Abstract
Background
Excessive adipose tissue, particularly with a centralized distribution, propagates hormonal and metabolic disturbance. The detrimental effects of adiposity may extend beyond the periphery and target the central nervous system, increasing vulnerability to cognitive decline. The aim of current study was to determine how central adiposity impacts the brain at midlife by examining the blood oxygen level-dependent (BOLD) response to a challenging cognitive task.
Methods
Seventy-three adults, aged 40-60 years, completed a 2-Back verbal working memory task during functional magnetic resonance imaging (fMRI). Central adiposity was assessed with waist circumference. The association between waist circumference and task-related activation in a priori regions of interest was modeled using bootstrapping regression models corrected for multiple-comparisons.
Results
Larger waist circumference was associated with diminished working-memory-related BOLD response in the right superior frontal gyrus (β=-0.008, p=0.001, 95% CI: -0.012 - -0.004) and left middle frontal gyrus (β=-0.009, p=0.002, 95% CI: -0.015 - -0.003), statistically adjusting for age, sex, systolic blood pressure, and total cholesterol. Reduced task-related activation in the right superior frontal gyrus (r=-0.369, p=0.002) and left middle frontal gyrus (r=-0.266, p=0.025) were related to slower reaction time on the task, controlling for age and education.
Conclusions
Larger waist circumference predicted alterations in the BOLD response that coupled with decrements in task performance. While future studies are necessary, the results suggest that similar to its role in the periphery, central adiposity may be a robust predictor of metabolic and hormonal alterations that impinge upon central nervous system functioning.
Keywords: waist circumference, obesity, fMRI, 2-Back task, working memory
Introduction
Prevalence rates of obesity have rapidly accelerated in developing countries, leading to its classification as one of the top global health problems (1). Obesity is associated with numerous disease states including increased risk of cardiovascular disease, cancer, and all-cause mortality (2). Moreover, recent evidence indicates that obesity may have a similarly detrimental impact on the brain (3–5), making it one of the most significant emerging threats to cognition. In particular, obesity at midlife is linked with increased dementia risk and accelerated cerebral atrophy in the elderly (3,6,7). Given the vast prevalence of obesity, identification of the pathogenic mechanisms that underlie obesity's deleterious impact on the brain is a public health imperative. Traditionally, the cognitive impact of obesity has been attributed to cardiovascular risk factors such as diabetes and hypertension. However, numerous reports indicate that the association between obesity and cognitive decline persists over and above adjustment for cardiovascular risk factors (6,7), indicating that adiposity itself may impinge upon central nervous system functioning (8).
Adipocytes, the primary cellular component of adipose tissue, may directly impact cerebral health. Adipocytes are metabolically active cells, capable of modulating hormonal, inflammatory, and growth factor pathways that affect central nervous system functioning (8). Moreover, the distribution of adiposity may be an even more important predictor of cognitive vulnerability than total adipose mass (9). Adipose tissue distributed on the arms and legs of the body contains subcutaneous fat, which accumulates under the skin and represents approximately 80% of total body fat (10). In contrast, adipose accumulation along the waistline is indicative of visceral fat, which surrounds the viscera and internal abdominal organs (10). In comparison to subcutaneous fat, visceral fat is more metabolically active (11) and thus may exert a larger influence on central nervous system functioning. In support of this hypothesis, higher midlife waist circumference, a proxy measure for visceral adiposity, predicts increased dementia risk in older adults, whereas thigh circumference, a measure of subcutaneous fat, has no predictive utility (6).
A few studies to date have examined the association between central adiposity and cognition in older adults (6,12). However, very little of this work has been translated to younger populations, leaving unanswered questions about how central adiposity may impact the brain in the absence of concomitant age-related cognitive decline and high rates of disease comorbidity. Determination of early adiposity-related brain changes is tantamount to preventive efforts as clinically significant cognitive decline is preceded by a latent period of degenerative changes that manifest in middle-aged and younger adults (13). In particular, fMRI may provide an efficacious method for assessing the impact of central adiposity in cognitively intact middle-aged adults as it can identity altered brain activation patterns indicative of cognitive vulnerability (14). Prior work in our laboratory and others has identified changes in brain activation during cognition in association with elevated body mass index (15–17). However, to our knowledge, no published studies have examined the impact of central adiposity on the BOLD response to cognitive challenge. Thus, the goal of the current study was to determine how a centralized distribution of adiposity relates to brain activation during a working memory task in a cognitively-intact middle-aged sample. Working memory was examined during fMRI as this cognitive domain has been shown to be particularly vulnerable to obesity in otherwise healthy older adult populations (4). The working memory paradigm consisted of a verbal n-Back task because this measure has demonstrated sensitivity for detecting alterations in association with cardiovascular and metabolic risk factors (16,18). Moreover, the expected pattern of activation for this task, including the prefrontal cortex and superior and inferior parietal lobes, has been well established in the literature (19,20). Central adiposity was assessed with waist circumference, the best anthropometric index of visceral adipose accumulation (21). Based on the robust metabolic properties of visceral adiposity (11), waist circumference was hypothesized to predict alterations in brain activation during the working memory challenge.
Materials and Methods
Adults, between the ages of 40-60 years, were recruited from the community through electronic and print advertisements. All potential participants underwent a telephone screening and completed a medical history questionnaire to establish eligibility. Eligibility criteria included a medical history free of overt coronary artery disease, neurological disease (e.g., Parkinson's disease, stroke, multiple sclerosis, clinically significant traumatic brain injury), major psychiatric illness (e.g. bipolar disorder, schizophrenia), and substance abuse (i.e., diagnosed abuse and/or treatment for substance abuse). Additionally, exclusionary criteria were left-hand dominance, current smoking, diabetes (fasting glucose >126 mg/dl), global cognitive impairment (Full Scale Intellectual Quotient, FSIQ < 85), and incomplete or unusable data due to excessive movement in the scanner (translational displacement >2.5 mm in any plane). The study sample consisted of seventy-three participants, who provided written informed consent before enrollment. Based on participants' self-report, the ethnic distribution of the sample was as follows: 71.2% - Caucasian, 17.8% - Hispanic, 4.1% - African American, and 6.8% - Other/Did Not Specify.
Procedures
The study was approved by the local institutional review board and completed in accordance with the Helsinki Declaration of 1975. Participants underwent two separate study visits, a general health assessment and a neuropsychological/brain imaging assessment.
