Abstract
Introduction:
Rabeprazole, a member of substituted benzimidazoles, inhibits the final step in gastric acid secretions. This drug claims to cause fastest acid separation (due to higher pKa), and more rapidly converts to the active species to aid gastric mucin synthesis. The most significant pharmacological action of Rabeprazole is dose dependent suppression of gastric acid secretion; without anticholinergic or H2-blocking action. It completely abolishes the hydrochloric acid secretion as it is powerful inhibitor of gastric acid. Rabeprazole is acid labile and hence commonly formulated as an enteric coated tablet. The absorption of rabeprazole occurs rapidly as soon as tablet leaves the stomach.
Aim:
In the present study an attempt was made to formulate and evaluate Rabeprazole sustained release matrix tablet using wet granulation technique incorporating various polymers like HPMC-E15, Carbopol934, and sodium carboxymethyl cellulose (CMC).
Materials and Methods:
The Formulated tablets were evaluated for different physicochemical properties like rheological properties, weight variation, thickness, hardness, % friability, in vitro release studies and drug content.
Results:
Studies revealed that all the physicochemical parameters comply with the official standards. The in vitro release studies exhibits the release up to 90%, over a prolonged period of time which confirms the extended release profile of formulation, having better bioavailability as well as decreased dosing frequency with reduced doses.
Conclusion:
The sustained release matrix tablets of rabiprazole shown better bioavailability, efficacy and potency, when compared with official standards.
KEY WORDS: Bioavailability, carcinogen, DENA, gatifloxacin, hepatocellular carcinoma, histology, HPMC-E15, matrix, rabeprazole, sustained release
Retention of drug delivery systems in the stomach prolongs overall gastrointestinal transit time improving oral bioavailability of the drugs that are having site specific absorption from the stomach or upper part of the small intestine. Therefore different approaches have been proposed to retain the dosage form in the stomach including bioadhesive systems, swelling, and expanding systems and delayed gastric emptying devices to achieve gastric residence time for sustained drug release.[1] The goal of any drug delivery system is to provide a therapeutic amount of drug to the proper site in the body to achieve promptly and then maintain the desired drug concentration.[2] This deliberate control of drug release is achieved in sustained release dosage form as it prolongs the therapeutic effect by continuously releasing medication over an extended time after administration of a single dose.[3]
The most employed method to modulate the sustained drug release is to include it in a matrix system. Because of their flexibility, hydrophilic polymer matrix systems are widely used in oral controlled drug delivery to obtain a desirable drug release profile, cost-effectiveness, and broad regulatory acceptance.[4] Matrix system are also favored because of their simplicity, patient compliance than traditional drug delivery (TDS), which have many drawbacks like repeated administration and fluctuation in blood concentration level. In this type of drug delivery system, the drug is homogeneously dispersed throughout the matrix of crosslink of linear polymer chain.[5] It is assumed that from this type of drug delivery system, drug molecule come out from matrix by dissolution and then diffusion through the polymer structure.[6] As the drug is released, the distance for diffusion becomes greater and solid particles began to deplete. Most of the highly water-soluble drugs, if not formulated properly, may readily release the drug at a faster rate, and are likely to produce toxic concentration.[6] Hydrophilic polymers have become product of choice as an important ingredient for formulating sustained release formulations of highly water soluble drugs.[7] As the dissolution medium or biological fluid penetrates the dosage form, the polymer swells and drug molecules begin to move out of the system by diffusion at a rate determined by the nature and composition of the polymer as well as formulation technology.[8]
Sustained release matrix tablets prepared by wet granulation technique using microcrystalline ethyl cellulose polymer and Bees wax showed good sustaining drug release concluding that sustained release tablet could be successfully combined with accurate control and prolongation of the drug release patterns.[9,10]
Chemically Rabeprazole is designated as RS)-2-([4-(3-methoxypropoxy)-3-methylpyridin-2-yl] methylsulfinyl)-1H-benzo[d] imidazole with molecular formula C18H21N3O3S. It is the member of substituted benzimidazoles which inhibits the final step in gastric secretion.
