Abstract
Component cognitive and motor processes contributing to diminished visuomotor procedural learning in HIV infection with comorbid chronic alcoholism (HIV+ALC) include problems with attention and explicit memory processes. The neural correlates associated with this constellation of cognitive and motor processes in HIV infection and alcoholism have yet to be delineated. Frontostriatal regions are affected in HIV infection, frontothalamocerebellar regions are affected in chronic alcoholism, and frontolimbic regions are likely affected in both; all three of these systems have the potential of contributing to both visuomotor procedural learning and explicit memory processes. Here, we examined the neural correlates of implicit memory, explicit memory, attention, and motor tests in 26 HIV+ALC (5 with comorbidity for nonalcohol drug abuse/dependence) and 19 age-range matched healthy control men. Parcellated brain volumes, including cortical, subcortical, and allocortical regions, as well as cortical sulci and ventricles, were derived using the SRI24 brain atlas. Results indicated that smaller thalamic volumes were associated with poorer performance on tests of explicit (immediate and delayed) and implicit (visuomotor procedural) memory in HIV+ALC. By contrast, smaller hippocampal volumes were associated with lower scores on explicit, but not implicit memory. Multiple regression analyses revealed that volumes of both the thalamus and the hippocampus were each unique independent predictors of explicit memory scores. This study provides evidence of a dissociation between implicit and explicit memory tasks in HIV+ALC, with selective relationships observed between hippocampal volume and explicit but not implicit memory, and highlights the relevance of the thalamus to mnemonic processes.
Keywords: visuomotor procedural learning, implicit memory, explicit memory, thalamus, hippocampus, HIV infection-alcoholism comorbidity
Introduction
Visuomotor skill learning is a complex function that depends on multiple, dissociable mnemonic and nonmnemonic component processes, including selective attention, working memory, explicit (declarative) memory, visuospatial abilities, and motor skills (Corkin, 1968; Fama et al., 2012). A recent behavioral study examining cognitive component processes of visuomotor procedural learning in HIV infection and chronic alcoholism (Fama et al., 2012) reported that attention and explicit memory contributed to overall visuomotor learning and retention in individuals with comorbid HIV infection and chronic alcoholism.
Over the past two decades, a number of studies have documented cognitive and motor deficits associated with HIV infection with and without concomitant alcoholism and their relationship with brain structure (Gonzalez et al., 2008; Heaton et al., 2011; [reviewed in Neuropsychology Review: Maki et al., 2009; Woods et al., 2009]); however, the neural correlates that contribute to implicit and explicit memory compromise in HIV-alcoholism comorbidity have not been fully explicated (Chang et al., 2001; Clifford and Ances, 2013; Heaton et al., 2011; Maki et al., 2009; Rothlind et al., 2005). Structural brain imaging studies have revealed regional brain volume deficits in the thalamus and frontal lobes in both HIV infection and chronic alcoholism and the cingulate cortices in chronic alcoholism (Pfefferbaum et al., 2012), structures intimately involved in mnemonic processes. Additionally, frontostriatal, frontothalamocerebellar, and frontolimbic structures are potentially compromised in HIV-alcoholism comorbidity: the striatum from HIV infection (Castelo et al., 2006; Chang et al., 2001), frontocerebellar sites from chronic alcoholism (Harper and Kril, 1993; Sullivan, 2003), and, thus, frontolimbic sites likely occurring in both conditions.
Having previously established behavioral relations between visuomotor procedural learning and attention and memory processes in HIV infection with alcoholism comorbidity (Fama et al., 2012), we now focused on the neural correlates of these cognitive processes. Accordingly, the aims of the present study were to identify brain-behavior relations in HIV with substance abuse comorbidity, primarily alcoholism, that are associated with visuomotor procedural memory and cognitive processes known to contribute to implicit memory performance, including explicit memory and attention.
We tested the following hypotheses: (1) impaired visuomotor procedural learning and retention would be related to structural deficits associated with HIV infection and alcoholism including regional volume deficits of the thalamus, cingulate, frontal lobes, and hippocampus and (2) brain regions would differentially contribute to dissociable cognitive component processes. We also tested the double dissociation hypothesis that implicit memory scores would be primarily predicted from thalamus volumes, whereas explicit memory scores would be primarily predicted from hippocampus volumes. We hypothesized that in individuals with HIV infection and chronic alcoholism, extra-hippocampal regions, particularly the thalamus, cingulate, and frontal regional volumes, would contribute more to visuomotor (implicit) memory performance than explicit memory performance, whereas the hippocampus would contribute more to explicit memory than visuomotor (implicit) memory performance.
Methods
Two groups of subjects were examined: (1) 26 HIV+ALC men who were recruited from HIV clinics to be positive for HIV infection and to meet criteria for alcohol dependence (n=22) or abuse (n=4) within 3 years of study entry; (2) 19 age-range matched normal control (NC) men who were neither positive for HIV infection nor met alcohol dependence or abuse criteria or any other Axis-I diagnosis in their lifetime based on laboratory tests and SCID interviews. Participants were part of an ongoing longitudinal study on the effects of HIV infection and alcohol on brain structure and function. These 45 study participants were a subset of a larger group of 48 HIV+ALC and NC subjects from our previous study (Fama et al., 2012); excluded were the 2 HIV+ALC and 1 NC participant who did not have imaging data. All subjects gave written informed consent to participate in this study, which was approved by the Institutional Review Boards of Stanford University, SRI International, and Santa Clara Valley Medical Center. Demographic data for the participant groups are in Table 1.
Table 1.
Demographic characteristics of subject groups (mean, standard deviation, range)
| Group | Age (yrs) | Education (yrs) | NART IQ† | Lifetime Alcohol Consumption (kg) |
Beck Depression Inventory-II |
|---|---|---|---|---|---|
| Control (NC n=19) | 43.4 (10.5) 22 to 57 |
15.1 (2.0) 12 to 18 |
111.4 (8.1) 92 to 123 |
32.3 (64.9) 0 to 264 |
3.1 (3.7) 0 to 12 |
| HIV and Alcoholism (HIV+ALC n=26) | 48.7 (9.7) 22 to 60 |
13.6 (1.8) 10 to 18 |
107.7 (7.5) 94 to 123 |
674.2 (502.1) 31* to 1637 |
10.1 (7.3) 0 to 24 |
| Two-tailed, unpaired t test | ns | p=.01 | ns | p=.0001 | p=.0004 |
NART - National Adult Reading Test
The HIV+ALC subject who had 31 kg lifetime alcohol consumption was 25 years old.
