Abstract
Aim:
To examine the preventive effect of Aloe vera gel ethanolic extract using diabetic foot ulcer (DFUs) protocol in Wistar rats.
Materials and Methods:
Male Wistar rats were divided into untreated control (Group I), untreated DFUs (Group II), DFUs treated with A. vera gel ethanolic extract (Group III), DFUs treated with topical A. vera gel (Group IV), DFUs treated with A. vera gel ethanolic extract and topical A. vera gel (Group V). The rats in the treatment groups were daily administered the A. vera gel and ethanolic extract for 9 days. Fasting blood glucose levels and percentage of wound ulcer contraction were measured on day 3, 6, and 9.
Statistical Analysis used:
The results are expressed as a mean ± Standard Error Mean (SEM). Data were analyzed using one-way analysis of variance (ANOVA) after Newman–Keuls test. P < 0.05 were considered statistically significant in all cases.
Results:
Oral administration of A. vera gel ethanolic extract at a dose of 300 mg/kg body weight per day to diabetic rats for a period of 9 days resulted in a significant reduction in fasting blood glucose and a significant improvement in plasma insulin. Topical application of A. vera gel at a dose 30 mg/kg body weight per day to streptozotocin (STZ)-induced diabetic rats for a period of 9 days resulted in no change in blood glucose and plasma insulin. Oral administration as well as topical application of A. vera gel ethanolic extract and gel significantly reduced the blood glucose, improved the plasma insulin, and significantly increased DNA and glycosaminoglycans (GAGs) to improve the wound ulcer healing as well as the breaking strength on day 9.
Conclusions:
Present findings provide a scientific rationale for the use of A. vera gel ethanolic extract, and showed that the gel attenuated the diabetic foot wound in rats.
KEY WORDS: Aloe vera, Ayurveda, ethanolic extract, hypoglycemia, streptozotocin
Aloe vera is a perennial succulent plant belonging to the lily (Liliaceae) family. This plant has been known as the “healing plant” or “silent healer.” It has been claimed that Aloe has several important therapeutic properties, including wound healing, thermal injury healing, anti-inflammation immunomodulation, antidiabetic, and hypoglycemic. Using these effects, Aloe is nowadays used in a variety of commercial products, including sun creams, cosmetics, and lotions. However, the whole extracts of Aloe are used, and the relationship between the various components and their effects has not been well elucidated. Therefore, in Aloe research, it is important to isolate single components with biological effects, to examine these effects, and to elucidate their functional mechanism.[1,2,3]
Wound healing is a complex and highly regulated process that can be compromised by both endogenous factors (pathophysiological) and exo genous factors (micro-organisms). Microbial colonization of both acute and chronic wounds is inevitable, and in most situations, endogenous bacteria predominate, many of which are potentially pathogenic in the wound environment.[4]
Diabetic foot ulcers (DFUs), a leading cause of amputations, affect 15% of people with diabetes. A series of multiple mechanisms, including decreased cell and growth factor response, lead to diminished peripheral blood flow and decreased local angiogenesis, all of which can contribute to lack of healing in persons with DFUs.[5]
Against this backdrop, the present study is aimed to determine the wound healing activity of A. vera gel ethanolic extract in experimental animal models.
Materials and Methods
Experimental procedure
Collection and authentification of A. vera
The medicinal plants were identified and collected locally during the month of June, and were authenticated by Botanical Survey of India, Wardha, India. Voucher specimen no. 2008/001 and herbarium sheet were kept in the institute for further references.
Preparation of A. vera gel and A. vera gel ethanol extract
A. vera gel was prepared according to method described earlier.[6] In brief, full-size mature leaves were cut from the plant and the rind removed. The colorless parenchyma was ground in a blender and centrifuged at 10000 rpm for 30 min at 4°C to remove the fibers. The supernatant was lyophilized and stored at room temperature until use. A. vera gel was mixed with 95% ethanol and the total volume was made up to 500 ml. After extraction, the volume becomes 385 ml, and after 1 week of drying, 12.6 g powder of A. vera gel ethanolic extract is obtained.
Grouping of animals
Young healthy male Wistar rats weighing 180-225 g were used in the present investigation. Animals were provided with standard pellets and drinking water ad libitum and were maintained in 12 h light and dark cycle. The protocol of the experiment was approved (approval no. ACP/01/08-2009) by Institutional Animal Ethics Committee (IAEC), and the experiment was conducted in accordance with the guidelines as per “Guide for the care and use of laboratory animals” and with permission from Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA). Male Wistar rats were divided into untreated control (Group I), untreated DFUs (Group II), DFUs treated with A. vera gel ethanolic extract (Group III), DFUs treated with topical A. vera gel (Group IV), and DFUs treated with A. vera gel ethanolic extract and topical A. vera gel (Group V) which were daily administered the A. vera gel and ethanolic extract for 9 days. Fasting blood glucose levels and percentage of wound ulcer contraction were measured on day 3, 6, and 9.
