Abstract
The hypothalamic kisspeptin signaling system is a major positive regulator of the reproductive neuroendocrine axis, and loss of Kiss1 in the mouse results in infertility, a condition generally attributed to its hypogonadotropic hypogonadism. We demonstrate that in Kiss1−/− female mice, acute replacement of gonadotropins and estradiol restores ovulation, mating, and fertilization; however, these mice are still unable to achieve pregnancy because embryos fail to implant. Progesterone treatment did not overcome this defect. Kiss1+/− embryos transferred to a wild-type female mouse can successfully implant, demonstrating the defect is due to maternal factors. Kisspeptin and its receptor are expressed in the mouse uterus, and we suggest that it is the absence of uterine kisspeptin signaling that underlies the implantation failure. This absence, however, does not prevent the closure of the uterine implantation chamber, proper alignment of the embryo, and the ability of the uterus to undergo decidualization. Instead, the loss of Kiss1 expression specifically disrupts embryo attachment to the uterus. We observed that on the day of implantation, leukemia inhibitory factor (Lif), a cytokine that is absolutely required for implantation in mice, is weakly expressed in Kiss1−/− uterine glands and that the administration of exogenous Lif to hormone-primed Kiss1−/− female mice is sufficient to partially rescue implantation. Taken together, our study reveals that uterine kisspeptin signaling regulates glandular Lif levels, thereby identifying a novel and critical role for kisspeptin in regulating embryo implantation in the mouse. This study provides compelling reasons to explore this role in other species, particularly livestock and humans.
Kisspeptins are currently best known for being central regulators of the reproductive endocrine system. Kisspeptins potently stimulate the hypothalamic release of GnRH, thereby activating the entire reproductive endocrine cascade, with pituitary release of FSH and LH and, in turn, gonadal secretion of sex steroids and gametogenesis (1, 2). In the absence of kisspeptin signaling, GnRH secretion is markedly impaired, resulting in hypogonadotropic hypogonadism, and human patients with mutations in the genes for kisspeptin (KISS1) or its receptor (KISS1R; formerly called GPR54) and mice carrying targeted deletions in Kiss1 or Kiss1r fail to undergo sexual maturation and are infertile (1–7).
Based on a number of studies, extrahypothalamic kisspeptin signaling has been proposed as a regulator of placentation and pregnancy. These studies include the observations that KISS1 and KISS1R mRNA are expressed at high levels in the placenta (8) and that in nonpregnant women, plasma concentrations of immunoreactive kisspeptin are very low (∼1 fmol/mL), whereas in pregnant women kisspeptin levels increase dramatically, 940-fold in the first trimester and about 7000-fold in the third trimester (9). Kisspeptin also increases extravillous trophoblast adhesion in vitro (10), and this might in part account for the decrease in extravillous trophoblast invasion also observed in vitro (8). In addition to being expressed in the human placenta (8–10), KISS1 and KISS1R are also expressed in the human female genital tract (11). Collectively these findings suggest that kisspeptin is important for embryonic implantation, placentation, and pregnancy, independent of its effects on GnRH secretion.
To assess the role of kisspeptin in pregnancy in vivo, we have examined the ability of Kiss1−/− and Kiss1r−/− mice to become pregnant. Because an intact hypothalamic-pituitary-gonadal axis is required both for ovulation and for pregnancy maintenance, exogenous hormone replacement regimens were used to overcome the hormonal deficiencies of these mutant mice. This allowed us to determine whether mice defective in kisspeptin signaling have intrinsic, hormone-independent defects in the ability to achieve and maintain pregnancy.
Materials and Methods
Mice
Kiss1−/− (Kiss1tm1Rla) and Kiss1r−/− (Kiss1rtm1Rla) mice were generated as previously described (5). Animals were housed at the London Regional Cancer Program Animal Facility (London, Ontario, Canada) at controlled temperature and a 12-hour light, 12-hour dark cycle. Animal care and handling were done according to the guidelines of the University of Western Ontario (Canada) Animal Care Committee approved by the Canadian Council on Animal Care.
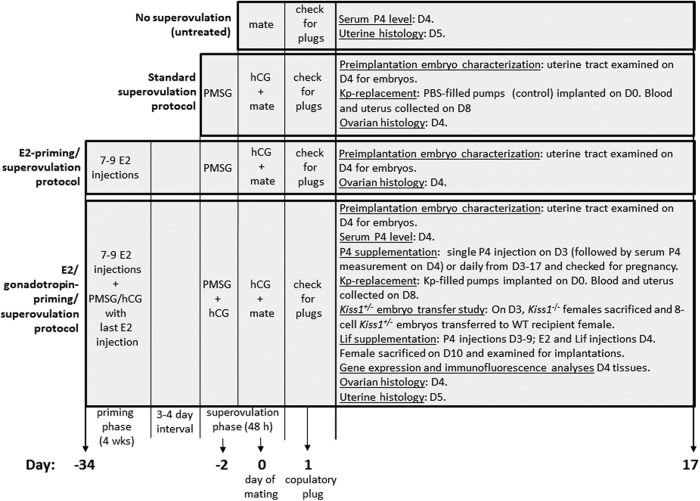
For the following methods, please refer to Figure 1.
Figure 1.
Chart summarizing the treatments used in Kiss1−/− female mice.
Standard superovulation protocol
Kiss1−/−, Kiss1r−/−, and wild-type (WT) female mice, 6–8 weeks old, were administered 7.5 IU pregnant mare serum gonadotrophin (PMSG; Folligon; Intervet) ip followed 48 hours later by 7.5 IU human chorionic gonadotropin (hCG; Chorulon; Intervet) ip Immediately after the hCG injection, mice were mated to WT males. The day of mating is defined as day 0. Only females that showed a copulatory plug (evidence of successful mating) on the morning of day 1 were studied further.
17β-Estradiol (E2) priming/superovulation protocol
Four-week-old Kiss1−/− and Kiss1r−/− female mice received seven to nine injections of E2 (Sigma) 1 μg in 100 μL sesame oil (Sigma) per injection sc every 3–4 days over a 4-week period. Three to four days after the last E2 injection, E2-primed mice underwent superovulation as described above. Mice were approximately 9 weeks old at the end of this treatment.
E2/gonadotropin priming/superovulation protocol
Four-week-old Kiss1−/−, Kiss1r−/−, and WT female mice received seven to nine E2 injections over a 4-week period as described above. Coincident with the last E2 injection, mice received 7.5 IU PMSG/7.5 IU hCG ip. Three to four days later, mice received 7.5 IU PMSG/1.0 IU hCG ip and 48 hours later 7.5 IU hCG ip. Immediately after the final hCG injection, mice were mated to WT males. Mice were approximately 9 weeks old at the end of this treatment.
Progesterone (P4) supplementation
E2/gonadotropin-primed/superovulated Kiss1−/− females received a single injection of P4 (1 mg in 100 μL peanut oil; Sigma) sc either once on the third day (day 3) after mating or daily from day 3 to day 17. E2 (20 ng in 100 μL peanut oil) was injected sc on day 4 to further induce uterine receptivity (12).
Isolation and characterization of preimplantation embryos
Time after hCG was used to determine the developmental age of the embryos. Preimplantation embryos were collected at 66 hours (eight cell stage) from both the oviduct and the uterus (for the Kiss1+/− embryo transfer study, see below), and on day 4 from the uterus (for characterizing preimplantation embryonic development, see Table 1) (13). Embryos were flushed from the reproductive tract using M2 medium (Sigma).
Table 1.
