Abstract
Introduction: The genesis of cancer appears to be a complex matter, which is not simply based upon few genetic abnormalities/alteration. In fact, irregular microvasculature and aberrant interstitium of solid tumors impose significant pathophysiologic barrier functions against cancer treatment modalities, hence novel strategies should holistically target bioelements of tumor microenvironment (TME). In this study, we provide some overview and insights on TME and important strategies used to control the impacts of such pathophysiologic barriers.
Methods: We reviewed all relevant literature for the impacts of tumor interstitium and microvasculature within the TME as well as the significance of the implemented strategies.
Results: While tumorigenesis initiation seems to be in close relation with an emergence of hypoxia and alterations in epigenetic/genetic materials, large panoplies of molecular events emerge as intricate networks during oncogenesis to form unique lenient TME in favor of tumor progression. Within such irregular interstitium, immune system displays defective surveillance functionalities against malignant cells. Solid tumors show multifacial traits with coadaptation and self-regulation potentials, which bestow profound resistance against the currently used conventional chemotherapy and immunotherapy agents that target solely one face of the disease.
Conclusion: The cancerous cells attain unique abilities to form its permissive microenvironment, wherein (a) extracellular pH is dysregulated towards acidification, (b) extracellular matrix (ECM) is deformed, (c) stromal cells are cooperative with cancer cells, (d) immune system mechanisms are defective, (e) non-integrated irregular microvasculature with pores (120-1200 nm) are formed, and (h) interstitial fluid pressure is high. All these phenomena are against cancer treatment modalities. As a result, to control such abnormal pathophysiologic traits, novel cancer therapy strategies need to be devised using multifunctional nanomedicines and theranostics.
Keywords: Targeted therapy of cancer, Tumor microenvironment, Tumor interstitium, Tumor microcirculation, Nanomedicines, Theranostics
Introduction
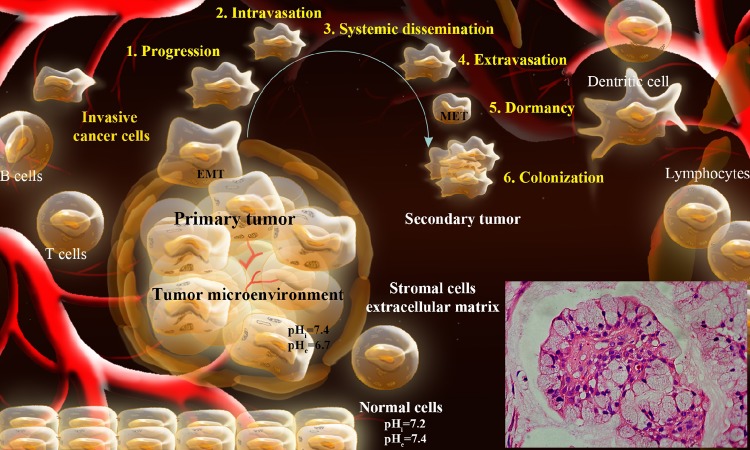
Over the past century, revolutionary investigations have highlighted malignancies as complex systems with auto-regulation, coadaptation and networking potentials. The emergence of cancer is not fully understood, hence we struggle to diagnose the disease as early as possible and treat it as effective as possible. We have learned that arrays of genes, transcription factors and signaling pathways are networked intriguingly in initiation, promotion and progression of cancer, by which cancerous cells appear to intelligently communicate with the surrounding biocomponents through intricate molecular means. In their long path, cancer cells are able to escape from the surveillance mechanisms of immune system and settle down deep within the normal tissues and create its unique permissive setting, the so-called “tumor microenvironment”. Fig. 1 schematically represents the formation of TME and colonization of metastatic cancer cells.
Fig. 1 .
Schematic representation of TME and colonization of metastatic cancer cells. Inset: Mucoepidermoid carcinoma. Figure is adapted with permission from our paper published in BioImpacts.1 TME: Tumor microenvironment.
To be able to undergo the unchecked cell division, the cancerous cells need to get necessary nutrients within such a hospitable bed in a co-operative manner with stromal cells. While the initiation and progression of cancer occurs as panoplies of molecular markers involved in both proliferation and differentiation phenomena. All these activities provide the cancerous cells abilities to modulate the extracellular pH, ECM, stromal cells, immune system, and angiogenesis. The distribution pattern of non-binding macromolecules within the TME appears to be irregular and heterogeneous. Part of settlement of macromolecules in the TME appears to occur based on irregular extravasation and some others are originated from cancer cells and/or cancer associated cells. Regardless of the origin of the macromolecular bioelements within the TME, they can inherently increase oncotic pressure of the tumor interstitial fluid (TIF). As a proximal fluid, TIF encompasses not only the array of extravasated blood-borne/-circulating proteins but also contains rebelliously externalized biocomponents of tumor cells and their aberrant metabolic byproducts as well as biomolecules, in part segregated form deformed ECM and stroma. In addition to tumor vasculature, many abnormally overexpressed carrier-mediated transporters are involved in formation of tumor interstitium. All these anomalous phenomena make TME to be exposed to cocktail of various enzymes with acidic pH, upon which ECM can be remodeled/deformed, resulting in emergence of a viscose TME with high oncotic pressure – a pathophysiologic obstacle against cancer therapy.2 In fact, TIF encompasses plethora of oncomarkers anomalously secreted during tumor development, progression and invasion.3 In a uniformly perfused tumor, based upon findings by numerical simulations, the elevated TIF pressure (TIFP) is the main driving force for heterogeneous dissemination of macromolecules, in part due to low pressure and non-uniform filtration of tumor microvasculature necessary for extravasation of fluid and macromolecules from microvasculature. As a result, such aberrant high pressure in TIF can hamper the interstitial traverse of therapeutic macromolecules such as monoclonal antibodies (mAbs) and multifunctional nanomedicines. To overcome these physiological barriers within TME, cancer treatment modalities need to be devised to lower the TIFP and to improve the blood flow. In the current study, we will discuss tumor development, TME, parameters involved in enhanced TIFP, strategies against pathophysiologic barriers of tumor and impacts of multifunctional nanosystems to circumvent the high oncotic pressure of TIF.
Complexity of tumor
At the early stage of tumorigenesis, a complex network of signaling pathways are initiated, mainly through danger signals and epigenetic remodeling (i.e., DNA methylation/demethylation and histone acetylation and deacetylation), and hence inadvertent enhanced hydrophobicity leading to inevitable over- and/or under-expression of array of genes. The hydrophobic portions of molecules (Hyppos) of genomic materials are intrinsically concealed with hydrophilic molecules. While there exists a hydrophobic and hydrophilic balance within all cellular bioelements in normal condition,4 any impulsively exposed Hyppos aggregates may induce undesired molecular injuries. It seems that molecular signaling paths are in close association with damage-associated molecular patterns (DAMPs), displaying unique pattern of HYPPOs derived from dead/dying and damaged cells. Such concept appears to be the same for nucleic acids, in which for example the unmethylated CPG sequences can be a sign for molecular/cellular damage(s).5 Although the mechanisms by which the malignant cells regulate gene expression differ in various solid tumors, remodeling of genomic materials seems to be the central dogma that can modulate array of transcription factors, resulting in up- and/or down-regulation of specific genes and hence alteration of biological functions in favor of the progression of tumor. Within such process, inimitable patterns of methylation of cytosine molecules as well as acetylation of histones appear to regulate the gene expression profiles.6
In fact, we believe cancers show all characteristics of the “complex adaptive systems” such as (a) unique emergence, (b) self-organization potential, (c) collective behavior, (d) networking capacity, (e) evolution and adaptation capacities, and (f) pattern formation ability. Such complexity has been well-delineated by Hanahan and Weinberg as the hallmarks of cancer by (a) self-sufficiency in growth signals, (b) insensitivity to anti-growth signals, (c) evading immunosurveillance and apoptosis, (d) sustained angiogenesis, (e) tissue invasiveness and metastasis, and (f) limitless replicative potential.7
Anomalous energetic metabolism among cancer cells and stromal cells
Inherently, the oxygen-mediated oxidative phosphorylation (OMOP) produce ATP in the mitochondria. In such process NADH and FADH2 are oxidized and ADP is phosphorylated, resulting in formation of ATP that can also be generated in the absence of oxygen through another pathway called “glycolysis” within the cytosol. In normal cells, ATP is largely produced through OMOP in mitochondria, while in the cancer cells the cytosolic glycolysis appears to be the dominant path for the production of ATP even in the presence of sufficient levels of oxygen. Such increased rate of glycolysis in the presence of oxygen (a process so-called “aerobic glycolysis”) is known as “Warburg effect”.8 In solid tumors, the cancerous epithelial cells show huge intercommunication with the neighboring stromal cells, in which the lactate shuttle bridging among cancer cells and stromal cells and is termed as “reverse Warburg effect”. Since the cancer cells are able to secrete high levels of hydrogen peroxide (H2O2),9 they can induce a local oxidative stress within TME, wherein the stromal cells are markedly affected. As a result, the autophagy within these cells are modulated and tumor-stroma interactions are shaped during carcinogenesis.10 It should be pointed out that the autophagy is known to suppress the tumorigenesis at the early stages; however, in established tumors, it functions in favor of cancer cells’ survival, bestowing chemoresistance potential to at least colonies of cells.11 The autophagy is believed to play compartment-specific roles in tumor metabolism, hence it along with mitochondrial dysfunction may promote cellular catabolism in stromal cells, resulting in production of recycled nutrients that can be reused as high-energy fuels by cancer cells. Such sources of fuels seem to dramatically promote the growth of tumor in association with oxidative mitochondrial metabolism.12 In fact, the co-culture of cancer cells with the fibroblasts was shown to enhance the mitochondrial mass in cancer cells but not in fibroblasts. This may imply existence of a parasitic life for cancer cells through exploitation of