In vitro and animal studies have demonstrated that rabeprazole is a more potent inhibitor of H+, K(+)-ATPase and acid secretion than omeprazole, and is a more rapid inhibitor of proton pumps than omeprazole, lansoprazole, or pantoprazole. This probably reflects rabeprazole's faster activation in the parietal cell canaliculus.[11]
It inhibits the final transport of hydrogen ions (via exchange with potassium ions) into the gastric lumen. Since the H+, K+ -ATPase enzyme system is regarded as the acid (proton) pump of the gastric mucosa, rabeprazole is known as a gastric acid pump inhibitor. It does not have anticholinergic or histamine H2 -receptor antagonist properties,[12] and is acid-labile. Once Rabeprazole has left the stomach, absorption occurs within 1 hour of administration.[12] Elimination of rabeprazole occurs very rapidly and almost entirely by metabolism to inactive or less active metabolites. The differences in the metabolism of these drugs may favorably or unfavorably affect their pharmacodynamics (e.g., acid suppression and plasma gastrin profile), pharmacokinetics, and potential for drug interactions, including those with the polymorphic cytochrome P450 (CYP) isoenzyme, CYP2C19.[13]
It has short biological half-life (1-2 h) that requires frequent daily dosing and therapeutic use in acid-related diseases that necessitates its formulation into sustained release dosage form.[14] This study is an attempt to formulate Rabeprazole as sustained release matrix tablet for extending its release rate for prolong period of time thus increasing the bioavailability.
Materials and Methods
Materials
Rabeprazole was received as a gift sample from Elder Pharmaceuticals Pvt Ltd, Dehradun (India). The polymer HPMC E-15, Carbopol 934, Sodium CMC was procured from Elder Pharmaceuticals Pvt Ltd, Dehradun (India). Talc, Magnesium stearate was from S.D. Fine Chem. Ltd. Mumbai. All the chemicals were of analytical grade.
Methods
Identification of pure drugs
Identification of Rabeprazole was examined by FT-IR and compared with the reference spectrum of drug.
Method used to estimate rabeprazole sodium
The drug Rabeprazole Sodium was dissolved in phosphate buffer 7.2 to obtain 10 μg/ml solutions. Further diluted with the same buffer and scanned for maximum absorbance (λmax) in a double beam UV-VIS Spectrophotometer, between the UV ranges from 200 to 400 nm against phosphate buffer pH 7.2 as blank; and λ max is found to be 287 nm.
Prepration of calibration curve
Rabeprazole in water
Accurately 25 mg of Rabeprazole was taken in a 100 ml volumetric flask. Sufficient amount of water was added to make up the mark (stock solution). 10 ml of the volume was made up to the mark with water using the standard solution 1 ml, 2 ml, 4 ml, 6 ml, 8 ml, 10 ml that was withdrawn individually and in each case the volume was made up to 10 ml. The absorbance of these solution were measured spectrophotometrically at a suitable wavelength. The observed absorbance was plotted against concentration [Table 1 and Figure 1].
Table 1.
Data for standard curve of rabeprazole in distilled water

Figure 1.
