Abstract
Evidence suggests that when presented with novel acute stress, animals previously exposed to chronic homotypic or heterotypic stressors exhibit normal or enhanced hypothalamic-pituitary-adrenal (HPA) response compared with animals exposed solely to that acute stressor. The molecular mechanisms involved in this effect remain unknown. The extracellular signal-regulated kinase (ERK) is one of the key pathways regulated in the hippocampus in both acute and chronic stress. The aim of this study was to examine the interaction of prior chronic stress, using the chronic variable stress model (CVS), with exposure to a novel acute stressor (2,5-dihydro-2,4,5-trimethyl thiazoline; TMT) on ERK activation, expression of the downstream protein BCL-2, and the glucocorticoid receptor co-chaperone BAG-1 in control and chronically stressed male rats. TMT exposure after chronic stress resulted in a significant interaction of chronic and acute stress in all 3 hippocampus subregions on ERK activation and BCL-2 expression. Significantly, acute stress increased ERK activation, BCL-2 and BAG-1 protein expression in the dentate gyrus (DG) of CVS-treated rats compared with control, CVS-treated alone, and TMT-only animals. Furthermore, CVS significantly increased ERK activation in medial prefrontal cortex, but acute stress had no significant effect. Inhibition of corticosterone synthesis with metyrapone had no significant effect on ERK activation in the hippocampus; therefore, glucocorticoids alone do not mediate the molecular effects. Finally, because post-translational modifications of histones are believed to play an important role in the stress response, we examined changes in histone acetylation. We found that, in general, chronic stress decreased K12H4 acetylation, whereas acute stress increased acetylation. These results indicate a molecular mechanism by which chronic stress-induced HPA axis plasticity can lead to neurochemical alterations in the hippocampus that influence reactivity to subsequent stress exposure. This may represent an important site of dysfunction that contributes to stress-induced pathology such as depression, anxiety disorders, and posttraumatic stress disorder.
The physiological and psychological reaction of an individual to stress can have dramatic implications for the resulting susceptibility to mental health disorders. Prior history of stressors is a vulnerability factor for development of posttraumatic stress disorder (PTSD) and other anxiety disorders in response to stress or trauma. Many studies have investigated the role of early life adversity in predisposition of individuals to the development of psychopathology in later life, especially depression and anxiety disorders (1). However, few studies have investigated the response to a traumatic stressor during a period of chronic stress.
In order to fully understand progression of chronic stress to depression and anxiety disorders, it is necessary to understand the physiological and molecular response to an acute stress during a high stress state in brain areas that are impacted by stress, including hippocampus and prefrontal cortex. The hypothalamic-pituitary-adrenal (HPA) axis is the main neuroendocrine system within the body responsible for restoring homeostasis in response to stress exposure and plays a critical role in determination of affective disorder susceptibility. Acute stress elicits release of the stress hormones, glucocorticoids, from the adrenal gland (2). These hormones mobilize energy necessary to respond to the stressor and regulate function of various brain areas; this response is generally considered an adaptive response. On the other hand, animals previously exposed to chronic homotypic or heterotypic stressors exhibit normal or enhanced HPA response compared with animals exposed solely to the same acute stressor (3–9).
Facilitation of the HPA axis to a novel acute stress in chronically stressed animals is postulated to include changes in activity of the central systems that modulate the stress response in the forebrain. Recent evidence suggests that certain brain areas are indeed differentially activated in response to acute stress in chronically stressed rats (10). Further, cellular changes are reported, including decreased expression or sensitivity of the glucocorticoid receptor (GR) in the hippocampus and hypothalamus (11, 12), decreased corticotropin-releasing hormone receptor expression in the anterior pituitary (6) as well as facilitation of the noradrenergic system particularly at the level of basolateral amygdala (3) and paraventricular nucleus (13). However, whereas several studies have investigated the neural circuitry mediating novel acute stress-induced enhancement of the HPA response, the molecular mechanisms involved have rarely been examined.
Extracellular signal-regulated kinase (ERK)1/2 is a member of the mitogen-activated protein kinase (MAPK) intracellular signaling cascade (14) and is implicated as one of the key pathways regulated in both acute (15–17) and chronic stress (15, 18–20). ERK1/2 is highly expressed throughout the central nervous system with particular prominence in all three regions of the hippocampus (21, 22), a brain region particularly susceptible to the effects of chronic stress-induced alterations in glucocorticoid feedback. Upon activation through phosphorylation, ERK1/2 regulates a multitude of neuronal processes through direct protein-protein interactions or through activation of transcriptions factors such as cAMP-response element binding protein including neuronal growth and proliferation, differentiation, apoptosis, and synaptic plasticity (23–25). Furthermore, ERK1/2 modulates histone phosphorylation and acetylation in hippocampus neurons (26–29) that is also implicated in both acute (17, 30–32) and chronic stress (20, 32–35). We previously showed decreased activation of the ERK/MAPK pathway and expression of the downstream neuroprotective target BCL-2, in response to chronic stress (20).
BCL2-associated athanogene (BAG-1) is a family of cochaperone proteins associated with the GR (45). BAG-1 has several known functions that may be relevant in the pathology of stress-induced depression. First, BAG-1 potentiates the antiapoptotic function of BCL-2, thereby enhancing cell survival (46). BAG-1 activates ERK/MAPK signaling through Raf-1 (47), and finally BAG-1 is a cochaperone protein involved in modulating the nuclear translocation of the GR, thereby limiting its transcriptional activity in the nucleus, and in particular the ability of the GR to promote transcription of the Bcl-2 gene (45). Additionally, chronic mild stress induced a decrease in Bag-1 mRNA levels in the hippocampus, which was reversed with chronic lithium treatment (48, 49), a treatment also shown to have an effect on the ERK/MAPK pathway (50). The effect of chronic and acute stress on BAG-1 protein, however, is unknown.
Understanding the molecular pathways involved in HPA facilitation to acute novel stress after chronic stress exposure is particularly important given that this mechanism may represent an important site of dysregulation that contributes to stress-induced pathology such as depression, anxiety disorders and PTSD. The aim of the current study was to expand on this research and examine regulation of these molecular markers in chronically stressed animals exposed to a novel acute stressor, 2,5-dihydro-2,4,5-trimethyl thiazoline (TMT), an innate stressor in rodents which has been shown to elicit strong activation of the HPA axis including a robust corticosterone response (36, 37). In addition, because post-translational modifications of histones are believed to play an important role in the stress response, we also examined changes in histone acetylation.
