with Myr (10 μM) for 24, 48, 72 h (B).
2. Inflammation increases ceramide de novo synthesis and Myriocin reduces the inflammatory response in IB3 cells
To understand the above reported different behaviour of the two cell lines upon sphingolipid de novo synthesis inhibition, we measured ceramide in Myr pre-treated (8 h) and/or TNFα treated (24 h) cells. First of all, we observed a higher content of ceramide in IB3 than in C38 unstimulated cells (Figure 2A). The higher ceramide levels found in IB3 may account for their reduced sensitivity to the anti-proliferative effect of sphingolipid synthesis inhibition (see Figure 1 A). Second, we observed that ceramide was elevated by two folds in TNFα treated IB3 but not in C38 cells (Figure 2A), indicating that de novo synthesis of ceramide is hyper-stimulated in the CFTR mutant cells upon inflammatory stimulation. As expected, Myr treatment drastically reduced ceramide levels in both IB3 and C38 cell lines. Notably, in TNFα stimulated IB3 cells, Myr reduced ceramide levels from 900 ± 68.3 to 187 ± 62.7 pmoles/mg protein. To further assess the correlation between sphingolipid de novo synthesis and inflammatory signalling, we stimulated IB3 and C38 cells with TNFα and measured SPT (the sphingolipid synthesis rate-limiting enzyme, SPT1 and 2 subunits) transcription by Real Time PCR. TNFα induced an increase in SPT 1 and 2 mRNA levels. Such an increase was significantly higher in IB3 than in C38 cells (Figure 2B). The transcript increase was confirmed by an augmented protein expression of both subunits of the enzyme (Figure 2 C and 2 D).We then evaluated if sphingolipid synthesis inhibition was able to modulate cytokines release upon TNFα stimulation. IB3 and C38 cells were treated with TNFα (24 h), either alone or in combination with a pre-treatment with Myr (8 h). Myr significantly reduced TNFα stimulated IL-8 mRNA expression and protein release in IB3 cells, whereas cytokines expression and release was slightly affected in C38 treated cells (Figure 3 A and C). IL-6 mRNA expression was down-regulated by Myr alone both in C38 and IB3 cells. When cells were stimulated with TNFα, Myr was able to reduce (about 2 folds) the IL-6 mRNA in IB3 but no significant reduction was observed in C38 cell line (Figure 3 B). IL-6 protein release was not affected by either TNFα or by Myr stimulation in C38 cells. Whereas Myr alone did not alter the levels of secreted IL-6 protein in IB3 cells, it was effective in decreasing cytokines release induced by TNFα in IB3 (Figure 3 D).
Figure 2. TNFα differently induces de novo ceramide synthesis in IB3 vs C38 cells.
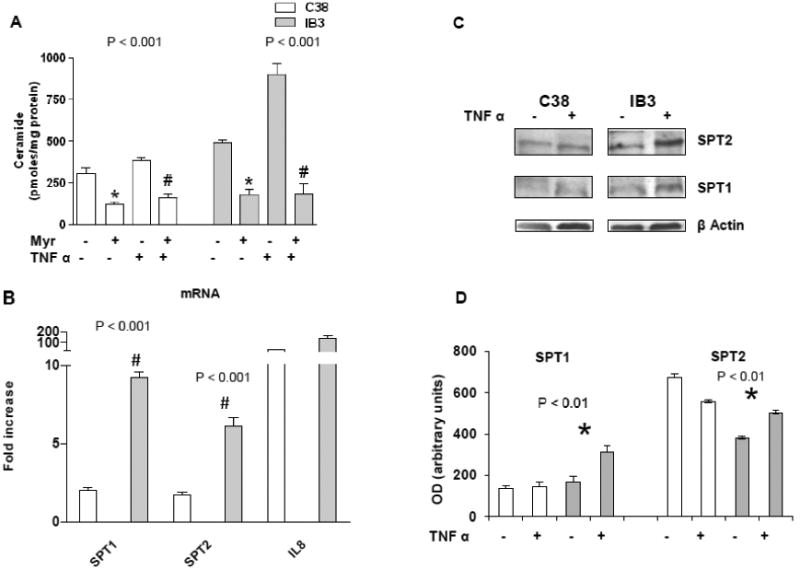
Cer quantitation, by LC–MS analysis, in IB3 and C38 cells stimulated with TNFα (20 ng/ml) either alone or in combination with Myr (10 μM, 8 h pre-treatment) for 24 h. Significance was evaluated by one-way ANOVA (value reported on the graphs). #, *, vs TNFα-treated and untreated cells (Bonferroni post-test: P <0.01 and P <0.001 in C38 and IB3, respectively) (A). Real time PCR of SPT 1 and 2 transcripts in C38 and IB3 cells cells stimulated with TNFα (20 ng/ml) for 24 hours. IL8 was evaluated as positive control of TNFα-induced inflammation. Data are expressed as fold increase of treated cells vs. untreated cells (B). SPT protein expression (SPT1, SPT2) in IB3 and C38 cells TNFα-treated and untreated for 24 h. β actin was used as loading control (C). Densitometric analysis of the SPT protein bands normalized on the corresponding β actin value. Medium value of three independent experiments. Significance was evaluated by one-way ANOVA (value reported on the graphs). SPT2: *, vs TNFα-treated IB3 and untreated C38 cells. SPT1: *, vs TNFα-treated C38 cells (Bonferroni post-test: P <0.05) (D).
