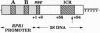Abstract
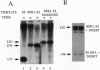
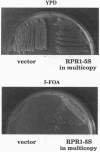
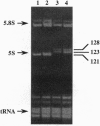
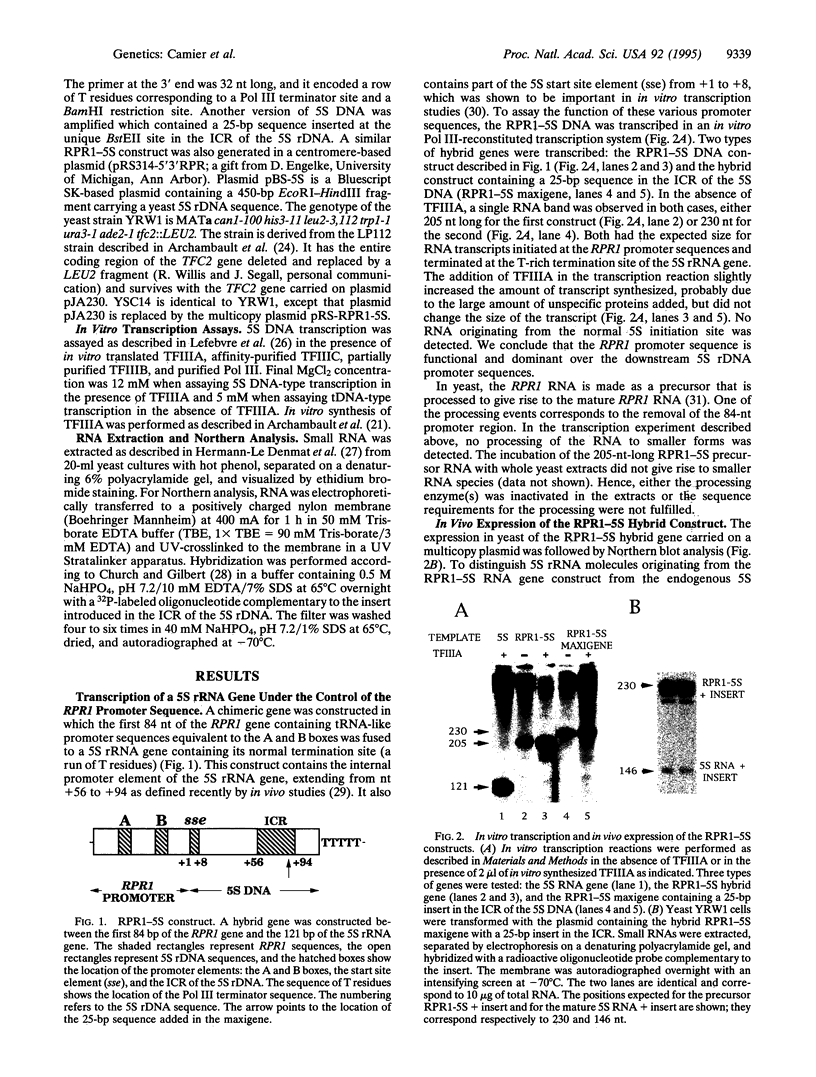
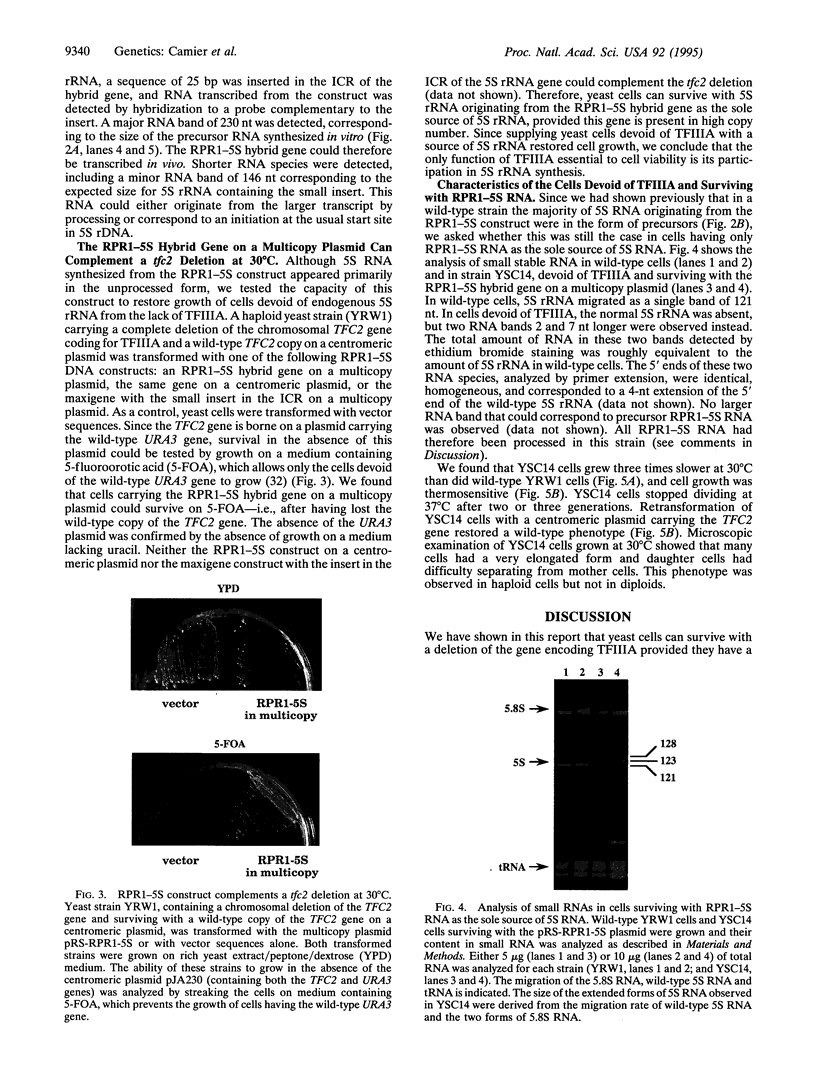
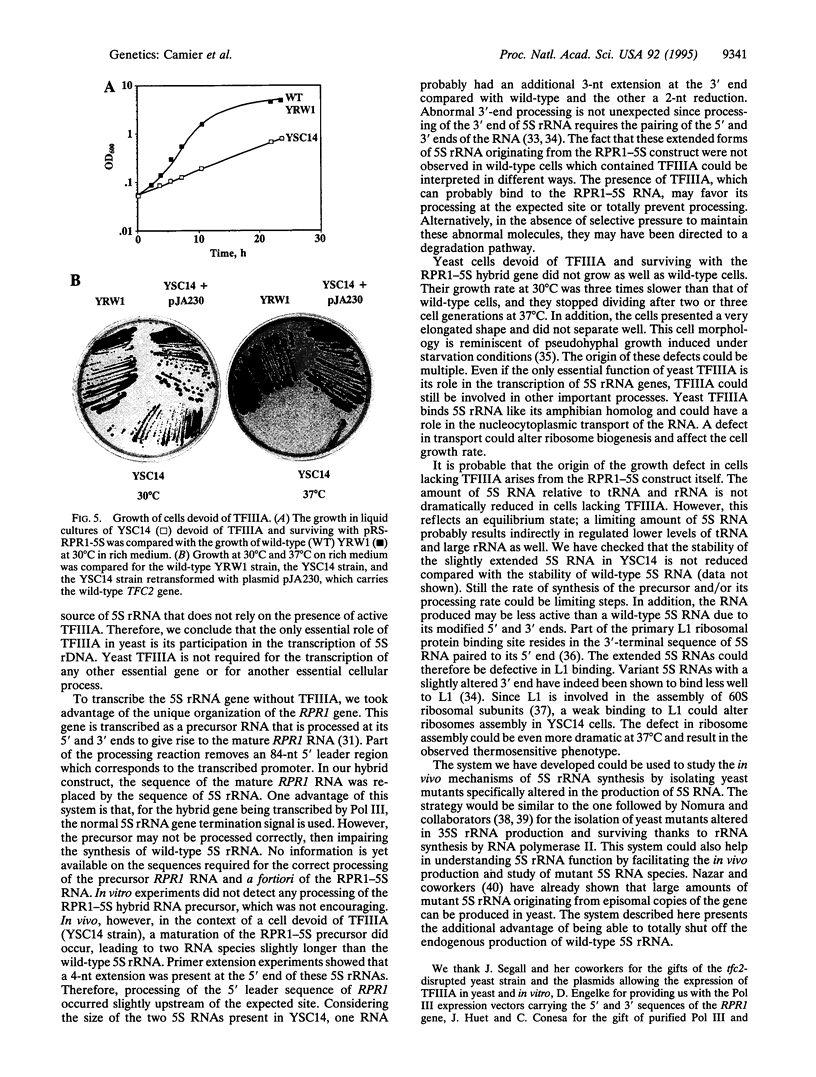
We have developed a system to transcribe the yeast 5S rRNA gene in the absence of the transcription factor TFIIIA. A long transcript was synthesized both in vitro and in vivo from a hybrid gene in which the tRNA-like promoter sequence of the RPR1 gene was fused to the yeast 5S RNA gene. No internal initiation directed by the endogenous 5S rDNA promoter or any processing of the hybrid transcript was observed in vitro. Yeast cells devoid of transcription factor TFIIIA, which, therefore, could not synthesize any 5S rRNA from the endogenous chromosomal copies of 5S rDNA, could survive if they carried the hybrid RPR1-5S construct on a multicopy plasmid. In this case, the only source of 5S rRNA was the precursor RPR1-5S transcript that gave rise to two RNA species slightly larger than wild-type 5S rRNA. This establishes that the only essential function of TFIIIA is to promote the synthesis of 5S rRNA. However, cells devoid of TFIIIA and surviving with these two RNAs grew more slowly at 30 degrees C compared with wild-type cells and were thermosensitive at 37 degrees C.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews M. T., Brown D. D. Transient activation of oocyte 5S RNA genes in Xenopus embryos by raising the level of the trans-acting factor TFIIIA. Cell. 1987 Nov 6;51(3):445–453. doi: 10.1016/0092-8674(87)90640-4. [DOI] [PubMed] [Google Scholar]
- Archambault J., Milne C. A., Schappert K. T., Baum B., Friesen J. D., Segall J. The deduced sequence of the transcription factor TFIIIA from Saccharomyces cerevisiae reveals extensive divergence from Xenopus TFIIIA. J Biol Chem. 1992 Feb 15;267(5):3282–3288. [PubMed] [Google Scholar]
