Abstract
Recently, initiatives have been undertaken to establish an islet transplantation program in Athens, Greece. A major hurtle is the high cost associated with the establishment and maintenance of a clinical-grade islet manufacturing center. A collaboration was established with the University Hospitals of Geneva, Switzerland, to enable remote islet cell manufacturing with an established and validated fully operational team. However, remote islet manufacturing requires shipment of the pancreas from the procurement to the islet manufacturing site (in this case from anywhere in Greece to Geneva) and then shipment of the islets from the manufacturing site to the transplant site (from Geneva to Athens). To address challenges related to cold ischemia time of the pancreas and shipment time of islets, a collaboration was initiated with the University of Arizona, Tucson, USA. An international workshop was held in Athens, December 2011, to mark the start of this collaborative project. Experts in the field presented in three main sessions: [1] Islet transplantation: state-of-the-art, and the “network approach”; [2] Technical aspects of clinical islet transplantation and outcomes; and [3] Islet manufacturing – from the donated pancreas to the islet product. This manuscript presents a summary of the workshop.
Keywords: clinical islet transplantation, continuous glucose monitoring, GRAGIL network, Greece, immunosuppression, islet shipment, National Commission Group Islet Transplant centre, National Scottish Islet Transplant centre, Network approach in islet transplantation, pancreas preservation, persufflation
Introduction (presentation by Theodore Karatzas, Associate Professor of Surgery, Medical School, University of Athens, Greece)
Type 1 diabetes currently affects approximately 5 million individuals in the world. In Greece about 1,000 new cases with type 1 diabetes are reported every year, with an increasing frequency afflicting especially children under school age. Tight control of blood glucose achieved by intensive insulin treatment, self blood glucose monitoring and patient education, could prevent the development of diabetic complications and retard the progression of the disease. Beta-cell replacement by islet transplantation is a potential treatment that can achieve insulin independence, constant normoglycemia, avoidance of life-threatening complications and severe hypoglycemic episodes.
There is no islet transplantation program in Greece. Many potential donor pancreata are available every year to cover the needs of the country, but the transplant community encounters difficulties in benefiting from these pancreatic grafts. As a result, the pancreata from deceased donors are not retrieved and are discarded. Therefore, there is a need for an islet transplant program.
The field of islet transplantation worldwide has evolved tremendously, since the introduction of the Edmonton Protocol in 2000 (1). Today, islet transplantation is a standard procedure for the treatment of type 1 diabetes in many centers (2-4). Substantial efforts have been initiated to establish collaborative clinical trials with centralized islet manufacturing centers. The results of successful clinical trials that come from international collaborations and assisted by innovative technologies, offer a great potential. Citing Dr Reinhart Bretzel et al.: “continued international collaboration will further stimulate excitement in the field as innovative solutions, such as preservation methods, isolated techniques etc, are created to overcome the problems with islet cell transplantation” (5).
The Swiss-French consortium GRAGIL (Groupe Rhin – Rhône – Alpes et Genève pour la greffe d'Îlots de Langerhans) (6), a multi-center islet transplant network consortium, constitutes an excellent example of a successful international collaboration. Multi-center networks covering large areas, with the possibility of crossing country borders, allow considerable expansion of the donor pool and the patient population eligible for islet transplantation at a given program. Also, the high cost and high level of expertise necessary for maintaining and operating islet manufacturing facilities, together with the economies of scale and improvement in success rates achieved by increasing the numbers of pancreata processed per facility, have prompted the establishment of consortia with centralized islet manufacturing. The Swiss-French consortium has raised the possibility of implementing an islet transplantation program in Greece, in collaboration with the centers of Geneva and Tucson- Arizona.
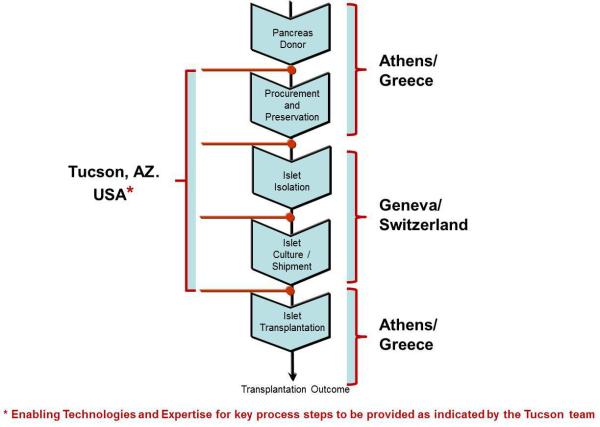
An international workshop was held in Athens, December 2011, to mark the start of this collaborative project. Experts in the field were invited to present in three main sessions: [1] Islet transplantation: state-of-the-art, and the “network approach”; [2] Technical aspects of clinical islet transplantation and outcomes; and [3] Islet manufacturing – from the donated pancreas to the islet product. The main objective of this workshop was to enable a clinical collaborative islet trial that would involve the following three main steps: [1] pancreas retrieval from deceased donors performed in Greece; [2] transferring the pancreas to Geneva for processing, islet isolation and assessment of islet quality; and [3] culture and shipment of islets from Geneva to Athens to be infused in diabetic patients (Fig. 1).
Fig. 1.
The Athens-Geneva-Tucson collaboration, showing the flow-chart of pancreas harvested in Greece transported to Switzerland for islet cell manufacturing, followed by shipment of islets to Greece. The long distance between Athens and Geneva requires the implementation of innovative new technologies and expertise for organ preservation and islet shipment that are contributed by the Tucson group.
