Abstract
Objective
To examine the association between exposure to traffic-related air pollution (TRAP) and hospital readmission for asthma or bronchodilator-responsive wheezing.
Study design
A population-based cohort of 758 children ages 1–16 years, admitted for asthma or bronchodilator-responsive wheezing was assessed for asthma readmission within 12 months. TRAP exposure was estimated using a land use regression model using the home address at index admission; TRAP was dichotomized at the sample median (0.37 μg/m3). Covariates included allergen-specific IgE, tobacco smoke exposure, and social factors obtained at enrollment. Associations between TRAP exposure and readmission were assessed using logistic regression and Cox proportional hazards.
Results
Study participants were 58% were African American (AA), 32% white; 19% were readmitted within 12 months. Children with higher TRAP exposure were readmitted at a higher rate overall (21% v. 16%, p = 0.05); this association was not significant after adjusting for covariates (adjusted OR 1.4; 95% CI 0.9–2.2). Race modified the observed association: white children with high TRAP exposure had three-fold increased odds of asthma readmission (OR 3.0; 95% CI 1.1–8.1), compared with low exposed whites. TRAP exposure among AA children was not associated with increased readmission (OR.1.1; 95% CI 0.6–1.8). TRAP exposure was associated with decreased time to readmission for high TRAP-exposed white children (HR 3.2; 95% CI 1.5–6.7) vs. AA children (HR 1.0; 95% CI 0.7–1.4); AA children had a higher readmission rate overall.
Conclusions
TRAP exposure is associated with increased odds of readmission in white children; this relationship was not observed in AA children.
Keywords: traffic-related air pollution, asthma, health-care utilization, children
Asthma is the most common chronic disease in children, (1) affecting approximately 7.1 million children in the United States. (2) Asthma-related admissions due to exacerbations make up 5.6% of all hospital admissions for children. (3) The cost of environmentally mediated childhood asthma is estimated at $2.2 billion annually in the United States. (4) A range of factors is known to be associated with asthma morbidity including race, viral infections, allergen exposure (in atopic individuals), medication adherence, and traffic-related air pollution (TRAP) exposure. (5,6)
TRAP is a complex mixture of carbon monoxide, carbon dioxide, hydrocarbons, oxides of nitrogen, particulate matter, and mobile source air toxics. (5) Studies in Southern California and Cincinnati suggest that diesel exhaust particles (DEP) make up a substantial portion of particulate matter in urban areas, and that the levels of DEP have significant spatial variability. (7,8) Prior studies have shown that DEP, which is predominately ultrafine (< 0.1 μm) in size, is associated with decreases in FEV1 and FVC in adult volunteers with asthma. (9) In urban areas, traffic emissions are the predominant source of intra-urban variability in air pollutants, and land-use regression (LUR) models have been previously shown to explain a large amount of this variability. (10,11) In Cincinnati, Ohio, estimated DEP, using a LUR model, was associated with infant wheeze (12) as well as nighttime cough during childhood. (13)
A systematic review found sufficient evidence to infer a causal relationship between TRAP exposure and exacerbation of asthma symptoms in children. (14) The evidence linking TRAP to asthma health care utilization, however, is insufficient to determine causality. In particular, the review noted that key limitations of the TRAP and asthma health care utilization literature included a reliance on ecological association studies and a relative absence of individual-level covariates beyond age and sex. (14) Though subsequent studies have found associations between TRAP exposure and asthma-related health care utilization, (15–17) these studies either lacked individual-level covariates or were conducted in subspecialty clinic settings.
The Greater Cincinnati Asthma Risks Study (GCARS) overall objective is to examine racial disparities in readmission for asthma and bronchodilator-responsive wheezing in children. In the present study, we sought to examine the association between TRAP exposure and asthma readmission within 12 months. The prospective cohort design offered an opportunity to address prior study limitations with extensive individual-level information on demographic factors, allergen sensitization, and medication use.
Methods
GCARS, a population-based, prospective, observational cohort, collected data from children, aged 1–16 years, admitted for asthma or bronchodilator-responsive wheezing to Cincinnati Children’s Hospital Medical Center (CCHMC), an urban, tertiary care hospital between August 2010 and October 2011. The cohort also included children admitted to a nearby satellite inpatient facility beginning November 2010. Given data from the Ohio Hospital Association indicating that nearly 85% of all asthma admissions for children aged 1–16 years within our 8-county primary service area occur at CCHMC facilities, our accrued admission sample was considered to be population-based. (18,19) Children not admitted to CCHMC tended to be older and to live farther from the main hospital in less urbanized areas. (18)
Patients were identified by use of the evidence-based clinical pathway for acute asthma or bronchodilator-responsive wheezing by the admitting physician. The pathway includes medications, delivery devices, education, and a standardized bronchodilator weaning protocol used by respiratory therapists. Quality assurance data shows the order set is used for over 98% of children admitted to CCHMC with asthma. Children who were removed from the asthma pathway prior to discharge, had significant respiratory or cardiovascular comorbidities (e.g., cystic fibrosis, congenital heart disease), who resided outside of the eight-county primary service area, or whose primary caregiver did not understand written or spoken English were excluded from the sample (roughly 2% of those otherwise eligible). The CCHMC Institutional Review Board approved this study.