Number and Stages of Preimplantation Embryos Obtained on Day 4 After Superovulated or E2/Gonadotropin-Primed/SO WT and Kiss1−/− Females Were Mated to WT Males
| Embryonic Stage | WT Females After Standard SO (n = 6) | WT Females After E2/Gonadotropin-Priming/SO (n = 6) | Kiss1−/− Females After Standard SO (n = 6) | Kiss1−/− Females After E2/Gonadotropin-Priming/SO (n = 9) |
|---|---|---|---|---|
| Two cell | 0 | 0 | 0 | 0 |
| Four cell | 0 | 0 | 0 | 0 |
| Eight cell | 0.3 ± 0.3 | 0 | 0 | 0 |
| Morula | 7.2 ± 1.4 | 7.7 ± 1.6 | 0 | 2.9 ± 1.0 |
| Blastocysts | 7.2 ± 1.7 | 11.0 ± 2.3 | 0 | 5.8 ± 1.3 |
| Degenerate | 8.0 ± 2.9 | 7.0 ± 0.7 | 0 | 8.7 ± 3.0 |
| Mean ± SEM for viable embryos (morula and blastocyst) per mouse | 14.7 ± 2.6 | 18.7 ± 3.7 | 0 | 8.7 ± 1.7 |
Abbreviation: SO, superovulated. Data are shown as mean ± SEM.
Leukemia inhibitory factor (Lif) supplementation
E2/gonadotropin-primed/superovulated Kiss1−/− females received single P4 (1 mg) injections sc daily from the third to the ninth day (day 3 to day 9) after mating. On day 4 at 10:00 am, mice received a single E2 (20 ng) injection sc that was coadministered with the P4 injection; 4–6 hours later they were given a single ip injection of Lif (Escherichia coli-derived recombinant mouse Lif; eBioscience, Inc) 10, 12.5, or 17 μg. Lif dosing was based on previous studies (14, 15). On day 10, mice were killed by CO2 exposure followed by cervical dislocation, and uteri were examined for embryo implantation sites.
LH, P4, and prolactin (PRL) assays
Serum LH, P4, and PRL were measured by the Endocrine Technology and Support Lab, Oregon National Primate Research Center (Beaverton, Oregon). Blood was allowed to clot at room temperature for 120 minutes. The clot was then removed with a wooden applicator stick and the remaining serum centrifuged at 2000 × g for 20 minutes at room temperature. LH was analyzed by a traditional double-antibody RIA procedure similar to that described previously (16). The detection limit of the assay was 0.1–0.2 ng/mL. The interassay variation was less than 12% and the intraassay variation was less than 8%. P4 was measured by ether extraction RIA (17). The detection range of the assay was 0.17–25 ng/mL. The overall interassay variation was less than 15% and the intraassay variations were 10% or less. PRL was determined using a commercial ELISA kit (Abcam catalog number 100736) calibrated specifically for the mouse. This assay has a range of 27.43–20 000 pg/mL. The intra- and interassay variations were less than 5% and 10%, respectively.
Kisspeptin replacement
E2/gonadotropin-primed/superovulated Kiss1−/− and superovulated WT mice were implanted sc with osmotic minipumps (Alzet model 2001) that delivered Kp-10 (Sigma) at a rate of 0.25 nmol/h (1 μL/h) or vehicle alone (PBS) for 8 days. Implantation was done on the morning of day 0 (the day of mating), 2 days after the mice received 7.5 IU PMSG/1.0 IU hCG. On the afternoon of day 0, mice were administered 7.5 IU hCG ip and mated immediately afterward to WT males. On the morning of day 8, the mice were anesthetized by CO2 exposure followed by cervical dislocation, and blood was collected immediately afterward by cardiac puncture. Uteri were examined for embryo implantation sites. In a companion study designed to determine the effect of prolonged kisspeptin (Kp)-10 on Kiss1r signaling, 6- to 8-week-old WT female mice were ovariectomized and one week later were separated into four groups. Each group was implanted sc with osmotic minipumps that delivered PBS to two groups for 8 days or Kp-10 (Sigma) 1.0 μl (0.25 nmol)/h to the remaining two groups for 8 days. On the morning of the eighth day, one PBS- and one Kp-10-infused group received 100 μL of PBS ip, whereas one PBS- and one Kp-10-infused group received 100 μL (50 nmol) of Kp-10 ip. Thirty minutes later, the mice were anesthetized by CO2 exposure followed by cervical dislocation, and blood was collected immediately after by cardiac puncture.
Artificial decidualization
Experiments were conducted similarly to that described previously (14). Six-week-old female Kiss1−/− (n = 4) and WT control (n = 4) mice were bilaterally ovariectomized under isoflurane anesthesia. Approximately 2 weeks later, the mice were given daily sc injections of 100 ng of E2 (Sigma) prepared in sesame oil for 3 days. After 2 days of rest, the mice received daily injections of 1 mg P4 (Sigma) plus 10 ng of E2 prepared in sesame oil for 2 days. On the third day, under isoflurane anesthesia, 50 μL of sesame oil was injected into the lumen of the left uterine horn as a decidual stimulus. After all of the surgical procedures, ketoprofen was given for 2 days for pain relief. Daily sc injections of P4 (1 mg) were continued for 4 days. On the fifth day, the uterus was removed and analyzed.
The following methods are described in the Supplemental Information: Kiss1+/− embryo transfer study; quantitative RT-PCR analysis of gene expression (Supplemental Table 3); histology; immunofluorescence analysis (Supplemental Table 4); immunohistochemistry (Supplemental Table 4); Western blotting (Supplemental Table 4); in situ analysis; and statistical analysis.
Results
Priming with both E2 and gonadotropins is required for follicular development in Kiss1−/− and Kiss1r−/− mice
In Kiss1−/− and Kiss1r−/− mice, follicular development stalls at the antral stage, and knockout females mated to WT males of proven fertility do not become pregnant (2–5). To determine whether the infertility in these animals was due solely to their neuroendocrine defects, we bypassed the gonadotropin deficiency of Kiss1−/− and Kiss1r−/− female mice by administering exogenous gonadotropins using a standard superovulation protocol (Figure 1). Treated female mice housed with fertile WT males (day 0) never displayed copulatory plugs, indicating that there was no successful mating. On day 4, gross examination revealed that the ovaries of gonadotropin-treated Kiss1−/− and Kiss1r−/− females were pale and indistinguishable from those of untreated Kiss1−/− and Kiss1r−/− mice. Additionally, they were approximately 75% smaller than ovaries from superovulated WT females [Supplemental Figure 1, A and B (data shown for Kiss1−/− females only)], and histological analysis revealed follicular development stalled at the early antral stage (Supplemental Figure 1, A and B), similar to what had previously been reported for untreated Kiss1−/− and Kiss1r−/− females (2–5). Upon flushing the dissected uterine tracts from superovulated Kiss1−/− and Kiss1r−/− females on day 4, neither oocytes nor embryos were recovered (Table 1, data shown for Kiss1−/− females only), and females never showed outward signs of being pregnant nor did they give birth. In striking contrast, WT females that underwent the superovulation protocol displayed copulatory plugs after mating and possessed larger genital tracts and an abundance of corpora lutea (CL) (Supplemental Figure 1A). Preimplantation embryos could be obtained from superovulated WT females (Table 1), and these mice exhibited successful pregnancies. We conclude that gonadotropin replacement administered through a standard superovulation protocol is not sufficient to restore follicular development and ovulation in Kiss1−/− and Kiss1r−/− mice. In subsequent studies we focused our attention on Kiss1−/− female mice only.
Because estradiol acts synergistically with FSH to enhance follicular proliferation and differentiation in hypophysectomized mice (18), we next determined whether E2 priming coupled to the standard superovulation protocol was sufficient to trigger follicular maturation and ovulation. We refer to this as the E2 priming/superovulation protocol (Figure 1). In two independent trials, we found this treatment was sufficient to trigger ovulation. However, upon histological examination, we observed a large number of CL in the ovaries that contained unreleased eggs (data not shown).