General Health Assessment
After an eight hour fast, a blood sample was collected from the antecubital vein by venipucture. Fasting glucose and total cholesterol levels were ascertained using standard enzymatic technique. Waist circumference was assessed by measuring the midpoint between the iliac crest and lower rib during exhalation as recommended by the World Health Organization (22). Height in centimeters and weight in kilograms were measured on a beam balance scale for body mass index calculations (kilograms divided by meters squared). Brachial systolic and diastolic blood pressure was assessed with a semi-automated device (VP-2000, Omron Healthcare, Bannockburn, IL) after fifteen minute period of rest.
Neuropsychological/Brain Imaging Assessment
Participants were administered a neuropsychological battery consisting of clinical instruments with established reliability and validity. The battery was administered by research assistants with training in standard administration and scoring criteria. In effort to limit the number of multiple comparisons, the cognitive tests were grouped into one of three cognitive domain scores as follows 1). Global: Mini Mental Status Exam (23) and Wechsler Abbreviated Scale of Intelligence II (WASI-II) Vocabulary and Matrix Reasoning raw subtest scores (24); 2) Memory: California Verbal Learning Test II (CVLT-II) short delay free recall, long delay recall, and recognition discriminability (25); 3) Executive: Trails A and B time to completion (26), Controlled Oral Word Associations Test (COWAT) (27), Wechsler Adult Intelligence Scale IV (WAIS-IV) Digit Span Subtest (28), and Stoop Color-Word Condition (29). Z-scores were computed from raw test scores using the study sample's mean and standard deviation. Scores from timed tests were multiplied by -1 so that higher scores indicated superior performance. Within each domain, the z-scores were averaged together to create a composite domain score.
Magnetic resonance imaging (MRI) was conducted using a 3T Siemens Skyra scanner equipped with a standard head-coil. Anatomical scans of the entire brain were collected in the sagittal plane using a high-resolution Spoiled Gradient Echo (SPGR) sequence (256 × 256 matrix, flip angle = 7°, FOV = 24 × 24 cm2, 1 mm slice thickness, 0 gap). Functional magnetic resonance imaging was performed during completion of the 2-Back verbal working memory using a using a whole brain echo-planer imaging (EPI) sequence (TR = 3000 ms, TE = 30 ms, flip angle = 90°, FOV = 24 × 24 cm2, 64 × 64 matrix, 42 axial slices, 3 mm slice thickness, 0.3 mm gap).
During fMRI scanning, participants completed two seven-minute runs of the verbal n-Back task (19,20), each containing three alternating 0-Back, 1-Back, and 2-Back blocks. Each block consisted of a visual presentation of twelve (0-Back condition) or fifteen (1-Back and 2-Back conditions) individual consonants, displayed in random order for 500 ms each with a 2500 ms inter-stimulus interval. Using an MRI-compatible two-button response box, participants indicated whether or not each letter was a target (33% in each block). The target for the 0-Back condition was a pre-specified letter (H). The targets for the 1-Back and 2-Back conditions were any letter identical to the one presented one or two stimuli before, respectively. E-Prime software (Psychology Software Tools, Inc., Pittsburgh, PA) was used to program and display the task. During scanning, the task was back-projected onto a screen located behind the participant's head and viewed through a double-mirror fixed to the head coil. Mean accuracy and reaction time for correct trials was calculated for each condition. All participants were given the opportunity to practice the task on a laptop prior to scanning.
Functional Imaging Analyses
fMRI data was processed with tools available from FSL v 4.1.2 (FMRIB's Software Library, www.fmrib.ox.ac.uk/fsl). During preprocessing, fMRI images underwent motion correction with MCFLIRT (30), removal of non-brain structures with BET (31), FILM prewhitening, high-pass filtering with a cut-off of 100 seconds, and spatial smoothing with a 5 mm full width half maximum Gaussian kernel. Non-brain structures were also extracted from the high-resolution anatomical images using BET (31). Each participant's functional images were aligned to his/her high-resolution anatomical images using a 7-parameter affine transformation with FMRIB's Linear Image Registration Tool (FLIRT) (30). These images were then registered to standard stereotaxic space template (MNI 152) using a 12-parameter affine transformation with FLIRT (30).
First level data analysis for the 2-Back task was conducted using the general linear model through FSL's Feat. The time series at each voxel was modeled with regressors for the block events (1-Back, 2-Back) against a baseline condition (0-Back), following convolution with a double-gamma hemodynamic response function. Additional covariates in the model included reaction time, missed trials, motion parameters, and the temporal derivates of all regressors. Given the interest in assessing working memory-related activation, the only examined contrast was 2-Back>0-Back. The contrast of interest was chosen based on meta-analytic studies revealing that the n-Back activates a consistent network of brain regions across 1- and 2-Back conditions (19), indicating that they engage similar processing strategies. However, higher load conditions (e.g., 2-Back) have superior psychometric properties, presumably due to the ceiling effect that often occurs during the 1-Back condition (32). Assessment of the interaction between peripheral metabolic parameters and working memory load was beyond the scope of the present study.
In the second level analysis, the parameter estimates were combined across task runs within each individual using a fixed effects design. Individual fMRI results from the second level analysis were combined across participants using FMRIB's Local Analysis of Mixed Effects (FLAME).
An a priori region of interest (ROI) analysis was performed to explore the impact of waist circumference on 2-Back-related BOLD response. To prevent circularity, the ROIs were created based on the published coordinates of 2-Back task-related activation in an independent sample (18). These ROIs were specifically selected due to their sensitivity for detecting alterations in response to metabolic and vascular factors (18). GingerAle 2.0 (www.brainmap.org) was used to convert the Talairach space coordinates published in Haley et al. (2007) into MNI stereotaxic space for the current analyses (33). Subsequently, 5 mm spheres were created around these coordinates and binarized into a mask. The mean 2-Back>0-Back BOLD response was extracted from each ROI and percent signal change was computed as described in http://mumford.bol.ucla.edu/perchange_guide.pdf. To calculate percent signal change, the 2-Back>0-Back parameter estimate was multiplied by a reference regressor height. This value was divided by the mean activation across the entire time series and then multiplied by 100.
Statistical Analyses
Descriptive statistics were used to calculate the study sample's means and standard deviations for the demographic, physiological, and cognitive domain variables. The relationship between cognitive domain performance and waist circumference was explored with linear regression, statistically adjusting for age and education.
