Abstract
Type II diabetes originates from various genetic and environmental factors. Recent studies showed that an adverse uterine environment such as that caused by a gestational low-protein (LP) diet can cause insulin resistance in adult offspring. The mechanism of insulin resistance induced by gestational protein restriction is not clearly understood. Our aim was to investigate the role of insulin signaling molecules in gastrocnemius muscles of gestational LP diet–exposed male offspring to understand their role in LP-induced insulin resistance. Pregnant Wistar rats were fed a control (20% protein) or isocaloric LP (6%) diet from gestational day 4 until delivery and a normal diet after weaning. Only male offspring were used in this study. Glucose and insulin responses were assessed after a glucose tolerance test. mRNA and protein levels of molecules involved in insulin signaling were assessed at 4 months in gastrocnemius muscles. Muscles were incubated ex vivo with insulin to evaluate insulin-induced phosphorylation of insulin receptor (IR), Insulin receptor substrate-1, Akt, and AS160. LP diet-fed rats gained less weight than controls during pregnancy. Male pups from LP diet–fed mothers were smaller but exhibited catch-up growth. Plasma glucose and insulin levels were elevated in LP offspring when subjected to a glucose tolerance test; however, fasting levels were comparable. LP offspring showed increased expression of IR and AS160 in gastrocnemius muscles. Ex vivo treatment of muscles with insulin showed increased phosphorylation of IR (Tyr972) in controls, but LP rats showed higher basal phosphorylation. Phosphorylation of Insulin receptor substrate-1 (Tyr608, Tyr895, Ser307, and Ser318) and AS160 (Thr642) were defective in LP offspring. Further, glucose transporter type 4 translocation in LP offspring was also impaired. A gestational LP diet leads to insulin resistance in adult offspring by a mechanism involving inefficient insulin-induced IR, Insulin receptor substrate-1, and AS160 phosphorylation and impaired glucose transporter type 4 translocation.
Insulin plays a vital role in glucose metabolism. It is well known that insulin resistance leads to the development of type II diabetes (1). Insulin resistance is characterized by the inability of insulin to maintain glucose homeostasis despite being produced at high levels. The etiology of insulin resistance is complex with various origins including genetic and environmental influences (2). Recent studies show that maternal nutrition during pregnancy plays an important role in the development and progression of type II diabetes in the adult offspring (3).
Experimental and epidemiological studies show that prenatal exposure to a low-protein (LP) diet programs the offspring to develop metabolic diseases in their adult life (4). There is a strong association between in utero growth restriction and subsequent development of type 2 diabetes (5). Maternal protein restriction has been shown to cause glucose intolerance and insulin resistance in rat offspring (6–9). Several studies have shown that the severity of the disease progresses with time, ultimately leading to the development of type 2 diabetes (6–11). Although the onset and severity of the disease differed between males and females (9, 12), it is clear from these studies that maternal protein restriction causes glucose intolerance and insulin resistance, leading to the development of type 2 diabetes.
Insulin regulates glucose homeostasis by acting on peripheral target tissues. Insulin binds to insulin receptor (IR), resulting in the phosphorylation of IR substrate (IRS) proteins, which activate the phosphatidylinositol 3-kinase (PI3K)–Akt/protein kinase B pathway (13). Akt activates glucose uptake by translocating glucose transporter 4 (Glut4) from cytoplasmic vesicles to plasma membrane (13), which is mediated by AS160 (14). Further, phosphorylation of IRS-1 at various tyrosine and serine residues plays decisive roles in regulating insulin signaling (15). Skeletal muscles dispose of up to 75% of glucose and hence impaired insulin signaling in skeletal muscles contributes to the development of insulin resistance (16). Earlier studies in LP rat skeletal muscles showed increased basal glucose transport, and insulin was less effective in increasing glucose transport (17). LP rats also showed higher expression of IR and increased basal membrane mobilization of Glut4 in skeletal muscles (17). Protein kinase Cζ, a protein that is important for glucose uptake was down-regulated in skeletal muscles of LP rats compared with those of controls (6, 8). However, the roles of IR, IRS-1, AS160, and their phosphorylation and its implication for insulin resistance in LP rats are not known.
The LP rat model is widely used to explore the relationship between in utero protein restriction and metabolic disorders, including hypertension and insulin resistance (18–22). We have used the LP rat model and have extensively demonstrated that the offspring from LP diet–fed mothers are hypertensive and show significant vascular dysfunctions (20–22). These include endothelial nitric oxide production and function (21) and angiotensin II–induced vascular smooth muscle contraction (20). However, the molecular mechanism of insulin resistance in skeletal muscles induced by in utero protein restriction is not clearly understood. We wanted to explore the possible mechanism of insulin resistance in gastrocnemius muscles by investigating signaling molecules involved in insulin-induced glucose transport. The objective of our present study was to investigate the role of insulin signaling molecules such as IR, IRS-1, AS160, and Glut4 in gastrocnemius muscles of gestational LP diet–exposed male offspring to understand their role in LP diet–induced insulin resistance.
Materials and Methods
Animals
Timed pregnant Wistar rats were purchased from Harlan Laboratories on day 4 of pregnancy. Rats were housed in a temperature-controlled room (23°C) with a 12:12-hour light/dark cycle with unlimited access to food and water. Pregnant rats were fed with a control (20% protein, n = 4) or isocaloric LP (6%, n = 4) diet from day 4 of pregnancy until delivery. Rats were allowed to deliver at term, and birth weights of pups were recorded. After delivery, mothers were given a normal diet. The number of pups in the control and LP litters were culled to 10 pups per mother (pups with weights at extremes were killed) to ensure equal nutrient access for all offspring. Pups were weaned at 3 weeks of age and after weaning, offspring were given a normal diet. All rats were killed at 4 months, and tissues were collected, snap-frozen, and stored at −80°C until analysis. Male offspring alone were used for this study. All experimental procedures were performed with approval by the Institutional Animal Care and Use Committee of the University of Texas Medical Branch.