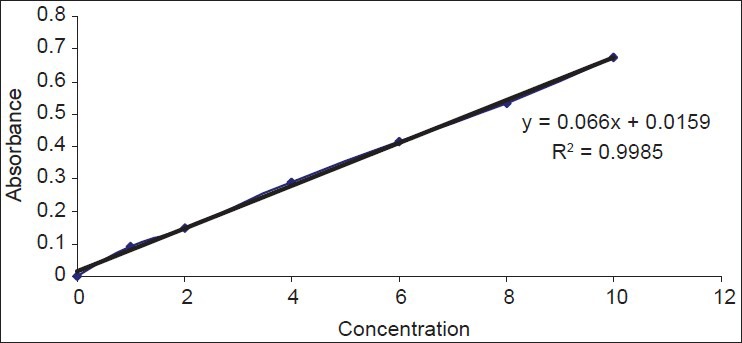
Standard curve of rabeprazole
Fabrication of tablets
Wet granulations
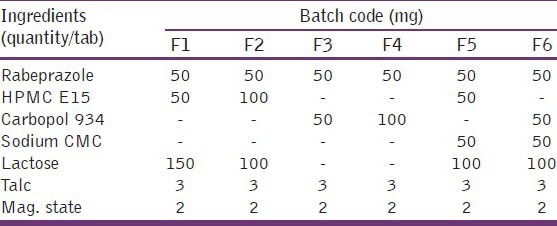
All the polymers and active ingredients were passed through sieve no. 80 separately. Accurately weighed amount of polymers and excipients were thoroughly mixed in glass mortar pestle. The granules were prepared by wet granulation technique and passed them to sieve no. 20 and dried in hot air oven at 45°C. The granules were then mixed properly with magnesium stearate, talc and punched with the help of automatic punching machine to a desired hardness, shape, and size [Table 2].
Table 2.
Formulation chart
Determination of hardness of tablet
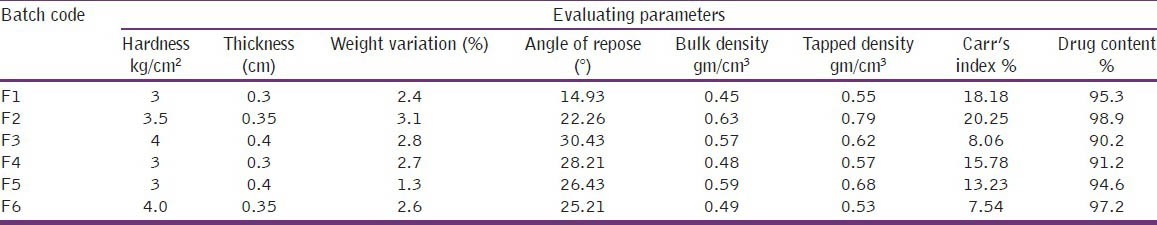
Randomly sampled 5 tablets in each batch of formulation were used for the determination of hardness with the help of Monsanto type hardness [Table 3].[15]
Table 3.
Evaluation of different parameters
Determination of friability
Roche friabilator is used in which approx. 6 gm of dedusted tablet are subjected to 100 freefalls of 6 inches in rotating drum at 25 rpm and then reweighed [Table 3].
F = 100 (1-w0 /w)
Determination of weight variation
20 tablets were selected at random and weighed accurately; the average weight of the tablet was calculated. Then the deviation of individual weight from the drug weight was calculated [Table 3].[15]
Determination of thickness of tablets
The individual crown to crown thickness of ten tablets was determined using slide calipers for each batch [Table 3].[16]
Measurement of the density of formulation
The approach densities of the tablet were calculated from the volumes and masses in triplicate. The volumes (v) of the cylindrical tablets were calculated from their heights (h) and radius (r) are both determined with micrometer gauze using the mathematical equation for a cylinder [Table 3].[17]
V = ∏ r2h
Determination of drug content in tablets
3 tablets from each batch were selected randomly and transferred to a 100 ml volumetric flask were, filled up with 0.1N HCL. Kept it for 48 hours then took 1ml from each of volumetric flask was transferred to the test tubes samples were then filtered, suitable diluted and analyzed spectrophotometrically at a suitable wavelength [Table 3].[9,12]
Angle of repose
It was determined by using funnel method. The accurately weighed spheres were taken in funnel, and were adjusted in such a way that the tip of funnel just touches the apex of the heap of blends. The blends were allowed to flow through the funnel, freely on the surface. The diameter of the powder concentration was measured; angle of repose was calculated by using following equation [Table 3].[15,16]
Tan ø = h/r
Where
h = height of pile
Ø = angle of repose
R = radius of base pile
25 = excellent flow
25-35 = good flow
30-40 = passable
>40 = very poor flow
Bulk density
Apparent bulk density was measured by pouring the pre-weighed blend into a graduated cylinder. The bulk volume of the blend was determined, and then the bulk density was calculated by using the formula [Table 3].[18]
Pb = M/VT
Tapped density
The measuring cylinder containing a known mass of blend was tapped for a fixed time and the min. Volume (Wt) occupied in the cylinder was measured; the tapped density (pt) was calculated by using the following formula [Table 3].[18]
Pt = M/vt
Consolidation index %
It is one method for determining flow properties and also called as carr's index of compressibility. It is indirectly related to the relative flow rate, cohesiveness, and particle size. It is simple, fast, and popular method of predicting powder flow characteristic [Table 3].[18]
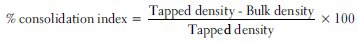
Results and Discussion
The possible interactions between Rabeprazole sodium and distinct polymers were investigated via FT-IR studies. Results proved that the drug was found to be compatible with excipients as wave numbers are almost similar for pure drug as well as drug excipients mixture.