Figure 3. Myriocin effect on IL-8 and IL-6 transcription and release in TNFα–stimulated IB3 and C38 cells.
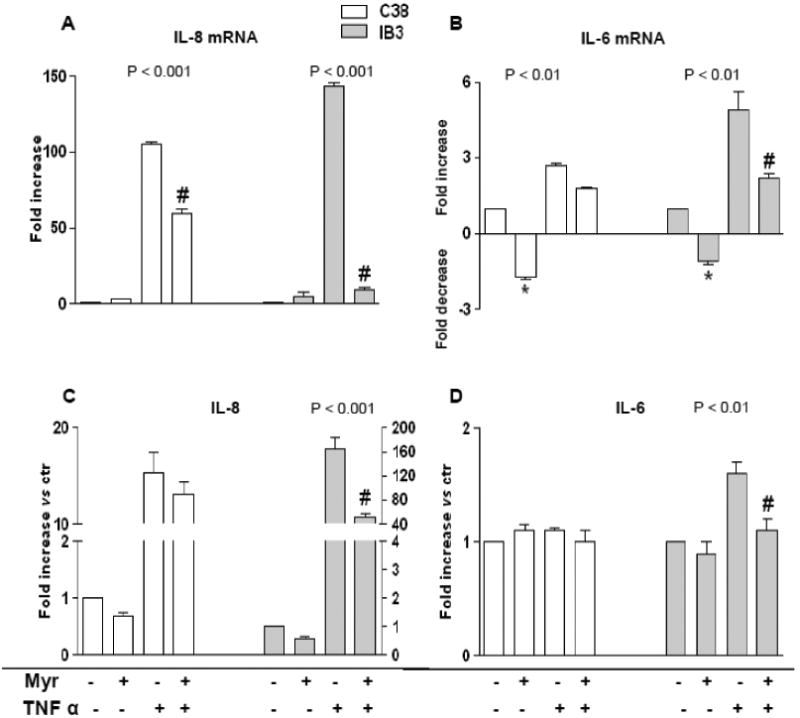
IL-8 and IL-6 mRNA expression (A and B, respectively) and protein release (C and D, respectively) in IB3 and C38 cells stimulated with TNFα (20 ng/ml) either alone or in combination with Myr (10 μM, 8 h pre-treatment) for 24 h. Significance was evaluated by one-way ANOVA (value reported on the graphs). Panel A, C: #, vs TNFα-treated cells (P <0.001, Bonferroni post-test). Panel B, D: #,*, vs TNFα-treated and untreated cells, respectively (P <0.05, Bonferroni post-test).
3. Myriocin downregulates NF-kB activation in IB3 cells
Human and murine CF epithelium was demonstrated to sustain inflammatory signaling via NF-kB activation (44-46).Therefore we investigated on Myr ability to alter NF-kB activation in human CF epithelial cell line. IB3 cells were treated with TNFα (24 h) either alone or in combination with Myr pre-treatment (8 h). Cytosolic fractions were separated from nuclear fractions. We observed that TNFα-induced degradation of the cytosolic Ik-Bα (the NF-kB inhibitor, Figure 4 A and B) and nuclear accumulation of NF-kB p65 (Figure 4 C and D), were both reversed by Myr.
Figure 4. Myriocin effect on NF-kB activation in TNFα–stimulated IB3.
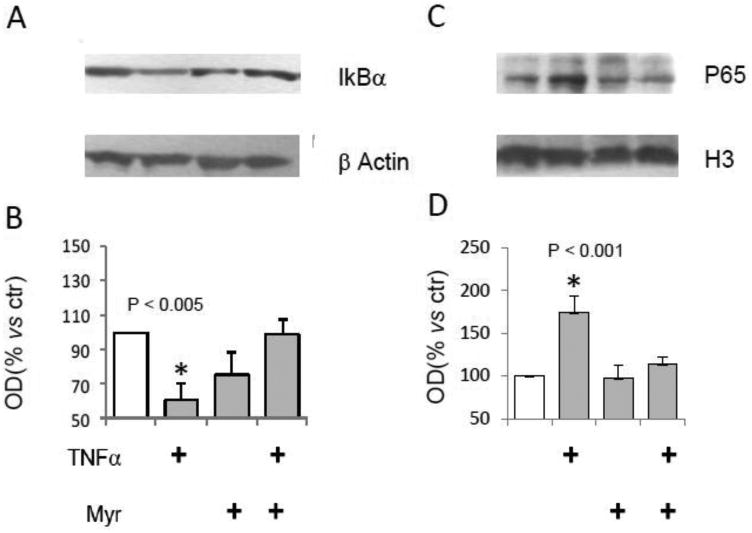
IB3 cells stimulated with TNFα (20 ng/ml) for 24 h. Western blotting evaluation of I-kB from cytosolic extracts, compared to β-actin expression (A). OD from optical densitometry of 3 independent experiments was reported (B). Western blotting evaluation of NF-kB p65 from nuclear extracts, compared to histone H3. Significance was evaluated by one-way ANOVA (value reported on the graphs, P <0.001, Bonferroni post-test).