Glucose tolerance test
Rats were fasted overnight and were given glucose orally (2 g/kg body weight) at 3 months of age. Blood was collected at 0, 30, 60, and 120 minutes by orbital sinus puncture. Plasma was isolated from the blood by centrifugation and stored at −80°C until analysis. Plasma glucose levels were measured using a glucose colorimetric assay (Cayman Chemical), following the manufacturer's protocol. In brief, the samples were diluted 1:10 with assay buffer. To 15 μL of samples and standards, 85 μL of assay buffer was added, mixed well, and incubated with 100 μL of colorimetric enzyme mixture for 5 minutes at 37°C. Absorbance was read at 514 nm.
Insulin measurements
Insulin was measured using a rat insulin ELISA kit (Mercodia), following the manufacturer's instruction. In brief, 10 μL of plasma or calibrators and 100 μL of enzyme conjugate were mixed and incubated for 2 hours at room temperature with constant shaking. After washing, 200 μL of 3,3′,5,5′-tetramethylbenzidine substrate was added and incubated for 15 minutes for color development. The reaction was then stopped by adding 50 μL of stop solution. Absorbance was read at 450 nm using a FLUOstar plate reader (BMG Labtech GmbH), and the results were calculated with cubic spline regression fit using FLUOstar Omega data analysis software.
Homeostasis model assessment
Homeostasis model assessment of insulin resistance (HOMA-IR) and homeostasis model assessment of insulin sensitivity (HOMA-IS) were calculated to assess insulin resistance and insulin sensitivity of control and LP rats using the following equations (23):
Ex vivo insulin treatment
During autopsy, gastrocnemius muscles were quickly dissected and split longitudinally into 2 strips of similar size for control and LP rats. Gastrocnemius muscles were chosen because they are more sensitive than soleus muscles for insulin resistance (24). The muscle strips were preincubated for 30 minutes with Krebs-Henseleit bicarbonate buffer (120 mM NaCl, 4.7 mM KCl, 1.25 mM MgSO4, 1.2 mM KH2PO4, 2.5 mM CaCl2, and 25 mM NaHCO3, pH 7.4) containing 5.5 mM glucose, 2 mM sodium pyruvate, and 0.1% BSA followed by incubation with and without insulin (10 mU/mL; Sigma-Aldrich) for 30 minutes (25). The incubations were gassed continually with 95% O2 with 5% CO2. After the incubations, the muscles were blotted rapidly on filter paper and frozen in liquid nitrogen and stored in −80°C until analysis.
Total and membrane protein extraction
All procedures were performed at 4°C. Muscle tissues were weighed and homogenized in 1× radioimmunoprecipitation assay buffer (Cell Signaling Technology) containing 1 mM phenylmethylsulfonyl fluoride, protease inhibitor cocktail (Roche), and phosphatase inhibitor cocktails (Sigma-Aldrich). The lysates were then sonicated with 3 bursts of 5 s each with 30% power and were spun at 14 000 × g for 10 minutes. The supernatants were collected and stored at −80°C until further analysis. Membrane proteins from gastrocnemius muscles were extracted using a plasma membrane protein extraction kit (BioVision), following the manufacturer's instruction for plasma membrane proteins. Total and membrane proteins were quantified using a BCA kit (Pierce Biotechnology).
Western blotting
Protein extracts (10–30 μg) from each sample were resolved on 4% to 12% precast gradient polyacrylamide gels (NuPAGE; Life Technologies). Proteins from the gel were transferred to a polyvinylidene difluoride membrane (Millipore) by electroblotting. After the membranes were blocked in 5% BSA or nonfat dried milk in Tris-buffered saline containing 0.1% Tween for 1 hour at room temperature, they were incubated (overnight at 4°C) with the primary antibodies. Details of the primary antibodies and their dilutions are as mentioned below. Antibodies for IRS-1 (1:1000; catalog no. 3407), phospho-IRS-1 (Ser307, 1:1000, catalog no. 2381; Ser318, 1:1000, catalog no. 5610; and Ser612, 1:1000, catalog no. 3203), Akt (1:1000, catalog no. 9272), phospho-AS160 (Thr642, 1:1000, catalog no. 4288), and phospho-Akt (Ser473, 1:1000, catalog no. 9271 and Thr308, 1:1000, catalog no. 9275) were obtained from Cell Signaling Technology. IRβ antibody (1:500, catalog no. 610109) was obtained from BD Biosciences. AS160 antibody (1:1000, catalog. no. 07–741) was obtained from Millipore. Glut4 (1:2000, catalog. no. ab654), phospho-IR Tyr972 (1:1000, catalog. no. ab5678), phospho-IRS-1 Tyr612 (1:500, catalog no. ab66153), phospho-IRS-1 Tyr896 (1:500, catalog no. ab46800), α1 sodium potassium ATPase (1:5000, catalog no. ab7671), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (1:2500, catalog no. ab9485) antibodies were obtained from Abcam. After primary antibody incubations, membranes were washed and incubated for 60 minutes at room temperature with horseradish peroxidase–conjugated secondary antibodies. Membranes were washed and incubated in ECL Western blotting detection reagents (Pierce Biotechnology) for 1 minute and exposed to film and developed. Membranes were then reprobed for GAPDH. Films were scanned, and densitometric analyses were performed using ImageJ software.
Real-time quantitative PCR
Total RNA was isolated from gastrocnemius muscles using TRIzol reagent (Life Technologies) and further purified with RNeasy clean-up kit (Qiagen). All RNA samples were treated with DNase. RNA concentration and purity were determined using an ND-1000 model NanoDrop spectrophotometer (Thermo Fisher Scientific). Then 2 μg of total RNA were reverse transcribed using a modified Maloney murine leukemia virus-derived RT (New England Biolabs Inc) and random hexamer primers (Life Technologies). The reaction was performed at 37°C for 60 minutes and stopped by heating at 95°C for 5 minutes, followed by 4°C, before storage at −20°C until further analysis. The resulting cDNA was diluted 1:10, and 1 μL of the resulting cDNA was amplified by real-time PCR using SYBR Green (Bio-Rad Laboratories) as the fluorophore in a CFX96 real-time thermal cycler (Bio-Rad Laboratories). Specific pairs of primers (Integrated Device Technology) (Supplemental Table 1) were used for each gene amplification. PCR conditions used were 10 minutes at 95°C for 1 cycle, 15 seconds at 95°C, 30 seconds at 60°C, and 15 seconds at 72°C for 40 cycles, followed by a melt curve analysis (0.5°C every 5 seconds from 65 to 95°C). Results were calculated using the 2−ΔΔCT method and expressed as fold changes of expression of genes of interest. All reactions were performed in duplicate, and cyclophilin A was used as an internal control.