Phytochemical screening and thin-layer chromatography
The extract obtained was subjected to various qualitative tests for identification of the constituents present, by using simple and standard qualitative methods described earlier.[6,7]
Thin-layer chromatography (TLC) was performed to investigate the authenticity of A. vera ingredients. Separation was performed on pre-coated 10 × 10 cm high-performance thin layer chromatography (HPTLC) glass plates (sorbent: Silica gel; pore size: 60). Sample and standard volumes of 20 μl were applied to the plates. The plates were developed using a freshly prepared mobile phase of n-butanol: n-propanol: Glacial acetic acid: Water (30:10:10:10, v/v/v/v). After drying at room temperature, the spots were stained with a solution of anisaldehyde reagent (0.5 ml of anisaldehyde, 10 ml acetic acid, 85 ml methanol, and 5 ml sulfuric acid) by dipping, followed by heating for 5 min at 105-110°C, and comparison was made between test and standard.[8]
Induction of experimental diabetes
Rats were fasted overnight before being injected with streptozotocin (STZ) at a dose of 60 mg/kg body weight (in 0.1 M citrate buffer, pH 4.5) intraperitoneally (i.p.). Seventy-two hours later, the blood samples were collected and glucose levels were determined to confirm the development of diabetes. Only those animals that showed hyperglycemia (blood glucose level >200 mg/dl) were used in the experiment.[9]
Surgical wounding procedures
Only those rats with severe diabetes on day 4 (fasting blood glucose >300 mg/dl) were selected for ulcer induction.[10] One week after glucose estimation, the ulcer induction was carried out by two methods, i.e. excision and incision wound models. The day of wound induction was considered as day 0. Animals were treated with A. vera gel ethanolic extract [300 mg/kg, per os (p.o.), twice daily] and A. vera gel (30 mg/kg, twice daily) from day 1 to day 9.
Diabetic wound ulcer models
Excision wound ulcer
Excision wound ulcer was produced by the method described earlier,[10] for determination of biochemical parameters and the rate of wound contraction. In brief, on day 0, rats were anesthetized with ketamine [100 mg/kg, intramuscularly (i.m.)] and xylazine (5 mg/kg, i.m.) combination. A rectangle was marked on the dorsal surface of the foot using a signet and then a layer of skin in full thickness (standard area 2 × 5 mm) was removed. One day after wound induction (day 1), the wound became slightly larger.
General parameter (scoring system)
The DEPA scoring system includes the depth of the ulcer (D), the extent of bacterial colonization (E), the phase of ulcer healing (P) and the associated underlying etiology (A) was used to evaluate three important parameters of DFUs in addition to the associated underlying cause. Each parameter was rated on a point scale according to the severity and complexity as given in Tables 1 and 2.[11]
Table 1.
DEPA score: General ulcer parameters
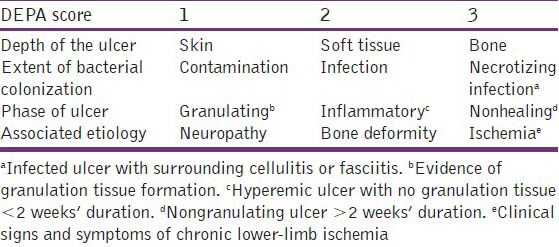
Table 2.
Grades of diabetic ulcers

Incision wound ulcer
Incision wound ulcer was produced with a slight modification of the earlier described method for excision wound,[12] for determination of tensile strength. In brief, on day 0, rats were anesthetized with ketamine (100 mg/kg, i.m.) and xylazine (5 mg/kg, i.m.) combination. A rectangle was marked on the dorsal surface of the foot using a signet and then a layer of skin in full thickness (standard area 2 × 5 mm) was removed. One day after wound induction (day 1), the wound became slightly larger, which was parted together by stitching the skin with sutures (non-absorbent) 1 mm apart; continuous threads on both wound edges were tightened for good adaptation of wound.
Measurement of metabolic parameters
Blood glucose monitoring
Blood samples were collected from the tail vein of overnight fasted rats and the blood glucose levels were estimated using Ascensia Entrust Blood Glucose Meter (Bayer Diagnostics, Dublin Ireland).
Plasma insulin
Fasting plasma insulin was measured using the insulin enzyme-linked immunosorbent assay (ELISA; American Laboratory Products Company, Windham, NH, USA). The regular insulin ELISA was used to analyze plasma from Wistar rats.
Biochemical analyses of excision wounds
Estimation of glycosaminoglycans
For extraction of total glycosaminoglycans (GAGs) from the granulation tissue, the tissue samples (10-20 mg) were suspended in 1% sodium dodecyl sulfate (SDS) and incubated in a boiling water bath for 10 min. After cooling to room temperature, proteinase K (0.5 mg/ml final concentration) digestion was carried out at 600°C for 18 h. The digested sample was precipitated with an equal volume of 10% tricholoroacetic acid (TCA) and the supernatant was subsequently extracted with cold chloroform: Methanol (2:1 v/v). GAGs were precipitated form the extracted aqueous phase by adding of 4-5 volumes of 95% ethanol saturated with 5% potassium acetate. The precipitate was dissolved in water and the amount of GAGs was determined spectrophotometrically at 224 nm. The results of GAGs estimation were statistically compared and the probability (P) values were calculated.[13]
Estimation of DNA
For the estimation of DNA, the chopped-off regenerated tissue was homogenized with 10% TCA, followed by serial washing with a mixture of alcohol and sodium acetate, alcohol and ether, and then ether alone. The pellet was suspended in NaOH and left for 20 h at room temperature and centrifuged. Finally, the pellet was resuspended in 10% TCA, and DNA was extracted at 90°C followed by centrifugation. Resultant supernatant was used for DNA estimation.[14,15]
Determination of wound area
On day 1, 3, 6, and 9, the wounds were traced on a transparent paper having a scale in millimeters, and the wound area was measured with a caliper using the method described earlier.[16] The wound of each animal was photographically documented and expressed in terms of the area calculated and percentage of wound area that had healed. The results of wound contraction studies of all groups were statistically compared and the P values were calculated. The wound contraction percentage was determined using the following formula:
Percentage of wound contraction = healed area/total area × 100
Breaking strength
For measurement of tensile strength, the sutures were removed from the stitched wounds of rats after recovery and the tensile strength was measured as follows. On day 9, the sutures were removed, and on day 10, wound ulcer breaking strength was determined. In brief, on day 10, the newly repaired tissue was excised and one of the edges of the wound was fixed while applying measurable force to the other one. The load (weight) in grams required to disrupt the wound was determined.