oxidative stress for extraction of nutrients from surrounding stromal cells (e.g., fibroblasts). In addition, another reason for such symbiotic relationship is the induction of the autophagic destruction of mitochondria in stromal cells which can lead these cells towards “aerobic glycolysis” that can produce energy-rich nutrients such as lactate and ketones in favor of cancer cells to be metastasized with no need to blood vessels as a food source.12-14 It should be emphasized that the autophagy is known as the major lysosomal pathway for recycling of intracellular biological components and even whole organelles, therefore it is considered as a pivotal key process that modulate the tumorigenesis. Likewise, we envision that the cancer cells are able to exploit the glycolysis path to acidify the extracellular fluid that can in turn intensify the release of lysosomal components (particularly various enzymes) into the TME. Given that the lysosomes are mixture of different enzymes, their release into the extracellular fluid will further intensity the oxidative stress within TME, resulting in enhanced deformation of ECM in favor of tumor progression and invasiveness.15 Of the aberrant metabolism within TME, production of acidic byproducts such as lactic acid can acidify the interacellular pH (pHi) and hence cancer cells must pump them out of the cytoplasm through pH related transport machineries, which results in alkalinized pHi but acidified extracellular pH (pHe).16 Several enzymes and transport machineries (e.g., MCT-1, NHE-1, CA IX and H+ pump V-ATPase) have been shown to be involved in maintenance of the permissiveness of the TME.15 The exocytosis of lysosomes together with acidic pH of extracellular fluid can cause profound failure in normal immunosurveillance functions,17 in which the infiltrating immune cell subsets play important roles for regulating the process of tumor angiogenesis and vascular remodeling – once again in favor of cancer progrssion.18 The “angiogenic switch” seems to be influenced by various cell types of both innate and adaptive immune system.18 Given the fact that the currently used chemotherapies have focused on targeted inhibition of some key signal transduction pathway molecules (e.g., EGFR, MAPK/Erk and Akt) important for the survival and progression of cancer cells, biomolecules involved in the formation of tumor interstitium may also act as the regulators of functions of immune cells. Therefore, it is imaginable that the conventional chemotherapy and radiotherapy modalities may further impair the immune system. Thus, biomolecules and their metabolic byproducts may be considered as new targets, in which for example the dysregulated pH can be inhibited by specific inhibitors (e.g., amiloride, troglitazone and cariporide),19-21 and hypoxia inducible factor-1 (HIF-1) can be targeted by several specific inhibitors.22
Angiogenic switch
As one of the key elements of Warburg phenomenon, HIF-l can be activated in various malignancies mainly because of emergence of hypoxia. Its upregulation may elicit functional expression of several important transcription factors and genes involved in glucose metabolism, apoptosis resistance, invasion, metastasis and angiogenesis. Of these, glucose metabolism is shifted towards increased glycolysis in orchestration with cancer energy needs in lack of oxygen, producing glycolytic metabolites. Once cancer cells reached a mass greater than 106 cells, they undergo some metabolic strains because the fast growing cancer cells are distanced (by 100–200 μm) from nutritive sources. As a result, the simple diffusion mechanism can no longer retain the necessary nutritional needs and oxygen demands for the malignant cells. Given that the cancer cells at oxygen pressure around 2.5–10 mmHg become hypoxic and anoxic, they need to switch on the neovascularization through various mechanisms to overcome the nutritive deprivation through adaptation or escape from such “hostile environment”. This is why the vascular endothelial growth factor (VEGF), which binds to its cognate receptors (VEGFR 1 and VEGFR2) on the surface of vascular endothelial cells, is upregulated to associate with initiation of the “angiogenic switch”.23
Formation of tumor microenvironment and impacts of neovascularization
Upon settlement of a group of malignant cells in a host tissue, various bioevents such as angiogenesis commence to form the capillary sprouts leading towards formation of a tumor microcirculatory network through (a) the preexisting network of the host tissue, and (b) those resultant from the angiogenic activities of tumors. Regarding the tumor neovascularization process, it should also be asserted that even in the most invasive forms of malignancies, there is no alteration in the architecture of the arterial wall and the malignant invasion of arterioles is extremely rare. For tumor capillary, however, both non-fenestrated and fenestrated capillaries have been shown in different types of cancers. Further, discontinuous capillaries have been observed in TME responsible for possible extravasation of plasma fibrinogen.24 In some tumors such as melanomas and some sarcomas, for microcirculation, cancerous epithelial cells may line up and resemble channels to connect to sinusoids/vessels (Fig. 2).
Fig. 2 .
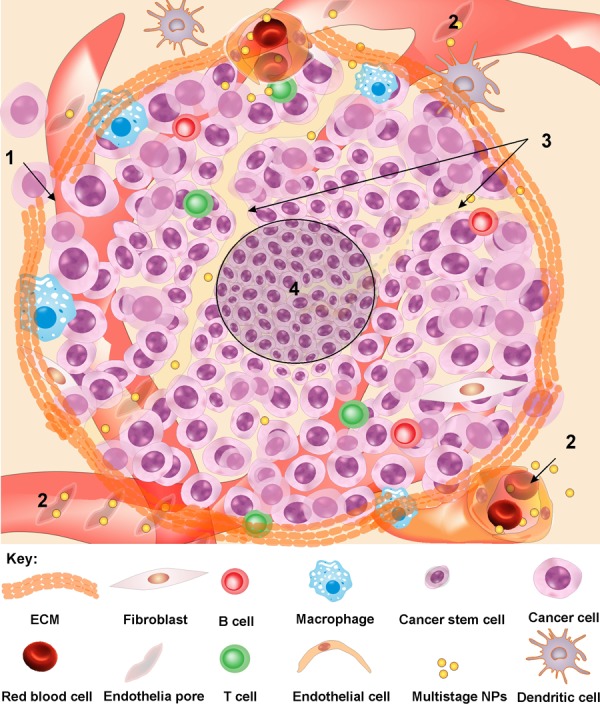
Tumor interstitium and microcirculation. 1: Deformed/ damaged ECM allows invasion and metastasis. 2: Non-integrated tumor microvasculature with pores (120-1200 nm) results in high pressure in TIF, extravasation of macromolecules and enhanced permeability and retention of NPs. 3: Cancer microcirculation can be attained by lined-up epithelial cancer cells. 4: In the core of TME, colonies of untouched cancer cells are located, in which anticancer agents fail to reach these cells. TIF: Tumor interstitial fluid. ECM: extracellular matrix. NPs: nanoparticles.
In most malignancies, the neovascularization can lead to capillary sprouts that appear to be very fragile and prone to hemorrhage. Within the TME, post-capillary venules, which are highly tortuous with no integrated basement membranes, display large diameter as giant capillaries and appear to be responsible for intravasation of cancer cells. Likewise, the venules and veins of solid tumors seem to be extremely tortuous and dilated, which can literally cause profound alteration in the flow of blood and hence delivery of oxygen within TME. As mentioned previously, the arteriovenous anastomoses that directly shunt blood from the arterial to the venous make intratumoral delivery of anticancer agents very problematic.25
While tumor cells secretes various vasoactive substances that supports neovascularization and tumor vessels spring from preexisting vascular cells, several other cell types (i.e., endothelial progenitor cells from the bone marrow and in circulation, hematopoietic stem cells, and differentiated cells of the monocytic lineage such as macrophages and dendritic cells) potentially contribute to the pathological neovascularization of solid tumors.26 Of these cells, bone marrow-derived endothelial progenitor cells contain unique subpopulations that do not become fully-differentiated vascular constituents and rather display immature myeloid or mesenchymal lineage nature, enhancing tumor angiogenic milieu in close relation with tumor vessels.27 These cells are able to migrate into pre-metastatic niche and hence elicit new vasculature settings. All these abnormal events, along with non-integrated irregular tumor microvasculature with pores and gaps (120-1200 nm),28 result in abnormal hemostasis that favors the progression of cancer and formation of a permissive milieu with high oncotic pressure interstitial fluid that will be discussed in the following sections. Further, TME encompasses network of different cell types, soluble factors, signaling molecules and ECM components, which orchestrate the progression and invasiveness of the tumor. Within the TME, cancer stem cells (CSCs) appear to retain the tumor mass. Fibroblasts and macrophages seem to favor the growth of tumor and HIFs (in particular HIF-1) and vascular endothelial growth factors (VEGFs), which function synergistically for the successful maintenance and outgrowth of metastasis.29
Morphological changes during epithelial–mesenchymal transition
During tumor development, the cancerous epithelial cells often undergo some unique morpho-physiological changes such as enhanced motility and diminished intercellular adhesion leading to mesenchymal cells, which enable them to impose profound invasiveness. During such process, a number of oncogenic pathways (e.g., peptide growth factors, Src, Ras, Ets, integrin, Wnt/β-catenin, and Notch) are activated.30-35 The upregulation and/or downregulation of these genes, together with aberrant metabolic changes, may create colonies of chemotherapy-resistant cells (CRCs), resulting in the failure of the conventional chemotherapy.
Biological barriers in cancer drug delivery
Having capitalized on various methods to study the tumor perfusion, scientists have recruited different techniques clinically including magnetic resonance image (MRI),36-38 ultrasound,39,40 positron emission tomography (PET)41-43 and volume computed tomography (VCT).44,45 These investigations have confirmed existence of a network of compromised and anisotropic blood supply as well as temporal heterogeneity in blood perfusion. Multiple layers of evidence have evinced pathophysiologic barrier functionalities of solid tumors to intratumoral drug delivery. Despite showing enhanced permeability and retention (EPR) effects due to pore size of 120-1200 nm among tumor microvasculature endothelial cells and accumulation of nanoparticles (NPs) within TME,46,47 solid tumors are able to show several pathophysiologic barriers to intratumoral drug delivery systems (DDSs) mainly through (a) high pressure zones and (b) collapsed blood vessels resultant from high tumor interstitial pressure (TIP) within TME.48,49 It seems that such irregular architecture creates profound difficulties to blood-borne anticancer agents to reach into the inner core of solid tumors. In particular, the convective penetration of macromolecular antineoplastic agents and diffusion of small molecules within tumor interstitium are largely dependent on the status of the TIF that will be discussed in the following sections.