The geographic peculiarities of Greece and the long distance between Athens and Geneva produce time limitations of pancreas preservation leading to prolonged cold ischemia time, that would consequently affect pancreas processing, having deleterious effect on the quality of isolated islets. These complications of prolonged cold ischemia time (longer than 10-12 hours), were also discussed in the workshop. Islets are very fragile and extremely susceptible to conditions of low oxygen supply, and many do not survive the procedure of enzymatic tissue digestion followed by purification and cell culture, after exposure of the pancreas to prolonged cold ischemia beyond 10 hours. The collaboration with the University of Arizona at Tucson was initiated to bring innovative technologies enabling prolonged pancreas preservation times (up to 24 hrs) and optimal shipment conditions of islets, to meet the demands presented by the long distance between Athens and Geneva (Fig. 1). During the workshop aspects of improved pancreas preservation with the emerging technology of the persufflation preservation method, that could circumvent the current limitations of a >10 hours cold ischemia time (7). Aspects of islet manufacturing with innovative technologies that may have the potential of maintaining islet quality and function were also highlighted in the workshop (8).
An opening introduction was given by Prof. Rainer Gruessner (Professor and Chair, Department of Surgery, University of Arizona, Tucson, USA), and a keynote lecture was given at the end of the workshop by Prof. David Sutherland (Professor of Surgery, Department of Surgery, Director Schulze Diabetes Institute, University of Minnesota, Minneapolis, USA). This manuscript presents a summary of the workshop.
Session 1: Islet transplantation: state-of-the-art, and the “network approach”
Islet transplantation: current state of the art (presentation by Thierry Berney, Professor of Surgery, Department of Visceral Surgery and Transplantation, University Hospitals Geneva, Switzerland)
This presentation started with a description of the procedure of processing the donor pancreas to yield the purified islet preparations which are infused into the portal vein and implant in the liver. Two groups of patients are nowadays eligible for receiving an islet transplant: patients with diabetic nephropathy, receiving a transplant simultaneously with or after a kidney transplant (aim: nephroprotection); and patients without diabetic nephropathy with severe hypoglycemia (aim: glycemic stability). Outcomes of the Collaborative Islet Transplant Registry show a high percentage of successful transplants with a gradual decline after transplantation, so that insulin independence is reduced to 25% of cases after 4 years (9). However this does not reflect a complete loss of graft function: C-peptide reflecting graft function is measurable in 60-70% of patients at 5 years after transplantation, and glycemic stability is evident by an almost absence of significant hypoglycemic insults 4-5 years after transplantation. Benefits of graft function are also evident in a reduced progression of retinopathy and a better function of a kidney graft.
The interest in islet transplantation, with subsequent higher numbers of patients transplanted, increased substantially after the immunosuppressive treatment following the Edmonton protocol was published in 2000, and with time thereafter the outcomes are improving, i.e., the incidence of insulin independence at 2 years after transplantation increased from appoximately 40% in transplants performed in the period 1999-2003 to 55% for transplants performed in the period 2007-2009; in selected protocols 50% of patients show insulin independence at 5 years after transplantation and 70% of patients show graft function (10,11).
New approaches besides immunosuppression include intervention to attenuate the “instant blood-mediated inflammatory reaction” occurring after infusion of islets into the blood circulation, and the use of GLP-1 analogues to protect islet beta cells from apoptosis. The increasingly favorable comparison with whole pancreas transplantation shows the enormous potential of islet cell transplantation for further expansion in the area of beta-cell therapy (Table 1).
Table 1.
Comparison of pancreas and islet transplantation
| Pancreas transplantation | Islet transplantation | |
|---|---|---|
| First case performed | 1966 (Minneapolis) | 1974 (Minneapolis) |
| Total world experience | ~30,000 cases | ~1,600 cases |
| Insulin independence | ||
| 1 year | 85% | 80% |
| 5 years | 70% | 25-50% |
| Graft function | ||
| 5 years | 70% | 70% |
| Surgical approach | Laparotomy | Interventional radiology |
| Major surgery | Minimally invasive | |
| Complications | Frequent - severe | Less frequent – less severe |
| Thrombosis | Bleeding | |
| Pancreatitis | Portal thrombosis | |
| Peritonitis | ||
| Mortality | Low (4%) | Exceptional (~0%) |
| Preferred indication | Simultaneous pancreas-kidney | Islet transplant alone |
| Number of donors | 1 | 1-4 |
| Potential | Achieved | Enormous |
Islet transplantation in networks: the Swiss-French GRAGIL experience (presentation by Christian Toso Senior Consultant, Department of Visceral Surgery and Transplantation, University Hospitals Geneva, Switzerland)
The GRAGIL Consortium is a multicenter network in islet transplantation, that was initiated in 1997 particularly to reduce costs associated with the infrastructure and operations of islet cell manufacturing (6,11-13). It also enabled centers to start islet transplantation without the need to build an infrastructure for islet cell manufacturing: hence, the network approach indirectly increases the donor and recipient population, and enables to combine efforts in clinical trials. Evidently, this network requires the feasibility of distant islet cell processing as shown in collaborative efforts by other groups in Europe and the US, keeping ischemia time of donor pancreata as short as possible. GRAGIL currently comprises 9 transplant centers in three regions in France and one in Switzerland, around one islet production group in Geneva: the waiting list, serum library, donor selection, islet processing and islet allocation is centralized, and patient screening, pancreas procurement, transplant procedure and patient follow-up is localized (Fig. 2).
Fig. 2.
