Abstract
The idea that what we eat, feel, and experience influences our physical and mental state and can be transmitted to our offspring and even to subsequent generations has been in the popular realm for a long time. In addition to classic gene mutations, we now recognize that some mechanisms for inheritance do not require changes in DNA. The field of epigenetics has provided a new appreciation for the variety of ways biological traits can be transmitted to subsequent generations. Thus, transgenerational epigenetic inheritance has emerged as a new area of research. We have four goals for this minireview. First, we describe the topic and some of the nomenclature used in the literature. Second, we explain the major epigenetic mechanisms implicated in transgenerational inheritance. Next, we examine some of the best examples of transgenerational epigenetic inheritance, with an emphasis on those produced by exposing the parental generation to endocrine-disrupting compounds (EDCs). Finally, we discuss how whole-genome profiling approaches can be used to identify aberrant epigenomic features and gain insight into the mechanism of EDC-mediated transgenerational epigenetic inheritance. Our goal is to educate readers about the range of possible epigenetic mechanisms that exist and encourage researchers to think broadly and apply multiple genomic and epigenomic technologies to their work.
The word epigenetics is loosely used and its meaning is still being debated (1). Here, we are using epigenetics to mean “DNA sequence-independent changes in chromosomal function that yield a stable and heritable phenotype” (2). Thus, transgenerational epigenetic inheritance covers changes in biological traits that are not mediated by changes in the DNA primary sequence and are passed on to subsequent generations. The result is a change in gene expression, which can be brought about by chemical modifications on DNA or histone proteins and/or by one of an increasing number of different noncoding RNAs (ncRNAs) that alter chromatin structure and DNA accessibility.
In this review, we will focus primarily on endocrine-disrupting compounds (EDCs) as the environmental agents causing transgenerationally inherited traits and epigenetic modifications (3, 4). Like other forms of inheritance, transgenerational inheritance must be coded in the genome and epigenome of primordial germ cells (PGCs, oocytes, and spermatozoa) (oocytes and spermatozoa). To be called transgenerational, expression of the trait has to persist for at least 3 generations after the initial exposure to the environmental agent. For transmission via either the maternal or the paternal parental generation (P0), a phenotype may be present in the first filial generation (F1), but this does not ensure that the trait is heritable. In maternal transmission, if a pregnant female is exposed to an EDC that passes through the placenta, her embryos (F1) and the germ cells they possess (F2) are also directly exposed. Thus, the transgenerational phenotype has to be displayed by F3 and subsequent generations (4–6). Importantly, a phenotype may be expressed to different degrees in F1, F2, and F3 generations. Exposures in the F1 can produce modifications caused by direct interactions of the EDC with the developing tissue (brain, mammary tissue, or prostate). Changes in F2 phenotypes may be influenced by temporary modification in germ cell genomes and epigenomes, again produced by direct contact with the EDC. Modifications observed in the F2 need not be identical to those in the F1 or to the final transgenerational phenotypes noted in F3 (and beyond) generations (7–9).
In contrast, if the trait is passed down via the paternal lineage, the transgenerational phenotype can be established earlier, in the F2 generation. For example, if an adult male (P0) is exposed to an EDC, it can produce epigenetic alterations in germ cells due to ongoing spermatogenesis, which represent the F1 generation. Because the F1 germ cells are not directly exposed when the P0 is exposed, alterations that are expressed in the F2 generation can be attributed to transgenerational epigenetic inheritance because the F2 is the first generation that is not directly exposed to EDC (10) (Figure 1). It is important to understand these definitions because there are many examples of reports claiming “transgenerational” effects, which in fact are inter-generational (F1 or F2) but are not strictly transgenerational.
Figure 1.
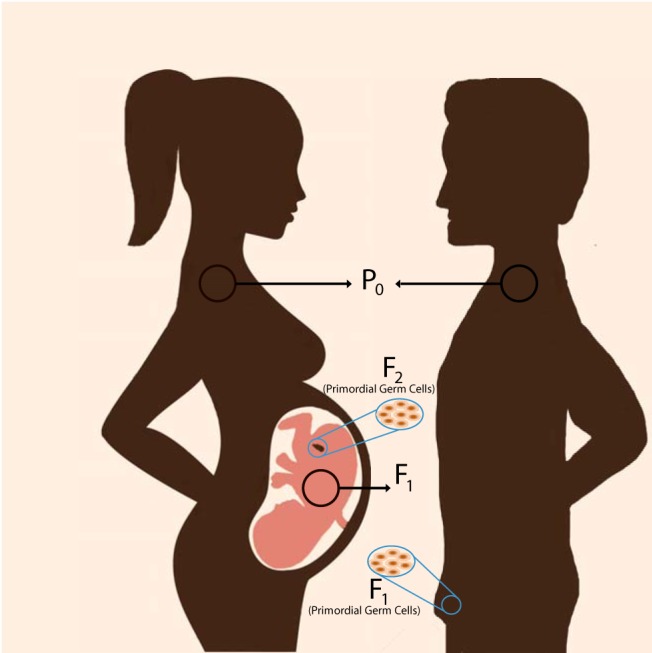
A cartoon of the multiple generations comprising transgenerational inheritance. A pregnant woman (P0) carries genetic and epigenetic information for up to the F2 generation. Her fetus (F1) and its germ cells (F2) may be affected by environmental exposures such as EDCs. For any changes in the F1 phenotype or epigenetic state to be considered transgenerational, the aberrant epigenetic state has to be sustained until at least the F3 generation. In contrast to females, EDC exposure in males (P0) will potentially change his germ cells, which will be the F1 generation. However, because the F2 germ cells are not exposed, any phenotypic or epigenetic changes noted in F2 can be considered transgenerational.
In addition to target tissues in the developing embryo, the pregnant female is also exposed to EDCs, which can change maternal behavior directed to the F1 offspring. Such context-dependent changes in behavior may last for the lifetime of the individual, or not, but do not persist for more than a few generations (11). The best-known example is maternal licking and grooming of rat pups, which changes the pups' own maternal behaviors when they themselves are dams. Fostering pups to dams that show high or low levels of the licking and grooming behaviors dictates the pups' maternal behaviors in adulthood, regardless of the phenotype of their biological dam (12). Although behaviors transmitted to offspring in this manner are not accepted as transgenerational epigenetic inheritance, they might be caused by epigenetic alterations of chromatin states of steroid hormone receptors and other related neurotransmitter genes in the brain (13). Thus, changes in parental behavior can produce transient changes in F1 behaviors, which are not heritable. In the next section, we will discuss and summarize the major mechanisms of epigenetic regulation.
Major Epigenetic Mechanisms
DNA modifications
DNA methylation is the most widely studied epigenetic modification. As the name suggests, it is a biochemical process in which “methyl” chemical groups are covalently attached to “cytosine” (C) residues by DNA methyltransferase enzymes. Although it was initially thought that only cytosines in cytosine-guanosine dinucleotide pairs (CpG) could be modified, later studies demonstrated that other non-CpG cytosine residues are methylated as well (14, 15). DNA methylation is historically associated with transcriptional repression. However, it should be noted that in addition to methylation, the cytosine residues are also hydroxymethylated, formylated, and carboxylated (16, 17). Although the functional roles of these modifications are not fully understood, they are considered intermediate modifications during the DNA demethylation process. The formation of these alternate modifications is facilitated by ten-eleven translocation (TET) enzymes (18). Transgenerational alterations in DNA methylation have been observed globally and at specific genomic loci in studies using EDCs and other environment-related factors in rodents (19–22). It is likely that DNA methylation has received more attention than other epigenetic modifications because, before discovery of TET enzymes, this modification was considered to be highly stable and permanent.