Materials and Methods
Animals and experimental groups
Male Wistar rats (42 days of age upon arrival) were purchased from Harlan Inc. On arrival, animals were housed in pairs on a 12:12 light/dark cycle (lights on at 7:00 AM) and received food and water ad libitum. Animals were given 14 days to acclimate prior to experimental manipulation. All experimental procedures were conducted during the light cycle and performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Tulane University Institutional Animal Care and Use Committee.
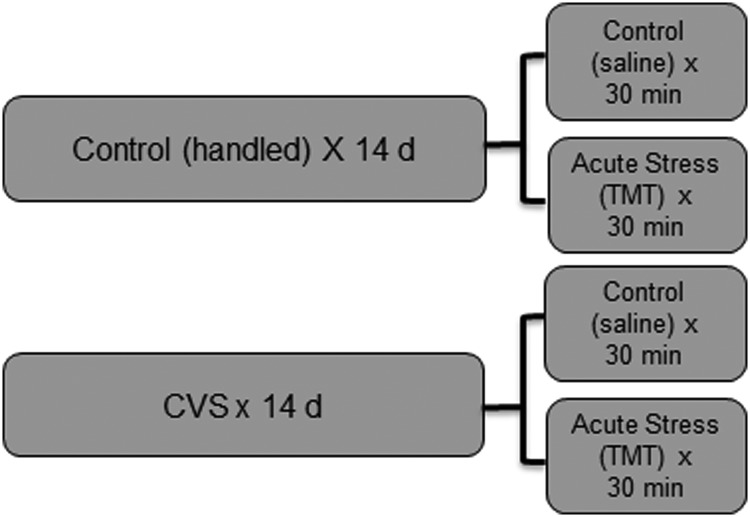
Animals were randomly assigned to one of four experimental conditions. Group assignment was by cage and both animals from one cage were assigned to the same test group. At 56 days of age, rats were either handled for 14 days (control conditions) or subjected to twice-daily exposure to chronic variable stress (CVS) as described below. On day 15, approximately 16 hours after the administration of the last stressor, animals were exposed to 30 minutes of either control (saline) or acute stress (2,5-dihydro-2,4,5-trimethyl thiazoline; TMT) conditions as described below. This resulted in 4 experimental groups: 1) control (unstressed), 2) acute stress, 3) CVS, and 4) CVS plus acute stress (see Figure 1). Animals were run in groups of 24 rats, with 6 animals per group. A total of 3 cohorts were run for a total of 72 rats.
Figure 1.
Schematic representation of the groups in our experimental design. Rats were divided into control and CVS treatment. Each of these groups was further divided into control and acute stress (TMT)-treated rats, resulting in 4 groups.
Chronic variable stress
CVS was conducted using a method previously reported (35, 38). Briefly, the CVS paradigm consisted of twice-daily exposure to randomly assigned stressors applied over 14 consecutive days. With the exception of the overnight stressors, the daily stressors were applied in a semi-randomized manner with each stressor being assigned equally over the 14 days. All animals were weighed every other day. On the morning of the 15th day, approximately 12 hours after the last stressor, animals were killed by rapid decapitation, trunk blood collected and adrenal glands weighed. The hippocampus was extracted and subdivided into CA1, CA3, and dentate gyrus (DG) regions for histone and protein extraction. Animals were killed between 9:00 AM and 10:00 AM, when corticosterone levels are lowest and at least 16 hours after administration after the last stressor.
Novel acute stress
The predatory odor TMT, an innate stressor in rodents which has been shown to elicit strong activation of the HPA axis and an acute increase in serum corticosterone levels was used for acute stress (36, 37). TMT application was based on methods previously described (37). Briefly, after 14 days of CVS or control condition, animals in the acute stress and the CVS + novel acute stress groups were exposed to 30 minutes of the stressor TMT. Animals in the control and CVS groups were exposed to 30 minutes of control (saline) conditions. Prior to exposure to TMT, all animals were removed from the vivarium and experimental and nonexperimental animals were placed in separate isolated rooms to prevent unintentional scent exposure to nonexperimental animals. Animals were given 2 hours to acclimate prior to TMT exposure. For experimental groups, a small Petri dish with filter paper soaked with 150 μl of undiluted TMT (Phero Tech Inc.) was placed in the center of the animal's home cage. Rats were exposed to TMT for 30 minutes. After the 30 minutes, all animals were immediately killed by rapid decapitation, trunk blood collected, and adrenal glands weighed. The prefrontal cortex and hippocampus was extracted and hippocampus was subdivided into CA1, CA3, and DG regions for histone and protein extraction.
Sucrose preference test
A separate cohort of rats (6 rats per group) was tested for sucrose preference. Rats were given 12 hours (7:00 AM to 7:00 PM) to habituate to a free choice between two bottles, both containing tap water. At 7:00 PM, one bottle was replaced with 3% sucrose. The other bottle remained as tap water. Rats were given free choice between sucrose and tap water for 12 hours (7:00 PM to 7:00 AM). To prevent possible effects of side preference, the position of the sucrose bottle was randomly distributed among the cages. Consumption was calculated as the percentage of sucrose fluid volume consumed to total fluid volume consumed. Chronically stressed rats were tested on day 14 of CVS protocol, approximately 12 hours after their final stressor. Animals exposed to acute stress were tested for sucrose preference approximately 12 hours after TMT exposure.
Corticosterone assay
Trunk blood was allowed to coagulate at room temperature for 90 minutes. Samples were centrifuged at 2000 × g for 15 minutes, serum collected and samples stored at −20°C. Samples were sent to the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core for corticosterone measurements.
Histone extraction
Because histone extraction requires an extraction that is different than the sample processing for Western blots described below, histone extraction was performed on hippocampi from a separate cohort of rats, 6 rats per group for a total of 24 rats. In some cases, lower numbers are reported due to unsuccessful dissection of an area. The brain was removed and immersed in ice-cold artificial cerebrospinal fluid. One millimeter coronal sections were made using a brain matrix (Braintree Scientific) and the hippocampus was subdivided into CA1, CA3, and DG regions under direct visualization for histone and protein extraction. Sub-dissected hippocampus tissue was processed in homogenization buffer containing (in mM): Sucrose 250, Tris pH 7.5 50, KCl 50, PMSF 5, Sodium Butyrate 20, Sodium Orthovanadate. Sample was centrifuged at 7700 G for 1 minute. Pellet was resuspended in 0.4 N H2SO4 and centrifuged at max speed for 10 minutes at 4°C. Trichloroacetic acid + 4 mg/ml deoxychloric acid was added to the supernatant and centrifuged at max speed for 30 minutes at 4°C. Histones were extracted with cold acidified acetone and centrifuged at max speed for 5 minutes at 4°C. The histone pellet was resuspended in 10 mM Tris, ph 8.0 and stored at −80°C. Protein concentration was determined through Lowry protein assay. Samples were normalized to 0.5 μg/μl and 4× sample buffer was added (0.3M Tris, 40% glycerol, 8% SDS, 200 mM DTT, and bromophenol blue). Histones were separated on a 15% gel and transferred to Immobilon for Western blotting.