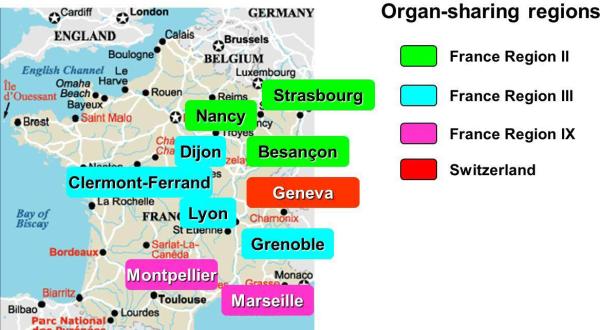
The centers participating in the GRAGIL (Groupe Rhin – Rhône – Alpes et Genève pour la greffe d'Îlots de Langerhans) network.
The review of the activities published in 2005 showed a clear increase with time of operations of both pancreas donors (up to 150/year) and islet isolations (up to 60/year), with a clear relationship between the number of donors and numbers of patients on the waiting list for the individual regions. Trials were conducted to validate the Edmonton protocol, and also to compare the outcome of islets that were shipped in the network with that of islets that were not shipped. The results of clinical outcomes were similar for islets prepared at distant location and islets that were not shipped. It is concluded that the multicenter network in GRAGIL works very well, with achievements being maximizing technical expertise, reaching of critical mass (donor pool and waiting list) and decreasing financial investments. The model is reproduced elsewhere (e.g., the Nordic network).
Centralized islet procurement for clinical transplants: key challenges for long-distance islet processing (presentation by James Shaw, Professor of Regenerative Medicine for Diabetes, Institute of Cellular Medicine, Medical School, Newcastle University, Newcastle upon Tyne, UK)
In the UK a network for islet cell transplantation was established, which was the first health service funded program as an established clinical intervention, guided by the National Health Service/ National Institute for Health and Clinical Excellence with the goal of preventing recurrent severe hypoglycemia and attaining HbA1c targets below 7% (14,15). The first transplant with transported islets was undertaken in Newcastle in 2008.
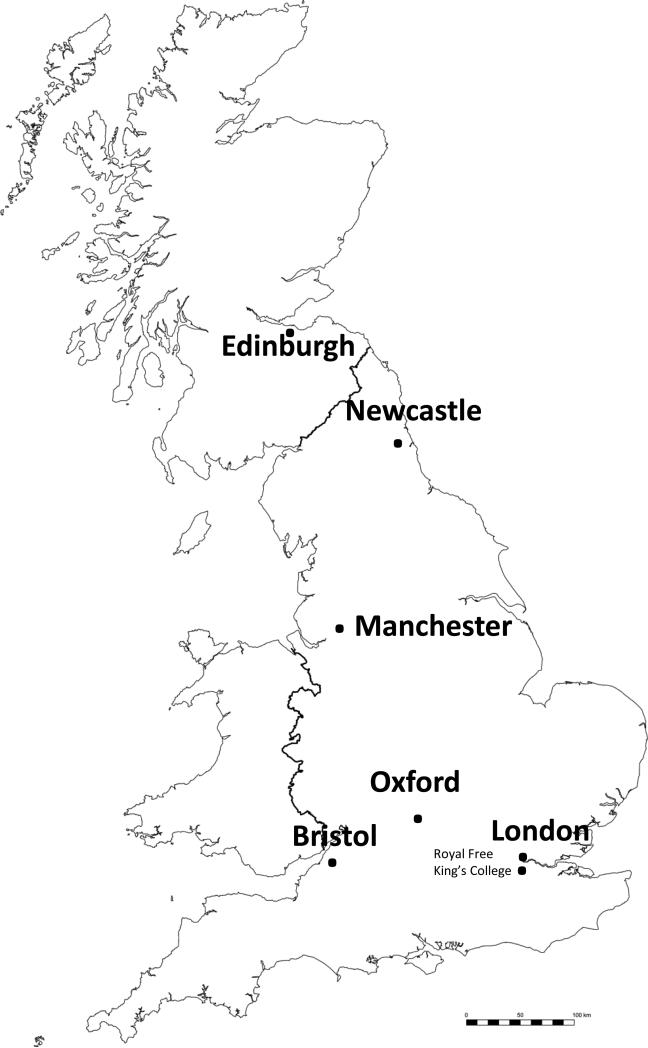
In this national collaborative initiative between 7 centers (Fig. 3), truly equitable sharing of organs between whole pancreas and isolated islet recipients has been achieved, maximizing donor organ use and ensuring optimal quality for islet patients wherever they live in the UK. This is now leading to increased awareness of inclusion criteria nationally and established referral pathways. The centrally commissioned target is to achieve transplantation of 80-100 patients each year. During the period 2008-2011, 38 transplants were performed in 24 patients without major side effects (no mortality and only 2 minor hepatic bleeds): 96% of recipients attained primary graft function with prevention of recurrent severe hypoglycaemia in all and average HbA1c level below 7% attained.
Fig. 3.
The National Commisioning Group (NCG) and National Scottish Islet Transplant Centres network for islet cell transplantation in the United Kingdom. Red circles, NCG Islet Transplant Centre; blue circle, National Scottish Islet Transplant Centre.
In this program there is a high focus on innovation and research (16-18). Amongst other areas a detailed experimental medicine approach to determine biomedical and psychosocial outcomes has been established. This includes continuous subcutaneous glucose monitoring, centralized assay of standardized mixed-meal tolerance tests and urinary C-peptide creatinine ratio; T-lymphocyte studies of recurrent autoimmunity and serum cytokines; in addition to protocol hepatic magnetic resonance imaging. Validated and novel patient-reported outcome questionnaires are completed at each time point.