4. Myriocin modulates P. aeruginosa airways inflammation in Cftrtm1UNCTgN(FABPCFTR) mice
At the aim of validating the results obtained on human cell lines in an in vivo model, Cftrtm1UNCTgN(FABPCFTR) mice (KO) and congenic control (WT) mice were treated with Myr dissolved in 10% DMSO, by intra-tracheal micro-sprayer nebulisation for 24 h prior to infection with P. aeruginosa (PAO1) by intra-tracheal injection. Animals were sacrificed 18 h after infection. Myr treatment was significantly effective in reducing lungs Cer level in infected KO mice (from 722 ± 9 to 313.3 ± 6.9 pmoles/mg protein) and small reduction was detected in infected WT mice lungs (from 784.5 ± 60.5 to 612.5 ± 129.5 pmoles/mg protein) (Figure 5).
Figure 5. Myriocin effect on ceramide lung content and infection in KO and WT mice.
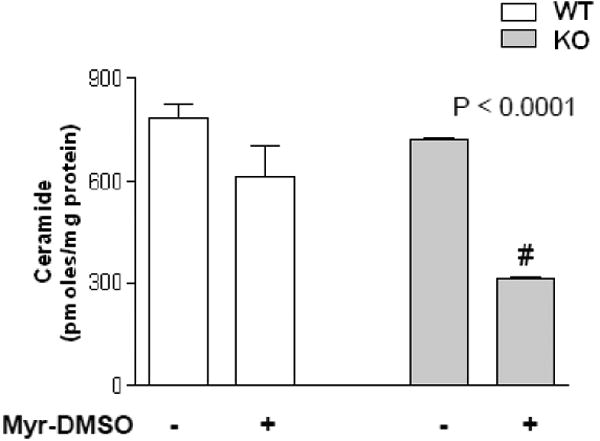
Cer quantitation, by LC–MS analysis, in KO and WT mice treated (or untreated, DMSO vehicle only) with DMSO-solved Myr (11.95 μg of Myr/mouse lungs) 24 h prior to infection with P. aeruginosa. Animals were sacrificed 18 h post infection. Significance was evaluated by unpaired two-tailed Student t-test. #, vs untreated mice.
On the contrary, sphingolipid synthesis inhibition was associated to a reduced KC mRNA content (Figure 6 A) as well as in KC protein level (Figure 6 C), both in KO and in WT mice lungs homogenate. IL-6 mRNA was down-modulated by Myr in both KO and WT animals and IL-6 protein release was reduced in both animal groups (Figure 6 B and D, respectively). All animal experiments have been repeated three times.
Figure 6. Myriocin effect on KC and IL-6 transcription and release in PAO1-infected lung of KO and WT mice.
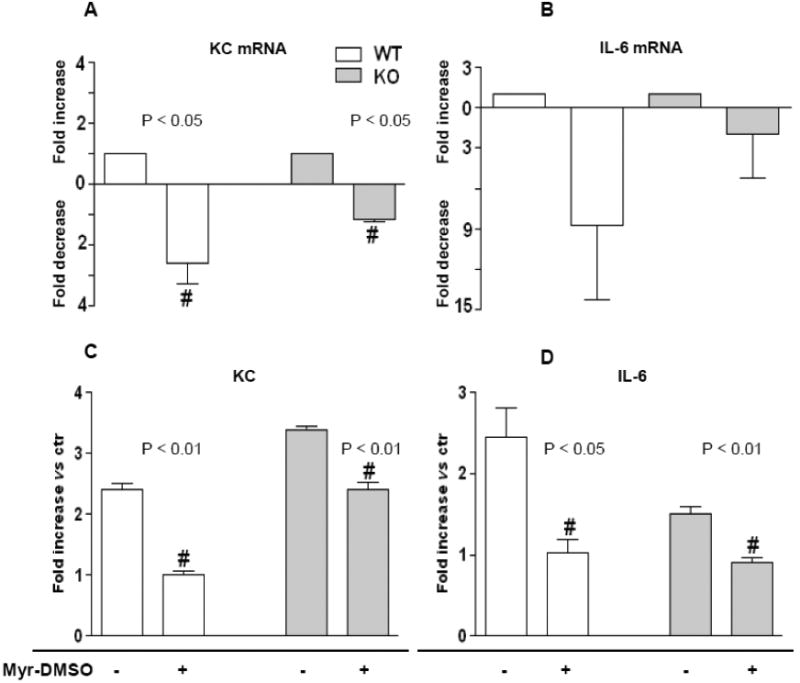
KC and IL-6 mRNA expression (A and B, respectively) and lung protein content (C and D, respectively) in KO and WT mice treated with DMSO-solved Myr (11.95 μg of Myr/mouse lungs) 24 h prior to infection with P. aeruginosa (PAO1). Animals were sacrificed 18 h post infection. mRNA values are expressed as fold change vs ctr (untreated mice). Protein values are expressed as fold increase vs ctr. Significance was evaluated by unpaired two-tailed Student t-test. #, vs ctr mice.
5. SLN delivery of Myriocin enhances its effects on infection and inflammation in murine airways
Myr is highly lipophilic and poorly soluble, even in a 10% DMSO solution. At the aim of optimizing lungs delivery and prolonging the effect of the inhibitor by increasing its half-life in the lungs, Myr was loaded into SLN and injected as aerosol solution, by intra-tracheal microsprayer nebulisation, 24 h prior to infection with P. aeruginosa. Animals were sacrificed at 18 h post infection. SLN- mediated Myr delivery ensured a high efficacy on sphingolipid synthesis inhibition as demonstrated by 37% or 60% of lungs ceramide reduction in WT and KO infected animals respectively (Figure 7 A). The inhibition of de novo ceramide synthesis in CF mouse airways was associated to a marked reduction of glucosyl-galactosyl ceramide pool. No changes were detected in the overall content of sphingomyelins (supplementary Figure 1). This suggests that in CF models, de novo generated ceramide can be preferentially metabolized by glycosylation. This hypothesis is in line with the anti-inflammatory effect of miglustat, an inhibitor of ceramide glycosylation, obtained by Dechecchi and coworkers (39).