Statistical analysis
The results are expressed as a mean ± Standard Error Mean (SEM). Data were analyzed using one-way analysis of variance (ANOVA) after Newman–Keuls test. P < 0.05 were considered statistically significant in all cases.
Results
General parameters
Phytochemical screening and TLC
Phytochemical analysis of the extract showed the presence of C-glycosides, viz. aloe emodin, anthranols, tannins, steroids, saponins, alkaloids, flavanoids, and polyphenol. Results of TLC proved the authentification of sample A. vera gel [Figure 1].
Figure 1.
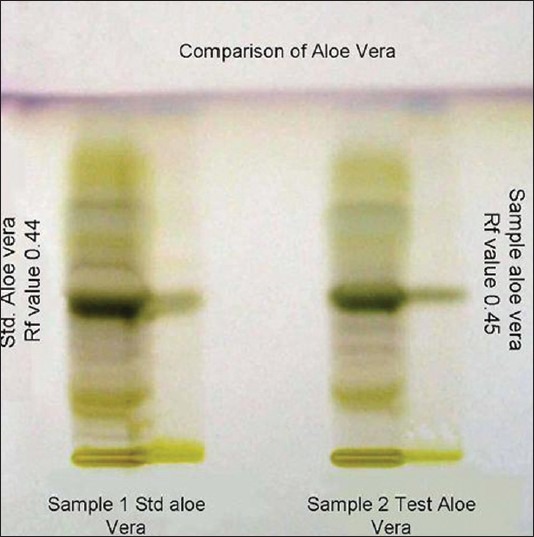
TLC of Aloe vera product and of authentic sample for comparison
Metabolic parameters
Fasting blood glucose
The administration of single dose of STZ produced significant increase in the blood glucose levels on day 1 and day 9, as compared to vehicle-treated group (P < 0.0001). Administration of A. vera ethanolic extract significantly reduced the blood glucose levels in diabetic rats (P < 0.05) on day 9. Topical application of A. vera gel did not influence the blood glucose levels in diabetic rats (P > 0.05) [Figure 2].
Figure 2.
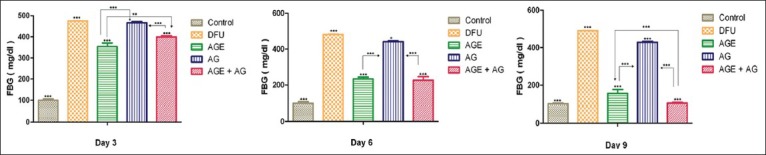
Fasting blood glucose levels of Aloe vera treated and untreated ulcers in diabetic rats. Data are given as mean ± SEM for six animals in each group. Statistically significant results are indicated as ***P < 0.0001
Fasting insulin and calculated insulin resistance
Measurement of fasting insulin on day 9 demonstrated relatively low level of insulin in animals with DFUs because increase in blood glucose levels for the excision and incision models with no statistical difference between these groups.
Biochemical analysis of excision wound
Determination of GAGs
Treated groups had significantly higher level of GAGs as compared to the untreated DFUs. GAGs level was significantly higher in the group treated with A. vera ethanolic gel extract when compared to the group administered A. vera gel. The increment in GAGs was observed to be higher in diabetic animals treated with both A. vera ethanolic extract and topical application of A. vera gel (P < 0.0001) compared to that in diabetic rats treated with vehicle [Figure 3].
Figure 3.
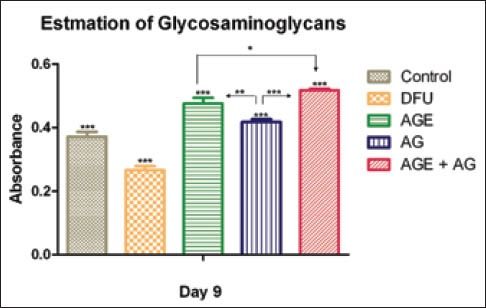
Glycosaminoglycan content of Aloe vera treated and untreated wound ulcers in diabetic rats. Data are given as mean ± SEM for six animals in each group. Statistically significant results are indicated as ***P < 0.0001
Estimation DNA
Treated groups had significantly higher level of DNA as compared to untreated DFUs. DNA level was significantly higher in the group treated with A. vera gel ethanolic extract when compared to the group treated with A. vera gel. The increment in DNA was observed to be higher in diabetic animals treated with both A. vera gel ethanolic extract and topical application of A. vera gel (P < 0.0001) compared to that in diabetic rats treated with vehicle [Figure 4].