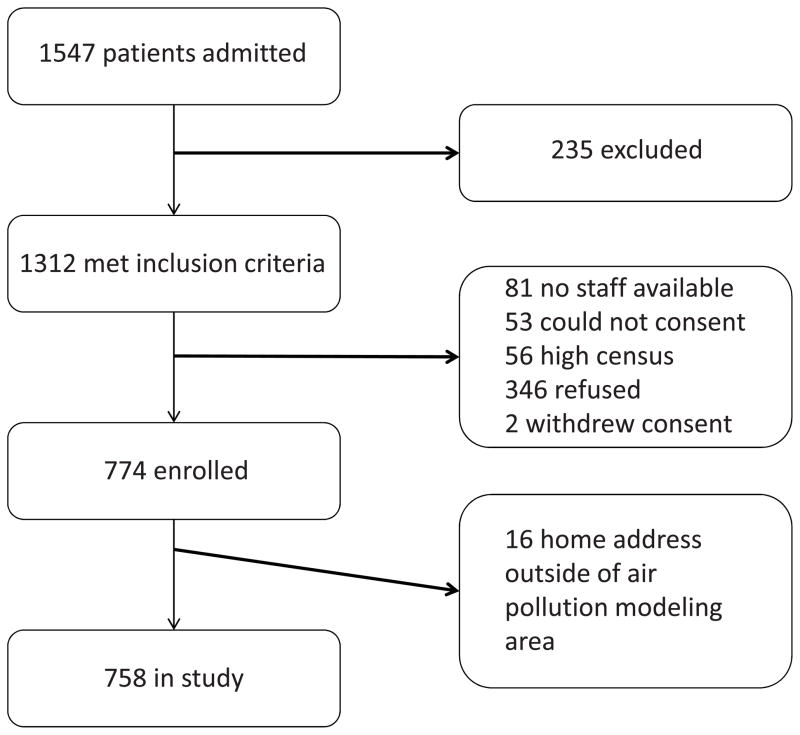
During the enrollment period, 1547 patients were admitted for asthma, 1312 (85%) of whom met inclusion criteria (Figure 1; available at www.jpeds.com). Of the 1312 eligible patients, 774 children (59%) enrolled in the study. Eighty-one patients (6%) were admitted when research staff were not available, 53 children (4%) had no parent or guardian available to provide consent, and 56 children (4%) could not be consented due to high census or competing patient care priorities. Of the 346 children (26%) who refused to participate in the study, the most common reasons given were discomfort with the blood draw or disinterest in research participation. Two children withdrew participation during the study. Recruitment was for fourteen months and included parts of two summer & autumn seasons.
Figure 1.
Recruitment diagram for children enrolled in the Greater Cincinnati Asthma Risks Study (August 2010 to October 2011)
To assess rates of loss-to-follow-up and potential readmissions to sites other than CCHMC, a 25% random sub-sample of the 774 children were contacted by telephone approximately 12 months after the index admission. If the family could not be reached, the participant’s current home address was identified using the electronic medical record (EMR) and/or public records. A total of 95.9% of the random sub-sample were confirmed as having maintained residence in CCHMC’s primary service area. Of those reached by telephone (n=164, 84% response), 0% reported an admission for asthma or wheezing to a hospital other than CCHMC during the follow up period.
Asthma readmission outcome
Our primary outcome, readmission to the hospital within 12 months of enrollment, was captured using the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) classification codes of primary or secondary discharge diagnoses (493.XX or 786.07 for asthma or wheeze, respectively) recorded in hospital billing data. Outcome accuracy was verified by review of the EMR to ensure that each readmission event met the same inclusion and exclusion criteria as the index admission.
Traffic-Related Air Pollution exposure
Exposure to TRAP was estimated by applying a previously developed and validated LUR (12,20) model to the reported primary residence of the enrolled child. A complete description of the LUR methodology used to derive TRAP exposure estimates has been described previously. (12,20) Briefly, ambient air sampling was conducted on a rotating basis at 27 sampling sites in the greater Cincinnati area from 2001–2006. The average daily concentration of elemental carbon was determined at each site. The final LUR model, including elevation, truck traffic within 400 meters, and length of bus routes within 100 meters, provided an estimate of elemental carbon attributed to traffic (ECAT), a specific component of TRAP, primarily related to diesel exhaust. (8,12,20) For this study, we used the average daily ECAT exposure as a surrogate for the TRAP mixture.
To estimate TRAP exposure for each enrolled patient, the primary home address at the time of the index admission was geocoded using ArcGIS software (Version 9.3, ESRI, Redlands, CA).). Using the previously described LUR model, a TRAP estimate was assigned to each patient’s address. Children who lived outside of the air pollution modeling area (n = 16) were excluded from the analyses.