We then tested whether E2 priming coupled to a brief period of gonadotropin priming followed by the standard superovulation protocol could rescue follicular development and trigger ovulation more efficiently. We refer to this as the E2/gonadotropin priming/superovulation protocol (Figure 1). On the day after mating, approximately half of Kiss1−/− females that underwent this protocol displayed a copulatory plug. Their ovaries contained multiple CL similar in size and number to those of age-matched WT females that had undergone either the standard superovulation protocol (Supplemental Figure 1, A and C) or the E2/gonadotropin priming/superovulation protocol (data not shown). Furthermore, these CL did not contain unreleased eggs. Upon flushing the dissected uterine tracts, embryos were detected ranging from the eight-cell to blastocyst stages (Table 1). The numbers of total and viable embryos recovered from E2/gonadotropin-primed/superovulated Kiss1−/− and superovulated WT or E2/gonadotropin-primed/superovulated WT females were comparable (P > .05) (Table 1).
Based on the results from the three trials just described, we used the E2/gonadotropin priming/superovulation protocol for conducting subsequent studies. Importantly and irrespective of the protocol used, our data clearly revealed that the defects in the folliculogenesis and ovulation observed in mice deficient for kisspeptin signaling are secondary to their hypogonadotropic state.
E2/gonadotropin-primed/superovulated Kiss1−/− mice fail to achieve pregnancy due to defects in embryo implantation
Although the E2/gonadotropin priming/superovulation protocol successfully induced follicular maturation and ovulation in Kiss1−/− mice, these mice failed to become pregnant after mating (n = 3). In contrast, WT females that underwent the E2/gonadotropin priming/superovulation protocol went on to have successful and normal pregnancies. Mating plugs were observed in approximately 60% of E2/gonadotropin-primed/superovulated Kiss1−/− females mated to WT males vs approximately 70% of WT females mated to WT males, and preimplantation embryos could be retrieved from the uterine tracts on day 4. Thus, the inability to achieve pregnancy could not be attributed to defects in mating, fertilization, or early development. Furthermore, because implantation sites were not detected on day 7 (n = 3) or day 14 (n = 2) in the dissected uterine tracts and unimplanted blastocysts could be flushed out of the uterus as late as day 7, failure to become pregnant was likely due to an implantation defect.
E2/gonadotropin-primed/superovulated Kiss1−/− mice produce normal amounts of P4
Secretion of P4 by the CL is essential for the development of endometrial receptivity for blastocyst implantation and the maintenance of pregnancy (19). To determine whether the implantation defect observed in Kiss1−/− mice could be due to deficiency of P4, measurements of P4 were performed in Kiss1−/− and WT mice on day 4 after mating (Table 2). The P4 levels in the untreated WT females were greater than that of untreated Kiss1−/− females (43.5 ± 10.1 vs 7.1 ± 0.6 ng/mL) (P < .05), an observation consistent with the absence of CL in the Kiss1−/− females (2–5). The P4 levels in the E2/gonadotropin-primed/superovulated Kiss1−/− mice were approximately 6-fold greater than those in untreated Kiss1−/− females (44.3 ± 10.3 vs 7.1 ± 0.6 ng/mL) (P < .05), indicating that hormone treatment produced functional CL in the Kiss1−/− females. Finally, we compared the P4 levels in the treated Kiss1−/− females with untreated WT females (44.3 ± 10.3 vs 43.5 ± 10.1 ng/mL) (P > .05) and superovulated WT females (44.3 ± 10.3 vs 109.0 ± 23.7 ng/mL) (P > .05). Although the P4 levels were statistically similar among the three groups, confirming that hormone treatment produced functional CL in the Kiss1−/− females, the P4 levels trended lower in the treated Kiss1−/− and untreated WT females vs superovulated WT females.
Table 2.
P4 Levels Measured on the Fourth Day After Female Mice From the Groups Were Mated to WT Males
| Hormone | WT, SO (n = 9) | WT, Untreated (n = 6) | Kiss1−/− E2/Gonadotropin-Primed/SO (n = 6) | Kiss1−/− Untreated (n = 4) | Kiss1−/− E2/Gonadotropin-Primed/SO+P4 (Day 3) +E2 (Day 4) (n = 5) |
|---|---|---|---|---|---|
| P4, ng/mL | 109.0 ± 23.7 | 43.5 ± 10.1 | 44.3 ± 10.3 | 7.1 ± 0.6 | 83.1 ± 7.9 |
Abbreviation: SO, superovulated. Groups are as follows: 1) SO WT females; 2) untreated WT females; 3) E2/gonadotropin-primed SO Kiss1−/− females; 4) untreated Kiss1−/− females; and 5) E2/gonadotropin-primed SO Kiss1−/− females that received a P4 injection (sc) (1 mg/100 μL) on the third day after mating followed by an E2 injection (sc) (20 ng/100 μL) on the morning of day 4. Data are shown as mean ± SEM.
P4 and E2 supplementation does not rescue implantation in E2/gonadotropin-primed/superovulated Kiss1−/− mice
Although no significant differences in serum P4 levels were found between the E2/gonadotropin-primed/superovulated Kiss1−/− and WT mice on day 4 after mating to WT males (Table 2), it remains possible that subtle differences in P4 production could have resulted in impaired endometrial receptivity. To address this possibility, E2/gonadotropin-primed/superovulated Kiss1−/− females were injected with P4 on the third day after mating followed by an E2 injection on day 4 (day of implantation) to potentiate uterine receptivity (12). Although the P4 injection resulted in P4 levels in animals that were nearly 2-fold higher than that in noninjected E2/gonadotropin-primed/superovulated Kiss1−/− females [83.1 ± 7.9 vs 44.3 ± 10.3 ng/mL (Table 2)], P4 and E2 treatment did not rescue implantation. To determine whether more prolonged exposure to P4 could rescue implantation, mice were injected repeatedly with P4 from day 4 through day 17; these mice also failed to become pregnant.
P4 and E2 receptors are expressed in the Kiss1−/− uterus
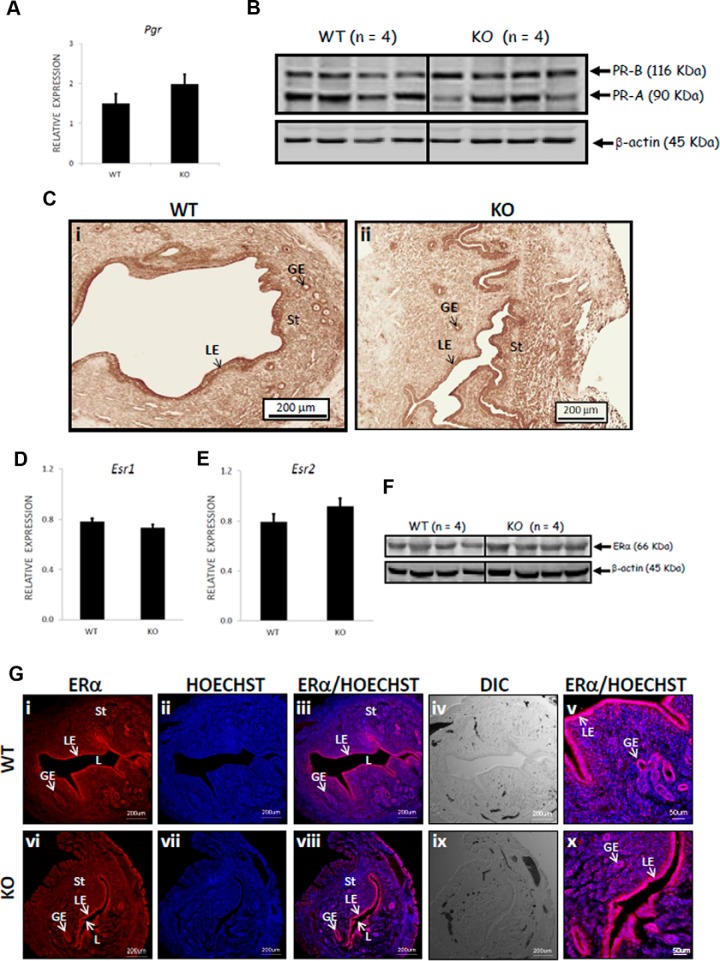
Given the observation that E2 and P4 supplementation failed to rescue implantation, we considered the possibility that the expression of Pgr, Esr1, and Esr2, the genes encoding for the progesterone and estrogen receptors (α and β), respectively, was down-regulated in the Kiss1−/− uterus, and thus, the uterus was unable to respond to these hormonal signals. However, in day 4 (1:00 pm) uterine tissue, RT-PCR analyses confirmed all genes were expressed at similar levels (P > .05) in both the superovulated WT and E2/gonadotropin-primed/superovulated Kiss1−/− females (Figure 2, A, D, and E). Western blot analyses revealed that both progesterone receptor (PR) isoforms and the estrogen receptor (ER)-α were also expressed at similar levels between WT and Kiss1−/− mice (Figure 2, B and F). Additionally, the levels and spatial distribution of PR and ERα, as determined by immunohistochemistry/immunofluorescence, were similar between the WT and Kiss1−/− mice, with both receptors localized to the luminal and glandular epithelia and endometrial stromal cells (Figure 2, C and G). Taken together, the data indicate that the expression of PR-A, PR-B, and ERα was similar between genotypes and therefore that changes in expression levels were not the cause of the implantation defect.
