Abstract
The serine protease inhibitor (SERPIN) family member corticosteroid-binding globulin (CBG) is the main carrier of glucocorticoids in plasma. Human CBG mediates the targeted release of cortisol at sites of inflammation through cleavage of its reactive center loop (RCL) by neutrophil elastase. The RCLs of SERPIN family members are targeted by diverse endogenous and exogenous proteases, including several bacterial proteases. We tested different bacteria for their ability to secrete proteases that disrupt CBG cortisol-binding activity, and characterized the responsible protease and site of CBG cleavage. Serum CBG integrity was assessed by Western blotting and cortisol-binding capacity assay. Effects of time, pH, temperature, and protease inhibitors were tested. Proteolytically active proteins from bacterial media were purified by fast protein liquid chromatography, and the active protease and CBG cleavage sites were identified by mass spectrometry. Among the bacteria tested, medium from Pseudomonas aeruginosa actively disrupted the cortisol-binding activity of CBG. This proteolytic activity was inhibited by zinc chelators and occurred most efficiently at pH 7 and elevated physiological temperature (ie, 41°C). Mass spectrometric analysis of a semi-purified fraction of P. aeruginosa media identified the virulence factor LasB as the responsible protease, and this was confirmed by assaying media from LasB-deficient P. aeruginosa. This metalloprotease cleaves the CBG RCL at a major site, distinct from that targeted by neutrophil elastase. Our results suggest that humoral responses to P. aeruginosa infection are influenced by this pathogen's ability to secrete a protease that promotes the release of the anti-inflammatory steroid, cortisol, from its plasma transport protein.
Human SERPINA6 or corticosteroid-binding globulin (CBG) is a clade A member of the serine protease inhibitor (SERPIN) family (1, 2) that binds 80–90% of cortisol in blood plasma (3). Like other SERPINs, CBG has a surface-exposed reactive center loop (RCL) that serves as a “protease bait domain” (4). Whereas most SERPINs act to inhibit proteases, CBG and several other SERPINs with hormone-binding properties are not known to be protease inhibitors (2). Instead, human CBG is a substrate for neutrophil elastase, which cleaves its RCL at a specific site (5–7). As in other SERPINs, the proteolytically cleaved RCL of human CBG inserts into the protein core to form a novel β-strand, resulting in a conformational change that greatly reduces its affinity for cortisol (7). This loss of CBG steroid-binding activity causes a substantial redistribution of plasma cortisol between the albumin-bound and unbound or “free” fractions, and thereby enhances glucocorticoid bioavailability at sites of infection or inflammation (6). The steroid-binding affinity of CBG is also reduced through undefined mechanisms in response to increased temperature (8), and this may further accentuate the actions of cortisol under pathological conditions (9).
A recent crystal structure of human CBG obtained in complex with progesterone displayed the typical “relaxed” conformation of a SERPIN that occurs after proteolytic cleavage of the RCL (10). This was unexpected because the protein had not been treated with a protease prior to crystallization. Furthermore, this crystal structure revealed that RCL cleavage had occurred at a position different from the known site of cleavage by neutrophil elastase, suggesting the presence of an unknown protease prior to or during the crystallization process.
When SERPINA6 structures are compared between species, the RCL represents one of the most poorly conserved regions, which is surprising given its important role in CBG function. However, it has been proposed that the RCL sequences of SERPINs have rapidly evolved as an adaptive response to proteases secreted by species-specific pathogens (11). It is known that the RCL of α1-antitrypsin (SERPINA1) is cleaved by bacterial proteases (12). We therefore set out to determine whether CBG is specifically targeted by proteases secreted by a variety of bacteria, including common pathogens such as Pseudomonas aeruginosa, Burkholderia cenocepacia, and Staphylococcus aureus, which cause acute disease in the airways, as well as chronic lung infections in cystic fibrosis (CF) patients (13). These and several other pathogens tested are also of major concern in skin infections and wound healing, especially in burn patients (14). Importantly, these clinical conditions are commonly treated with glucocorticoids to control inflammation.
Whereas cleavage of CBG by neutrophil elastase may contribute to a rapid loss of CBG from the blood circulation in patients with systemic infections (15) or severe burns (16), we have explored the possibility that proteases produced by pathogens may act in a similar manner to accentuate the release of CBG-bound cortisol locally at sites of infections.
Materials and Methods
Assays of the levels and electrophoretic properties of CBG
Serum CBG levels were measured using a steroid-binding capacity assay with [3H]cortisol (American Radiolabeled Chemicals, Inc.) as the labeled ligand (17). In the experiments described below, this assay was used to test the ability of proteins secreted by bacteria to disrupt the cortisol binding of CBG in diluted human serum. The same assay was used to test the effects of LasB (pseudolysin, or P. aeruginosa elastase; EC 3.4.24.26; from Elastin Products Co, Inc), as well as protease inhibitors, including phenylmethanesulfonyl fluoride (PMSF), ethylenediaminetetraacetic acid (EDTA), N,N,N′,N′-tetrakis(2-pyridylmethyl)ethylenediamine (TPEN), and N-α-tosyl-L-lysine chloromethyl ketone hydrochloride (TLCK) (all from Sigma-Aldrich).
For Western blot analysis, 50 ng of purified CBG was incubated with 5 μl of P. aeruginosa medium or 20 μl of active chromatographic fractions for 16 hours at 37°C, or with 300 ng of neutrophil elastase (Elastin Products Co, Inc) for 10 minutes at room temperature, in a total volume of 50 μl using 20 mM Tris buffer. Ten microliters of each reaction was subjected to SDS-PAGE and transferred by Western blotting. Membranes were incubated with polyclonal rabbit antihuman CBG antiserum (18) (1:5,000) and a horseradish peroxidase-labeled goat antirabbit IgG antibody (Sigma-Aldrich). Detection was performed using ECL Prime and an ImageQuant LAS4000 (GE Healthcare).