Figure 7. Myriocin-SLN effect on ceramide and infection in KO and WT mice.
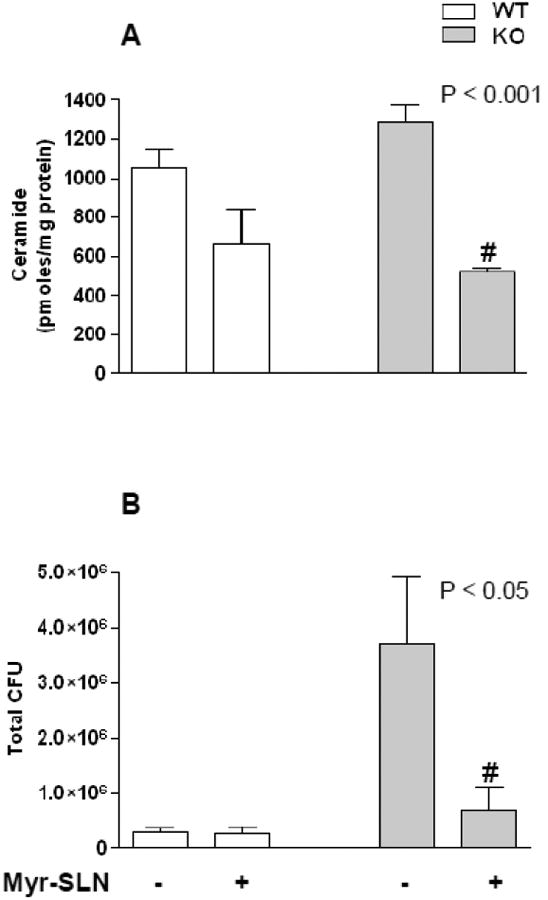
KO and WT mice treated with Myr-SLN (1.7 μg of Myr/mouse lungs) or untreated (empty SLN), 24 h prior to infection with P. aeruginosa (PAO1). Animals were sacrificed 18 h post infection. Cer quantitation, by LC–MS analysis (A). PAO1 colonies formation assay from lungs homogenate of infected KO and WT mice treated or untreated (B). Significance was evaluated by unpaired two-tailed Student t-test. #, vs untreated mice.
Notably, SLN- mediated Myr delivery reduced P.aeruginosa lung colonies 6 times in treated KO treated compared to untreated KO mice, while no differences were found between treated and untreated WT mice (Figure 7 B). Sphingolipid synthesis inhibition via Myr-loaded SLN was associated to a significant reduction in lungs KC and IL-6 mRNA expression (Figure 8 A and B). Lung KC and IL-6 protein levels were similarly down-regulated by Myr both in KO and WT infected mice. All animal experiments have been repeated three times.
Figure 8. Myriocin-SLN effect on KC and IL-6 transcription and release in PAO1-infected lung of KO and WT mice.
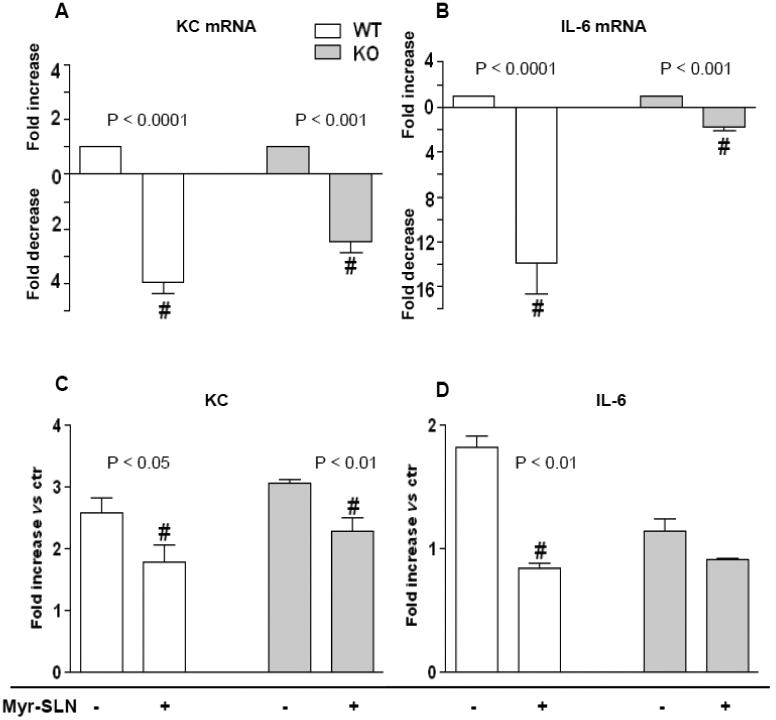
KO and WT mice treated with Myr-SLN (1.7 μg of Myr/mouse lungs) or untreated (empty SLN), 24 h prior to infection with P. aeruginosa (PAO1). Animals were sacrificed 18 h post infection. KC and IL-6 mRNA expression (A and B, respectively) and lungs protein content (C and D, respectively) in KO and WT mice. mRNA values are expressed as fold change vs ctr (untreated mice). Protein values are expressed as fold increase vs ctr. Significance was evaluated by unpaired two-tailed Student t-test. #, vs ctr.