Histone modifications
Histone proteins are the building blocks of genomic DNA organization. Importantly, histone proteins undergo various posttranslational modifications in their tails (23). The modifications change the local chromatin structure by altering the electrical charge of the histone tails. At last count, there are >100 histone modifications, only a handful of which have been studied. Recent mapping of various histone modifications at the whole-genome level reveal important insights into the biological function of these modifications (24–26). Unique sets of histone modifications are associated with distinct regulatory regions and genomic activity (Figure 2). For example, active enhancer regions are marked with histone 3 lysine 27 acetylation and histone 3 lysine 4 monomethylation (H3K4me1), active promoters are marked with histone 3 lysine 4 trimethylation (H3K4me3), and repressive genomic regions are marked with H3K27me3 (24, 27, 28). Importantly, mutations in histone modifiers (enzymes that add or remove various histone marks) are recurrently found in many diseases states including cancer (29–31).
Figure 2.
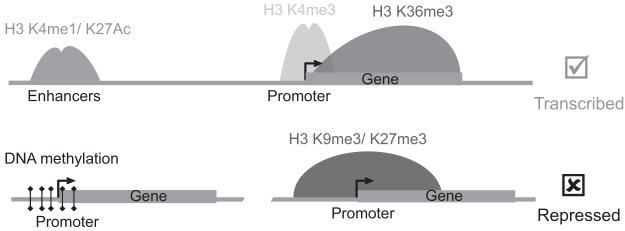
Epigenomic features of expressed and silenced chromatin states. Genomic positions and patterns of various histone modifications are based on chromatin immunoprecipitation-sequencing–mediated epigenome mapping. Active genes are marked with H3K4me3 in their promoters and H3K36me3 in the gene body. Active enhancers are marked with H3K4me1 and H3K27 acetylation (ac). On the other hand, silenced genes have repressive H3K9me3 and H3K27me3 marks as well as DNA methylation in their promoter regions.
Probably the most complete dataset on transgenerational epigenetic inheritance of histone modifications comes from studies in Caenorhabditis elegans. It is important to keep in mind that these animals do not have DNA methylation. Genetically wild-type descendants of mutants for the H3K4me3 methyltransferase complex (COMPASS) display significantly longer life spans (32, 33). Another set of data implicates mutations in the worm homolog of lysine specific demethylase 1 (LSD1), the H3K4me2 histone demethylase enzyme. This mutation causes progressive sterility and smaller brood sizes over many generations of worms (34). Moreover, in the LSD1 mutant animals, the H3K4me2 mark increases in germ cells, leading to aberrant regulation of spermatogenesis genes and sterility over generations. LSD1 interacts with the androgen receptor, and interestingly, when C. elegans develop on medium containing testosterone, the behavioral responses of the offspring to gentle touch are reduced (31). This behavioral modification persists for 4 generations after the initial exposure. Several potential androgen receptor gene sequences were identified in this organism, and after consumption of bacteria containing RNA interference (RNAi) for a subset of these genes, the behavioral effects of testosterone are blocked (35). These data suggest that natural hormones, such as EDCs, can have transgenerational actions.
In Drosophila, stress-induced alterations in dATF2 (Drosophila activating transcription factor-2) function, which involves HP1 (heterochromatin protein 1), results in a defective chromatin state over multiple generations (36). HP1 is an essential mediator of heterochromatin formation, requiring H3K9me3 methylation by the enzyme SUV39 methyltransferase (37). Polycomb-induced repressive chromatin formation by H3K27me3 is another pattern altered over many generations in response to diet or stress in flies and mice (38, 39). Thus, there is precedence for a role of the histone modification proteins and enzymes in transgenerational inheritance.
ncRNAs
ncRNAs are being increasingly recognized as major players in epigenetic gene regulation and global chromatin organization. Thousands of short and long ncRNAs have been discovered; however, the molecular function of the vast majority of these is yet to be discovered (40–42). Seminal work on several long ncRNAs has shown their vital functions in gene regulation and chromatin organization. The best example is Xist ncRNA, an essential player in X-chromosome inactivation in mammals (43). X-chromosome inactivation is a dosage compensation process in females (XX) by which one of the X chromosomes is randomly silenced by Xist-mediated polycomb group proteins. The orchestrated recruitment of key components of polycomb group proteins such as enhancer of zeste homolog 2 (EZH2) by Xist is one of best characterized repressive epigenetic mechanisms coordinated by a ncRNA and a histone modifier, EZH2 (44). EZH2 is the major enzymatic subunit of the polycomb repressive complex that catalyzes the H3K27me3 modification.
In addition to long ncRNA, small ncRNAs such as short interfering RNA, microRNA (miRNA), small nucleolar RNA, and piwi-interacting RNA are important regulators of gene expression in various organisms ranging from yeast to human (45). Whether small ncRNAs (small interfering RNA and miRNA) mediate classic epigenetic mechanisms in mammalian systems is still being debated (46, 47). However, there is compelling evidence in plants that they mediate epigenetic gene regulation through DNA methylation (48, 49). For example, in higher plants interactions between short RNA and genomic DNA triggers de novo DNA methylation, a process known as RNA-directed DNA methylation (50). Furthermore, RNAi-directed assembly of heterochromatin formation is well established in fission yeast (51) and C. elegans (52). Importantly, small RNA-mediated H3K9me3 methylation is implicated in the transgenerational inheritance of the RNAi mechanism in C. elegans (53, 54). These examples highlight how the epigenetic regulation of gene expression is orchestrated with layers of mechanisms coordinated through ncRNAs, DNA methylation, and histone modification.
Transmission of Epigenetic Information
Epigenetic mechanisms control how DNA sequence information is being used. Modifications in DNA and histone proteins and in ncRNA molecules are implicated in chromatin structure and function and are major forms of epigenetic information. The exact mechanism(s) of transmission for epigenetic information through mitotic or meiotic cell cycle division is yet to be understood (55). DNA methylation is mitotically transmitted in a semiconservative fashion (56). However, there is no clear evidence that histone modifications are transmitted the same way. Indeed, recent studies suggest an alternative scenario that the histone modifiers, ie, the enzymes that catalyze the modification, are transmitted: they remain bound to DNA during DNA replication and hence reestablish the histone marks on the sister chromatids (57, 58).
For germline-dependent transmission, epigenetic information must be transmitted during meiosis. Thus, the mechanism of transgenerational epigenetic inheritance is more complex than mitotic transmission. This is because the transmitted epigenetic information has to be maintained during the 2 major waves of global reprograming events at the onset of each generation, fertilization, and PGC development, associated with epigenome resetting events. Studies of DNA methylation patterns shed important light on the dynamics of these epigenomic-resetting events. For example, right after fertilization, before the sperm and oocyte pronuclei merge, DNA methylation is actively erased globally from the paternal genome (59, 60). Moreover, the maternal genome undergoes a replication-dependent, passive DNA demethylation process that is completed by the blastocyst stage. As a result of this major wave of DNA methylation erasure, the genome of the inner cell mass is substantially hypomethylated. In the inner cell mass, only 20% of CpG sites are methylated, but in sperm and oocytes nearly 90% and 40% of the CpG sites (respectively) are methylated (56). As embryonic development progresses from the blastocyst to epiblast stage (around embryonic day [E] 6.5 in the mouse), the genome undergoes de novo methylation whereby nearly 70% of the genome is methylated. Around this developmental stage, subsets of epiblast cells follow specific sets of developmental cues and become PGCs in which somatic transcription is silenced (61, 62). The second major period for epigenomic reprograming occurs during development of the PGCs. As the PGCs begin to migrate through the developing hindgut to colonize the undifferentiated gonads (about E7.25 in mice), the genome undergoes a global DNA methylation erasure (61). These 2 waves of global epigenomic reprograming during preimplantation development and PGC specification are well orchestrated. During this process, major components of de novo DNA methylation are inhibited and key players of DNA demethylation program such as the recently discovered TET enzymes are activated (56). Similar to DNA methylation, there is extensive reprograming of histone modifications during preimplantation and PGC development (63, 64).