Bacterial cultures
Cultures were prepared from: Pseudomonas aeruginosa (PAO1), Staphylococcus aureus (RN4220), Burkholderia cenocepacia (ATCC 25416), Micrococcus luteus (Davies' lab collection, UBC), Enterococcus faecalis (OG1RF), Escherichia coli (K12 MG1655), Acinetobacter baumannii (ATCC 19606), and Mycobacterium smegmatis (MC2 155). Cultures of LasB-deficient PAO1 strains (PW7302 and PW7303, from the PA two-allele library), as well as the parental PAO1 strain, were also prepared (19). Three ml cultures in Luria Bertani broth containing 10 g/L NaCl, 5 g/L yeast extract and 10 g/L tryptone were started from a single colony for incubation overnight (two days for E. faecalis and M. smegmatis) at 37°C with agitation. Then, 1 ml was transferred to a 20 ml culture and incubated in the same conditions. Cultures were considered to have reached a stationary phase of growth when harvested. Bacteria were pelleted and supernatants were filter-sterilized.
Time, temperature, and pH dependence and effects of protease inhibitors
In time-course experiments, 1 μl of human serum was incubated in the presence or absence of 1 μl P. aeruginosa-conditioned medium in a volume of 100 μl with Dulbecco's phosphate-buffered saline (DPBS) (pH 7.4) for 5 minutes, 15 minutes, 1 hour, 4 hours, 8 hours, and 16 hours at 37°C or 41°C. Reactions were stopped by the addition of 5 mM EDTA and samples were stored at 4°C until analysis of CBG cortisol-binding capacity. To test temperature and pH effects, human serum samples were incubated as above with DPBS (pH 6, 7, or 8) for 8 hours at 37°C, 39°C, or 41°C. Human serum (1 μl) was also incubated in the presence or absence of 1 μl of P. aeruginosa medium in the presence or absence of the following protease inhibitors: 5 mM EDTA, 1 mM PMSF, or 50 μM TPEN in a volume of 100 μl with DPBS for 16 hours at 37°C.
Purification of proteins secreted by P. aeruginosa that disrupt the cortisol binding of CBG
Fast protein liquid chromatography (FPLC) was performed using an AKTA Explorer chromatography system (GE Healthcare) to purify the protein(s) secreted by P. aeruginosa that disrupt the cortisol-binding activity of CBG. Medium from a P. aeruginosa culture was dialyzed in 20 mM Tris (pH 8), then subjected to ion exchange chromatography on a HiTrap Q FF column and eluted using a 1M NaCl gradient (GE Healthcare). Proteins capable of disrupting the cortisol binding of CBG were observed only in the flow-through solution, which was then concentrated approximately 100-fold and subjected to size exclusion chromatography on a Superdex75 (GE Healthcare) column.
Identification of bacterial proteases and proteolytic cleavage sites within the RCL of CBG
Protein bands separated by SDS-PAGE were excised and in-gel digested with L-1-tosylamide-2-phenylethyl chloromethyl ketone (TPCK)-treated bovine trypsin, after denaturation with dithiothreitol and alkylation of free thiols with iodoacetamide (all from Sigma-Aldrich). Extracted, concentrated peptide mixtures were desalted and fractionated with μC18-ZipTips (Millipore) then analyzed by matrix-assisted laser desorption/ionization time-of-flight (tandem) mass spectrometry on a 4700 Proteomics Analyzer (Applied Biosystems). The peptide maps and peptide fragmentation data were analyzed with the MASCOT software (Matrixscience) for protein identification. Human CBG and its proteolytic fragments were analyzed on a Voyager Biospectrometry Workstation (Applied Biosystems). Details are provided in the Supplemental Data.
Inhibition of protease IV
Protease IV is irreversibly inhibited by TLCK (20). We preincubated 0.05 mM TLCK with 1 μl of P. aeruginosa medium in a volume of 100 μl in DPBS, or with DPBS alone for 1 hour at 37°C. The reactions were applied to 0.5 ml 10K Amicon Ultra Centrifugal filter spin columns (Millipore) and washed with 10 volumes of DPBS, in order to remove excess TLCK because it has an inhibitory effect on CBG cortisol-binding. P. aeruginosa medium was filtered and washed, as above, as the reference. Retentates were adjusted to 50 μl with DPBS and incubated with 1 μl human serum in 50 μl of DPBS for 16 hours at 37°C prior to steroid-binding capacity assays.
Elastolytic activity assay
The elastolytic activities of P. aeruginosa media from the parental and LasB-deficient PAO1 strains, and of commercial LasB, were tested using elastin-congo red (Elastin Products Co, Inc) as the substrate (21). In brief, elastin-congo red (5 mg) in 400 μl reaction buffer (10 mM Tris-HCl pH 8) was added to 100 μl of 5-fold concentrated media samples, or 15 μl of commercial LasB (5 μg in 50 mM Tris-HCl pH 7.5, 10 mM CaCl2) and 85 μl reaction buffer, and the mixtures were incubated for 24 hours at 37°C. As controls, 100 μl of 5-fold concentrated Luria Bertani culture medium or 15 μl of LasB buffer and 85 μl reaction buffer were also tested. Samples were then centrifuged at 18,000g for 15 minutes and the absorbance of supernatants was determined at 490 nm using a Victor X4 plate reader (Perkin Elmer).
Statistical analysis
Two-way ANOVA was performed using GraphPad Prism 6 software (GraphPad Software, Inc) to evaluate the effect of time and temperature in time-course experiments, as well as the effect of pH and temperature in characterization experiments. A P value < .05 was considered significant.
Results
P. aeruginosa culture medium specifically disrupts human CBG cortisol binding
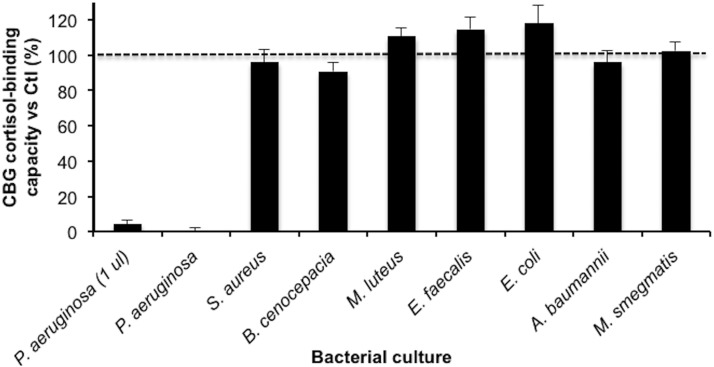
Culture media from isolates of different bacteria were first examined to determine if they were capable of disrupting the cortisol-binding activity of human CBG (Figure 1). This assay involved measuring the cortisol-binding capacity of CBG in 10 μl of a diluted (1:10) human serum sample after 16 hours incubation at 37°C with either 90 μl of culture medium from each organism, or the same amount of DPBS. Under these conditions, only medium from P. aeruginosa reduced the cortisol-binding capacity of human CBG (Figure 1). We then tested the P. aeruginosa medium at a 90 times lower concentration, and this demonstrated that the activity in the P. aeruginosa medium was substantially greater than that in any of the other pathogens tested. In a titration assay using 0.5, 1.0, 2.5, or 5.0 μl of P. aeruginosa medium, approximately 10%, 5%, 0%, and 0% of CBG cortisol-binding capacity remained, respectively.