Discussion
The role of sphingolipid de novo synthesis pathway in inflammation is still largely unexplored. The data presented in this article demonstrate for the first time that de novo sphingolipid synthesis is enhanced during inflammation and, in turn, it promotes the production of inflammatory mediators (Figure 2 and 4). In CF mice model, we proved that blocking SPL de novo synthesis can reduce hyper-inflammation favouring the recovery of an effective response to P.aeruginosa infection in the respiratory tract. It is important to consider that CF is a chronic inflammatory disease, prior to become an infective chronic disease: noteworthy even CF uninfected infants exhibit high levels of pulmonary inflammation (27). Hence, it is mandatory to cure innate immunity in CF patients and immunomodulatory agents are required to reduce the persistent inflammation that favours infection stabilization. From literature evidences it is clear that acute and chronic inflammation modulate sphingolipids and are, in turn, tuned by sphingolipid metabolites (2; 31; 39; 47). Accordingly, in CF human cells we showed: i) a basal higher amount of endogenously synthesized ceramide, that provides the cells with the ability to partially overcome the antiproliferative effect of sphingolipid de novo synthesis inhibition; ii) an increased expression of the rate limiting enzyme of sphingolipid de novo synthesis upon TNFα stimulation (Figure 2 B). We also proved that de novo sphingolipid synthesis inhibition in this cells reduced NF-kB activation (Figure 4) and IL8 and IL6 release (Figure 3) upon TNFα stimulation. These evidences demonstrate the existence of a noxious loop between ceramide de novo synthesis and inflammation in CF. Gulbins and colleagues demonstrated first in CF mice and then in CF human patients, that inhalation of an acid sphingomyelinase inhibitor reduces ceramide accumulation and lung burden of inflammation and infection (31). On the other side, in pulmonary infections, rapid acid sphingomyelinase activation and formation of ceramide-enriched membrane platforms was shown to be required for the internalization/clearence of different bacteria, including P.aeruginosa (17; 48). It was also found that infection of acid sphingomyelinase-deficient mice with P. aeruginosa caused increased bacterial load, cytokine storm (49) and finally death of the mice (50). Thus the activity of acid sphingomylinase seems to be required for a proper inflammatory response and for pathogens clearance (48; 51), casting doubt on a long term use of acid sphingomyelinase inhibitor, which may negatively impact on the immunity system of the respiratory tract. Petrache and co-workers demonstrated that increased rate of sphingolipid de novo synthesis is associated with inflammation and, importantly, with an increase in the secretion of acid sphingomyelinase, which is responsible for a high release of ceramide in respiratory mucosa of COPD mice. This ceramide contributes to mucus thickness and inflammation (10). Our hypothesis is that CFTR mutation, possibly by inducing endogenous stress, associates with an enhanced rate of de novo sphingolipid synthesis and with an increase in the content of ceramide within the respiratory mucosa. In addition to the block of ceramide release from secreted sphingomyelinase activity by amitriphteline, the correction of the hyper-stimulated de novo synthesis, which may be responsible of increased sphingomyelinase activity (10) by Myr, is able to counteract the excessive inflammatory reaction upon infection and to favour bacteria clearance.
Sphingolipid targeting for human therapy has the problem of hydrophobicity of most of the known metabolism inhibitors. Lipophilic ceramide was previously delivered in vivo by means of liposomes carriers (52). Recently, the use of solid lipid nanocarriers was successfully experienced in mouse eye, by external administration of eye drops containing Myr-loaded SLN (35). We here demonstrate that surfactant like containing nanoparticles allow a good uptake and delivery of Myr. Comparing the efficacy of the compound in terms of ceramide content reduction, we observed approximately a 7 fold decrease in the effective drug concentration when using SLN delivery in respect to the concentration required when Myr was dissolved in DMSO and directly diluted into saline. It is feasible that aqueous suspensions and perhaps dry powder formulations of SLN can be used for pulmonary inhalation. We think that SLN may be a very important tool for any drug targeting sphingolipid metabolism in vivo.
Supplementary Material
Highlights.