Figure 4.
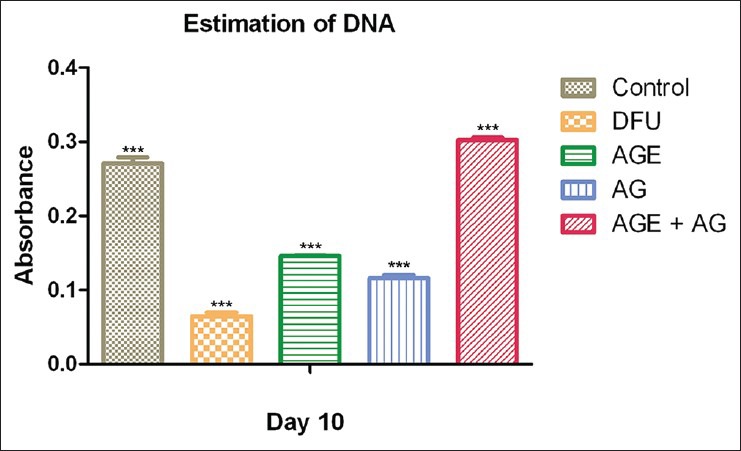
DNA content of Aloe vera treated and untreated wound ulcers in diabetic rats. Data are given as mean ± SEM for six animals in each group. Statistically significant results are indicated as ***P < 0.0001
Rate of wound contraction and period of epithelialization
On day 1, all the groups treated with STZ (diabetic groups) exhibited significantly increased wound size compared to control animals (P < 0.05). There was decrease in wound size in the control group (non-diabetic) which showed complete healing on day 9. There was gradual increase in the wound size in vehicle-treated diabetic rats. The wound size was gradually decreased in diabetic animals treated with A. vera gel ethanolic extract (P < 0.01) and topical application of A. vera gel (P < 0.01) [Figure 5].
Figure 5.
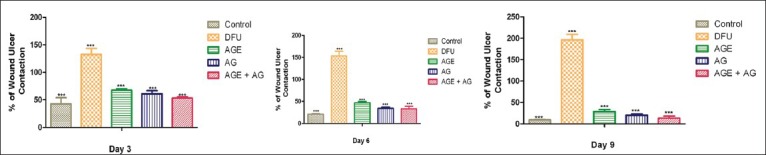
Rate of wound ulcer contraction of Aloe vera treated and untreated ulcers in diabetic rats. Data are given as mean ± SEM for six animals in each group. Statistically significant results are indicated as ***P < 0.0001
Breaking strength (tensile strength)
The treated diabetic group exhibited significantly lower breaking strength compared to vehicle treatment in the non-diabetic group (P < 0.0001). There was increase in breaking strength in the diabetic animals treated with A. vera extract (P < 0.0001) and topical application of A. vera gel (P < 0.0001) [Figure 6].
Figure 6.
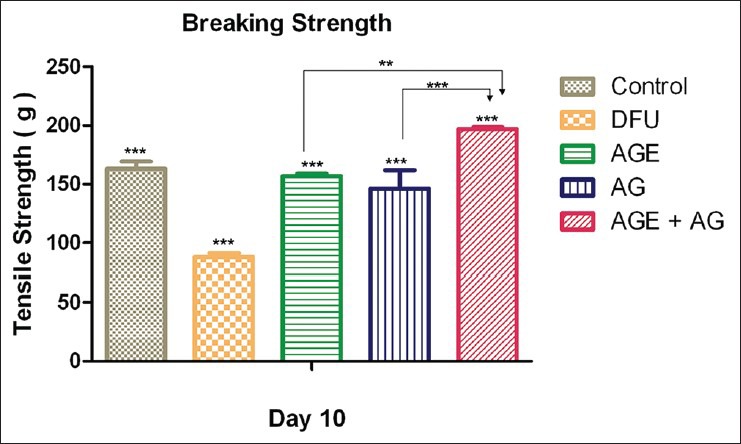
Tensile strength of Aloe vera treated and untreated wound ulcers in diabetic rats. Data are given as mean ± SEM for six animals in each group. Statistically significant results are indicated as ***P < 0.0001
Discussion
STZ causes diabetes by rapid depletion of β-cells, which leads to a reduction of insulin release. It is well established that A. vera causes hypoglycemia by stimulating the secretion of insulin and insulin synthesis from the existing pancreatic β-cells, and that this compound is active in moderate STZ-induced diabetes, whereas it is inactive in intense STZ diabetes (in which nearly all β-cells have been destroyed). Results show that A. vera gel ethanolic extract significantly reduced the blood glucose levels in diabetic rats (P < 0.05) on day 9, compared to other treatments [Figure 2]. Treatment of moderate STZ diabetic rats with medicinal plant extract resulted in the activation of β-cells and granulation returning to normal, showing an insulinogenic effect. The antihyperglycemic activity of A. vera was associated with an increase in plasma insulin, suggesting that the antihyperglycemic activity of A. vera could be due to an insulinogenic activity of the A. vera gel ethanolic extract. The increased levels of insulin observed in the present study indicate that the A. vera gel ethanolic extract stimulates insulin secretion from the remnant β-cells and from regenerated β-cells.[7,12,13] Effect of pseudoprototinosaponin AIII and prototinosaponin AIII on glucose uptake and insulin release suggested their hypoglycemic effects are due to their action on hepatic gluconeogenesis or glycogenolysis. Single as well as repeated doses of the bitter principle of A. vera showed hypoglycemic effect in diabetic rats, which was through stimulation of synthesis or release of insulin from pancreatic β-cells.[16]
Processed A. vera gel was found to suppress the expression of the adipogenic genes, sterol regulatory element-binding proteins (SREBP-1a), fatty acid synthase (FAS), and glycerol-3-phosphate acyltransferase (GPAT), suggesting that the gel improves insulin resistance by reducing the toxic effects of lipids in the liver.[17] Based on the findings of another study on STZ-induced diabetic rats, it was suggested that the mechanism of action of A. vera extracts in reducing blood glucose levels is by enhancing the glucose metabolism. It was further proposed that the glucose-lowering effect could be explained by an antioxidant mechanism because it attenuated oxidative damage in the brains of STZ-induced mice and reduced peroxidation levels in the kidneys of STZ-induced diabetic rats.[18]