Figure 1.
Changes in CBG cortisol-binding capacity after incubation of human serum with culture media from a range of different bacteria. The cortisol-binding capacity of CBG was measured using [3H]cortisol after human serum (1 μl) was incubated for 16 hours at 37°C with media (90 μl) from bacterial cultures. A marked decrease in cortisol-binding capacity was also observed after incubation with only 1 μl of P. aeruginosa medium. Data are presented as mean percentage ± SD of cortisol-binding capacity relative to the serum incubated with 90 μl of DPBS instead of bacterial culture media. Incubations were performed in triplicates.
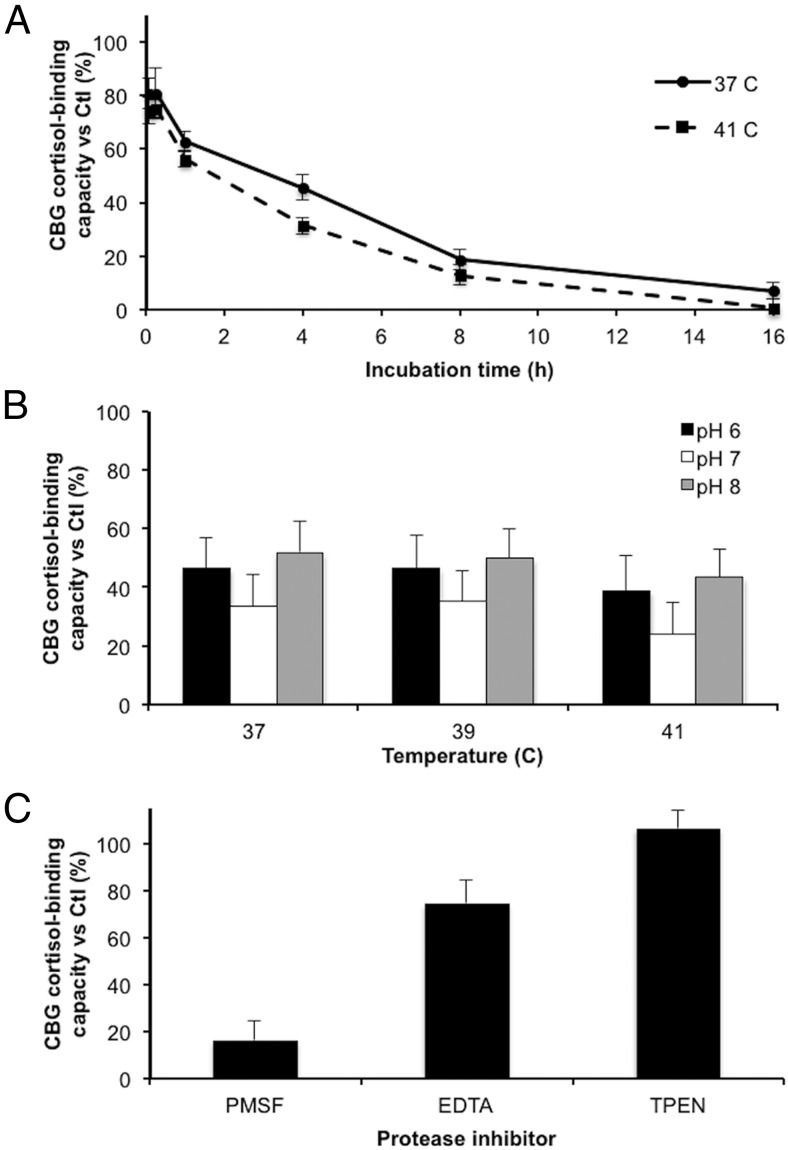
Loss of CBG cortisol binding after incubation with P. aeruginosa medium is temperature, pH, and zinc dependent
In time-course incubations of human serum and P. aeruginosa culture medium at different physiological temperatures, the CBG cortisol-binding capacity decreased progressively between 1 hour and 16 hours (Figure 2A). Over the time points studied, the CBG cortisol-binding capacity was significantly lower (P < .001) after incubations at 41°C than at 37°C, with a loss of approximately 45% and approximately 70% occurring by 1 hour and 4 hours, respectively, in the samples incubated at 41°C. In addition, after an 8-hour incubation, the remaining activity was significantly lower (P = .0065) at pH 7 than at pH 6 and 8 (Figure 2B). At all pH conditions, a greater decrease in cortisol-binding capacity was observed at 41°C than at 37°C and 39°C, which is consistent with results presented in Figure 2A. It was also determined that incubation of the P. aeruginosa medium for 30 minutes at 65°C destroyed its ability to disrupt CBG cortisol binding.
Figure 2.
Reduction in the cortisol-binding capacity of CBG in human serum after incubation with P. aeruginosa media is time-, temperature-, and pH-dependent, and requires the presence of zinc. A, The cortisol-binding capacity of CBG was measured after incubation of human serum with P. aeruginosa media for 5 minutes, 15 minutes, 1 hour, 4 hours, 8 hours, and 16 hours at 37°C or 41°C. Incubation at 41°C led to a greater decrease (P < .001) in activity than incubation at 37°C. B, Human serum was incubated with P. aeruginosa media for 8 hours at pH 6, 7, or 8, and at 37, 39, or 41°C. The cortisol-binding capacity of CBG was lower after incubations at pH 7 than at pH 6 and 8 for all temperatures tested (P = .0065). C, Human serum was incubated with P. aeruginosa media in the presence of 1 mM PMSF (serine/cysteine protease inhibitor), 5 mM EDTA (divalent cation inhibitor), or 20 ng/μl TPEN (specific zinc chelator) for 16 hours at 37°C. All incubations were performed in triplicates and data are presented as mean percentage ± SD of steroid-binding activity relative to a control.