Statistical analyses
Statistical analyses were performed using GraphPad Prism or SigmaPlot 11 software. Data are presented as means ± SEM. Comparisons between 2 groups were performed using unpaired Student t tests. Comparisons between multiple groups were performed using ANOVAs followed by Newman-Keuls tests. When 2 factors were involved, statistical analyses were performed with two-way ANOVAs followed by Bonferroni tests. Differences were considered significant when P was <.05.
Results
LP diet–fed pregnant rats gain less weight during late pregnancy
Control and LP diet–fed pregnant rats gained weight similarly until day 17 of pregnancy. However, from day 18 onward the body weight gain of LP diet–fed pregnant rats were significantly lower compared with normal diet–fed controls. The weight difference between control and LP rats widened as pregnancy progressed and by day 22, LP rats weighed ∼55 g less than the controls, which corresponds to ∼17% of the total body weight (Figure 1A).
Figure 1.
Maternal and pup weights and plasma glucose and insulin levels in control and LP offspring. A, Maternal weight gain in control and LP diet–fed rats from day 4 to day 22 of pregnancy (n = 4 for each group). B, Weight gained by pups (n = 9–16) born to control and LP diet–fed mothers. C and D, Glucose (C; Δ AUC) and insulin levels (D, Δ AUC) for control and gestational LP diet-fed offspring. Blood samples were collected at 0, 30, 60, and 120 minutes after oral glucose (2 g/kg body weight) administration. The overall plasma glucose levels are expressed as Δ AUC calculated by trapezoidal method. Δ AUC is calculated as a difference between the total AUC and the baseline values calculated after an OGTT at 3 months. Results are presented as means ± SEM (n = 4–5/group) *, P < .05; **, P < .01; ***, P < .001.
LP pups weigh less at birth but catch up by 4 weeks
Pups born to LP diet–fed mothers were significantly smaller (5.3 ± 0.1 g) compared with those born to control mothers (6.2 ± 0.1 g) when weighed on day 1. Similar weight differences were observed until 3 weeks. However, LP pups showed rapid catch-up growth, and their weights matched with those of controls (86.6 ± 1.6 g [control] vs 85.3 ± 0.9 g [LP]) in 4 weeks, and they maintained similar body weights (553 ± 18 g [control] vs 544 ± 18 g [LP]) until killing at 4 months of age (Figure 1B).
LP offspring show elevated plasma glucose and insulin levels in glucose tolerance tests
Fasting plasma glucose levels in LP offspring (6.0 ± 0.8 mmol/L) were similar to those of controls (6.2 ± 0.7 mmol/L); however, after administration of glucose during oral glucose tolerance tests (OGTTs), plasma glucose levels in LP offspring were significantly elevated at 30 minutes (9.6 ± 1.2 mmol/L vs 7.2 ± 0.7 mmol/L, P < .01) and 60 minutes (10.1 ± 1.9 mmol/L vs 7.9 ± 0.9 mmol/L, P < .05) followed by no difference at 120 minutes (8.9 ± 0.2 mmol/L vs 8.1 ± 0.2 mmol/L). The overall plasma glucose levels after oral glucose administration were analyzed by calculating the Δ glycemia area under the curve (AUC) using the trapezoidal method, and these levels were significantly (P < .01) higher (glycemia, 383 ± 36 mmol/L · 120 minutes) in LP than in control offspring (glycemia, 163 ± 30 mmol/L · 120 minutes) (Figure 1C). Fasting insulin levels were similar in control (66 ± 7 pmol/L) and LP (60 ± 9 pmol/L) offspring. However, plasma insulin levels at 30 minutes after glucose administration were significantly (P < .001) higher in LP rats (496 ± 63 pmol/L) than in controls (325 ± 31 pmol/L). Insulin levels did not show any difference at 60 minutes (control, 226 ± 18 pmol/L; LP, 151 ± 24 pmol/L) or 120 minutes (control, 136 ± 13 pmol/L; LP, 164 ± 33 pmol/L). The overall plasma insulin responses, expressed as Δ insulin AUC after oral glucose administration, were greater in LP (21 970 ± 2729 pmol/L · 120 minutes) (P < .05) than in control offspring (14 470 ± 353 pmol/L · 120 minutes) (Figure 1D). HOMA-IR (1.11 ± 0.21 [control] and 0.95 ± 0.18 [LP]) and HOMA-IS (24.8 ± 4.7 [control] and 30.66 ± 6.3 [LP]) did not show any difference between the control and LP offspring.
Expression of IRβ and AS160 is up-regulated in the gastrocnemius muscles of LP offspring
The basal expression of the β subunit of IR and its downstream signaling molecules such as IRS-1, Akt, Glut4, and AS160 were evaluated in gastrocnemius muscles. IRβ and AS160 mRNA and protein levels were up-regulated in LP rats compared with those in controls. In LP rats, IRβ mRNA expression was ∼22% (P < .01) and the protein level was ∼53% (P < .01) higher than in controls (Figure 2). Similarly, LP rats showed greater mRNA (∼35%, P < .05) and protein (∼67%, P < .05) expression for AS160 than controls. However, IRS-1, Akt, and Glut4 levels were not different between controls and LP offspring (Figure 2).
Figure 2.
Basal mRNA and protein expression levels of molecules involved in insulin signaling pathway were quantified by quantitative PCR and Western blotting, respectively. Panel A, Figure showing the mRNA expression of IRβ subunit (IRβ), IRS-1, Akt, Glut4, and AS160. Quantitative PCR data were normalized with the housekeeping gene cyclophilin A. Panel B, Representative blots and their densitometric analyses showing the protein levels of IRβ, IRS-1, Akt, Glut4, and AS160. GAPDH was used as a loading control for Western blots, and its values were used for normalization. C, control. Results are presented as means ± SEM (n = 4–5/group) *, P < .05; **, P < .01.