Western blotting
Molecular analysis was performed on the brains of another cohort of animals, 6 animals per group for a total of 24 rats. In some cases, the numbers reported are lower due to loss of an animal during an experiment, unsuccessful dissection or processing of an area. Proteins were extracted using Chemicon's compartmental protein extraction kit. Protein concentration was determined through Lowry protein assay. Samples were normalized to 0.5 μg/μl and 4× sample buffer was added. Proteins were separated on a 10% SDS-PAGE gel. Antibodies were acquired from Cell Signaling unless otherwise indicated. Blots were probed for antiphospho ERK antibody (1:1000), anti-ERK antibody (1:1000), anti-Bcl-2 antibody (1:1000), anti-BAG-1 (1:1000, Santa Cruz), and anti-GAPDH antibody (1:1000).
Histone fractions were probed for the following antibodies: antiacetyl lysine 12 (K12) H4 (1:1000) and antihistone 3 (1:1000). Blots for modified histone antibody were stripped and reprobed for total histone 3. The histone 4 antibody was undetectable; therefore the histone modifications (K12H4) are normalized to total H3 protein expression. Chemiluminescence was detected with either ECL (Amersham) or Super Signal (Pierce). Blots were analyzed using Image J.
Statistical analysis
Statistical analyses were performed using 2-way ANOVA (chronic condition × acute condition) or (acute stress × metyrapone injection) for the metyrapone experiment followed by post hoc test comparisons when an interaction was found. The Student t test was used for pair-wise comparisons. All analysis was performed using Graph Pad 6.
Results
Physiological signs of chronic stress
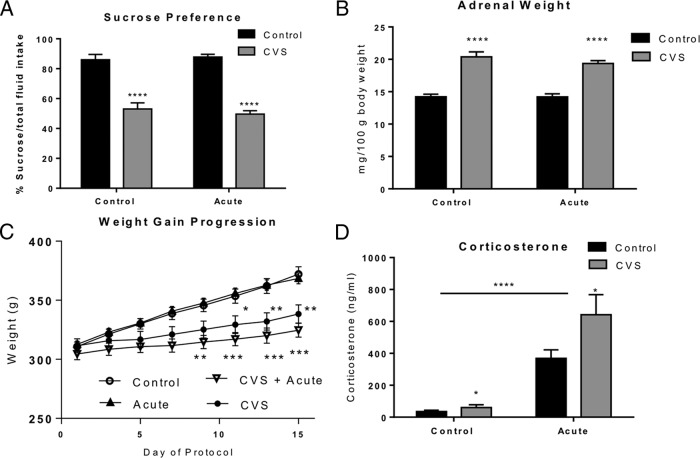
The sucrose preference test, which measures the rat's consumption of a palatable rewarding substance over a less appetitive choice, is a behavioral measure of anhedonia in rats that is affected during exposure to chronic stress (39, 40). Two-way ANOVA revealed a significant decrease of sucrose consumption in the CVS-treated animals [F(1, 13) = 105.5, P < .0001), but no effect of acute stress (F(1, 13) = 0.06, P = .82] and no significant interaction [F(1, 13) = 0.59; P = .46]. Figure 2A shows that both control animals and acute stress only animals demonstrated a clear preference for sucrose whereas animals exposed to CVS alone or CVS + acute stress failed to show preference for the sucrose solution. No significant difference was observed between all groups in total fluid intake (P = .3; data not shown). This pattern suggests that CVS exposure alone or in combination with a subsequent acute stress exposure does not affect fluid consumption but does alter preference for sucrose fluid.
Figure 2.
Physiological measures of chronic stress and acute stress. Quantification of sucrose preference test and physical parameters from animals subjected to either control conditions, 30 minutes of acute stress alone, 14 days of chronic variable stress (CVS) or 14 days of CVS plus 30 minutes of acute stress. A, Results from sucrose preference test administered as described in the Materials and Methods. CVS treatment caused a significant reduction in sucrose preference compared with control and acute stress rats (****, P < .0001, n = 6 per group). Acute stress had no significant effect on sucrose preference. B, Adrenal gland weight. CVS treatment caused a significant increase in adrenal weight relative to body weight compared with control animals (****, P < .0001; measured as mg/100g body weight; n = 12 per group). C, Change in body weight across stress paradigm. Both CVS (n = 12) and CVS plus novel acute stress (n = 12) rats showed a significant decrease in weight gain compared with control rats (n = 12). No significant difference in body weight gain was seen between CVS and CVS plus novel acute stress animals. D, CVS treatment resulted in a significant increase in serum levels of corticosterone from trunk blood compared with control animals (*, P < .05; n = 12). Furthermore, acute stress significantly increased serum levels of corticosterone compared with both control animals and CVS animals (****, P < .0001). Data are shown as means ± SE.
The adrenal weight of each animal was also recorded (Figure 2B). A significant main effect of chronic stress was observed [F(1, 44) = 106.9; P < .0001; 2-way ANOVA]. No effect of acute stress [F(1, 44) = 0.9; P = .35] and no interaction of chronic and acute stress [F(1, 44) = 0.84; P = .36] was observed. These results show that as predicted, CVS caused adrenal hypertrophy, whereas acute stress had no effect on adrenal weight.
As expected, both the CVS and CVS + novel acute stress groups demonstrated a significant decrease in body weight gain (Figure 2C) compared with handled controls and animals receiving acute stress only. Two-way ANOVA (repeated measures over days) revealed a significant overall interaction [F(21,352) = 1.76, P < .05] as well as a significant effect of experimental chronic stress condition [F(3,352) = 51.87, P < .001] and day of protocol [F(7,352) = 25.49, P < .0001]. Subsequent Bonferroni post hoc tests confirmed a significant reduction in body weight gain in the CVS animals by day 11 (P < .05), which persisted throughout the remainder of the experiment (day 13 P < .01; day 15 P < .01). In addition, CVS plus novel acute stress animals which were treated similarly prior to novel stress exposure demonstrated a similar decreased weight gain which was significant by day 9 of the experiment (P < .01) and persisted throughout the remainder of the experiment (day 11, 13, 15, P < .001). No significant difference in body weight gain was seen between CVS and CVS plus novel acute stress animals (Figure 2C). These results suggest that there were no differences in these two groups prior to TMT exposure. Furthermore, no significant difference in body weight gain was seen between control and acute stress only animals.