- Archambault J., Schappert K. T., Friesen J. D. A suppressor of an RNA polymerase II mutation of Saccharomyces cerevisiae encodes a subunit common to RNA polymerases I, II, and III. Mol Cell Biol. 1990 Dec;10(12):6123–6131. doi: 10.1128/mcb.10.12.6123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeke J. D., LaCroute F., Fink G. R. A positive selection for mutants lacking orotidine-5'-phosphate decarboxylase activity in yeast: 5-fluoro-orotic acid resistance. Mol Gen Genet. 1984;197(2):345–346. doi: 10.1007/BF00330984. [DOI] [PubMed] [Google Scholar]
- Bouvet P., Dimitrov S., Wolffe A. P. Specific regulation of Xenopus chromosomal 5S rRNA gene transcription in vivo by histone H1. Genes Dev. 1994 May 15;8(10):1147–1159. doi: 10.1101/gad.8.10.1147. [DOI] [PubMed] [Google Scholar]
- Braun B. R., Bartholomew B., Kassavetis G. A., Geiduschek E. P. Topography of transcription factor complexes on the Saccharomyces cerevisiae 5 S RNA gene. J Mol Biol. 1992 Dec 20;228(4):1063–1077. doi: 10.1016/0022-2836(92)90315-b. [DOI] [PubMed] [Google Scholar]
- Brow D. A., Geiduschek E. P. Modulation of yeast 5 S rRNA synthesis in vitro by ribosomal protein YL3. A possible regulatory loop. J Biol Chem. 1987 Oct 15;262(29):13953–13958. [PubMed] [Google Scholar]
- Brow D. A. In vitro transcripts of a yeast variant 5 S rRNA gene exhibit alterations in 3'-end processing and protein binding. J Biol Chem. 1987 Oct 15;262(29):13959–13965. [PubMed] [Google Scholar]
- Challice J. M., Segall J. Transcription of the 5 S rRNA gene of Saccharomyces cerevisiae requires a promoter element at +1 and a 14-base pair internal control region. J Biol Chem. 1989 Nov 25;264(33):20060–20067. [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens K. R., Wolf V., McBryant S. J., Zhang P., Liao X., Wright P. E., Gottesfeld J. M. Molecular basis for specific recognition of both RNA and DNA by a zinc finger protein. Science. 1993 Apr 23;260(5107):530–533. doi: 10.1126/science.8475383. [DOI] [PubMed] [Google Scholar]