Relevant to this symposium, pancreas preservation and islet shipment is also core to the integrated UK program with only three central islet isolation facilities (King's College London, Oxford and Edinburgh) providing isolation and distribution for all seven transplant centers (King's College London, Oxford, Edinburgh, Bristol, Manchester, Newcastle and Royal Free London). With the aim of increasing successful transplant conversion from Extended Criteria Donors, pancreas persufflation technology is undergoing testing in pancreata affected by ischemia due to donor circulatory failure in donors, in collaboration with the University of Arizona at Tucson. National Health Service commissioning of the UK islet program underpins collaborative research towards novel innovations in the field and facilitates early funded adoption of these once validated.
Session 2: Technical aspects of clinical islet transplantation and outcomes
Current immunosuppressive protocols in islet transplantation (presentation by Thierry Berney, Professor of Surgery, Department of Visceral Surgery and Transplantation, University Hospitals Geneva, Switzerland)
An overview of various immunosuppressants was presented with the associated goals: prevention of rejection, prevention of recurrence of autoimmunity, and prevention of non-immune graft loss (19). Besides efficacy, a major aspect is the emergence of adverse side effects, in particular islet toxicity that is particularly well-known for glucocorticosteroids and the calcineurin inhibitor tacrolimus. But, this is also demonstrated for rapamycin and cyclosporine: mycophenolic mofetil is an exception amongst traditional immunosuppressants because it does not have diabetogenic properties. The success of the Edmonton protocol was the use of islets from 2-3 donors per recipient, the absence of glucocorticosteroids and use of low-dose tacrolimus in combination with rapamycin. Other adverse events, such as leukopenia, anemia, thrombocytopenia and cholesterolemia in over 80% of patients, warranted the development of alternative strategies.
Post-Edmonton regimens include T-cell depletion (anti-thymocyte globulin, anti-CD3 antibody, anti-CD52 antibody), anti-inflammatory reagents including anti-TNFα and anti-IL1 reagents, and costimulatory blockade using anti-LFA1 and CTLA4Ig reagents (20,21).
Current standard immunosuppression includes induction with a T-cell depleting agent, a TNFα inhibitor and a calcineurin inhibitor, and maintenance immunosuppression with a calcineurin inhibitor and either mycophenolic mofetil or rapamycin. With the innovations and optimizations of immunosuppressive regimens, in particular T-cell depletion, the survival of islet transplants measured in insulin independence of the recipient is approaching the same level as survival of pancreas transplants (4).
Radiological guidance for islet infusion (presentation by Paris Pappas, Director of Radiology, University of Athens, Greece)
The procedure of islet infusion into the portal vein using the percutaneous transhepatic approach under radiological guidance (22) was described in detail, using data and illustrations provided by the Geneva group. The intraportal islet infusion procedure has excellent results in terms of islet graft function and has a low morbidity. This procedure is performed under monitoring of the portal pressure. The rise in portal pressure correlates with the tissue volume infused: but, neither the volume infused nor the rise in portal pressure is associated with the occurrence of complications. However, an increase in portal pressure exceeding 20 cm H2O warrants to interrupt the procedure. For 93 islet infusions there were 9 complications recorded: 3 perihepatic hematomas, 2 portal branch thrombosis, and 4 intra-abdominal hemorrhages.
Metabolic monitoring of the islet graft – continuous glucose monitoring (presentation by François Pattou, Professor and Chair, Department of General and Endocrine Surgery, University of Lille, France)
After an introduction of the islet program in Lille (23,24) including islet manufacturing and quality assessment, a study was presented that evaluated the influence of restoring endogenous insulin secretion by islet transplantation on various components of dysglycemia, focusing on continuous glucose monitoring (25). This was done in 23 consecutive type 1 diabetes patients, 23 consecutive patients with type 1 diabetes (14 islet-alone, 9 islet-after-kidney) receiving 2 or 3 islet infusions within 3 months using the Edmonton protocol. At the 3-year visit, graft function persisted in 19 patients (82%), and 10 (43%) remained insulin-independent with an HbA1c below 6.5%. Graft function was estimated via ß-score, a previously validated index (range 0–8) based on treatment requirements, C-peptide, blood glucose and HbA1c. The four components of the glucose profile (mean glucose, glucose standard deviation, hyperglycemia and hypoglycemia) were significantly improved after transplantation (Table 2). All these outcomes were related to ß-score (p<0.001). However, partial function (ß-score>3) was sufficient to abrogate hypoglycemia, and sub-optimal function (ß-score>5) was necessary to significantly improve mean glucose, glucose standard deviation, hyperglycemia. When graft function was optimal (ß-score 8) the four components of glucose profile became identical to healthy controls.
Table 2.
Glucose profile after islet transplantation
| Baseline (n=23)a | 6 months (n=23)a | 3 years (n=23)a | B-score 8 (n=39)b | |
|---|---|---|---|---|
| HbAlc, % | 8.3 (7.3-9.0) | 6.0 (5.7-6.4)** | 6.7 (5.9-7.7)** | 5.6 (5.0-5.8)** |
| Insulin requirements, IU/kg/day | 0.63 (0.40-0.75) | 0 (0-0)** | 0 (0-0.28)** | 0 (0-0)** |
| Mean glucose, mmol/L | 8.0 (7.3-11.6) | 6.0 (5.5-7.8)** | 6.2 (5.8-7.3)* | 5.7 (5.3-6.2)** |
| Glucose standard deviation, mmol/L | 3.2 (2.3-4.3) | 0.9 (0.7-1.3)** | 1.8 (0.8-2.8)* | 0.8 (0.6-1.1)** |
| HYPER, % of time above 10 mmol/L | 33 (19-51) | 0 (0-5)** | 6 (0-15)* | 0 (0-0)** |
| HYPO, % of time under 3 mmol/L | 5 (1-8) | 0 (0-3.5)* | 0 (0-2)* | 0 (0-0)** |
Data presented are median values(interquartile range)
analysis per intention to treat of continuous glucose measurement outcomes in the 23 patients throughout the 3-year study
combined analysis of continued glucose measurement outcomes of the 39 tests associated with excellent graft function (ß-score 8) at any given time following transplantation
p< 0.01 versus baseline
p< 0.05 versus baseline
Taken together, these results showed that all components of dysglycemia are not equally affected by the degree of islet graft function, which could have important implications for future development of beta cell replacement. Continuous glucose monitoring allows the objective assessment of metabolic control related to graft function.