Figure 2.
Pgr, Esr, PR, and ER are expressed at similar levels in the uteri of Kiss1−/− and WT mice. Relative to the expression of two housekeeping genes (Hprt, Sdha), the expression of Pgr, Esr1, and Esr2 (A, D, and E) was determined by quantitative RT-PCR [for superovulated WT female mice, n = 6; for E2/gonadotropin-primed/superovulated KO (Kiss1−/−) mice, n = 4]. The spatial distribution and expression levels of PR-A and PR-B (the PR antibody detects both isoforms) were determined by immunohistochemistry, whereas that of ERα was determined by immunofluorescence (C and G) (for WT, n = 4; for KO (Kiss1−/−), n = 4). The expression level of PR-A, PR-B, and ERα was also determined by Western blotting (B and F) (for WT, n = 4; for knockout (KO) (Kiss1−/−), n = 4). Error bars represent SEM. DIC, differential interference contrast; GE, glandular epithelium; L, lumen; LE, luminal epithelium; St, stroma; HOECHST, nucleic acid stain that identifies nuclei.
Implantation failure in Kiss1−/− mice is due to a maternal defect
We found that E2/gonadotropin priming/superovulation restored follicular development, triggered ovulation, and generated functional CL in Kiss1−/− females. Additionally, Kiss1−/− oocytes were successfully fertilized in vivo. Despite their normal appearance, it was possible that some impairment in Kiss1+/− blastocysts was the underlying cause of implantation failure. To test this possibility, eight-cell Kiss1+/− embryos generated from E2/gonadotropin-primed/superovulated Kiss1−/− females and eight-cell WT control embryos from superovulated WT females (both mated to WT males) were transferred into the oviducts of a recipient WT female (n = 3). Both WT and Kiss1+/− embryos implanted successfully and developed at a similar rate (Supplemental Figure 2), leading us to conclude that the failure of Kiss1+/− embryos to implant in a Kiss1−/− female is a maternal and not an embryonic defect.
The Kiss1/Kiss1r signaling system is expressed in the ovaries, oviducts, and uterus of mice
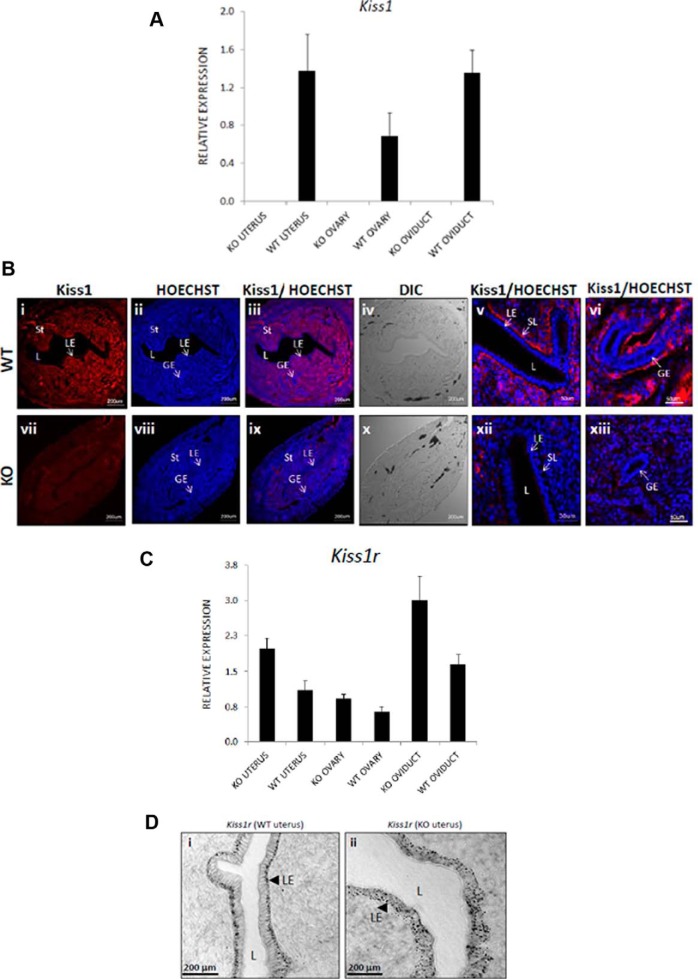
Because implantation failure could be attributed to a maternal defect, the expression of Kiss1 and Kiss1r in the female reproductive tract was examined on day 4 (1:00 pm) using RT-PCR. The expression of Kiss1 and Kiss1r was demonstrated in the ovary, oviduct, and uterus of WT mice (Figure 3). Kiss1 expression, as expected, was absent from these tissues in the Kiss1−/− mouse (Figure 3A). Uterine kisspeptin expression was also determined by immunofluorescence analysis (Figure 3B). Again, as expected, kisspeptin was not expressed in the Kiss1−/− mouse (Figure 3B, panels vii-xiii). In the WT mouse, however, kisspeptin was detected at high levels throughout the endometrium (Figure 3B, panels i-vi) and upon closer investigation was found to be strongly localized to the subluminal epithelial layer (Figure 3B, panel v) and on the outside of the glandular epithelium (Figure 3B, panel vi). Based on quantitative RT-PCR analysis of gene expression, relative to WT mice, Kiss1−/− mice demonstrated a nonsignificant trend toward increased Kiss1r expression in the ovary (P = .094) and a significant increase in expression in the oviduct (P = .020) and uterus (P = .023) (Figure 3B). Based on in situ analysis, we observed that in the WT uterus, Kiss1r is distributed to the distal face of the luminal epithelial layer (Figure 3D, panel i), whereas in the Kiss1−/− uterus, the distribution is more dispersed within the epithelial layer (Figure 3D, panel ii). Overall, based on visual inspection of the in situ data, it appears as though Kiss1r expression is elevated in Kiss1−/− luminal epithelial cells, a finding that is consistent with the quantitative RT-PCR analysis. These findings demonstrate that the kisspeptin/Kiss1r signaling system is expressed in the WT female reproductive tract.