Because preimplantation and PGC development are coupled with extensive epigenomic reprograming, it is important to ask how an epigenetic chromatin state is transmitted from parent to offspring. A partial answer to this question comes from genomic imprinting studies in which only 1 allele is expressed based on its parent of origin. The epigenetic state of imprinted genes escapes from both waves of epigenome reprograming (55, 65). Interestingly, in addition to the imprinted genes, the chromatin state of pericentromeric heterochromatin and certain genomic repeat regions such as intracisternal A particles (IAPs) escape the global reprograming process (66).
Indeed, the best-studied mouse model of epigenetic inheritance includes insertion of an IAP element distal to the Agouti locus (67). These studies suggest that certain genomic regions, such as imprinted genes and IAP elements, are more susceptible to epigenetic alterations that persist in subsequent generations. Several EDCs can disrupt the normal imprinting process when exposure occurs during either the fertilization and/or germ cell migratory periods. Two (Igf2r and Peg3) of 3 imprinted genes examined are hypomethylated in germ cells from gestational day 12.5 in mice treated with a low dose of diethylhexyl phthalate or bisphenol A (BPA) (68, 69). These genes are still hypomethylated in 21-day-old oocytes compared with controls. Even more interestingly, hypomethylation persists in F2 offspring germ cells. Rats treated with BPA for the first 5 days after birth possess hypomethylation at the imprinted H19 gene promoter in their spermatozoa (70). Unfortunately, transcript levels of these genes have not been quantified. The only truly transgenerational data (F1, F2, and F3) on imprinted genes in sperm show that vinclozolin decreases the methylation of H19, Peg3, and Snrpn (71).
One aspect of sperm maturation that complicates identification of epigenetic modifications produced by EDCs is that the chromatin undergoes a remarkable remodeling process during spermatogenesis. During this process, protamine proteins replace the vast majority of histone proteins. The current view is that only a fraction of the nucleosomes (1%) are retained in mouse spermatozoa; however, in humans a greater percentage (approximately 15%) of nucleosomes are retained (72). Importantly, recent epigenome mapping studies show unique patterns of histone modifications in human and mouse spermatozoa (72–74). For example, H3K27me3 and H3K4me2 are retained at the promoters of developmentally critical genes (73, 74). Moreover, detailed mapping studies also show that the retained nucleosomes are particularly enriched in CpG-rich gene promoters that lack DNA methylation in mouse spermatozoa (72). Whether these histone modifications are transmitted transgenerationally is yet to be determined. However, whole-genome mapping studies suggest that the H3K27me3 mark in sperm is implicated in gene repression during early stages of preimplantation development (72). During fertilization, the entire sperm is fused to the ovum. Therefore, in addition to the histones, coding and ncRNAs such as miRNAs present in sperm are also transferred to the ovum (75). It is also likely that sperm RNA and protamine modifications contribute to intergenerational as well as transgenerational epigenetic inheritance (75–77). Further studies are needed to elucidate the epigenetic roles for histone, DNA methylation, and other mechanisms.
EDCs, Steroids, and Epigenetics
The classic case of an EDC is diethylstilbestrol (DES), an estrogen agonist and androgen receptor antagonist synthesized first in the 1930s and prescribed to at least 5 million women at risk for miscarriage or experiencing other reproductive problems, from 1938 up to 1975. Instead of the desired effects, use of this compound lead to increased incidence of breast, vaginal, and cervical cancers (78–81). In addition, maternal exposure has documented adverse affects on daughters. These include the same types of cancers as well as a variety of difficulties conceiving and maintaining pregnancies, reproductive tract malformations, abnormal menstrual cycles, early puberty, and behavioral issues (81–83). The vast variety of effects is probably related to complexities introduced by the timing of DES treatment and doses. For example, a recent large study of women exposed prenatally to DES revealed a strong correlation between DES, particularly in the first trimester, and noncancerous uterine fibroids (84). Offspring of rodents exposed to DES during pregnancy recapitulate many of these effects (85–87).
Although the initial clinical studies were limited to female offspring, correlations between DES exposure and hypospadias, cryptorchidism, and testicular cancer have been reported in F1 and F2 sons and grandsons of women given DES (88–90). Analyses of the clinical studies suggest that the male reproductive illnesses are related to but not necessarily caused by estrogen actions. An alternative hypothesis is that DES produces low-birth-weight babies, and these infants are more prone to testicular dysgenesis syndrome (90, 91). Multigenerational work in mice has demonstrated that high, but clinically relevant, doses of DES increase the incidence of uterine and other reproductive tract tumors in females and lesions in the male rete testes in F2 offspring (86, 92, 93). Few data on the critical F3 generation in humans are available nor are there experimental data from rodent models (81).
EDCs, such as DES, share many properties with steroid hormones: they act at low doses (picograms) and can act in a nonmonotonic manner (94, 95). Like hormones, they are particularly effective during development, at which time they can modify the course of reproductive tract and brain development (96–98). Importantly, the EDCs are more promiscuous than steroids and bind to a larger variety of receptors than normal ligands, albeit with reduced affinities (95).
Steroid receptor proteins are transcription factors (TFs) and typically act in association with clusters of protein complexes that include coactivators or corepressors, all of which are required to access DNA. Many of these coregulators (ie, NCor [nuclear receptor corepressor], CBP [cAMP response element–binding protein–binding protein], and others) are also epigenetic factors (99). Numerous interactions between steroids, their receptors, and epigenetic mechanisms are known. For example, estradiol stimulation of breast cancer cells alters a number of chromatin modifications as well as DNA methylation and chromosome interactions (100). Estrogen receptor α interacts with and suppresses the Drosha enzyme, which in turn affects miRNA expression (101), and estradiol affects methylation of its α receptor (102). Therefore, EDCs may alter the epigenetic state by regulating the activity of various TFs, thereby changing expression of chromatin regulators such as DNA or histone methyl transferases and/or ncRNAs. Moreover, TFs can also directly change the local chromatin states by recruiting epigenetic regulators (Figure 3).
Figure 3.
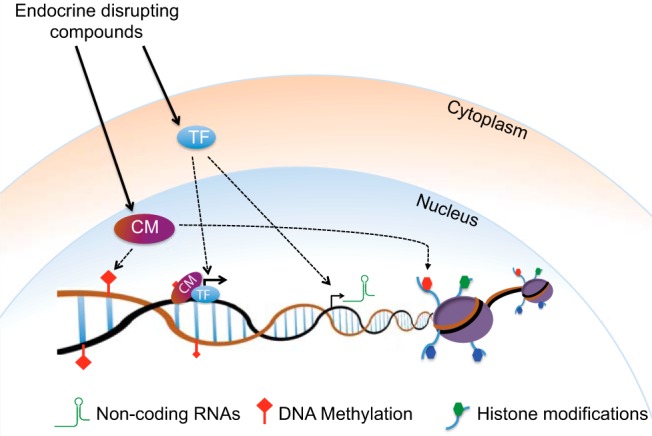
A general model depicting epigenetic alterations upon exposure to EDCs. EDCs bind to steroid receptors, and other TFs, thereby changing the local chromatin states and expression of various chromatin modifiers (CM) such as histone and DNA modifiers or ncRNAs. Moreover, EDCs can change the composition or the activity of epigenetic chromatin regulator complexes and thereby act directly on the global epigenome.