Finally we examined circulating levels of corticosterone at the time of sacrifice (Figure 2D). Two-way ANOVA revealed an overall significant effect of acute stress [F(1, 42) = 53.74; P < .0001] and CVS [F(1, 42) = 5.7; P = .02], and a trend to a significant interaction [F(1, 42) = 4; P = .054]. The pattern of corticosterone regulation suggests that exposure to a novel acute stress differentially regulates the response to the HPA axis and specifically corticosterone secretion after prior chronic stress exposure. It also suggests that modulation of the HPA axis by prior chronic stress exposure produces an exaggerated response in corticosterone to a novel acute stressor even compared with exposure to the same novel acute stressor alone as previously reported (3–9).
Regulation of extracellular signal-regulated kinase 1/2 in CVS after a subsequent novel stressor
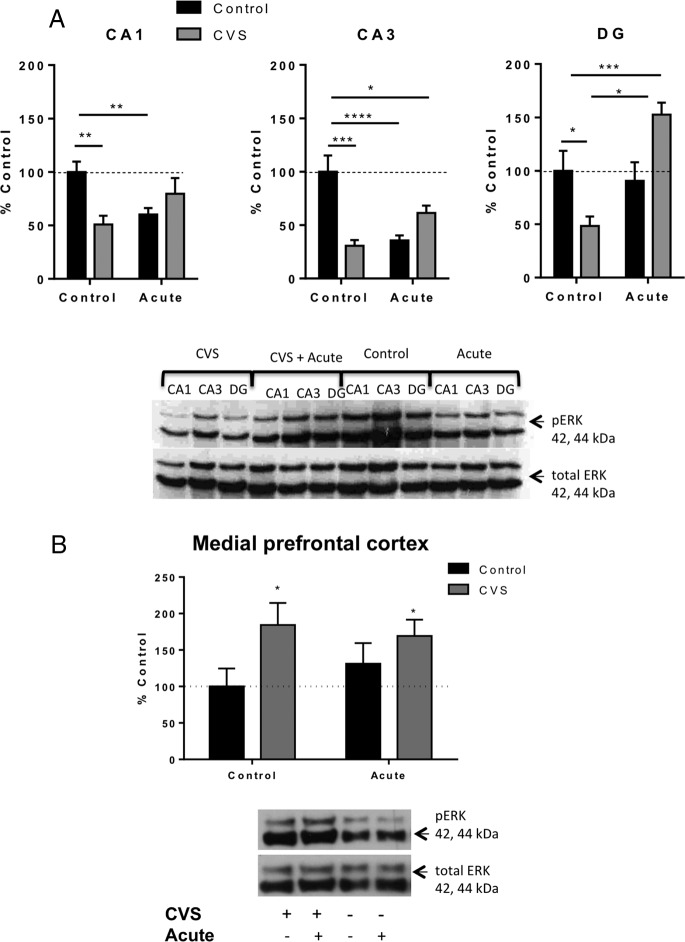
We previously showed decreased ERK1/2 activation in all areas of the hippocampus in response to CVS exposure (20). Therefore in the present study, we examined activation of ERK 1/2 in the subregions of the hippocampus of CVS, acute stress alone, CVS + novel acute stress and control animals (Figure 3A). Two-way ANOVA (chronic condition × acute condition) revealed a significant interaction in each area of the hippocampus [CA1: F(1, 18) = 10.27; P = .005), CA3: F(1, 18) = 23.63; P = .0001), and DG: F(1, 15) = 16.97; P = .0009]. Subsequent pair-wise comparisons showed a significant reduction in immunoreactivity of phospho-ERK antibody relative to total ERK in all three regions of the hippocampus of CVS animals [CA1 (51 ± 8%, n = 5 compared with control animals at 100 ± 10%, n = 6, P < .01); CA3 (31 ± 5%, n = 5 compared with control animals at 100 ± 15%, n = 6, P < .001); and DG (48 ± 9%, n = 5 compared with control animals at 100 ± 19%, n = 4, P < .05)]. By comparison, acute stress exposure resulted in a significant reduction in phospho-ERK reactivity in the CA1 (60 ± 6%, n = 5, P < .05) and CA3 (36 ± 5%, n = 5, P < .0001) compared with control animals, with no significant change in phospho-ERK immunoreactivity in the DG region of the hippocampus. CVS-treated animals exposed to the novel acute stress prior to killing showed a similar significant decrease in phospho-ERK immunoreactivity relative to total ERK in the CA3 region compared with control (61 ± 7%, n = 6, P < .05). On the other hand, CVS plus novel acute stress resulted in a significant increase from control animals in activation of ERK in the DG region of the hippocampus (153 ± 11%, n = 6, P < .001). Furthermore, animals exposed to CVS plus novel acute stress showed a significant increase in immunoreactivity of phospho-ERK antibody in the DG compared with animals exposed to CVS only (122 ± 13%, n = 6). No significant differences were observed between CVS and CVS plus novel acute stress animals in CA1 and CA3. The pattern of ERK1/2 regulation observed suggests that exposure to an acute novel stress after CVS exposure results in an increase in ERK1/2 activation specifically in the DG within the hippocampus. The mechanism for the acute stress effect was further investigated (see Supplemental Materials).
Figure 3.
Regulation of ERK in the hippocampus and medial prefrontal cortex of control, acute stress, CVS, CVS plus novel acute stress animals. A, Quantification and representative Western blots of phosphorylated ERK relative to total ERK in the separate hippocampus subregions of control, acutely stressed, chronic variable stressed (CVS) animals, and CVS plus novel acute stress (*, P < .05; **, P < .01; ***, P < .001; ****, P < .0001). B, Quantification and representative Western blots of phosphorylated ERK relative to total ERK in the mPFC. Chronic stress significantly increased ERK phosphorylation (*, P < .05, n = 3–4 per group), but acute stress had no effect. Data are shown as mean ± SEM.