IRβ phosphorylation at tyrosine 974 is enhanced by insulin in controls, but LP offspring have higher basal phosphorylation levels
Insulin phosphorylates IRβ at various sites including Tyr974 (Tyr972 in human), and this position is important for the recruitment of IRS-1 substrate. Our data show that insulin increases (P < .05) phosphorylation of IRβ at Tyr974 in control rats. In LP animals, the basal phosphorylation is significantly higher than that of controls (P < .05), and insulin did not further increase the phosphorylation levels (Figure 3). Insulin did not affect the protein levels of IRβ (Figure 3), and IRβ levels tended to be higher in LP rats than in controls (Figure 3).
Figure 3.
Ex vivo incubation of gastrocnemius muscles with insulin (10 mU/mL) for 30 minutes increases the phosphorylation of insulin receptor (IRβ) Tyr974 (Tyr972 in human) in controls but not in LP offspring and the increased basal IRβ Tyr974 phosphorylation in LP rats. (A) Representative blots showing bands for phospho IRβ Tyr974 and total IR levels along with GAPDH. Densitometric analysis of (B) IRβ Tyr974 and (C) total IRβ levels in control and LP rat tissues treated with insulin. Values were normalized with loading control, GAPDH. Results are presented as mean ± SEM (n = 3–4/group) *, P < .05.
Insulin induced tyrosine and serine phosphorylation of IRS-1 at multiple sites in control but not in LP offspring
Insulin phosphorylates IRS-1 in several tyrosine and serine residues. Our study shows that insulin increased phosphorylation of IRS-1 at Tyr608 (Tyr612 in human; P < .05) (Figure 4, A and B), Tyr895 (Tyr896 in human; P < .05) (Figure 4, A and C), Ser307 (Ser312 in human) (Figure 4, A and D), and Ser318 (Ser 323 in human; P < .001) (Figure 4, A and E) positions in control animals. This increase was greater in the Ser318 position with an ∼3-fold surge in phosphorylation (Figure 4, A and E). However in LP rats, insulin could not increase the phosphorylation in any of these positions. Consequently, there were significant differences in the insulin-induced phosphorylation of controls compared with those in LP rats in Tyr608 (P < .05), Tyr895 (P < .01), Ser307 (P < .05), and Ser318 (P < .001) (Figure 4, A–E). A similar tendency was observed in the phosphorylation at the Ser612 (human Ser616) position, but it did not reach statistical significance (Figure 4, A and F). Incubation with insulin did not alter protein levels of IRS-1 in either control or LP rats, and there were no differences in the basal levels of IRS-1 protein between control and LP rats (Figure 4, A and G).
Figure 4.
Insulin-activated tyrosine and serine phosphorylation of IRS-1 in gastrocnemius muscles after incubation with insulin (10 mU/mL) for 30 minutes. A, Representative blots showing bands for pIRS-1 (Tyr608), pIRS-1 (Tyr895), pIRS-1 (Ser307), pIRS-1 (Ser318), pIRS-1 (Ser612), total IRS-1, and GAPDH in the presence or absence of insulin. B–G, Histograms from the densitometric analyses of blots of showing IRS-1 (Tyr608) (B), pIRS-1 (Tyr895) (C), pIRS-1 (Ser307) (D), pIRS-1 (Ser318) (E), pIRS-1 (Ser612) (F), and total IRS-1 (G) levels in the presence or absence of insulin. Values were normalized with GAPDH. Results are presented as means ± SEM (n = 3–4/group) *, P < .05.
Insulin induced Akt phosphorylation at serine 473 in both control and LP offspring
Akt is a downstream effector of insulin action, which is mediated by IRS-1 acting via the PI3K pathway. Insulin facilitates the phosphorylation of Akt/protein kinase B at Ser473 in both control and LP rats (Figure 5, A and B). Our data show that the phosphorylation is significantly increased (∼2 fold, P < .05) in both rat groups. Insulin-induced phosphorylation at the Thr308 position was not apparent in either group (data not shown). Total Akt levels, however, remained unaltered by insulin treatment. Further, there were no differences in the protein levels of Akt between control and LP rats (Figure 5, A and C).
Figure 5.
Incubation of gastrocnemius muscles with insulin (10 mU/mL) for 30 minutes increases the phosphorylation of Akt Ser473 in both control and LP offspring. A, Representative blots showing bands for phospho-Akt Ser473 and total Akt levels along with GAPDH. B and C, Densitometric analyses of Akt Ser473 (B) and total Akt (C) levels in control and LP rat tissues treated with vehicle and insulin. Values were normalized with the loading control, GAPDH. Results are presented as means ± SEM (n = 4–5/group) *, P < .05).
Insulin-induced membrane localization of Glut4 is less efficient in LP rats
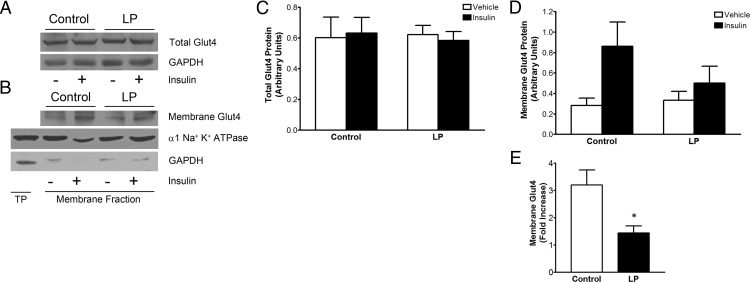
Glut4 facilitates glucose uptake by migrating to plasma membrane. Glut4 localization in plasma membrane was assessed using the membrane fraction isolated from gastrocnemius muscles after incubation with insulin. Insulin did not alter total Glut4 levels in control and LP rats (Figure 6, A and C). Our data show that the membrane localization of Glut4 in control rats tended to be greater than that in LP rats treated with 10 mU/mL of insulin for 30 minutes (Figure 6, B and D). Consequently, the fold increase of insulin-stimulated Glut4 membrane localization is significantly lower in LP rats (∼1.4 fold) than in controls (∼3.2 fold), suggesting inefficient membrane localization of Glut4 in LP rats (Figure 6E).