The association between 2-Back-related activation in the a priori ROIs and waist circumference was assessed with linear regression models, statistically adjusting for age, sex, systolic blood pressure, and total cholesterol levels. The models were further adjusted for fasting glucose values in an exploratory follow-up step, despite the fact that none of the participants fulfilled criteria for diabetes. Covariates were selected based on their documented association with adiposity. Shapiro-Wilk tests revealed non-normal residuals for six of these models (p<0.05) so non-parametric bootstrapping was employed. Bootstrapping does not necessitate reliance on parametric assumptions for the underlying distribution and is thus appropriate for non-Gaussian distributed data. Moreover, it provides a method to correct for bias between the sample statistic and population parameter. In order to estimate the sampling distribution, 1,000 samples of n=73 were drawn with replacement. This distribution was then used to estimate regression statistics (standard errors and 95% bias-corrected confidence intervals) for each model. In order to preserve the type 1 error across multiple comparisons, a Bonferroni corrected alpha level of 0.0062 was used as the criterion for statistical significance.
As a follow-up analysis, partial correlations were used to explore the association between 2-Back task performance and BOLD response in the two ROIs with significant effects for waist circumference after controlling for age and education. Given the exploratory nature of these analyses, a two-tailed alpha level of 0.05 was used as the criterion for significance. All statistical analyses were conducted using SPSS 21.0 (SPSS Inc., Chicago, IL).
Results
Means and standard deviations of demographic and physiological characteristics are presented in Table 1. According to the World Health Organization's guidelines for waist circumference (22), 24 participants (32.9%) were at low risk (<94 cm for men and <80 cm for women), 11 (15.1%) were at increased risk (>94 to <102 cm for men and >80 to <88 cm for women), and 38 (52.1%) were at substantially increased risk (>102 cm for men and >88 cm for women) of future metabolic complications. Table 2 presents the means and standard deviations for the raw neuropsychological test scores and 2-Back task performance indices. Descriptive statistics revealed a highly educated (mean education = 16.6 years), cognitively-intact middle-aged sample.
Table 1. Selected demographic & physiological characteristics.
| Characteristic | Mean±SD |
|---|---|
| Male/Female | 26/47 |
| Education, years | 16.6±2.6 |
| Age, years | 49.02±6.1 |
| Systolic Blood Pressure, mmHg | 121.3±14.1 |
| Diastolic Blood Pressure, mmHg | 72.4±10.0 |
| Total Cholesterol, mg/dl | 203.5±39.7 |
| Blood Glucose, mg/dl | 94.1±10.6 |
| Body Mass Index, kg/m2 | 28.0±5.7 |
| Waist Circumference, cm | 94.0±14.5 |
Table 2. Raw neuropsychological test scores & 2-Back task performance.
| Test Scores | Mean±SD |
|---|---|
|
| |
| Global Cognition | |
| Mini Mental Status Exam (MMSE) | 28.8±1.3 |
| Weschler Abbreviated Scale of Intelligence II (WASI-II) | |
| Vocabulary Subtest | 45.3±5.5 |
| Matrix Reasoning Subtest | 21.9±3.6 |
| Full Scale IQ - 2 subtests | 116.3±13.6 |
|
| |
| Memory | |
| California Verbal Learning Test II (CVLT-II) | |
| Short Delay Free Recall | 11.2±3.2 |
| Long Delay Free Recall | 11.7±3.0 |
| Recognition Discriminability | 2.9±0.7 |
|
| |
| Executive Function | |
| Controlled Oral Word Association Test (COWAT) | 41.6±11.8 |
| Trail Making Test A, sec | 28.6±9.6 |
| Trail Making Test B, sec | 61.7±35.2 |
| Stroop Color-Word Subtest | 41.4±9.5 |
| Weschler Adult Intelligence Scale III (WAIS-III) Digit | 20.8±4.3 |
| Span Subtest, total | |
|
| |
| 2-Back Task Performance | |
| 0-Back reaction time, ms | 97.8±2.8 |
| 2-Back reaction time, ms | 79.6±10.4 |
| 0-Back accuracy, % correct | 671.9±143.2 |
| 2-Back accuracy, % correct | 1081.3±249.1 |
Table 3 displays the results of the linear regression analyses examining the relation between cognitive domain performance and waist circumference, after statistical adjustment for age and education. Only the overall model for the global cognitive domain attained statistical significance (p=0.014) with higher education predictive of better performance (p=0.002). Age and waist circumference did not account for any unique variance the model. The non-significant associations between cognitive performance and waist circumference are not surprising given the relatively young, high functioning (mean FSIQ = 116) sample.
Table 3. Linear Regression Models Examining the Association Between Cognitive Domain Scores & Waist Circumference.
| Cognitive Domain | Estimate ± SE | p-value | 95% CIs |
|---|---|---|---|
|
| |||
| Global Cognition | 0.014* | ||
| Age | -0.017±0.014 | 0.234 | -0.044 – 0.011 |
| Education | 0.102±0.032 | 0.002* | 0.039 – 0.165 |
| Waist Circumference | -0.002±0.006 | 0.766 | -0.013 – 0.010 |
|
| |||
| Memory | 0.087 | ||
| Age | -0.038±0.018 | 0.034 | -0.073 – -0.003 |