The medial prefrontal cortex, another region of the brain implicated in the negative effects of chronic stress, was also investigated for changes in ERK phosphorylation (Figure 3B). Interestingly, ERK activation increased in response to CVS but acute stress had no effect. Two-way ANOVA revealed no significant interaction [F(1, 14) = 0.77, P = .4] or effect of acute stress [F(1, 14) = 0.09, P = .8, but a significant main effect of chronic stress [F(1, 14) = 5.438, P = .04]. This suggests that ERK activation in different brain areas is differentially regulated in response to both acute and chronic stress.
BCL-2 regulation in the hippocampus of CVS animals exposed to acute stress
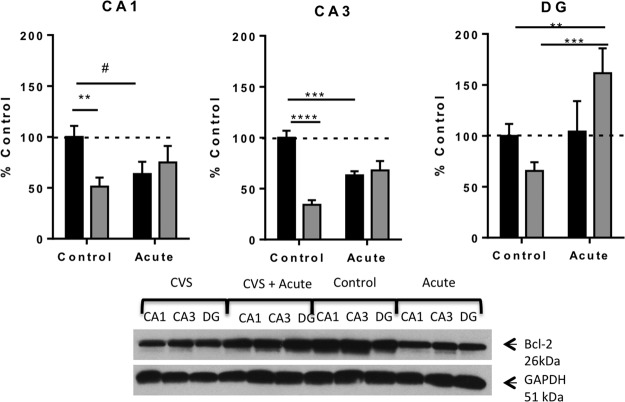
We previously showed that BCL-2 protein expression was decreased by chronic stress (20). We further investigated this regulation by acute stress (Figure 4). Two-way ANOVA revealed a significant interaction in each area of the hippocampus [CA1: F(1, 18) = 5.60; P = .03, CA3: F(1, 15) = 29.36; P < .0001), DG: F(1, 18) = 4.67; P = .04]. Furthermore, pair-wise comparisons demonstrated that there was a significant reduction in immunoreactivity of BCL-2 antibody relative to GAPDH antibody in the CA1 (51 ± 9%, n = 6), CA3 (34 ± 4%, n = 5), and DG (66 ± 8%, n = 5) regions of the hippocampus of CVS animals compared with control animals [CA1 (100 ± 11%, n = 6, P = .008); CA3 (100 ± 7%, n = 4, P < .0001); and DG (100 ± 12%, n = 6, P = .05)]. Similarly, acute stress resulted in a significant reduction in BCL-2 immunoreactivity in CA3 (63 ± 4%, n = 5, P < .001) and approached a significant reduction in the CA1 region of the hippocampus (64 ± 12%, n = 5, P = .051). No significant difference was observed between acutely stressed animals and control animals in the DG region. By contrast, acute stress exposure after CVS resulted in a significant increase in BCL-2 antibody immunoreactivity only in the DG region (162 ± 24%, n = 5) compared with control animals (P < .01) as well as compared with CVS animals (P < .001). No significant difference in BCL-2 immunoreactivity was observed in the CA1 and CA3 regions of the hippocampus of CVS plus novel acute stress animals compared with either control or CVS animals. The BCL-2 results were very similar to the ERK1/2 results and suggest a parallel interaction of ERK1/2 and BCL-2 expression particularly after in the DG in response to an acute stressor after chronic stress.
Figure 4.
Regulation of expression of the neuroprotective ERK target, BCL-2 by chronic and acute stress. This figure shows quantification and representative Western blots of BCL-2 relative to GAPDH protein from the hippocampus of control, acutely stressed, CVS animals, and CVS plus novel acute stress. Chronic and acute stress resulted in decreased BCL-2 expression in CA1 and CA3, but chronic plus acute stress increased BCL-2 expression in the DG. Data are shown as means ± SE; (*, P < .05; **, P < .01; ***, P < .0001; #, P = .054 (n = 4–6 animals per group).
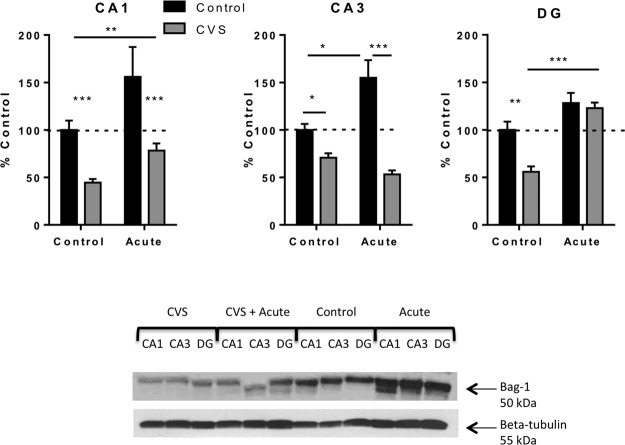
BAG-1 expression was also investigated in the subregions of the hippocampus (Figure 5). In CA1, there was no significant interaction [F(1, 11) = 0.63; P = .44], but a significant effect of acute stress [F(1, 11) = 10.31; P = .008], as well as chronic stress [F(1, 11) = 22.69; P = .0006]. A significant interaction was revealed in CA3 [F(1, 10) = 16.10; P = .003] and in the DG [F(1, 12) = 5.92; P = .03]. Subsequent pairwise comparisons revealed BAG-1 protein levels in CVS-treated rats were significantly decreased compared with control animals in CA3 (71 ± 5%, n = 4, P < .05) and DG (56 ± 6%, n = 4, P < .01). Furthermore, BAG-1 protein levels were significantly decreased in the DG region of CVS only animals (56 ± 6%, n = 4) compared with CVS animals that experienced a novel acute stress (123 ± 6%, n = 4, P < .001. Acute stress resulted in a significant increase in BAG-1 protein levels in the CA3 (155 ± 19%, n = 3) compared with control animals (100 ± 6%, n = 3, P < .05). The levels of BAG-1 protein expression after acute stress were also significantly elevated in CA3 compared with CVS-treated animals exposed to acute stress (71 ± 5%, n = 4, P < .001). These results suggest that BAG-1 is differentially regulated by acute and chronic stress.
Figure 5.
Regulation of BAG-1 protein expression in the hippocampus. This figure shows quantification and representative Western blots of BAG-1 relative to beta tubulin protein from the hippocampus of control, acute stress only, CVS, and CVS plus novel acute stress animals. CVS resulted in a significant decrease in BAG-1 protein relative to beta tubulin protein in all three regions of the hippocampus compared with control rats. CVS plus novel acute stress resulted in a significant increase of BAG-1 in the CA1 and DG of the hippocampus compared with CVS animals and a significant decrease in the CA3 compared with control animals. Data are shown as means ± SE; *, P < .05; **, P < .01; ***, P < .001 (n = 4 animals per group).