Diabetes mellitus is known to be associated with a variety of alterations in connective tissue metabolism, as a result of which diabetics face the problem of poor wound healing. Loss of collagen observed in diabetes may be due to decreased levels of synthesis or enhanced catabolism of newly synthesized collagen or both.[19] As A. vera was reported to cause hypoglycemic effects, it was felt that it would be interesting to study its influence on the healing of wounds in diabetic conditions. A. vera gel ethanolic extract significantly reduced the wound size in excision wound ulcer model, as well as increased the breaking strength in incision wound ulcer model. Topical application of A. vera gel also significantly reduced the wound size and increased the breaking strength [Figures 5 and 6]. The reduction in wound size and increase in breaking strength was significantly higher in diabetic rats treated with both oral A. vera gel ethanolic extract and topical A. vera gel in combination.[16,20] Results obtained in the present study suggest that treatment of diabetic rats with A. vera gel may have a beneficial influence on wound healing.
Collagen is the predominant extracellular protein in the granulation tissue of a healing wound and there is a rapid increase in the synthesis of this protein in the wound area soon after an injury. In addition to providing strength and integrity to the tissue matrix, collagen also plays an important role in hemostasis. Subsequent epithelialization also requires collagen. In the present study, we examined the influence of A. vera gel and extract on the collagen content in the granulation tissues of healing full-thickness wounds in diabetic rats. Treatment of wounds with A. vera gel ethanolic extract and gel increased the collagen to maximum levels in the granulation tissue, as compared to the untreated diabetic controls [Figure 3].
GAGs and proteoglycans are synthesized by fibroblasts in the wound area. These substances form a highly hydrated gel-like ground substance, a provisional matrix on which collagen fibers are embedded. As collagen accumulates, hexosamine levels decrease. Treatment with A. vera gel increases the content of ground substance in the granulation tissues. It may be seen that the decrease in hexosamine content was associated with a concomitant increase in collagen content.[21] Hyaluronic acid, a GAG, is a major component of the extracellular matrix found on cell surface and is the first component of the extracellular matrix to be synthesized during wound healing process.[3] It is well documented that the levels of GAGs decreased in the diabetic rats as well as in diabetic wound.[22] Literature reports suggest treatment with hyaluronic acid based products significantly improve healing of DFUs.[23] Interestingly, oral administration as well as topical application of A. vera is reported to increase the hyaluronic acid. Therefore, the reduction in DFUs by A. vera could be subsequent to its effect on GAGs [Figure 3].[3]
The protein and DNA content of granulation tissues indicates the level of protein synthesis and cellular proliferation. Higher protein and DNA content (compared to the untreated controls) of the treated wounds suggests that A. vera, through an as yet unknown mechanism, stimulates cellular proliferation. The collagen: DNA ratio of the granulation tissues also suggests that A. vera gel may increase the synthesis of collagen per cell. The DNA and protein are the major components to be synthesized during wound healing process. It is well documented that DNA and protein levels increase in the diabetic treated rats as well as in diabetic wounds. Interestingly, oral administration as well as topical application of A. vera is reported to increase the DNA level [Figure 6]. Therefore, the reduction in diabetic foot wound by A. vera could be subsequent to its effect on DNA.[3]
Wound healing is a response to the injured tissue that results in the restoration of tissue integrity. It was shown in several studies that A. vera gel could improve wound healing after topical and systemic administration, while others claimed no effect or even a delay in wound healing. Conflicting results obtained may be explained by the stability of the active ingredients, as it was shown that the time of treatment after harvesting was an important factor that determined the activity. Several mechanisms have been proposed for the wound healing effects of A. vera gel, which include keeping the wound moist, increasing epithelial cell migration, more rapid maturation of collagen, and reduction in inflammation.[24] Rodent models of diabetes display impaired wound repair, with decreased wound tensile strength and poor collagen deposition, and have decreased levels of DNA and protein. Moreover, A. vera is reported to exhibit anti-inflammatory effect, and increases collagen deposition in wound tissue, tensile strength, DNA content, and protein synthesis.[3,25] Treatment with A. vera has been shown to reduce tissue loss and improve healing in frost bite and electrical injury under non-diabetic conditions.[25,26] Its beneficial influence on the healing of wounds in both normal and diabetic rats was also observed by Davis et al.[27,28] Certain triterpenes from other plants have been demonstrated to enhance wound healing.[29,30]