BPA is one of the most heavily manufactured EDCs worldwide (103). BPA is commonly used as a plastic hardener in bottles and for epoxy resins that line metal-based food and beverage cans, and most recently it has been added to thermal cash receipts. Globally, approximately 6 billion tons are produced each year. BPA has been shown to alter various epigenetic mechanisms, for example, it promotes hypomethylation and hypermethylation (104, 105). Moreover, supplementing mice with a methyl donor–rich diet can reverse the ability of BPA ability to hypomethylate. BPA also modifies expression of a number of the estrogen receptors in brain (7, 106) and DNA methylation of both Esr1 and Esr2 (107, 108). Immunocytochemical analysis has been conducted on testes from F1 and F3 rats, steroid receptors, and several coactivators (androgen receptor, estrogen receptor β, steroid receptor coactivator 1 [SRC1], and NCor) are reduced by BPA exposure across all generations (109). These types of relationships have been noted for other EDCs. Given these interactions between the epigenome and steroid hormones and their receptors, it is not surprising that EDCs can promote transgenerational change.
EDCs and Transgenerational Epigenetic Inheritance
EDCs have transgenerational actions on multiple target tissues in rodents, particularly germ cells, gonads, and brain (4, 19). Exposure in midgestation to the phthalate diethylhexyl phthalate produces puberty delay in males both in the F1 and F3 generations, and F3 males have lower sperm counts, testicular germ cell function, and increased incidence of abnormal seminiferous tubules (8). These phenotypes are noted in F3 males regardless of the parent of origin. Dioxins (eg, 2,3,7,8-tetrachlorodibenzo-p-dioxin) are another class of EDCs found throughout the world. In mice, exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin during pregnancy reduces fertility in F1 and F3 female offspring (110). Mixtures of EDCs can also have transgenerational actions (111, 112). The combination of phthalates and BPA, at 2 doses, several dioxins, and jet fuels causes puberty advancement female rats 3 generations removed from the initial gestational exposure and reduces the numbers of ovarian follicles. In F3 males, serum testosterone is lower in adults of all EDC lineages, with the exception of the pesticides.
The first EDC shown to possess transgenerational actions was vinclozolin, a fungicide in use since 1984 in high-production agriculture (9). Aged male rats and mice exposed to high levels in utero have higher rates of germ cell death and disease-like phenotypes in prostate, kidneys, and other tissues (through at least the F3 generation). In rats, the effects on sperm are more extreme than those in mice, including decreased numbers and motility (9, 113). Female F3 rats and mice exposed to vinclozolin as embryos in the P0 generation have increased numbers of ovarian cysts (113, 114). Vinclozolin lineages have fewer numbers of newborn rat oocytes and fewer primary follicles in mature ovaries, suggesting that germ cell death in embryonic PGCs is increased by this EDC (114). Behavioral effects are present as well and include an effect of vinclozolin on female mate preference and heightened anxiety-like behavior in females but less anxiety in males, and this phenotype is modified by stress during the pubertal period (115, 116).
DNA methylation analyses have been conducted with materials from F3 vinclozolin and control rats and mice (9, 21, 113, 117). Immunoprecipitation (methylated DNA immunoprecipitation-chromatin immunoprecipitation) followed by hybridization to promoter tiling arrays, bisulfite, and/or pyrosequencing has produced a series of articles showing differentially methylated regions (DMRs) between material from F3 control and vinclozolin lineages. Vinclozolin has both hypomethylating and hypermethylating actions. Many of the identified DMRs contain known genes, and recently one such region displayed a copy number variation (117). A consensus sequence motif, which is associated with genes containing low CpG density (1%–8%), is present in most of the confirmed promoters. The DMRs in F3 vinclozolin-exposed mice and rats are not conserved, but the motif identified in rats is also present in affected mouse DMRs (113). Interestingly, the largest gene pathway affected in E13 germ cells and in brain is an olfactory transduction pathway (116, 117). This may be related to some of the transgenerational behaviors reported.
BPA has transgenerational effects on behavior and sperm in rodents (7, 118, 119). Pregnant mouse dams ingested BPA at a low concentration in their chow throughout pregnancy and on the day of birth all pups were transferred to control dams. This was done to block the actions of BPA on maternal behavior and to ensure that exposure was ended at birth. F1 and either F3 or F4 juveniles were tested on several types of behavioral tasks. The social interaction tasks provided to be sensitive to BPA. One of the tasks used was an unrestrained interaction between nonfamilial juveniles of the same age and sex and given the same treatment. In this task, F1 mice were less socially interactive than controls if they had been exposed to BPA in utero. However, in the F2 and F4 generations, the BPA lineage mice were more interactive than the controls (7). In the well-established social recognition task, juveniles exposed to BPA in utero did not habituate as rapidly to repeatedly presented adult females compared with controls, and this behavior persisted in F3 mice (118). This hyperactivity phenotype is noted by others also giving mice low doses of BPA (120). Finally during the dishabituation portion of the social recognition task, control juveniles display heightened interested in a novel adult, whereas F3 mice in the BPA lineage fail to do so. This recognition failure is not caused by lack of olfactory abilities, and taken together, the data show that social interactions are affected transgenerationally by BPA exposure during gestation.
BPA can increase or decrease DNA methylation, depending on the dose (94). In young girls, urinary concentrations of BPA are associated with hypermethylation and hypomethylation of DNA, and, as in mice, higher BPA levels are correlated with hypomethylation (121). In addition, BPA increases miRNA expression in immortalized placenta cells (122). BPA enhances expression and protein for the histone methyltransferase, Ezh2 in human cancer cells and mouse mammary tissues (123). This methyltransferase is part of the polycomb group complex 2, which is essential for genetic imprinting during germ cell development. Enhanced H3K27me3 is likewise a result of BPA exposure. Although the studies are in their infancy, it is certain that more than one epigenetic mechanism underlies the transgenerational actions of BPA.
Moving Ahead
As described above for BPA and vinclozolin, EDCs can alter the epigenome via multiple pathways. Likewise the known examples of epigenetic transgenerational inheritance use a number of epigenetic mechanisms. Of course, although the types of epigenetic information are typically explained separately, these have to act together to establish a particular chromatin and cellular state. For example, the epigenetic mechanisms in X chromosome inactivation require interaction between Xist ncRNA and EZH2, which recruits polycomb group proteins and then deposits repressive H3K37me3 histone marks to silence the inactive X chromosome via DNA methylation (44). Therefore, it is safe to say that it is the total net effect of the combination of these epigenetic processes that establishes a unique chromatin structure and function.
Further studies are needed to address several important questions in the field. The major question that remains unanswered is how to detect transgenerational changes. We suggest that unbiased whole-genome approaches using next-generation or newer sequencing technologies, are essential for discovery (also see Ref. 124). Because the epigenetic mechanisms work together, whole-genome mapping of histone modifications and DNA methylation will reveal aberrant chromatin state(s) at specific loci in normal as well as malignant development (24, 27, 125). To this end, studying ncRNAs is an essential complementary approach. Although it is relatively straightforward to measure differentially regulated coding and ncRNAs across many generations, identification of genomic regions regulated by RNA continues to present a major technical challenge. Unlike histone and DNA modifications, which act in cis, RNA may act in both cis and trans. Therefore, quantification of an ncRNAs does not necessarily reveal the essential genomic region. However, DNA or histone modification mapping will reveal specific positional information, and when combined with RNA expression profiles (RNA sequencing), these unbiased genomic approaches will yield a complete picture of both the epigenetic and the expression states of genic as well as nongenic genomic regions.