The HIV+ALC and NC groups did not differ significantly in age or estimated IQ score based on the National Adult Reading Test (Nelson, 1982). The HIV+ALC group had fewer years of education (t(43)=2.69, p=.01) and a higher level of depression symptoms as assessed with the Beck Depression Inventory-II (Beck et al., 1996; Sassoon et al., 2012) than the NC group. As expected, the HIV+ALC group drank significantly more alcohol in their lifetime than the controls.
All participants were screened using the Structured Clinical Interview for DSM-IV (SCID) (First et al., 1998) and structured questionnaires on health status. Upon initial assessment, exclusionary criteria for HIV+ALC were history of schizophrenia or bipolar disorder, neurological disorder unrelated to HIV infection or chronic alcoholism, HIV-related opportunistic infection, or drug dependence or abuse within the past 3 months. Normal control participants were excluded from the study if they met criteria for any Axis I disorder or had a neurological disorder or serious medical condition. Non-removable ferrous metal was an additional exclusion for both subject groups. All participants admitted to the study underwent a semi-structured interview to quantify lifetime alcohol consumption (Skinner, 1982; Skinner and Sheu, 1982). All HIV+ALC subjects had a CD4 cell count >100 cells per mm3 and a Karnofsky score (Karnofsky, 1949) >90 at time of study entry. At the time of testing, 4 HIV+ALC subjects had CD4 cell counts below 200 and 14 subjects had CD4 cell counts above 500. Almost all HIV+ALC subjects (23 of 26 individuals) were on HAART; 10 HIV+ALC subjects tested positive for Hepatitis C.
Of the 26 HIV+ALC participants, 23 individuals (88.5%) had a lifetime history of DSM-IV drug abuse or dependence at the time of testing. Although an exclusion criterion for study entry was a drug-related diagnosis within the past 3 months, the subjects included in this study were generally tested during follow-up. For those participants with drug history, mean length of remission (time since last met DSM-IV criteria for any drug abuse or dependence including marijuana) was approximately 100.7+93.9 months (median = 69.0 months; range 0.5 to 330.8 months) at time of testing. Only two participants met DSM-IV criteria for drug abuse or dependence within the year prior to their testing visit: one was 0.5 months sober and the other was 7.1 months sober. The most common drugs of abuse were cocaine, reported by 17 out of the 23 HIV+ALC individuals with drug history, followed by amphetamines, reported by 10 out of the 23 individuals with drug history. Use of opioids or hallucinogens was less frequently reported. Of the 26 HIV+ALC subjects, 21 had a longer reported duration of alcohol than non-marijuana drug abuse or dependence.
Rotary Pursuit Test
The Rotary Pursuit Test (Buxton and Grant, 1939; Corkin, 1968) has often been used to assess visuomotor procedural learning and memory. Using a stylus, subjects track a circle of light rotating counterclockwise on a turntable. The stylus is held directly over the circle of light without touching the glass surface. A clicking sound indicates to subjects when they go on and off the light target. Using their preferred hand, subjects were titrated to a turntable speed at which they could keep contact with the target for 5 seconds of a 20 second trial (cf. Heindel et al., 1988). This speed was retained for all trials for a given subject. Each participant received 4 learning sessions spaced over 2 test days (2 learning sessions per day), and each session consisted of 8 trials lasting 20 seconds each. A 1-minute rest period was given between trials 4 and 5 of each session. Sessions 1 and 2 and sessions 3 and 4 were separated by at least 1 hour. Sessions 2 and 3 were separated by at least 1 day; the median time being 1 day, with 2 subjects having testing days separated by more than one week, the longest being 32 days. Time on target was recorded for each trial. Total learning score was operationally defined as the difference between time on target for Session 4 (average of the 8 trials) and Session 1 (average of the 8 trials).
Ancillary Cognitive Tests
To examine the neural correlates of cognitive component processes associated with implicit memory processes subjects completed tests of attention (Wechsler Memory Scale – Revised (WMS-R); Wechsler, 1987) simple motor (Fine Finger Movement Test; Corkin, 1968), psychomotor (Symbol Digit Test; Smith, 1973), verbal and visual, immediate and delayed explicit memory (WMS-R), and balance (Walk-a-Line Ataxia Battery; Fregly et al., 1972) abilities.
MRI Scanning and Quantification
MRI data were acquired on a 3T GE scanner with a volumetric SPGR (Spoiled Gradient recalled) sequence (1.25-mm thick slices; skip=0 mm; TR=6.5s; TE=1.54ms; matrix=256×256). Dual-echo FSE (fast spin-echo) sequences were also obtained for brain extraction and automated fluid-tissue compartmentalization. All scans were read by a clinical neuroradiologist to ensure that there were no space-occupying lesions or other dysmorphology precluding automatic quantification. Brain regions of interest (ROIs) were selected based on previous reports concerning brain-behavior relationships related to visuospatial learning and motor abilities in humans. These regions were chosen from a parcellated template (http://nitc.org/projects/sri24), which was based on a published brain atlas of 24 control subjects spanning the adult age range (Rohlfing et al., 2010), both of which were created in our laboratory.