Figure 3.
The Kiss1/Kiss1r signaling system is expressed in the ovaries, oviducts, and uteri of Kiss1−/− and WT mice. In the ovaries, oviducts, and uteri of E2/gonadotropin-primed/superovulated KO (Kiss1−/−) female mice, relative to the expression of two housekeeping genes (Hprt, Sdha), the expression of Kiss1 and Kiss1r (A and C) [for WT, n = 6; for KO (Kiss1−/−), n = 4] was determined by quantitative RT-PCR. The expression of Kiss1r was further determined by in situ analysis (D, arrowheads show Kiss1r is localized to the luminal epithelium) [for WT, n = 4; for KO (Kiss1−/−), n = 4], whereas the expression of Kiss1 was determined by immunofluorescence (B) [for WT, n = 4; for KO (Kiss1−/−), n = 4]. Error bars represent SEM. DIC, differential interference contrast; GE, glandular epithelium; KO, knockout; L, lumen; LE, luminal epithelium; SL, subluminal epithelial layer; St, stroma; HOECHST, nucleic acid stain that identifies nuclei.
Kisspeptin replacement fails to rescue implantation in E2/gonadotropin-primed/superovulated Kiss1−/− mice
To determine whether restoring kisspeptin to Kiss1−/− mice could rescue implantation, we delivered Kp-10 sc for 8 days at a rate of 0.25 nmol/h, a dose based on published studies using Kp-10 in a rat model (20) and adjusted based on weight. First, to exclude the possibility that exogenous continuous kisspeptin administration itself could disrupt pregnancy, superovulated WT females received either Kp-10 or PBS. In both groups examined on day 8, implanted WT embryos were present in both horns of the examined uteri, and pregnancy outcomes for the WT females receiving Kp-10 were comparable with WT females receiving PBS (five of seven WT females receiving Kp-10 became pregnant with 12, 23, 12, 9, and 18 embryo implantations per mouse vs four of four WT females receiving PBS became pregnant with 14, 12, 23, and 20 embryo implantations per mouse, P > .05). In contrast, on day 8, E2/gonadotropin-primed/superovulated Kiss1−/− females that received infusions of either Kp-10 or vehicle alone displayed no evidence of implantation. Hormone measurements revealed that P4 levels were not significantly different between superovulated WT and E2/gonadotropin-primed/superovulated Kiss1−/− females (Supplemental Table 1) and that LH levels (Supplemental Table 1) were comparable in magnitude with levels reported for unstimulated WT and Kiss1−/− mice (5). PRL, a major luteotropic hormone essential for CL function in mice, was comparable across treatments and genotypes (Supplemental Table 1).
Because both WT and Kiss1−/− mice were chronically infused with kisspeptin for the same period of time (8 d) and WT mice became pregnant at a rate similar to WT mice receiving PBS, uterine Kiss1r desensitization could not account for the implantation failure in the Kiss1−/− mice. To investigate this further, we determined whether hypothalamic Kiss1r displayed evidence of desensitization after 8 days of Kp-10 infusion. The ovariectomized mice (with effective gonadectomy demonstrated by low P4 levels, Supplemental Table 2) were infused with either Kp-10 or PBS for 8 days and then received a single ip injection of either PBS or 50 nmol Kp-10. The LH levels were significantly (P < .05) higher in the animals that received the Kp-10 ip injection, regardless of whether the baseline infusion was PBS or Kp-10 (Supplemental Table 2). Thus, assuming that the hypothalamic Kiss1r function is an appropriate surrogate for uterine Kiss1r function, we concluded that the chronic infusion of Kp did not desensitize Kiss1r.
Kiss1−/− and WT uteri are histologically similar
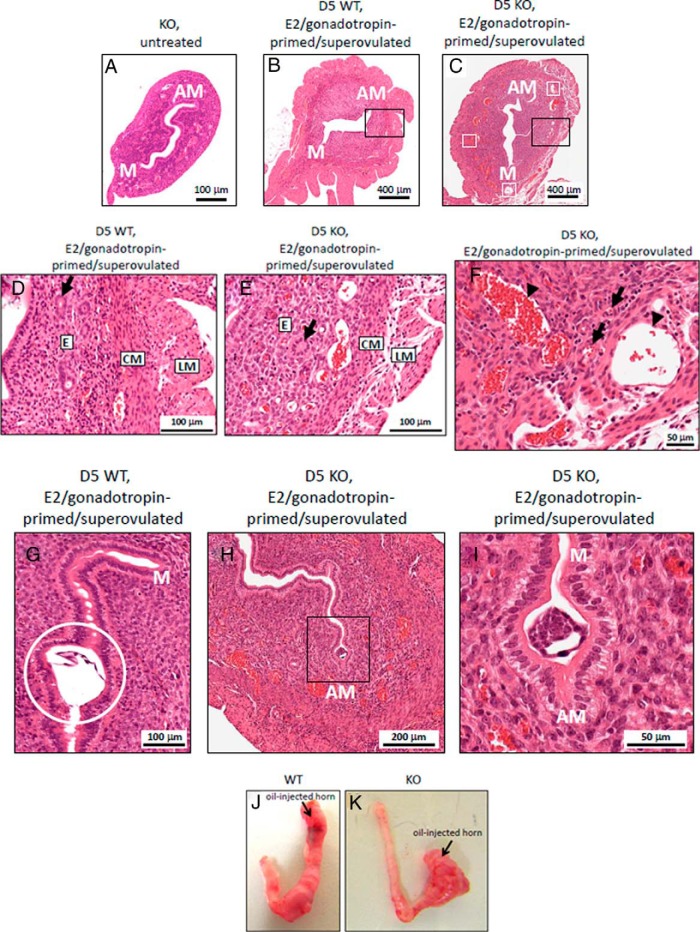
We next examined whether the underlying cause of implantation failure was an anatomical defect of the Kiss1−/− uterus. The uterine horns from 6- to 12-week-old untreated Kiss1−/− females were threadlike as previously reported (4, 5), whereas the horns from age-matched E2/gonadotropin-primed/superovulated Kiss1−/− and WT females were approximately 4 times larger in diameter (P < .001) (Figure 4, A–C). Despite the difference in size, uterine anatomy between the WT and Kiss1−/− mice was very similar (Figure 4, A–C). The only striking difference detected was the greater presence of dilated blood vessels localized to the basal endometrium at the interface with circular smooth muscle layer (Figure 4, C, E, and F). On higher-power images (Figure 4F), numerous tightly constricted arterioles are present, identified by surrounding vascular smooth muscle (black arrows). This suggests that the dilated structures (black arrowheads) are veins. The presence of increased numbers of these dilated endometrial vessels was not due to E2/gonadotropin priming/superovulation because uteri from the WT mice that had undergone the same treatment had fewer such vessels (Figure 4, B and D).
Figure 4.
Histology and morphology of Kiss1−/− and WT mice uteri. Anatomy of the representative uteri of the age-matched female mice is as follows: untreated KO (Kiss1−/−) female mice (A), E2/gonadotropin-primed/superovulated WT female mice (B), and E2/gonadotropin-primed/superovulated KO female mice (C) on day 5 after mating; white boxes show dilated blood vessels. Please note the different magnifications in panel A vs panels B and C. D–F, Higher-magnification images of the uterine sections corresponding to E2/gonadotropin-primed/superovulated WT and knockout (KO) female mice on day 5 after mating. D, Normal uterine architecture (corresponds to black outlined box in panel B) from E2/gonadotropin-primed/superovulated WT female mice on day 5 after mating. E, Abnormal uterine architecture (corresponds to black outlined box in panel C) from E2/gonadotropin-primed/superovulated KO female mice on day 5 after mating. F, Dilated vessels (corresponds to lower part of image shown in panel C) of E2/gonadotropin-primed/superovulated KO female mice on day 5 after mating. G, An implanted WT embryo (enclosed within white outlined circle) is observed near the antimesometrial (AM) pole of an E2/gonadotropin-primed/superovulated WT female mouse on day 5 after mating. H, Nonimplanted Kiss1+/− embryo (seen within black outlined box) is observed near the AM pole of an E2/gonadotropin-primed/superovulated KO female mouse on day 5 after mating. I, Nonimplanted Kiss1+/− embryo (as seen within black outlined box in panel H) is shown at higher-power magnification. Please note the different magnifications shown in panels G–I. J, Representative images of uterine horns after the injection of oil into the left horn of the hormone-primed ovariectomized WT (J) (n = 4) and KO female (K) (n = 4). CM, circular muscle of the myometrium; D, endometrium; GO, gland opening to uterine lumen; LM, longitudinal muscle; M, mesometrial pole.