Herbal products that are rich in phenolic compounds, flavonoids, terpenoids, coumarins, glycosides, saponins, bitter principles, and carbohydrates usually show positive effects.[31,32,33,34,35,36] These compounds were shown to possess potent hypoglycemic, antihyperglycemic, and glucose-suppressive activities. Saponins were confirmed to be bioactive for diabetes and/or diabetic complications by regulating the activity of enzymes related to glucose metabolism and promoting insulin secretion.[33] Polyphenolic compounds, especially flavonoids, are among the classes of compounds that have received the most attention with regard to their antidiabetic properties.[37] Flavonoids are natural polyphenolic molecules of plant origin known for their antioxidant, anti-inflammatory, and anticarcinogenic properties.[38] Dietary intake of flavonoids might prove to be important for alternative diabetes treatments or reducing the risk of the disease. Attempts have been made to determine their potential in improving the function of β-cells of pancreas,[33] preventing β-cell apoptosis, promoting β-cell proliferation and insulin secretion,[38] and enhancing insulin activity.[39] An alcoholic extract of the A. vera gel contains compounds such as saponins, naftoquinones, anthroquinones, sterols, and triterpenoids.[40] These compounds may mediate the beneficial effects of A. vera. However, each of these compounds needs to be tested separately to identify the putative active principle of the gel. As reported by Davis et al., among the two major constituents of Aloe, glucose-6-phosphate and mannose-6-phosphate, mannose-6-phosphate is the important structural constituent that promotes wound healing and has anti-inflammatory activity.[41] Glucomannan, a mannose-rich polysaccharide, and gibberellin, a growth hormone, interact with growth factor receptors on the fibroblast, thereby stimulating their activity and proliferation, which in turn significantly increase collagen synthesis after topical and oral administration of A. vera gel. A. vera gel not only increased collagen content of the wound but also changed collagen composition (more type III) and increased the degree of collagen cross-linking. Due to this, it accelerated wound contraction and increased the breaking strength of resulting scar tissue. An increased synthesis of hyaluronic acid and dermatan sulfate in the granulation tissue of a healing wound following oral or topical treatment has been reported.[42]
The present study demonstrates that the A. vera gel ethanolic extract accelerate wound healing in diabetes. The results suggest that treatment with A. vera gel may have a beneficial influence on the various phases of wound healing, i.e. fibroplasia, collagen synthesis and contraction, resulting in faster healing. It is quite possible that the enhanced healing of wounds in diabetic rats by A. vera gel is a result of its hypoglycemic activity (since a control over blood glucose levels has been shown to improve wound healing in diabetics) and/or its capacity to stimulate fibroblast function during the healing process. Oral administration of A. vera gel ethanolic extract at a dose of 300 mg/kg body weight per day to STZ-induced diabetic rats for a period of 9 days resulted in a significant reduction in fasting blood glucose and a significant improvement in plasma insulin. Topical application of A. vera gel at a dose 30 mg/kg body weight per day to STZ-induced diabetic rats for a period of 9 days resulted in no change in blood glucose and plasma insulin levels.
Conclusion
On the basis of the results obtained, we conclude that oral administration as well as topical application of A. vera gel ethanolic extract significantly reduces blood glucose level and improves the plasma insulin. It also significantly increases the DNA content and the level of GAGs to improve the wound ulcer healing as well as the breaking strength on day 9. Thus, the results of the present study provide a scientific rationale for the use of A. vera gel ethanolic extract, and show that the gel attenuated the diabetic foot wound in rats.
Footnotes
Source of Support: Department of Pharmacology, Agnihotri College of Pharmacy, Wardha, Maharashtra, India
Conflict of Interest: None declared.