Session 3: Islet manufacturing – from the donated pancreas to the islet product
Organ preservation: pre-clinical and clinical experience with persufflation in whole organ preservation prior to transplantation (presentation by Thomas Minor, Professor of Surgical Research, Head of Surgical Research Division, Clinic of Surgery, University of Bonn, Germany)
This presentation addressed other organs than the pancreas, in particular the liver (Fig. 4). There are various techniques of organ preservation, such as static cold storage, machine perfusion, and oxygen persufflation. Many studies on persufflation were performed on livers, showing a better preservation regarding energy metabolism, integrity of mitochondria and endothelial cells, prevention of Kupffer cell activation and reduction of proteolysis (27-29). In animal models, mainly in pigs, livers subjected to venous systemic oxygen persufflation showed better survival compared to organs subjected to conventional cold storage, thanks to a better preservation of metabolism and organ integrity (30-33). The improved quality of the organ upon gaseous oxygenation during cold storage is most likely related to a better mitochondrial and cellular homeostasis prior to the challenge of warm reperfusion.
Fig. 4.
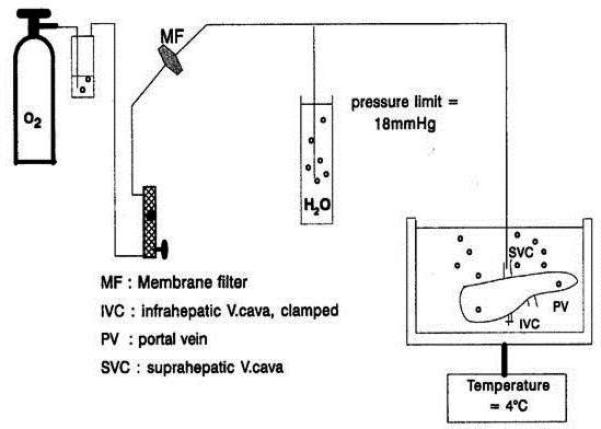
Schematic representation of the gaseous oxygen persufflation set-up used for the condition of livers during hypothermic preservation. From ref 26.
Based on the preclinical evidence, venous systemic oxygen persufflation was tested in the clinical liver transplantation, at first in a pilot study to document the feasibility of the approach. These studies are presently ongoing.
Enabling technologies for shipment and assessment of pancreas and islet products (presentation by Klearchos K Papas, Professor of Surgery, Scientific Director, Institute for Cellular Transplantation, University of Arizona, Tucson, United States)
Islet potency testing
real-time islet potency testing is critical for widespread clinical application of islet transplantation as it will minimize the chances of transplanting non-viable and nonfunctional preparations, further improving success rates. A substantial investment has been made by the Juvenile Diabetes Research Foundation and National Institutes of Health on this topic with fruitful results (8,34-39). A variety of tests have been proposed and evaluated: some with very good to excellent predictive capabilities in small animal models. As an islet potency assay, the oxygen consumption measurement normalized to DNA has been developed and validated in various islet transplantation models such as the rat, pig and human-to-mouse models, and more recently in pig-to-nonhuman primate islet transplantation and single donor human allo- and auto-transplantation (8). This test is currently being evaluated through assessments of islet products transplanted in patients participating in the NIH sponsored phase III registration islet transplantation trials in the Clinical Islet Transplant consortium. This test will also be implemented for the assessment of clinical islet products in the Athens-Geneva-Tucson project, after isolation and before shipment from Geneva and prior to transplantation in Athens.
Pancreas Preservation
Prolonged cold ischemia time during pancreas preservation has a detrimental impact on islet yield and viability. Even 8-12 hours of cold ischemia time can have a significant and negative impact on viable islet yield (40-43). Methods that improve oxygenation during pancreas preservation are believed to have a substantial impact extending the preservation time window to up to 24 hours and to improve viable islet yield (7). Pancreas tissue is particularly susceptible to damage during prolonged warm and/or cold ischemia. The impact of hypoxia on viable islet yield may be in part due to the exquisite sensitivity of beta cells to hypoxia as they do not have the ability to generate appreciable levels of ATP via anaerobic glycolysis because they express very low levels of LDHα (40).
The two-layer cold storage method was implemented as it was believed to improve islet integrity and graft functional outcome, thanks to enhanced tissue oxygenation. Methods to enhance oxygenation during pancreas preservation via static cold storage (such as the two-layer-method) have been proven largely ineffective and incapable of oxygenating porcine and human pancreata (7,41-45), with a large volume fraction (~85%) of the human pancreas remaining hypoxic (44). Although the two-layer-method may be effective for thin-sized pancreata, e.g. those from small animals, alternative approaches for oxygenation are required for larger-sized pancreata. Persufflation is such an alternative, as has been documented in experimental studies including islet potency in the diabetic mouse model (a potency assay mentioned above). Persufflation is currently being evaluated in the UK islet transplant network mentioned above through funding provided by the DWRF, and will be included in the Athens-Geneva-Tucson project.