De novo synthesis of ceramide is increased in CF respiratory tract
Myriocin reduces ceramide in CF respiratory tract
Myriocin reduces inflammation in CF respiratory tract
Nanocarriers delivery of myriocin in vivo reduces lung infection in CF mice
Acknowledgments
We thank the Italian Cystic Fibrosis Research Fundation for financial, technical, administrative support and post-doctoral fellowship. We thank Dr Horneman University Hospital Zürich, Switzerland for kindly providing us with anti-SPT antibodies. We thank Dr. Marco Trinchera, Università dell'Insubria, Italy for kind support and advices. We thank Eva Dalmau for her excellent technical assistance. We thank Dr.Giovanna Riccio for setting up HPLC-RI method for myriocin analysis and quantitation and for her valuable technical contribution to the project. Financial support from the Institutional Grants of University of Milan and the PhD program in Molecular Medicine, Università di Milano, Italy is acknowledged. Financial support from Generalitat de Catalunya (grant SGR2009 1072), Ministerio de Economía y Competitividad (grant SAF2011 22444) and Fundació La Marató de TV3 (grant 112130) is acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chalfant CE, Spiegel S. Sphingosine 1-phosphate and ceramide 1-phosphate: expanding roles in cell signaling. Journal of cell science. 2005;118:4605–12. doi: 10.1242/jcs.02637. [DOI] [PubMed] [Google Scholar]
- 2.El Alwani M, Wu BX, Obeid LM, Hannun YA. Bioactive sphingolipids in the modulation of the inflammatory response. Pharmacol Ther. 2006;112:171–83. doi: 10.1016/j.pharmthera.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 3.Miyake Y, Kozutsumi Y, Nakamura S, Fujita T, Kawasaki T. Serine palmitoyltransferase is the primary target of a sphingosine-like immunosuppressant, ISP-1/myriocin. Biochemical and biophysical research communications. 1995;211:396–403. doi: 10.1006/bbrc.1995.1827. [DOI] [PubMed] [Google Scholar]
- 4.Schutze S, Potthoff K, Machleidt T, Berkovic D, Wiegmann K, Kronke M. TNF activates NF-kappa B by phosphatidylcholine-specific phospholipase C-induced “acidic” sphingomyelin breakdown. Cell. 1992;71:765–76. doi: 10.1016/0092-8674(92)90553-o. [DOI] [PubMed] [Google Scholar]
- 5.Sawai H, Okazaki T, Yamamoto H, Okano H, Takeda Y, et al. Requirement of AP-1 for ceramide-induced apoptosis in human leukemia HL-60 cells. The Journal of biological chemistry. 1995;270:27326–31. doi: 10.1074/jbc.270.45.27326. [DOI] [PubMed] [Google Scholar]
- 6.Kim MY, Linardic C, Obeid L, Hannun Y. Identification of sphingomyelin turnover as an effector mechanism for the action of tumor necrosis factor alpha and gamma-interferon. Specific role in cell differentiation. The Journal of biological chemistry. 1991;266:484–9. [PubMed] [Google Scholar]
- 7.Kajander K, Myllyluoma E, Kyronpalo S, Rasmussen M, Sipponen P, et al. Elevated pro-inflammatory and lipotoxic mucosal lipids characterise irritable bowel syndrome. World J Gastroenterol. 2009;15:6068–74. doi: 10.3748/wjg.15.6068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu DY, Chen HC, Yang MS, Hsu YM, Lin HJ, et al. Ceramide and Toll-like receptor 4 are mobilized into membrane rafts in response to Helicobacter pylori infection in gastric epithelial cells. Infection and immunity. 2012;80:1823–33. doi: 10.1128/IAI.05856-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bodas M, Min T, Mazur S, Vij N. Critical modifier role of membrane-cystic fibrosis transmembrane conductance regulator-dependent ceramide signaling in lung injury and emphysema. J Immunol. 2011;186:602–13. doi: 10.4049/jimmunol.1002850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petrache I, Natarajan V, Zhen L, Medler TR, Richter AT, et al. Ceramide upregulation causes pulmonary cell apoptosis and emphysema-like disease in mice. Nature medicine. 2005;11:491–8. doi: 10.1038/nm1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Milhas D, Clarke CJ, Hannun YA. Sphingomyelin metabolism at the plasma membrane: implications for bioactive sphingolipids. FEBS letters. 2010;584:1887–94. doi: 10.1016/j.febslet.2009.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grassme H, Becker KA. Bacterial infections and ceramide. Handbook of experimental pharmacology. 2013:305–20. doi: 10.1007/978-3-7091-1511-4_15. [DOI] [PubMed] [Google Scholar]
- 13.Oda M, Hashimoto M, Takahashi M, Ohmae Y, Seike S, et al. Role of sphingomyelinase in infectious diseases caused by Bacillus cereus. PloS one. 2012;7:e38054. doi: 10.1371/journal.pone.0038054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dhami R, He X, Schuchman EH. Acid sphingomyelinase deficiency attenuates bleomycin-induced lung inflammation and fibrosis in mice. Cellular physiology and biochemistry: international journal of experimental cellular physiology, biochemistry, and pharmacology. 2010;26:749–60. doi: 10.1159/000322342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gulbins E, Kolesnick R. Raft ceramide in molecular medicine. Oncogene. 2003;22:7070–7. doi: 10.1038/sj.onc.1207146. [DOI] [PubMed] [Google Scholar]