References
- 1.Choi SW, Son BW, Son YS, Park YI, Lee SK, Chung MH. The wound-healing effect of a glycoprotein fraction isolated from aloe vera. Br J Dermatol. 2001;145:535–45. doi: 10.1046/j.1365-2133.2001.04410.x. [DOI] [PubMed] [Google Scholar]
- 2.Chithra P, Sajithlal GB, Chandrakasan G. Influence of Aloe vera on collagen characteristics in healing dermal wounds in rats. Mol Cell Biochem. 1998;181:71–6. doi: 10.1023/a:1006813510959. [DOI] [PubMed] [Google Scholar]
- 3.Chithra P, Sajithlal GB, Chandrakasan G. Influence of Aloe vera on the glycosaminoglycans in the matrix of healing dermal wounds in rats. J Ethnopharmacol. 1998;59:179–86. doi: 10.1016/s0378-8741(97)00112-8. [DOI] [PubMed] [Google Scholar]
- 4.Bowler PG. Wound pathophysiology, infection and therapeutic options. Ann Med. 2002;34:419–27. doi: 10.1080/078538902321012360. [DOI] [PubMed] [Google Scholar]
- 5.Brem H, Tomic-Canic M. Cellular and molecular basis of wound healing in diabetes. J Clin Invest. 2007;117:1219–22. doi: 10.1172/JCI32169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rajasekaran S, Sivagnanam K, Ravi K, Subramanian S. Hypoglycemic effect of Aloe vera gel on streptozotocin-induced diabetes in experimental rats. J Med Food. 2004;7:61–6. doi: 10.1089/109662004322984725. [DOI] [PubMed] [Google Scholar]
- 7.Rajasekaran S, Sivagnanam K, Subramanian S. Antioxidant effect of Aloe vera gel extract in streptozotocin-induced diabetic in experimental rats. Pharmacol Rep. 2005;57:90–6. [PubMed] [Google Scholar]
- 8.Lachenmeier K, Kuepper U, Musshoff F, Madea B, Reusch H, Lachenmeier DW. Quality control of Aloe vera beverages. Electron J Environ Agric Food Chem. 2005;4:1033–42. [Google Scholar]
- 9.Wang J, Wang H, Hao P, Xue L, Wei S, Zhang Y, et al. Inhibition of aldehyde dehydrogenase 2 by oxidative stress is associated with cardiac dysfunction in diabetic rats. Mol Med. 2011;17:172–9. doi: 10.2119/molmed.2010.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lau TW, Sahota DS, Lau CH, Chan CM, Lam FC, Ho YY, et al. An in vivo investigation of the wound-healing effect of two medicinal herbs using an animal model with foot. Eur Surg Res. 2008;41:15–23. doi: 10.1159/000122834. [DOI] [PubMed] [Google Scholar]
- 11.Younes NA, Albsoul AM. The DEPA scoring system and its correlation with the healing rate of diabetic foot ulcers. J Foot Ankle Surg. 2004;43:209–13. doi: 10.1053/j.jfas.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 12.Mukherjee PK, Verpoorte R, Suresh B. Evaluation of in-vivo wound healing activity of Hypericum patulum (Family: Hypericaceae) leaf extract on different wound model in rats. J Ethnopharmacol. 2000;70:315–21. doi: 10.1016/s0378-8741(99)00172-5. [DOI] [PubMed] [Google Scholar]
- 13.Smith RL, Gilkerson E, Kohatsu N, Merchant T, Schurman DJ. Quantitative microanalysis of synovial fluid and articular cartilage glycosaminoglycans. Anal Biochem. 1980;103:191–200. doi: 10.1016/0003-2697(80)90255-9. [DOI] [PubMed] [Google Scholar]
- 14.Burton K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956;62:315–23. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burton K. The relation between the synthesis of deoxyribonucleic acid and the synthesis of protein in the multiplication of bacteriophage T2. Biochem J. 1955;61:473–83. doi: 10.1042/bj0610473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ajabnoor MA. Effect of Aloes on blood glucose levels in normal and alloxan diabetic mice. J Ethnopharmacol. 1990;28:215–20. doi: 10.1016/0378-8741(90)90031-n. [DOI] [PubMed] [Google Scholar]
- 17.Kim K, Kim H, Kwon J, Lee S, Kong H, Im SA, et al. Hypoglycemic and hypolipidemic effects of processed A. vera gel in a mouse model of non-insulin-dependent diabetes mellitus. Phytomedicine. 2009;16:856–63. doi: 10.1016/j.phymed.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 18.Boudreau MD, Beland FA. An Evaluation of the biological and toxicological properties of A. barbadensis (Miller), A. vera. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2006;24:103–54. doi: 10.1080/10590500600614303. [DOI] [PubMed] [Google Scholar]
- 19.Lien YH, Tseng MM, Stern R. Glucose and glucose analogs modulate collagen metabolism. Exp Mol Pathol. 1992;57:215–21. doi: 10.1016/0014-4800(92)90012-z. [DOI] [PubMed] [Google Scholar]
- 20.Ghannam N, Kingston M, Al-Meshaal IA, Tariq M, Parman NS, Woodhouse N. The antidiabetic activity of Aloes: Preliminary clinical and experimental observations. Horm Res. 1986;24:288–94. doi: 10.1159/000180569. [DOI] [PubMed] [Google Scholar]
- 21.Dunphy JE, Udupa KN. Chemical and histochemical sequences in the normal healing wounds. N Engl J Med. 1955;253:847–51. doi: 10.1056/NEJM195511172532002. [DOI] [PubMed] [Google Scholar]