Many have shown that EDCs mediate both short-term direct effects as well as long-term transgenerational effects. Utilization of state-of-the-art genomic and epigenomic approaches in well-controlled treated and nontreated experimental settings will allow identification of such effects. Moreover to truly assert that an effect is transgenerational, F3 and later generations have to be compared with F1, a step that is often skipped. EDCs and other environmental toxicants may alter the chromatin state in critical genomic regions by changing both histone modifications and DNA methylation patterns. Furthermore, the chromatin features at some of these differentially regulated genomic regions (DRGRs), if not all, may persist over generations, inherited transgenerationally. We would like to use the term “transgenerational epigenetically regulated regions” (TERRs) to describe such critical genomic sites. Our working model is illustrated in Figure 4. Unbiased whole-genome genomic and epigenomic data acquisition and comparative analyses are required to identify both DRGRs and TERRs associated with EDC exposure. Such studies will also reveal the epigenetic mechanisms contributing to establishment of DRGRs and TERRs.
Figure 4.
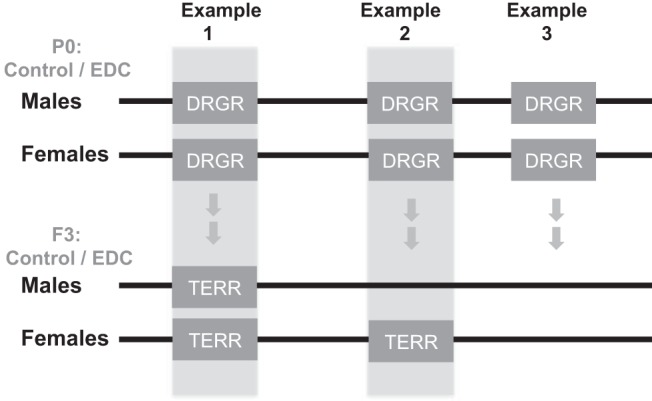
Hypothetical model for potential short-term and long-term effects of EDCs on the genome of germ cells. Direct exposure (in P0) to EDCs induces DRGRs. The chromatin features at some or all of these DRGRs may persist over generations (as pictured here in the F3) and be inherited transgenerationally. We are calling such regions “transgenerational epigenetically regulated regions” (TERRs). In example 1, a DRGR is present in both sexes after exposure to an EDC and retained transgenerationally. Example 2 illustrates a sex difference in establishment of a TERR. Example 3 is a transient DRGR that is not retained as a TERR.
Other mechanistic questions that remain are how epigenomic changes at specific loci are maintained via the germline and what determines the duration of transgenerational epigenetic traits. To this end, epigenome mapping in the germ cells is essential. However, this also poses significant technical challenges due to limited material. Therefore, new genomic and epigenomic methods that require less material are in great need (27, 126). Another important area of research needed is comparative epigenetic mechanisms of action. Most transgenerational studies use mice, flies, or worms and so far even the limited data available suggest divergence of mechanisms. If improving human health is our ultimate goal, we must be able to pinpoint which mechanisms are conserved in different organisms. The time is ripe for technology development, comparative epigenetic studies, and their applications to transgenerational inheritance of traits in human health and disease.
Acknowledgments
This work was supported by the National Institutes of Health (Grant R01ES022759).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BPA
- bisphenol A
- DES
- diethylstilbestrol
- DMR
- differentially methylated region
- DRGR
- differentially regulated genomic region
- E
- embryonic day
- EDC
- endocrine-disrupting compound
- EZH2
- enhancer of zeste homolog 2
- H3K4me1
- histone 3 lysine 4 monomethylation
- H3K4me2
- histone 3 lysine 27 dimethylation
- H3K4me3
- histone 3 lysine 4 trimethylation
- H3K27me3
- histone 3 lysine 27 trimethylation
- IAP
- intracisternal A particle
- miRNA
- microRNA
- ncRNA
- noncoding RNA
- PGC
- primordial germ cell
- RNAi
- RNA interference
- TERR
- transgenerational epigenetically regulated regions
- TET
- ten-eleven translocation
- TF
- transcription factor.
References
- 1. Ptashne M. On the use of the word 'epigenetic.' Curr Biol. 2007;17:R233–236 [DOI] [PubMed] [Google Scholar]
- 2. Berger SL, Kouzarides T, Shiekhattar R, Shilatifard A. An operational definition of epigenetics. Genes Dev. 2009;23:781–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Skinner MK, Manikkam M, Guerrero-Bosagna C. Epigenetic transgenerational actions of endocrine disruptors. Reprod Toxicol. 2011;31:337–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jirtle RL, Skinner MK. Environmental epigenomics and disease susceptibility. Nat Rev Genet. 2007;8:253–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Feil R, Fraga MF. Epigenetics and the environment: emerging patterns and implications. Nat Rev Genet. 2011;13:97–109 [DOI] [PubMed] [Google Scholar]
- 6. Sharma A. Transgenerational epigenetic inheritance: focus on soma to germline information transfer. Prog Biophys Mol Biol. 2013;113:439–446 [DOI] [PubMed] [Google Scholar]
- 7. Wolstenholme JT, Edwards M, Shetty SR, et al. Gestational exposure to bisphenol a produces transgenerational changes in behaviors and gene expression. Endocrinology. 2012;153:3828–3838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Doyle TJ, Bowman JL, Windell VL, McLean DJ, Kim KH. Transgenerational effects of di-(2-ethylhexyl) phthalate on testicular germ cell associations and spermatogonial stem cells in mice. Biol Reprod. 2013;88:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Anway MD, Cupp AS, Uzumcu M, Skinner MK. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science. 2005;308:1466–1469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schmidt CW. Uncertain inheritance transgenerational effects of environmental exposures. Environ Health Perspect. 2013;121:A298–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Crews D. Epigenetics and its implications for behavioral neuroendocrinology. Front Neuroendocrinol. 2008;29:344–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Francis D, Diorio J, Liu D, Meaney MJ. Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science. 1999;286:1155–1158 [DOI] [PubMed] [Google Scholar]
- 13. Walker DM, Goetz BM, Gore AC. Dynamic postnatal developmental and sex-specific neuroendocrine effects of prenatal polychlorinated biphenyls in rats. Mol Endocrinol. 2014;28:99–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lister R, Pelizzola M, Dowen RH, et al. Human DNA methylomes at base resolution show widespread epigenomic differences. Nature. 2009;462:315–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lister R, Mukamel EA, Nery JR, et al. Global epigenomic reconfiguration during mammalian brain development. Science. 2013;341:1237905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kriaucionis S, Heintz N. The nuclear DNA base 5-hydroxymethylcytosine is present in Purkinje neurons and the brain. Science. 2009;324:929–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tahiliani M, Koh KP, Shen Y, et al. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science. 2009;324:930–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kohli RM, Zhang Y. TET enzymes, TDG and the dynamics of DNA demethylation. Nature. 2013;502:472–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Skinner MK. Environmental epigenetic transgenerational inheritance and somatic epigenetic mitotic stability. Epigenetics. 2011;6:838–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Padmanabhan N, Jia D, Geary-Joo C, et al. Mutation in folate metabolism causes epigenetic instability and transgenerational effects on development. Cell. 2013;155:81–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Guerrero-Bosagna C, Settles M, Lucker B, Skinner MK. Epigenetic transgenerational actions of vinclozolin on promoter regions of the sperm epigenome. PLoS One. 2010;5(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Carone BR, Fauquier L, Habib N, et al. Paternally induced transgenerational environmental reprogramming of metabolic gene expression in mammals. Cell. 2010;143:1084–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705 [DOI] [PubMed] [Google Scholar]