Covariates
During the index admission, a research assistant verbally administered a questionnaire and serum samples were collected. Race of the child was determined through caregiver report and classified as white, African American, multiracial, or other. Caregivers reported if their child was on a controller medication for their asthma (yes/no), and whether their child was exposed to tobacco smoke at their primary home or other places that the child spent his/her time (yes/no). They also reported the presence of cracks or holes in the walls, and the presence of carpeting in the child’s bedroom. Caregivers were also surveyed about their psychological distress over the past four weeks using the Kessler-6 (K6) questionnaire. (21,22) Allergen-specific IgE testing was done for the following allergens: ragweed, white oak, cat, dog, and mouse dander, American cockroach, and two types of dust mite using Quantitative ImmunoCAP® Fluorescent Enzyme Immunoassay (ARUP Laboratories, Salt Lake City, Utah). Demographic data from the EMR was also used for comparisons of the enrolled and unenrolled.
Statistical analyses
The primary objective was to assess the association between TRAP exposure and hospital readmission for asthma or bronchodilator-responsive wheezing within 12 months of the index admission. For all analyses, TRAP exposure was dichotomized at the median (0.37 μg/m3) and defined as high (above the median estimated exposure) and low (below the median estimated exposure). Dichotomization of the TRAP exposure was consistent with prior studies in Cincinnati, OH. (23,24) In addition, experimental studies of diesel exhaust suggest a threshold effect with health effects observed only at higher concentrations.(25)
Logistic regression was used to examine the association between TRAP exposure and readmission within 12 months. Covariates included asthma controller use, age, sex, race (black vs. white), income (dichotomized at ≤ or >$60000 based on a evident breakpoint in the income-readmission association), and maternal education (high school graduate or higher vs. non-high school graduate). In addition, allergen sensitization was included because there is evidence that TRAP exposure can act as an adjuvant to allergen exposure. (26) Additional interactions between the primary exposure of interest, TRAP exposure, and tobacco smoke exposure, suboptimal housing (rodents, cockroaches, cracks in walls, report of mold/mildew, water leaks, or plumbing problems), and allergen sensitization were also examined.
Subsequent analyses, driven by the focus of GCARS, addressed the possible role of TRAP exposure on racial disparities in asthma readmission. The potential for effect modification by race was examined in stratified analysis and by the inclusion of a TRAP by race interaction term in the logistic model. To examine whether TRAP and race influenced the time to readmission, the time to readmission was analyzed using a Cox proportional hazards regression model while stratifying by race and TRAP exposure.
A post hoc analysis involved variables not included in the initial multivariable model, chosen to provide a more detailed understanding of what might underlie effect modification by race. Given a much higher admission rate in African Americans, we hypothesized that they experienced multiple other risk factors that might make the TRAP-readmission association harder to discern. Specifically, analyses stratified the cohort by both race and TRAP exposure. The children in the four strata (white/high TRAP, white/low TRAP, African-American/high TRAP, African-American/low TRAP) were compared using Chi square analysis with respect to the following variables: high caregiver psychological distress on the K6, report of cockroaches in the home, report of rodents in the home, the presence of carpet in the bedroom, report of cracks/holes in the walls of the home, and the percentage of families in their home’s census tract below 50% of the federal poverty limit.
All analyses were conducted using SAS version 9.3 (SAS Institute, Cary, NC). In all models, main effects were considered statistically significant at p < 0.05 and interactions considered statistically significant at p < 0.10.
Results
A total of 774 children were enrolled in the GCARS cohort and exposure to TRAP could be estimated for 98% (n = 758). Demographic, environmental, social, and medical characteristics of the study population are presented in Table I. Of the 758 children, 38.3% were less than four-years-old, 64.6% were male, and 58.4% were African American. The median estimated TRAP exposure for the sample was 0.37 μg/m3 (interquartile range: 0.31–0.49, minimum 0.23, maximum 0.87). Tobacco exposure was reported for 33.9% and asthma controller medication use was reported in 41.7%. Outdoor allergen sensitivity (ragweed and white oak) was present in 38.6% and indoor allergen sensitivity (cat/dog/mouse dander, dust mites, and cockroach) was present in 66.0%.
Table 1.