| Education | 0.058±0.039 | 0.145 | -0.020 – 0.136 |
| Waist Circumference | -0.003±0.007 | 0.711 | -0.017 – 0.012 |
|
| |||
| Executive Function | 0.075 | ||
| Age | -0.008±0.013 | 0.528 | -0.034 – 0.018 |
| Education | 0.074±0.030 | 0.015 | 0.015 – 0.133 |
| Waist Circumference | -0.004±0.005 | 0.437 | -0.015 – 0.007 |
p<0.05
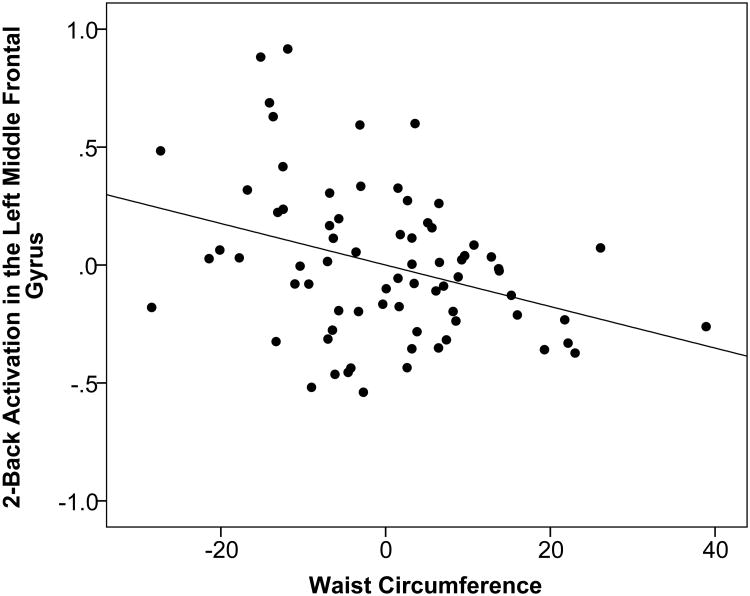
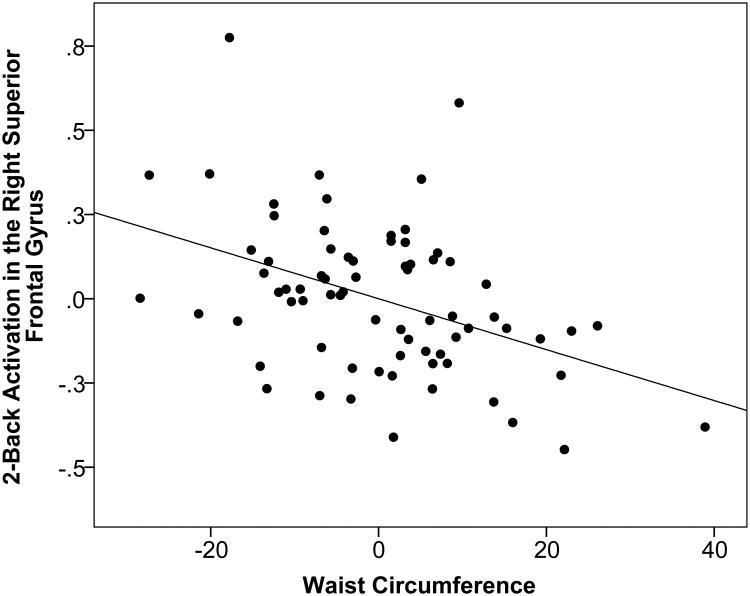
Multiple linear regression with bootstrapping was used to explore the associations between 2-Back-related activation in the a priori ROIs and waist circumference, controlling for age, sex, systolic blood pressure, and total cholesterol. As displayed in Table 4, the overall models for left middle frontal gyrus (p=0.003) and right superior frontal gyrus (p<0.001) were statistically significant even after adjustment for multiple comparisons (p<0.0062). For the left middle frontal gyrus, sex (p=0.002) and waist circumference (p=0.002) accounted for significant variance. As displayed in Figure 1, higher waist circumference predicted lower 2-Back activation in the left middle frontal gyrus. For the right superior frontal gyrus, sex (p=0.001), systolic blood pressure (p=0.006) and waist circumference (p=0.001) were significant. Higher waist circumference predicted lower 2-Back activation in the right superior frontal gyrus (Figure 2). Despite the fact that none of the participants fulfilled criteria for diabetes, the models were further adjusted for fasting glucose values in a follow-up analysis due to the documented effects of obesity on fasting glucose. The main effect of waist circumference on activation in the left middle frontal gyrus and right superior frontal gyrus was maintained, although slightly attenuated by the inclusion of fasting glucose levels in the model (left middle frontal gyrus: overall p-value = 0.006, age β=-0.009 p=0.184, sex β=0.282 p=0.002, systolic blood pressure β=0.006 p=0.065, total cholesterol β=-0.001 p=0.307, glucose β=-0.003 p=0.373, waist circumference β=-0.008 p=0.010; right superior frontal gyrus: overall p-value < 0.001, age β=-0.006 p=0.132, sex β=0.230 p=0.002, systolic blood pressure β=0.006 p=0.002, total cholesterol β=0.000 p=0.789, glucose β=-0.005 p=0.063, waist circumference β=-0.006 p=0.004).
Table 4. Bootstrapped Linear Regression Models Examining the Association Between a Priori ROI 2-Back>0-Back Activation & Waist Circumference.
| Brain Region & MNI Coordinates (X, Y, Z) | Estimate ± SE | p-value | 95% CIs |
|---|---|---|---|
|
| |||
| Left Middle Frontal Gyrus (-33, 8, 58) | 0.025 | ||
| Age | -0.025±0.009 | 0.008 | -0.042 – -0.009 |
| Sex | 0.137±0.112 | 0.219 | -0.074 – 0.379 |
| Systolic Blood Pressure | 0.011±0.005 | 0.018 | 0.002 – 0.021 |
| Total Cholesterol | 0.001±0.001 | 0.432 | -0.002 – 0.004 |
| Waist Circumference | -0.009±0.005 | 0.095 | -0.020 – 0.001 |
|
| |||
| Left Medial Frontal Gyrus (-4, 24, 43) | 0.032 | ||
| Age | -0.009±0.005 | 0.080 | -0.020 – 0.000 |
| Sex | 0.180±0.089 | 0.052 | 0.024 – 0.366 |
| Systolic Blood Pressure | 0.004±0.002 | 0.085 | 0.000 – 0.009 |
| Total Cholesterol | 0.000±0.001 | 0.966 | -0.002 – 0.002 |
| Waist Circumference | -0.006±0.003 | 0.045 | -0.012 – -0.001 |
|
| |||
| Right Superior Parietal Lobule (40, -62, 59) | 0.061 | ||
| Age | -0.011±0.011 | 0.275 | -0.032 – 0.011 |
| Sex | 0.382±0.152 | 0.016 | 0.101 – 0.683 |
| Systolic Blood Pressure | 0.010±0.005 | 0.047 | 0.001 – 0.021 |
| Total Cholesterol | 0.002±0.002 | 0.379 | -0.002 – 0.005 |
| Waist Circumference | -0.009±0.005 | 0.100 | -0.019 – 0.001 |
|
| |||
| Left Inferior Parietal Lobule (-50, -51, 49) | 0.450 | ||
| Age | -0.007±0.007 | 0.316 | -0.020 – 0.006 |
| Sex | 0.094±0.086 | 0.280 | -0.082 – 0.261 |
| Systolic Blood Pressure | 0.004±0.004 | 0.341 | -0.003 – 0.012 |
| Total Cholesterol | -0.001±0.001 | 0.317 | -0.003 – 0.001 |
| Waist Circumference | -0.005±0.004 | 0.140 | -0.013 – 0.002 |
|
| |||
| Left Middle Frontal Gyrus (-45, 48, 8) | 0.003* | ||
| Age | -0.009±0.007 | 0.191 | -0.021 – 0.005 |
| Sex | 0.275±0.085 | 0.002* | 0.099 – 0.437 |
| Systolic Blood Pressure | 0.005±0.003 | 0.096 | -0.001 – 0.012 |
| Total Cholesterol | -0.001±0.001 | 0.280 | -0.003 – 0.001 |
| Waist Circumference | -0.009±0.003 | 0.002* | -0.015 – -0.003 |
|
| |||
| Right Superior Frontal Gyrus (36, 52, 9) | <0.001* | ||
| Age | -0.005±0.004 | 0.243 | -0.013 – 0.003 |
| Sex | 0.219±0.052 | 0.001* | 0.121 – 0.325 |
| Systolic Blood Pressure | 0.006±0.002 | 0.006* | 0.002 – 0.009 |
| Total Cholesterol | 0.000±0.001 | 0.810 | -0.001 – 0.001 |
| Waist Circumference | -0.008±0.002 | 0.001* | -0.012 – -0.004 |
|
| |||
| Right Middle Frontal Gyrus (35, 10, 56) | 0.300 | ||
| Age | -0.017±0.010 | 0.099 | -0.038 – 0.005 |
| Sex | 0.208±0.142 | 0.132 | -0.080 – 0.494 |
| Systolic Blood Pressure | 0.009±0.006 | 0.151 | -0.003 – 0.020 |
| Total Cholesterol | 0.001±0.001 | 0.693 | -0.002 – 0.003 |
| Waist Circumference | -0.004±0.006 | 0.449 | -0.016 – 0.008 |
|
| |||
| Right Inferior Frontal Gyrus (51, 16, -2) | 0.654 | ||