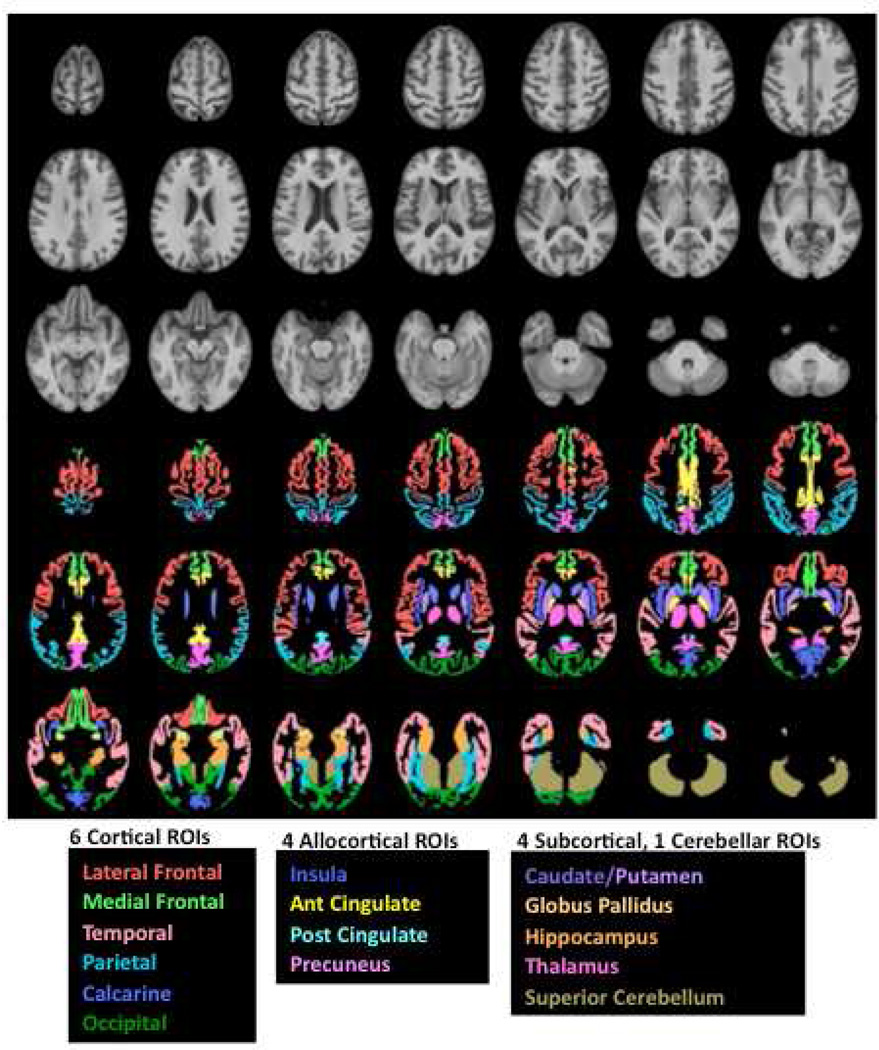
This brain atlas was semi-automatically parcellated using an already published description of anatomical brain regions (Sullivan et al., 2011) (Figure 1). Here, we examined 15 regions of interest: Six bilateral regions of cortex (lateral frontal, medial frontal, temporal, parietal, calcarine, and occipital), four allocortical regions (insula, anterior cingulate, posterior cingulate, and precuneus), and four subcortical structures (caudate/putamen, globus pallidus, hippocampus, and thalamus), as well as one cerebellar region (superior cerebellum) (Pfefferbaum et al., 2012). Gray matter volume was computed separately for each cortical region, and tissue (gray+white matter) volume was computed for each subcortical region. CSF-filled volumes of the lateral ventricles, third ventricles, frontal sulci, and Sylvian fissures were also measured. Scores are presented as age- and head size-corrected Z-scores based on a control group of 154 subjects ranging in age from 18 to 82 years (by definition, control group mean=0, sd=1). The subjects used in this study all had rotary pursuit testing (Fama et al., 2012) and these individuals consisted of a subset of the participants included in a previous MRI study (Pfefferbaum et al., 2012).
Figure 1.
Top panel in gray scale: Axial slices from the SRI24 atlas of the superior (top left) to inferior (bottom right) brain regions. Bottom panel in color: Six bilateral cortical and four allocortical gray matter regions and four subcortical tissue regions overlaid on the SRI24 atlas and color-coded by structure name. Ant, anterior; Glob, globus; Post, posterior; ROI, region of interest.
Statistical Analyses
Group differences were examined with two-tailed, unpaired t-tests. Brain-behavior relationships between visuomotor procedural learning scores and brain volumes were assessed with Pearson product-moment correlations. In light of the multiple comparisons made between ancillary cognitive and motor scores and brain volumes, a relationship was reported as significant only if it met criteria for Benjamini and Hochberg’s false discovery rate (FDR) procedure, which protects against Type I errors while also limiting Type II errors (Benjamini et al., 2001; Benjamini and Hochberg, 1995). The FDR for within-group correlational analyses was based on 8 test scores and set to an alpha level of 5% to denote significant differences and a rate of 10% to denote statistical trends. To assess the unique contribution and amount of variance in cognitive scores accounted for by selective brain regional volumes, multiple regression analyses were conducted. Prior to these analyses, scatterplots depicting the single-order correlations between statistically significant brain-behavior relationships were examined to ensure there were no outliers and that the range of both cognitive and brain scores were adequately distributed (e.g., no ceiling or floor effects).
Results
Brain Volume and Cognitive Measures
HIV+ALC had significantly smaller thalamus (t(43)=2.85, p=.007) and larger Sylvian fissure (t(43)=2.14, p=.038) volumes than controls. Modest deficits of lateral frontal (t(43)=1.92, p=.062) and parietal (t(43)=1.83, p=.074) volumes were also observed (Table 2).
Table 2.
HIV+ALV vs. NC: T-tests for regional brain volume Z scores
| t value | p | ||
|---|---|---|---|
| Lateral Frontal | 1.92 | .062 @ | HIV+ALC<NC |
| Medial Frontal | 0.06 | .955 | |
| Temporal | 0.80 | .429 | |
| Parietal | 1.83 | .074 @ | HIV+ALC<NC |
| Calcarine | 1.62 | .112 | |
| Occipital | 0.36 | .724 | |
| Superior Cerebellum | 0.62 | .540 | |
| Caudate/Putamen | 0.19 | .852 | |
| Globus Pallidus | 1.33 | .192 | |
| Insula | 0.06 | .955 | |
| Anterior Cingulum | 1.28 | .209 | |
| Posterior Cingulum | 0.27 | .789 | |
| Precuneus | 1.59 | .120 | |
| Hippocampus | 1.03 | .308 | |
| Thalamus | 2.85 | .007 ** | HIV+ALC<NC |
| Frontal Sulci | 0.98 | .335 | |
| Sylvian Fissure | 2.14 | .038 * | HIV+ALC>NC |
| Lateral Ventricles | 1.15 | .258 | |
| Third Ventricle | 0.33 | .744 |
p≤.01;
p≤.05;
p≤.10
Consistent with performance results based on the full sample of 28 HIV+ALC and 20 NC (Fama et al., 2012), this subset of HIV+ALC subjects with MRI data demonstrated visuomotor speed deficits, assessed by calibrated rpm, (t(43)=2.74, p=.009) but no impairment on rotary pursuit learning scores across the four sessions compared with controls. Deficits were observed, however, in simple motor [fine finger movement t(43)=2.55, p=.015], psychomotor [symbol-digit substitution t(30)=3.96, p=.0004], and attention and explicit memory [WMS-R subtests: attention t(29)=2.29, p=.03, verbal memory t(30)=2.79, p=.009, immediate memory t(30)2.7, p=.011, delayed memory t(30)2.71, p=.011] scores.