All the formulated matrix tablets of Rabeprazole were mainly prepared by using different polymers like HPMC-E15, Carbopol934, sodium CMC either alone or in combination. The matrix tablet mainly fabricated using wet granulation method. As such all the formulated matrix tablets were of good quality respect to size, hardness, and drug content.
The hardness measured by Monsanto type hardness tester was found within the range of 3 to 4.5 g/cm2 and it was ideally suited with requirement of matrix formulation [Table 3].
The weight variation study was performed on 20 individuals on randomly selected samples from each batch; the weight uniformity results of prepared matrix tablets indicate no significant difference in the weight of individual tablet from average value and the variation was found within the limit [Table 3].
The thickness of the formulated tablets was within the range of 0.3 to 0.4 cm [Table 3].
The drug content study was performed on 3 randomly selected samples from each batch. The result indicates that the content of Rabeprazole of all batches was found within the range of 90% to 97%.
The formulated granules of different formulations were evaluated for their rheological properties like angle of repose, bulk density, tapped density, and Carr's index [Table 3]. The result of angle of repose indicates the good flow properties of all the formulated granules. The bulk density, tapped density, and Carr's index values also suggested that the prepared granules have good property regarding flow ability.
In vitro releases of rabeprazole from different batches were studied using USP-II paddle type dissolution apparatus in acidic pH for 2 hrs and in phosphate buffer for 10 hrs.
The different concentrations of different polymers showed different release patterns. From the release data of different formulations [Tables 4 and 5], it was observed that the release rate of most of the formulation were more than 80% to 90% in 10 hours study period [Figures 2 and 3]. The higher concentration of Carbapol 934 showed better sustained release properties (F4) than HPMC (F1). The combination of different polymers like HPMC, NaCMC, with Rabeprazole also showed better sustained release than the combination with Carbapol 934 and NaCMC. By observing the release data it may be concluded that the release rate of drug from matrix formulations largely depends upon type of polymer and its amount.
Table 4.
Zero order and higuchi release for the following formulations
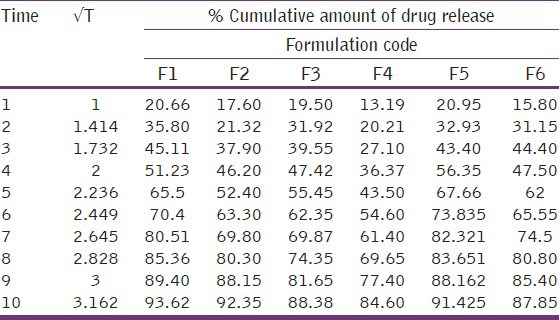
Table 5.