To characterize the type of protease activity involved in disrupting CBG cortisol binding, different protease inhibitors were tested, including PMSF as a serine and cysteine protease inhibitor, EDTA as a nonspecific chelator of divalent cations, and TPEN as a potent and specific zinc chelator (Figure 2C). The results indicated that PMSF failed to protect the loss of CBG cortisol-binding capacity in human serum after incubation with 1 μl P. aeruginosa culture medium at 37°C for 16 hours, whereas EDTA was partially protective and TPEN was fully protective. These results suggest that a zinc-dependent metalloprotease is responsible for CBG cleavage.
LasB is the protease responsible for CBG cleavage at a specific location within the RCL
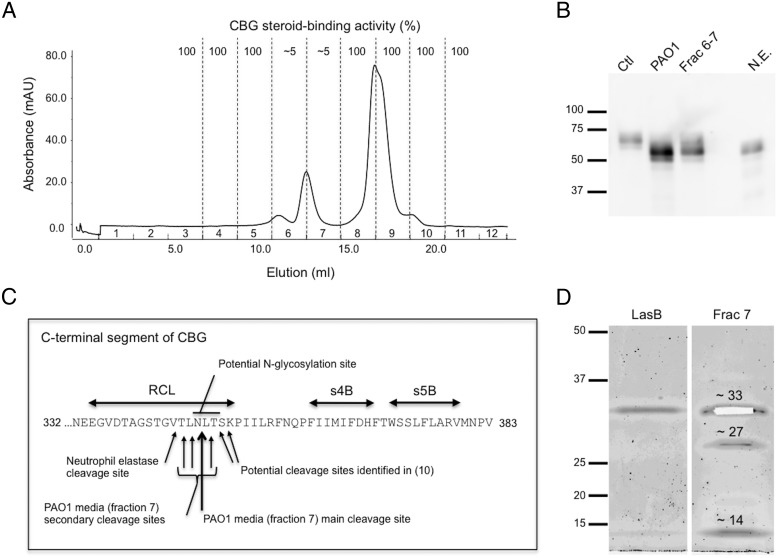
To identify the protease(s) responsible for CBG cleavage, several FPLC purification steps were performed on samples of P. aeruginosa culture media. First, culture medium was injected onto a HiTrap Q FF strong anion exchange column. None of the eluted fractions influenced CBG cortisol binding, but the flow-through material did, thus suggesting that the protease(s) responsible for CBG cleavage are not negatively charged at physiological pH. The flow-through solution was concentrated and injected on a Superdex75 size exclusion column. Proteolytic activity was observed in two fractions containing proteins eluting within a 14–43 kDa size range, with a main absorbance peak spanning them (Figure 3A).
Figure 3.
Chromatographic separation and identification of candidate proteases within P. aeruginosa media that target and cleave the CBG reactive center loop (RCL). A, Elution profile of proteins from a semipurified extract of P. aeruginosa medium after Superdex75 chromatography (see Methods). Fractions 6 and 7 disrupted the cortisol-binding activity of CBG. B, Purified CBG was incubated with P. aeruginosa media (PAO1), with active chromatographic fractions of P. aeruginosa media (Frac 6–7), or with neutrophil elastase (N.E.), and subjected to SDS-PAGE. A shift in molecular weight of 5–10 kDa is observed in all treatment conditions. Protein ladder (kDa) is shown on the left. C, The RCL and adjacent β-sheets (s4B and s5B) are indicated within the C-terminal region of human CBG. Mass spectrometry was performed after incubation of human CBG with fraction 7 (panel A). The main cleavage site was located between N347 and L348 (large arrow). The consensus sequence for N-glycosylation, as well as the neutrophil elastase cleavage site (5, 6) and the cleavage sites observed previously in a human CBG crystal structure (10) are indicated. D, Sypro Ruby stained SDS-PAGE of fraction 7 revealed the presence of 3 bands that were excised for analysis by mass spectrometry (see Methods and Supplemental Data). The approximately 33 kDa band (excised from the gel) was identified as LasB, the approximately 27 kDa band as protease IV, and the approximately 14 kDa band as a C-terminal fragment of LasB. LasB obtained commercially is also shown as a reference.
To confirm that the loss of CBG cortisol binding after incubation with P. aeruginosa media was not due to random proteolysis, Western blotting was used to assess the integrity of CBG (Figure 3B). A shift of 5–10 kDa was observed between the intact and cleaved CBG, suggesting that the protease(s) targeted a specific region of CBG, similar to that observed after incubation with neutrophil elastase (Figure 3B) (7). Further, the proteolytic fragment seen after incubation of CBG with unfractionated P. aeruginosa medium (Figure 3B) was also observed after incubation with the two chromatographic fractions that disrupted its cortisol-binding activity (Figure 3A).
To determine the protease cleavage site(s), purified CBG (6) was incubated with semipurified proteins isolated from P. aeruginosa media (fraction 7), and the product was analyzed by mass spectrometry. Four peptides generated from adjacent cleavage sites in the RCL of CBG were identified, with most cleavage occurring between Asn347 and Leu348 (Figure 3C and Supplemental Figures 1–3 and Supplemental Table 1).
Gel electrophoresis of fraction 7 that only contained a portion of the main peak (Figure 3A) revealed the presence of three bands of approximately 33, 27, and 14 kDa (Figure 3D and Supplemental Figure 4). For those three bands, tryptic in-gel digestion and peptide extraction were performed and followed by mass spectrometry analysis. The approximately 33 kDa band was identified as LasB (pseudolysin or P. aeruginosa elastase), the approximately 27 kDa was identified as lysyl endopeptidase, or protease IV, and the approximately 14 kDa band was identified as a C-terminal portion of LasB (see Supplemental Figures 5 and 6 and Supplemental Tables 2 and 3).