The Kiss1−/− uterus remains partially responsive to the embryo and can undergo decidualization in response to an artificial stimulus
Histological analysis clearly revealed that on the afternoon of day 5 in E2/gonadotropin-primed/superovulated WT mothers, WT embryos had implanted (n = 5) (Figure 4G), whereas Kiss1+/−embryos had not (n = 5) in their E2/gonadotropin-primed/superovulated Kiss1−/− mothers (Figure 4, H and I). Nevertheless, the Kiss1+/− embryo was oriented properly with its embryonic-antiembryonic axis aligned to the mesometrial-antimesometrial axis of the Kiss1−/− uterine horn (Figure 4, H and I) (21, 22), and closure of the implantation chamber around the Kiss1+/− embryo was clearly observed (Figure 4, H and I). Additionally, an artificial decidualization study revealed that the hormone-primed uterus of the Kiss1−/− mouse (n = 4) could undergo decidualization as effectively as the WT uterus (n = 4) (Figure 4, J and K), suggesting the uterus could support a pregnancy.
Lif supplementation partially rescues implantation in hormone-primed Kiss1−/− mice
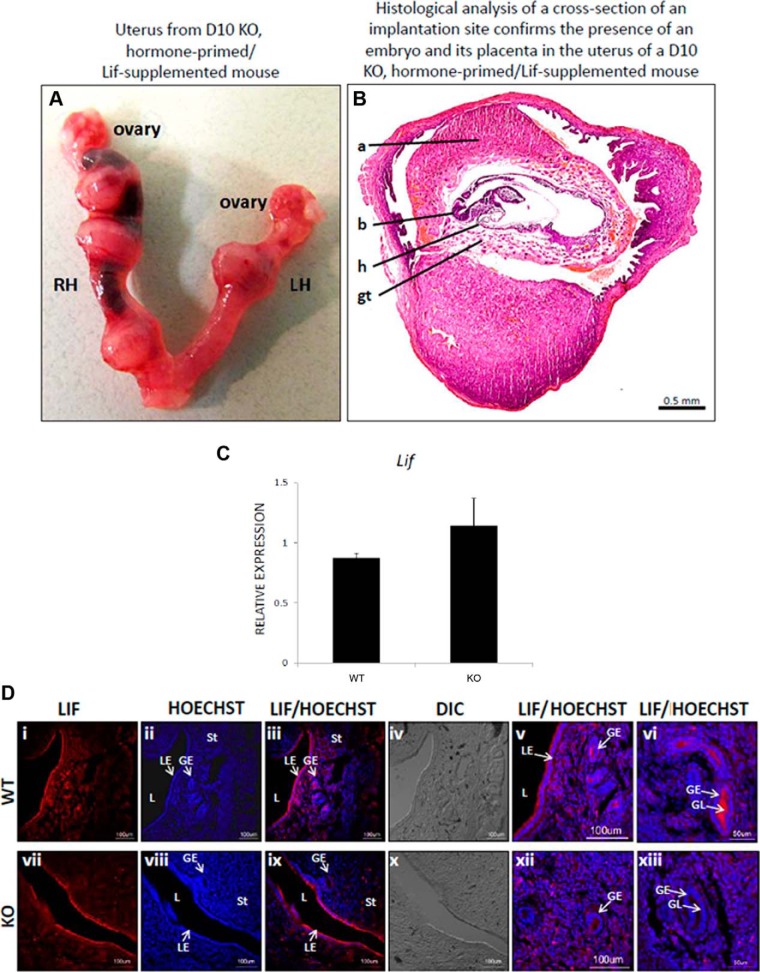
Because events upstream of implantation (ovulation, mating, fertilization, embryonic maturation, embryo orientation in the implantation chamber, and chamber closure) and downstream of implantation (decidualization) were all intact in E2/gonadotropin-primed/superovulated Kiss1−/− mice, these results collectively suggest that Kiss1−/− mice lack a signal required for embryonic implantation. Because the Lif is essential for implantation in mice (19, 23–25), we determined whether exogenous recombinant Lif, administered in conjunction with P4 and E2 to E2/gonadotropin-primed/superovulated Kiss1−/− females, could rescue the implantation defect. Examination of Kiss1−/− uteri on day 10 revealed no implantations in the five mice that received Lif 10 μg and in one mouse that received Lif 17 μg. However, in the two mice that received Lif 12.5 μg, two implanted and two resorbed embryos were observed, whereas in two mice that received Lif 17 μg, four implanted and one resorbed embryos were observed (Table 3 and Figure 5A). Histological analysis of a cross-section of an implantation site (from a mouse that received Lif 17 μg) revealed the presence of a developing embryo (Figure 5B).
Table 3.
Effect of Lif Administration on Embryo Implantation in Hormone-Primed Kiss1−/− Female Mice Mated to WT Male Mice
| Lif (μg) | Number of Mice | Number of Uteri With Viable and Nonviable Implantations (Total Number of Viable Embryos/Total Number of Resorbed Embryos) | Number of Uteri With No Implantations |
|---|---|---|---|
| 10.0 | 5 | 0 | 5 |
| 12.5 | 2 | 2 (2/2) | 0 |
| 17.0 | 3 | 2 (4/1) | 1 |
Figure 5.
Lif rescues implantation in a hormone-primed Kiss1−/− mouse uterus. A, Right uterine horn (RH) of a Lif-treated, hormone-primed Kiss1−/− mouse bears three implanted embryos, whereas the left uterine horn (LH) bears one. B, Histological analysis of a cross-section of an implantation site confirms the presence of an embryo and its placenta. C–E, Relative to the expression of two housekeeping genes (Hprt, Sdha), the expression of Lif (C) was determined by quantitative RT-PCR [for WT, n = 6; for knockout (KO; Kiss1−/−), n = 4], whereas the expression of Lif was determined by immunofluorescence (D) [for WT, n = 4; for KO (Kiss1−/−), n = 4]. Error bars represent SEM. a, amnion; b, brain; gt, giant trophoblast cells; h, heart; DIC, differential interference contrast; GE, glandular epithelium; L, lumen; LE, luminal epithelium; St, stroma; HOECHST, nucleic acid stain that identifies nuclei.
Kiss1−/− mice exhibit lower levels of Lif in glandular lumen
Based on this Lif-dependent rescue of implantation, we hypothesized Lif expression is reduced in the Kiss1−/− female mouse and proceeded to test this. RT-PCR and immunofluorescence analyses of gene and protein expression, respectively, in the day 4 uterus of E2/gonadotropin-primed/superovulated WT and Kiss1−/− females that did not receive exogenous Lif revealed no difference in Lif mRNA expression between Kiss1−/− and WT hormone-primed control females (P = .35) (Figure 5C). However, at the protein level, although both WT and Kiss1−/− mice expressed Lif in the endometrium (Figure 5D) with high levels on the luminal face of the luminal epithelium (Figure 5D, panels iii and ix), Kiss1−/− mice exhibited noticeably lower levels of Lif in the glandular lumen (Figure 5D, panels v and vi vs xii and xiii). Thus, it appears that, unlike WT mice, Kiss1−/− secretes lower levels of glandular Lif on the afternoon of day 4.