Correlational and Multiple Regression Analyses in HIV+ALC
Visuomotor Procedural Learning and Retention
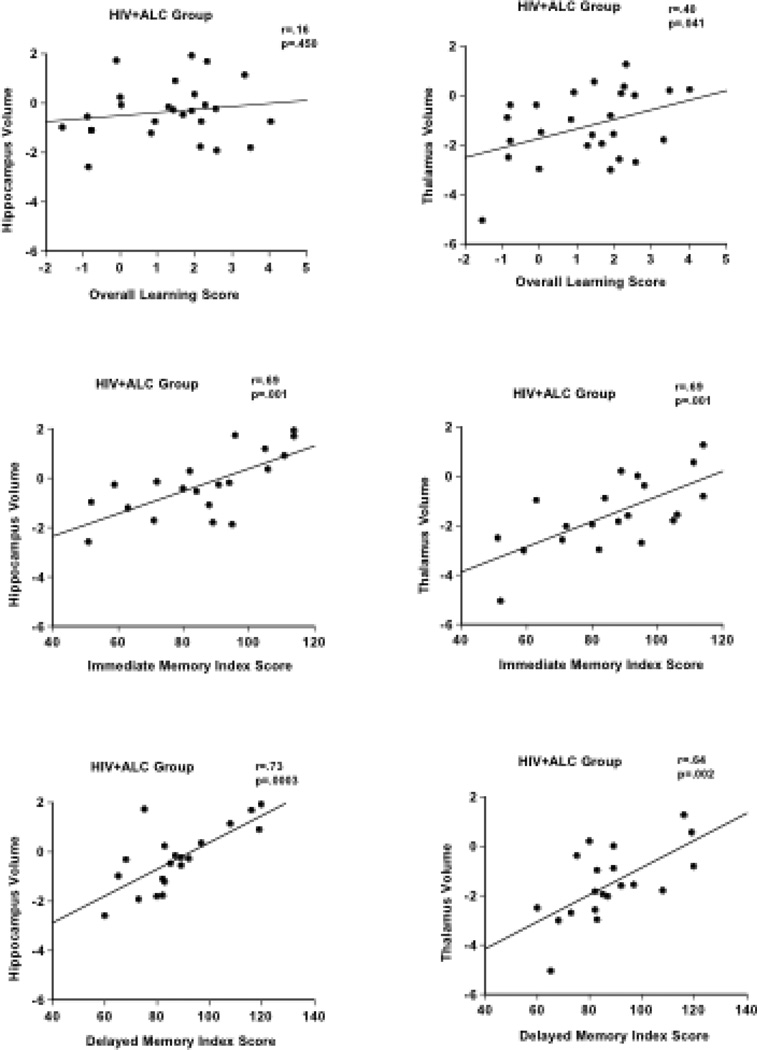
In the HIV+ALC group, lower overall learning score (Session 4 – Session 1) on rotary pursuit was significantly correlated with smaller thalamus volume (r=.40, p=.04) (Figure 2). A correlational trend was observed between overall visuomotor learning score and superior cerebellum volume (p=.36, p=.07).
Figure 2.
Scatterplots depicting the relationships between implicit and explicit memory scores and hippocampus and thalamus volumes in the HIV+ALC group. Overall Learning Scores represent the difference in seconds between Session 4 and Session 1 on the Rotary Pursuit Task. Immediate Memory Index Score and Delayed Memory Index Score are from the Wechsler Memory Scale – Revised (WMS-R).
Multiple regression analyses indicated that together the volumes of the thalamus and superior cerebellum accounted for 24.5% of the variance in overall learning scores in the HIV+ALC group. Of this portion of the variance, thalamus volume, a modest unique predictor of overall learning score, accounted for 11.3% and superior cerebellum accounted for 8.2%.
To examine the relative contributions of frontostriatal and frontocerebellar structures to overall learning on the rotary pursuit we conducted post-hoc multiple regression analysis predicting behavior score from volumes of the globus pallidus and superior cerebellum. Results indicated that neither was a unique predictor of rotary pursuit overall learning score.
Ancillary Cognitive Tests
We next investigated brain-behavior relationships in components of attention and explicit memory contributing to visuomotor procedural learning and their dissociability from other cognitive and motor processes. Accordingly, correlational analyses (FDR-corrected) were conducted between parcellated brain volumes and attention, explicit memory, simple motor, psychomotor, and balance scores in HIV+ALC (Table 3).
Table 3.
Pearson correlations between Ancillary Measures and Brain ROIs (Z-scores)
| HIV+ALC group | ||||||||
|---|---|---|---|---|---|---|---|---|
| Immediate Memory | Delayed Memory | Verbal Memory | Visual Memory | Attention | Symbol Digit | Fine Finger | Balance | |
| Lateral Frontal | −.24 | −.13 | −.25 | −.12 | −.10 | .14 | .03 | .15 |
| Medial Frontal | −.20 | −.04 | −.29 | .04 | −.01 | .02 | .01 | .17 |
| Temporal | .31 | .41 | .24 | .33 | .44 | .45 | .06 | .20 |
| Parietal | .39 | .39 | .26 | .47 | −.03 | .50 | .26 | .06 |
| Calcarine | .23 | .42 | .15 | .18 | .08 | .45 | .29 | .09 |
| Occipital | .06 | .06 | .23 | −.23 | −.34 | .06 | .02 | −.05 |
| Superior Cerebell | .02 | .19 | −.04 | .10 | .35 | −.01 | .23 | .24 |
| Caudate/Putamen | −.21 | −.31 | −.24 | −.10 | −.15 | −.13 | −.03 | −.06 |
| Globus Pallidus | .34 | .24 | .39 | .13 | .37 | .26 | .11 | .47 |
| Insula | −.13 | .00 | −.08 | −.17 | −.02 | .22 | .03 | .00 |
| Anterior Cingulum | −.10 | −.02 | −.07 | −.13 | −.23 | .02 | −.10 | −.03 |
| Posterior Cingulu | −.13 | .10 | −.17 | −.03 | −.22 | −.07 | .07 | −.07 |
| Precuneus | .38 | .63 * | .24 | .53 * | .06 | .57 * | .15 | .39 |
| Hippocampus | .69 ** | .73 ** | .67 ** | .52 @ | .22 | .52 * | .27 | .24 |
| Thalamus | .69 ** | .64 ** | .59 * | .60 * | .46 @ | .62 ** | .36 | .03 |
| Frontal Sulci | −.02 | −.13 | .02 | −.06 | .12 | −.22 | −.01 | .00 |
| Sylvian Fissure | −.21 | −.15 | −.25 | −.07 | −.04 | −.35 | −.03 | .14 |
| Lateral Ventricles | −.18 | −.35 | −.18 | −.13 | −.08 | −.21 | −.12 | −.21 |
| Third Ventricle | −.51 @ | −.30 | −.45 | −.40 | −.46 | −.54 @ | −.15 | −.26 |
False Discovery Rate implemented for ancillary measures:
p≤.01,
p≤.05,
p≤.10
n=20 for WMS-R subtests; n=26 for Symbol Digit, Fine Finger Movement, and Balance
Lower WMS-R immediate memory and delayed memory index scores were associated with smaller volumes of the hippocampus (immediate r=.69, p=.001; delayed r=.73, p=.0004) and thalamus (immediate r=.69, p=.001; delayed r=.64, p=.002). Lower delayed memory scores were also associated with smaller precuneus volumes (r=.63, p=.003). Lower WMS-R verbal memory (r=.59, p=.007) and visual memory (r=.60, p=.006) index scores were significantly correlated with smaller volumes of the thalamus. Lower verbal memory scores were also related to smaller hippocampus (r=.67, p=.001) and larger third ventricle volumes (r=−.45, p=.046), and lower visual memory scores were related to smaller precuneus volumes (r=.53, p=.02). Poorer symbol digit substitution scores correlated with smaller volumes of the precuneus (r=.57, p=.01), hippocampus (r=.52, p=.02), and thalamus (r=.62, p=.004).