Tumor interstitium
Solid tumors create an important sub-compartment known as “tumor interstitium”, which is restricted by the walls of blood vessels on one side and by the membranes of cells on the other, forming a unique space in favor of tumor progression and invasion. It can be divided into (a) the colloid-rich gel space containing the hydrophilic hyaluronate and proteoglycans, and (b) the colloid-poor and free-fluid space. Using extracellular/paracellular markers such as D-mannitol, the tumor interstitial space has been found to be abnormally large, in which the large “free-fluid” space seem to display less resistance to the interstitial transport and may function as a sink/reservoir for anticancer chemotherapy agents and other blood-borne macromolecules. This phenomenon seems to play a key role in terms of the enhanced pressure of TIF, which has been confirmed by methods such as wick-in-needle technique or micropipette. For detailed information on the tumor interstitium and its composition and role in anticancer drug delivery, reader is directed to see excellent papers published previously by Jane’s group.24,25,50,51
A glance at blood flow
While the efficacy of radiotherapy of tumor is dependent on the local oxygen concentration maintained by the local blood flow, the effectiveness of chemotherapy and immunotherapy depends upon blood flow important for delivery of anticancer agents.25 Hence, we briefly will discuss the blood flow in normal and malignant tissues.
In general, the blood flow rate (Q) in both normal and diseased tissues is proportional to the pressure difference (ΔP) between the arterial and venous ends of the tissue circulation and inversely proportional to the flow resistance (R).25 The R depends on the vascular morphologic aspects (e.g., the number, diameter, length, and volume) of blood vessels and the viscosity of blood. For the simplest case of laminar flow through a circular rigid vessel with the diameter/radius of r and the length of L, the R may be delineated by the Hagen-Poiseuille Law (Eq. 1):
| (Eq. 1) |
where, R, η,L and r denote the resistance of the blood flow, the viscosity of blood, the length of vessel and the radius of vessel, respectively. And, π is the mathematical constant.
As shown in Eq. 1, the flow resistance is proportional to the viscosity of blood and the length of vessel and inversely proportional to the radius of vessel. One may consider the resistance of the blood flow as a product of the viscosity for a network of blood vessels, in which a geometrical resistance (Z) should also be taken into account. Thus, the blood flow rate through a tissue may be given by Eq. 2:
| (Eq. 2) |
It should be noted that, unlike normal tissue, the blood flow in solid tumors is characterized by temporal and spatial heterogeneity, resulting in profound leakiness.52 It should be noted that, in the normal tissue, even trivial amount of the extravasated fluid is quickly drained by the lymphatic vessels resulting in very low interstitial fluid pressure (IFP), in solid tumor leakiness of the tumor vasculature and lack of lymphatic vessel as well as increased oncotic pressure and viscosity dramatically enhance the IFP.53,54
The hematocrit and the shear rate of blood appear to be the main factors by which the apparent viscosity of blood in large blood vessels is primarily governed. While the hematocrit has direct impact(s) on the viscosity, the aggregation of red blood cells (RBCs) at low shear rates tend to increase the viscosity that can be exacerbated in the presence of biomacromolecules such as globulins and proteins/peptides. Such phenomena in microvessels can enhance the migration of RBCs toward center and hence the hematocrit is lessened – a phenomenon known as “Fahraeus effect”. However, as the diameter of the blood vessels decreases to be comparable to the diameter of RBCs, both the hematocrit and the viscosity begin to increase – a trend reversal in the Fahraeus and the Fahraeus-Lindqvist effects.25,55 It appears that, within the TME, factors modulating the apparent viscosity of blood are changed because of the irregular setting of the tumor microvasculature as well as the acidification of the extracellular fluid due to the enhanced glycolysis, which in turn can affect the circulation of blood and hence distribution of oxygen and nutrients. Further, in both healthy and malignant tissues, the blood flow pressure of the microvessels in the arterial side are nearly equal, while the venous pressures in tumors are significantly lower than that of the healthy tissue.25 In fact, such decreased pressure in venular vessels with enhanced TIFP can result in reduced extravasation of fluid and solute molecules in tumors. This phenomenon tends to be due to the increased tortuous vessels in solid tumors and the enhanced viscosity of TIF, which can act as a physiologic barrier.
Capillary system and water traverse
The basic structural bioelements of normal capillary wall include: (a) the endothelium, (b) the basal lamina and (c) the pericytes, in which the inner diameter is between 5 to 10 μm with length around 20 to 100 mm. Such basic architecture of the interstitium shows similar structures in all tissues, wherein the collagens form the fiber framework that encompasses a gel phase composed of (a) 70% water, (b) glycosaminoglycans (GAGs), (c) salt solution and (d) proteins derived from plasma.56 In a solid tumor, however, there exists an altered reactive tumor ECM that encompasses an enhanced capillary density, myofibroblasts and VEGF. The latter biomolecule can increase the permeability of the tumor microvasculature and hence enhance the extravasation of the blood-circulating macromolecules, resulting in filtration of plasma proteins such as fibrinogen and hence further leakiness. Of note, the water transition across capillaries depends on a balance between filtration and reabsorption following changes in capillary and tissue hydrostatic and oncotic pressure. Based on Starling Law,57 for the normal interstitial fluid (NIF), the quantitative expression of these interrelations can be expressed as the equation (Eq.) 3:
| (3) |
where, Jv, LP and S/V denotes the amount of net fluid movement per unit time, the fluid permeability of the blood capillary and the surface area of capillaries per unit tissue volume, respectively. Pc and Pi respectively denote the capillary and the interstitium hydrostatic pressures, and σ is the reflection coefficient representing the ease of solute penetration through the capillary wall (which is almost zero for small molecules and approximately 1 for macromolecules). Пc and Пi denote the oncotic pressures of the capillary plasma and the tissue interstitium, respectively. L represents the lymphatic drainage, which is absent within the TME.
As shown in Fig. 3, two hydrostatic and oncotic phases act to control the water exchange in all tissues. In the normal tissues microvasculature (Fig. 3A), at the arterial side of the capillary, where water is filtered, the capillary hydrostatic pressure is the dominant phase and Pc is significantly greater than Πc. However, at the venous end of the capillary, where water is reabsorbed, the oncotic pressure is the dominant phase and Πc is greater than Pc and Pi. For NIF, as shown in Fig. 3A, Pc values at the arterial and the venous ends are respectively about 32 mmHg and 15 mmHg (with ∆Pc ~18). For TIF, as shown in Fig. 3B, ∆Pc is about 8, which allows development of a gradient at the venous end for Πi compared to Pi, perhaps due to the greater presence of arteriovenous anastomoses and enhanced leakiness and tortuosity of the tumor microvasculature.
Fig. 3.
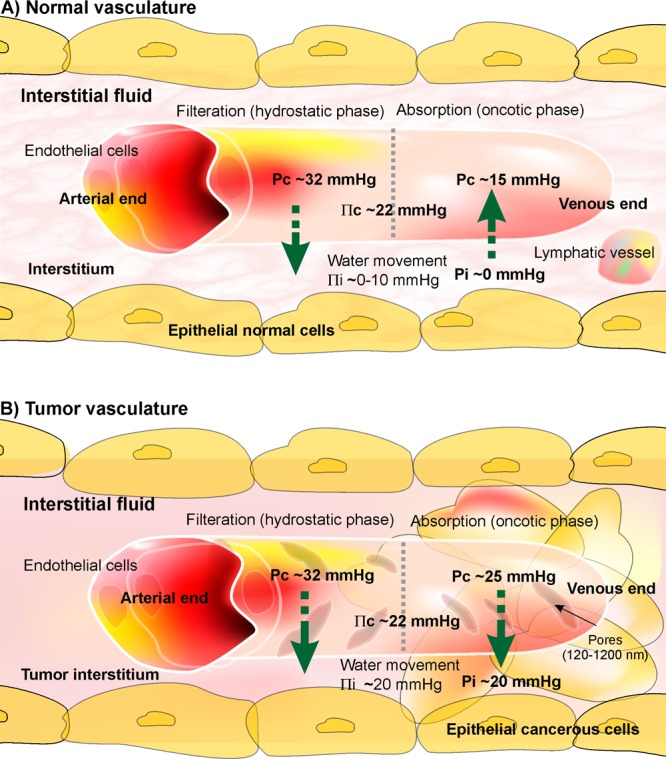
Blood flow in normal and cancerous tissues. A) In normal tissue, the Pc (capillary hydrostatic pressure) values at the arterial and the venous ends are respectively about 32 mmHg and 15 mmHg (with ΔPc ~18). B) In solid tumors, Pc values at the arterial and the venous ends are respectively about 32 mmHg and 20 mmHg (with ΔPc ~8), which allows development of a gradient at the venous end for the Πi (tissue oncotic pressure) compared to the Pi (interstitium hydrostatic pressure).
Such phenomenon, together with lack of lymphatic path, create a flow of macromolecules toward the interstitium, in which plasma albumin (responsible for ~70% of the oncotic pressure of blood flow) is lost, resulting in enhanced Πi in the interstitium of TME (i.e., ~20 mmHg) in comparison with the interstitium of the normal tissues (i.e., ~0-10 mmHg). For detailed information, reader is directed to see some following references.24,25,50,53,56,58
Importance of tumor flow and interstitium
The tumor vasculature endothelia display loose non-integrated interconnections with intercellular openings, which can be worsened by proangiogenic factors (e.g., VEGF) and vasoactive factors such as bradykinin, nitric oxide (NO) and peroxynitrite (ONOO−) produced by tumor cells. These events, together with defected functions of pericytes, are able to make tumor microvasculature more permeable and leaky, resulting in intensified fluid exchange between the tumor vasculature and the interstitial space within TME.56
In short, as shown in Fig. 3, tumor vasculature endothelia appear to be non-obedience from the Starling Law because they show irregular behavior within the TME, wherein the arterial venous pressure difference shows significant less difference than that of the normal vasculature, resulting in decreased reabsorption rate. Such diminished reabsorption together with marked leakiness in tumor vasculature can inevitably originate an inadvertent flow of macromolecules into tumor interstitium, resulting in high pressure TIF with increased viscosity.24,25,59 Thus, the accumulation of the blood flow and preservation of macromolecules carries an increase in TIF pressure, which can in turn hamper the delivery and distribution of chemotherapies in particular macromolecular drugs such as immunotherapy agents.
