Abstract
PURPOSE
To identify favored choice of transplantation in patients with acute promyelocytic leukemia in second complete remission.
PATIENTS
We studied 294 acute promyelocytic leukemia (APL) patients receiving allogeneic (n=232) or autologous (62) hematopoietic cell transplantation (HCT) in second complete remission (CR2) reported to the Center for International Blood and Marrow Transplantation Research (CIBMTR) from 1995 to 2006 including pre-HCT PML/RAR∝ status in 155 (49% of allogeneic and 66% of autologous).
METHODS
Patient characteristics and transplant characteristics including treatment related mortality, overall survival, and disease free survival were collected and analyzed for both univariate and multivariate outcomes.
RESULTS
With median follow-up of 115 (allogeneic) and 72 months (autologous), 5-year disease-free survival (DFS) favored autologous 63% (49-75%) compared to allogeneic 50% (44-57%) (p=0.10) and overall survival (OS) 75% (63-85%) vs. 54% (48-61%) (p=.002) Multivariate analysis showed significantly worse DFS after allogeneic HCT (HR=1.88, 95% CI=1.16-3.06, p=0.011) and age >40 years (HR=2.30, 95% CI 1.44-3.67, p=0.0005). OS was significantly worse after allogeneic HCT (HR=2.66, 95%CI 1.52-4.65, p=0.0006; age >40 (HR=3.29, 95% CI 1.95-5.54, p<0.001) and CR1<12 months (HR=1.56 95% CI 1.07-2.26, p=0.021). Positive pre-HCT PML-RAR∝ status in 17/114 allogeneic and 6/41 autologous transplants did not influence relapse, treatment failure or survival in either group. The survival advantage for autografting was attributable to increased 3 years TRM: allogeneic 30%; autologous 2%, and GVHD.
CONCLUSION
We conclude that autologous HCT yields superior overall survival for APL in CR2. Long term DFS in autologous recipients, even with MRD+ grafts remains an important subject for further study.
Introduction
Acute promyelocytic leukemia (APL) accounts for 10-15% of de novo acute myeloid leukemia in younger adults [1]. Therapeutic strategies of ATRA, anthracycline-based induction, and recently arsenic trioxide (ATO) yield remission rates of approximately 90%, most of which are durable with 2-year DFS of 79% and overall survival (OS) of 92%, respectively [2, 3]. For the 10-20% of patients who relapse, recent evidence suggests that ATO re-induction can lead to second complete remission (CR2) in up to 85% of relapsed, previously arsenic naive patients [4, 5]. Although hematopoietic cell transplantation (HCT) is the generally accepted therapy for APL in CR2, the choice of allogeneic vs. autologous HCT remains controversial. It is uncertain whether the increased treatment-related mortality (TRM) generally associated with allografts is compensated for by a lower relapse rate due to a graft vs. APL effect. It has also been suggested that the outcome for autologous HCT is best if molecularly negative cells are collected [6, 7], the relative impact of persistent PML-RAR∝ transcripts prior to allograft or autologous HCT on outcome is uncertain In this retrospective study, we analyzed allogeneic and autologous HCT in CR2 to compare their toxicities and survival outcomes and to evaluate the impact of residual molecularly detectable pre-transplant marrow disease on outcome.
Methods
This multi-institutional, international, retrospective study used data on autologous and allogeneic HCT for APL in CR2 reported to the Center for International Blood and Marrow Transplant Research (CIBMTR) from 79 centers in 18 countries during the years 1995-2006. Variables analyzed for their influence on outcome included age, time from CR1 to relapse (< or >12 months), and for allografts type of donor. In addition to the information available from standard CIBMTR report forms, supplemental data were retrieved from the HCT centers to determine molecular and cytogenetic results prior to HCT, time from CR2 to transplant and therapy used to attain CR2. Evidence of positivity of disease status just prior to transplantation defined minimal residual disease (MRD). Supplemental forms collected both quantitative and qualitative molecular and cytogenetic data for establishment of MRD. Supplemental data were retrieved in 114/232 (49%) of allogeneic and 41/62 (66%) of autologous cases.