- Del Rio S., Setzer D. R. The role of zinc fingers in transcriptional activation by transcription factor IIIA. Proc Natl Acad Sci U S A. 1993 Jan 1;90(1):168–172. doi: 10.1073/pnas.90.1.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshmukh M., Tsay Y. F., Paulovich A. G., Woolford J. L., Jr Yeast ribosomal protein L1 is required for the stability of newly synthesized 5S rRNA and the assembly of 60S ribosomal subunits. Mol Cell Biol. 1993 May;13(5):2835–2845. doi: 10.1128/mcb.13.5.2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimeno C. J., Ljungdahl P. O., Styles C. A., Fink G. R. Unipolar cell divisions in the yeast S. cerevisiae lead to filamentous growth: regulation by starvation and RAS. Cell. 1992 Mar 20;68(6):1077–1090. doi: 10.1016/0092-8674(92)90079-r. [DOI] [PubMed] [Google Scholar]
- Guddat U., Bakken A. H., Pieler T. Protein-mediated nuclear export of RNA: 5S rRNA containing small RNPs in xenopus oocytes. Cell. 1990 Feb 23;60(4):619–628. doi: 10.1016/0092-8674(90)90665-2. [DOI] [PubMed] [Google Scholar]
- Hanas J. S., Hazuda D. J., Wu C. W. Xenopus transcription factor A promotes DNA reassociation. J Biol Chem. 1985 Oct 25;260(24):13316–13320. [PubMed] [Google Scholar]
- Hazuda D. J., Wu C. W. DNA-activated ATPase activity associated with Xenopus transcription factor A. J Biol Chem. 1986 Sep 15;261(26):12202–12208. [PubMed] [Google Scholar]
- Hermann-Le Denmat S., Werner M., Sentenac A., Thuriaux P. Suppression of yeast RNA polymerase III mutations by FHL1, a gene coding for a fork head protein involved in rRNA processing. Mol Cell Biol. 1994 May;14(5):2905–2913. doi: 10.1128/mcb.14.5.2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klekamp M. S., Weil P. A. Specific transcription of homologous class III genes in yeast-soluble cell-free extracts. J Biol Chem. 1982 Jul 25;257(14):8432–8441. [PubMed] [Google Scholar]
- Lee J. Y., Evans C. F., Engelke D. R. Expression of RNase P RNA in Saccharomyces cerevisiae is controlled by an unusual RNA polymerase III promoter. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):6986–6990. doi: 10.1073/pnas.88.16.6986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y., Erkine A. M., Van Ryk D. I., Nazar R. N. In vivo analyses of the internal control region in the 5S rRNA gene from Saccharomyces cerevisiae. Nucleic Acids Res. 1995 Feb 25;23(4):634–640. doi: 10.1093/nar/23.4.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre O., Rüth J., Sentenac A. A mutation in the largest subunit of yeast TFIIIC affects tRNA and 5 S RNA synthesis. Identification of two classes of suppressors. J Biol Chem. 1994 Sep 16;269(37):23374–23381. [PubMed] [Google Scholar]