Loss of CBG cortisol binding after incubation with purified LasB and absence of effect of the protease IV inhibitor TLCK
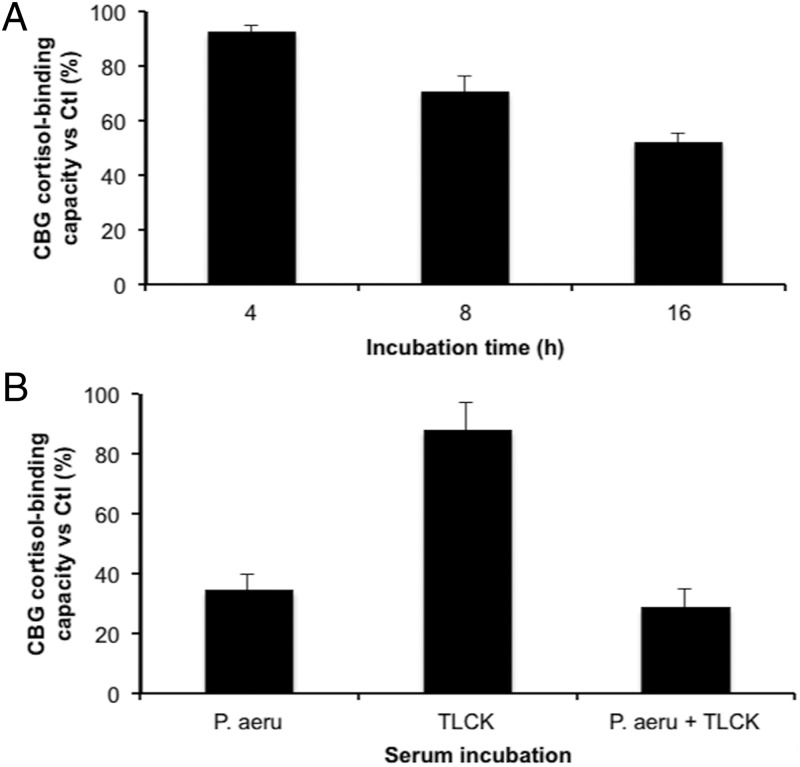
The ability of 1 μg purified LasB to cleave CBG was examined in a time-course experiment, and approximately 50% of CBG cortisol-binding capacity in human serum was lost after 16 hours of incubation at 37°C (Figure 4A), thus confirming the involvement of this metalloprotease in the proteolytic activity of P. aeruginosa media. The relatively low activity of the commercial LasB on CBG when compared with the P. aeruginosa medium was addressed in an elastin-congo red assay that indicated that the elastolytic activity of 5 μg of purified enzyme was lower than the activity of 1 μl of P. aeruginosa medium, consistent with the differences observed in the reductions in CBG cortisol-binding capacity after incubations with commercial LasB (Figure 4A) versus P. aeruginosa media (Figure 1).
Figure 4.
The cortisol-binding capacity of CBG in human serum is disrupted by purified LasB but not by protease IV. A, The cortisol-binding capacity of CBG in human serum was determined in 1 μl human serum after incubation with 1 μg purified LasB for 4 hours, 8 hours, or 16 hours at 37°C. A progressive decrease in cortisol-binding capacity was observed with approximately 50% remaining after 16 hours of incubation. Incubations were performed in triplicates. B, P. aeruginosa medium (P.aeru) was preincubated with 0.05 mM TLCK as an inhibitor of protease IV for 1 hour at 37°C, filtered and then incubated with human serum for 16 hours at 37°C (see Methods for details). The cortisol-binding capacity of CBG was not protected by TLCK, suggesting that protease IV is not involved in CBG cleavage. The data are presented as mean percentage ± SD of steroid-binding activity relative to a control that did not include P. aeruginosa media.
Protease IV was the only other protein detectable in the chromatographic fraction of P. aeruginosa medium that disrupted CBG cortisol binding (Figure 3D). As a serine protease, protease IV also acts as a virulence factor (20). To determine if protease IV targets CBG, experiments using TLCK, a known irreversible inhibitor of protease IV, were performed (Figure 4B). The results excluded any direct action of protease IV on CBG, as TLCK did not influence the ability of P. aeruginosa media to disrupt cortisol-binding activity.
LasB-deficient strains of PAO1 do not disrupt CBG cortisol-binding capacity
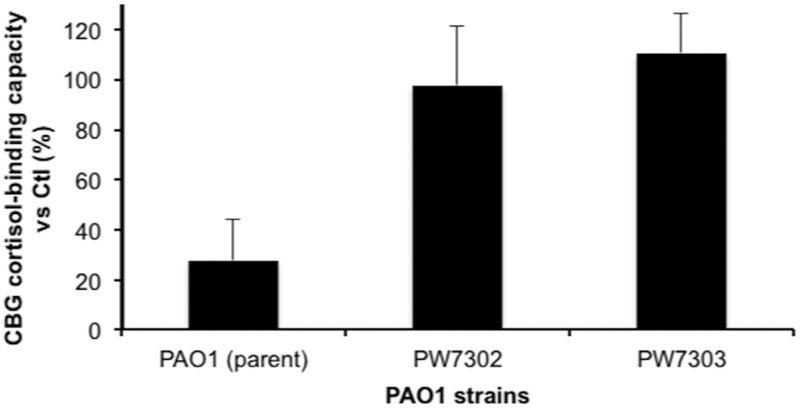
To further establish the role of LasB in the disruption of CBG cortisol binding, human serum was incubated with media from cultures of LasB-deficient strains of PAO1 and the effect on CBG cortisol-binding capacity was compared with that of the parent strain (Figure 5). These results confirm that LasB is the protease secreted by PAO1 that is mainly responsible for disrupting the cortisol-binding activity of human CBG. In addition, the elastolytic activity of the media from LasB-deficient strains, as determined by an elastin-congo red assay, was not different from the assay background.
Figure 5.
The cortisol-binding capacity of CBG in human serum is not disrupted by culture media from LasB-deficient P. aeruginosa. Human serum (1 μl) was incubated with 90 μl of 5-fold concentrated conditioned media from 2 LasB-deficient clones (ie, PW7302 and PW7303) of P. aeruginosa and from the parental PAO1 strain. No loss of cortisol-binding activity was observed with the 2 LasB-deficient clones, confirming the main role of LasB in the disruption of CBG cortisol-binding capacity. All incubations were performed in triplicate and data are presented as mean percentage ± SD of the steroid-binding capacity relative to serum control diluted in 5-fold concentrated bacterial culture media.