Islet Culture and Shipment
Oxygen supply is of particular importance for islets during culture and shipment. Theoretical models confirmed with experimental data indicate a direct relationship between cell density and oxygen deprivation of cultured islets (46-47). A relevant improvement in culture flasks with gas-permeable silicon rubber membranes was presented: experimental studies have shown a much better outcome for islets cultured in such devices (46). The same applies to islet shipment for which devices with gas permeable membranes in pressure regulated gyroscopic shipping containers are to be used (Fig. 5). These containers fit within specialized shipping boxes containing phase change materials capable of maintaining temperature within them at 22°C irrespective of external temperature (in the range of -20°C to 60°C) over 48 hours (47).
Fig. 5.
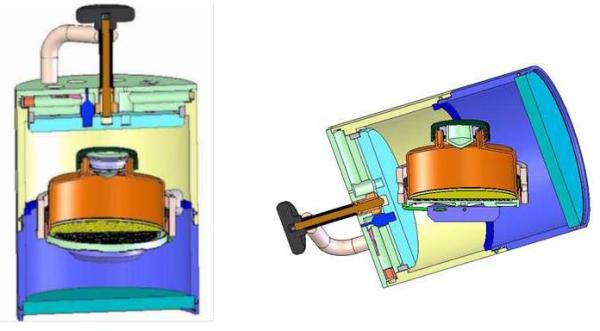
Schematic depicting a silicone rubber vessel within specially designed gyroscopic container enabling upright positioning of the silicone rubber vessel irrespective of the position and orientation of the gyroscopic container.
Conclusions
In this international workshop a series of expert presentations were given that together gave an excellent overview of the present status of clinical islet transplantation. First, the Edmonton protocol has given a substantial boost in islet transplantation now more than 10 years ago, and during this decade the transplant outcomes have gradually increased. Besides adaptations to immunosuppressive regimens, with the aim to further reduce diabetogenic drugs, improved outcomes are also ascribed to improved islet cell manufacturing and quality assessments, as well as patient monitoring. To avoid substantial investments, in particular in islet cell manufacturing, a number of islet cell transplantation networks have been established: the experiences of the GRAGIL group and the UK network show that such networks are successful, with the perspective that more organs become available and more patients can be transplanted.
However, besides the field of immunosuppression, major improvements are warranted in pancreas preservation and islet culture and shipment. This is particularly relevant for the building of an islet transplantation program in Greece, to avoid damage to organ and cells related to the distance between Athens and Geneva where islet cell manufacturing is planned. Organ persufflation is one approach, and cell culture and shipment in devices with gas permeable membranes is another. The overview of various aspects of islet transplantation during this workshop included a comprehensive description of all aspects to be considered in the Athens-Geneva-Tucson project. This aside, this workshop presented the ingredients for other islet transplant centers and networks as well to enhance achievements in transplant outcomes.
Acknowledgements
This workshop was financially supported by Giner, Inc (www.ginerinc.com), Wilson Wolf Manufacturing Corporation (www.wilsonwolf.com) and Instech Laboratories, Inc (www.instechlabs.com). The collaborations described in this workshop have been supported by the Diabetes Research and Wellness Foundation (http://www.diabeteswellness.net).
Financial Support for Research: NIH/NIDDK SBIR GRANTS: R43DK070400, R44DK070400, R43DK06965, R44DK069865.
References
- 1.SHAPIRO AM, LAKEY JR, RYAN EA, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230–238. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 2.SUTHERLAND DE, GRUESSNER A, HERING BJ. Beta-cell replacement therapy (pancreas and islet transplantation) for treatment of diabetes mellitus: an integrated approach. Endocrinol Metab Clin North Am. 2004;33:135–148. doi: 10.1016/S0889-8529(03)00099-9. [DOI] [PubMed] [Google Scholar]
- 3.SHAPIRO AM, RICORDI C, HERING BJ, et al. International trial of the Edmonton protocol for islet transplantation. N Engl J Med. 2006;355:1318–1330. doi: 10.1056/NEJMoa061267. [DOI] [PubMed] [Google Scholar]
- 4.BARTON FB, RICKELS MR, ALEJANDRO R, et al. Improvement in outcomes of clinical islet transplantation: 1999-2010. Diabetes Care. 2012;35:1436–1445. doi: 10.2337/dc12-0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.BRETZEL RG, JAHR H, ECKHARD M, MARTIN I, WINTER D, BRENDEL MD. Islet cell transplantation today. Langenbecks Arch Surg. 2007;392:239–253. doi: 10.1007/s00423-007-0183-4. [DOI] [PubMed] [Google Scholar]
- 6.BERNEY T, BENHAMOU P, KESSLER L, MOREL P. Islet transplantation in multicenter networks: the GRAGIL example. Cur Opin Organ Transpl. 2004;9:72–76. [Google Scholar]
- 7.IWANAGA Y, SUTHERLAND DER, HARMON JV, PAPAS KK. Pancreas preservation for pancreas and islet transplantation. Cur Opin Organ Transpl. 2008;13:445–451. doi: 10.1097/MOT.0b013e328303df04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.PAPAS KK, SUSZYNSKI TM, COLTON CK. Islet assessment for transplantation. Cur Opin Organ Transpl. 2009;14:674–682. doi: 10.1097/MOT.0b013e328332a489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. [December 5, 2011];Collaborative Islet Transplant Registry (CITR) http://www.citregistry.org/.