Regulation of histone acetylation after chronic stress exposure in response to a novel acute stress
We have previously shown that chronic stress decreases levels of acetylation of K12H4 in the hippocampus (20, 35). We looked at acetylation of K12H4 in the hippocampus of animals exposed to control, acute, CVS, and CVS plus novel acute stress conditions (Figure 6). A significant interaction of chronic and acute stress was observed in CA1 [F(1, 19) = 5.023; P = .04] and CA3 [F(1, 18) = 8.99, P = .008]. In DG, there was no significant interaction [F(1, 16) = 3.18; P = .09], but there was a significant effect of acute stress [F(1, 16) = 19.09; P = .0005] and no significant effect of chronic stress [F(1, 16) = 1.28; P = .27]. Subsequent pairwise comparisons revealed a significant decrease in immunoreactivity for the acetylated H4 (K12) antibody in the CA3 (53 ± 8%, n = 6) after CVS exposure compared with control animals CA3 (100 ± 18%, n = 5, P < .05). No significant change in acetylated H4 (K12) immunoreactivity was observed in CA1 although a trend was observed (P = .06). No significant change was observed in CA1 or CA3 of the hippocampus in animals exposed to TMT only compared with control animals. In addition, acute stress resulted in a significant increase in acetylated K12H4 in the CVS-treated animals in CA1 (P < .01). This pattern suggests that in the DG and CA1, the increase in acetylation of K12 H4 seen in the CVS plus novel acute stress animals not only rescues the deficit in K12H4 acetylation seen in the CVS animals but is actually enhanced compared with control animals.
Figure 6.
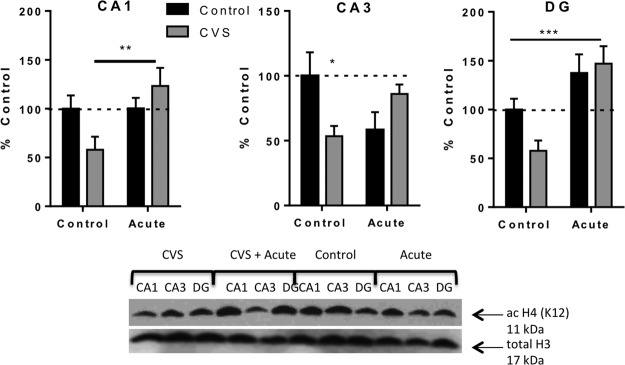
Changes in histone acetylation in response to acute stress, CVS, or CVS plus novel acute stress within the hippocampus are subregion-specific. This figure shows quantification and representative Western blots of acetylation of lysine 12 on histone 4 relative to total histone 3 protein in the hippocampus of control, acutely stressed, CVS, and CVS plus acute stress. Data are shown as mean ± SE; *, P < .05; **, P < .01; ***, P < .001 (n = 5–6 animals per group).
Discussion
We examined the molecular actions of HPA facilitation to novel acute stress in animals exposed to CVS compared with animals with a single exposure to either CVS alone or acute stress alone. We demonstrated a significant effect of chronic and acute stress such that when exposed to novel stress, animals that were previously exposed to CVS had an enhanced corticosterone response compared with CVS-exposed animals alone as well as animals that received acute stress alone. In addition, we showed for the first time that although CVS down-regulated ERK1/2 and its downstream target, BCL-2 in the subregions of the hippocampus, novel acute stress exposure in animals with previous CVS was able to reverse some of these deficits that were associated with concomitant regulation of acetylation of K12H4. These results suggest that activation of ERK1/2 may be upstream of both BCL-2 expression and K12H4 acetylation. In addition, although CVS activated ERK1/2 in the medial prefrontal cortex, acute stress had no effect. This shows that novel acute stress exposure in CVS animals is able to produce profound changes in chromatin regulation in several brain areas, with particularly interesting effects within the DG region of the hippocampus.
The levels of corticosterone that we observed in response to acute TMT exposure suggest that despite dysregulation of negative feedback onto the HPA axis subsequent to CVS exposure, the system was able to respond to the novel acute stress. In fact, the levels of corticosterone that we observed were significantly higher than the corticosterone levels of animals exposed acutely to TMT without prior stress experience, a finding that is consistent with other findings of HPA facilitation (3–9). Not surprisingly, CVS-treated animals exhibited reduced sucrose preference, whereas acute stress exposure, even after CVS, did not affect the sucrose preference. This pattern suggests that a single exposure to an acute stress has no effect on anhedonic behavior and the facilitation of the HPA axis and molecular changes observed in response to acute stress after prior CVS exposure had no effect on anhedonic behavior observed in CVS-treated animals.
In general, acute stress increased BAG-1 expression independent of prior stress experience, whereas chronic stress decreased BAG-1 protein expression. The exception was in the DG, where BAG-1 expression was increased in CVS-treated rats exposed to acute stress, suggesting a rapid differential response to acute stress of BAG-1 expression in the DG. BAG-1 and another GR cochaperone, FK506 binding protein 51 (FKBP5), are associated with mood disorders and act as negative modulator cochaperones of the GR and mainly act to modulate glucocorticoid sensitivity through inhibiting GR nuclear translocation. Polymorphisms in FKBP5 gene are associated with maladaptive responses to stress and development of mental disorders (41–43). Whereas less is known about BAG-1, it has been shown that under acute stress conditions GR activation can rapidly induce the transcription of its negative cochaperone proteins within an ultrashort negative feedback loop (44). The observed regulation of BAG-1 in the hippocampus following acute stress may represent a rapid GR-mediated feedback mechanism to attenuate the negative consequences of elevated corticosterone in regions of the hippocampus particularly vulnerable to glucocorticoids, specifically the DG. Further studies are necessary to determine the role of hippocampal BAG-1 expression in HPA axis regulation and the role the different subregions of the hippocampus play in this regulation.