First order drug release for following formulations
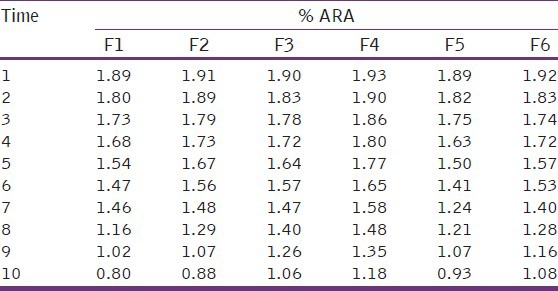
Figure 2.
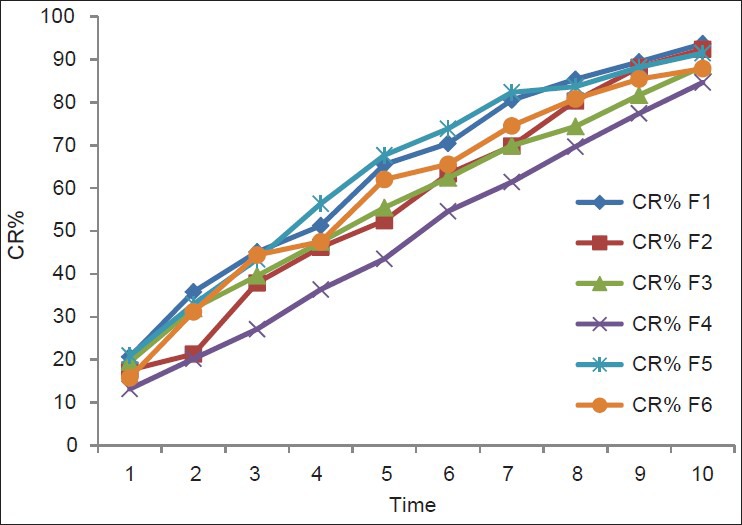
Zero order release
Figure 3.
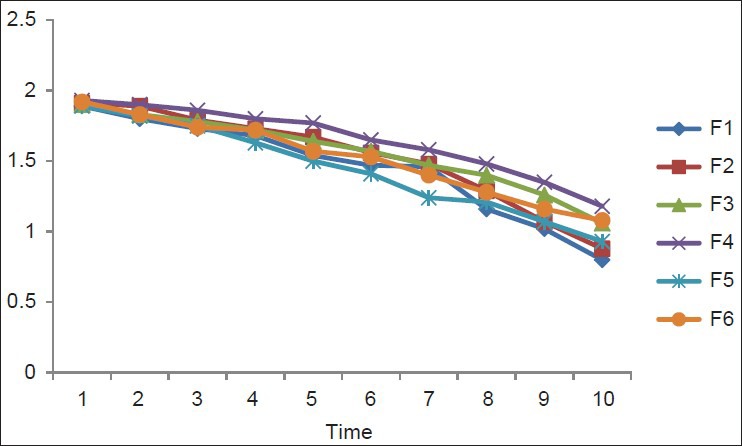
First order release
Conclusion
In our study, our observation shows that the Rabeprazole matrix tablet extends the release rate of drug for a prolong period of time at least 10 hrs and shows to increase the bioavailability and simultaneously decrease the dosing interval as well as dosing amount. The formulation minimizes the blood level oscillations, dose related adverse effects and cost and ultimately improve the patient compliance and drug efficiency.