- Mao X., Darby M. K. A position-dependent transcription-activating domain in TFIIIA. Mol Cell Biol. 1993 Dec;13(12):7496–7506. doi: 10.1128/mcb.13.12.7496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J., McLachlan A. D., Klug A. Repetitive zinc-binding domains in the protein transcription factor IIIA from Xenopus oocytes. EMBO J. 1985 Jun;4(6):1609–1614. doi: 10.1002/j.1460-2075.1985.tb03825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne C. A., Segall J. Mapping regions of yeast transcription factor IIIA required for DNA binding, interaction with transcription factor IIIC, and transcription activity. J Biol Chem. 1993 May 25;268(15):11364–11371. [PubMed] [Google Scholar]
- Nazar R. N. The ribosomal protein binding site in Saccharomyces cerevisiae ribosomal 5 S RNA. A conserved protein binding site in 5 S RNA. J Biol Chem. 1979 Aug 25;254(16):7724–7729. [PubMed] [Google Scholar]
- Nogi Y., Vu L., Nomura M. An approach for isolation of mutants defective in 35S ribosomal RNA synthesis in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7026–7030. doi: 10.1073/pnas.88.16.7026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogi Y., Yano R., Nomura M. Synthesis of large rRNAs by RNA polymerase II in mutants of Saccharomyces cerevisiae defective in RNA polymerase I. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3962–3966. doi: 10.1073/pnas.88.9.3962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagán-Ramos E., Tranguch A. J., Kindelberger D. W., Engelke D. R. Replacement of the Saccharomyces cerevisiae RPR1 gene with heterologous RNase P RNA genes. Nucleic Acids Res. 1994 Jan 25;22(2):200–207. doi: 10.1093/nar/22.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Brown D. D. A specific transcription factor that can bind either the 5S RNA gene or 5S RNA. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4170–4174. doi: 10.1073/pnas.77.7.4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper P. W., Bellatin J. A., Lockheart A. Altered maturation of sequences at the 3' terminus of 5S gene transcripts in a Saccharomyces cerevisiae mutant that lacks a RNA processing endonuclease. EMBO J. 1983;2(3):353–359. doi: 10.1002/j.1460-2075.1983.tb01430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds W. F., Gottesfeld J. M. 5S rRNA gene transcription factor IIIA alters the helical configuration of DNA. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1862–1866. doi: 10.1073/pnas.80.7.1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steitz J. A., Berg C., Hendrick J. P., La Branche-Chabot H., Metspalu A., Rinke J., Yario T. A 5S rRNA/L5 complex is a precursor to ribosome assembly in mammalian cells. J Cell Biol. 1988 Mar;106(3):545–556. doi: 10.1083/jcb.106.3.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stünkel W., Kober I., Kauer M., Taimor G., Seifart K. H. Human TFIIIA alone is sufficient to prevent nucleosomal repression of a homologous 5S gene. Nucleic Acids Res. 1995 Jan 11;23(1):109–116. doi: 10.1093/nar/23.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremethick D., Zucker K., Worcel A. The transcription complex of the 5 S RNA gene, but not transcription factor IIIA alone, prevents nucleosomal repression of transcription. J Biol Chem. 1990 Mar 25;265(9):5014–5023. [PubMed] [Google Scholar]
- Van Ryk D. I., Lee Y., Nazar R. N. Efficient expression and utilization of mutant 5 S rRNA in Saccharomyces cerevisiae. J Biol Chem. 1990 May 25;265(15):8377–8381. [PubMed] [Google Scholar]
- Wang C. K., Weil P. A. Purification and characterization of Saccharomyces cerevisiae transcription factor IIIA. J Biol Chem. 1989 Jan 15;264(2):1092–1099. [PubMed] [Google Scholar]
- Woychik N. A., Young R. A. Genes encoding transcription factor IIIA and the RNA polymerase common subunit RPB6 are divergently transcribed in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1992 May 1;89(9):3999–4003. doi: 10.1073/pnas.89.9.3999. [DOI] [PMC free article] [PubMed] [Google Scholar]