Demographic, Environmental, and Medical Characteristics of GCARS Subjects
| Variable | Overall (n = 758) n (%) |
Readmitted (n = 141) n (%) |
Non-readmitted (n = 617) n (%) |
p-value* |
|---|---|---|---|---|
| Age (Years) | 0.06 | |||
| <4 | 290 (38.3) | 57 (40.4) | 233 (37.8) | |
| 4–11 | 389 (51.3) | 77 (54.6) | 312 (50.6) | |
| ≥12 | 79 (10.4) | 7 (5.0) | 72 (11.7) | |
| Sex | 0.72 | |||
| Male | 490 (64.6) | 93 (66.0) | 397 (64.3) | |
| Female | 268 (35.4) | 48 (34.0) | 220 (35.7) | |
| Race | < 0.01 | |||
| White | 239 (31.7) | 25 (17.7) | 214 (34.9) | |
| African American | 441 (58.4) | 102 (72.3) | 339 (55.2) | |
| Multiracial & Other | 75 (9.9) | 14 (9.9) | 61 (9.9) | |
| Income ($) | 0.02 | |||
| < 14,999 | 259 (34.8) | 53 (38.4) | 206 (34.0) | |
| 15 – 29,999 | 208 (28.0) | 43 (31.2) | 165 (27.2) | |
| 30 – 44,999 | 106 (14.3) | 23 (16.7) | 83 (13.7) | |
| 45 – 59,999 | 43 (5.8) | 10 (7.3) | 33 (5.5) | |
| 60 – 89,999 | 74 (10.0) | 7 (5.1) | 67 (11.1) | |
| 90 – 119,999 | 34 (4.6) | 2 (1.5) | 32 (5.3) | |
| > 120,000 | 20 (2.7) | 0 (0.0) | 20 (3.3) | |
| Maternal Education | 0.03 | |||
| ≤ High School | 322 (44.2) | 70 (52.6) | 252 (42.3) | |
| > High School | 407 (55.8) | 63 (47.4) | 344 (57.7) | |
| Exposure to TRAP | 0.05 | |||
| High (≥ 0.37 μg/m3) | 379 (50.0) | 81 (57.5) | 298 (48.3) | |
| Low (< 0.37 μg/m3) | 379 (50.0) | 60 (42.6) | 319 (51.7) | |
| Reported Exposure to Tobacco Smoke | 257 (33.9) | 54 (38.3) | 203 (32.9) | 0.22 |
| Asthma Controller Use | 314 (41.7) | 79 (56.4) | 235 (38.3) | < 0.01 |
| Outdoor Allergen Sensitivity | 253 (38.6) | 48 (40.3) | 205 (38.2) | 0.66 |
| Indoor Allergen Sensitivity | 442 (66.0) | 87 (70.2) | 355 (65.0) | 0.28 |
| Index Admission Season** | ||||
| Summer | 101 (13.1) | |||
| Autumn | 389 (50.3) | |||
| Winter | 108 (14.0) | |||
| Spring | 176 (22.7) | |||
p-value for Chi-square test comparing % characteristic between readmitted versus non-readmitted subjects
Overall, 18.6% of children were readmitted to the hospital for asthma or bronchodilator-responsive wheezing within 12 months of their index admission. Children who were readmitted for asthma within 12 months were significantly more likely to be African American, have lower reported household income, have a mother with a high school education or less, and be on an asthma controller medication than children who were not readmitted within 12 months (Table I). Children with higher TRAP exposure were readmitted at a higher rate overall (21% v. 16%, p = 0.05); unadjusted logistic model Odds Ratio (OR) = 1.5; 95% Confidence Interval [CI] 1.0–2.1 (Table II). After adjusting for covariates, the association between TRAP exposure and readmission was not statistically significant (adjusted OR 1.4; 95% CI 0.9–2.2). The TRAP x Race interaction term (OR 1.5; 95% CI 0.9–2.5) was marginally significant at p=0.07. Covariates that remained in the model and were associated with readmission included controller medication use (OR 0.5; 95% CI 0.3–0.83) and household income < $60,000/year (OR 3.1; 95% CI 1.1–8.2). The other covariates (age, sex, caregiver education, and allergen sensitization) were not associated with readmission. Interactions between TRAP exposure and tobacco smoke exposure, poor housing quality (cockroaches, water damage, etc.), and allergen sensitization were not statistically significant.
Table 2.
Association Between TRAP and Asthma Readmission
| Model | OR (95% CI) | p-value |
|---|---|---|
| Unadjusted | 1.5 (1.0 – 2.1) | 0.05 |
| Adjusted* | 1.4 (0.9 – 2.2) | 0.08 |
| Model Including Race x TRAP Interaction** | 0.07*** | |
| White | 3.0 (1.1 – 8.1) | 0.03 |
| African-American | 1.1 (0.6 – 1.8) | 0.7 |
n = 621, adjusted for age, race, gender, household income, maternal education, controller medication use, and allergic sensitization
n = 556, includes white and African-American only, adjusted for age, gender, household income, maternal education, controller medication use, allergic sensitization
p-value for interaction term
We also examined whether the observed association between TRAP and asthma readmission varied between African American and white children by including a TRAP by race interaction term in the adjusted model. Thirty-eight percent of white children had TRAP estimates above the median compared with 58% of African American children. As shown in Table II, the effect of TRAP exposure on asthma readmission varied by race (p-value for the interaction term = 0.07). White children exposed to high levels of TRAP had a three-fold increased odds of asthma readmission (OR = 3.0; 95% CI 1.1 – 8.1) compared with low exposed white children. In contrast, exposure to TRAP among African-American children was not significantly associated with increased asthma readmissions (Table II).