Selectivity of Thalamus and Hippocampus Volumes to Memory Scores
Multiple regression analyses examined the independent contributions of brain correlates observed to be simple predictors of implicit and explicit memory scores in HIV+ALC (Table 4). Thalamus volume was modestly predictive (p=.07) of overall visuomotor procedural learning score, accounting for 14% of the variance, whereas hippocampus volume added no predictive value (0%). By contrast, both the thalamus and the hippocampus volumes were each unique predictors of immediate memory and delayed memory scores. The hippocampus accounted for 18.6% of the variance in immediate memory score (p=.008) and 24.3% of the variance in delayed memory score (p=.003); the thalamus accounted for 17.7% of the variance in immediate memory score (p=.009) and 12.3% of the variance in delayed memory score (p=.026). Although precuneus and third ventricle volumes were associated with immediate and delayed memory scores, neither made independent contributions to their prediction.
Table 4.
Multiple regressions predicting episodic and visuomotor memory in HIV+ALC
| Dependent Measure | Predictors | Beta-Coefficient | R-squared change |
|---|---|---|---|
| Rotary Pursuit | |||
| Overall Learning | Hippocampus | .02 | .00 |
| Thalamus | .40 | .14 @ | |
| Wechsler Memory Scale | |||
| Immediate Memory | Hippocampus | .48 | .19** |
| Thalamus | .47 | .18** | |
| Delayed Memory | Hippocampus | .55 | .24** |
| Thalamus | .39 | .12* | |
p<.01;
p<.05,
<.10
Examination of the relative contribution of the hippocampus and thalamus volumes to verbal memory and visual memory scores from the WMS-R indicated that the hippocampus accounted for a higher portion of the variance than the thalamus for verbal explicit memory score (hippocampus: 20.6%, p=.013; thalamus: 10.3%, p=.065), whereas the thalamus accounted for a higher portion of the variance for visual explicit memory score than the hippocampus (thalamus: 16.6%, p=.040; hippocampus: 7.9%, p=.144).
Further multiple regressions showed no significant contribution of CD4 count or lifetime alcohol consumption to these brain-behavior relationships.
Discussion
Poorer visuomotor procedural (implicit) memory in HIV+ALC was associated with smaller volumes of the thalamus and superior cerebellum, central nodes of the frontothalamocerebellar system. By contrast, explicit memory was selectively related to the hippocampus and the thalamus, nodes of the limbic system. To the extent that tissue volume contributes to function, smaller volumes of notes of these systems support these association in HIV infection with alcoholism comorbidity, a condition not marked by a frank lesion but affected by subtler brain dysmorphology.
A dissociation between implicit and explicit memory has been previously documented in other clinical populations compared with controls (Buckner et al., 1995; Fama et al., 2006; Heindel et al., 1989). Fama and colleagues (Fama et al., 2006) demonstrated this type of dissociation in Korsakoff’s Syndrome, where despite severe explicit memory impairment, KS subjects showed relatively intact visuoperceptual implicit memory across sessions compared with controls. Thus, even in instances where deficits in individual cognitive component processes are observable, overall learning on implicit memory tasks can be relatively intact. Further, dissociations regarding the relevance of the hippocampus to implicit and explicit memory processes within clinical groups have been reported (Bondi and Kaszniak, 1991; Bylsma et al., 1991; Carlesimo and Oscar-Berman, 1992). The present study extends this brain-behavior dissociation, a selective relationship between the normal age-expected volume of the hippocampus and explicit memory, but not implicit memory, to HIV infection with alcoholism comorbidity.
The results of this study are also consistent with previous reports of thalamic lesions affecting nondeclarative motor skill learning and explicit memory processes (Exner et al., 2001). To the extent that the thalamus is a neural substrate of attentional processes (Sturm and Willmes, 2001), these results suggest that attentional processes are likely relevant to visuomotor procedural memory and are consistent with our behavioral findings in visuospatial procedural (implicit) memory in HIV+ALC (Fama et al., 2012).
Although dissociable processes, implicit and explicit memory often work in tandem in the learning of information, supporting different phases of acquisition, storage, and retention. The thalamus and medial temporal lobe, particularly the hippocampus and surrounding structures, have rich connections that have been identified in human and animal studies (Aggleton et al., 2010; Aggleton and Saunders, 1997). Together, these diencephalic and medial temporal lobe structures are considered the “extended hippocampal system.” Connectivity between the thalamus and hippocampus has also been demonstrated with functional MRI (Metzger et al., 2013; Stein et al., 2000). It has further been hypothesized that the interactions between the thalamus and the hippocampus are reciprocal, with the thalamus having influence over hippocampal function and being vital for episodic memory processes (Aggleton et al., 2010; Van der Werf et al., 2000). The present study demonstrates that, although interconnected, the thalamus and the hippocampus make independent contributions to episodic memory processes.
Speculation on the role of the thalamus in mnemonic processes has precedent (Cohen, 1984; Crosson, 1992; Exner et al., 2001; Harding et al., 2000; Squire, 1982). Individuals with Wernicke-Kosakoff’s Syndrome (WKS), a neurological condition arising from thiamine depletion (Thomson et al. 2010; Victor et al., 1989) and resulting in neuronal damage (Harding et al., 2000), and survivors of thalamic strokes suffer as severe an explicit memory impairment as observed in individuals with strokes invading the hippocampal region, further supporting a role of the thalamus in explicit mnemonic processes (Carlesimo et al. 2011; Harding et al., 2000; Serra et al., 2013). Additionally, evidence for the contribution of the thalamus and related networks to memory processes was demonstrated in a resting state functional MRI study, which reported positive and selective relationship between functional connectivity of the left mammillothalamic tract and verbal memory scores in an individual with KS after high-dose thiamine replacement therapy (Kim et al., 2010). Taken together, these results demonstrate that the thalamus, previously heralded as a sensory and motor relay station between cerebellar and subcortical structures and cortical association areas (Alexander et al., 1986), may also play a vital role in memory functioning.
The absence of selective brain-behavior relationships observed herein between regional brain volumes and implicit memory compared with explicit memory is consistent with the position that implicit memory processes may depend more on disturbance within processing systems across the brain rather than to a single circumscribed neural system (Reber, 2013). Reber proposed that the inability to demonstrate a true double dissociation between implicit and explicit memory may be due to an absence of an “implicit memory system” per se; implicit learning would thus occur as a result of a general, pervasive plasticity within processing networks, demonstrated by improved functions after repeated experience. This neural plasticity allows an individual to learn from experiences, adapting more efficient cognitive and motor responses (Reber, 2013). Thus, learning and memory of visuomotor procedural skills may rely more on neural mechanisms at the local level of neural networks subserving the component processes of the task to be learned than tied to a particular brain region or structure.
Examination of individuals with HIV infection and alcoholism allows testing dissociable neural structures and systems implicated in visuomotor procedural learning. Because of the common occurrence of non-alcohol drug-related abuse and dependence in individuals recruited for their alcohol use disorder, caution is warranted in interpreting the selective contribution of alcohol to the deficits observed. In an effort to disentangle the relative contributions of alcohol versus other substances to the brain-behavior relationships observed, we conducted several post-hoc analyses. Of the 26 HIV+ALC subjects, 21 reported use of both alcohol and illicit substances (excluding marijuana), but alcohol use disorder was primary in that it was of longer duration than the non-alcohol substance-related diagnosis. The remaining 5 subjects had a duration of substance-related diagnosis (excluding marijuana) that was similar or exceeded the length of their alcohol-related diagnosis. Statistical analysis excluding these 5 subjects yielded the identical pattern of results reported: hippocampal volumes were related to explicit but not implicit memory scores, whereas thalamus volumes were related to both explicit and implicit memory scores. Similarly, multiple regressions again indicated that both the thalamus and the hippocampus were unique and independent predictors of immediate and delayed explicit memory scores. Although the relative contribution of alcohol vs. drug use disorders to the memory deficits observed in HIV+ALC here cannot be determined, the pattern of results was the same with and without individuals whose duration of drug use rivaled the duration of their alcohol use. Thus, we cannot rule out the possibility that non-alcohol drug use affects memory processes, but these supplementary analyses provide support to the dissociation between implicit and explicit memory processes and associated structural brain volumes in HIV with primary alcoholism comorbidity.
In conclusion, this study demonstrates the dissociability of neural compromise underlying deficits in procedural memory and explicit memory processes in HIV infection with primary chronic alcoholism. Further, this study provides evidence of the selectivity of brain-behavior relationships in HIV infection with substance use comorbity, and highlights the relevance of extra-hippocampal structures, namely the thalamus, to memory functions.
Acknowledgment
This research was supported by grants from the National Institute on Alcohol Abuse and Alcoholism AA017347, AA005965, AA010723, and AA017168.
References
- Aggleton JP, O'Mara SM, Vann SD, Wright NF, Tsanov M, Erichsen JT. Hippocampal-anterior thalamic pathways for memory: uncovering a network of direct and indirect actions. Eur J Neurosci. 2010;31(12):2292–2307. doi: 10.1111/j.1460-9568.2010.07251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggleton JP, Saunders RC. The relationships between temporal lobe and diencephalic structures implicated in anterograde amnesia. Memory. 1997;5(1–2):49–71. doi: 10.1080/741941143. [DOI] [PubMed] [Google Scholar]
- Alexander G, DeLong M, Strick P. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annual Review of Neuroscience. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Benjamini Y, Drai D, Elmer G, Kafkafi N, Golani I. Controlling the false discovery rate in behavior genetics research. Behav Brain Res. 2001;125(1–2):279–284. doi: 10.1016/s0166-4328(01)00297-2. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to mulitple testing. J Royal Stat Soc Ser B. 1995;57(1):289–300. [Google Scholar]
- Bondi MW, Kaszniak AW. Implicit and explicit memory in Alzheimer's disease and Parkinson's disease. Journal of Clinical and Experimental Neuropsychology. 1991;13:339–358. doi: 10.1080/01688639108401048. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Petersen SE, Ojemann JG, Miezin FM, Squire LR, Raichle ME. Functional anatomical studies of explicit and implicit memory retrieval tasks. Journal of Neuroscience. 1995;15(1 Part 1):12–29. doi: 10.1523/JNEUROSCI.15-01-00012.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxton CE, Grant DA. Retroaction and gains in motor learning: II. Sex differences, and a further analysis of gains. Journal of Experimental Psychology. 1939;25(2):198–208. [Google Scholar]
- Bylsma FW, Rebok GW, Brandt J. Long-term retention of implicit learning in Huntington's disease. Neuropsychologia. 1991;29:1213–1221. doi: 10.1016/0028-3932(91)90035-7. [DOI] [PubMed] [Google Scholar]
- Carlesimo G, Oscar-Berman M. Memory deficits in Alzheimer patients: A comprehensive review. Neuropsychology Review. 1992;3:119–169. doi: 10.1007/BF01108841. [DOI] [PubMed] [Google Scholar]
- Carlesimo GA, Lombardi MG, Caltagirone C. Vascular thalamic amnesia: a reappraisal. Neuropsychologia. 2011;49(5):777–789. doi: 10.1016/j.neuropsychologia.2011.01.026. [DOI] [PubMed] [Google Scholar]
- Castelo JM, Sherman SJ, Courtney MG, Melrose RJ, Stern CE. Altered hippocampal-prefrontal activation in HIV patients during episodic memory encoding. Neurology. 2006;66(11):1688–1695. doi: 10.1212/01.wnl.0000218305.09183.70. [DOI] [PubMed] [Google Scholar]
- Chang L, Speck O, Miller EN, Braun J, Jovicich J, Koch C, Itti L, Ernst T. Neural correlates of attention and working memory deficits in HIV patients. Neurology. 2001;57(6):1001–1007. doi: 10.1212/wnl.57.6.1001. [DOI] [PubMed] [Google Scholar]
- Clifford DB, Ances BM. HIV-associated neurocognitive disorder. Lancet Infect Dis. 2013;13:976–986. doi: 10.1016/S1473-3099(13)70269-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen NJ. Preserved learning capacity in amnesia: Evidence for multiple memory systems. In: Squire LR, Butters N, editors. Neuropsychology of Memory. New York: Guilford Press; 1984. [Google Scholar]
- Corkin S. Acquisition of motor skills after bilateral medial temporal lobe excision. Neuropsychologia. 1968;6:255–256. [Google Scholar]
- Crosson B. Subcortical Functions in Language and Memory. New York: The Guilford Press; 1992. [Google Scholar]
- Doyon J, Penhune V, Ungerleider LG. Distinct contribution of the corticostriatal and cortico-cerebellar systems to motor skill learning. Neuropsychologia. 2003;41(3):252–262. doi: 10.1016/s0028-3932(02)00158-6. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Cohen NJ. From Conditioning to Conscious Recollection. Oxford: Oxford University Press; 2001. [Google Scholar]
- Eslinger PJ, Damasio AR. Preserved motor learning in Alzheimer's disease: Implications for anatomy and behavior. Journal of Neuroscience. 1986;6:3003–3009. doi: 10.1523/JNEUROSCI.06-10-03006.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exner C, Weniger G, Irle E. Implicit and explicit memory after focal thalamic lesions. Neurology. 2001;57:2054–2063. doi: 10.1212/wnl.57.11.2054. [DOI] [PubMed] [Google Scholar]
- Fama R, Pfefferbaum A, Sullivan EV. Visuoperceptual priming in alcoholic Korsakoff Syndrome. Alcoholism: Clinical and Experimental Research. 2006;30:680–687. doi: 10.1111/j.1530-0277.2006.00085.x. [DOI] [PubMed] [Google Scholar]
- Fama R, Rosenbloom MJ, Sassoon SA, Pfefferbaum A, Sullivan EV. Differential effect of alcoholism and HIV infection on visuomotor procedural learning and retention. Alcohol Clin Exp Res. 2012;36(10):1738–1747. doi: 10.1111/j.1530-0277.2012.01790.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID) Version 2.0. New York, NY: Biometrics Research Department, New York State Psychiatric Institute; 1998. [Google Scholar]
- Fregly AR, Graybiel A, Smith MS. Walk on floor eyes closed (WOFEC): A new addition to an ataxia test battery. Aerospace Medicine. 1972;43(4):395–399. [PubMed] [Google Scholar]
- Gonzalez R, Jacobus J, Amatya AK, Quartana PJ, Vassileva J, Martin EM. Deficits in complex motor functions, despite no evidence of procedural learning deficits, among HIV+ individuals with history of substance dependence. Neuropsychology. 2008;22(6):776–786. doi: 10.1037/a0013404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafton ST, Mazziotta JC, Presty S, Friston KJ, Frackowiak RSJ, Phelps ME. Functional Anatomy of Human Procedural Learning Determined with Regional Cerebral Blood Flow and PET. Journal of Neuroscience. 1992;12(7):2542–2548. doi: 10.1523/JNEUROSCI.12-07-02542.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding A, Halliday G, Caine D, Kril J. Degeneration of anterior thalamic nuclei differentiates alcoholics with amnesia. Brain. 2000;123(Pt 1):141–154. doi: 10.1093/brain/123.1.141. [DOI] [PubMed] [Google Scholar]
- Harper CG, Kril JJ. Neuropathological changes in alcoholics. In: Hunt WA, Nixon SJ, editors. Alcohol Induced Brain Damage: NIAAA Research Monograph No. 22. Rockville, MD: National Institute of Health; 1993. pp. 39–69. [Google Scholar]
- Heaton R, Franklin D, Ellis R, McCutchan J, Letendre S, LeBlanc S, Corkran S, Duarte N, Clifford D, Woods S, Collier A, Marra C, Morgello C, Mindt M, Taylor M, Marcotte T, JH A, Wolfson T, Gelman B, McArthur J, Simpson D, Abramson I, Gamst A, Fennema-Notestine C, Jernigan T, Wong J, Grant I. HIVassociated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. Journal of Neurovirology. 2011;17:3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heindel WC, Butters M, Salmon DP. Impaired learning of a motor skill in patients with Huntington's disease. Behavioral Neuroscience. 1988;102(1):141–147. doi: 10.1037//0735-7044.102.1.141. [DOI] [PubMed] [Google Scholar]
- Heindel WC, Salmon DP, Shults CW, Walicke PA, Butters N. Neuropsychological evidence for multiple implicit memory systems: A comparison of Alzheimer's, Huntington's, and Parkinson's disease patients. The Journal of Neuroscience. 1989;9:582–587. doi: 10.1523/JNEUROSCI.09-02-00582.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnofsky DA. The clinical evaluation of chemotherapeutic agents in cancer. In: MacLeod CM, editor. Evaluation of Chemotherapeutic Agents. New York: Columbia University Press; 1949. pp. 191–205. [Google Scholar]
- Kim E, Ku J, Jung Y-C, Lee H, Kim SI, Kim J-J, Namkoong K, Song D-H. Restoration of mammilothalamic functional connectivity through thiamine replacement therapy in Wernicke's encephalopathy. Neuroscience Letters. 2010;479:257–261. doi: 10.1016/j.neulet.2010.05.074. [DOI] [PubMed] [Google Scholar]