- 16.Kowalski MP, Pier GB. Localization of cystic fibrosis transmembrane conductance regulator to lipid rafts of epithelial cells is required for Pseudomonas aeruginosa-induced cellular activation. J Immunol. 2004;172:418–25. doi: 10.4049/jimmunol.172.1.418. [DOI] [PubMed] [Google Scholar]
- 17.Grassme H, Gulbins E, Brenner B, Ferlinz K, Sandhoff K, et al. Acidic sphingomyelinase mediates entry of N. gonorrhoeae into nonphagocytic cells. Cell. 1997;91:605–15. doi: 10.1016/s0092-8674(00)80448-1. [DOI] [PubMed] [Google Scholar]
- 18.Ndengele MM, Cuzzocrea S, Masini E, Vinci MC, Esposito E, et al. Spinal ceramide modulates the development of morphine antinociceptive tolerance via peroxynitrite-mediated nitroxidative stress and neuroimmune activation. The Journal of pharmacology and experimental therapeutics. 2009;329:64–75. doi: 10.1124/jpet.108.146290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu H, Valerio M, Bielawski J. Fenretinide Inhibited de novo Ceramide Synthesis and Pro-inflammatory Cytokines Induced by A. actinomycetemcomitans. Journal of lipid research. 2012 doi: 10.1194/jlr.M031427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kroesen BJ, Pettus B, Luberto C, Busman M, Sietsma H, et al. Induction of apoptosis through B-cell receptor cross-linking occurs via de novo generated C16-ceramide and involves mitochondria. The Journal of biological chemistry. 2001;276:13606–14. doi: 10.1074/jbc.M009517200. [DOI] [PubMed] [Google Scholar]
- 21.Maiuri L, Raia V, De Marco G, Coletta S, de Ritis G, et al. DNA fragmentation is a feature of cystic fibrosis epithelial cells: a disease with inappropriate apoptosis? FEBS letters. 1997;408:225–31. doi: 10.1016/s0014-5793(97)00347-5. [DOI] [PubMed] [Google Scholar]
- 22.Rowe SM, Miller S, Sorscher EJ. Cystic fibrosis. The New England journal of medicine. 2005;352:1992–2001. doi: 10.1056/NEJMra043184. [DOI] [PubMed] [Google Scholar]
- 23.McKone EF, Emerson SS, Edwards KL, Aitken ML. Effect of genotype on phenotype and mortality in cystic fibrosis: a retrospective cohort study. Lancet. 2003;361:1671–6. doi: 10.1016/S0140-6736(03)13368-5. [DOI] [PubMed] [Google Scholar]
- 24.Cohen TS, Prince A. Cystic fibrosis: a mucosal immunodeficiency syndrome. Nature medicine. 2012;18:509–19. doi: 10.1038/nm.2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clunes LA, Davies CM, Coakley RD, Aleksandrov AA, Henderson AG, et al. Cigarette smoke exposure induces CFTR internalization and insolubility, leading to airway surface liquid dehydration. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2012;26:533–45. doi: 10.1096/fj.11-192377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verhaeghe C, Delbecque K, de Leval L, Oury C, Bours V. Early inflammation in the airways of a cystic fibrosis foetus. Journal of cystic fibrosis: official journal of the European Cystic Fibrosis Society. 2007;6:304–8. doi: 10.1016/j.jcf.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 27.Khan TZ, Wagener JS, Bost T, Martinez J, Accurso FJ, Riches DW. Early pulmonary inflammation in infants with cystic fibrosis. American journal of respiratory and critical care medicine. 1995;151:1075–82. doi: 10.1164/ajrccm/151.4.1075. [DOI] [PubMed] [Google Scholar]
- 28.Muhlebach MS, Noah TL. Endotoxin activity and inflammatory markers in the airways of young patients with cystic fibrosis. American journal of respiratory and critical care medicine. 2002;165:911–5. doi: 10.1164/ajrccm.165.7.2107114. [DOI] [PubMed] [Google Scholar]
- 29.Gangell C, Gard S, Douglas T, Park J, de Klerk N, et al. Inflammatory responses to individual microorganisms in the lungs of children with cystic fibrosis. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2011;53:425–32. doi: 10.1093/cid/cir399. [DOI] [PubMed] [Google Scholar]
- 30.Riethmuller J, Anthonysamy J, Serra E, Schwab M, Doring G, Gulbins E. Therapeutic efficacy and safety of amitriptyline in patients with cystic fibrosis. Cellular physiology and biochemistry: international journal of experimental cellular physiology, biochemistry, and pharmacology. 2009;24:65–72. doi: 10.1159/000227814. [DOI] [PubMed] [Google Scholar]
- 31.Becker KA, Riethmuller J, Luth A, Doring G, Kleuser B, Gulbins E. Acid sphingomyelinase inhibitors normalize pulmonary ceramide and inflammation in cystic fibrosis. American journal of respiratory cell and molecular biology. 2010;42:716–24. doi: 10.1165/rcmb.2009-0174OC. [DOI] [PubMed] [Google Scholar]
- 32.Vilela RM, Lands LC, Meehan B, Kubow S. Inhibition of IL-8 release from CFTR-deficient lung epithelial cells following pre-treatment with fenretinide. International immunopharmacology. 2006;6:1651–64. doi: 10.1016/j.intimp.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 33.Brodlie M, McKean MC, Johnson GE, Gray J, Fisher AJ, et al. Ceramide is increased in the lower airway epithelium of people with advanced cystic fibrosis lung disease. American journal of respiratory and critical care medicine. 2010;182:369–75. doi: 10.1164/rccm.200905-0799OC. [DOI] [PubMed] [Google Scholar]
- 34.Teichgraber V, Ulrich M, Endlich N, Riethmuller J, Wilker B, et al. Ceramide accumulation mediates inflammation, cell death and infection susceptibility in cystic fibrosis. Nature medicine. 2008;14:382–91. doi: 10.1038/nm1748. [DOI] [PubMed] [Google Scholar]