| Age | 0.005±0.005 | 0.267 | -0.005 – 0.016 |
| Sex | 0.031±0.070 | 0.663 | -0.108 – 0.167 |
| Systolic Blood Pressure | 0.001±0.002 | 0.570 | -0.003 – 0.006 |
| Total Cholesterol | -0.001±0.001 | 0.282 | -0.003 – 0.001 |
| Waist Circumference | -0.002±0.002 | 0.274 | -0.007 – 0.002 |
p<0.0062 based on bootstrapping results
Figure 1.
Residual regression plot displaying the association between waist circumference (range: 64 – 135 centimeters) and percent signal change for 2-Back activation in the left middle frontal gyrus after adjustment for age, sex, systolic blood pressure, and total cholesterol. X- and Y-axis units are arbitrary. The strength of the association was assessed using non-parametric bootstrapping.
Figure 2.
Residual regression plot displaying the association between waist circumference (range: 64 – 135 centimeters) and percent signal change for 2-Back activation in the right superior frontal gyrus after adjustment for age, sex, systolic blood pressure, and total cholesterol. X- and Y-axis units are arbitrary. The strength of the association was assessed using non-parametric bootstrapping.
Additionally, partial correlations were used to explore the association between 2-Back task performance and BOLD response in the two ROIs with significant effects for waist circumference after controlling for age and education. Higher activation in the left middle frontal gyrus was associated with faster 2-Back reaction time (r=-0.266, p=0.025), as well as trends towards faster 0-Back reaction time (r=-0.217, p=0.070) and higher 0-Back accuracy (r=0.222, p=0.062). There was no association with 2-Back accuracy (r=0.161, p=0.181). Within the right superior frontal gyrus, higher activation was associated with faster 0-Back reaction time (r=-0.369, p=0.002). There were no significant associations with 2-Back reaction time (r=-0.115, p=0.338), 0-Back accuracy (r=0.174, p=0.147), and 2-Back accuracy (r=0.084, p=0.486).
Discussion
The primary finding from the current study was that higher waist circumference was associated with decreased working memory-related BOLD response in the left middle frontal gyrus and right superior frontal gyrus, a pattern of activation that corresponded with poorer task performance. Moreover, the main effect of waist circumference was independent of systolic blood pressure and total cholesterol. In the periphery, visceral adiposity is robust and independent indicator of future metabolic and cardiovascular dysfunction (11). Our results suggest that central adiposity may similarly exert a detrimental influence on central nervous system functioning.
In the current study, larger waist circumference was associated with alterations in the BOLD response during cognition, but not performance on neuropsychological tests. A well-established link exists between midlife obesity and late life cognitive decline (6,7). However, the impact of obesity on cognition within middle-aged populations has been less widely examined. Prior research on middle-aged adults with a similar sample size has also failed to find obesity-related cognitive changes on cross-sectional examination (5). Moreover, cognitive effects may be particularly difficult to detect in our sample given the high level educational attainment (mean = 16.6 years) and above average IQ (mean FSIQ = 116.3). These cognitive reserve factors have been found to diminish observed neuropsychological deficits in individuals with elevated body mass index (34). In contrast to the null cognitive effects, waist circumference was associated with alterations brain activation that predicted poorer task performance. Thus, fMRI may provide greater sensitivity for detecting early adiposity-related changes in neural efficiency as documented in healthy individuals at genetic risk for Alzheimer's disease (14).
fMRI was performed during completion of a working memory task in the current study. Working memory, a process that involves the short-term storage and manipulation of information, governs planning and reasoning processes that are fundamental to most daily activities (35). In our study, higher waist circumference predicted diminished BOLD response in the right superior frontal gyrus and left middle frontal gyrus, integral areas for working memory performance (19,20). The middle frontal gyrus governs executively-mediated components of working memory such as monitoring and manipulation of incoming information, whereas the superior frontal gyrus regulates attentional allocation (19). Numerous studies have reported that higher BOLD response in these regions is necessary to negate age-related declines in neural efficiency and maintain task performance (36,37). Not surprisingly, waist circumference-related decrements in the BOLD response in these regions predicted slower reaction time. Diminished activation in the frontal lobes observed in association with larger waist circumference may be indicative of inability to flexibly recruit neural resources during challenging cognitive tasks, resulting in slower information processing (37).