Discussion
Bacterial pathogens secrete an array of proteases as virulence factors that accentuate their ability to cause disease (22, 23). It is also known that the RCL of SERPINA1 is targeted by several bacterial proteases, including LasB secreted by P. aeruginosa (12). It was therefore remarkable that among the bacteria tested, only P. aeruginosa potently disrupted the cortisol-binding activity of CBG through cleavage of its RCL. Our identification of LasB as the P. aeruginosa protease responsible for cleavage of the CBG RCL is supported by several key observations. First, the reaction was inhibited by the removal of zinc ions that are essential for the activity of this metalloprotease. Second, LasB was identified by mass spectrometry as one of only two proteins in a proteolytically active chromatographic fraction of P. aeruginosa medium. Third, and most importantly, media from LasB-deficient P. aeruginosa strains were unable to disrupt the cortisol-binding activity of CBG. Moreover, whereas a commercial preparation of LasB was less active than semipurified LasB from P. aeruginosa media, in terms of its ability to disrupt the cortisol-binding activity of CBG, the commercial preparation of LasB was also found to be far less active than P. aeruginosa media samples in a biochemical assay of elastolytic activity. Finally, whereas protease IV was also detected in the active chromatographic fraction of P. aeruginosa medium, our results indicate that this protease did not contribute to the disruption of CBG cortisol binding.
LasB is a zinc-metalloprotease that has been extensively studied as a virulence factor secreted by P. aeruginosa (24, 25). It is the most abundant extracellular endopeptidase secreted by this pathogen and is commonly found in sputum samples of chronically infected CF patients (26). It shows optimum proteolysis at pH 7–8, and its activity and stability are zinc- and calcium-dependent, respectively. Its proteolytic action causes tissue destruction as well as modulation of the host immune response. For example, LasB is known to degrade matrix components, complement factors, cytokines, and to inactivate host protease inhibitors such as α1-antitrypsin and α2-macroglobulin ([25, 27]; and references therein). It also acts intracellularly in bacteria to promote biofilm formation (28) that contributes to sustained inflammation in chronic infection (13). Different inhibitors of LasB have been developed as a potential second-generation class of antibiotics that might minimize the emergence of resistant strains (25, 29). Our results suggest that these inhibitors might also modulate CBG cleavage and the release of bound cortisol.
Our results revealed that CBG incubated with semipurified P. aeruginosa medium was preferentially cleaved within its RCL domain between Asn347 and Leu348. It has been reported that LasB favors hydrophobic or aromatic amino acid residues at the P1′ position (24, 30), and the main cleavage site we identified is consistent with this. In addition, we identified adjacent secondary cleavage sites that also differ from the one between Val344 and Thr345, observed after cleavage by neutrophil elastase (5, 6) and the one after Thr349 or Ser350, observed in the recent crystal structure of human CBG bound with progesterone (10). Cleavage of the CBG RCL in this structure was unexpected, and a protease produced by another microorganism might have been responsible for this. However, it is also possible that presentation of the RCL of E. coli-produced recombinant CBG bound to progesterone may differ from the RCL of serum CBG bound to cortisol and may therefore be targeted differently by proteases. In this regard, occupancy of the CBG steroid-binding site may also alter the conformation and surface presentation of the RCL (7). In addition, human plasma CBG is extensively glycosylated (31), whereas CBG expressed in E. coli is not glycosylated. Importantly, the human CBG RCL contains a consensus sequence for N-glycosylation that is utilized in about 80% of CBG molecules (31). Furthermore, the composition of the N-glycans attached at this site varies (31) and the proteolytic cleavage of the CBG RCL may therefore be modulated by the presence and/or type of N-glycan within the RCL. Heterogeneity in glycosylation at this site may explain why we observed several secondary cleavage sites adjacent to the main one. In support of the idea that post-translational modifications of the RCL sequence might alter its recognition and cleavage by proteases, oxidation of Met358 in the RCL of α1-antitrypsin changes the cleavage specificity of several bacterial proteases as well as the efficiency of proteolysis (12).
After incubation with neutrophil elastase, CBG loses its cortisol-binding activity rapidly, even at room temperature (6). By contrast, although a rapid decrease of about 20% of the cortisol-binding capacity of CBG in human serum was observed within 5 minutes of incubation with P. aeruginosa medium at physiological temperatures, this was followed by a more progressive loss. The explanation for this might be related to differences in the presentation or recognition of the CBG RCL or to the presence of inhibitors in serum, such as α2-macroglobulin, which limit the activities of bacterial proteases (32). The loss of CBG cortisol-binding activity after incubation with P. aeruginosa media was, however, consistently more efficient at 41°C when compared with 37°C, and this is of interest because increases in temperature over this range also decrease the steroid-binding activity of CBG (9). In cases of infections accompanied by fever, any increase in proteolytic activity would act in concert with a decreased steroid-binding activity to enhance free cortisol levels. In addition, the LasB-induced cortisol release from CBG may vary according to the pH of an infected area, which will likely be different, for example between infected burn wounds (33) and airways (34).
As one of the most common opportunistic pathogens, P. aeruginosa is involved in a variety of pathologic complications involving wounds (35), burn infections (36), septicemia (37), and pneumonia (38), and is a major cause of chronic lung infections in CF patients (13). P. aeruginosa infections lead to a proinflammatory environment with reduction of bacterial clearance, leukotaxis, and excess immune, inflammatory, oxidative, and proteolytic activities that trigger further inflammation and tissue damage (39). The recruited, activated neutrophils secrete neutrophil elastase that can rapidly cleave the RCL of CBG, thus increasing the local concentration of free cortisol that will be available to target cells (5, 6); in addition, we have shown here that secreted protease, LasB, is also able to cleave CBG. The combined effects of neutrophil elastase and LasB on CBG at sites of infection/inflammation will lead to an increased release of cortisol and to an accelerated depletion of the cortisol reservoir. Further, LasB might also modulate the cleavage of CBG by neutrophil elastase through its ability to cleave α1-antitrypsin without forming inhibitory complexes, thereby allowing neutrophil elastase to escape inhibition by α1-antitrypsin (40). However, the production of free radicals by neutrophils has been shown to cause the reversible oxidation of Met358 in the RCL of α1-antitrypsin and greatly reduce its ability to inhibit neutrophil elastase (41). Similarly, the ability of LasB to cleave oxidized α1-antitrypsin has been shown to be reduced (12). Therefore, a complex interplay between various protease/SERPIN interactions most likely occurs, and would ultimately modulate free cortisol levels. In addition, other factors will influence the impact of LasB on cortisol release, including the presence of other substrates and the level of LasB production, which will likely vary according to the mode of growth and the strain of the pathogen (42). Changes in plasma CBG levels could also be a factor, as they decrease rapidly during acute inflammatory states caused by sepsis (15) or severe burns (16).