Figure 6.
Insulin-induced membrane localization of Glut4 is less efficient in LP. A, Representative blots showing bands for total Glut4 along with GAPDH (loading control). B, Blot showing membrane Glut4 and the α subunit of Na+,K+-ATPase (loading control) along with GAPDH (cytoplasmic marker). TP, total protein. C and D, Densitometric analysis for total Glut4 (C) and membrane Glut4 (D), showing their levels in control and LP rats in the presence or absence of insulin. Values were normalized with the loading controls, GAPDH for total Glut4 and the α subunit of Na+,K+ ATPase for membrane Glut4. (E) Plot showing the fold increase of membrane Glut4 in response to insulin treatment in controls and LP rats. Results are presented as means ± SEM (n = 3–5/group) *, P < .05.
Insulin increased AS160 threonine 642 phosphorylation in control but not in LP offspring
AS160 is an Akt substrate involved in the trafficking of Glut4. Ex vivo insulin treatment significantly induced the phosphorylation of AS160 at Thr642 (P < .001) by ∼2-fold in the gastrocnemius muscles of control offspring but failed to elicit a similar response in LP rats (Figure 7, A and B). Insulin-induced phosphorylation of AS160 in LP rats was significantly less (P < .05) than that of controls. Insulin did not alter the basal levels of AS160 protein, but LP offspring displayed an increasing tendency (Figure 7, A and C).
Figure 7.
Ex vivo insulin treatment increased AS160 Thr642 phosphorylation in control but not in LP offspring. A, Representative blots showing bands for phospho-AS160 Thr642 and total AS160 along with GAPDH (loading control). B and C, Densitometric analysis of AS160 Thr642 (B) and total AS160 (C) levels in control and LP rat tissues treated with vehicle or insulin. Results are presented as means ± SEM (n = 4–5/group). *, P < .05; ***, P < .001.
Discussion
Gestational protein restriction is well documented to cause metabolic syndrome including hypertension (20–22) and insulin resistance (5, 9, 12). We show that gestational protein restriction affects insulin function at various stages of the insulin signaling pathway in the gastrocnemius muscle of adult male offspring. In this study, we examined the basal glucose and insulin levels and responses to OGTTs. We also studied the downstream insulin signaling mechanisms, especially those pertaining to glucose transport after ex vivo insulin treatment of gastrocnemius muscles. Results show that the basal levels of both glucose and insulin are similar in LP and control rats with comparable HOMA-IR and HOMA-IS. However, upon glucose administration, LP rats, unlike controls, showed high plasma glucose levels despite higher insulin secretion. Thus, although the basal glucose and insulin levels seem unaltered with LP programming, the induced responses are severely altered, leading to metabolic disturbances. We also show for the first time that insulin-induced phosphorylation of IR, IRS-1, and AS160 is affected in LP rats and their implications in insulin signaling.
Nutritional insults such as protein restriction during pregnancy cause developmental programming in offspring in human and animal models, leading to various metabolic diseases in their adult lives (5). Our study demonstrates that pregnant rats fed with an LP diet gained less weight than controls in late pregnancy, and, consequently, the pup weights are significantly less at birth. However, these pups exhibited rapid catch-up growth similar to that reported in rodents and humans (18, 26, 27). Recent reports suggest that rapid catch-up growth after gestational protein deficiency could exacerbate metabolic disturbances including insulin resistance (3, 18). Our data show hyperglycemia and hyperinsulinemia after a glucose tolerance test in LP rats, indicating glucose intolerance and insulin resistance. Similar observations have been reported earlier in LP diet–programmed rats of different ages and both sexes (6–9).
Skeletal muscle is a major contributor of insulin resistance because it is the major source of glucose utilization (13). We studied the mechanism of gestational LP-induced insulin resistance in gastrocnemius muscles. Basal IR mRNA and protein expressions were increased in LP rats, indicating insulin resistance. Such increased IR levels were observed in tibialis anterior muscles (17); however, other studies reported no such changes (6, 8). These variations could be attributed to variations in age, sex, animal model, and tissues studied. Further, basal IR phosphorylation of LP rats at Tyr974 position was ∼2-fold higher than that of controls, and insulin did not cause any further increase, indicating a blunted response to insulin. The failure of insulin to increase Tyr974 phosphorylation in LP rats indicates a defect in the autophosphorylation of IR, and similar defects have been reported in humans and rodent diabetic models (28–30). The increase in basal phosphorylation in LP rats could be attributed to high basal IR expression. Tyrosine 974 in IR is present in the juxtamembrane region, and its phosphorylation is important to facilitate the binding of IR to the phosphotyrosine-binding domains of IRS-1 (30, 31). Thus, our data show that recruitment of IRS-1 to IR could be affected in LP offspring.
IRS-1 is a downstream substrate of IR and is phosphorylated by the IR and other kinases at various tyrosine and serine residues (15). Tyrosine phosphorylation of IRS-1 is essential for normal insulin-induced responses (15). Several tyrosine and serine phosphorylation sites have been identified in IRS-1 with distinct functions (32). Our data show increased insulin-stimulated tyrosine phosphorylation of IRS-1 at Tyr608 and Tyr895 positions in control but not in LP rats, indicating a significantly blunted response to insulin in LP rats. Such reduced insulin-stimulated IRS-1 tyrosine phosphorylation has been reported in other insulin-resistant models also (15, 27, 33). Tyrosine phosphorylation of IRS-1 is essential for the activation of the PI3K signaling cascade to initiate the metabolic functions of insulin, and this pathway involves the activation of Akt (32). Our observations show increased Akt phosphorylation (Ser473) by insulin in control and LP rats. Interestingly, despite faulty IRS-1 tyrosine phosphorylation, LP rats also show increased insulin-induced Akt phosphorylation, suggesting the existence of other compensatory mechanisms to phosphorylate Akt; thereby, Akt signaling is unaffected in LP rats. This could be due to the involvement of Akt in various other key signaling pathways in skeletal muscles (34).