Visceral adiposity, which accumulates along the waistline, has hormonal and molecular properties that may govern its pathogenic effects on central nervous system functioning. In comparison to total mass, visceral adiposity is a stronger predictor of elevated levels of pro-inflammatory cytokines such as TNF-alpha, interleukin 6 (IL-6), and C-reactive protein (CRP) (38). These cytokines are capable of crossing the blood brain barrier, where they can propagate a local inflammatory response (39). In the brain, inflammation stimulates microglial reactivity, which may directly damage neuronal tissue by inducing oxidative damage (40). Multiple reports indicate that elevated levels of pro-inflammatory cytokines are linked to poorer cognitive performance (41), cerebral atrophy (42), and white matter damage (43). Moreover, systemic and central nervous system inflammation is a hallmark of Alzheimer's disease and may contribute to its progression (44,45). Thus, the alterations in the BOLD response observed in response to larger waist circumference may be driven by central nervous system damage secondary to inflammation.
The significance of central adiposity on the BOLD response may also be governed by the direct impact of adipokines on the brain. Adipokines are metabolically active peptides that are secreted by adipose tissue. One of the most widely studied adipokines is leptin, which serves to regulate energy homeostasis and is secreted in higher levels with greater adipose tissue mass (46). Leptin uses saturable transport systems to access the central nervous system (47), where it modulates pre- and post-synaptic neurotransmitter release to facilitate learning and memory (48). Elevations in triglycerides, which are directly linked to visceral adiposity (49), impair the transport of leptin to the brain (50). Reductions in leptin are associated with poorer cognitive performance (51) and diminished brain volume, particularly in the frontal lobes (52). Similarly, in the current study, larger waist circumference was predictive of BOLD signal alterations in the highly vulnerable frontal lobe regions, indicating that leptin dysregulation may be a contributing mechanism.
Finally, central adiposity may affect central nervous system functioning by modulating neurotransmitter synthesis, particularly in the dopamingeric system. Obesity is associated diminished dopamine receptor availability in both human and animal studies (53,54). Moreover, adipokines such as leptin and resistin directly suppress dopamine release in the central nervous system (55,56). Disruptions to the dopamine system induce prefrontal cortex hypometabolism (57) and impair executive function performance (58), presumably due to reduced dopamingeric regulation of pyramidal and GABAergic inhibitory neurons in the frontal lobes (59). Diminished left prefrontal cortex activation during working memory has been shown to be mediated by dopamine receptor availability (60). In the current study, larger waist circumference was associated with lower BOLD response in this region, suggesting that adiposity-related reductions in dopaminergic function may be a governing factor.
Adiposity-induced changes in inflammation, leptin levels, and dopamingeric activity may act synergistically with one another and other unmeasured physiological factors to perturb central nervous system functioning. The proposed mechanisms are speculative at this point and will necessitate validation from future studies that directly assess these components. Additional limitations of the current study must also be considered when interpreting the findings. The study used a cross-sectional design, which precludes the ability to assess causation. While we predict that a centralized distribution of adiposity impacts the central nervous system, it is possible that individuals with higher waist circumference have inherit alterations in the brain that contributes both to cognitive performance and the development of obesity. Additionally, waist circumference is a proxy measure for visceral adiposity. While prior research has indicated that waist circumference is the best anthropometric index of visceral adiposity (21), future studies will benefit from more direct assessments of adipose mass such as magnetic resonance imaging and computed tomography. Another methodological consideration is the inclusion of non-cognitive and hypercapnia fMRI tasks to determine if the observed BOLD changes are specific to cognition or more globally indicate cerebrovascular disturbance. Finally, the sample was relatively healthy and homogenous in terms of educational achievement and global cognitive functioning. Future studies should include larger, more diverse samples to more fully assess how central adiposity affects brain activation across the general population.
In summary, larger waist circumference was associated with diminished working-memory-related BOLD signal in the left middle frontal gyrus and right superior frontal gyrus, a pattern of activation that corresponded with poorer task performance. Moreover, the main effect of waist circumference was observed over and above control for systolic blood pressure and total cholesterol. While future studies are necessary, the results suggest that similar to its role in the periphery (10,11), central adiposity may be a robust predictor of metabolic and hormonal alterations that impinge upon central nervous system functioning.
Acknowledgments
This work was funded in part by grants from the National Institute of Neurological Disorders and Stroke (R01NS75565, A.P.H.) and the National Institute on Aging (F31AG040890, M.M.G.).
Footnotes
Conflict of Interest: The authors declare no conflict of interest.
References
- 1.World Health Organization. Obesity: Preventing and managing the global epidemic Report of a WHO consultation. World Health Organization; Geneva, Switzerland: 2000. [PubMed] [Google Scholar]
- 2.Kopelman PG. Obesity as a medical problem. Nature. 2000;404:635–643. doi: 10.1038/35007508. [DOI] [PubMed] [Google Scholar]
- 3.Gustafson D, Lissner L, Bengtsson C, Bjorkelund C, Skoog I. A 24-year follow-up of body mass index and cerebral atrophy. Neurology. 2004;63:1876–1881. doi: 10.1212/01.wnl.0000141850.47773.5f. [DOI] [PubMed] [Google Scholar]