Clinical and demographic characteristics of the two groups were examined using Chi-square statistics. Additional analyses compared the characteristics of patients with or without the requested supplemental data to insure generalizability of data from the smaller cohort with this added detail available. Defined end points included disease free survival (DFS) as the time from HCT to death or relapse. Relapse was defined as time to hematologic recurrence and TRM was defined as death within the first 21 days or death prior to relapse. Overall survival (OS) was calculated as the time from HCT to death or last contact alive. Statistical analysis was performed using proportional hazards regression models. The proportional hazards assumptions for all variables were examined and all variables met the assumptions. A stepwise selection procedure was used to select variables significantly associated with the outcomes. Since the type of transplant was the main effect of interest, it was included in all steps of the model building process. Risk factors with a significance level of 0.05 were included in the final models. The interaction between the main effect of transplant type and all significant covariates was examined and no interaction was found to be statistically significant. All analyses were conducted using SAS V9.3.
Results
Patient characteristics and transplant related factors are shown in Table 1. The numbers of allogeneic HCT decreased over time, while the relative number of autologous HCT increased in more recent years.
Table 1.
Patient and Transplant Characteristics
| Patient Characteristics | Allogeneic | Autologous | p-value |
|---|---|---|---|
| Number of Patients | 232 (%) | 62 (%) | |
| Age at Transplant | <0.001 | ||
| <20 years | 70 (30) | 9 (15) | |
| 20-40 years | 93 (40) | 19 (31) | |
| >40 years | 69 (30) | 34 (55) | |
| Year of HCT | <0.001 | ||
| 1995-2000 | 145 (63) | 25 (40) | |
| 2001-2006 | 87 (38) | 37 (60) | |
| Sex | 0.18 | ||
| Male | 116 (50) | 37 (60) | |
| Female | 116 (50) | 25 (40) | |
| KPS | 0.91 | ||
| >90% | 178 (77) | 46 (74) | |
| <90% | 48(21) | 14 (23) | |
| Missing | 6 (3) | 2 (3) | |
| Time from CR1 to Relapse (median) | 14 (<1-87) | 17 (<1-85) | 0.08 |
| <12 months | 83 (36) | 18 (29) | |
| >12 Months | 130 (56) | 33 (53) | |
| Missing | 19 (8) | 11 (18) | |
| Time from CR2 to HCT (median) | 2 (<1-45) | 2 (<1-10) | 0.19 |
| <6 months | 202 (87) | 59 (95) | |
| >6 months | 28 (12) | 3 (5) | |
| Missing | 2 (<1) | -- | |
| Conditioning Regimen | |||
| Myeloablative | 213 (92) | 55 (89) | |
| RIC | 15 (7) | 5 (8) | |
| Missing | 4 (2) | 2 (4) | |
| TBI containing | 116 (50) | 47 (76) | 0.001 |
| Non-TBI | 114 (49) | 15 (24) | |
| Donor Type | N/A | ||
| HLA identical Sibling | 124 (53) | ||
| URD well/partially matched | 63 (27) | ||
| Other related | 45 (19) | ||
| Stem cell source | <0.001 | ||
| Marrow | 154 (66) | 8 (12) | |
| Peripheral blood | 78 (34) | 54 (88) | |
| GVHD prophylaxis CSA/Tac±MMF | 203 (88) | N/A | |
| T cell depletion+post-HCT immunosuppression | 24 (10) | ||
| Other | 5 (2) |
†KPS - Karnofsky performance score prior to conditioning, RIC - reduced intensity transplant, TBI- Total Body Irradiation, HLA - human leukocyte antigen, URD - unrelated donor, GVHD - graft vs host disease, CSA - cyclosporine, Tac - tacrolimus, MMF – mycophenolate mofetil.
Patient demographics including KPS, and sex were not statistically different between allogeneic and autologous recipients. However, allogeneic recipients were significantly younger. Disease-related factors including histologic subtype, white blood cell (WBC) count at diagnosis, cytogenetic and/or molecular positivity at diagnosis, time from CR1 to relapse, and time from CR2 to transplant were not significantly different. The use of arsenic trioxide (ATO) occurred during the period of study, representing a major change in the treatment of APL. For this reason, we evaluated the impact of ATO therapy prior to transplantation. In univariate and multivariate analysis we observed no impact of ATO-containing vs. non-ATO pre-HCT therapy on the risk of relapse after HCT [HR 0.598 (0.230-1.456) p=0.25].