We have previously shown that chronic stress results in a down-regulation of acetylation of K12H4 across the subregions of the hippocampus (20, 35). In this study, we found that K12H4 acetylation was significantly increased specifically in the DG in response to acute stress, suggesting differential regulation of H4 (K12) acetylation in the DG with acute and chronic stress. Furthermore, acute stress exposure subsequent to CVS was associated with concomitant increase of acetylation of K12H4, suggesting that this pathway demonstrates dynamic and reversible regulation despite chronic stress-induced down regulation. This change was specifically seen in the DG suggesting that in the DG, the increase in acetylation of K12H4 seen in the CVS plus novel acute stress animals not only rescues the effect seen in the CVS animals but is actually enhanced compared with control animals. This is consistent with recent studies that show that exposure to a novel acute stress (45) or corticosterone (46) following 21 days of chronic restraint stress leads to changes in patterns of gene expression compared with naïve exposed animals, particularly in the DG region of the hippocampus. Our findings here indicate that the increased gene expression seen in the DG of acutely stressed animals following chronic stress exposure may be associated with increased acetylation of K12 on histone 4, and increased epigenetic flexibility. This may represent an adaptive response to maintain function in an area that is particularly susceptible to glucocorticoids. Future studies will determine the specific genes regulation by this post-translational modification to determine the contribution these alterations have to the development of neuropsychological disorders associated with chronic stress exposure.
We further investigated possible mechanisms for the effect of acute stress on ERK activation in the hippocampus (Supplemental Materials). We were surprised to find that blockade of corticosterone synthesis with metyrapone did not block the effect of acute stress on ERK activation in CA1 and DG. Together, these results indicate that glucocorticoids alone do not mediate the molecular effects in the hippocampus. Other possible mechanisms include noradrenergic activity or endocannabinoids. Previous studies implicate noradrenergic activity in glucocorticoid-mediated memory retrieval impairments (48–50). In addition, it appears that endocannabinoids are also involved in the memory impairments mediated by glucocorticoids (51). Therefore, noradrenaline or cannabinoids may contribute to the reduced ERK activation observed in acutely stressed animals. Further investigation is necessary to determine the mechanism of this regulation.
Given that the magnitude of the response of the HPA axis to a novel acute stress is mediated by the efficiency of the negative feedback system and depends upon the pre-existing glucocorticoids tone, particularly in the hippocampus (47), the facilitation of the HPA axis to a novel acute stress seen in animals with prior stress experience may play a critical role in exaggerated stress-related memory formation and may contribute to psychopathology such as anxiety disorders and PTSD. The enhanced glucocorticoid response and increase in ERK1/2 phosphorylation and downstream pathways following an acute stressor may be a mechanism of formation of an intense distressing memory that results in a series of adaptations, including hypervigilance, personality changes, and cognitive impairments (48). Future studies will determine the role of interaction of chronic and acute stress in development of PTSD-like symptoms.
Acknowledgments
We would like to thank Dan Liu for her excellent technical assistance.
This work was supported by the NSF grant 1146853 and NIH/COBRE grant P20RR016816. Mentoring Neuroscience in Louisiana and Tulane Research Enhancement funds to L.A.S.
Present address for E.P.H.: Neuroscience Graduate Program, University of Virginia, Charlottesville, Virginia.
Disclosure Summary: C.L.F., E.P.H., M.L., and L.A.S. have nothing to declare. M.L. and E.P.H. were undergraduate students and C.L.F. was a graduate student at the time the work was performed. L.A.S. was employed by Tulane University.
Footnotes
- CVS
- chronic variable stress
- DG
- dentate gyrus
- ERK
- extracellular signal-regulated kinase
- GR
- glucocorticoid receptor
- HPA
- hypothalamic-pituitary-adrenal
- MAPK
- mitogen-activated protein kinase
- PTSD
- posttraumatic stress disorder.
References
- 1. Davidson RJ, McEwen BS. Social influences on neuroplasticity: stress and interventions to promote well-being. Nat Neurosci. 2012;15:689–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Herman JP, Cullinan WE. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci. 1997;20:78–84 [DOI] [PubMed] [Google Scholar]
- 3. Pardon MC, Ma S, Morilak DA. Chronic cold stress sensitizes brain noradrenergic reactivity and noradrenergic facilitation of the HPA stress response in Wistar Kyoto rats. Brain Res. 2003;971:55–65 [DOI] [PubMed] [Google Scholar]
- 4. Vining C, Iyer V, Bhatnagar S. Intracerebroventricular administration of corticotrophin-releasing hormone receptor antagonists produces different effects on hypothalamic pituitary adrenal responses to novel restraint depending on the stress history of the animal. J Neuroendocrinol. 2007;19:198–207 [DOI] [PubMed] [Google Scholar]
- 5. Bhatnagar S, Vining C. Facilitation of hypothalamic-pituitary-adrenal responses to novel stress following repeated social stress using the resident/intruder paradigm. Horm Behav. 2003;43:158–165 [DOI] [PubMed] [Google Scholar]
- 6. Hauger RL, Lorang M, Irwin M, Aguilera G. CRF receptor regulation and sensitization of ACTH responses to acute ether stress during chronic intermittent immobilization stress. Brain Res. 1990;532:34–40 [DOI] [PubMed] [Google Scholar]
- 7. Bhatnagar S, Dallman M. Neuroanatomical basis for facilitation of hypothalamic-pituitary-adrenal responses to a novel stressor after chronic stress. Neuroscience. 1998;84:1025–1039 [DOI] [PubMed] [Google Scholar]
- 8. Ostrander MM, Ulrich-Lai YM, Choi DC, Richtand NM, Herman JP. Hypoactivity of the hypothalamo-pituitary-adrenocortical axis during recovery from chronic variable stress. Endocrinology. 2006;147:2008–2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ottenweller JE, Natelson BH, Pitman DL, Drastal SD. Adrenocortical and behavioral responses to repeated stressors: toward an animal model of chronic stress and stress-related mental illness. Biol Psychiatry. 1989;26:829–841 [DOI] [PubMed] [Google Scholar]