- 4.Walther K, Birdsill AC, Glisky EL, Ryan L. Structural brain differences and cognitive functioning related to body mass index in older females. Hum Brain Mapp. 2009;31:1052–1064. doi: 10.1002/hbm.20916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ward M, Carlsson C, Trivedi M, Sager M, Johnson S. The effect of body mass index on global brain volume in middle-aged adults: a cross sectional study. BMC Neurol. 2005;5:23. doi: 10.1186/1471-2377-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whitmer RA, Gustafson DR, Barrett-Connor E, Haan MN, Gunderson EP, Yaffe K. Central obesity and increased risk of dementia more than three decades later. Neurology. 2008;71:1057–1064. doi: 10.1212/01.wnl.0000306313.89165.ef. [DOI] [PubMed] [Google Scholar]
- 7.Gustafson D, Rothenberg E, Blennow K, Steen B, Skoog I. An 18-year follow-up of overweight and risk of Alzheimer disease. Arch Inter Med. 2003;163:1524–1528. doi: 10.1001/archinte.163.13.1524. [DOI] [PubMed] [Google Scholar]
- 8.Gustafson DR. Adiposity hormones and dementia. J Neurol Sci. 2010;299:30–34. doi: 10.1016/j.jns.2010.08.036. [DOI] [PubMed] [Google Scholar]
- 9.Cereda E, Sansone V, Meola G, Malavazos AE. Increased visceral adipose tissue rather than BMI as a risk factor for dementia. Age Ageing. 2007;36:488–491. doi: 10.1093/ageing/afm096. [DOI] [PubMed] [Google Scholar]
- 10.Ibrahim MM. Subcutaneous and visceral adipose tissue: structural and functional differences. Obs Rev. 2010;11:11–18. doi: 10.1111/j.1467-789X.2009.00623.x. [DOI] [PubMed] [Google Scholar]
- 11.Wisse BE. The inflammatory syndrome: the role of adipose tissue cytokines in metabolic disorders linked to obesity. J Am Soc Nephrol. 2004;15:2792–2800. doi: 10.1097/01.ASN.0000141966.69934.21. [DOI] [PubMed] [Google Scholar]
- 12.Wolf PA, Beiser A, Elias MF, Au R, Vasan RS, Seshadri S. Relation of obesity to cognitive function: importance of central obesity and synergistic influence of concomitant hypertension. The Framingham Heart Study. Curr Alzheimer Res. 2007;4:111–116. doi: 10.2174/156720507780362263. [DOI] [PubMed] [Google Scholar]
- 13.Gustafson D. A life course of adiposity and dementia. Eur J Pharmacol. 2008;585:163–175. doi: 10.1016/j.ejphar.2008.01.052. [DOI] [PubMed] [Google Scholar]
- 14.Bondi MW, Houston WS, Eyler LT, Brown GG. fMRI evidence of compensatory mechanisms in older adults at genetic risk for Alzheimer disease. Neurology. 2005;64:501–508. doi: 10.1212/01.WNL.0000150885.00929.7E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Braskie MN, Small GW, Bookheimer SY. Vascular health risks and fMRI activation during a memory task in older adults. Neurobiol Aging. 2010;31:1532–1542. doi: 10.1016/j.neurobiolaging.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonzales M, Tarumi T, Miles S, Tanaka H, Shah F, Haley A. Insulin Sensitivity as a mediator of the relationship between body mass index and working memory-related brain activation. Obesity. 2010;18:2131–2137. doi: 10.1038/oby.2010.183. [DOI] [PubMed] [Google Scholar]
- 17.Volkow ND, Wang GJ, Telang F, Fowler JS, Goldstein RZ, Alia-Klein N, et al. Inverse association between BMI and prefrontal metabolic activity in healthy adults. Obesity. 2008;17:60–65. doi: 10.1038/oby.2008.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haley AP, Sweet LH, Gunstad J, Forman DE, Poppas A, Paul RH, et al. Verbal working memory and atherosclerosis in patients with cardiovascular disease: an fMRI study. J Neuroimaging. 2007;17:227–233. doi: 10.1111/j.1552-6569.2007.00110.x. [DOI] [PubMed] [Google Scholar]
- 19.Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp. 2005;25:46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Braver TS, Cohen JD, Nystrom LE, Jonides J, Smith EE, Noll DC. A parametric study of prefrontal cortex involvement in human working memory. Neuroimage. 1997;5:49–62. doi: 10.1006/nimg.1996.0247. [DOI] [PubMed] [Google Scholar]
- 21.Pouliot MC, Després JP, Lemieux S, Moorjani S, Bouchard C, Tremblay A, et al. Waist circumference and abdominal sagittal diameter: best simple anthropometric indexes of abdominal visceral adipose tissue accumulation and related cardiovascular risk in men and women. Am J Cardiol. 1994;73:460–468. doi: 10.1016/0002-9149(94)90676-9. [DOI] [PubMed] [Google Scholar]
- 22.World Health Organization. Waist Circumference and Waist-Hip Ratio: Report of a WHO Expert Consulation. World Health Organization; Geneva, Switzerland: 2008. [Google Scholar]
- 23.Folstein MF, Folstein SE, McHugh PR. Mini-Mental State: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 24.Wechsler D. WASI II: Wechsler Abbreviated Scale of Intelligence. 2nd. Psychological Corporation; San Antonio, TX, USA: 2011. [Google Scholar]
- 25.Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test. 2nd. The Psychological Corporation; San Antonio, TX, USA: 2000. [Google Scholar]
- 26.Reitan RM. Validity of the Trail Making Test as an indicator of organic brain damage. Percept Mot Skills. 1958;8:271–276. [Google Scholar]
- 27.Ruff RM, Light RH, Parker SB, Levin HS. Benton Controlled Oral Word Association Test: reliability and updated norms. Arch Clin Neuropsychol. 1996;11:329–338. [PubMed] [Google Scholar]
- 28.Wechsler D. Wechsler Adult Intelligence Scale. 4th. Psychological Corporation; San Antonio, TX, USA: 2008. [Google Scholar]
- 29.Golden CJ. Stroop Color and Word Test: A Manual for Clinical and Experimental Uses. Skoelting; Chicago, IL, USA: 1978. [Google Scholar]
- 30.Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- 31.Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jaeggi SM, Buschkuehl M, Perrig WJ, Meier B. The concurrent validity of the N-back task as a working memory measure. Memory. 2010;18:394–412. doi: 10.1080/09658211003702171. [DOI] [PubMed] [Google Scholar]
- 33.Laird AR, Robinson JL, McMillan KM, Tordesillas-Gutiérrez D, Moran ST, Gonzales SM, et al. Comparison of the disparity between Talairach and MNI coordinates in functional neuroimaging data: Validation of the Lancaster transform. Neuroimage. 2010;51:677–683. doi: 10.1016/j.neuroimage.2010.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Galioto RM, Alosco ML, Spitznagel MB, Stanek KM, Gunstad J. Cognitive reserve preserves cognitive function in obese individuals. Aging Neuropsychol C. 2013;20:684–699. doi: 10.1080/13825585.2012.762972. [DOI] [PubMed] [Google Scholar]