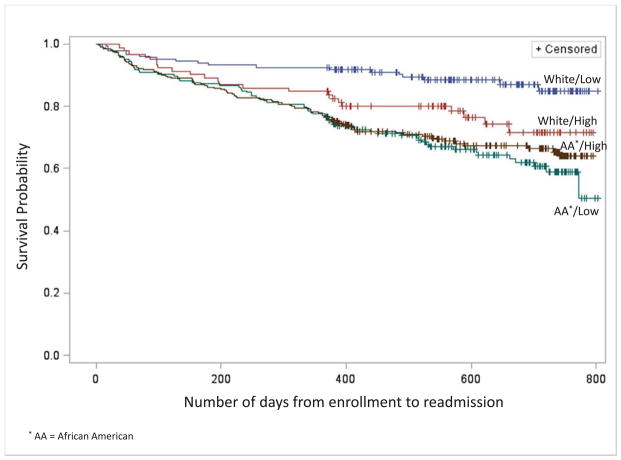
Additional analyses examining the association between TRAP exposure and time to readmission were conducted using a Cox proportional hazards regression analysis (Figure 2). This analysis demonstrated a significant association between TRAP exposure and time to readmission in white children (Hazard Ratio [HR] = 3.2 95% Confidence Interval [CI] 1.5–6.70) but not in African American children (HR 1.0, 95% CI 0.7–1.4). Regardless of TRAP exposure status, African American children had a shorter the time to readmission than white children (Figure 2 and Table III; Table III available at www.jpeds.com).
Figure 2.
Cox Regression Modeling of Time to Hospital Readmission for Asthma or Bronchodilator-Responsive Wheezing within 12 months by Race (African American or White) and by TRAP exposure, N=680
Table III.
Proportion of children not readmitted at three month intervals following index admission, stratified by race and TRAP exposure
| Race | ECAT | 3 Month | 6 Month | 9 Month | 12 Month |
|---|---|---|---|---|---|
| White | Hi | 0.99 | 0.95 | 0.88 | 0.85 |
| Low | 0.99 | 0.95 | 0.93 | 0.92 | |
|
| |||||
| African American | Hi | 0.995 | 0.91 | 0.87 | 0.81 |
| Low | 0.996 | 0.91 | 0.86 | 0.82 | |
To further investigate the different TRAP effects by race, we conducted a post hoc analysis comparing caregiver psychological distress, housing factors and neighborhood-level income data among the four-race/TRAP strata (Table IV). African American caregivers (both high and low TRAP exposed) reported significantly higher rates of psychological distress compared with caregivers of white children. In addition, African American children with high TRAP exposure were more likely to live in poor housing conditions (cockroaches or visible cracks/holes). Even low TRAP exposed African-American children lived in census tracts with two fold higher rates of extreme poverty compared with high TRAP exposed white children (8.9% vs. 4.4%).
Table IV.
Post hoc Analysis of Potential Factors Related to Asthma Readmission by TRAP and Race
| TRAP Exposure/Race Strata | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| White | African American | ||||||||
| High TRAP (n=92) | Low TRAP (n=147) | High TRAP (n=254) | Low TRAP (n=187) | p-value* | |||||
| n | % | n | % | n | % | n | % | ||
| High psychological stress in caregiver (K6 questionnaire) | 6 | 6.5 | 8 | 5.4 | 43 | 17 | 31 | 16.6 | < 0.01 |
| Observed Cockroaches | 4 | 4.4 | 8 | 5.4 | 49 | 19.5 | 22 | 11.2 | < 0.01 |
| Observed Rodents | 15 | 16.3 | 14 | 9.5 | 18 | 7.2 | 9 | 4.8 | < 0.01 |
| Carpeting in Child’s Bedroom | 68 | 73.9 | 109 | 74.2 | 161 | 64.1 | 149 | 80.1 | < 0.01 |
| Cracks/holes in Walls | 15 | 16.5 | 25 | 17.2 | 69 | 27.9 | 43 | 23.1 | 0.04 |
| TRAP Median (Q1, Q3) | 0.47 | (0.42, 0.54) | 0.3 | (0.28, 0.33) | 0.49 | (0.43, 0.57) | 0.31 | (0.29, 0.34) | < 0.01 |
| % Families in Census Tract < 50% of Poverty Limit Median (Q1, Q3) | 4.4 | (2.9, 7.7) | 3.3 | (1.4, 5.4) | 19.7 | (7.6, 34.4) | 8.9 | (4.0, 15.7) | < 0.01 |
p value calculated across all strata
Discussion
In this population-based cohort of children admitted to the hospital for asthma or bronchodilator-responsive wheezing, TRAP exposure was significantly associated with subsequent hospital readmission, for white children only. We found no association between TRAP exposure and readmission among African American children, despite their overall higher rates of asthma readmission as compared with white children. Post hoc analyses found African American children had significantly higher concentrations of housing risks, environmental factors, and neighborhood poverty and these may have obscured TRAP effects in this group.
Exposure to TRAP has been consistently associated with adverse respiratory health effects in children including the exacerbation of existing asthma, wheeze, medication use, and asthma pathogenesis. (12,14,27) Prior studies looking at asthma health care utilization (emergency department visits, hospitalizations) have primarily had ecological study designs examining correlations between pollution levels and health care administrative databases; they have lacked more detailed data regarding individual-level socioeconomic, medication, or residential exposure histories. (16,28,29) A strength of this study was the population-based nature of the cohort in conjunction with individual-level data on asthma medication use, allergen sensitivity, and other variables related to both asthma exacerbation and health care utilization. After adjustment for these covariates, TRAP remained significantly associated with asthma readmission in white children. Examination of readmission is important, because the admission to the hospital illustrates the severity of their disease as well as creating an opportunity for a change in treatment plan.