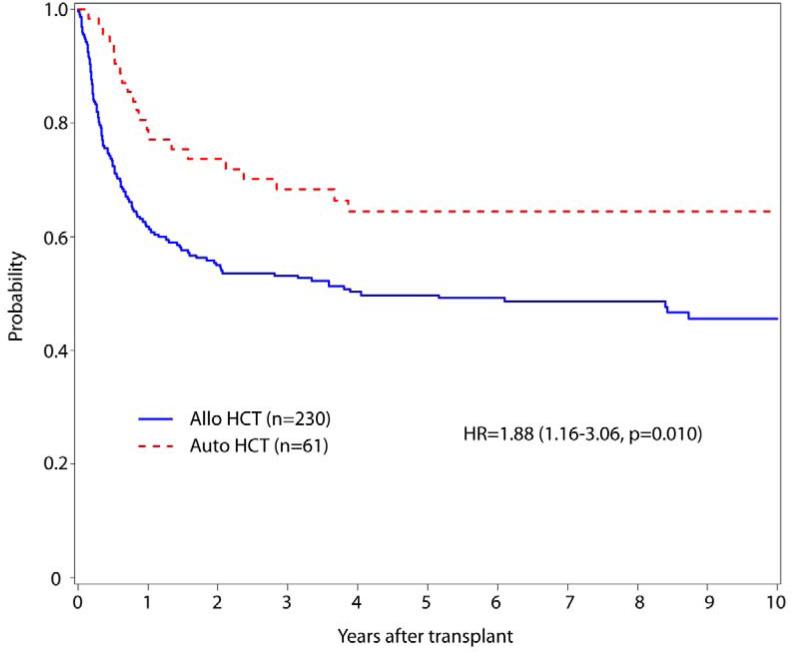
The vast majority of autologous transplants were myeloablative using standard regimens, most often using total body irradiation (TBI, Table 1). Univariate outcomes are shown in Table 2; with favorable OS, DFS and TRM observed in autologous HCT. Only a modest and not significant reduction in relapse followed allogeneic HCT. Five-year OS for the autologous 75% (63-85%) vs. allogeneic cohort was 54% (48-61%)(Figure 2). The risk of overall mortality (inverse of OS) was higher after allogeneic transplant compared to autologous [HR 2.66 (1.52-4.65) p=0.0006]. DFS at five years also favored autologous HCT 63% (49-75%) compared to 50% (44-57%) in allogeneic cohort (Figure 3). Equivalently, the risk of treatment failure (inverse of DFS) was higher after allogeneic transplant [HR 1.88 (1.16-3.06), p=0.011). Multivariate analysis showed that age >40 also adversely influenced overall mortality [HR of 3.29(1.95-5.54) p<0.001] and treatment failure with a HR 2.30(1.44-3.67) p=0.0005. A shorter duration of CR1 (<12 months) associated with significantly higher mortality [HR 1.56(1.07-2.26), p=0.02].
Table 2.
Univariate Probability of Outcome
| Allogeneic (N = 232) | Autologous (N = 62) | ||||
|---|---|---|---|---|---|
| Outcomes | N Eval | Prob (95% CI) | N Eval | Prob (95% CI) | P-value |
| Relapse | 230 | 61 | |||
| 1 -year | 10 (6-14)% | 21 (11-32)% | 0.14 | ||
| 3-year | 16 (12-22)% | 28 (17-40)% | 0.34 | ||
| 5-year | 18 (14-24)% | 30 (19-42)% | 0.40 | ||
| Treatment related mortality | 230 | 61 | |||
| 1 -year | 28 (22-34)% | 2 (0-8)% | <0.001 | ||
| 3-year | 30 (24-36)% | 5 (1-14)% | <0.001 | ||
| 5-year | 31 (25-37)% | 7 (2-17)% | <0.001 | ||
| Disease free survival | 230 | 61 | |||
| 1 -year | 62 (56-68)% | 78 (66-87)% | 0.02 | ||
| 3-year | 54 (47-60)% | 67 (54-78)% | 0.07 | ||
| 5-year | 50 (44-57)% | 63 (49-75)% | 0.10 | ||
| Overall survival | 232 | 62 | |||
| 1 -year | 66 (60-72)% | 93 (85-98)% | <0.001 | ||
| 3-year | 58 (52-65)% | 79 (67-88)% | 0.002 | ||
| 5-year | 54 (48-61)% | 75 (63-85)% | 0.002 | ||
Figure 2.