In addition to the tumor interstitium barrier functionality, using various technologies (e.g., MS-based proteomics, LC–MS/MS or “shotgun” strategy, array-based proteomics), the TIF represents upregulation of some important proteins such as 14-3-3 zeta, AKR1A, AR, Calreticulin, CRABP-II, CLIC1, EF 1β, EF-2, Galectin1, GSTP-1, Importin β1, Ppase, IDH, LDH-B, PRDX2, PGAM-B, PD-ECGF, PCNA, PDI, DJ-1, TCP 1-epsilon, TCP 1-theta, Trx, TIM, TPMα4, UCTH 5. Of these TIF markers, 9 proteins (i.e., Calreticulin, CRABP-II, CLIC1, EF 1β, Galectin1, PRDX2, PD-ECGF, PDI and UCTH 5) seem to be the blood circulating molecular markers which can be used for early detection of various solid tumors.3
Modulation of tumor interstitial fluid pressure
To control the hypoxia and the formation of aberrant TIF as well as the increased TIFP, several strategies have so far been designed (a) to improve the oxygenation process and/or (b) to decrease the oxygen consumption in solid tumors.
Targeting VEGF
In addition to carbogen breathing during radiotherapy of solid tumors, blood vessel normalization approach through inhibition of VEGF (using bevacizumab) and VEGFR2 (using DC-101) seems to significantly increase the oxygenation of tumor, sensitizing malignant cells to treatment modalities. Anti-VEGF therapy were shown to alter the structure of the immature vessels and improve the perivascular cell coverage and structure of ECM, resulting in normalization of tumor vessels.60
Nevertheless, there exists some controversy on the impacts of VEGFs in tumor vasculature systems. For example, Hafmann et al. (2013) recently reported that peritumorally injection of VEGF-C in xenograft mice could induce lymphangiogenesis, and thereby resulting in significant reduction of the tumor growth and the TIFP.61
Targeting PDGF
Imatinib (STI-571), as a tyrosine kinase inhibitor, was also shown to suppress the platelet derived growth factor (PDGF) receptor beta (PDGF-Rβ), resulting in the lowered TIFP in some solid tumors such as thyroid carcinoma and colon carcinoma.62 In addition to the lessened TIFP, the inhibition of PDGF was reported to increase the penetration of paclitaxel (PTX) in KAT-4 tumors in SCID mice and fluorouracil (5-FU) in PROb tumors in syngeneic BDIX rats.63
Nicotinamide
The amide form of vitamin B3, nicotinamide, has been used for its capacity to decrease the TIFP in tumor-bearing mice.64 The mechanism of lessened TIFP appeared to be related to the reduction of vascular resistance in a tumor-size dependent manner.64
Chemotherapies
Of the chemotherapy agents, it was reported that PTX is able to reduce TIFP in some tumors such as murine mammary carcinoma, human soft tissue sarcoma and breast cancer.65,66 Importantly, Taghian et al. capitalized on some important clinical investigations and confirmed such effects of PTX in breast carcinoma.66
Dexamethasone
In 1986, Braunschweiger and Schifferd reported that dexamethasone (DEX) can have profound effects on vascular function and water compartmentalization in RIF-1 tumors.67 Later, Kristjansen et al. evaluated the effect of DEX on TIFP in a human colonic adenocarcinoma in vivo model. They reported that the daily i.p. injection of DEX to LS174T tumor-bearing SCID mice (with 3-, 10-, and 30-mg/kg) was able to significantly reversibly reduce the TIFP, in part as a result of decreased permeability of tumor microvascular and vascular hydraulic conductivity.68
Remodeling of ECM
Some studies have shown that remodeling of ECM may also lead to an increased microcirculation of tumor with lowered TIFP. Of various bioelements of ECM within TME, hyaluronan (the so-called hyaluronic acid (HA) or hyaluronate) plays a key role in tumor cell adhesion and migration. HA, which is largely networked with GAGs, is deemed to modulate the extracellular water. Having interacted with cellular elements such as receptors, it plays a pivotal role in signaling pathways and biofunctions of cancerous cells. Removal of HA can lead to remodeling of the tumor stroma, reduction of TIFP, development of tumor blood vessels, acceleration of anticancer agents penetration into tumor, which literally result in inhibition of tumor.69, 70 There exist compelling evidence that the administration of exogenous hyaluronidase can impose significant anticancer activity in HA-overexpressing tumors,71 however local endogenous hyaluronidase expression within the TME appears to function as a cancer promoting agent in various solid tumors.72-74 Direct intratumoral injection of bovine testicular hyaluronidase in orthotopic osteosarcoma xenograft nude mice was shown to reduce the TIFP by 63-84% in a non-linear concentration-dependent manner.71 Recombinant human hyaluronidase, halozyme (Hylenex™) also known as rHuPH20, is a FDA-approved enzyme that can reversibly degrade the HA after s.c. administration and hence facilitate the absorption and dispersion of injected chemotherapy agents. While it is rapidly inactivated in the body and also not survived in the blood, its PEGylated form (PEGPH20) has recently been introduced into clinical trials (trial IDs: NCT00834704, NCT01170897, NCT01959139). It is expected these trials provide promising outcomes.
Prostaglandin
To control the TIFP, Rubin et al. have capitalized on the administration of prostaglandin E1 methyl ester (PGE1) in the s.c. tissue surrounding transplanted rat colonic (PROb) carcinomas or chemically-induced rat mammary carcinomas and looked at the transport of ethylenediaminetetraacetic acid (EDTA). Upon such intervention, these researchers showed a lowered TIFP by 30% together with an enhanced transcapillary traverse of EDTA into the interstitium of PROb tumors (up to 39.6%).75 These findings have been reconfirmed in a study conducted by Salnikov et al., who coadministered PGE1 and 5-FU in tumors xenograft rats and showed a significant tumor inhibitory effect of 5-FU mainly due to the lowered TIFP, and hence purported a synergistic anticancer effect by these two agents.76
Hyperthermia
The basis of hyperthermia treatment modality is the use of heat to decrease the TIFP and to increase the blood flow within TME. Leunig et al. applied a localized hyperthermia in Amelanotic melanoma bearing Syrian golden hamsters and showed a thermal dose-dependent decrease in IFP, in which the hyperthermia treatment at 43 °C for 30–60 min was able to significantly lower the TIFP.77 Likewise, to study the delivery of anticancer immunotherapy agents, Hauck et al. used a local hyperthermia (41.8 °C for 4 h) and showed significantly enhanced uptake of radiolabeled mAbs in D-54 MG glioma xenograft athymic mice.78 Although these scientists rejected the involvement of reduced TIFP in the enhanced uptake of mAbs in the human glioma xenograft model, it can be speculated that possible increased blood flow could be responsible for such an enhanced uptake of mAbs.
Fighting tumor interstitium by seamless nanomedicines
Technically, to reach the cancerous cells within the TME in solid tumors, chemotherapy and immunotherapy agents need to (a) enter tumor blood vessels, (b) cross the vessel wall, and (c) migrate through the interstitium. While the tumor microvessels are in general more permeable to macromolecules than normal vessels, the TIFP often acts against such molecular movement. Further, the traverse of hydrophilic and macromolecular therapeutics seem to be much more problematic because of the acidic microenvironment of solid tumors that can change the ionization pattern of the therapeutics.
Proof of concept
There exists a relationship between the molecular weight (MW) and the permeability of the tumor microvasculature, and a positive relationship between the plasma half- life and MW increase. In general, it can be stated that the greater the plasma half-life, the higher the chance of the extravasation and accumulation of therapeutics in TME. Dreher et al. (2006) reported the greater accumulation of dextrans with MW of 40 and 70 kDa, but homogeneous and deeper penetration of dextrans with MW of 3.3 and 10 kDa in tumor. They inferred that the increasing the MW of macromolecules improved the accumulation of macromolecules within solid tumors but significantly lowered the vascular permeability because macromolecules appeared to largely concentrate at the vicinity of the vascular surface unable to penetrate deeper into the core of tumor. This is an important finding which may lead us towards designing much more efficient and advanced multifunctional nanomedicines and nanoscaled theranostics.79 In addition, it is now well understood that NPs are able to penetrate the mucus barriers abundant in solid tumors through their multitude of traits (size, charge density, and surface functional groups) that can all be tailored to achieve optimal penetration of NPs into the thick and fibrous mucus barriers.80 On the basis that the delivery of NPs into solid tumors is dependent on not only the TIFP but also the vessel density and collagen content within the TME, Torosean et al. showed that the quantitative uptake of 40 nm fluorescent beads in tumor-bearing mice correlate with the vascular permeability through the growth of the neovasculature, while it inversely correlate with the function of collagen density and TIFP.81 Hence, all these influencing parameters have to be taken into account in order to achieve better clinical outcome of the advanced nanomedicines.