Though African American children had a higher asthma readmission rate, we found the association between TRAP exposure and readmission was significant only among white children. These results are consistent with Delfino et al (2009) who reported the association between TRAP exposure and asthma readmission in children was significantly modified by race/ethnicity with lower risk for non-white children. Other studies, however, have found lower SES to increase susceptibility to air pollution. (30,31) These inconsistent findings may be, in part, attributable to unmeasured covariates in these studies including tobacco smoke exposure, allergen exposure, psychological stress, and medication use. In addition, different racial and economic groups may be exposed to different subcomponents of air pollution. (32) However, we did not measure all components of TRAP and are unable to directly address this.
To address the issue of differences in individual-level variables by race, we performed a post hoc analysis. Caregiver psychological distress, visible cockroaches, and having cracks/holes in the walls of the home were significantly more likely to be reported by African American caregivers compared with white caregivers and the proportion was greatest among African American children with high TRAP exposure. Census tract rates of extreme poverty were also much higher among African American children. These associations are similar to those reported by our group previously. (33) Collectively, this intense concentration of additional asthma risk factors among African American children are strongly associated with readmission and may have hidden the association between TRAP and readmission in these children.
There are limitations to our study. First, the population was recruited from a single institution and it is possible that some enrolled children may have been readmitted for asthma elsewhere. However, CCHMC admits approximately 85% of the children in its 8-county primary service area. Those admitted to other facilities tend to be older and less urban and may limit generalizability. Notably, no re-hospitalizations were reported outside of CCHMC during the 12-month follow up. Although the population was racially diverse in terms of African American and white races, we had an insufficient sample of other race and ethnic subgroups and therefore the findings may not be generalizable to them. Error in TRAP exposure assessment is also possible due to the mobility of our population. We were only able to estimate TRAP for the address reported at the time of the index asthma admission, which may not be the same as the address at the time of readmission. TRAP exposure assessment was conducted using a LUR model developed from ambient air sampling conducted prior to this study. Thus, estimated ECAT concentrations may not be reflective of current concentrations. However, spatial contrasts in TRAP due to traffic and elevation are likely to remain stable over time. Furthermore, a recent study found that LUR models were able to predict spatial contrasts in pollution up to 8 years later. (11) Both residential mobility and changes in TRAP concentrations would likely bias our findings toward the null hypothesis and lead to underestimates of TRAP effects. The TRAP estimates were for the year of follow-up, not broken down by season, thus limiting analysis of a TRAP by allergen interaction. In addition, there is no home sampling to document each child’s allergen exposure. As the association between TRAP exposure and readmission in the overall study population did not achieve statistical significance, the study may be inadequately powered to detect this association. Finally, the GCARS population includes children from age one through 16. In young children, a diagnosis of asthma is difficult and misclassification is possible. However, other studies have found TRAP to be associated with wheezing and asthma symptoms in young children. (35,36)
In conclusion, our study suggests that exposure to TRAP is significantly associated with increased readmission rates for asthma or bronchodilator-responsive wheezing among white children in our population-based, prospective cohort study. The African American children in the cohort had a higher rate of readmission overall, suggesting that social and environmental factors other than TRAP are particularly relevant in this subset of the GCARS population. Future research should consider whether TRAP estimates could be available in real-time during a hospital admission and, if so, how this information might change asthma care, such as modifying asthma controller recommendations. At a population level such data could lead to broad policy approaches regarding pollution regulation.
Acknowledgments
Funded by the National Institutes of Health/the National Institute of Allergy and Infectious Diseases (Greater Cincinnati Asthma Risks Study R01AI088116 [PI: R.S.K.]) and the Cincinnati Center for Clinical and Translational Sciences and Training UL1 RR026314 PI: James E Heubi.
Abbreviations
- TRAP
Traffic-related air pollution
- ECAT
Elemental carbon attributed to traffic
- GCARS
Greater Cincinnati Asthma Risks Study
- DEP
diesel exhaust particles
- CCHMC
Cincinnati Children’s Hospital Medical Center
- LUR
Land use regression
Footnotes
The authors declare no conflicts of interest.
Portions of this study were presented at the Pediatric Academic Societies’ Meeting, Washington, DC, May 4–7, 2013.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.World Health Organization. [Accessed 3/5/2013];WHO | Asthma. 2013 Available at: http://www.who.int/mediacentre/factsheets/fs307/en/
- 2.Centers for Disease Control and Prevention. [Accessed 3/5/2013];CDC - Asthma - Data and Surveillance - Asthma Surveillance Data. 2013 Available at: http://www.cdc.gov/asthma/asthmadata.htm.