In summary, we have discovered that the P. aeruginosa virulence factor, LasB, specifically cleaves the RCL of human CBG at novel sites leading to a loss of its cortisol-binding activity. In the control of infectious and inflammatory diseases of tissues like the lung and skin, where P. aeruginosa infections are common, LasB cleavage of CBG may augment the actions of neutrophil elastase, promoting the localized release of cortisol from the CBG steroid-binding site. Increases in free cortisol levels at these sites will have a broad range of actions, including modifying the production of cytokines, free radicals, prostaglandins, and chemotactic factors by various cell types involved in the inflammatory response, as well as controlling the activity of infiltrating immune cells (43). Understanding the relative contribution of LasB and neutrophil elastase to these processes may be relevant in optimizing the dosage and route of glucocorticoid administration to treat inflammation associated with severe P. aeruginosa infections.
Acknowledgments
We thank Jürgen Kast, Suzanne Perry, and Jason Rogalski of the UBC-CHiBi (Centre for High Throughput Biology) for their access to and help with the mass spectrometric equipment, as well as Manjeet Bains for his technical assistance with bacterial cultures. We also thank Dr Julian Davies for providing bacterial strains and for his insightful comments.
This work was supported by an operating grant (MOP-111102) from the Canadian Institutes of Health Research (to G.L.H.). G.L.H. is a Canada Research Chair in Reproductive Health. M.S. holds a Postdoctoral Fellowship from the Fonds de Recherche du Québec en Santé and the Michael Smith Foundation for Health Research. B.O.K. thanks the Child and Family Research Institute for an Establishment Award. R.E.W.H. is a Canada Research Chair in Health and Genomics and is funded by Cystic Fibrosis Canada. We acknowledge the National Institutes of Health Grant P30 DK089507 for the use of P. aeruginosa strains.
Disclosure Summary: All authors have nothing to disclose.
Footnotes
- CBG
- corticosteroid-binding globulin
- CF
- cystic fibrosis
- DPBS
- Dulbecco's phosphate-buffered saline
- EDTA
- ethylenediaminetetraacetic acid
- PMSF
- phenylmethanesulfonyl fluoride
- RCL
- reactive center loop
- SERPIN
- serine protease inhibitor
- TLCK
- tosyl-L-lysine chloromethyl ketone hydrochloride
- TPEN
- tetrakis(2-pyridylmethyl)ethylenediamine.
References
- 1. Hammond GL, Smith CL, Goping IS, et al. Primary structure of human corticosteroid binding globulin, deduced from hepatic and pulmonary cDNAs, exhibits homology with serine protease inhibitors. Proc Natl Acad Sci USA. 1987;84:5153–5157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Law RH, Zhang Q, McGowan S, et al. An overview of the serpin superfamily. Genome Biol. 2006;7:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Siiteri PK, Murai JT, Hammond GL, Nisker JA, Raymoure WJ, Kuhn RW. The serum transport of steroid hormones. Recent Prog Horm Res. 1982;38:457–510 [DOI] [PubMed] [Google Scholar]
- 4. Lin HY, Muller YA, Hammond GL. Molecular and structural basis of steroid hormone binding and release from corticosteroid-binding globulin. Mol Cell Endocrinol. 2010;316:3–12 [DOI] [PubMed] [Google Scholar]
- 5. Pemberton PA, Stein PE, Pepys MB, Potter JM, Carrell RW. Hormone binding globulins undergo serpin conformational change in inflammation. Nature. 1988;336:257–258 [DOI] [PubMed] [Google Scholar]
- 6. Hammond GL, Smith CL, Paterson NA, Sibbald WJ. A role for corticosteroid-binding globulin in delivery of cortisol to activated neutrophils. J Clin Endocrinol Metab. 1990;71:34–39 [DOI] [PubMed] [Google Scholar]
- 7. Lin HY, Underhill C, Gardill BR, Muller YA, Hammond GL. Residues in the human corticosteroid-binding globulin reactive center loop that influence steroid binding before and after elastase cleavage. J Biol Chem. 2009;284:884–896 [DOI] [PubMed] [Google Scholar]
- 8. Westphal U. Corticosteroid-binding globulin. A review of some recent aspects. Mol Cell Biochem. 1983;55:145–157 [DOI] [PubMed] [Google Scholar]
- 9. Cameron A, Henley D, Carrell R, Zhou A, Clarke A, Lightman S. Temperature-responsive release of cortisol from its binding globulin: a protein thermocouple. J Clin Endocrinol Metab. 2010;95:4689–4695 [DOI] [PubMed] [Google Scholar]
- 10. Gardill BR, Vogl MR, Lin HY, Hammond GL, Muller YA. Corticosteroid-binding globulin: structure-function implications from species differences. PLoS One. 2012;7:e52759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hill RE, Hastie ND. Accelerated evolution in the reactive centre regions of serine protease inhibitors. Nature. 1987;326:96–99 [DOI] [PubMed] [Google Scholar]
- 12. Rapala-Kozik M, Potempa J, Nelson D, Kozik A, Travis J. Comparative cleavage sites within the reactive-site loop of native and oxidized alpha1-proteinase inhibitor by selected bacterial proteinases. Biol Chem. 1999;380:1211–1216 [DOI] [PubMed] [Google Scholar]
- 13. Ciofu O, Hansen CR, Høiby N. Respiratory bacterial infections in cystic fibrosis. Curr Opin Pulm Med. 2013;19:251–258 [DOI] [PubMed] [Google Scholar]
- 14. Rafla K, Tredget EE. Infection control in the burn unit. Burns. 2011;37:5–15 [DOI] [PubMed] [Google Scholar]
- 15. Pugeat M, Bonneton A, Perrot D, et al. Decreased immunoreactivity and binding activity of corticosteroid-binding globulin in serum in septic shock. Clin Chem. 1989;35:1675–1679 [PubMed] [Google Scholar]
- 16. Bernier J, Jobin N, Emptoz-Bonneton A, Pugeat MM, Garrel DR. Decreased corticosteroid-binding globulin in burn patients: relationship with interleukin-6 and fat in nutritional support. Crit Care Med. 1998;26:452–460 [DOI] [PubMed] [Google Scholar]