IRS-1 is also phosphorylated on serine residues at multiple locations to perform contrasting roles by positively or negatively regulating insulin signaling (15, 35). Our data show increased phosphorylation in control rats at Ser307 and Ser318 when treated with insulin, but LP rats failed to show increased phosphorylation in these sites. Phosphorylation at Ser307 of IRS-1 has been shown to reduce the efficiency of IR to couple with IRS-1, thereby reducing the insulin signaling (35, 36). Similarly, down-regulation of insulin signaling by Ser318 phosphorylation of IRS-1 has also been reported (32, 37). However, other studies show contrasting findings. Insulin-induced phosphorylation of IRS-1 at Ser307 in human skeletal muscles and cultured skeletal muscle cells promotes insulin signaling (38, 39). Further, Ser307 has been shown to promote insulin sensitivity in mice by maintaining PI3K binding to IRS-1 (40). Insulin treatment has also been shown to increase Ser318 phosphorylation of IRS-1, Akt phosphorylation, and glucose uptake (39, 41, 42). These discrepancies are explained by a study by Weigert et al (39), suggesting that serine phosphorylation of IRS-1 plays opposing roles, depending on the different simultaneous phosphorylation sites rather than a single site in a temporal fashion. Thus, the temporal changes of serine phosphorylation could positively regulate insulin signaling (39). By studying the phosphorylation status of IRS-1 at 30 minutes of insulin treatment as in our present study, we capture a snapshot of the phosphorylation status at that moment, which suggests that Ser307 and Ser318 phosphorylation could play a promoting role in the insulin signaling of control rats. The absence of such insulin-induced phosphorylation in LP animals, which are insulin resistant and glucose intolerant, indicates the importance of serine phosphorylation in positive insulin signaling. Our study shows that there are differences in the insulin-induced phosphorylation patterns of tyrosine and serine residues between control and LP rats with functional consequences showing their connection in causing insulin resistance in LP rats.
Glucose uptake into the skeletal muscle is facilitated by the glucose transporter, Glut4 (43). Although a family of glucose transporters has been identified in various tissues, Glut4 is the major glucose transporter in skeletal muscle (43). Insulin stimulates the delivery of Glut4 from intracellular vesicles to the cell surface and facilitates glucose transport from plasma into the cell (43). Thus, the regulation of subcellular distribution of Glut4 is essential for maintaining normal glucose homeostasis. Our study shows altered insulin-induced membrane distribution of Glut4 in LP rats, indicating compromised Glut4 transport. Similarly, impaired distribution was observed in vivo in tibialis anterior muscles of rats and in skeletal muscles from type 2 diabetic patients (17, 44). Insulin-induced Glut4 transport is assisted by an Akt substrate of 160 kDa called AS160 (45). In response to insulin, AS160 is phosphorylated, and it facilitates the movement of Glut4 vesicle to dock and fuse with plasma membrane, thereby making glucose accessible for Glut4 for transportation (45). A recent study using AS160 knockout mice showed that membrane translocation of Glut4 was affected in gastrocnemius muscles, indicating the importance of AS160 in Glut4 trafficking and glucose homeostasis (46). This study also showed that in the absence of AS160, insulin failed to increase the membrane-associated Glut4 (46). Similar observations have been reported in adipocyte and skeletal muscle cell lines (47–49). Our findings show increased mRNA and protein expression of AS160 in LP rats; however, its phosphorylation in response to insulin is severely reduced. Phosphorylation of AS160 is shown to be essential for its function (50, 51), and, therefore, we suggest that AS160 is functionally impaired in LP rats. AS160 retains Glut4 in the vesicles and prevents it from docking and fusing with plasma membrane in the absence of insulin (46, 52). Because AS160 is functionally impaired in LP rats, it leads to compromised Glut4 translocation to the plasma membrane, behaving similarly to its behavior the AS160 knockout mice. Increased expression of AS160 in LP rats could be an attempt to compensate for the compromised Glut4 translocation.
In conclusion, our study shows that gestational protein restriction alters insulin signaling mechanisms related to glucose transport in adult offspring rats (Figure 8). Although the basal levels of glucose and insulin appear normal, the system is under strain and struggles to maintain homeostasis when there is a glucose overload. Apparent homeostasis is achieved by compensating for the inefficient insulin signaling by increased levels of insulin, IR, and AS160. We also show that insulin-induced phosphorylation of IR, IRS-1, and AS160 is severely hampered in LP rats. The expression and function of various kinases need to be probed to understand the reasons for compromised phosphorylation in LP offspring. The question of how a gestational LP diet programs the signaling still remains to be answered. Elucidating the programing mechanism may help in identifying novel targets that could be used to reverse this metabolic disease.
Figure 8.
Schematic diagram showing the impaired insulin signaling cascade in gastrocnemius muscles of 4-month-old maternal protein–restricted male offspring. *, Impaired insulin-induced phosphorylation. Phosphorylation of IR (tyrosine 974), IRS-1 (serine 307 and 318 and tyrosine 608 and 895), and AS160 (threonine 642) is severely decreased in LP rats. Increased levels of IR and AS160 (open arrows) is seen in LP rats. #, Insulin regulation of Glut4 translocation in LP offspring.
Acknowledgments
This work was supported by National Institutes of Health (Grants HL102866 and HL58144 to C.Y. and HD069750 and HL119869 to K.S.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AUC
- area under the curve
- IR
- insulin receptor
- IRS
- insulin receptor substrate
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- Glut4
- glucose transporter type 4
- HOMA-IR
- homeostasis model assessment of insulin resistance
- HOMA-IS
- homeostasis model assessment of insulin assessment
- LP
- low protein
- OGTT
- oral glucose tolerance test
- PI3K
- phosphatidylinositol 3-kinase.