- 24. Zhu J, Adli M, Zou JY, et al. Genome-wide chromatin state transitions associated with developmental and environmental cues. Cell. 2013;152:642–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. ENCODE Project Consortium, Bernstein BE, Birney E, et al. An integrated encyclopedia of DNA elements in the human genome. Nature. 2012;489:57–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bernstein BE, Stamatoyannopoulos JA, Costello JF, et al. The NIH Roadmap Epigenomics Mapping Consortium. Nat Biotechnol. 2010;28:1045–1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Adli M, Zhu J, Bernstein BE. Genome-wide chromatin maps derived from limited numbers of hematopoietic progenitors. Nat Methods. 2010;7:615–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Heintzman ND, Hon GC, Hawkins RD, et al. Histone modifications at human enhancers reflect global cell-type-specific gene expression. Nature. 2009;459:108–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Berdasco M, Esteller M. Aberrant epigenetic landscape in cancer: how cellular identity goes awry. Dev Cell. 2010;19:698–711 [DOI] [PubMed] [Google Scholar]
- 30. Brower V. Epigenetics: Unravelling the cancer code. Nature. 2011;471:S12–S13 [DOI] [PubMed] [Google Scholar]
- 31. Shen H, Laird PW. Interplay between the cancer genome and epigenome. Cell. 2013;153:38–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Greer EL, Maures TJ, Hauswirth AG, et al. Members of the H3K4 trimethylation complex regulate lifespan in a germline-dependent manner in C. elegans. Nature. 2010;466:383–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Greer EL, Maures TJ, Ucar D, et al. Transgenerational epigenetic inheritance of longevity in Caenorhabditis elegans. Nature. 2011;479:365–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Katz DJ, Edwards TM, Reinke V, Kelly WG. A C. elegans LSD1 demethylase contributes to germline immortality by reprogramming epigenetic memory. Cell. 2009;137:308–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gamez-Del-Estal MM, Contreras I, Prieto-Perez R, Ruiz-Rubio M. Epigenetic effect of testosterone in the behavior of C. elegans. A clue to explain androgen-dependent autistic traits? Front Cell Neurosci. 2014;8:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Seong KH, Li D, Shimizu H, Nakamura R, Ishii S. Inheritance of stress-induced, ATF-2-dependent epigenetic change. Cell. 2011;145:1049–1061 [DOI] [PubMed] [Google Scholar]
- 37. Canzio D, Liao M, Naber N, et al. A conformational switch in HP1 releases auto-inhibition to drive heterochromatin assembly. Nature. 2013;496:377–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Stern S, Fridmann-Sirkis Y, Braun E, Soen Y. Epigenetically heritable alteration of fly development in response to toxic challenge. Cell Rep. 2012;1:528–542 [DOI] [PubMed] [Google Scholar]
- 39. Chong S, Vickaryous N, Ashe A, et al. Modifiers of epigenetic reprogramming show paternal effects in the mouse. Nat Genet. 2007;39:614–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: insights into functions. Nat Rev Genet. 2009;10:155–159 [DOI] [PubMed] [Google Scholar]
- 41. Cheng J, Kapranov P, Drenkow J, et al. Transcriptional maps of 10 human chromosomes at 5-nucleotide resolution. Science. 2005;308:1149–1154 [DOI] [PubMed] [Google Scholar]
- 42. Derrien T, Johnson R, Bussotti G, et al. The GENCODE v7 catalog of human long noncoding RNAs: analysis of their gene structure, evolution, and expression. Genome Res. 2012;22:1775–1789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lee JT, Bartolomei MS. X-inactivation, imprinting, and long noncoding RNAs in health and disease. Cell. 2013;152:1308–1323 [DOI] [PubMed] [Google Scholar]
- 44. Zhao J, Sun BK, Erwin JA, Song JJ, Lee JT. Polycomb proteins targeted by a short repeat RNA to the mouse X chromosome. Science. 2008;322:750–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Costa FF. Non-coding RNAs, epigenetics and complexity. Gene. 2008;410:9–17 [DOI] [PubMed] [Google Scholar]
- 46. Saetrom P, Snove O, Jr, Rossi JJ. Epigenetics and microRNAs. Pediatr Res. 2007;61(5 Pt 2):17R–23R [DOI] [PubMed] [Google Scholar]
- 47. Saab BJ, Mansuy IM. Neuroepigenetics of memory formation and impairment: the role of microRNAs. Neuropharmacology. 2014;80:61–69 [DOI] [PubMed] [Google Scholar]
- 48. Pélissier T, Thalmeir S, Kempe D, Sänger HL, Wassenegger M. Heavy de novo methylation at symmetrical and non-symmetrical sites is a hallmark of RNA-directed DNA methylation. Nucleic Acids Res. 1999;27:1625–1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wassenegger M, Heimes S, Riedel L, Sänger HL. RNA-directed de novo methylation of genomic sequences in plants. Cell. 1994;76:567–576 [DOI] [PubMed] [Google Scholar]
- 50. Zhang H, Zhu JK. RNA-directed DNA methylation. Curr Opin Plant Biol. 2011;14:142–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Verdel A, Moazed D. RNAi-directed assembly of heterochromatin in fission yeast. FEBS Lett. 2005;579:5872–5878 [DOI] [PubMed] [Google Scholar]
- 52. Buckley BA, Burkhart KB, Gu SG, et al. A nuclear Argonaute promotes multigenerational epigenetic inheritance and germline immortality. Nature. 2012;489:447–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Burton NO, Burkhart KB, Kennedy S. Nuclear RNAi maintains heritable gene silencing in Caenorhabditis elegans. Proc Natl Acad Sci USA. 2011;108:19683–19688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gu SG, Pak J, Guang S, Maniar JM, Kennedy S, Fire A. Amplification of siRNA in Caenorhabditis elegans generates a transgenerational sequence-targeted histone H3 lysine 9 methylation footprint. Nat Genet. 2012;44:157–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lange UC, Schneider R. What an epigenome remembers. BioEssays. 2010;32:659–668 [DOI] [PubMed] [Google Scholar]
- 56. Wu H, Zhang Y. Reversing DNA methylation: mechanisms, genomics, and biological functions. Cell. 2014;156:45–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Francis NJ, Follmer NE, Simon MD, Aghia G, Butler JD. Polycomb proteins remain bound to chromatin and DNA during DNA replication in vitro. Cell. 2009;137:110–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Petruk S, Sedkov Y, Johnston DM, et al. TrxG and PcG proteins but not methylated histones remain associated with DNA through replication. Cell. 2012;150:922–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mayer W, Niveleau A, Walter J, Fundele R, Haaf T. Demethylation of the zygotic paternal genome. Nature. 2000;403:501–502 [DOI] [PubMed] [Google Scholar]
- 60. Oswald J, Engemann S, Lane N, et al. Active demethylation of the paternal genome in the mouse zygote. Curr Biol. 2000;10:475–478 [DOI] [PubMed] [Google Scholar]