Magnetic nanofluid hyperthermia
Of various techniques used, salvage therapy of solid tumors has been conducted using magnetic fluid hyperthermia modality.82-85 In a clinical investigation, Wust et al. applied such modality in 22 patients suffering from heavily pretreated recurrences of different tumors. They used the hyperthermia in conjunction with irradiation and/or chemotherapy, capitalizing on three implantation methods of (a) infiltration under CT fluoroscopy, (b) transrectal ultrasound – guided implantation with X-fluoroscopy, and (c) intra-operative infiltration under visual control. The results showed that the instillation of such treatment modality was well tolerated without or with only moderate side effects with a 40°C heat-coverage of 86%. They also purported that an improved temperature distribution can be attained through refining of the implantation techniques, increasing of the nanofluid amount, or elevating of the magnetic field strength. And, on the basis of the actual NPs distribution and derived temperatures, they inferred that a reasonable escalation of the H-field (by only 2 kA/m) could markedly improve the 42°C coverage towards ~100%, which indicates excellent applicability of the nanofluid-based hyperthermia.82
For rationalized utilization of magnetic nanoparticles (MNPs), in 2011, Dutz et al. conceptualized that the movement and relaxation behavior of MNPs within TME is an important matter because it determines their specific heating potential. To this end, tumor xenograft mice were injected with multicore MNPs and their behaviors were evaluated using magnetic field (H = 25 kA m(-1), f = 400 kHz). Their findings resulted in compelling evidence for MNPs immobilization and hence improved relaxation behavior of MNPs, at which they extrapolated that the mobility of MNPs must be taken into account for much more accuracy of the hyperthermia.84
Improved interstitial transport of NPs by enlargement of extracellular space
To improve the penetration of macromolecules into tumor interstitium, McGuire and Yuan aimed to improve the transport through enlargement of extracellular space. Using tumor xenograft rat, they theorized that treating tumors with hypertonic solution of mannitol and cytotoxic agents such as ethacrynic acid (ECA) could respectively shrink and kill the cancerous cells. For improved interstitial penetration of dextran (with various MW), they showed that the impact of hypertonic solution was more effective than ECA, in which the impact was largely dependent upon the size of macromolecules. Based on these findings, they proposed that increases in both size and connectedness of interstitial pathways are important factors towards improved interstitial transport of macromolecules and NPs.86
Multistage nanosystems
For selective and improved delivery of NPs into tumors, Stylianopoulos et al. devised multistage NPs with initial size of ~100 nm. However, once in TME, the engineered NPs were able to shrink to ~10 nm and hence could effectively cross the tumor interstitial space and penetrate into the deeper core of solid tumors. They capitalized on a simple methodology based upon the ECM characteristics of solid tumors. To achieve such size transition within TME, they exploited the matrix metalloproteinase (MMP) enzymes overexpressed in the TME for degradation of the gelatin-based NPs to smaller NPs. They engineered gelatin NPs (~100 nm in diameter) and grafted them with amine-functionalized PEGylated quantum dots (amino-PEG-QDs). Once inside the TME, these NPs were degraded by MMP into smaller NPs (~10 nm in diameter), which were able to release the cargo into the TME. Using the intravital microscopy (IVM), the smaller NPs were shown to penetrate into deeper core of the tumor in human tumor xenograft animal models. These results confirmed the proof of principle of the strategy, which is in accord with the studies reported by Wong et al. who showed that the activity of the enzyme in HT1080 soft tissue sarcomas was sufficient to degrade the gelatin and release the QDs (10 nm) conjugated to NPs.87
Enzyme responsive nanomedicines
In 2012, Kim et al. reported development of esterase-responsive NPs labeled with 111 In and loaded with a high content of dexamethasone palmitate (DEX-P) which is a chemotherapeutic adjuvant for lowering the TIFP in solid tumors. While no apparent hepatic or renal toxicity of the DEX-P NPs were observed, the conversion of DEX-P to DEX was shown to occur upon exposure of NPs to the tumor homogenate and ascites from the tumor bearing mice, but not the human plasma.88
Improved extravasation of nanoliposomes
The impact(s) of hyperthermia was also studied for improved tumor vasculature permeability and subsequent extravasation of liposomes as well as interstitial penetration in tumor-bearing mice.83 Using IVM, it was found that implementation of a local hyperthermia at 41°C for 30 min was able to impose the extravasation liposome (~85 nm) through permeable tumor vasculature in all tumor models studied. It should be highlighted that the tumor vasculature was shown to remain permeable for 8h after applying the hyperthermia. The researcher concluded that a thermal dose of 41°C for 1h could effectively elicit a long-lasting permeability to tumor microvasculature for the extravasation and interstitial penetration of the engineered nanoliposomes.83
Targeted nanomedicines and theranostics
On the ground that low MW HA (<10 kDa) is able to elicit inadvertent inflammatory responses (e.g., induction of cytokines, chemokines, reactive nitrogen species and growth factors), Mizrahy et al. devised low and high MW HA coated lipid-based NPs to target the tumor cells expressing HA receptor (CD44). Once administered in tumor-bearing mice, they found that low MW HA decorated NPs displayed low binding affinity to the CD44 and high MW HA decorated NPs exhibited high binding affinity to the receptor. However, none of them appeared to induce the biosynthesis of cytokine(s) after intravenous injection of NPs to the healthy C57BL/6 mice, indicating no activation of immune system. Further, administration of high MW HA decorated NPs resulted in an enhanced circulation time and tumor targeting specificity, in part due to greater accumulation within TME. These researchers also showed high encapsulation capacity of high MW HA decorated NPs for anticancer cytotoxic agent methotrexate (MTX), whose liberation from NPs tend to be slow with a half-life of 13.75 days, mainly because of the active cellular targeting rather than EPR effect.89
Gold nanoparticles (AuNPs), which possess important multi-wavelength photoacoustic characteristics used for simultaneous imaging and photodynamic/thermal therapy,90-93 can be easily tailored towards seamless multimodal nanomedicines and theranostics. In one study, the pharmacokinetics and micro-distribution of Ab-mediated active targeting AuNPs were investigated in mice with subcutaneous lung carcinoma.90 AuNPs were conjugated with cetuximab (C225) for active targeting of EGFR and labeled with 111In. Once administered as PEGylated AuNps in A549 tumor xenograft mouse model, MicroSPECT/CT imaging revealed a high biodistribution of Ab-armed AuNPs in the tumor cells, in which majority of AuNPs appeared to linger within the tumor interstitium through active binding to EGFR expressing cells.90 Since AuNPs possess photoacoustic properties, they are deemed to be one the most applicable tools for implementation of hyperthermia in order to elevate the blood flow and lower the TIFP.
Final remarks and expert opinions
Functional presence of pathophysiologic barriers of solid tumors is now an incontrovertible issue. Such barrier functionalities arise from (a) aberrant tumor microvasculature, (b) low blood flow and microcirculation, (c) high interstitial fluid pressure, and (d) abnormal tumor cell layers’ architecture. As a result, effective delivery of anticancer agents into solid tumors is hampered, and even if delivered, their penetration into deeper core of TME is hindered by several opposing pathophysiologic mechanisms of tumor. Despite being partially effective, due to these complex physiologic mechanisms emerged within TME, the currently used conventional chemotherapies and even targeted molecular modalities may fail to completely reach the cancer stem cells reclined within the deeper core of tumor interstitium waiting to wake up and invade. Hence, ultimate therapy of cancer needs advanced DDSs and novel strategies.79,94-102 Among various DDSs, we have previously reported that the synthetic polymers and lipids can inevitably induce inadvertent alterations in genomic and proteomic levels.103-109 Therefore, developing biocompatible smart multifunctional nanosystems may be one of the few chances left. In addition to high pressure of TIF, of the typical characteristics of tumor microvasculature is their discontinuous and fenestrated morphology with gaps and pores between endothelial cells with pore sizes ranging from 120 to 1200 nm.28 Thus, NPs at a range of 100-250 nm can present substantial extravasation via EPR effect.110 However, even after accumulation of NPs within TME, they often fail to penetrate into deeper sections of tumor resulting in untouched remaining group of cells with ability to materialize lethal relapse in patients who underwent the conventional chemotherapies. Hence, we need to devise ingeniously effective cancer treatment modalities such as seamless multimodal nanomedicines and theranostics. We are now aware of the impacts of the physicochemical properties of NPs (i.e., size, shape and surface charge and properties), surface chemistry (PEGylation, ligand conjugation) as well as the composition of the entrapped drugs in NPs and nature of the delivery system (e.g., polymer, lipid, MNPs, AuNPs, micelles) used for the formulation of NPs, which literally influence the pharmacokinetics, biodistribution, intratumoral penetration and even tumor bioavailability. However, on the other hand, it seems that we have underestimated the impacts of tumor biology (i.e., blood flow, perfusion, permeability, interstitial fluid pressure and stroma contents) as well as patient-based parameters, which can also pose profound influences on the final aims of cancer treatment strategies.111 We have previously discussed that pH dysregulation within TME can hamper the cancer therapy, no matter what the treatment modality is! In addition, the pathophysiology traits of the tumor microvasculature and interstitium should be highlighted,54,112-119 It must be noted that a high interstitial tension inside TME may result in at least two important clinical implications as (a) prognostication of tumor cell seeding into the blood and lymphatic circulation, and (b) possible failure of cancer therapy modalities through hampering the traverse and penetration of anticancer macromolecular therapeutics in tumor. Therefore, our next step for cancer therapy seems to be the combination therapy using specific inhibitors of TME bioelements involved in pH dysregulation, TIFP and aberrant microcirculation using advanced multifunctional nanomedicines and theranostics.
Acknowledgements
The authors express their sincere gratitude to Prof. George Coukos (Ovarian Cancer Research Center at University of Pennsylvania and Ludwig Center for Cancer Research at the University of Lausanne) for his remarkably constructive comments and insights on cancer biology and therapy.
Ethical issues
The authors declare no ethical issues.
Competing interests
The authors declare no conflict of interests.
References
- 1.Barar J, Omidi Y. Dysregulated pH in tumor microenvironment checkmates cancer therapy modalities. Bioimpacts. 2013;3:149–62. doi: 10.5681/bi.2013.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heldin CH, Rubin K, Pietras K, Ostman A. High interstitial fluid pressure - an obstacle in cancer therapy. Nat Rev Cancer. 2004;4:806–13. doi: 10.1038/nrc1456. [DOI] [PubMed] [Google Scholar]
- 3.Gromov P, Gromova I, Olsen CJ, Timmermans-Wielenga V, Talman ML, Serizawa RR. et al. Tumor interstitial fluid - a treasure trove of cancer biomarkers. Biochim Biophys Acta. 2013;1834:2259–70. doi: 10.1016/j.bbapap.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 4. Omidi Y, Gumbleton M. Biological Membranes and Barriers. In: Mahato RI, editor. Biomaterials for Delivery and Targeting of Proteins Nucleic Acids. New York: CRC Press; 2005. p. 232-74.