Acknowledgement
The authors are humbly thankful to Mr. Durga Verma (Chairman) and Mr. R. R. Aggarwal (Director) of Siddhartha Institute of Pharmacy, Dehradun, and D. S. Chauhan (Vice-chancellor, Uttarakhand Technical University, Dehradun) for providing lab and library facilities.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Gambhire MN, Ambade KW, Kurmi SD, Kadam VJ, Jadhav KR. Development and in vitro evaluation of an oral floating matrix tablet formulation of diltiazem hydrochloride. AAPS Pharm Sci Tech. 2007;8:E73. doi: 10.1208/pt0803073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gordon ER, Rosanke TW, Fonner OE, Anderson NR, Baker GS. Pharmaceutical dosage forms: Tablets. In: Lieberman HA, Lachman L, Schwartz, editors. New York: Marcel Dekker; 1990. p. 83. [Google Scholar]
- 3.Vayas SP, Khar RK. 1st ed. New Delhi: Vallabh Prakashan; 2002. Controlled drug delivery concepts and advances; p. 100. [Google Scholar]
- 4.Reddy KR, Mutalik S, Reddy S. Once-daily sustained release matrix tablets of nicorandil: Formulation and in vitro valuation. AAPS Pharm Sci Tech. 2003;4:E61. doi: 10.1208/pt040461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siddique S, Mohd Y. Formulation of sustained release matrix of highly water soluble drugs. Pharma Review. 2008 [Google Scholar]
- 6.King RE. Tablets in remington's pharmaceutical sciences. In: Coy MP, editor. 15th ed. Vol. 1. Pennsylvania: 1975. [Google Scholar]
- 7.Jain NK. 1st ed. Vol. 1. CBS Publishers and Distributers; 1975. Pharmaceutical Product Development”; pp. 420–21. (428-29). [Google Scholar]
- 8.Krishnaiah YS, Karthikeyan RS, Gouri Sankar V, Satyanarayana V. Three-layer guar gum matrix tablet formulations for oral controlled delivery of highly soluble trimetazidine dihydrochloride. J Control Release. 2002;81:45–56. doi: 10.1016/s0168-3659(02)00031-7. [DOI] [PubMed] [Google Scholar]
- 9.Subal CB, Kesevan SK, Murugesan R. Design and release characteristics of sustained release tablet containing metformin HCl. Braz J Pharm Sci. 2008;44:477–83. [Google Scholar]
- 10.Sugawara S, Imai T, Otagiri M. The controlled release of prednisolone using alginate gel. Pharm Res. 1994;11:272–7. doi: 10.1023/a:1018963626248. [DOI] [PubMed] [Google Scholar]
- 11.Williams MP, Pounder RE. Review article: The pharmacology of rabeprazole. Aliment Pharmacol Ther. 1999;13(Suppl 3):3–10. doi: 10.1046/j.1365-2036.1999.00019.x. [DOI] [PubMed] [Google Scholar]
- 12.Chambers HF. General considerations of antimicrobial therapy. In: Brunton LL, Lazo JS, Parker KL, editors. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 11th ed. New York: McGraw-Hill Medical Publishing Division; 2006. pp. 969–73. [Google Scholar]
- 13.Ishizaki T, Horai Y. Review article: Cytochrome P450 and the metabolism of proton pump inhibitors-emphasis on rabeprazole. Aliment Pharmacol Ther. 1999;13:27–36. doi: 10.1046/j.1365-2036.1999.00022.x. [DOI] [PubMed] [Google Scholar]
- 14.Welage LS, Berardi RR. Evaluation of omeprazole, lansoprazole, pantoprazole, and rabeprazole in the treatment of acid-related diseases. J Am Pharm Assoc (Wash) 2000;40:52–62. doi: 10.1016/s1086-5802(16)31036-1. [DOI] [PubMed] [Google Scholar]
- 15.Vidyadhara S, Rao PR, Prasad JA. Development and in vitro kinetics of propanolol hydrochloride controlled release matrix tablets. Indian Pharm. 2006;5:66–70. [Google Scholar]
- 16.Vidyadhara S, Choudhary YA, Murthy TE, Rao MV, Reddy KN. Influence of electrolyte on controlled release of Ambroxol hydrochloride from methocel matrix tablet. Pharma Review. 2006:101–04. [Google Scholar]
- 17.Varshosaz J, Tavakoli N, Roozbahani F. Formulation and in vitro characterization of Captopril floating extended release tablet. Drug Deliv. 2006;13:277–85. doi: 10.1080/10717540500395106. [DOI] [PubMed] [Google Scholar]
- 18.Rahman Z, Ali M, Khar R. Design and evaluation of bilayer floating tablets of captopril. Acta Pharm. 2006;56:49–57. [PubMed] [Google Scholar]