References
- 1. DeFronzo RA. Insulin resistance, lipotoxicity, type 2 diabetes and atherosclerosis: the missing links. The Claude Bernard Lecture 2009. Diabetologia. 2010;53:1270–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Moller DE, Kaufman KD. Metabolic syndrome: a clinical and molecular perspective. Annu Rev Med. 2005;56:45–62 [DOI] [PubMed] [Google Scholar]
- 3. Martin-Gronert MS, Ozanne SE. Metabolic programming of insulin action and secretion. Diabetes Obes Metab. 2012;14(suppl 3):29–39 [DOI] [PubMed] [Google Scholar]
- 4. Stocker CJ, Arch JR, Cawthorne MA. Fetal origins of insulin resistance and obesity. Proc Nutr Soc. 2005;64:143–151 [DOI] [PubMed] [Google Scholar]
- 5. Barker DJ. The origins of the developmental origins theory. J Intern Med. 2007;261:412–417 [DOI] [PubMed] [Google Scholar]
- 6. Ozanne SE, Olsen GS, Hansen LL, et al. Early growth restriction leads to down regulation of protein kinase Cζ and insulin resistance in skeletal muscle. J Endocrinol. 2003;177:235–241 [DOI] [PubMed] [Google Scholar]
- 7. Petry CJ, Dorling MW, Pawlak DB, Ozanne SE, Hales CN. Diabetes in old male offspring of rat dams fed a reduced protein diet. Int J Exp Diabetes Res. 2001;2:139–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fernandez-Twinn DS, Wayman A, Ekizoglou S, Martin MS, Hales CN, Ozanne SE. Maternal protein restriction leads to hyperinsulinemia and reduced insulin-signaling protein expression in 21-mo-old female rat offspring. Am J Physiol Regul Integr Comp Physiol. 2005;288:R368–R373 [DOI] [PubMed] [Google Scholar]
- 9. Zambrano E, Bautista CJ, Deas M, et al. A low maternal protein diet during pregnancy and lactation has sex- and window of exposure-specific effects on offspring growth and food intake, glucose metabolism and serum leptin in the rat. J Physiol. 2006;571(Pt 1):221–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sugden MC, Holness MJ. Gender-specific programming of insulin secretion and action. J Endocrinol. 2002;175:757–767 [DOI] [PubMed] [Google Scholar]
- 11. Hales CN, Desai M, Ozanne SE, Crowther NJ. Fishing in the stream of diabetes: from measuring insulin to the control of fetal organogenesis. Biochem Soc Trans. 1996;24:341–350 [DOI] [PubMed] [Google Scholar]
- 12. Aiken CE, Ozanne SE. Sex differences in developmental programming models. Reproduction. 2013;145:R1–R13 [DOI] [PubMed] [Google Scholar]
- 13. Abdul-Ghani MA, DeFronzo RA. Pathogenesis of insulin resistance in skeletal muscle. J Biomed Biotechnol. 2010;2010:476279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Choi K, Kim YB. Molecular mechanism of insulin resistance in obesity and type 2 diabetes. Korean J Intern Med. 2010;25:119–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gual P, Le Marchand-Brustel Y, Tanti JF. Positive and negative regulation of insulin signaling through IRS-1 phosphorylation. Biochimie. 2005;87:99–109 [DOI] [PubMed] [Google Scholar]
- 16. Klip A, Pâquet MR. Glucose transport and glucose transporters in muscle and their metabolic regulation. Diabetes Care. 1990;13:228–243 [DOI] [PubMed] [Google Scholar]
- 17. Ozanne SE, Wang CL, Coleman N, Smith GD. Altered muscle insulin sensitivity in the male offspring of protein-malnourished rats. Am J Physiol. 1996;271:E1128–E1134 [DOI] [PubMed] [Google Scholar]
- 18. Duque-Guimarães DE, Ozanne SE. Nutritional programming of insulin resistance: causes and consequences. Trends Endocrinol Metab. 2013;24:525–535 [DOI] [PubMed] [Google Scholar]
- 19. McArdle HJ, Andersen HS, Jones H, Gambling L. Fetal programming: causes and consequences as revealed by studies of dietary manipulation in rats—a review. Placenta. 2006;27(suppl A):S56–S60 [DOI] [PubMed] [Google Scholar]
- 20. Sathishkumar K, Balakrishnan M, Chinnathambi V, Gao H, Yallampalli C. Temporal alterations in vascular angiotensin receptors and vasomotor responses in offspring of protein-restricted rat dams. Am J Obstet Gynecol. 2012;206:507.e501–507.e510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sathishkumar K, Elkins R, Yallampalli U, Yallampalli C. Protein restriction during pregnancy induces hypertension and impairs endothelium-dependent vascular function in adult female offspring. J Vasc Res. 2009;46:229–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sathishkumar K, Elkins R, Yallampalli U, Yallampalli C. Protein restriction during pregnancy induces hypertension in adult female rat offspring—influence of oestradiol. Br J Nutr. 2012;107:665–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Aref AB, Ahmed OM, Ali LA, Semmler M. Maternal rat diabetes mellitus deleteriously affects insulin sensitivity and β-cell function in the offspring. J Diabetes Res. 2013;2013:429154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gómez-Pérez Y, Gianotti M, Proenza AM, Lladó I. Age-related decline of skeletal muscle insulin sensitivity in rats: effect of sex and muscle type. Rejuvenation Res. 2011;14:153–161 [DOI] [PubMed] [Google Scholar]
- 25. Lai YC, Liu Y, Jacobs R, Rider MH. A novel PKB/Akt inhibitor, MK-2206, effectively inhibits insulin-stimulated glucose metabolism and protein synthesis in isolated rat skeletal muscle. Biochem J. 2012;447:137–147 [DOI] [PubMed] [Google Scholar]
- 26. Berends LM, Fernandez-Twinn DS, Martin-Gronert MS, Cripps RL, Ozanne SE. Catch-up growth following intra-uterine growth-restriction programmes an insulin-resistant phenotype in adipose tissue. Int J Obes (Lond). 2013;37:1051–1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vo TX, Revesz A, Sohi G, Ma N, Hardy DB. Maternal protein restriction leads to enhanced hepatic gluconeogenic gene expression in adult male rat offspring due to impaired expression of the liver X receptor. J Endocrinol. 2013;218:85–97 [DOI] [PubMed] [Google Scholar]