- 3.Akinbami LJ, Moorman JE, Garbe PL, Sondik EJ. Status of childhood asthma in the United States, 1980–2007. Pediatrics. 2009 Mar;123(Suppl 3):S131–45. doi: 10.1542/peds.2008-2233C. [DOI] [PubMed] [Google Scholar]
- 4.Trasande L, Liu Y. Reducing The Staggering Costs Of Environmental Disease In Children, Estimated At $76.6 Billion In 2008. Health Affairs. 2011 May 01;30:863–870. doi: 10.1377/hlthaff.2010.1239. [DOI] [PubMed] [Google Scholar]
- 5.HEI Panel on the Health Effects of Traffic-Related Air Pollution. Traffic-Related Air Pollution: A Critical Review of the Literature on Emissions, Exposure, and Health Effects. HEI Special Report. 2010;17 [Google Scholar]
- 6.Forno E, Celedon JC. Predicting asthma exacerbations in children. Curr Opin Pulm Med. 2012 Jan;18:63–69. doi: 10.1097/MCP.0b013e32834db288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manchester-Neesvig JB, Schauer JJ, Cass GR. The distribution of particle-phase organic compounds in the atmosphere and their use for source apportionment during the Southern California Children’s Health Study. J Air Waste Manag Assoc. 2003 Sep;53:1065–1079. doi: 10.1080/10473289.2003.10466265. [DOI] [PubMed] [Google Scholar]
- 8.Sahu M, Hu S, Ryan PH, Le Masters G, Grinshpun SA, Chow JC, et al. Chemical compositions and source identification of PM(2).(5) aerosols for estimation of a diesel source surrogate. Sci Total Environ. 2011 Jun 1;409:2642–2651. doi: 10.1016/j.scitotenv.2011.03.032. [DOI] [PubMed] [Google Scholar]
- 9.McCreanor J, Cullinan P, Nieuwenhuijsen MJ, Stewart-Evans J, Malliarou E, Jarup L, et al. Respiratory effects of exposure to diesel traffic in persons with asthma. N Engl J Med. 2007 Dec 6;357:2348–2358. doi: 10.1056/NEJMoa071535. [DOI] [PubMed] [Google Scholar]
- 10.Hoek G, Beelen R, Kos G, Dijkema M, Zee SCvd, Fischer PH, et al. Land Use Regression Model for Ultrafine Particles in Amsterdam. Environ Sci Technol. 2011 Jan 15;45:622–628. doi: 10.1021/es1023042. 2013/06. [DOI] [PubMed] [Google Scholar]
- 11.Eeftens M, Beelen R, Fischer P, Brunekreef B, Meliefste K, Hoek G. Stability of measured and modelled spatial contrasts in NO2 over time. Occupational and Environmental Medicine. 2011 Oct 01;68:765–770. doi: 10.1136/oem.2010.061135. [DOI] [PubMed] [Google Scholar]
- 12.Ryan PH, Lemasters GK, Biswas P, Levin L, Hu S, Lindsey M, et al. A comparison of proximity and land use regression traffic exposure models and wheezing in infants. Environ Health Perspect. 2007 Feb;115:278–284. doi: 10.1289/ehp.9480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sucharew H, Ryan PH, Bernstein D, Succop P, Khurana Hershey GK, Lockey J, et al. Exposure to traffic exhaust and night cough during early childhood: the CCAAPS birth cohort. Pediatr Allergy Immunol. 2010 Mar;21:253–259. doi: 10.1111/j.1399-3038.2009.00952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Panel on the Health Effects of Traffic-Related Air Pollution. Traffic-Related Air Pollution: A Critical Review of the Literature on Emissions, Exposure, and Health Effects. SR-17 2010. [Google Scholar]
- 15.Brown MS, Sarnat SE, DeMuth KA, Brown LA, Whitlock DR, Brown SW, et al. Residential proximity to a major roadway is associated with features of asthma control in children. PLoS One. 2012;7:e37044. doi: 10.1371/journal.pone.0037044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang J, Delfino RJ, Gillen D, Tjoa T, Nickerson B, Cooper D. Repeated respiratory hospital encounters among children with asthma and residential proximity to traffic. Occup Environ Med. 2009 Feb;66:90–98. doi: 10.1136/oem.2008.039412. [DOI] [PubMed] [Google Scholar]
- 17.Cook AG, deVos AJ, Pereira G, Jardine A, Weinstein P. Use of a total traffic count metric to investigate the impact of roadways on asthma severity: a case-control study. Environ Health. 2011 Jun 2;10 doi: 10.1186/1476-069X-10-52. 52-069X-10-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bosnjakovic E. INSIGHT Database. 2009. [Google Scholar]
- 19.Szklo M. Population-based cohort studies. Epidemiol Rev. 1998;20:81–90. doi: 10.1093/oxfordjournals.epirev.a017974. [DOI] [PubMed] [Google Scholar]
- 20.Ryan PH, Lemasters GK, Levin L, Burkle J, Biswas P, Hu S, et al. A land-use regression model for estimating microenvironmental diesel exposure given multiple addresses from birth through childhood. Sci Total Environ. 2008 Oct 1;404:139–147. doi: 10.1016/j.scitotenv.2008.05.051. [DOI] [PubMed] [Google Scholar]