Overall Survival in patients undergoing allogeneic and autologous transplantation with APL in CR2
Figure 3.
Disease Free Survival in Patients Undergoing Allogeneic and Autologous Transplantation for APL in CR2
The molecular status of PML-RAR∝ transcripts or cytogenetic translocations detectable in the pre-HCT bone marrow were studied for their influence on relapse and other HCT outcomes. The demographics of the cohorts with these additional data are shown in supplemental Table S1. Surprisingly, in both autologous (n=6 of 41) and allogeneic HCT (n=17 of 114), molecular or cytogenetically positive grafts were not associated with increased risks. The 3 year risks of relapse were: Autologous positive pre-HCT (17%(95% CI, 0-55) vs. negative grafts 24%(10-41%), p=0.69; Allogeneic positive (27%(8-51). vs. negative 16%(9-25), p=0.38, disease free survival with Autologous positive pre-HCT was (83%(95% CI,46-100) vs. negative grafts 69%(51-84%), p=0.41; Allogeneic positive pre-HCT (60%(35-82) vs. negative 62%(51-73), p=0.87. Overall survival with Autologous positive pre-HCT (83%(95% CI,46-100) vs. negative grafts 77%(60-90),p=0.70; Allogeneic positive (65%(41-85) vs. negative 66%(55-76), p=0.90.
Multivariate analysis confirmed that positive molecular or cytogenetic status was not significantly associated with overall mortality (positive HR 0.73 (95%CI 0.318 – 1.651), p=0.44). In the allogeneic group with positive molecular or cytogenetically detectable disease pre-HCT, the relapse rate at 5 years was 27% (8-51), disease free survival was 60% (35-82) and overall survival was 65% (41-85). All were similar to these corresponding outcomes in those who had negative pre-HCT findings. Notably, in the autologous group, only six patients had positive cytogenetic or molecular testing prior to HCT, yet of these six, one relapsed at 6 months and died 11 months post-HCT. Five remain alive and disease free at 47, 60, 61, 72, and 129 months post HCT. We acknowledge the small numbers of patients with positive grafts makes any firm conclusions difficult.
In the allogeneic group, the cumulative incidence of acute GVHD grade II-IV at 100 days was 33% (95%CI 27-40), while chronic GVHD at 5 years was 45%(95%CI 38-52), consistent with established rates in allogeneic HCT. Figure 1 shows the cumulative incidence of TRM in the 2 cohorts. Causes of death for all patients (123/294) were primarily leukemia recurrence (n=33), new malignancy (4), idiopathic pneumonia syndrome (17), infection (17), and organ failure (21). The causes of death in allogeneic transplants (n=107/232) were less often APL recurrence (27), but more often was due to toxicities including organ toxicity/failure (18), GVHD (16), infection (15), idiopathic pneumonia syndrome (15) and others (2 graft failure, 2 hemorrhage, 1 thromboembolic, 6 other specified).
Figure 1.
Treatment related mortality in patients undergoing allogeneic and autologous transplants for APL in CR2
Discussion
This large, multinational series demonstrated that the preferred therapy for APL in CR2 is autologous over allogeneic HCT. The significantly greater TRM of the allogeneic HCT is not overcome by the modest, but not significantly better protection against relapse accompanying the allogeneic graft process, even when adjusted for the molecular or cytogenetic status of the patient prior to HCT in CR2. [7] An earlier analysis by the European Cooperative Group for Blood and Marrow Transplantation (EBMT) reviewed 625 patients with APL transplanted by either autologous or allogeneic HSCT in either CR1 or CR2 from 1993-2003.[8] Patients in CR2 (n=195) had lower relapse rates after allogeneic HCT (37±4% autologous vs. 17±3%) and 5 year leukemia free survival (LFS) of 51±4% autologous vs. 59±4% after allografts. A substantially higher TRM, particularly in autologous HCT 16±3% vs. 24±4% in allogeneic was noted, compromising survival. Allogeneic HCT was recommended in CR2 when a sibling donor was available.[8]
Few data on molecular positive patients transplanted during CR2 are available. Meloni et al. analyzed 15 patients with relapsed APL undergoing autologous HCT and PCR negative pre-HCT marrow led to 45% (6/15) of patients remaining in remission [9]. However, 7 of 7 patients with PCR positive marrow relapsed within 9 months of autologous HCT. They recommended allogeneic HCT if the pre-HCT marrow was cytogenetically or molecularly positive. Sanz et al described a single patient in CR2 with molecular positivity on preconditioning marrow who received an autologous HCT and survived leukemia free 22 months post HCT[10].