Discussion
The infertility of Kiss1−/− female mice has generally been attributed to their hypogonadotropic hypogonadism. We have now demonstrated that acute replacement of gonadotropins and estradiol can restore ovulation, mating, fertilization, and preimplantation development, but these mice are unable to achieve pregnancy, even after administration of progesterone and kisspeptin. This infertility arises from defective implantation, which is due to a maternal defect because embryos transferred to a WT mouse can implant successfully. The role of extrahypothalamic kisspeptin signaling in regulating reproduction has been largely recognized through indirect correlation, such as the rapid rise in plasma kisspeptin levels during pregnancy (9) and in vitro studies, such as those demonstrating kisspeptin regulates extravillous trophoblast adhesion and migration in culture (8, 10). Our study is the first to provide direct evidence that extrahypothalamic kisspeptin signaling regulates reproduction using an in vivo model.
Our study also identifies a previously unappreciated but very important relationship between kisspeptin and Lif in the mouse uterus. Lif is a member of the IL-6 cytokine family, which also includes IL-6 and IL-11. In the WT mouse, from day 1 to day 3 Lif mRNA is expressed in the uterine epithelium, whereas on day 4, the day of receptivity, Lif is transiently and exclusively expressed in glandular epithelia (19, 23–25). Glandular Lif is then secreted into the lumen of the uterus in which it binds the Lif receptor/gp130 heterodimeric complex on uterine epithelial cells to phosphorylate and activate signal transducer and activator of transcription 3, thereby triggering the expression of a number of genes critical to implantation (25). On the afternoon of day 4, we found that within the glandular lumen, Lif was more readily detected in the WT than Kiss1−/− uterus. This suggests that uterine kisspeptin signaling regulates glandular Lif levels and activation of the Lif receptor/gp130/signal transducer and activator of transcription 3 pathway to thereby mediate embryo implantation. Reduced endometrial Lif levels may also be the result of a reduced number of uterine glands in the Kiss1−/− mouse, as previously reported (4).
In the Lif supplementation studies, the fact that the rescue of implantation was only partial might be due to inadequate penetration of Lif to the sites of implantation. Alternatively, Kiss1−/− female mice may lack other factors essential for implantation. To date, in mice and rats, a number of maternal factors have been demonstrated to be essential for implantation. These include estrogen, P4, cytokines including Lif, and growth factors including TGF-β and epidermal growth factor (26).
Surprisingly, although we could partially rescue implantation in Kiss1−/− mice with Lif, we could not do so through chronic administration of Kp-10. Because Kp-10 is rapidly degraded in serum (27), we might have failed to generate sufficient local concentrations needed to restore implantation. Future studies using intrauterine administration of Kp may overcome this potential issue.
Our study adds to prior reports on the expression of the Kp/Kiss1r signaling system in the ovary and oviduct/fallopian tube (11, 28–30) and is among the first to report on its expression in the uterus (11). Based on these expression patterns, kisspeptin has been proposed to have direct roles in the ovarian regulation of follicular development and ovulation and in the prevention of tubal pregnancies resulting from premature and inappropriate implantation of embryos in the oviduct (11, 28–30). However, because we could rescue the defects in follicular development and ovulation in Kiss1−/− mice by priming with estradiol followed by superovulation, the ovulatory defect of Kiss1−/− mice can be attributed to their hypogonadotropic hypogonadism without the need to invoke a direct ovarian function of kisspeptin, although such a role is not entirely ruled out by the current results. Similarly, the fact that we did not observe implantation of embryos in the oviduct demonstrates that kisspeptin is not essential for preventing tubal pregnancies, although again a modulatory role cannot be excluded.
Just as male and female Kiss1r−/− mice have abnormal sexual maturation, human patients with mutations in the kisspeptin signaling pathway also display an absence of or delayed pubertal development (1, 2, 6, 7, 31). Patients with mutations in KISS1R have successfully achieved fertility with either exogenous GnRH or gonadotropins, but few data are available on the pregnancies of the few patients that have been reported. One female patient bearing homozygous L148S mutations in KISS1R achieved pregnancy through the use of exogenous gonadotropins (32). The first pregnancy, a twin pregnancy, was lost at 6 months of gestation, raising the possibility that kisspeptin signaling is required for the maintenance of pregnancy. However, this patient was subsequently able to carry a singleton pregnancy to term (32). As more patients with mutations in KISS1R are reported, closer examination of their gonadal structure and function, fertility, pregnancy course, and delivery will be required.
Defects in uterine receptivity account for most preclinical pregnancy loss (33). Although we acknowledge that there may be subtle to significant differences in the kisspeptin-dependent regulation of embryo implantation in the mouse vs the human, our study reveals a novel and critical role of kisspeptin signaling in the mouse and provides compelling reasons to explore this role in other species, including livestock and humans.
Acknowledgments
We thank Dr Anne B. Croy (Queen's University, Kingston, Ontario, Canada) for her expert analysis and discussion of the data presented in Figure 4, A–F. We also thank Karen Oppenheimer (Molecular Core Lab, Department of Obstetrics and Gynecology and Reproductive Sciences, University of Vermont College of Medicine, Burlington, Vermont) for her assistance in the quantitative real-time PCR studies.
This work was supported by Grant RGPIN/327334-2011 from the Natural Sciences and Engineering Research Council of Canada (to A.V.B.) and the Department of Obstetrics and Gynaecology, University of Western Ontario Academic Enrichment Fund (to A.V.B.); the Canadian Institutes of Health Research (to A.J.W.); Grant MOP 93697 from the Canadian Institutes for Health Research (to N.G.B.); Grant MOP107972 from the Canadian Institutes of Health Research (to M.B.); Grants R01 HD043341, U54 HD028138, and K24 HD067388 from the Eunice Kennedy Shriver National Institute for Child Health and Human Development (to S.B.S.); a Boston Children's Hospital Career Development Award and a Charles A. King Trust Postdoctoral Fellowship (to Y.-M.C.); and Training Grant 5K12HD063082-04 from the National Institutes of Health Women's Reproductive Health Research (to R.R.). M.B. and A.E. are currently supported by a Canadian Institutes for Health Research New Investigator's Award and a Vanier Canada Graduate Scholarship, respectively.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- CL
- corpora lutea
- E2
- 17β-estradiol
- ER
- estrogen receptor
- hCG
- human chorionic gonadotropin
- KISS1
- kisspeptin gene
- KISS1R
- receptor
- Kp
- kisspeptin
- Lif
- leukemia inhibitory factor
- P4
- progesterone
- PMSG
- pregnant mare serum gonadotrophin
- PR
- progesterone receptor
- PRL
- prolactin
- WT
- wild type.