- 5.Matzinger P. Friendly and dangerous signals: is the tissue in control? Nat Immunol. 2007;8:11–3. doi: 10.1038/ni0107-11. [DOI] [PubMed] [Google Scholar]
- 6.Gray SG, Eriksson T, Ekstrom TJ. Methylation, gene expression and the chromatin connection in cancer (review) Int J Mol Med. 1999;4:333–50. doi: 10.3892/ijmm.4.4.333. [DOI] [PubMed] [Google Scholar]
- 7.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 8.Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer. 2004;4:891–9. doi: 10.1038/nrc1478. [DOI] [PubMed] [Google Scholar]
- 9.Martinez-Outschoorn UE, Balliet RM, Lin Z, Whitaker-Menezes D, Howell A, Sotgia F. et al. Hereditary ovarian cancer and two-compartment tumor metabolism: Epithelial loss of BRCA1 induces hydrogen peroxide production, driving oxidative stress and NFkappaB activation in the tumor stroma. Cell Cycle. 2012;11:4152–66. doi: 10.4161/cc.22226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maes H, Rubio N, Garg AD, Agostinis P. Autophagy: shaping the tumor microenvironment and therapeutic response. Trends Mol Med. 2013;19:428–46. doi: 10.1016/j.molmed.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 11.Xu Y, Xia X, Pan H. Active autophagy in the tumor microenvironment: A novel mechanism for cancer metastasis. Oncol Lett. 2013;5:411–6. doi: 10.3892/ol.2012.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salem AF, Whitaker-Menezes D, Lin Z, Martinez-Outschoorn UE, Tanowitz HB, Al-Zoubi MS. et al. Two-compartment tumor metabolism: autophagy in the tumor microenvironment and oxidative mitochondrial metabolism (OXPHOS) in cancer cells. Cell Cycle. 2012;11:2545–56. doi: 10.4161/cc.20920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martinez-Outschoorn UE, Pavlides S, Howell A, Pestell RG, Tanowitz HB, Sotgia F. et al. Stromal-epithelial metabolic coupling in cancer: integrating autophagy and metabolism in the tumor microenvironment. Int J Biochem Cell Biol. 2011;43:1045–51. doi: 10.1016/j.biocel.2011.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martinez-Outschoorn UE, Lin Z, Trimmer C, Flomenberg N, Wang C, Pavlides S. et al. Cancer cells metabolically “fertilize” the tumor microenvironment with hydrogen peroxide, driving the Warburg effect: implications for PET imaging of human tumors. Cell Cycle. 2011;10:2504–20. doi: 10.4161/cc.10.15.16585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barar J, Omidi Y. Dysregulated pH in Tumor Microenvironment Checkmates Cancer Therapy. Bioimpacts. 2013;3:149–62. doi: 10.5681/bi.2013.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reshkin SJ, Cardone RA, Harguindey S. Na+-H+ exchanger, pH regulation and cancer. Recent Pat Anticancer Drug Discov. 2013;8:85–99. doi: 10.2174/15748928130108. [DOI] [PubMed] [Google Scholar]
- 17.Barar J. Targeting tumor microenvironment: the key role of immune system. Bioimpacts. 2012;2:1–3. doi: 10.5681/bi.2012.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stockmann C, Schadendorf D, Klose R, Helfrich I. The Impact of the Immune System on Tumor: Angiogenesis and Vascular Remodeling. Front Oncol. 2014;4:69. doi: 10.3389/fonc.2014.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Friday E, Oliver R, Welbourne T, Turturro F. Glutaminolysis and glycolysis regulation by troglitazone in breast cancer cells: Relationship to mitochondrial membrane potential. J Cell Physiol. 2011;226:511–9. doi: 10.1002/jcp.22360. [DOI] [PubMed] [Google Scholar]
- 20.Wong P, Lee C, Tannock IF. Reduction of intracellular pH as a strategy to enhance the pH-dependent cytotoxic effects of melphalan for human breast cancer cells. Clin Cancer Res. 2005;11:3553–7. doi: 10.1158/1078-0432.CCR-04-2472. [DOI] [PubMed] [Google Scholar]
- 21.Steffan JJ, Snider JL, Skalli O, Welbourne T, Cardelli JA. Na+/H+ exchangers and RhoA regulate acidic extracellular pH-induced lysosome trafficking in prostate cancer cells. Traffic. 2009;10:737–53. doi: 10.1111/j.1600-0854.2009.00904.x. [DOI] [PubMed] [Google Scholar]
- 22.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–32. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 23.Nyberg P, Salo T, Kalluri R. Tumor microenvironment and angiogenesis. Front Biosci. 2008;13:6537–53. doi: 10.2741/3173. [DOI] [PubMed] [Google Scholar]
- 24.Jain RK. Transport of molecules across tumor vasculature. Cancer Metastasis Rev. 1987;6:559–93. doi: 10.1007/BF00047468. [DOI] [PubMed] [Google Scholar]
- 25.Jain RK. Determinants of tumor blood flow: a review. Cancer Res. 1988;48:2641–58. [PubMed] [Google Scholar]
- 26.Furuya M, Nishiyama M, Kasuya Y, Kimura S, Ishikura H. Pathophysiology of tumor neovascularization. Vasc Health Risk Manag. 2005;1:277–90. doi: 10.2147/vhrm.2005.1.4.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Furuya M, Yonemitsu Y, Aoki I. III. Angiogenesis: complexity of tumor vasculature and microenvironment. Curr Pharm Des. 2009;15:1854–67. doi: 10.2174/138161209788453275. [DOI] [PubMed] [Google Scholar]
- 28.Adiseshaiah PP, Hall JB, McNeil SE. Nanomaterial standards for efficacy and toxicity assessment. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2010;2:99–112. doi: 10.1002/wnan.66. [DOI] [PubMed] [Google Scholar]
- 29.Catalano V, Turdo A, Di Franco S, Dieli F, Todaro M, Stassi G. Tumor and its microenvironment: a synergistic interplay. Semin Cancer Biol. 2013;23:522–32. doi: 10.1016/j.semcancer.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 30.Chung AS, Ferrara N. Targeting the tumor microenvironment with SRC kinase inhibition. Clin Cancer Res. 2010;16:775–7. doi: 10.1158/1078-0432.CCR-09-3081. [DOI] [PubMed] [Google Scholar]
- 31.Liang W, Kujawski M, Wu J, Lu J, Herrmann A, Loera S. et al. Antitumor activity of targeting SRC kinases in endothelial and myeloid cell compartments of the tumor microenvironment. Clin Cancer Res. 2010;16:924–35. doi: 10.1158/1078-0432.CCR-09-1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martinez-Outschoorn UE, Curry JM, Ko YH, Lin Z, Tuluc M, Cognetti D. et al. Oncogenes and inflammation rewire host energy metabolism in the tumor microenvironment: RAS and NFkappaB target stromal MCT4. Cell Cycle. 2013;12:2580–97. doi: 10.4161/cc.25510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seguin L, Weis SM, Cheresh DA. Variety in the tumor microenvironment: integrin splicing regulates stemness. Cell Stem Cell. 2014;14:557–8. doi: 10.1016/j.stem.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 34.Macheda ML, Stacker SA. Importance of Wnt signaling in the tumor stroma microenvironment. Curr Cancer Drug Targets. 2008;8:454–65. doi: 10.2174/156800908785699324. [DOI] [PubMed] [Google Scholar]
- 35. Bailey JM, Leach SD. Signaling pathways mediating epithelial- mesenchymal crosstalk in pancreatic cancer: Hedgehog, Notch and TGFbeta. In: Grippo PJ, HG Munshi, editors. Pancreatic Cancer and Tumor Microenvironment. Trivandrum (India)2012. [PubMed]
- 36.Li SP, Padhani AR. Tumor response assessments with diffusion and perfusion MRI. J Magn Reson Imaging. 2012;35:745–63. doi: 10.1002/jmri.22838. [DOI] [PubMed] [Google Scholar]
- 37.Jordan BF, Sonveaux P. Targeting tumor perfusion and oxygenation to improve the outcome of anticancer therapy. Front Pharmacol. 2012;3:94. doi: 10.3389/fphar.2012.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matsuo M, Matsumoto S, Mitchell JB, Krishna MC, Camphausen K. Magnetic Resonance Imaging of the Tumor Microenvironment in Radiotherapy: Perfusion, Hypoxia, and Metabolism. Semin Radiat Oncol. 2014;24:210–7. doi: 10.1016/j.semradonc.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gee MS, Saunders HM, Lee JC, Sanzo JF, Jenkins WT, Evans SM. et al. Doppler ultrasound imaging detects changes in tumor perfusion during antivascular therapy associated with vascular anatomic alterations. Cancer Res. 2001;61:2974–82. [PubMed] [Google Scholar]
- 40.Ta CN, Kono Y, Barback CV, Mattrey RF, Kummel AC. Automating tumor classification with pixel-by-pixel contrast-enhanced ultrasound perfusion kinetics. J Vac Sci Technol B Nanotechnol Microelectron. 2012;30:2C103. doi: 10.1116/1.3692962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Langen AJ, Lubberink M, Boellaard R, Spreeuwenberg MD, Smit EF, Hoekstra OS. et al. Reproducibility of tumor perfusion measurements using 15O-labeled water and PET. J Nucl Med. 2008;49:1763–8. doi: 10.2967/jnumed.108.053454. [DOI] [PubMed] [Google Scholar]
- 42.Mertens J, Ham H, De Zutter A, Depicker A, Van de Wiele C, Smeets P. et al. Tumor perfusion using first-pass F-18 FDG PET images. Clin Nucl Med. 2012;37:166–7. doi: 10.1097/RLU.0b013e31823ea188. [DOI] [PubMed] [Google Scholar]
- 43.Apostolova I, Hofheinz F, Buchert R, Steffen IG, Michel R, Rosner C. et al. Combined measurement of tumor perfusion and glucose metabolism for improved tumor characterization in advanced cervical carcinomaA PET/CT pilot study using [15O]water and [18F]fluorodeoxyglucose. Strahlenther Onkol. 2014;190:575–81. doi: 10.1007/s00066-014-0611-7. [DOI] [PubMed] [Google Scholar]
- 44.Hermans R, Van den Bogaert W. Outcome prediction after surgery and chemoradiation of head-and-neck squamous cell carcinoma (HNSCC), using baseline perfusion computed tomography (CT) microcirculatory parameters vstumor volume. Int J Radiat Oncol Biol Phys. 2009;74:1307. doi: 10.1016/j.ijrobp.2009.03.038. [DOI] [PubMed] [Google Scholar]
- 45.Petralia G, Preda L, Giugliano G, Jereczek-Fossa BA, Rocca A, D’Andrea G. et al. Perfusion computed tomography for monitoring induction chemotherapy in patients with squamous cell carcinoma of the upper aerodigestive tract: correlation between changes in tumor perfusion and tumor volume. J Comput Assist Tomogr. 2009;33:552–9. doi: 10.1097/RCT.0b013e31818d446e. [DOI] [PubMed] [Google Scholar]
- 46.Maeda H. The enhanced permeability and retention (EPR) effect in tumor vasculature: the key role of tumor-selective macromolecular drug targeting. Adv Enzyme Regul. 2001;41:189–207. doi: 10.1016/s0065-2571(00)00013-3. [DOI] [PubMed] [Google Scholar]
- 47.Iyer AK, Khaled G, Fang J, Maeda H. Exploiting the enhanced permeability and retention effect for tumor targeting. Drug Discov Today. 2006;11:812–8. doi: 10.1016/j.drudis.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 48.Flessner MF, Choi J, Credit K, Deverkadra R, Henderson K. Resistance of tumor interstitial pressure to the penetration of intraperitoneally delivered antibodies into metastatic ovarian tumors. Clin Cancer Res. 2005;11:3117–25. doi: 10.1158/1078-0432.CCR-04-2332. [DOI] [PubMed] [Google Scholar]
- 49.Wu M, Frieboes HB, Chaplain MA, McDougall SR, Cristini V, Lowengrub JS. The effect of interstitial pressure on therapeutic agent transport: Coupling with the tumor blood and lymphatic vascular systems. J Theor Biol. 2014;355:194–207. doi: 10.1016/j.jtbi.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jain RK. Transport of macromolecules in tumor microcirculation. Biotechnol Prog. 1985;1:81–94. doi: 10.1002/btpr.5420010205. [DOI] [PubMed] [Google Scholar]
- 51.Jain RK. Transvascular and interstitial transport in tumors. Adv Exp Med Biol. 1988;242:215–20. doi: 10.1007/978-1-4684-8935-4_24. [DOI] [PubMed] [Google Scholar]
- 52.Baish JW, Netti PA, Jain RK. Transmural coupling of fluid flow in microcirculatory network and interstitium in tumors. Microvasc Res. 1997;53:128–41. doi: 10.1006/mvre.1996.2005. [DOI] [PubMed] [Google Scholar]
- 53.Shieh AC, Swartz MA. Regulation of tumor invasion by interstitial fluid flow. Phys Biol. 2011;8:015012. doi: 10.1088/1478-3975/8/1/015012. [DOI] [PubMed] [Google Scholar]
- 54.Wiig H, Swartz MA. Interstitial fluid and lymph formation and transport: physiological regulation and roles in inflammation and cancer. Physiol Rev. 2012;92:1005–60. doi: 10.1152/physrev.00037.2011. [DOI] [PubMed] [Google Scholar]
- 55.Fahraus R, Lindqvist T. The viscosity of the blood in narrow capillary tubes. American Journal of Physiology. 1931;96:562–8. [Google Scholar]
- 56. Baronzio G, Freitas I, Hau K. Significance of Tumor Microenvironment on the Genesis of: Interstitial Fluid, Angiogenesis, Haemostatic/Haemorheologic Abnormalities. Pathogenesis and Therapeutic Aspects. In: Baronzio G, CR Cogle, editors. Cancer Microenvironment and Therapeutic Implications: Tumor Pathophysiology Mechanisms and Therapeutic Strategies. New York: Springer; 2009.