- 10. Bangasser DA, Lee CS, Cook PA, Gee JC, Bhatnagar S, Valentino RJ. Manganese-enhanced magnetic resonance imaging (MEMRI) reveals brain circuitry involved in responding to an acute novel stress in rats with a history of repeated social stress. Physiol Behav. 2013;122:228–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mizoguchi K, Yuzurihara M, Ishige A, Sasaki H, Chui DH, Tabira T. Chronic stress differentially regulates glucocorticoid negative feedback response in rats. Psychoneuroendocrinology. 2001;26:443–459 [DOI] [PubMed] [Google Scholar]
- 12. Buwalda B, Felszeghy K, Horváth KM, Nyakas C, de Boer SF, Bohus B, Koolhaas JM. Temporal and spatial dynamics of corticosteroid receptor down-regulation in rat brain following social defeat. Physiol Behav. 2001;72:349–354 [DOI] [PubMed] [Google Scholar]
- 13. Ma S, Morilak DA. Chronic intermittent cold stress sensitises the hypothalamic-pituitary-adrenal response to a novel acute stress by enhancing noradrenergic influence in the rat paraventricular nucleus. J Neuroendocrinol. 2005;17:761–769 [DOI] [PubMed] [Google Scholar]
- 14. Schaeffer HJ, Weber MJ. Mitogen-activated protein kinases: specific messages from ubiquitous messengers. Mol Cell Biol. 1999;19:2435–2444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Meller E, Shen C, Nikolao TA, et al. Region-specific effects of acute and repeated restraint stress on the phosphorylation of mitogen-activated protein kinases. Brain Res. 2003;979:57–64 [DOI] [PubMed] [Google Scholar]
- 16. Pardon MC, Roberts RE, Marsden CA, et al. Social threat and novel cage stress-induced sustained extracellular-regulated kinase1/2 (ERK1/2) phosphorylation but differential modulation of brain-derived neurotrophic factor (BDNF) expression in the hippocampus of NMRI mice. Neuroscience. 2005;132:561–574 [DOI] [PubMed] [Google Scholar]
- 17. Chandramohan Y, Droste SK, Arthur JS, Reul JM. The forced swimming-induced behavioural immobility response involves histone H3 phospho-acetylation and c-Fos induction in dentate gyrus granule neurons via activation of the N-methyl-D-aspartate/extracellular signal-regulated kinase/mitogen- and stress-activated kinase signalling pathway. Eur J Neurosci. 2008;27:2701–2713 [DOI] [PubMed] [Google Scholar]
- 18. Gourley SL, Wu FJ, Kiraly DD, et al. Regionally specific regulation of ERK MAP kinase in a model of antidepressant-sensitive chronic depression. Biol Psychiatry. 2008;63:353–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Qi X, Lin W, Li J, Pan Y, Wang W. The depressive-like behaviors are correlated with decreased phosphorylation of mitogen-activated protein kinases in rat brain following chronic forced swim stress. Behav Brain Res. 2006;175:233–240 [DOI] [PubMed] [Google Scholar]
- 20. Ferland CL, Hawley WR, Puckett RE, et al. Sirtuin activity in dentate gyrus contributes to chronic stress-induced behavior and extracellular signal-regulated protein kinases 1 and 2 cascade changes in the hippocampus. Biol Psychiatry. 2013;74:927–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Flood DG, Finn JP, Walton KM, et al. Immunolocalization of the mitogen-activated protein kinases p42MAPK and JNK1, and their regulatory kinases MEK1 and MEK4, in adult rat central nervous system. J Comp Neurol. 1998;398:373–392 [DOI] [PubMed] [Google Scholar]
- 22. Ortiz J, Harris HW, Guitart X, Terwilliger RZ, Haycock JW, Nestler EJ. Extracellular signal-regulated protein kinases (ERKs) and ERK kinase (MEK) in brain: regional distribution and regulation by chronic morphine. J Neurosci. 1995;15:1285–1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Davis S, Vanhoutte P, Pages C, Caboche J, Laroche S. The MAPK/ERK cascade targets both Elk-1 and cAMP response element-binding protein to control long-term potentiation-dependent gene expression in the dentate gyrus in vivo. J Neurosci. 2000;20:4563–4572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sweatt JD. The neuronal MAP kinase cascade: a biochemical signal integration system subserving synaptic plasticity and memory. J Neurochem. 2001;76:1–10 [DOI] [PubMed] [Google Scholar]
- 25. Impey S, Obrietan K, Wong ST, et al. Cross talk between ERK and PKA is required for Ca2+ stimulation of CREB-dependent transcription and ERK nuclear translocation. Neuron. 1998;21:869–883 [DOI] [PubMed] [Google Scholar]
- 26. Reul JM, Hesketh SA, Collins A, Mecinas MG. Epigenetic mechanisms in the dentate gyrus act as a molecular switch in hippocampus-associated memory formation. Epigenetics. 2009;4:434–439 [DOI] [PubMed] [Google Scholar]
- 27. Levenson JM, O'Riordan KJ, Brown KD, Trinh MA, Molfese DL, Sweatt JD. Regulation of histone acetylation during memory formation in the hippocampus. J Biol Chem. 2004;279:40545–40559 [DOI] [PubMed] [Google Scholar]
- 28. Chwang WB, Arthur JS, Schumacher A, Sweatt JD. The nuclear kinase mitogen- and stress-activated protein kinase 1 regulates hippocampal chromatin remodeling in memory formation. J Neurosci. 2007;27:12732–12742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chwang WB, O'Riordan KJ, Levenson JM, Sweatt JD. ERK/MAPK regulates hippocampal histone phosphorylation following contextual fear conditioning. Learn Mem. 2006;13:322–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chandramohan Y, Droste SK, Reul JM. Novelty stress induces phospho-acetylation of histone H3 in rat dentate gyrus granule neurons through coincident signalling via the N-methyl-D-aspartate receptor and the glucocorticoid receptor: relevance for c-fos induction. J Neurochem. 2007;101:815–828 [DOI] [PubMed] [Google Scholar]
- 31. Bilang-Bleuel A, Ulbricht S, Chandramohan Y, De Carli S, Droste SK, Reul JM. Psychological stress increases histone H3 phosphorylation in adult dentate gyrus granule neurons: involvement in a glucocorticoid receptor-dependent behavioural response. Eur J Neurosci. 2005;22:1691–1700 [DOI] [PubMed] [Google Scholar]
- 32. Tsankova NM, Kumar A, Nestler EJ. Histone modifications at gene promoter regions in rat hippocampus after acute and chronic electroconvulsive seizures. J Neurosci. 2004;24:5603–5610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tsankova NM, Berton O, Renthal W, Kumar A, Neve RL, Nestler EJ. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci. 2006;9:519–525 [DOI] [PubMed] [Google Scholar]
- 34. Weaver IC, Cervoni N, Champagne FA, et al. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–854 [DOI] [PubMed] [Google Scholar]
- 35. Ferland CL, Schrader LA. Regulation of histone acetylation in the hippocampus of chronically stressed rats: a potential role of sirtuins. Neuroscience. 2011;174:104–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tanapat P, Hastings NB, Rydel TA, Galea LA, Gould E. Exposure to fox odor inhibits cell proliferation in the hippocampus of adult rats via an adrenal hormone-dependent mechanism. J Comp Neurol. 2001;437:496–504 [DOI] [PubMed] [Google Scholar]
- 37. Thomas RM, