Abstract
Fasting in rodents is characterized by decreases in serum T4 and T3 levels but no compensatory increase in serum TSH level. The types 1 and 2 deiodinases (D1 and D2) are postulated to play key roles in mediating these changes. However, serum T4 and T3 levels in fasted 5′-deiodinase-deficient mice decreased by at least the same percentage as that observed in wild-type mice, whereas serum TSH level was unaffected. D3 activity was increased in kidney, muscle, and liver up to 4-fold during fasting, and the mean serum rT3 level was increased 3-fold in fasted D1-deficient mice, compared with fed animals. In wild-type mice, the tissue contents of T4 and T3 in liver, kidney, and muscle were unchanged or increased in fasted animals, and after the administration of [125I]T4 or [125I]T3, the radioactive content in the majority of tissues from fasted mice was increased 2- or 4-fold, respectively. These findings suggest that the observed fasting-induced reductions in the circulating T3 and T4 levels are mediated in part by increased D3 activity and by the sequestration of thyroid hormone and their metabolites in tissues. Studies performed in D3-deficient mice demonstrating a blunting of the fasting-induced decrease in serum T4 and T3 levels were consistent with this thesis. Thus, the systemic changes in thyroid hormone economy as a result of acute food deprivation are not dependent on the D1 or D2 but are mediated in part by sequestration of T4 and T3 in tissues and their enhanced metabolism by the D3.
Numerous studies have shown that fasting in humans and rodents results in marked changes in thyroid hormone (TH) economy. These include decreases in total and free levels of T3 in serum accompanied by an inappropriately normal or reduced serum TSH level (1–7). In rodents, fasting also results in decreases in total and free T4 levels in serum (8, 9). Changes have also been reported in the transcriptional activity of several TH-responsive genes in the fasted rodent liver that are similar to those observed in hypothyroidism (10, 11), suggesting that the T3 content in at least some tissues is also reduced in fasting (10). Because fasting also results in a decrease in the basal metabolic rate in both humans (12) and rodents (13), it has been suggested that these changes are an adaptive response aimed at preserving energy reserves. However, the actual physiological consequences of these alterations in TH economy remain poorly defined.
The mechanisms responsible for the decrease in serum TH levels during fasting remain enigmatic. However, evidence in fasted rodents suggests that thyroidal secretion of T4 is decreased (5, 14, 15), due in part to the leptin/neuropeptide Y pathway mediating suppression of TRH and, hence, TSH secretion (16). In the hypothalamus, increased activity of the type 2 deiodinase (D2), which converts T4 to T3 via 5′-deiodination (5′D), has been observed in fasting and postulated to augment TRH suppression (17, 18).
Changes in the handling and/or metabolism of TH in peripheral tissues have also been implicated. However, it appears that the clearance rates of TH from serum are not enhanced in fasting humans or rodents (2, 14, 19, 20), and indeed, the uptake of T3 into some tissues may be impaired (21). Thus, if changes in peripheral TH economy do contribute to the fasting-induced fall in circulating TH levels, this must result from a diminished flux of T4 and/or T3 from tissues to plasma, due to sequestration and/or alterations in their metabolism in tissues.
In liver, fasting results in a decrease in D1 activity, which catalyzes the 5′D of T4 to T3 (22, 23). This finding has been postulated to contribute to the decrease in serum T3 level during fasting (22, 23). In addition, increased activity of the D3, which catalyzes 5-deiodination (5D), could result in accelerated inactivation of T4 and T3 by converting them to rT3 and 3,3′-diiodothyronine, respectively (24). Finally, the expression of several sulfotransferases and the uridine diphosphate-glucuronosyltransferase 1 family polypeptide A1 (UGT1A1) are also increased in the liver by fasting (16, 25), and this may further enhance the metabolism and inactivation of T4. The extent to which these changes in enzymatic activity, as determined in vitro, influence tissue TH content and metabolism and contribute to the serum TH changes observed in fasting is unknown.
The goal of the present study was to determine the contribution of peripheral TH metabolism in the fasting response using wild-type (WT) mice and mice deficient in 1 or more of the 3 deiodinases.
Materials and Methods
Animal and treatments
Experiments were performed in 10- to 16-week-old WT mice and mice completely deficient in the D1, the D2, or both (D1 knockout [D1KO], D2KO, and D1/D2KO mice). These animals were in the C57Bl/6 background, and WT and homozygous mutant mice were bred from parents of the corresponding genotype. In other studies, D3-deficient (D3KO) mice were used. Because D3−/− mice in the C57Bl/6 background generally do not survive birth, D3KO and WT mice were F1 littermates generated by the breeding of a D3+/− C57Bl/6 father and a D3+/− 129/SvJ mother. Male mice were used in all experiments.
Mice were bred and housed in the barrier section of Dartmouth Medical School's animal facility, under conditions of controlled lighting, 12-hour light, 12-hour dark cycle, and temperature (22 ± 1°C). Animal protocols were approved by the Institutional Animal Care and Use Committee.
In one series of experiments, mice were fasted for periods ranging from 16 to 36 hours. Comparable mice received food ad libitum. All mice had free access to water. After the fasting period, the mice were euthanized with CO2, exsanguinated via the inferior vena cava, and serum, kidneys, liver, muscle, skin, brown adipose tissue (BAT), pituitary, and brain were harvested. In some cases, the hypothalamus, cerebellum, and cerebral cortex (CCx) were separated from the rest of the brain. All tissues were frozen on dry ice and stored at −80°C for subsequent study.
In a second series of experiments, WT mice were place in wire-bottomed cages that permitted the separate collection of urine and feces. Half the mice were fasted and half had free access to food. All had access to water. In the first set of these experiments, food was withdrawn from fasted animals at 9 am, and the fed and fasted animals were injected at 1 pm the same day with [125I]T4, [125I]T3, or [125I]rT3 (specific activities ∼969, ∼2200, and ∼546 Ci/mmol, respectively). Mice were then euthanized at 9 am the day after, 24 hours after food withdrawal from the experimental group. In the second set of these experiments, all animals were injected with the designated radiolabeled hormone at 9 am followed by food withdrawal from fasted animals at 11 am and killed 24 hours later. In this series of studies, all tissues, including blood, were harvested, and the total radioactivity in tissues, urine, and feces determined in a γ-counter (model 1195; Amersham Searle). Because these compounds were labeled only at the outer ring position, any release of [125I]iodide represented 5′D. The labeled hormones were obtained from PerkinElmer, Inc and purified by chromatography using Sephadex LH-20 (Sigma) before injection.
Determination of 5′D and 5D activities
5′D and 5D activities were assayed according to published methods (26, 27). D1 activity was determined using [125I]rT3 as substrate plus or minus 1mM 6-n-propylthiouracil (PTU) to inhibit D2 activity. D2 and D3 activities were determined using [125I]T4 and [125I]T3 as substrates, respectively. Deiodination is expressed as pmol (D1) or fmol (D2 and D3) of product generated/h·mg of protein, and in pituitary as fmol/h per pituitary.
Extraction of TH from tissues for RIA
THs were extracted from pieces of liver, kidney, and muscle into 95% methanol containing 0.1mM PTU, as previously described (28). To release TH from their glucuronide conjugates (eg, T4-glucuronide [T4g]) in kidney and liver (28, 29), the dried extracts were resuspended in 1 mL of 75mM sodium phosphate buffer (pH 6.5) and incubated with 100 U of β-glucuronidase (type 1X-A from Escherichia coli, sulfatase free; Sigma) at 37°C for 1 hour. The mixture was then evaporated to dryness.
For extraction of TH from hypothalamus and pituitary, same procedure was scaled down appropriately.
Assay of iodothyronines in serum and tissue samples
Total serum T4 and T3 concentrations were determined using the Coat-A-Count RIA total T4 and total T3 kits (Diagnostic Services Laboratory, Inc). Relative levels of serum TH binding activity were determined using the Coat-A-Count T3 uptake kit (Diagnostic Services Laboratory, Inc). The values obtained for %T3 uptake were used with the corresponding serum T4 and T3 concentrations to calculate the free T4 and T3 indices. All kits were used according to the manufacturer's instructions.
Serum rT3 concentration was measured using the same RIA procedures used to measure TH content in the tissue extracts, as previously described (30). The rT3 antibody was a gift from Dr L. Braverman (31) and ultrapure unlabeled rT3 (Henning Co) was used as the standard. The assay sensitivity was approximately 2 pg per tube. Serum TSH levels were determined by Dr A. F. Parlow (32).
The T4 and T3 contents of tissue extracts were determined using our highly sensitive RIA procedures (3). The antibodies were obtained from a commercial source (T3, catalog no. 20-TR45, cross-reactivity with T4, 0.38%; T4, catalog no. 20-TR40, cross-reactivity with T3, 7.5%; Fitzgerald Industries International, Inc). The dried tissue extract from 25-mg wet tissue weight was dissolved in 1.0 mL of RIA buffer. Preliminary tests indicated that up to 20 μL of sample could be assayed before linearity with the standard curve was lost. Thus, 10 μL of sample was used in the assay. Assay sensitivity was approximately 2 pg per tube for T3 and 4 pg per tube for T4.
Isolation of labeled iodothyronines from serum and tissues for analysis by paper chromatography
The labeled iodothyronines present in serum, liver, and kidney were purified and concentrated for chromatographic analysis using a Dowex-1 anion exchange resin as previously described (33). The distribution of the radioactivity was tracked throughout by counting aliquots of the original homogenate and eluted fractions. Chromatographic analysis on paper strips was carried out using tertiary amyl alcohol/2N NH3 (1:1) as the solvent system. Additional strips containing [125I]T3 and [125I]T4 as markers were included. The strips were dried and the locations of the labeled compounds on the strips determined by exposure to x-ray film.
Statistical analysis
Statistical significance between groups was determined by the two-tailed Student's t test. Comparisons between more than 2 groups were performed by one-way ANOVA followed by Fisher's least significant difference (protected t test) test or Dunnett's analysis. Comparison of the differences in the decreases in serum TH levels in fasted vs fed WT and D3KO animals was performed using a two-way ANOVA.
Results
Deiodinase activities in tissues of fed and fasted mice
To confirm and extend previous reports concerning the effects of fasting on deiodinase activities, the levels of D1 and D2 activity were determined in 9 tissues obtained from WT-fed mice and mice fasted for 30 hours. As in previous studies (22, 23), the level of D1 activity was significantly reduced in liver from fasted mice (fed vs fasted, 29.2 ± 3.5 vs 9.7 ± 2.7 pmol/h·mg of protein; P < .005), and D1 activity was also reduced in the pituitary (fed vs fasted, 69.0 ± 8.0 vs 30.0 ± 6.3 fmol/h per pituitary; P < .005). In contrast, fasting did not significantly affect the level of D1 activity in kidney (fed vs fasted, 2.20 ± 0.24 vs 2.03 ± 0.23 pmol/h·mg of protein). No D1 activity was detected in skeletal muscle, skin, BAT, cerebrum, CCx, or hypothalamus.
Fasting resulted in a significantly reduction in the level of D2 activity in pituitary (fed vs fasted, 199 ± 19 vs 56 ± 14 fmol/h per pituitary; P < .001) and BAT (fed vs fasted, 58.3 ± 6.0 vs 18.9 ± 7.2 fmol/h·mg of protein; P < .005) but had no significant effect on the level in the cerebrum (fed vs fasted, 24.5 ± 1.0 vs 31.0 ± 5.8 fmol/h·mg of protein) and CCx (fed vs fasted, 24.0 ± 3.6 vs 17.0 ± 1.3 fmol/h·mg of protein). The level of D2 activity was also unaffected after fasting in the hypothalamus (fed vs fasted, 32.0 ± 8.3 vs 30.0 ± 3.6 fmol/h·mg of protein), even though the corresponding mRNA level was significantly increased (fed vs fasted, 0.9 ± 0.4 vs 2.0 ± 0.1 D2 mRNA/cyclophilin mRNA; P < .025). No D2 activity was detected in liver, kidney, muscle, or skin.
Fasting resulted in a marked and significant increase in the level of D3 activity in skin, kidney, and muscle of WT mice and in kidney and muscle of D1/D2KO mice (Figure 1). These tissues normally exhibit a low level of D3 activity. In contrast, D3 activity in several brain regions that express a relatively high level of this enzyme was not increased in the fasted WT mouse. In the liver, D3 activity was undetectable in WT animals and minimal during fasting (<1 fmol/h·mg of protein).
Figure 1.
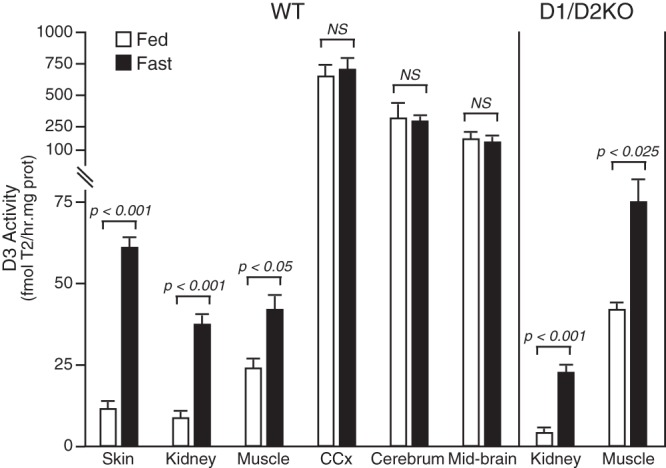
D3 activity in tissues of fed and fasted WT and D1/D2KO mice. Fasted mice were without food for 30 hours. Bars represent the mean ± SEM of values obtained in a minimum of 5 mice per group. NS, not significant.
Serum iodothyronine and TSH levels in fed and fasted WT, D1KO, D2KO, and D1/D2KO mice
As shown in Table 1, a 30-hour fast resulted in approximately a 16% decrease in body weight in all 4 genotypes. The serum T4 level was reduced by 42% in the fasted WT mice, and a comparable decrease occurred in all 3 5′-deiodinase-deficient genotypes. The serum T3 level was also decreased in the fasted WT mice, and a decrease at least as marked occurred in the deiodinase-deficient mice. Despite these substantial fasting-induced decreases in the serum TH levels, the serum TSH level was not increased, relative to fed animals, in any of the genotypes. As shown previously (30), the serum rT3 level is elevated in D1KO and D1/D2KO mice (Table 1). In these mice, the level was elevated an additional 3-fold during fasting (Table 1). In the WT and D2KO mice, in which the basal serum rT3 level was relatively low, fasting did result in a nonsignificant increase in the mean serum rT3 level. However, in a subsequent experiment with WT mice, a significant increase was observed in this parameter in fasted animals (WT fed vs WT fasted, 33 ± 17 vs 169 ± 50 ng/100 mL; P < .05). In this additional experiment, serum T3 and T4 levels again declined to a degree comparable with those shown in Table 1.
Table 1.
Serum Iodothyronine and TSH Levels in Fed and Fasted (30 h) WT Mice and Mice Lacking D1 and/or D2 Activity
| Group | Body weight (% decrease) | T4 (μg/100 mL) | T3 (ng/100 mL) | rT3 (ng/100 mL) | TSH (ng/mL) |
|---|---|---|---|---|---|
| WT fed (5) | 0 | 3.7 ± 0.1 | 91 ± 3 | 15 ± 3.3 | 132 ± 12 |
| WT fast (5) | 16.5 ± 0.9 | 2.1 ± 0.2 | 70 ± 7 | 32 ± 18.1 | 120 ± 16 |
| P value | P < .001 | P < .001 | P < .025 | ns | ns |
| Fast-induced change | 42% decrease | 23% decrease | |||
| D1KO fed (6) | 0 | 4.7 ± 0.4 | 86 ± 5 | 140 ± 24 | 116 ± 8 |
| D1KO fast (7) | 16.5 ± 1.0 | 2.9 ± 0.4 | 44 ± 8 | 414 ± 22 | 118 ± 7 |
| P value | P < .001 | P < .005 | P < .001 | P < .001 | ns |
| Fast-induced change | 38% decrease | 49% decrease | 3-fold increase | ||
| D2KO fed (6) | 0 | 4.5 ± 0.1 | 116 ± 10 | 18 ± 7.6 | 719 ± 182 |
| D2KO fast (7) | 15.0 ± 0.7 | 2.7 ± 0.2 | 67 ± 3 | 31 ± 4.0 | 590 ± 120 |
| P value | P < .001 | P < .001 | P < .001 | ns | ns |
| Fast-induced change | 40% decrease | 42% decrease | |||
| D1/D2KO fed (6) | 0 | 6.4 ± 0.6 | 97 ± 6 | 190 ± 21 | 546 ± 84 |
| D1/D2KO fast (7) | 16.0 ± 0.7 | 3.6 ± 0.6 | 70 ± 7 | 581 ± 64 | 463 v 47 |
| P value | P < .001 | P < .001 | P < .01 | P < .001 | ns |
| Fast-induced change | 44% decrease | 28% decrease | 3-fold increase |
Abbreviation: ns, not significant.
To determine whether the decrease in serum TH levels that occurred during the 30-hour fast merely reflected a decrease in the TH-binding activity of serum proteins, a competitive T3 uptake test was performed (Supplemental Table 1). Fasting had no effect on the %T3 uptake in serum from D1KO and D2KO mice. In WT mice, the %T3 uptake was slightly, but significantly, increased (fed vs fasted, 52.0 ± 0.8 vs 57.0 ± 0.8; P < .001), and in the D1/D2KO mice it was slightly decreased (fed vs fasted, 58.0 ± 0.7 vs 55.0 ± 1.1; P < .025). Nevertheless, the calculated Free T3 Index was markedly decreased by fasting in all 4 genotypes and paralleled the decreases in the total serum T3 levels, thus indicating that the latter were not the result of a change in the T3 binding capacity of the serum proteins but rather was indicative of a reduction in the free serum T3 concentration.
Serum iodothyronine levels in fed and fasted WT and D3KO mice
The serum levels of T4 and T3 determined in WT and D3KO mice at the end of a 30-hour fast are shown in Figure 2. As reported previously, D3KO mice have baseline levels of T4 and T3 that are significantly less than those in WT animals (34). As in WT mice, fasting of D3KO mice resulted in a significant reduction in serum T4 and T3 levels. However, the relative incremental decline in the D3-deficient animals was significantly less than in the WT animals (serum T3 decrement, WT 55.3 ± 5.0% vs D3KO 34.9 ± 3.3%; P < .005 and serum T4 decrement, WT 71.8 ± 4.1% vs 54.8 ± 5.9%; P < .001; n = 6–9 mice per group).
Figure 2.
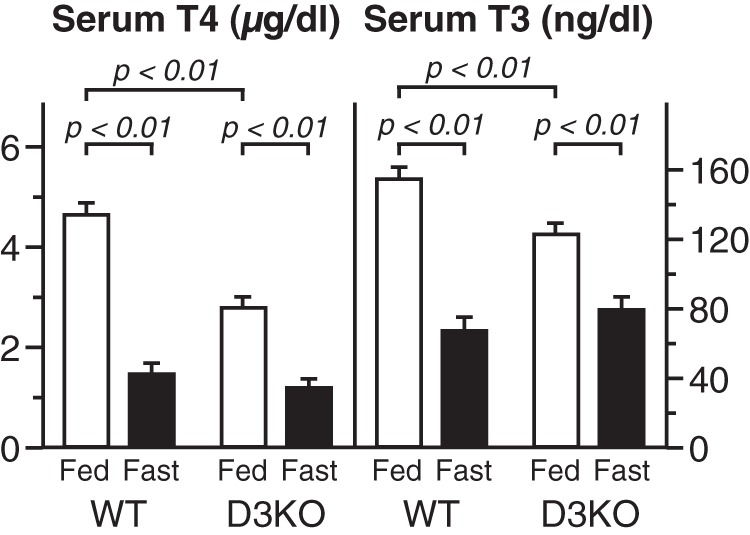
Serum T4 and T3 levels in WT and D3-deficient (D3KO) mice. Bars represent the mean ± SE of values obtained in 6–10 mice per group.
T4 and T3 contents in tissues of fed and fasted WT mice
The effects of fasting for 16, 28, and 36 hours on the TH contents in serum, liver, and kidney of WT mice are shown in Figure 3. Mean values of serum T4 and T3 levels declined steadily throughout the fast, and with the exception of the T3 level after 16 hours of fasting, the decreases were all statistically significant. In contrast, the T4 and T3 contents in kidney were either unchanged (T4) or increased (T3) by fasting. The T3 content in liver was also significantly increased after 28 and 36 hours of fasting. The T4 content in liver, although very low compared with that in kidney, was variable and underwent little change, although the values at 16 and 36 hours of fasting were slightly reduced relative to the fed state.
Figure 3.
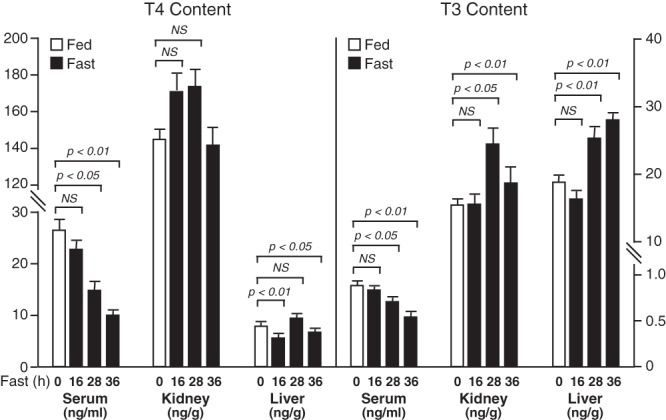
T4 and T3 levels in serum, and T4 and T3 content in liver and kidney of mice fasted for 0, 16, 28, or 36 hours. Bars represent the mean ± SEM of values obtained in a minimum of 6 mice per group. NS, not significant.
The T3 content in muscle after a 30-hour fast was unchanged (WT fed vs WT fasted, 3.9 ± 0.6 vs 3.5 ± 1.4 ng/g wet weight [wt]).
In the hypothalamus, the T4 content after a 30-hour fast was significantly reduced (WT fed vs WT fasted, 3.7 ± 0.3 vs 2.4 ± 0.4 ng/g wet wt; P < .05), but a decrease in the mean pituitary T4 content was not significant (WT fed vs WT fasted, 37 ± 5 vs 27 ± 2 pg per pituitary). The T3 content of both these tissues was unaltered by fasting: hypothalamus, fed vs fasted 2.8 ± 0.3 vs 2.6 ± 0.2 ng/g wet wt; pituitary 74 ± 7 vs 67 ± 4 pg per pituitary.
Excretion of radioactivity in urine and feces after injection of [125I]T4, [125I]T3, or [125I]rT3 to WT and D1/D2KO mice
The percentages of the injected radioactivity present in urine and feces 24 hours after injection of [125I]T4, [125I]T3, or [125I]rT3 to WT and D1/D2KO mice are compared in Figure 4. After correction for the [125I]iodide that contaminated the injected iodothyronines, iodide was the only labeled substance detected by chromatographic analysis in the urine of WT mice, and no radioactive iodide (or other compounds) was detected in urine from D1/D2KO mice, reflecting their inability to carry out 5′D. T3 and rT3 were degraded and/or eliminated from the body more rapidly than T4 regardless of genotype. Indeed, after injection of [125I]rT3 to WT or D1/D2KO mice, approximately 90% of the radioactivity was eliminated within 24 hours. It is also notable that in the WT mice, most the radioactivity after [125I]rT3 appears as iodide in the urine, whereas in the absence of D1 and D2, the same efficiency in elimination is noted, but elimination is entirely via the feces, presumably as intact rT3 and it conjugates and metabolites.
Figure 4.
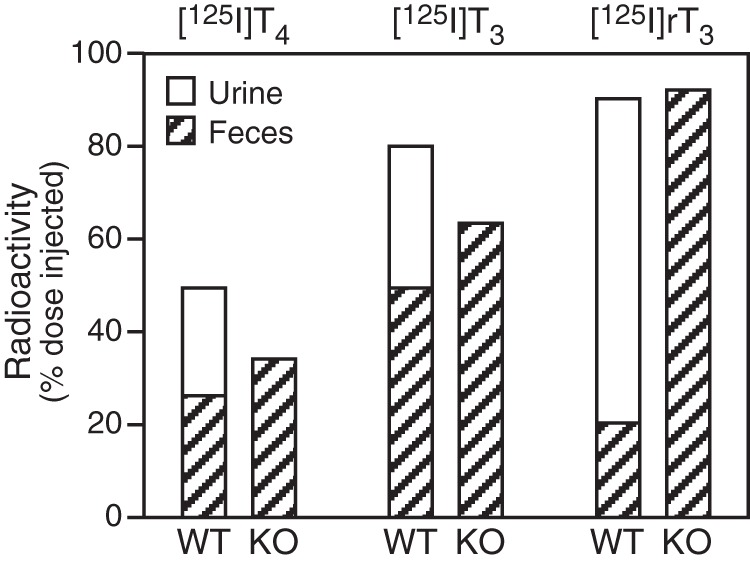
Radioactivity in urine and feces 24 hours after injection of [125I]T4, [125I]T3, or [125I]rT3 in WT and D1/D2KO mice. Urinary radioactivity in WT mice was exclusively in the form of [125I]iodide (see text). The data shown for each hormone were obtained in a single pair of mice, but comparable values were obtained in a second experiment.
Tissue distribution of radioactivity after administration of [125I]iodothyronines to fed and fasted WT and D1/D2KO mice
To assess the fate of T3 in the serum after the initiation of fasting, the distribution of radioactivity in blood, liver, kidney, testis plus fat pad, spleen, stomach, intestine (including contents), heart plus lung, brain, urine, and feces was determined 20 hours after an injection of [125I]T3 into 6 WT mice. Half the mice were fasted for 24 hours (starting 4 h before administration of the [125I]T3), the others had free access to food throughout the experimental period. In all tissues except spleen, the total radioactivity, expressed as a percentage of that injected, was higher in the fasted than the fed mice, and except in brain, the increase was at least 2-fold (Figure 5). At the same time, excretion of radioactivity by the mice was decreased, in part because the amount of feces eliminated in fasted mice was greatly reduced.
Figure 5.
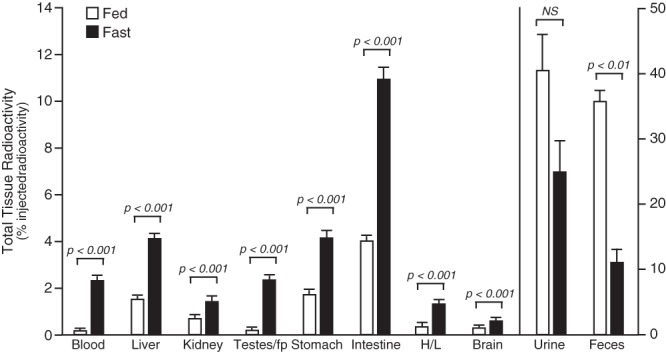
Comparison of total radioactivity in tissues, urine, and feces in fasted and fed mice 20 hours after an injection of [125I]T3. Data are expressed as a percentage of the total amount of radioactivity injected, Mice were fasted for 24 hours, and the [125I]T3 was administered after 4 hours of fasting. Bars represent the mean ± SEM of values obtained in 3 mice per group. H/L, heart + lung; Test/fp, testes + epidydimal fat pad. NS, not significant.
Not all tissues were included in this initial experiment, and thus, a significant amount of the injected radioactivity was unaccounted for: 14% in the fed mice and 37% in the fasted mice. Accordingly, additional experiments were carried out, in which the effect of fasting on the distribution of the radioactivity in the entire body was determined after administration of 3 different [125I]iodothyronines: [125I]T3, [125I]T4, and [125I]rT3. Typical results obtained in 3 pairs of WT mice are shown in Figure 6A. One member of each pair was fed and the other fasted for 24 hours, with both animals injected with [125I]T3 at 4 hours into the experimental period. The other pairs was treated identically but injected with [125I]T4 or [125I]rT3. Confirming the previous findings (Figure 5), after the injection of [125I]T3 (first panel), all tissues from the fasted mouse, except brain, contained more radioactivity than those from the fed mouse; the average tissue content of radioactivity in the fasted animal was 4.1-fold greater than in the fed animal. After [125I]T4 or [125I]rT3 administration, the radioactive content was again elevated in essentially all tissues, except the brain, of the fasted mice by an average of 1.9- and 2.0-fold, respectively. Notable was the markedly higher content of radioactivity in the gastrointestinal tract, skin, carcass, blood, and limbs of fasted animals after the injection of all 3 tracers and the significantly higher level of radioactivity in the liver after injection of [125I]T3. Comparable increases were obtained when the [125I]T3 or [125I]T4 were injected 2 hours before the start of the fast.
Figure 6.
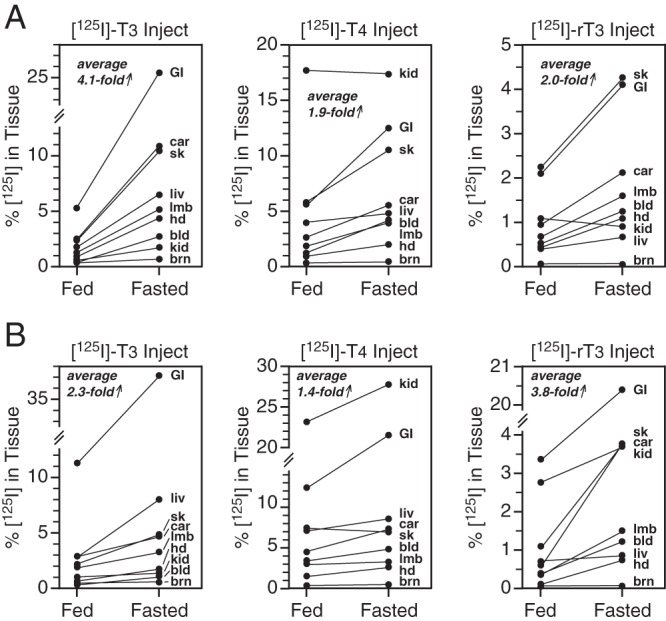
Effect of a 24-hour fast on the tissue distribution of radioactivity after administration of [125I]T3, [125I]T4, or [125I]rT3 in (A) WT or (B) D1/D2KO mice. Each labeled hormone was injected into animals that were fed or had been fasting for 4 hours. Animals were killed 20 hours later. The percentage of injected radioactivity present in each tissue for fed and fasted animals is presented for each tracer. The data shown for each hormone were obtained in a single pair of mice (1 fed, 1 fasted), but comparable values were obtained in confirmatory experiments (see text). The average fold increase in radioactive content due to fasting of the nine tissues is presented. bld, whole blood; brn, brain; car, carcass; GI, gastrointestinal tract; hd, head; kd, kidney; liv, liver; lmb, limbs; sk, skin.
It is notable that the radioactive content of the kidneys after [125I]T4 administration was much higher than that in the other tissues in both WT fed and fasted mice, and much higher than after the injection of [125I]T3 or [125I]rT3 (Figure 6A). In addition, after injection of either [125I]T4 or [125I]T3, a significant fraction of the radioactivity in whole blood was retained in the nonserum fraction (22%–30% and 50%—56% in fed and fasted mice, respectively). In concurrent studies performed in D1/D2KO mice (Figure 6B), the radioactive content in tissues after fasting was again generally higher than that in the fed mice, with average increases of 2.3-, 1.4-, and 3.8-fold after injection of radiolabeled T3, T4, and rT3, respectively.
Identification of labeled compounds in serum and tissues after injection of [125I]T4
In order to begin to identify the radioactive compounds in the serum and tissues of fed and fasted mice injected with radiolabeled [125I]T4, the labeled compounds were extracted from serum and liver and kidney homogenates and then concentrated and subjected to paper chromatographic analysis. The extraction procedure employed adsorption on a Dowex-1 anion exchange resin. As described previously (33), when diluted serum or a tissue homogenate is passed through a column of this resin equilibrated at pH 5.6, the iodothyronines and the iodide are adsorbed on the resin. The resin is then washed thoroughly with buffer at pH 5.6 to remove any retained tissue components, and then the iodothyronines are eluted with acetic acid (pH 1.4). The iodide remains on the resin. This technique permits the extraction of iodothyronines from a considerably greater amount of tissue than does the methanol/PTU method described above.
Using this extraction technique, it was determined that 46%–60% of the total radioactivity in serum, liver, and kidney was eluted at pH 1.4, suggesting that these represented intact iodothyronines. Percentages in serum from fed and fasted WT mice were 57 ± 1 and 51 ± 2, respectively, in liver, 55 ± 4 and 46 ± 3, and in kidney 60 ± 6 and 56 ± 3. These differences were not statistically significant.
Chromatographic analysis of the eluted radioactive iodothyronines from both fed and fasted animals demonstrated that only [125I]T4 was present in the serum and liver, whereas in the kidney, both [125I]T4 and [125I]T4g were found (Figure 7). No [125I]T3 was detected in these eluates because of limitations in the sensitivity of this technique. Indeed, extraction of iodothyronines from tissues after injection of [125I]T3 and [125I]rT3 proved to be impractical as well, because insufficient radioactivity remained in the tissues 20 hours after injection.
Figure 7.
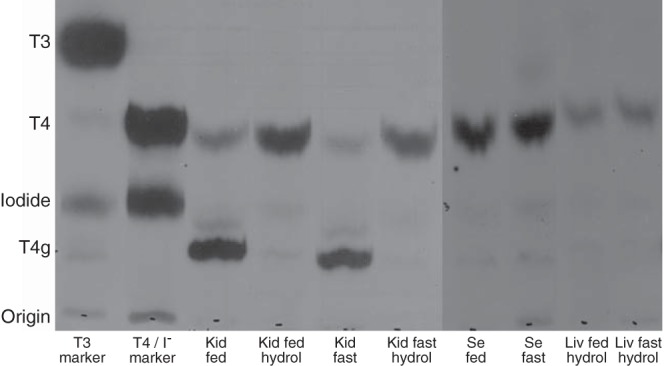
Paper chromatographic analysis of the radioactive compounds present in the iodothyronine fraction eluted over Dowex columns from kidney homogenates (Kid), serum (Se), and liver homogenates (Liv) from fed and fasted mice. Before chromatography, some aliquots of the kidney and liver elutes were hydrolized with β-glucuronidase (lanes designated by hydrol) in order to convert T4g to T4. The location of T3, T4, and iodide are demonstrated in the first 2 lanes where radiolabeled T3 and T4 have been applied to those strips. (Note that the marker radiolabeled T4 was not purified before application to the chromatography paper in order to identify the location of the iodide band.) Treatment of the kidney homogenates with β-glucuronidase results is complete conversion of the radiolabeled T4g band to T4. Essentially, no T4g was apparent in liver homogenates, and only the β-glucuronidase-treated samples are shown. The locations of the labeled compounds on the paper strips were visualized by exposure to x-ray film.
Discussion
The studies described here demonstrate clearly that the marked changes in serum TH and TSH profiles that occur in fasted WT mice are obtained also in mice deficient in the D1 and/or the D2. Therefore, regardless of whether they result from fasting-induced effects in peripheral tissues or the hypothalamic/pituitary/thyroid (HPT) axis or both, they are not dependent on the actions of the 5′-deiodinases. This striking finding necessitates a reassessment of the role of TH metabolism in the response to fasting.
Previous investigators have suggested that a fasting-induced increase in hypothalamic D2 expression, with a presumed increase in T3 content in this brain region, is critical to the suppression of the HPT axis in this condition (35). Our studies call this hypothesis into question; D2 activity and T3 content were not increased in the hypothalamus of fasted WT animals, despite a 2-fold increase in D2 mRNA expression in this region. Furthermore, as in WT animals, TSH levels did not rise above baseline levels in fasted D2KO and D1/D2KO animals, indicating that a functioning D2 is not essential for the suppression of the HPT axis in the fasted condition. Similarly, the consistently observed marked decrease in D1 activity observed in the liver of fasted rodents is not essential for the decreased serum T3 level observed in this condition.
These findings also point out the fallacy of extrapolating in vitro determined deiodinase mRNA or activity levels to in vivo rates of T3 production and/or degradation. With the D2 in particular, such extrapolation is ill-advised, because posttranslational processes impact enzyme content, and local cofactor and substrate availability, both indeterminate factors with present methodologies, are key determinants of enzyme activity.
With these caveats in mind, the present studies strongly suggest that the D3 plays a role in the fasting-induced alterations in TH homeostasis. Thus, D3 activity, although normally expressed at very low levels in adult peripheral tissues, was increased to significant levels in the skin, kidney, and muscle of both fasted WT and D1/D2KO mice. That such increases in D3 activity in these sizable organs were accompanied by increases in the in vivo rate of production of rT3 from T4 is supported by the increased serum rT3 level observed in fasted WT mice and especially in fasted mice lacking the D1. The D1 plays a major role in the metabolism of rT3, as demonstrated by the lack of urinary [125I]iodide excretion in D1/D2KO mice administered [125I]rT3 (Figure 4) and the markedly elevated serum rT3 level observed in the 2 groups of D1-deficient fed mice relative to the level in the WT or D2KO fed mice (Table 1). Thus, given that rT3 clearance is already markedly impaired in D1-deficient mice, the 3-fold increase in the serum rT3 level induced by fasting in these mice supports an enhanced rate of rT3 production by the D3 in this setting.
A direct assessment of the role of the D3 in the fasting response, by using D3-deficient mice, provides further evidence that this enzyme plays an important role in this condition. Thus, the reductions in serum T4 and T3 levels induced by fasting are significantly less in the D3KO animal than in the corresponding WT mice. However, the finding that some degree of reduction still occurs in the mutant animal points to additional factors influencing the response.
Another key finding here is that the TH content of liver, kidney, muscle, and pituitary was not reduced in the fasting state. Indeed, the T3 content of the liver and kidney increased during fasting. These findings were supported by the results of the [125I]T4 and [125I]T3 studies that showed highly significant increases in the radioactive content of most tissues after a 24-hour fast. These findings reinforce the growing recognition that circulating TH levels may not be indicative of the thyroid status of individual cells and tissues, especially under pathological conditions. Thus, in fasting, these tissues appear not to be hypothyroid despite significant decreases in circulating T4 and T3 levels, and hence changes reported previously in gene expression patterns appear to be attributable directly to changes in the nutritional status of the animal rather than changes in tissue TH levels per se.
Earlier studies have demonstrated that the decrease in serum TH levels during fasting cannot be attributed to their enhanced clearance from serum (2, 14, 19, 20). However, experimental evidence suggests that reduced thyroidal secretion of T4 plays a significant role (14, 15). Furthermore, in 2 studies using athyreotic rats isotopically equilibrated with either [125I]T4 and [131I]T3 (5) or [131I]T4 alone (20), neither partial nor complete food deprivation, respectively, led to a decline in the serum T4 level. In the former study, the serum T3 level was also decreased in fasting, suggesting that peripheral factors contributed to this observed change.
A fasting-induced sequestration of TH in peripheral tissues has received little attention as a possible contributor to the observed systemic changes in TH homeostasis. This might occur as a result of enhanced binding of TH in tissues, an increase in their metabolism, or impairment in their transport from tissues back into the circulation. Given that T3 is predominantly an intracellular hormone, its fate in tissues could have a major impact on the levels observed in the serum. In this regard, the results of our studies employing the administration of radiolabeled THs are intriguing. Thus, when WT mice were injected with [125I]T4, [125I]T3, or [125I]rT3, either 2 hours before or 4 hours after the start of a 24-hour fast, the radioactive content of almost all tissues at the end of the fast was greatly increased in the fasted vs fed mice. For [125I]T3, the increase was a remarkable 4-fold. Because clearance of T4 and T3 from the serum into tissues is not enhanced during fasting, these results suggest that THs, their metabolites, and/or iodide are preferentially retained, and thus sequestered in cells and undergo an enhanced rate of metabolism by the D3 and other processes. Such homeostatic alterations likely contribute significantly to the lowering of TH levels in serum.
There are several limitations in the interpretation of these radioactive studies. First, they are nonequilibrium studies with the endogenous hormonal pool labeled at a single time point. Second, to the extent that thyroidal secretion of endogenous hormone during the experimental period differed between the fed and fasted mice, the specific activity of the labeled hormone could have been altered to a different extent in fed and fasted mice. Third, fasted mice experience a significant degree of dehydration despite unlimited access to water. This could have resulted in a greater concentration of radioactivity (expressed as the amount of radioactivity/milligram of wet weight tissue) by the end of the experimental period in the fasted animals. To counter this, we have expressed the radioactivity measurements as the total amount in each whole tissue. Our observations thus demonstrate that both tissue and total body retention of radioactivity is much greater in fasted animals and is not secondary to a concentration effect. Nevertheless, the dehydrated state of the fasted animals likely reduced the excretion of iodide in the urine. Fourth, the labeled hormones were administered by ip injection, and there was likely an important first pass effect on their metabolism as they traversed the liver that could have differed between fed and fasted mice. However, administration of [125I]T3 2 hours before beginning the fast gave the same results as when the [125I]T3 was given 4 hours into the fast, suggesting that this was not a significant factor. Fifth, neither the individual mice nor the tissues were perfused before determining tissue radioactive content. This was done intentionally, so that a complete accounting of the injected radioactivity could be made. In summary, although these several factors may complicate the interpretation of these studies, it is highly unlikely that, even combined, they explain the dramatic 2- to 4-fold differences in tissue retention of radioactivity observed between the fed and fasted mice. Furthermore, our findings are entirely consistent with the study of Yen et al (15), who demonstrated that the plasma T3 appearance rate was markedly reduced in fasted rats, despite an increased rate of T4 to T3 conversion in extrathyroidal tissues.
However, the marked fasting-induced increase in radioactivity noted in whole blood after administration of the labeled compounds is problematic. These findings appear inconsistent with the results of direct determinations of serum TH using specific RIAs. In the case of both T4 and T3, a partial explanation results from our finding that there is an approximate 2-fold increase in the radioactivity associated with the nonserum components of the blood in fasted animals, a finding consistent with observations of others (15). Nevertheless, this cannot explain the finding that the radioactive counts in the serum were consistently greater in the fasted animals by approximately 50% for T4 and 100% for T3 and that, in both fed and fasted animals, the proportion of counts that represented the intact iodothyronine injected was about 50%. Although hemoconcentration in the fasted animal could explain at least part of this effect, it would also be expected to have resulted in increased concentrations of TH in the serum based on RIA determination, which is not observed. Thus, at the present time, the increased levels of radioactive hormone in the serum of the fasted animals remain an enigma.
As a final caveat regarding our experimental methodology, our estimation of free T3 levels using a T3 uptake assay and the calculated Free T3 Index might hypothetically underestimate the free hormone concentration in serum during fasting due to the dilution of a TH binding inhibitor, as has been shown to be the case in nonthyroidal illness (36). However, a modest inaccuracy in this measurement is unlikely to have been a major factor in these results. Furthermore, although we did not use the T3 uptake determination to calculate a Free T4 Index and estimate changes in the free T4 levels, previous studies in rats with chronic nutritional deprivation have demonstrated decreases in both free T4 and T3 levels as determined by equilibrium dialysis techniques (37), consistent with our findings. We thus conclude that alterations in the levels of free T4 and T3 in fasting do not explain the normal to increased content of tissue T4 and T3 that we have observed in the fasted animal, nor the increased levels of tissue radioactivity observed after the injection of radiolabeled hormones.
Thus, based on both previous studies (5, 14, 15, 20) and those described here, the principal changes observed in fasted animals include: 1) a diminished secretion of T4, and likely T3, from the thyroid gland; 2) an increase in the activity and action of the D3; and 3) an enhanced retention of T4, T3, rT3, lesser iodothyronines, and iodide in tissues. In at least some tissues (eg, liver, kidney, hypothalamus, and pituitary), the enhanced retention and metabolism of T3 appears to be balanced such that tissue hormone content is not diminished and tissues remain essentially euthyroid, as evidenced in the hypothalamic/pituitary axis by the maintenance of the TSH at euthyroid levels.
In summary, the present studies demonstrate that the actions of the D1 and D2 5′-deiodinases are not essential to the systemic alterations in TH homeostasis observed in fasted animals and suggest that sequestration and enhanced metabolism of T4 and T3 in tissues, in part by an increase in the activity of the D3, play important roles in the decreased serum levels of these hormones observed in this condition.
Acknowledgments
This work was supported by National Institutes of Health Grant 5R01DK79946.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BAT
- brown adipose tissue
- CCx
- cerebral cortex
- 5D
- 5-deiodination
- 5′D
- 5′-deiodination
- D2
- type 2 deiodinase
- HPT
- hypothalamic/pituitary/thyroid
- KO
- knockout
- PTU
- 6-n-propylthiouracil
- T4g
- T4-glucuronide
- TH
- thyroid hormone
- wt
- weight
- WT
- wild type.
References
- 1. Carlson HE, Drenick EJ, Chopra IJ, Hershman JM. Alterations in the basal and TRH stimulated serum levels of thyrotropin, prolactin, and thyroid hormones in starved obese men. J Clin Endocrinol Metab. 1977;45:707–713 [DOI] [PubMed] [Google Scholar]
- 2. Suda AK, Pittman CS, Shimizu T, Chambers JB., Jr The production and metabolism of 3,5,3′-triiodothyronine and 3,3′,5′-triiodothyronine in normal and fasting subjects. J Clin Endocrinol Metab. 1978;47:1311–1319 [DOI] [PubMed] [Google Scholar]
- 3. St. Germain DL, Galton VA. Comparative study of pituitary-thyroid hormone economy in fasting and hypothyroid rats. J Clin Invest. 1985;75:679–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chopra IJ. Alterations in monodeiodination of iodothyronines in the fasting rat: effects of reduced nonprotein sulfhydryl groups and hypothyroidism. Metabolism. 1980;29:161–167 [DOI] [PubMed] [Google Scholar]
- 5. van Doorn J, van der Heide D, Roelfsema F. The influence of partial food deprivation on the quantity and source of triiodothyronine in several tissues of athyreotic thyroxine-maintained rats. Endocrinology. 1984;115:705–711 [DOI] [PubMed] [Google Scholar]