- 21.Kessler RC, Andrews G, Colpe LJ, Hiripi E, Mroczek DK, Normand SL, et al. Short screening scales to monitor population prevalences and trends in non-specific psychological distress. Psychol Med. 2002 Aug;32:959–976. doi: 10.1017/s0033291702006074. [DOI] [PubMed] [Google Scholar]
- 22.Kessler RC, Barker PR, Colpe LJ, Epstein JF, Gfroerer JC, Hiripi E, et al. Screening for serious mental illness in the general population. Arch Gen Psychiatry. 2003 Feb;60:184–189. doi: 10.1001/archpsyc.60.2.184. [DOI] [PubMed] [Google Scholar]
- 23.Ryan PH, Bernstein DI, Lockey J, Reponen T, Levin L, Grinshpun S, et al. Exposure to traffic-related particles and endotoxin during infancy is associated with wheezing at age 3 years. Am J Respir Crit Care Med. 2009 Dec 1;180:1068–1075. doi: 10.1164/rccm.200808-1307OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Epstein TG, Ryan PH, LeMasters GK, Bernstein CK, Levin LS, Bernstein JA, et al. Poor asthma control and exposure to traffic pollutants and obesity in older adults. Ann Allergy Asthma Immunol. 2012 Jun;108:423–428.e2. doi: 10.1016/j.anai.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghio AJ, Smith CB, Madden MC. Diesel exhaust particles and airway inflammation. Curr Opin Pulm Med. 2012 Mar;18:144–150. doi: 10.1097/MCP.0b013e32834f0e2a. [DOI] [PubMed] [Google Scholar]
- 26.Diaz-Sanchez D, Proietti L, Polosa R. Diesel fumes and the rising prevalence of atopy: an urban legend? Curr Allergy Asthma Rep. 2003 Mar;3:146–152. doi: 10.1007/s11882-003-0027-4. [DOI] [PubMed] [Google Scholar]
- 27.McConnell R, Islam T, Shankardass K, Jerrett M, Lurmann F, Gilliland F, et al. Childhood incident asthma and traffic-related air pollution at home and school. Environ Health Perspect. 2010 Jul;118:1021–1026. doi: 10.1289/ehp.0901232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Delfino RJ, Chang J, Wu J, Ren C, Tjoa T, Nickerson B, et al. Repeated hospital encounters for asthma in children and exposure to traffic-related air pollution near the home. Ann Allergy Asthma Immunol. 2009 Feb;102:138–144. doi: 10.1016/S1081-1206(10)60244-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li S, Batterman S, Wasilevich E, Wahl R, Wirth J, Su FC, et al. Association of daily asthma emergency department visits and hospital admissions with ambient air pollutants among the pediatric Medicaid population in Detroit: time-series and time-stratified case-crossover analyses with threshold effects. Environ Res. 2011 Nov;111:1137–1147. doi: 10.1016/j.envres.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 30.O’Neill MS, Jerrett M, Kawachi I, Levy JI, Cohen AJ, Gouveia N, et al. Health, wealth, and air pollution: advancing theory and methods. Environ Health Perspect. 2003 Dec;111:1861–1870. doi: 10.1289/ehp.6334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yap PS, Gilbreath S, Garcia C, Jareen N, Goodrich B. The Influence of Socioeconomic Markers on the Association Between Fine Particulate Matter and Hospital Admissions for Respiratory Conditions Among Children. Am J Public Health. 2013 Feb 14; doi: 10.2105/AJPH.2012.300945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bell ML, Ebisu K. Environmental Inequality in Exposures to Airborne Particulate Matter Components in the United States. Environ Health Perspect. 2012 Aug 10; doi: 10.1289/ehp.1205201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beck AF, Simmons JM, Huang B, Kahn RS. Geomedicine: area-based socioeconomic measures for assessing risk of hospital reutilization among children admitted for asthma. Am J Public Health. 2012 Dec;102:2308–2314. doi: 10.2105/AJPH.2012.300806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu S, McDonald R, Martuzevicius D, Biswas P, Grinshpun SA, Kelley A, et al. UNMIX modeling of ambient PM(2.5) near an interstate highway in Cincinnati, OH, USA. Atmos Environ (1994) 2006;40:378–395. doi: 10.1016/j.atmosenv.2006.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ryan PH, LeMasters G, Biagini J, Bernstein D, Grinshpun SA, Shukla R, et al. Is it traffic type, volume, or distance? Wheezing in infants living near truck and bus traffic. J Allergy Clin Immunol. 2005 Aug;116:279–284. doi: 10.1016/j.jaci.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 36.Brauer M, Hoek G, Smit HA, de Jongste JC, Gerritsen J, Postma DS, et al. Air pollution and development of asthma, allergy and infections in a birth cohort. Eur Respir J. 2007 May;29:879–888. doi: 10.1183/09031936.00083406. [DOI] [PubMed] [Google Scholar]