After 1st relapse, several highly effective strategies for APL include ATO for re-induction and transplantation during CR2 [11]. Our data highlight that despite continued improvements in supportive care, and improvements in management of GVHD, the substantial excess of TRM limits survival for allogeneic HCT patients with APL in CR2. Somewhat surprisingly, pre-transplant molecular and cytogenetic positivity identifying detectable residual disease at HCT had no significant influence on treatment failure. Different from Meloni et al, and in an admittedly small cohort, residual detectable disease rarely led to failure in the autologous group with 5 of 6 patients remaining disease free. This observation suggests that effective eradication of residual disease in vivo, preferential mobilization into the autograft of short term repopulating cells, but too few leukemia stem cells to induce relapse [12], a modest purging effect of cryopreservation on unstable leukemic clones or other mechanisms may be protective and offers opportunities for further investigation.
The remarkable success obtained with the newest strategy, ATRA plus ATO for initial therapy will likely lead to less relapse and decrease in the need for any kind of transplantation.[13] the rate of However, these data further clarify that APL exhibits unique characteristics making it amenable to autologous HCT, compared to other types of leukemia where allogeneic HCT may be preferred. These data provide evidence that autologous HCT for APL is the superior treatment strategy, leading to long term remission and survival for a large proportion of patients in CR2.
Supplementary Material
Acknowledgments
CIBMTR Funding Support:
The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement U24-CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 5U10HL069294 from NHLBI and NCI; a contract HHSH250201200016C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-12-1-0142 and N00014-13-1-0039 from the Office of Naval Research; and grants from *Actinium Pharmaceuticals; Allos Therapeutics, Inc.; *Amgen, Inc.; Anonymous donation to the Medical College of Wisconsin; Ariad; Be the Match Foundation; *Blue Cross and Blue Shield Association; *Celgene Corporation; Chimerix, Inc.; Fred Hutchinson Cancer Research Center; Fresenius-Biotech North America, Inc.; *Gamida Cell Teva Joint Venture Ltd.; Genentech, Inc.;*Gentium SpA; Genzyme Corporation; GlaxoSmithKline; Health Research, Inc. Roswell Park Cancer Institute; HistoGenetics, Inc.; Incyte Corporation; Jeff Gordon Children's Foundation; Kiadis Pharma; The Leukemia & Lymphoma Society; Medac GmbH; The Medical College of Wisconsin; Merck & Co, Inc.; Millennium: The Takeda Oncology Co.; *Milliman USA, Inc.; *Miltenyi Biotec, Inc.; National Marrow Donor Program; Onyx Pharmaceuticals; Optum Healthcare Solutions, Inc.; Osiris Therapeutics, Inc.; Otsuka America Pharmaceutical, Inc.; Perkin Elmer, Inc.; *Remedy Informatics; *Sanofi US; Seattle Genetics; Sigma-Tau Pharmaceuticals; Soligenix, Inc.; St. Baldrick's Foundation; StemCyte, A Global Cord Blood Therapeutics Co.; Stemsoft Software, Inc.; Swedish Orphan Biovitrum; *Tarix Pharmaceuticals; *TerumoBCT; *Teva Neuroscience, Inc.; *THERAKOS, Inc.; University of Minnesota; University of Utah; and *Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration (HRSA) or any other agency of the U.S. Government.
*Corporate Members
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosure: None of the authors has a relevant financial conflict of interest to disclose.