- 10.BERNEY T, FERRARI-LACRAZ S, BÜHLER L, et al. Long-term insulin-independence after allogeneic islet transplantation for type 1 diabetes: over the 10-year mark. Am J Transpl. 2009;9:419–423. doi: 10.1111/j.1600-6143.2008.02481.x. [DOI] [PubMed] [Google Scholar]
- 11.BOROT S, NICLAUSS N, WOJTUSCISZYN A, et al. GRAGIL Network. Impact of the number of infusions on 2-year results of islet-after-kidney transplantation in the GRAGIL network. Transplantation. 2011;92:1031–1038. doi: 10.1097/TP.0b013e318230c236. [DOI] [PubMed] [Google Scholar]
- 12.KEMPF MC, ANDRES A, MOREL P, et al. GRAGIL group. Logistics and transplant coordination activity in the GRAGIL Swiss-French multicenter network of islet transplantation. Transplantation. 2005;79:1200–1205. doi: 10.1097/01.tp.0000161224.67535.41. [DOI] [PubMed] [Google Scholar]
- 13.BERNEY T, BÜHLER LH, MOREL P. Pancreas allocation in the era of islet transplantation. Transpl Int. 2005;18:763–767. doi: 10.1111/j.1432-2277.2005.00143.x. [DOI] [PubMed] [Google Scholar]
- 14.ALDIBBIAT A, HUANG GC, ZHAO M, et al. Validation of islet transport from a geographically distant islet isolation center enabling equitable access and National Health Service funding of a clinical islet transplant program for England. Cell Medicine (Part B of Cell Transplantation) 2012;2:97–104. doi: 10.3727/215517911X617905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.CHOUDHARY P, BIRTLES L, PARROTT N, RUTTER M. Islet cell transplantation: current stus in the UK. Pract Diab. 2012 in press. [Google Scholar]
- 16.SPEIGHT J, REANEY MD, WOODCOCK AJ, et al. Patient-reported outcomes following islet cell or pancreas transplantation (alone or after-kidney) in Type 1 diabetes: a systematic review. Diab Med. 2010;27:812–22. doi: 10.1111/j.1464-5491.2010.03029.x. [DOI] [PubMed] [Google Scholar]
- 17.RIDGWAY D, MANAS D, SHAW J, WHITE S. Preservation of the donor pancreas for whole pancreas and islet transplantation. Clin Transplant. 2010;24:1–19. doi: 10.1111/j.1399-0012.2009.01151.x. [DOI] [PubMed] [Google Scholar]
- 18.BROOKS AMS, AMIEL SA, FORBES S, et al. Resolution of hypoglycaemia, improvement in glycaemic control and assessment of metabolic graft function in islet transplant recipients in the UK islet transplant consortium programme. Diab Med. 2012;29(Suppl 1):6. [Google Scholar]
- 19.BERNEY T, BUHLER LH, MAJNO P, MENTHA G, MOREL P. Immunosuppression for pancreatic islet transplantation. Transplant Proc. 2004;36:362S–366S. doi: 10.1016/j.transproceed.2003.12.035. [DOI] [PubMed] [Google Scholar]
- 20.BELLIN MD, BARTON FB, HEITMAN A, et al. Potent induction immunotherapy promotes long-term insulin independence after islet transplantation in type 1 diabetes. Am J Transplant. 2012;12:1576–1583. doi: 10.1111/j.1600-6143.2011.03977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.POSSELT AM, SZOT GL, FRASSETTO LA, et al. Islet transplantation in type 1 diabetic patients using calcineurin inhibitor-free immunosuppressive protocols based on T-cell adhesion or costimulation blockade. Transplantation. 2010;90:1595–1601. doi: 10.1097/TP.0b013e3181fe1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.OWEN RJ, RYAN EA, O'KELLY K, et al. Percutaneous transhepatic pancreatic islet cell transplantation in type 1 diabetes mellitus: radiologic aspects. Radiology. 2003;229:165–170. doi: 10.1148/radiol.2291021632. [DOI] [PubMed] [Google Scholar]
- 23.VANTYGHEM MC, MARCELLI-TOURVIEILLE S, FERMON C, et al. Intraperitoneal insulin infusion versus islet transplantation: comparative study in patients with type 1 diabetes. Transplantation. 2009;87:66–71. doi: 10.1097/TP.0b013e31818bbdab. [DOI] [PubMed] [Google Scholar]
- 24.VANTYGHEM MC, KERR-CONTE J, ARNALSTEEN L, et al. Primary graft function, metabolic control, and graft survival after islet transplantation. Diabetes Care. 2009;32:1473–1478. doi: 10.2337/dc08-1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.VANTYGHEM MC, RAVERDY V, BALAVOINE AS, et al. Continuous glucose monitoring after islet transplantation in type 1 diabetes: An excellent graft function (ß score greater than 7) is required to abrogate hyperglycemia while a minimal function is necessary to suppress severe hypoglycemia (ß score greater than 3). J Clin Endocrinol Metab. 2012 doi: 10.1210/jc.2012-2115. published ahead of print, 2012 Sept 20, PMID-22996144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.MINOR T, SAAD S, NAGELSCHMIDT M, et al. Successful transplantation of porcine livers after warm ischemic insult in situ and cold preservation including postconditioning with gaseous oxygen. Transplantation. 1998;65:1262–1264. doi: 10.1097/00007890-199805150-00019. [DOI] [PubMed] [Google Scholar]
- 27.MINOR T, AKBAR S, TOLBA R, DOMBROWSKI F. Cold preservation of fatty liver grafts: prevention of functional and ultrastructural impairments by venous oxygen persufflation. J Hepatol. 2000;32:105–111. doi: 10.1016/s0168-8278(00)80196-8. [DOI] [PubMed] [Google Scholar]