- 57.Starling EH. On the Absorption of Fluids from the Connective Tissue Spaces. J Physiol. 1896;19:312–26. doi: 10.1113/jphysiol.1896.sp000596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jain RK. Transport of molecules in the tumor interstitium: a review. Cancer Res. 1987;47:3039–51. [PubMed] [Google Scholar]
- 59.McDonald DM, Foss AJ. Endothelial cells of tumor vessels: abnormal but not absent. Cancer Metastasis Rev. 2000;19:109–20. doi: 10.1023/a:1026529222845. [DOI] [PubMed] [Google Scholar]
- 60.Minchinton AI, Tannock IF. Drug penetration in solid tumours. Nat Rev Cancer. 2006;6:583–92. doi: 10.1038/nrc1893. [DOI] [PubMed] [Google Scholar]
- 61.Hofmann M, Pflanzer R, Zoller NN, Bernd A, Kaufmann R, Thaci D. et al. Vascular endothelial growth factor C-induced lymphangiogenesis decreases tumor interstitial fluid pressure and tumor. Transl Oncol. 2013;6:398–404. doi: 10.1593/tlo.13274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pietras K, Ostman A, Sjoquist M, Buchdunger E, Reed RK, Heldin CH. et al. Inhibition of platelet-derived growth factor receptors reduces interstitial hypertension and increases transcapillary transport in tumors. Cancer Res. 2001;61:2929–34. [PubMed] [Google Scholar]
- 63.Pietras K, Rubin K, Sjoblom T, Buchdunger E, Sjoquist M, Heldin CH. et al. Inhibition of PDGF receptor signaling in tumor stroma enhances antitumor effect of chemotherapy. Cancer Res. 2002;62:5476–84. [PubMed] [Google Scholar]
- 64.Lee I, Boucher Y, Jain RK. Nicotinamide can lower tumor interstitial fluid pressure: mechanistic and therapeutic implications. Cancer Res. 1992;52:3237–40. [PubMed] [Google Scholar]
- 65.Griffon-Etienne G, Boucher Y, Brekken C, Suit HD, Jain RK. Taxane-induced apoptosis decompresses blood vessels and lowers interstitial fluid pressure in solid tumors: clinical implications. Cancer Res. 1999;59:3776–82. [PubMed] [Google Scholar]
- 66.Taghian AG, Abi-Raad R, Assaad SI, Casty A, Ancukiewicz M, Yeh E. et al. Paclitaxel decreases the interstitial fluid pressure and improves oxygenation in breast cancers in patients treated with neoadjuvant chemotherapy: clinical implications. J Clin Oncol. 2005;23:1951–61. doi: 10.1200/JCO.2005.08.119. [DOI] [PubMed] [Google Scholar]
- 67.Braunschweiger PG, Schiffer LM. Effect of dexamethasone on vascular function in RIF-1 tumors. Cancer Res. 1986;46:3299–303. [PubMed] [Google Scholar]
- 68.Kristjansen PE, Boucher Y, Jain RK. Dexamethasone reduces the interstitial fluid pressure in a human colon adenocarcinoma xenograft. Cancer Res. 1993;53:4764–6. [PubMed] [Google Scholar]
- 69.Toole BP, Hascall VC. Hyaluronan and tumor growth. Am J Pathol. 2002;161:745–7. doi: 10.1016/S0002-9440(10)64232-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kultti A, Li X, Jiang P, Thompson CB, Frost GI, Shepard HM. Therapeutic targeting of hyaluronan in the tumor stroma. Cancers (Basel) 2012;4:873–903. doi: 10.3390/cancers4030873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brekken C, de Lange Davies C. Hyaluronidase reduces the interstitial fluid pressure in solid tumours in a non-linear concentration-dependent manner. Cancer Lett. 1998;131:65–70. doi: 10.1016/s0304-3835(98)00202-x. [DOI] [PubMed] [Google Scholar]
- 72.Lokeshwar VB, Lokeshwar BL, Pham HT, Block NL. Association of elevated levels of hyaluronidase, a matrix-degrading enzyme, with prostate cancer progression. Cancer Res. 1996;56:651–7. [PubMed] [Google Scholar]
- 73.Tamakoshi K, Kikkawa F, Maeda O, Suganuma N, Yamagata S, Yamagata T. et al. Hyaluronidase activity in gynaecological cancer tissues with different metastatic forms. Br J Cancer. 1997;75:1807–11. doi: 10.1038/bjc.1997.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pham HT, Block NL, Lokeshwar VB. Tumor-derived hyaluronidase: a diagnostic urine marker for high-grade bladder cancer. Cancer Res. 1997;57:778–83. [PubMed] [Google Scholar]
- 75.Rubin K, Sjoquist M, Gustafsson AM, Isaksson B, Salvessen G, Reed RK. Lowering of tumoral interstitial fluid pressure by prostaglandin E(1) is paralleled by an increased uptake of (51)Cr-EDTA. Int J Cancer. 2000;86:636–43. doi: 10.1002/(sici)1097-0215(20000601)86:5<636::aid-ijc6>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 76.Salnikov AV, Iversen VV, Koisti M, Sundberg C, Johansson L, Stuhr LB. et al. Lowering of tumor interstitial fluid pressure specifically augments efficacy of chemotherapy. FASEB J. 2003;17:1756–8. doi: 10.1096/fj.02-1201fje. [DOI] [PubMed] [Google Scholar]
- 77.Leunig M, Goetz AE, Dellian M, Zetterer G, Gamarra F, Jain RK. et al. Interstitial fluid pressure in solid tumors following hyperthermia: possible correlation with therapeutic response. Cancer Res. 1992;52:487–90. [PubMed] [Google Scholar]
- 78.Hauck ML, Coffin DO, Dodge RK, Dewhirst MW, Mitchell JB, Zalutsky MR. A local hyperthermia treatment which enhances antibody uptake in a glioma xenograft model does not affect tumour interstitial fluid pressure. Int J Hyperthermia. 1997;13:307–16. doi: 10.3109/02656739709023538. [DOI] [PubMed] [Google Scholar]
- 79.Omidi Y. Smart multifunctional theranostics: simultaneous diagnosis and therapy of cancer. Bioimpacts. 2011;1:145–7. doi: 10.5681/bi.2011.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Aljayyoussi G, Abdulkarim M, Griffiths P, Gumbleton M. Pharmaceutical nanoparticles and the mucin biopolymer barrier. Bioimpacts. 2012;2:173–4. doi: 10.5681/bi.2012.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Torosean S, Flynn B, Axelsson J, Gunn J, Samkoe KS, Hasan T. et al. Nanoparticle uptake in tumors is mediated by the interplay of vascular and collagen density with interstitial pressure. Nanomedicine. 2013;9:151–8. doi: 10.1016/j.nano.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wust P, Gneveckow U, Johannsen M, Bohmer D, Henkel T, Kahmann F. et al. Magnetic nanoparticles for interstitial thermotherapy--feasibility, tolerance and achieved temperatures. Int J Hyperthermia. 2006;22:673–85. doi: 10.1080/02656730601106037. [DOI] [PubMed] [Google Scholar]
- 83.Li L, ten Hagen TL, Bolkestein M, Gasselhuber A, Yatvin J, van Rhoon GC. et al. Improved intratumoral nanoparticle extravasation and penetration by mild hyperthermia. J Control Release. 2013;167:130–7. doi: 10.1016/j.jconrel.2013.01.026. [DOI] [PubMed] [Google Scholar]
- 84.Dutz S, Kettering M, Hilger I, Muller R, Zeisberger M. Magnetic multicore nanoparticles for hyperthermia--influence of particle immobilization in tumour tissue on magnetic properties. Nanotechnology. 2011;22:265102. doi: 10.1088/0957-4484/22/26/265102. [DOI] [PubMed] [Google Scholar]
- 85.Johannsen M, Thiesen B, Wust P, Jordan A. Magnetic nanoparticle hyperthermia for prostate cancer. Int J Hyperthermia. 2010;26:790–5. doi: 10.3109/02656731003745740. [DOI] [PubMed] [Google Scholar]
- 86.McGuire S, Yuan F. Improving interstitial transport of macromolecules through reduction in cell volume fraction in tumor tissues. Nanomedicine. 2012;8:1088–95. doi: 10.1016/j.nano.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 87.Wong C, Stylianopoulos T, Cui J, Martin J, Chauhan VP, Jiang W. et al. Multistage nanoparticle delivery system for deep penetration into tumor tissue. Proc Natl Acad Sci U S A. 2011;108:2426–31. doi: 10.1073/pnas.1018382108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kim JK, Yuan H, Nie J, Yang YT, Leggas M, Potter PM. et al. High payload dual therapeutic-imaging nanocarriers for triggered tumor delivery. Small. 2012;8:2895–903. doi: 10.1002/smll.201200437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mizrahy S, Goldsmith M, Leviatan-Ben-Arye S, Kisin-Finfer E, Redy O, Srinivasan S. et al. Tumor targeting profiling of hyaluronan-coated lipid based-nanoparticles. Nanoscale. 2014;6:3742–52. doi: 10.1039/c3nr06102g. [DOI] [PubMed] [Google Scholar]