- 61. Seisenberger S, Peat JR, Reik W. Conceptual links between DNA methylation reprogramming in the early embryo and primordial germ cells. Curr Opin Cell Biol. 2013;25:281–288 [DOI] [PubMed] [Google Scholar]
- 62. Sasaki H, Matsui Y. Epigenetic events in mammalian germ-cell development: reprogramming and beyond. Nat Rev Genet. 2008;9:129–140 [DOI] [PubMed] [Google Scholar]
- 63. Seki Y, Hayashi K, Itoh K, Mizugaki M, Saitou M, Matsui Y. Extensive and orderly reprogramming of genome-wide chromatin modifications associated with specification and early development of germ cells in mice. Dev Biol. 2005;278:440–458 [DOI] [PubMed] [Google Scholar]
- 64. Daujat S, Weiss T, Mohn F, et al. H3K64 trimethylation marks heterochromatin and is dynamically remodeled during developmental reprogramming. Nat Struct Mol Biol. 2009;16:777–781 [DOI] [PubMed] [Google Scholar]
- 65. Lawson HA, Cheverud JM, Wolf JB. Genomic imprinting and parent-of-origin effects on complex traits. Nat Rev Genet. 2013;14:609–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lane N, Dean W, Erhardt S, et al. Resistance of IAPs to methylation reprogramming may provide a mechanism for epigenetic inheritance in the mouse. Genesis. 2003;35:88–93 [DOI] [PubMed] [Google Scholar]
- 67. Morgan HD, Sutherland HG, Martin DI, Whitelaw E. Epigenetic inheritance at the agouti locus in the mouse. Nat Genet. 1999;23:314–318 [DOI] [PubMed] [Google Scholar]
- 68. Li L, Zhang T, Qin XS, et al. Exposure to diethylhexyl phthalate (DEHP) results in a heritable modification of imprint genes DNA methylation in mouse oocytes. Mol Biol Rep. 2014;41:1227–1235 [DOI] [PubMed] [Google Scholar]
- 69. Zhang XF, Zhang LJ, Feng YN, et al. Bisphenol A exposure modifies DNA methylation of imprint genes in mouse fetal germ cells. Mol Biol Rep. 2012;39:8621–8628 [DOI] [PubMed] [Google Scholar]
- 70. Doshi T, D'Souza C, Vanage G. Aberrant DNA methylation at Igf2–H19 imprinting control region in spermatozoa upon neonatal exposure to bisphenol A and its association with post implantation loss. Mol Biol Rep. 2013;40:4747–4757 [DOI] [PubMed] [Google Scholar]
- 71. Stouder C, Paoloni-Giacobino A. Transgenerational effects of the endocrine disruptor vinclozolin on the methylation pattern of imprinted genes in the mouse sperm. Reproduction. 2010;139:373–379 [DOI] [PubMed] [Google Scholar]
- 72. Erkek S, Hisano M, Liang CY, et al. Molecular determinants of nucleosome retention at CpG-rich sequences in mouse spermatozoa. Nat Struct Mol Biol. 2013;20:868–875 [DOI] [PubMed] [Google Scholar]
- 73. Hammoud SS, Nix DA, Zhang H, Purwar J, Carrell DT, Cairns BR. Distinctive chromatin in human sperm packages genes for embryo development. Nature. 2009;460:473–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Brykczynska U, Hisano M, Erkek S, et al. Repressive and active histone methylation mark distinct promoters in human and mouse spermatozoa. Nat Struct Mol Biol. 2010;17:679–687 [DOI] [PubMed] [Google Scholar]
- 75. Krawetz SA. Paternal contribution: new insights and future challenges. Nat Rev Genet. 2005;6:633–642 [DOI] [PubMed] [Google Scholar]
- 76. Rando OJ. Daddy issues: paternal effects on phenotype. Cell. 2012;151:702–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Brunner AM, Nanni P, Mansuy IM. Epigenetic marking of sperm by post-translational modification of histones and protamines. Epigenetics Chromatin. 2014;7:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Gibson DA, Saunders PT. Endocrine disruption of oestrogen action and female reproductive tract cancers. Endocr Relat Cancer. 2014;21:T13–T31 [DOI] [PubMed] [Google Scholar]
- 79. Giusti RM, Iwamoto K, Hatch EE. Diethylstilbestrol revisited: a review of the long-term health effects. Ann Intern Med. 1995;122:778–788 [DOI] [PubMed] [Google Scholar]
- 80. Herbst AL, Ulfelder H, Poskanzer DC. Adenocarcinoma of the vagina. Association of maternal stilbestrol therapy with tumor appearance in young women. N Engl J Med. 1971;284:878–881 [DOI] [PubMed] [Google Scholar]
- 81. Reed CE, Fenton SE. Exposure to diethylstilbestrol during sensitive life stages: a legacy of heritable health effects. Birth Defects Res C Embryo Today. 2013;99:134–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Newbold RR, Padilla-Banks E, Jefferson WN. Adverse effects of the model environmental estrogen diethylstilbestrol are transmitted to subsequent generations. Endocrinology. 2006;147(6 Suppl):S11–S17 [DOI] [PubMed] [Google Scholar]
- 83. Newbold RR, Padilla-Banks E, Snyder RJ, Jefferson WN. Developmental exposure to estrogenic compounds and obesity. Birth Defects Res A Clin Mol Teratol. 2005;73:478–480 [DOI] [PubMed] [Google Scholar]
- 84. Mahalingaiah S, Hart JE, Wise LA, Terry KL, Boynton-Jarrett R, Missmer SA. Prenatal diethylstilbestrol exposure and risk of uterine leiomyomata in the Nurses' Health Study II. Am J Epidemiol. 2014;179:186–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Hines M, Alsum P, Roy M, Gorski RA, Goy RW. Estrogenic contributions to sexual differentiation in the female guinea pig: influences of diethylstilbestrol and tamoxifen on neural, behavioral, and ovarian development. Horm Behav. 1987;21:402–417 [DOI] [PubMed] [Google Scholar]
- 86. Newbold RR, Hanson RB, Jefferson WN, Bullock BC, Haseman J, McLachlan JA. Increased tumors but uncompromised fertility in the female descendants of mice exposed developmentally to diethylstilbestrol. Carcinogenesis. 1998;19:1655–1663 [DOI] [PubMed] [Google Scholar]
- 87. Zulfahmi S, Yazan LS, Ithnin H, Armania N. The improvement of in vivo model (Balb/c mice) for cervical carcinogenesis using diethylstilbestrol (DES). Exp Toxicol Pathol. 2013;65:1083–1089 [DOI] [PubMed] [Google Scholar]
- 88. Kalfa N, Paris F, Soyer-Gobillard MO, Daures JP, Sultan C. Prevalence of hypospadias in grandsons of women exposed to diethylstilbestrol during pregnancy: a multigenerational national cohort study. Fertil Steril. 2011;95:2574–2577 [DOI] [PubMed] [Google Scholar]
- 89. Klip H, Verloop J, van Gool JD, et al. Hypospadias in sons of women exposed to diethylstilbestrol in utero: a cohort study. Lancet. 2002;359:1102–1107 [DOI] [PubMed] [Google Scholar]
- 90. Storgaard L, Bonde JP, Olsen J. Male reproductive disorders in humans and prenatal indicators of estrogen exposure. A review of published epidemiological studies. Reprod Toxicol. 2006;21:4–15 [DOI] [PubMed] [Google Scholar]
- 91. Brouwers MM, van der Zanden LF, de Gier RP, et al. Hypospadias: risk factor patterns and different phenotypes. BJU Int. 2010;105:254–262 [DOI] [PubMed] [Google Scholar]
- 92. Newbold RR, Hanson RB, Jefferson WN, Bullock BC, Haseman J, McLachlan JA. Proliferative lesions and reproductive tract tumors in male descendants of mice exposed developmentally to diethylstilbestrol. Carcinogenesis. 2000;21:1355–1363 [PubMed] [Google Scholar]
- 93. Walker BE, Haven MI. Intensity of multigenerational carcinogenesis from diethylstilbestrol in mice. Carcinogenesis. 1997;18:791–793 [DOI] [PubMed] [Google Scholar]