References
- 1. de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor KISS1R. Proc Natl Acad Sci USA. 2003;100:10972–10976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Seminara SB, Messager S, Chatzidaki EE, et al. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349:1614–1627 [DOI] [PubMed] [Google Scholar]
- 3. Funes S, Hedrick JA, Vassileva G, et al. The KiSS-1 receptor GPR54 is essential for the development of the murine reproductive system. Biochem Biophys Res Commun. 2003;312:1357–1363 [DOI] [PubMed] [Google Scholar]
- 4. d'Anglemont de Tassigny X, Fagg LA, Dixon JP, et al. Hypogonadotropic hypogonadism in mice lacking a functional Kiss1 gene. Proc Natl Acad Sci USA. 2007;104:10714–10719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lapatto R, Pallais JC, Zhang D, et al. Kiss1−/− mice exhibit more variable hypogonadism than Gpr54−/− mice. Endocrinology. 2007;148:4927–4936 [DOI] [PubMed] [Google Scholar]
- 6. Chan YM, Broder-Fingert S, Paraschos S, et al. GnRH-deficient phenotypes in humans and mice with heterozygous variants in KISS1/Kiss1. J Clin Endocrinol Metab. 2011;96:E1771–E1781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Topaloglu AK, Tello JA, Kotan LD, et al. Inactivating KISS1 mutation and hypogonadotropic hypogonadism. N Engl J Med. 2012;366:629–635 [DOI] [PubMed] [Google Scholar]
- 8. Bilban M, Ghaffari-Tabrizi N, Hintermann E, et al. Kisspeptin-10, a KiSS-1/metastin-derived decapeptide, is a physiological invasion inhibitor of primary human trophoblasts. J Cell Sci. 2004;117:1319–1328 [DOI] [PubMed] [Google Scholar]
- 9. Horikoshi Y, Matsumoto H, Takatsu Y, et al. Dramatic elevation of plasma metastin concentrations in human pregnancy: metastin as a novel placenta-derived hormone in humans. J Clin Endocrinol Metab. 2003;88:914–919 [DOI] [PubMed] [Google Scholar]
- 10. Taylor J, Pampillo M, Bhattacharya M, Babwah AV. Kisspeptin/KISS1R signaling potentiates extravillous trophoblast adhesion to type I collagen in a PKC- and ERK1/2-dependent manner. Mol Reprod Dev. 2014;81:42–54 [DOI] [PubMed] [Google Scholar]
- 11. Cejudo Roman A, Pinto FM, Dorta I, et al. Analysis of the expression of neurokinin B, kisspeptin, and their cognate receptors NK3R and KISS1R in the human female genital tract. Fertil Steril. 2012;97:1213–1219 [DOI] [PubMed] [Google Scholar]
- 12. Paria BC, Tan J, Lubahn DB, Dey SK, Das SK. Uterine decidual response occurs in estrogen receptorα-deficient mice. Endocrinology. 1999;140:2704–2710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bell CE, Watson AJ. p38 MAPK regulates cavitation and tight junction function in the mouse blastocyst. PLoS One. 2013;8:e59528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen JR, Cheng JG, Shatzer T, Sewell L, Hernandez L, Stewart CL. Leukemia inhibitory factor can substitute for nidatory estrogen and is essential to inducing a receptive uterus for implantation but is not essential for subsequent embryogenesis. Endocrinology. 2000;141:4365–4372 [DOI] [PubMed] [Google Scholar]
- 15. Terakawa J, Watanabe T, Obara R, et al. The complete control of murine pregnancy from embryo implantation to parturition. Reproduction. 2012;143:411–415 [DOI] [PubMed] [Google Scholar]
- 16. Pau K-YF, Orstead KM, Hess DL, Spies HG. Feedback effects of ovarian steroids on the hypothalamic-hypophyseal axis in the rabbit. Biol Reprod. 1986;3:1009–1023 [DOI] [PubMed] [Google Scholar]
- 17. Boyd-Leinen P, Gosse B, Rasmussen K, Martin-Dani G, Spelsberg TC. Regulation of nuclear binding of the avian oviduct progesterone receptor. Changes during estrogen-induced oviduct development, withdrawal, and secondary stimulation. J Biol Chem. 1984;259:2411–2421 [PubMed] [Google Scholar]
- 18. Wang XN, Greenwald GS. Synergistic effects of steroids with FSH on follicullogenesis, steroidogenesis and FSH- and hCG-receptors in hypophysectomized mice. J Reprod Fertil. 1999;99:403–413 [DOI] [PubMed] [Google Scholar]
- 19. Dey SK, Lim H, Das SK, et al. Molecular cues to implantation. Endocr Rev. 2004;25:341–373 [DOI] [PubMed] [Google Scholar]
- 20. Thompson EL, Murphy KG, Patterson M, et al. Chronic subcutaneous administration of kisspeptin-54 causes testicular degeneration in adult male rats. Am J Physiol Endocrinol Metab. 2006;291:E1074–E1082 [DOI] [PubMed] [Google Scholar]
- 21. Kirby DR, Potts DM, Wilson IB. On the orientation of the implanting blastocyst. J Embryol Exp Morphol. 1967;17:527–532 [PubMed] [Google Scholar]
- 22. Smith LJ. Embryonic axis orientation in the mouse and its correlation with blastocyst relationships to the uterus. II. Relationships from 4¼ to 9½ days. J Embryol Exp Morphol. 1985;89:15–35 [PubMed] [Google Scholar]
- 23. Bhatt H, Brunet LJ, Stewart CL. Uterine expression of leukemia inhibitory factor coincides with the onset of blastocyst implantation. Proc Natl Acad Sci USA. 1991;88:11408–11412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sun X, Bartos A, Whitsett JA, Dey SK. Uterine deletion of gp130 or stat3 shows implantation failure with increased estrogenic responses. Mol Endocrinol. 2013;27:1492–1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pawar S, Starosvetsky E, Orvis GD, Behringer RR, Bagchi IC, Bagchi MK. STAT3 regulates uterine epithelial remodeling and epithelial-stromal crosstalk during implantation. Mol Endocrinol. 2013;227:1996–2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Singh M, Chaudhry P, Asselin E. Bridging endometrial receptivity and implantation: network of hormones, cytokines, and growth factors. J Endocrinol. 2011;210:5–14 [DOI] [PubMed] [Google Scholar]
- 27. Liu Z, Ren C, Jones W, et al. LC-MS/MS quantification of a neuropeptide fragment kisspeptin-10 (NSC 741805) and characterization of its decomposition product and pharmacokinetics in rats. J Chromatogr B Analyt Technol Biomed Life. 2013;926:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Castellano JM, Gaytan M, Roa J, et al. Expression of KiSS-1 in rat ovary: putative local regulator of ovulation? Endocrinology. 2006;147:4852–4862 [DOI] [PubMed] [Google Scholar]
- 29. Gaytán M, Castellano JM, Roa J, Sánchez-Criado JE, Tena-Sempere M, Gaytán F. Expression of KiSS-1 in rat oviduct: possible involvement in prevention of ectopic implantation? Cell Tissue Res. 2007;329:571–579 [DOI] [PubMed] [Google Scholar]
- 30. Gaytán F, Gaytán M, Castellano JM, et al. KiSS-1 in the mammalian ovary: distribution of kisspeptin in human and marmoset and alterations in KiSS-1 mRNA levels in a rat model of ovulatory dysfunction. Am J Physiol Endocrinol Metab. 2009;296:E520–E531 [DOI] [PubMed] [Google Scholar]
- 31. Tenenbaum-Rakover Y, Commenges-Ducos M, Iovane A, Aumas C, Admoni O, de Roux N. Neuroendocrine phenotype analysis in five patients with isolated hypogonadotropic hypogonadism due to a L102P inactivating mutation of GPR54. J Clin Endocrinol Metab. 2007;92:1137–1144 [DOI] [PubMed] [Google Scholar]
- 32. Pallais JC, Bo-Abbas Y, Pitteloud N, Crowley WF, Jr, Seminara SB. Neuroendocrine, gonadal, placental, and obstetric phenotypes in patients with IHH and mutations in the G-protein coupled receptor, GPR54. Mol Cell Endocrinol. 2006;254–255:70–77 [DOI] [PubMed] [Google Scholar]
- 33. Coulam CB, Chapman C, Rinehart JS. What is a preclinical pregnancy loss? J Assist Reprod Genet. 1998;15:184–187 [DOI] [PMC free article] [PubMed] [Google Scholar]