References
- 1.Nabhan C, Mehta J, Tallman MS. The role of bone marrow transplantation in acute promyelocytic leukemia. Bone Marrow Transplant. 2001;28(3):219–26. doi: 10.1038/sj.bmt.1703119. [DOI] [PubMed] [Google Scholar]
- 2.Tallman MS. Arsenic trioxide: its role in acute promyelocytic leukemia and potential in other hematologic malignancies. Blood. 2001;15(3):133–42. doi: 10.1054/blre.2001.0160. [DOI] [PubMed] [Google Scholar]
- 3.Mandelli F, Diverio D, Awisati G, et al. Molecular remission in PML/RAR alpha-positive acute promyelocytic leukemia by combined all-trans retinoic acid and idarubicin (AIDA) therapy. Gruppo Italiano-Malattie Ematologiche Maligne dell'Adulto and Associazione Italiana di Ematologia ed Oncologia Pediatrica Cooperative Groups. Blood. 1997;90(3):1014–21. [PubMed] [Google Scholar]
- 4.Soignet SL, Frankel SR, Douer D, et al. United States multicenter study of arsenic trioxide in relapsed acute promyelocytic leukemia. J Clin Oncol. 2001;19(18):3852–60. doi: 10.1200/JCO.2001.19.18.3852. [DOI] [PubMed] [Google Scholar]
- 5.Soignet SL, Maslak P, Wang ZG, et al. Complete remission after treatment of acute promyelocytic leukemia with arsenic trioxide. N Engl J Med. 1998;339(19):1341–8. doi: 10.1056/NEJM199811053391901. [DOI] [PubMed] [Google Scholar]
- 6.Jurcic JG, Nimer SD, Scheinberg DA, et al. Prognostic significance of minimal residual disease detection and PML/RAR-alpha isoform type: long-term follow-up in acute promyelocytic leukemia. Blood. 2001;98(9):2651–6. doi: 10.1182/blood.v98.9.2651. [DOI] [PubMed] [Google Scholar]
- 7.Grimwade D, Tallman MS. Should minimal residual disease monitoring be the standard of care for all patients with acute promyelocytic leukemia? Leuk Res. 2011;35(1):3–7. doi: 10.1016/j.leukres.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 8.Sanz MA, Labopin M, Gorin NC, et al. Hematopoietic stem cell transplantation for adults with acute promyelocytic leukemia in the ATRA era: a survey of the European Cooperative Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 2007;39(8):461–9. doi: 10.1038/sj.bmt.1705620. [DOI] [PubMed] [Google Scholar]
- 9.Meloni G, Diverio D, Vignetti M, et al. Autologous bone marrow transplantation for acute promyelocytic leukemia in second remission: prognostic relevance of pretransplant minimal residual disease assessment by reverse-transcription polymerase chain reaction of the PML/RAR alpha fusion gene. Blood. 1997;90(3):1321–5. [PubMed] [Google Scholar]
- 10.Sanz MA, de la Rubia J, Bonanad S, et al. Prolonged molecular remission after PML/RAR alpha-positive autologous peripheral blood stem cell transplantation in acute promyelocytic leukemia: is relevant pretransplant minimal residual disease in the graft? Leukemia. 1998;12(6):992–5. doi: 10.1038/sj.leu.2401024. [DOI] [PubMed] [Google Scholar]
- 11.Yanada M, Tsuzuki M, Fujita H, et al. Phase 2 study of arsenic trioxide followed by autologous hematopoietic cell transplantation for relapsed acute promyelocytic leukemia. Blood. 2013;121(16):3095–102. doi: 10.1182/blood-2012-11-466862. [DOI] [PubMed] [Google Scholar]
- 12.Wang JC, Dick JE. Cancer stem cells: lessons from leukemia. Trends Cell Biol. 2005;15(9):494–501. doi: 10.1016/j.tcb.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 13.Estey E, Garcia-Manero G, Ferrajoli A, et al. Use of all-trans retinoic acid plus arsenic trioxide as an alternative to chemotherapy in untreated acute promyelocytic leukemia. Blood. 2006;107(9):3469–73. doi: 10.1182/blood-2005-10-4006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.