- 94. Anderson OS, Nahar MS, Faulk C, et al. Epigenetic responses following maternal dietary exposure to physiologically relevant levels of bisphenol A. Environ Mol Mutagen. 2012;53:334–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Vandenberg LN, Colborn T, Hayes TB, et al. Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr Rev. 2012;33:378–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Walker DM, Gore AC. Transgenerational neuroendocrine disruption of reproduction. Nat Rev Endocrinol. 2011;7:197–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Jefferson WN, Patisaul HB, Williams CJ. Reproductive consequences of developmental phytoestrogen exposure. Reproduction. 2012;143:247–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Nahar MS, Liao C, Kannan K, Dolinoy DC. Fetal liver bisphenol A concentrations and biotransformation gene expression reveal variable exposure and altered capacity for metabolism in humans. J Biochem Mol Toxicol. 2013;27:116–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Zhou W, Liu J, Liao L, Han S, Liu J. Effect of bisphenol A on steroid hormone production in rat ovarian theca-interstitial and granulosa cells. Mol Cell Endocrinol. 2008;283:12–18 [DOI] [PubMed] [Google Scholar]
- 100. Dasgupta S, Lonard DM, O'Malley BW. Nuclear receptor coactivators: master regulators of human health and disease. Annu Rev Med. 2014;65:279–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Department of Health and Human Services Centers for Disease Control and Prevention. Fourth National Report on Human Exposure to Environmental Chemicals. Atlanta, GA: Centers for Disease Control and Prevention; 2013 [Google Scholar]
- 102. Westberry JM, Trout AL, Wilson ME. Epigenetic regulation of estrogen receptor alpha gene expression in the mouse cortex during early postnatal development. Endocrinology. 2010;151:731–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. National Institute of Environmental Health Sciences. Fourth National Report on Human Exposure to Environmental Chemicals. Vol. 4 Atlanta, GA: Centers for Disease Control and Prevention; 2009 [Google Scholar]
- 104. Dolinoy DC, Huang D, Jirtle RL. Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proc Natl Acad Sci USA. 2007;104:13056–13061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Kim JH, Sartor MA, Rozek LS, et al. Perinatal bisphenol A exposure promotes dose-dependent alterations of the mouse methylome. BMC Genomics. 2014;15:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Cao J, Rebuli ME, Rogers J, et al. Prenatal bisphenol A exposure alters sex-specific estrogen receptor expression in the neonatal rat hypothalamus and amygdala. Toxicol Sci. 2013;133:157–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Doshi T, Mehta SS, Dighe V, Balasinor N, Vanage G. Hypermethylation of estrogen receptor promoter region in adult testis of rats exposed neonatally to bisphenol A. Toxicology. 2011;289:74–82 [DOI] [PubMed] [Google Scholar]
- 108. Kundakovic M, Gudsnuk K, Franks B, et al. Sex-specific epigenetic disruption and behavioral changes following low-dose in utero bisphenol A exposure. Proc Natl Acad Sci USA. 2013;110:9956–9961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Salian S, Doshi T, Vanage G. Impairment in protein expression profile of testicular steroid receptor coregulators in male rat offspring perinatally exposed to bisphenol A. Life Sci. 2009;85:11–18 [DOI] [PubMed] [Google Scholar]
- 110. Bruner-Tran KL, Osteen KG. Developmental exposure to TCDD reduces fertility and negatively affects pregnancy outcomes across multiple generations. Reprod Toxicol. 2011;31:344–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Manikkam M, Tracey R, Guerrero-Bosagna C, Skinner MK. Plastics derived endocrine disruptors (BPA, DEHP and DBP) induce epigenetic transgenerational inheritance of obesity, reproductive disease and sperm epimutations. PLoS One. 2013;8:e55387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Manikkam M, Guerrero-Bosagna C, Tracey R, Haque MM, Skinner MK. Transgenerational actions of environmental compounds on reproductive disease and identification of epigenetic biomarkers of ancestral exposures. PLoS One. 2012;7:e31901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Guerrero-Bosagna C, Covert TR, Haque MM, et al. Epigenetic transgenerational inheritance of vinclozolin induced mouse adult onset disease and associated sperm epigenome biomarkers. Reprod Toxicol. 2012;34:694–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Nilsson E, Larsen G, Manikkam M, Guerrero-Bosagna C, Savenkova MI, Skinner MK. Environmentally induced epigenetic transgenerational inheritance of ovarian disease. PloS One. 2012;7:e36129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Skinner MK, Anway MD, Savenkova MI, Gore AC, Crews D. Transgenerational epigenetic programming of the brain transcriptome and anxiety behavior. PLoS One. 2008;3:e3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Crews D, Gillette R, Scarpino SV, Manikkam M, Savenkova MI, Skinner MK. Epigenetic transgenerational inheritance of altered stress responses. Proc Natl Acad Sci USA. 2012;109:9143–9148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Skinner MK, Haque CG, Nilsson E, Bhandari R, McCarrey JR. Environmentally induced transgenerational epigenetic reprogramming of primordial germ cells and the subsequent germ line. PLoS One. 2013;8:e66318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Wolstenholme JT, Goldsby JA, Rissman EF. Transgenerational effects of prenatal bisphenol A on social recognition. Horm Behav. 2013;64:833–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Salian S, Doshi T, Vanage G. Perinatal exposure of rats to bisphenol A affects the fertility of male offspring. Life Sci. 2009;85:742–752 [DOI] [PubMed] [Google Scholar]
- 120. Anderson OS, Peterson KE, Sanchez BN, Zhang Z, Mancuso P, Dolinoy DC. Perinatal bisphenol A exposure promotes hyperactivity, lean body composition, and hormonal responses across the murine life course. FASEB J. 2013;27:1784–1792 : [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Kim JH, Rozek LS, Soliman AS, et al. Bisphenol A-associated epigenomic changes in prepubescent girls: a cross-sectional study in Gharbiah, Egypt. Environ Health. 2013;12:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Avissar-Whiting M, Veiga KR, Uhl KM, et al. Bisphenol A exposure leads to specific microRNA alterations in placental cells. Reprod Toxicol. 2010;29:401–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Doherty LF, Bromer JG, Zhou Y, Aldad TS, Taylor HS. In utero exposure to diethylstilbestrol (DES) or bisphenol-A (BPA) increases EZH2 expression in the mammary gland: an epigenetic mechanism linking endocrine disruptors to breast cancer. Horm Cancer. 2010;1:146–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Rozek LS, Dolinoy DC, Sartor MA, Omenn GS. Epigenetics: relevance and implications for public health. Annu Rev Public Health. 2014;35:105–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Abdel-Wahab O, Adli M, LaFave LM, et al. ASXL1 mutations promote myeloid transformation through loss of PRC2-mediated gene repression. Cancer Cell. 2012;22:180–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Adli M, Bernstein BE. Whole-genome chromatin profiling from limited numbers of cells using nano-ChIP-seq. Nat Protoc. 2011;6:1656–1668 [DOI] [PMC free article] [PubMed] [Google Scholar]