- 35.Strettoi E, Gargini C, Novelli E, Sala G, Piano I, et al. Inhibition of ceramide biosynthesis preserves photoreceptor structure and function in a mouse model of retinitis pigmentosa. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:18706–11. doi: 10.1073/pnas.1007644107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zeitlin PL, Lu L, Rhim J, Cutting G, Stetten G, et al. A cystic fibrosis bronchial epithelial cell line: immortalization by adeno-12-SV40 infection. American journal of respiratory cell and molecular biology. 1991;4:313–9. doi: 10.1165/ajrcmb/4.4.313. [DOI] [PubMed] [Google Scholar]
- 37.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. Journal of immunological methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 38.Munoz-Olaya JM, Matabosch X, Bedia C, Egido-Gabas M, Casas J, et al. Synthesis and biological activity of a novel inhibitor of dihydroceramide desaturase. ChemMedChem. 2008;3:946–53. doi: 10.1002/cmdc.200700325. [DOI] [PubMed] [Google Scholar]
- 39.Dechecchi MC, Nicolis E, Mazzi P, Cioffi F, Bezzerri V, et al. Modulators of sphingolipid metabolism reduce lung inflammation. American journal of respiratorycell and molecular biology. 2011;45:825–33. doi: 10.1165/rcmb.2010-0457OC. [DOI] [PubMed] [Google Scholar]
- 40.Hornemann T, Richard S, Rutti MF, Wei Y, von Eckardstein A. Cloning and initial characterization of a new subunit for mammalian serine-palmitoyltransferase. The Journal of biological chemistry. 2006;281:37275–81. doi: 10.1074/jbc.M608066200. [DOI] [PubMed] [Google Scholar]
- 41.Bragonzi A, Paroni M, Nonis A, Cramer N, Montanari S, et al. Pseudomonas aeruginosa microevolution during cystic fibrosis lung infection establishes clones with adapted virulence. American journal of respiratory and critical care medicine. 2009;180:138–45. doi: 10.1164/rccm.200812-1943OC. [DOI] [PubMed] [Google Scholar]
- 42.Paroni M, Moalli F, Nebuloni M, Pasqualini F, Bonfield T, et al. Response of CFTR-Deficient Mice to Long-Term chronic Pseudomonas aeruginosa Infection and PTX3 Therapy. The Journal of infectious diseases. 2012 doi: 10.1093/infdis/jis636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bragonzi A. Murine models of acute and chronic lung infection with cystic fibrosis pathogens. International journal of medical microbiology: IJMM. 2010;300:584–93. doi: 10.1016/j.ijmm.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 44.Finotti A, Borgatti M, Bezzerri V, Nicolis E, Lampronti I, et al. Effects of decoy molecules targeting NF-kappaB transcription factors in Cystic fibrosis IB3-1 cells:recruitment of NF-kappaB to the IL-8 gene promoter and transcription of the IL-8 gene. Artificial DNA, PNA & XNA. 2012;3:97–296. doi: 10.4161/adna.21061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ziady AG, Sokolow A, Shank S, Corey D, Myers R, et al. Interaction with CREB binding protein modulates the activities of Nrf2 and NF-kappaB in cystic fibrosis airway epithelial cells. American journal of physiology Lung cellular and molecular physiology. 2012;302:L1221–31. doi: 10.1152/ajplung.00156.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Venkatakrishnan A, Stecenko AA, King G, Blackwell TR, Brigham KL, et al. Exaggerated activation of nuclear factor-kappaB and altered IkappaB-beta processing in cystic fibrosis bronchial epithelial cells. American journal of respiratory cell and molecular biology. 2000;23:396–403. doi: 10.1165/ajrcmb.23.3.3949. [DOI] [PubMed] [Google Scholar]
- 47.von Bismarck P, Winoto-Morbach S, Herzberg M, Uhlig U, Schutze S, et al. IKK NBD peptide inhibits LPS induced pulmonary inflammation and alters sphingolipid metabolism in a murine model. Pulm Pharmacol Ther. 2012;25:228–35. doi: 10.1016/j.pupt.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 48.Utermohlen O, Karow U, Lohler J, Kronke M. Severe impairment in early host defense against Listeria monocytogenes in mice deficient in acid sphingomyelinase. J Immunol. 2003;170:2621–8. doi: 10.4049/jimmunol.170.5.2621. [DOI] [PubMed] [Google Scholar]
- 49.Jbeily N, Suckert I, Gonnert FA, Acht B, Bockmeyer CL, et al. Hyperresponsiveness of mice deficient in plasma-secreted sphingomyelinase reveals its pivotal role in early phase of host response. Journal of lipid research. 2013;54:410–24. doi: 10.1194/jlr.M031625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grassme H, Jendrossek V, Riehle A, von Kurthy G, Berger J, et al. Host defense against Pseudomonas aeruginosa requires ceramide-rich membrane rafts. Nature medicine. 2003;9:322–30. doi: 10.1038/nm823. [DOI] [PubMed] [Google Scholar]
- 51.Schramm M, Herz J, Haas A, Kronke M, Utermohlen O. Acid sphingomyelinase is required for efficient phago-lysosomal fusion. Cellular microbiology. 2008;10:1839–53. doi: 10.1111/j.1462-5822.2008.01169.x. [DOI] [PubMed] [Google Scholar]
- 52.Zolnik BS, Stern ST, Kaiser JM, Heakal Y, Clogston JD, et al. Rapid distribution of liposomal short-chain ceramide in vitro and in vivo. Drug metabolism and disposition: the biological fate of chemicals. 2008;36:1709–15. doi: 10.1124/dmd.107.019679. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