- 17. Hammond GL, Lähteenmäki PL. A versatile method for the determination of serum cortisol binding globulin and sex hormone binding globulin binding capacities. Clin Chim Acta. 1983;132:101–110 [DOI] [PubMed] [Google Scholar]
- 18. Robinson PA, Langley MS, Hammond GL. A solid-phase radioimmunoassay for human corticosteroid binding globulin. J Endocrinol. 1985;104:259–267 [DOI] [PubMed] [Google Scholar]
- 19. Jacobs MA, Alwood A, Thaipisuttikul I, et al. Comprehensive transposon mutant library of Pseudomonas aeruginosa. Proc Natl Acad Sci USA. 2003;100:14339–14344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Engel LS, Hill JM, Caballero AR, Green LC, O'Callaghan RJ. Protease IV, a unique extracellular protease and virulence factor from Pseudomonas aeruginosa. J Biol Chem. 1998;273:16792–16797 [DOI] [PubMed] [Google Scholar]
- 21. Caballero AR, Moreau JM, Engel LS, Marquart ME, Hill JM, O'Callaghan RJ. Pseudomonas aeruginosa protease IV enzyme assays and comparison to other Pseudomonas proteases. Anal Biochem. 2001;290:330–337 [DOI] [PubMed] [Google Scholar]
- 22. Lebrun I, Marques-Porto R, Pereira AS, Pereira A, Perpetuo EA. Bacterial toxins: an overview on bacterial proteases and their action as virulence factors. Mini Rev Med Chem. 2009;9:820–828 [DOI] [PubMed] [Google Scholar]
- 23. Bleves S, Viarre V, Salacha R, Michel GP, Filloux A, Voulhoux R. Protein secretion systems in Pseudomonas aeruginosa: A wealth of pathogenic weapons. Int J Med Microbiol. 2010;300:534–543 [DOI] [PubMed] [Google Scholar]
- 24. Morihara K. Pseudolysin and other pathogen endopeptidases of thermolysin family. Methods Enzymol. 1995;248:242–253 [DOI] [PubMed] [Google Scholar]
- 25. Cathcart GR, Quinn D, Greer B, et al. Novel inhibitors of the Pseudomonas aeruginosa virulence factor LasB: a potential therapeutic approach for the attenuation of virulence mechanisms in pseudomonal infection. Antimicrob Agents Chemother. 2011;55:2670–2678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Azghani AO, Bedinghaus T, Klein R. Detection of elastase from Pseudomonas aeruginosa in sputum and its potential role in epithelial cell permeability. Lung. 2000;178:181–189 [DOI] [PubMed] [Google Scholar]
- 27. Leduc D, Beaufort N, de Bentzmann S, et al. The Pseudomonas aeruginosa LasB metalloproteinase regulates the human urokinase-type plasminogen activator receptor through domain-specific endoproteolysis. Infect Immun. 2007;75:3848–3858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kamath S, Kapatral V, Chakrabarty AM. Cellular function of elastase in Pseudomonas aeruginosa: role in the cleavage of nucleoside diphosphate kinase and in alginate synthesis. Mol Microbiol. 1998;30:933–941 [DOI] [PubMed] [Google Scholar]
- 29. Fullagar JL, Garner AL, Struss AK, et al. Antagonism of a zinc metalloprotease using a unique metal-chelating scaffold: tropolones as inhibitors of P. aeruginosa elastase. Chem Commun (Camb). 2013;49:3197–3199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Miyoshi S, Shinoda S. Microbial metalloproteases and pathogenesis. Microbes Infect. 2000;2:91–98 [DOI] [PubMed] [Google Scholar]
- 31. Sumer-Bayraktar Z, Kolarich D, Campbell MP, Ali S, Packer NH, Thaysen-Andersen M. N-glycans modulate the function of human corticosteroid-binding globulin. Mol Cell Proteomics. 2011;10:M111.009100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Armstrong PB. Proteases and protease inhibitors: a balance of activities in host-pathogen interaction. Immunobiology. 2006;211:263–281 [DOI] [PubMed] [Google Scholar]
- 33. Sharpe JR, Booth S, Jubin K, Jordan NR, Lawrence-Watt DJ, Dheansa BS. Progression of wound pH during the course of healing in burns. J Burn Care Res. 2013;34:e201–e208 [DOI] [PubMed] [Google Scholar]
- 34. McShane D, Davies JC, Davies MG, Bush A, Geddes DM, Alton EW. Airway surface pH in subjects with cystic fibrosis. Eur Respir J. 2003;21:37–42 [DOI] [PubMed] [Google Scholar]
- 35. Bjarnsholt T, Kirketerp-Møller K, Jensen PØ, et al. Why chronic wounds will not heal: a novel hypothesis. Wound Repair Regen. 2008;16:2–10 [DOI] [PubMed] [Google Scholar]
- 36. Nichols DP, Caceres S, Caverly L, et al. Effects of azithromycin in Pseudomonas aeruginosa burn wound infection. J Surg Res. 2013;183:767–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. van Delden C. Pseudomonas aeruginosa bloodstream infections: how should we treat them? Int J Antimicrob Agents. 2007;30 Suppl 1:S71–S75 [DOI] [PubMed] [Google Scholar]
- 38. Roux D, Ricard JD. Novel therapies for Pseudomonas aeruginosa pneumonia. Infect Disord Drug Targets. 2011;11:389–394 [DOI] [PubMed] [Google Scholar]
- 39. Gellatly SL, Hancock RE. Pseudomonas aeruginosa: new insights into pathogenesis and host defenses. Pathog Dis. 2013;67:159–173 [DOI] [PubMed] [Google Scholar]
- 40. Morihara K, Tsuzuki H, Harada M, Iwata T. Purification of human plasma alpha 1-proteinase inhibitor and its inactivation by Pseudomonas aeruginosa elastase. J Biochem. 1984;95:795–804 [DOI] [PubMed] [Google Scholar]
- 41. Taggart C, Cervantes-Laurean D, Kim G, et al. Oxidation of either methionine 351 or methionine 358 in alpha 1-antitrypsin causes loss of anti-neutrophil elastase activity. J Biol Chem. 2000;275:27258–27265 [DOI] [PubMed] [Google Scholar]
- 42. Jimenez PN, Koch G, Thompson JA, Xavier KB, Cool RH, Quax WJ. The multiple signaling systems regulating virulence in Pseudomonas aeruginosa. Microbiol Mol Biol Rev. 2012;76:46–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Annane D, Cavaillon JM. Corticosteroids in sepsis: from bench to bedside? Shock. 2003;20:197–207 [DOI] [PubMed] [Google Scholar]