- 28.TRECKMANN J, MINOR T, SAAD S, OZCELIK A, et al. Retrograde oxygen persufflation preservation of human livers: a pilot study. Liver Transpl. 2008;14:358–364. doi: 10.1002/lt.21373. [DOI] [PubMed] [Google Scholar]
- 29.STEGEMANN J, MINOR T. Energy charge restoration, mitochondrial protection and reversal of preservation induced liver injury by hypothermic oxygenation prior to reperfusion. Cryobiology. 2009;58:331–336. doi: 10.1016/j.cryobiol.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 30.KOETTING M, LÜER B, EFFERZ P, PAUL A, MINOR T. Optimal time for hypothermic reconditioning of liver grafts by venous systemic oxygen persufflation in a large animal model. Transplantation. 2011;91:42–47. doi: 10.1097/TP.0b013e3181fed021. [DOI] [PubMed] [Google Scholar]
- 31.MINOR T, KOETTING M, KOETTING M, et al. Hypothermic reconditioning by gaseous oxygen improves survival after liver transplantation in the pig. Am J Transplant. 2011;11:2627–2634. doi: 10.1111/j.1600-6143.2011.03731.x. [DOI] [PubMed] [Google Scholar]
- 32.MINOR T, SCOTT WE, RIZZARI MD, et al. Energetic recovery in porcine grafts by minimally invasive liver oxygenation. J Surg Res: published ahead of print. 2012 Mar 10; doi: 10.1016/j.jss.2012.01.018. PMID-22445459. [DOI] [PubMed] [Google Scholar]
- 33.KOETTING M, MINOR T. Donation after cardiac death: dynamic graft reconditioning during or after ischemic preservation? Artif Organs. 2011;35:565–571. doi: 10.1111/j.1525-1594.2010.01138.x. [DOI] [PubMed] [Google Scholar]
- 34.CAIAZZO R, GMYR V, KREMER B, et al. Quantitative In vivo Islet potency assay in normoglycemic nude mice correlates with primary graft function after clinical transplantation. Transplantation. 2008;86:360–363. doi: 10.1097/TP.0b013e31817ef846. [DOI] [PubMed] [Google Scholar]
- 35.HANSON, MATTHEW S, PARK E, et al. A simplified approach to human islet quality assessment. Transplantation. 2010;89:1178–1188. doi: 10.1097/TP.0b013e3181d54bce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.SWEET IR, GILBERT M, JENSEN R, et al. Glucose stimulation of cytochrome C reduction and oxygen consumption as assessment of human islet quality. Transplantation. 2005;80:1003–1011. doi: 10.1097/01.tp.0000178381.35014.37. [DOI] [PubMed] [Google Scholar]
- 37.TODOROV IVAN, NAIR INDU, AVAKIAN-MANSOORIAN ALINA, et al. Quantitative assessment of ß-cell apoptosis and cell composition of isolated, undisrupted human islets by laser scanning cytometry. Transplantation. 2010;90:836–842. doi: 10.1097/TP.0b013e3181f1db5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.PAPAS KK, COLTON CK, NELSON RA, et al. Human islet oxygen consumption rate and DNA measurements predict diabetes reversal in nude mice. Am Jtransplant. 2007;7:707–13. doi: 10.1111/j.1600-6143.2006.01655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.PAPAS KK, PISANIA A, WU H, WEIR GC, COLTON CK. A stirred microchamber for oxygen consumption rate measurements with pancreatic islets. Biotechnol Bioeng. 2007;98:1071–1082. doi: 10.1002/bit.21486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.PAPAS KK, COLTON CK, GOUNARIDES JS, et al. NMR spectroscopy in β-cell engineering and islet transplantation. Ann NY Acad Sci. 2001;944:96–119. doi: 10.1111/j.1749-6632.2001.tb03826.x. [DOI] [PubMed] [Google Scholar]
- 41.CABALLERO-CORBALÁN J, EICH T, LUNDGREN T, et al. No beneficial effect of two-layer storage compared with UW-storage on human islet isolation and transplantation. Transplantation. 2007;84:864–869. doi: 10.1097/01.tp.0000284584.60600.ab. [DOI] [PubMed] [Google Scholar]
- 42.PAPAS KK, HERING BJ, GUNTHER L, et al. Pancreas oxygenation is limited during preservation with the two-layer method. Transplant Proc. 2005;37:3501–3504. doi: 10.1016/j.transproceed.2005.09.085. [DOI] [PubMed] [Google Scholar]
- 43.AVGOUSTINIATOS ES, HERING BJ, PAPAS KK. The rat pancreas is not an appropriate model for testing the preservation of the human pancreas with the two-layer method. Transplantation. 2006;81:1471–1472. doi: 10.1097/01.tp.0000215389.64186.3f. [DOI] [PubMed] [Google Scholar]
- 44.SCOTT WEW, III, FERRER-FABREGA J, AVGOUSTINIATOS ES, et al. Persufflation improves pancreas preservation compared with the two-layer method”. Transplant Proc. 2010;42:2016–2019. doi: 10.1016/j.transproceed.2010.05.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.SCOTT WEW, III, FERRER-FABREGA J, ANAZAWA T, et al. Pancreas oxygen persufflation increases ATP levels as shown by NMR”. Transplant Proc. 2010;42:2011–2015. doi: 10.1016/j.transproceed.2010.05.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.PAPAS KK, AVGOUSTINIATOS ES, TEMPELMAN LA, et al. High-density culture of human islets on top of silicone rubber membranes. Transplant Proc. 2005;37:3412–3414. doi: 10.1016/j.transproceed.2005.09.086. [DOI] [PubMed] [Google Scholar]
- 47.ROZAK PR, WEEGMAN BP, AVGOUSTINIATOS ES, et al. Devices and methods for maintenance of temperature and pressure during islet shipment. Transplant Proc. 2008;40:407–410. doi: 10.1016/j.transproceed.2008.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]