- 22.Baie SH, Sheikh KA. The wound healing properties of Channa striatus-cetrimide cream-wound contraction and glycosaminoglycan measurement. J Ethnopharmacol. 2000;73:15–30. doi: 10.1016/s0378-8741(00)00253-1. [DOI] [PubMed] [Google Scholar]
- 23.Vázquez B, Avila G, Segura D, Escalante B. Antiinflammatory activity of extracts from A. vera gel. J Ethnopharmacol. 1996;55:69–75. doi: 10.1016/s0378-8741(96)01476-6. [DOI] [PubMed] [Google Scholar]
- 24.Reynolds T, Dweck AC. Aloe vera leaf gel: A review update. J Ethnopharmacol. 1999;68:3–37. doi: 10.1016/s0378-8741(99)00085-9. [DOI] [PubMed] [Google Scholar]
- 25.Heggers JP, Robson MC, Zachary LS. Thromboxane inhibitors for the prevention of progressive dermal ischemia due to the thermal injury. J Burn Care Rehabil. 1985;6:466–8. doi: 10.1097/00004630-198511000-00002. [DOI] [PubMed] [Google Scholar]
- 26.Miller MB, Koltai PJ. Treatment of experimental frostbite with pentoxifylline and A. vera cream. Arch Otolaryngol Head Neck Surg. 1995;121:678–80. doi: 10.1001/archotol.1995.01890060076015. [DOI] [PubMed] [Google Scholar]
- 27.Davis RH, Kabbani JM, Maro NP. Aloe vera and wound healing. J Am Podiatr Med Assoc. 1987;77:165–9. doi: 10.7547/87507315-77-4-165. [DOI] [PubMed] [Google Scholar]
- 28.Davis RH, Leitner MG, Russo JM. Aloe vera. A natural approach for treating wounds, edema, and pain in diabetes. J Am Podiatr Med Assoc. 1988;78:60–8. doi: 10.7547/87507315-78-2-60. [DOI] [PubMed] [Google Scholar]
- 29.Maquart FX, Bellon G, Gillery P, Wegrowski Y, Borel JP. Stimulation of collagen synthesis in fibroblast cultures by a triterpene extracted from Centella asiatica. Connect Tissue Res. 1990;24:107–20. doi: 10.3109/03008209009152427. [DOI] [PubMed] [Google Scholar]
- 30.Suguna L, Sivakumar P, Chandrakasan G. Effects of Centella asiatica extract on dermal wound healing in rats. Indian J Exp Biol. 1996;34:1208–11. [PubMed] [Google Scholar]
- 31.Marles JR, Farnsworth NR. Antidiabetic plants and their active constituents. Phytomedicine. 1995;2:123–89. doi: 10.1016/S0944-7113(11)80059-0. [DOI] [PubMed] [Google Scholar]
- 32.Tabatabaei-Malazy O, Larijani B, Abdollahi M. A systematic review of in vitro studies conducted on effect of herbal products on secretion of insulin from langerhans islets. J Pharm Pharmaceut Sci. 2012;15:447–66. doi: 10.18433/j32w29. [DOI] [PubMed] [Google Scholar]
- 33.Li WL, Zheng HC, Bukru J, Dekimpe N. Natural Medicines used in traditional Chinese medicine system for therapy of diabetes mellitus. J Ethnopharmacol. 2004;92:1–21. doi: 10.1016/j.jep.2003.12.031. [DOI] [PubMed] [Google Scholar]
- 34.Tanko Y, Yaro AH, Isa AI, Erima M, Saleh MI, Mohammed A. Toxicological and hypoglycemic studies on the leaves of Cissampelos Mucronata (Menispermaceae) on blood glucose levels of streptozocin–induced diabetic wistar rats. J Med Plants Res. 2007;1:113–6. [Google Scholar]
- 35.Sherma RD, Sarkhar DK, Hazra MB. Toxicological evaluation of fenugreek seeds: A long term feeding experiment in diabetic patients. Phytother Res. 2010;10:519–20. [Google Scholar]
- 36.Sikarwar MS, Patil MB. Antidiabetic activity of Crateva nurvala stem bark extracts in alloxan-induced diabetic rats. J Pharm Bioallied Sci. 2010;2:18–21. doi: 10.4103/0975-7406.62700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coman C, Rugina OD, Socaciu C. Plants and natural compounds with antidiabetic action. Not Bot Horti Agrobo. 2012;40:314–25. [Google Scholar]
- 38.Pinent M, Castell A, Baiges I, Montagut G, Arola L. Bioactivity of flavonoids on insulin-secreting cells. Compr Rev Food Sci Food Safety. 2008;7:299–308. doi: 10.1111/j.1541-4337.2008.00048.x. [DOI] [PubMed] [Google Scholar]
- 39.Ahmed OM, Moneim AA, Yazid IA, Mahmoud AM. Antihyperglicemic antihyperlipidemic and antioxidant effects and the probable mechanisms of action of Ruta graveolens infusion and rutin in nicotinamide-streptozotocin-induced diabetic rats. Diabetologia Croatica. 2010;39:15–35. [Google Scholar]
- 40.Rosenthal SP. Acceleration of primay wound healing by insulin. Arch Surg. 1968;96:53–5. doi: 10.1001/archsurg.1968.01330190055012. [DOI] [PubMed] [Google Scholar]
- 41.Davis RH, Didonato JJ, Hartman GM, Haas RC. Anti-inflammatory and wound healing activity of a growth substance in aloe vera. J Amer Podiatric Med Assoc. 1994;84:77–81. doi: 10.7547/87507315-84-2-77. [DOI] [PubMed] [Google Scholar]
- 42.Surjushe A, Vasani R, Saple DG. Aloe vera: A short review. Indian J Dermatol. 2008;53:163–6. doi: 10.4103/0019-5154.44785. [DOI] [PMC free article] [PubMed] [Google Scholar]