- 6. Connors JM, DeVito WJ, Hedge G. Effects of food deprivation on the feedback regulation of the hypothalamic-pituitary-thyroid axis of the rat. Endocrinology. 1985;117:900–906 [DOI] [PubMed] [Google Scholar]
- 7. Harris AR, Fang SL, Azizi F, Lipworth L, Vagenakis AG, Barverman LE. Effect of starvation on hypothalamic-pituitary-thyroid function in the rat. Metabolism. 1978;27:1074–1083 [DOI] [PubMed] [Google Scholar]
- 8. Kaplan MM. Regulatory influences on iodothyronine deiodination in animal tissues. In: Hennemann G, ed. Thyroid Hormone Metabolism. New York, NY: Marcel Dekker, Inc; 1986:231–253 [Google Scholar]
- 9. Légrádi G, Emerson CH, Ahima RS, Flier JS, Lechan RM. Leptin prevents fasting-induced suppression of prothyrotropin-releasing hormone messenger ribonucleic acid in neurons of the hypothalamic paraventricular nucleus. Endocrinology. 1997;138:2569–2576 [DOI] [PubMed] [Google Scholar]
- 10. Carr FE, Seelig S, Mariash CN, Schwartz HL, Oppenheimer JH. Starvation and hypothyroidism exert an overlapping influence on rat hepatic messenger RNA activity profiles. J Clin Invest. 1983;72:154–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Carr FE, Bingham C, Oppenheimer JH, Kistner C, Mariash CN. Quantitative investigation of hepatic genomic response to hormonal and pathophysiological stimuli by multivariate analysis of two-dimensional mRNA activity profiles. Proc Natl Acad Sci USA. 1984;81:974–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wells SL, Campbell RG. Decrease in resting metabolic rate during rapid weight loss is reversed by low dose thyroid hormone treatment. Metabolism. 1986;35:289–291 [DOI] [PubMed] [Google Scholar]
- 13. Forsum E, Hillman PE, Nesheim MC. Effect of energy restriction on total heat production, basal metabolic rate, and specific dynamic action of food in rats. J Nutr. 1981;111:1691–1697 [DOI] [PubMed] [Google Scholar]
- 14. Kinlaw WB, Schwartz HL, Oppenheimer JH. Decreased serum triiodothyronine in starving rats is due primarily to diminished thyroidal secretion of thyroxine. J Clin Invest. 1985;75:1238–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yen YM, Distefano JJ, 3rd, Yamada H, Nguyen TT. Direct measurement of whole body thyroid hormone pool sizes and interconversion rates in fasted rats: hormone regulation implications. Endocrinology. 1994;134:1700–1709 [DOI] [PubMed] [Google Scholar]
- 16. Vella KR, Ramadoss P, Lam FS, et al. NPY and MC4R signaling regulate thyroid hormone levels during fasting through both central and peripheral pathways. Cell Metab. 2011;14:780–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kaplan MM, Yaskoski KA. Effects of congenital hypothyroidism and partial and complete food deprivation on phenolic and tyrosyl ring iodothyronine deiodination in rat brain. Endocrinology. 1982;110:761–767 [DOI] [PubMed] [Google Scholar]
- 18. Diano S, Naftolin F, Goglia F, Horvath TL. Fasting-induced increase in type II iodothyronine deiodinase activity and messenger ribonucleic acid levels is not reversed by thyroxine in the rat hypothalamus. Endocrinology. 1998;139:2879–2884 [DOI] [PubMed] [Google Scholar]
- 19. Vagenakis AG, Portnay GI, O'Brian JT, et al. Effect of starvation on the production and metabolism of thyroxine and triiodothyronine in euthyroid obese patients. J Clin Endocrinol Metab. 1977;45:1305–1309 [DOI] [PubMed] [Google Scholar]
- 20. Ingbar DH, Galton VA. The effect of food deprivation of the peripheral metabolism of thyroxine in rats. Endocrinology. 1975;96:1525–1532 [DOI] [PubMed] [Google Scholar]
- 21. De Jong M, Docter R, Van Der Hoek HJ, Vos RA, Krenning EP, Hennemann G. Transport of 3,5,3′-triiodothyronine into the perfused rat liver and subsequent metabolism are inhibited by fasting. Endocrinology. 1992;131:463–470 [DOI] [PubMed] [Google Scholar]
- 22. Harris AR, Fang SL, Vagenakis AG, Braverman LE. Effect of starvation, nutriment replacement, and hypothyroidism on in vitro hepatic T4 to T3 conversion in the rat. Metabolism. 1978;27:1680–1690 [DOI] [PubMed] [Google Scholar]
- 23. O'Mara BA, Dittrich W, Lauterio TJ, St. Germain DL. Pretranslational regulation of type I 5′-deiodinase by thyroid hormones and in fasted and diabetic rats. Endocrinology. 1993;133:1715–1723 [DOI] [PubMed] [Google Scholar]
- 24. Boelen A, van Beeren M, Vos X, et al. Leptin administration restores the fasting-induced increase of hepatic type 3 deiodinase expression in mice. Thyroid. 2012;22:192–199 [DOI] [PubMed] [Google Scholar]
- 25. Maglich JM, Watson J, McMillen PJ, Goodwin B, Willson TM, Moore JT. The nuclear receptor CAR is a regulator of thyroid hormone metabolism during caloric restriction. J Biol Chem. 2004;279:19832–19838 [DOI] [PubMed] [Google Scholar]
- 26. Sharifi J, St. Germain DL. The cDNA for the type I iodothyronine 5′-deiodinase encodes an enzyme manifesting both high Km and low Km activity. Evidence that rat liver and kidney contain a single enzyme which converts thyroxine to 3,5,3′-triiodothyronine. J Biol Chem. 1992;267:12539–12544 [PubMed] [Google Scholar]
- 27. Galton VA, Martinez E, Hernandez A, St. Germain EA, Bates JM, St. Germain DL. Pregnant rat uterus expresses high levels of the type 3 iodothyronine deiodinase. J Clin Invest. 1999;103:979–987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Buitendijk M, Galton VA. Is the kidney a major storage site for thyroxine as thyroxine glucuronide? Thyroid. 2012;22:187–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Galton VA, Pitt-Rivers R. Thyroid-hormone metabolism in the kidney. Biochem J. 1959;72:314–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Galton VA, Schneider MJ, Clark AS, St Germain DL. Life without thyroxine to 3,5,3′-triiodothyronine conversion: studies in mice devoid of the 5′-deiodinases. Endocrinology. 2009;150:2957–2963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Roti E, Gnudi A, Braverman LE, et al. Human cord blood concentrations of thyrotropin, thyroglobulin, and iodothyronines after maternal administration of thyrotropin-releasing hormone. J Clin Endocrinol Metab. 1981;53:813–817 [DOI] [PubMed] [Google Scholar]
- 32. Schneider MJ, Fiering SN, Pallud SE, Parlow AF, St Germain DL, Galton VA. Targeted disruption of the type 2 selenodeiodinase gene (DIO2) results in a phenotype of pituitary resistance to T4. Mol Endocrinol. 2001;15:2137–2148 [DOI] [PubMed] [Google Scholar]
- 33. Galton VA, Pitt-Rivers R. A quantitative method for the separation of thyorid hormones and related compounds from serum and tissues with an anion-exchange resin. Biochem J. 1959;72:310–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hernandez A, Martinez ME, Fiering S, Galton VA, St Germain D. Type 3 deiodinase is critical for the maturation and function of the thyroid axis. J Clin Invest. 2006;116:476–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Coppola A, Hughes J, Esposito E, Schiavo L, Meli R, Diano S. Suppression of hypothalamic deiodinase type II activity blunts TRH mRNA decline during fasting. FEBS Lett. 2005;579:4654–4658 [DOI] [PubMed] [Google Scholar]
- 36. Chopra IJ. Clinical review 86: euthyroid sick syndrome: is it a misnomer? J Clin Endocrinol Metab. 1997;82:329–334 [DOI] [PubMed] [Google Scholar]
- 37. van Haasteren GA, Linkels E, van Toor H, et al. Effects of long-term food deprivation on the hypothalamic-pituitary-thyroid axis in male and female rats. J Endocrinol. 1996;150:169–178 [DOI] [PubMed] [Google Scholar]