- 90.Kao HW, Lin YY, Chen CC, Chi KH, Tien DC, Hsia CC. et al. Biological characterization of cetuximab-conjugated gold nanoparticles in a tumor animal model. Nanotechnology. 2014;25:295102. doi: 10.1088/0957-4484/25/29/295102. [DOI] [PubMed] [Google Scholar]
- 91.Jing L, Liang X, Deng Z, Feng S, Li X, Huang M. et al. Prussian blue coated gold nanoparticles for simultaneous photoacoustic/CT bimodal imaging and photothermal ablation of cancer. Biomaterials. 2014;35:5814–21. doi: 10.1016/j.biomaterials.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 92.Gutrath BS, Beckmann MF, Buchkremer A, Eckert T, Timper J, Leifert A. et al. Size-dependent multispectral photoacoustic response of solid and hollow gold nanoparticles. Nanotechnology. 2012;23:225707. doi: 10.1088/0957-4484/23/22/225707. [DOI] [PubMed] [Google Scholar]
- 93.Mallidi S, Larson T, Tam J, Joshi PP, Karpiouk A, Sokolov K. et al. Multiwavelength photoacoustic imaging and plasmon resonance coupling of gold nanoparticles for selective detection of cancer. Nano Lett. 2009;9:2825–31. doi: 10.1021/nl802929u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Matthaiou EI, Barar J, Sandaltzopoulos R, Li C, Coukos G, Omidi Y. Shikonin-loaded antibody-armed nanoparticles for targeted therapy of ovarian cancer. Int J Nanomedicine. 2014;9:1855–70. doi: 10.2147/IJN.S51880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Najar AG, Pashaei-Asl R, Omidi Y, Farajnia S, Nourazarian AR. EGFR antisense oligonucleotides encapsulated with nanoparticles decrease EGFR, MAPK1 and STAT5 expression in a human colon cancer cell line. Asian Pac J Cancer Prev. 2013;14:495–8. doi: 10.7314/apjcp.2013.14.1.495. [DOI] [PubMed] [Google Scholar]
- 96.Barar J, Omidi Y. Targeted Gene Therapy of Cancer: Second Amendment toward Holistic Therapy. Bioimpacts. 2013;3:49–51. doi: 10.5681/bi.2013.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yari Khosoushahi A, Naderi-Manesh H, Yeganeh H, Barar J, Omidi Y. Novel water-soluble polyurethane nanomicelles for cancer chemotherapy: physicochemical characterization and cellular activities. J Nanobiotechnology. 2012;10:2. doi: 10.1186/1477-3155-10-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nourazarian AR, Pashaei-Asl R, Omidi Y, Najar AG. c-Src antisense complexed with PAMAM denderimes decreases of c-Src expression and EGFR-dependent downstream genes in the human HT-29 colon cancer cell line. Asian Pac J Cancer Prev. 2012;13:2235–40. doi: 10.7314/apjcp.2012.13.5.2235. [DOI] [PubMed] [Google Scholar]
- 99.Nourazarian AR, Najar AG, Farajnia S, Khosroushahi AY, Pashaei-Asl R, Omidi Y. Combined EGFR and c-Src Antisense Oligodeoxynucleotides Encapsulated with PAMAM Denderimers Inhibit HT-29 Colon Cancer Cell Proliferation. Asian Pac J Cancer Prev. 2012;13:4751–6. doi: 10.7314/apjcp.2012.13.9.4751. [DOI] [PubMed] [Google Scholar]
- 100.Barar J, Omidi Y. Translational Approaches toward Cancer Gene Therapy: Hurdles and Hopes. BioImpacts. 2012;2:127–43. doi: 10.5681/bi.2012.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Omidi Y. CNT Nanobombs for Specific Eradication of Cancer Cells: A New Concept in Cancer Theranostics. Bioimpacts. 2011;1:199–201. doi: 10.5681/bi.2011.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Majidi J, Barar J, Baradaran B, Abdolalizadeh J, Omidi Y. Target therapy of cancer: implementation of monoclonal antibodies and nanobodies. Hum Antibodies. 2009;18:81–100. doi: 10.3233/HAB-2009-0204. [DOI] [PubMed] [Google Scholar]
- 103. Omidi Y, Kafil V, Barar J, editors. Toxicogenomics of Nonviral Cationic Gene Delivery Nanosystems. Non-Viral Gene Therapy; 2011: InTech.
- 104.Barar J, Hamzeiy H, Mortazavi-Tabatabaei SA, Hashemi-Aghdam SE, Omidi Y. Genomic signature and toxicogenomics comparison of polycationic gene delivery nanosystems in human alveolar epithelial A549 cells. Daru. 2009;17:139–47. [Google Scholar]
- 105.Omidi Y, Barar J, Heidari HR, Ahmadian S, Yazdi HA, Akhtar S. Microarray analysis of the toxicogenomics and the genotoxic potential of a cationic lipid-based gene delivery nanosystem in human alveolar epithelial a549 cells. Toxicol Mech Methods. 2008;18:369–78. doi: 10.1080/15376510801891286. [DOI] [PubMed] [Google Scholar]
- 106.Hollins AJ, Omidi Y, Benter IF, Akhtar S. Toxicogenomics of drug delivery systems: Exploiting delivery system-induced changes in target gene expression to enhance siRNA activity. J Drug Target. 2007;15:83–8. doi: 10.1080/10611860601151860. [DOI] [PubMed] [Google Scholar]
- 107.Omidi Y, Barar J, Akhtar S. Toxicogenomics of cationic lipid-based vectors for gene therapy: impact of microarray technology. Curr Drug Deliv. 2005;2:429–41. doi: 10.2174/156720105774370249. [DOI] [PubMed] [Google Scholar]
- 108.Omidi Y, Hollins AJ, Benboubetra M, Drayton R, Benter IF, Akhtar S. Toxicogenomics of non-viral vectors for gene therapy: a microarray study of lipofectin- and oligofectamine-induced gene expression changes in human epithelial cells. J Drug Target. 2003;11:311–23. doi: 10.1080/10611860310001636908. [DOI] [PubMed] [Google Scholar]
- 109.Barar J, Omidi Y. Intrinsic bio-signature of gene delivery nanocarriers may impair gene therapy goals. Bioimpacts. 2013;3:105–9. doi: 10.5681/bi.2013.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Li Y, Wang J, Wientjes MG, Au JL. Delivery of nanomedicines to extracellular and intracellular compartments of a solid tumor. Adv Drug Deliv Rev. 2012;64:29–39. doi: 10.1016/j.addr.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ernsting MJ, Murakami M, Roy A, Li SD. Factors controlling the pharmacokinetics, biodistribution and intratumoral penetration of nanoparticles. J Control Release. 2013;172:782–94. doi: 10.1016/j.jconrel.2013.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Swartz MA, Hubbell JA, Reddy ST. Lymphatic drainage function and its immunological implications: from dendritic cell homing to vaccine design. Semin Immunol. 2008;20:147–56. doi: 10.1016/j.smim.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 113.Swartz MA, Fleury ME. Interstitial flow and its effects in soft tissues. Annu Rev Biomed Eng. 2007;9:229–56. doi: 10.1146/annurev.bioeng.9.060906.151850. [DOI] [PubMed] [Google Scholar]
- 114.Netti PA, Berk DA, Swartz MA, Grodzinsky AJ, Jain RK. Role of extracellular matrix assembly in interstitial transport in solid tumors. Cancer Res. 2000;60:2497–503. [PubMed] [Google Scholar]
- 115.Fukumura D, Jain RK. Tumor microenvironment abnormalities: causes, consequences, and strategies to normalize. J Cell Biochem. 2007;101:937–49. doi: 10.1002/jcb.21187. [DOI] [PubMed] [Google Scholar]
- 116.Fukumura D, Jain RK. Tumor microvasculature and microenvironment: targets for anti-angiogenesis and normalization. Microvasc Res. 2007;74:72–84. doi: 10.1016/j.mvr.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 118.Jain RK. Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat Med. 2001;7:987–9. doi: 10.1038/nm0901-987. [DOI] [PubMed] [Google Scholar]
- 119.Boucher Y, Leunig M, Jain RK. Tumor angiogenesis and interstitial hypertension. Cancer Res. 1996;56:4264–6. [PubMed] [Google Scholar]