- 35.Baddeley A. Working memory. Science. 1992;255:556–559. doi: 10.1126/science.1736359. [DOI] [PubMed] [Google Scholar]
- 36.Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging gracefully: compensatory brain activity in high-performing older adults. Neuroimage. 2002;17:1394–1402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- 37.Rypma B, D'Esposito M. Isolating the neural mechanisms of age-related changes in human working memory. Nat Neurosci. 2000;3:509–515. doi: 10.1038/74889. [DOI] [PubMed] [Google Scholar]
- 38.Malavazos AE, Corsi MM, Ermetici F, Coman C, Sardanelli F, Rossi A, et al. Proinflammatory cytokines and cardiac abnormalities in uncomplicated obesity: relationship with abdominal fat deposition. Nutr Metab Cardiovasc Dis. 2007;17:294–302. doi: 10.1016/j.numecd.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 39.Banks WA, Kastin AJ, Broadwell RD. Passage of cytokines across the blood-brain barrier. Neuroimmunomodulation. 1995;2:241–248. doi: 10.1159/000097202. [DOI] [PubMed] [Google Scholar]
- 40.Koutsilieri E, Scheller C, Tribl F, Riederer P. Degeneration of neuronal cells due to oxidative stress—microglial contribution. Parkinsonism Relat Disord. 2002;8:401–406. doi: 10.1016/s1353-8020(02)00021-4. [DOI] [PubMed] [Google Scholar]
- 41.Weaver JD, Huang MH, Albert M, Harris T, Rowe JW, Seeman TE. Interleukin-6 and risk of cognitive decline MacArthur Studies of Successful Aging. Neurology. 2002;59:371–378. doi: 10.1212/wnl.59.3.371. [DOI] [PubMed] [Google Scholar]
- 42.Jefferson AL, Massaro JM, Wolf PA, Seshadri S, Au R, Vasan RS, et al. Inflammatory biomarkers are associated with total brain volume The Framingham Heart Study. Neurology. 2007;68:1032–1038. doi: 10.1212/01.wnl.0000257815.20548.df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Satizabal CL, Zhu YC, Mazoyer B, Dufouil C, Tzourio C. Circulating IL-6 and CRP are associated with MRI findings in the elderly The 3C-Dijon Study. Neurology. 2012;78:720–727. doi: 10.1212/WNL.0b013e318248e50f. [DOI] [PubMed] [Google Scholar]
- 44.Tan ZS, Beiser AS, Vasan RS, Roubenoff R, Dinarello CA, Harris TB, et al. Inflammatory markers and the risk of Alzheimer disease The Framingham Study. Neurology. 2007;68:1902–1908. doi: 10.1212/01.wnl.0000263217.36439.da. [DOI] [PubMed] [Google Scholar]
- 45.Hull M, Strauss S, Berger M, Volk B, Bauer J. The participation of interleukin-6, a stress-inducible cytokine, in the pathogenesis of Alzheimer's disease. Behav Brain Res. 1996;78:37–41. doi: 10.1016/0166-4328(95)00213-8. [DOI] [PubMed] [Google Scholar]
- 46.Maffei M, Halaas J, Ravussin E, Pratley RE, Lee GH, Zhang Y, et al. Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat Med. 1995;1:1155–1161. doi: 10.1038/nm1195-1155. [DOI] [PubMed] [Google Scholar]
- 47.Banks WA, Kastin AJ, Huang W, Jaspan JB, Maness LM. Leptin enters the brain by a saturable system independent of insulin. Peptides. 1996;17:305–311. doi: 10.1016/0196-9781(96)00025-3. [DOI] [PubMed] [Google Scholar]
- 48.Oomura Y, Aou S, Fukunaga K. Prandial increase of leptin in the brain activates spatial learning and memory. Pathophysiology. 2010;17:119–127. doi: 10.1016/j.pathophys.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 49.Banerji MA, Buckley MC, Chaiken RL, Gordon D, Lebovitz HE, Kral JG. Liver fat, serum triglycerides and visceral adipose tissue in insulin-sensitive and insulin-resistant black men with NIDDM. Int J Obs Relat Metab Disord. 1995;19:846–850. [PubMed] [Google Scholar]
- 50.Banks WA, Coon AB, Robinson SM, Moinuddin A, Shultz JM, Nakaoke R, et al. Triglycerides induce leptin resistance at the blood-brain barrier. Diabetes. 2004;53:1253–1260. doi: 10.2337/diabetes.53.5.1253. [DOI] [PubMed] [Google Scholar]
- 51.Holden KF, Lindquist K, Tylavsky FA, Rosano C, Harris TB, Yaffe K. Serum leptin level and cognition in the elderly: Findings from the Health ABC Study. Neurobiol Aging. 2009;30:1483–1489. doi: 10.1016/j.neurobiolaging.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pannacciulli N, Le DSN, Chen K, Reiman EM, Krakoff J. Relationships between plasma leptin concentrations and human brain structure: a voxel-based morphometric study. Neurosci Lett. 2007;412:248–253. doi: 10.1016/j.neulet.2006.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang XF, Zavitsanou K, Huang X, Yu Y, Wang H, Chen F, et al. Dopamine transporter and D2 receptor binding densities in mice prone or resistant to chronic high fat diet-induced obesity. Behav brain Res. 2006;175:415–419. doi: 10.1016/j.bbr.2006.08.034. [DOI] [PubMed] [Google Scholar]
- 54.Wang GJ, Volkow ND, Logan J, Pappas NR, Wong CT, Zhu W, et al. Brain dopamine and obesity. Lancet. 2001;357:354–357. doi: 10.1016/s0140-6736(00)03643-6. [DOI] [PubMed] [Google Scholar]
- 55.Brunetti L, Orlando G, Recinella L, Michelotto B, Ferrante C, Vacca M. Resistin, but not adiponectin, inhibits dopamine and norepinephrine release in the hypothalamus. Eur J Pharmacol. 2004;493:41–44. doi: 10.1016/j.ejphar.2004.04.020. [DOI] [PubMed] [Google Scholar]
- 56.Brunetti L, Michelotto B, Orlando G, Vacca M. Leptin inhibits norepinephrine and dopamine release from rat hypothalamic neuronal endings. Eur J Pharmacol. 1999;372:237–240. doi: 10.1016/s0014-2999(99)00255-1. [DOI] [PubMed] [Google Scholar]
- 57.Vernaleken I, Buchholz HG, Kumakura Y, Siessmeier T, Stoeter P, Bartenstein P, et al. “Prefrontal” cognitive performance of healthy subjects positively correlates with cerebral FDOPA influx: An exploratory [18F]-fluoro-L-DOPA-PET investigation. Hum Brain Mapp. 2007;28:931–939. doi: 10.1002/hbm.20325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mozley LH, Gur RC, Mozley PD, Gur RE. Striatal dopamine transporters and cognitive functioning in healthy men and women. Am J Psychiatry. 2001;158:1492–1499. doi: 10.1176/appi.ajp.158.9.1492. [DOI] [PubMed] [Google Scholar]
- 59.Seamans JK, Yang CR. The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog Neurobiol. 2004;74:1–58. doi: 10.1016/j.pneurobio.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 60.Bäckman L, Karlsson S, Fischer H, Karlsson P, Brehmer Y, Rieckmann A, et al. Dopamine D(1) receptors and age differences in brain activation during working memory. Neurobiol Aging. 2011;32:1849–1856. doi: 10.1016/j.neurobiolaging.2009.10.018. [DOI] [PubMed] [Google Scholar]