- 28. Meyer MM, Levin K, Grimmsmann T, Beck-Nielsen H, Klein HH. Insulin signalling in skeletal muscle of subjects with or without type II-diabetes and first degree relatives of patients with the disease. Diabetologia. 2002;45:813–822 [DOI] [PubMed] [Google Scholar]
- 29. Sesti G, Federici M, Hribal ML, Lauro D, Sbraccia P, Lauro R. Defects of the insulin receptor substrate (IRS) system in human metabolic disorders. FASEB J. 2001;15:2099–2111 [DOI] [PubMed] [Google Scholar]
- 30. Youngren JF. Regulation of insulin receptor function. Cell Mol Life Sci. 2007;64:873–891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gustafson TA, He W, Craparo A, Schaub CD, O'Neill TJ. Phosphotyrosine-dependent interaction of SHC and insulin receptor substrate 1 with the NPEY motif of the insulin receptor via a novel non-SH2 domain. Mol Cell Biol. 1995;15:2500–2508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Boura-Halfon S, Zick Y. Phosphorylation of IRS proteins, insulin action, and insulin resistance. Am J Physiol Endocrinol Metab. 2009;296:E581–E591 [DOI] [PubMed] [Google Scholar]
- 33. Timmers S, Schrauwen P, de Vogel J. Muscular diacylglycerol metabolism and insulin resistance. Physiol Behav. 2008;94:242–251 [DOI] [PubMed] [Google Scholar]
- 34. Glass DJ. PI3 kinase regulation of skeletal muscle hypertrophy and atrophy. Curr Top Microbiol Immunol. 2010;346:267–278 [DOI] [PubMed] [Google Scholar]
- 35. Copps KD, White MF. Regulation of insulin sensitivity by serine/threonine phosphorylation of insulin receptor substrate proteins IRS1 and IRS2. Diabetologia. 2012;55:2565–2582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Aguirre V, Werner ED, Giraud J, Lee YH, Shoelson SE, White MF. Phosphorylation of Ser307 in insulin receptor substrate-1 blocks interactions with the insulin receptor and inhibits insulin action. J Biol Chem. 2002;277:1531–1537 [DOI] [PubMed] [Google Scholar]
- 37. Hennige AM, Stefan N, Kapp K, et al. Leptin down-regulates insulin action through phosphorylation of serine-318 in insulin receptor substrate 1. FASEB J. 2006;20:1206–1208 [DOI] [PubMed] [Google Scholar]
- 38. Yi Z, Langlais P, De Filippis EA, et al. Global assessment of regulation of phosphorylation of insulin receptor substrate-1 by insulin in vivo in human muscle. Diabetes. 2007;56:1508–1516 [DOI] [PubMed] [Google Scholar]
- 39. Weigert C, Kron M, Kalbacher H, et al. Interplay and effects of temporal changes in the phosphorylation state of serine-302, -307, and -318 of insulin receptor substrate-1 on insulin action in skeletal muscle cells. Mol Endocrinol. 2008;22:2729–2740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Copps KD, Hancer NJ, Opare-Ado L, Qiu W, Walsh C, White MF. Irs1 serine 307 promotes insulin sensitivity in mice. Cell Metab. 2010;11:84–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Weigert C, Hennige AM, Brischmann T, et al. The phosphorylation of Ser318 of insulin receptor substrate 1 is not per se inhibitory in skeletal muscle cells but is necessary to trigger the attenuation of the insulin-stimulated signal. J Biol Chem. 2005;280:37393–37399 [DOI] [PubMed] [Google Scholar]
- 42. Weigert C, Hennige AM, Lehmann R, et al. Direct cross-talk of interleukin-6 and insulin signal transduction via insulin receptor substrate-1 in skeletal muscle cells. J Biol Chem. 2006;281:7060–7067 [DOI] [PubMed] [Google Scholar]
- 43. Bryant NJ, Govers R, James DE. Regulated transport of the glucose transporter GLUT4. Nat Rev Mol Cell Biol. 2002;3:267–277 [DOI] [PubMed] [Google Scholar]
- 44. Karlsson HK, Zierath JR, Kane S, Krook A, Lienhard GE, Wallberg-Henriksson H. Insulin-stimulated phosphorylation of the Akt substrate AS160 is impaired in skeletal muscle of type 2 diabetic subjects. Diabetes. 2005;54:1692–1697 [DOI] [PubMed] [Google Scholar]
- 45. Miinea CP, Sano H, Kane S, et al. AS160, the Akt substrate regulating GLUT4 translocation, has a functional Rab GTPase-activating protein domain. Biochem J. 2005;391(Pt 1):87–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lansey MN, Walker NN, Hargett SR, Stevens JR, Keller SR. Deletion of Rab GAP AS160 modifies glucose uptake and GLUT4 translocation in primary skeletal muscles and adipocytes and impairs glucose homeostasis. Am J Physiol Endocrinol Metab. 2012;303:E1273–E1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Eguez L, Lee A, Chavez JA, et al. Full intracellular retention of GLUT4 requires AS160 Rab GTPase activating protein. Cell Metab. 2005;2:263–272 [DOI] [PubMed] [Google Scholar]
- 48. Zaid H, Antonescu CN, Randhawa VK, Klip A. Insulin action on glucose transporters through molecular switches, tracks and tethers. Biochem J. 2008;413:201–215 [DOI] [PubMed] [Google Scholar]
- 49. Larance M, Ramm G, Stöckli J, et al. Characterization of the role of the Rab GTPase-activating protein AS160 in insulin-regulated GLUT4 trafficking. J Biol Chem. 2005;280:37803–37813 [DOI] [PubMed] [Google Scholar]
- 50. Consitt LA, Van Meter J, Newton CA, et al. Impairments in site-specific AS160 phosphorylation and effects of exercise training. Diabetes. 2013;62:3437–3447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Funai K, Schweitzer GG, Sharma N, Kanzaki M, Cartee GD. Increased AS160 phosphorylation, but not TBC1D1 phosphorylation, with increased postexercise insulin sensitivity in rat skeletal muscle. Am J Physiol Endocrinol Metab. 2009;297:E242–E251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Koumanov F, Holman GD. Thrifty Tbc1d1 and Tbc1d4 proteins link signalling and membrane trafficking pathways. Biochem J. 2007;403:e9–e11 [DOI] [PMC free article] [PubMed] [Google Scholar]