- Maki PM, Cohen MH, Weber K, Little DM, Fornelli D, Rubin LH, Perschler P, Gould F, Martin E. Impairments in memory and hippocampal function in HIVpositive vs HIV-negative women: a preliminary study. Neurology. 2009;72(19):1661–1668. doi: 10.1212/WNL.0b013e3181a55f65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki PM, Martin-Thormeyer E. HIV, cognition and women. Neuropsychology Review. 2009;19:204–214. doi: 10.1007/s11065-009-9093-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Thompson RF. Cerebellum: Essential involvement in the classically conditioned eyelid response. Science. 1984;223:296–299. doi: 10.1126/science.6701513. [DOI] [PubMed] [Google Scholar]
- Metzger CD, van der Werf YD, Walter M. Functional mapping of thalamic nuclei and their integration into cortico-striatal-thalamo-cortical loops via ultra-high resolution imaging-from animal anatomy to in vivo imaging in humans. Front Neurosci. 2013;7:24. doi: 10.3389/fnins.2013.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson HE. The National Adult Reading Test (NART) Windsor, Canada: Nelson Publishing Company; 1982. [Google Scholar]
- Pfefferbaum A, Rosenbloom MJ, Sassoon SA, Kemper CA, Deresinski S, Rohlfing T, Sullivan EV. Regional brain structural dysmorphology in human immunodeficiency virus infection: effects of acquired immune deficiency syndrome, alcoholism, and age. Biol Psychiatry. 2012;72(5):361–370. doi: 10.1016/j.biopsych.2012.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reber P. The neural basis of implicit learning and memory: a review of neuropsychological and neuroimaging research. Neuropsychologia. 2013;51:2026–2042. doi: 10.1016/j.neuropsychologia.2013.06.019. [DOI] [PubMed] [Google Scholar]
- Rohlfing T, Zahr NM, Sullivan EV, Pfefferbaum A. The SRI24 multi-channel atlas of normal adult human brain structure. Human Brain Mapping. 2010;31(5):798–819. doi: 10.1002/hbm.20906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothlind JC, Greenfield TM, Bruce AV, Meyerhoff DJ, Flenniken DL, Lindgren JA, Weiner MW. Heavy alcohol consumption in individuals with HIV infection: effects on neuropsychological performance. J Int Neuropsychol Soc. 2005;11(1):70–83. doi: 10.1017/S1355617705050095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint-Cyr JA, Taylor AE, Lang AE. Procedural learning and neostriatal dysfunction in man. Brain. 1988;111:941–959. doi: 10.1093/brain/111.4.941. [DOI] [PubMed] [Google Scholar]
- Sassoon SA, Rosenbloom MJ, Fama R, Sullivan EV, Pfefferbaum A. Selective neurocognitive deficits and poor life functioning are associated with significant depressive symptoms in alcoholism-HIV infection comorbidity. Psychiatry Res. 2012;199(2):102–110. doi: 10.1016/j.psychres.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidtke K, Manner H, Kaufmann R, Schmolck H. Cognitive procedural learning in patients with fronto-striatal lesions. Learning and Memory. 2002;9(6):419–429. doi: 10.1101/lm.47202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra L, Cercignani M, Carlesimo GA, Fadda L, Tini N, Giulietti G, Caltagirone C, Bozzali M. Connectivity-based parcellation of the thalamus explains specific cognitive and behavioural symptoms in patients with bilateral thalamic infarct. PLoS One. 2013;8(6):e64578. doi: 10.1371/journal.pone.0064578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner HA. Development and Validation of a Lifetime Alcohol Consumption Assessment Procedure. Toronto, Canada: Addiction Research Foundation; 1982. [Google Scholar]
- Skinner HA, Sheu WJ. Reliability of alcohol use indices: The lifetime drinking history and the MAST. Journal of Studies on Alcohol. 1982;43:1157–1170. doi: 10.15288/jsa.1982.43.1157. [DOI] [PubMed] [Google Scholar]
- Smith A. The Symbol Digit Modalities Test Manual. Los Angeles: Western Psychological Services; 1973. [Google Scholar]
- Squire LR. Comparisons between forms of amnesia: some deficits are unique to Korsakoff's syndrome. Journal of Experimental Psychology: Learning, Memory and Cognition. 1982;8:560–571. doi: 10.1037//0278-7393.8.6.560. [DOI] [PubMed] [Google Scholar]
- Stein T, Moritz C, Quigley M, Cordes D, Haughton V, Meyerand E. Functional connectivity in the thalamus and hippocampus studied with functional MR imaging. AJNR Am J Neuroradiol. 2000;21(8):1397–1401. [PMC free article] [PubMed] [Google Scholar]
- Sturm W, Willmes K. On the functional neuroanatomy of intrinsic and phasic alertness. Neuroimage. 2001;14(1 Pt 2):S76–S84. doi: 10.1006/nimg.2001.0839. [DOI] [PubMed] [Google Scholar]
- Sullivan EV. Compromised pontocerebellar and cerebellothalamocortical systems: speculations on their contributions to cognitive and motor impairment in nonamnesic alcoholism. Alcoholism: Clinical and Experimental Research. 2003;27(9):1409–1419. doi: 10.1097/01.ALC.0000085586.91726.46. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A, Rohlfing T, Baker FC, Padilla ML, Colrain IM. Developmental change in regional brain structure over 7 months in early adolescence: Comparison of approaches for longitudinal atlas-based parcellation. NeuroImage. 2011;57:214–224. doi: 10.1016/j.neuroimage.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson AD, Marshall EJ, Guerrini I. Biomarkers for detecting thiamine deficiency--improving confidence and taking a comprehensive history are also important. Alcohol Alcohol. 2010;45(2):213. doi: 10.1093/alcalc/agq004. [DOI] [PubMed] [Google Scholar]
- Tranel D, Damasio AR, Damasio H, Brandt JP. Sensorimotor skill learning in amnesia: additional evidence for the neural basis of nondeclarative memory. Learning and Memory. 1994;1(3):165–179. [PubMed] [Google Scholar]
- Van der Werf YD, Witter MP, Uylings HB, Jolles J. Neuropsychology of infarctions in the thalamus: a review. Neuropsychologia. 2000;38(5):613–627. doi: 10.1016/s0028-3932(99)00104-9. [DOI] [PubMed] [Google Scholar]
- Victor M, Adams RD, Collins GH. The Wernicke-Korsakoff Syndrome and Related Neurologic Disorders Due to Alcoholism and Malnutrition. 2nd edition. Philadelphia: F.A. Davis Co; 1989. [Google Scholar]
- Wechsler D. Wechsler Memory Scale - Revised. San Antonio, TX: The Psychological Corporation; 1987. [Google Scholar]
- Woods S, Moore D, Weber E, Grant I. Cognitive neuropsychology of HIV associated neurocognitive disorders. Neuropsychology Review. 2009;19(2):152–168. doi: 10.1007/s11065-009-9102-5. [DOI] [PMC free article] [PubMed] [Google Scholar]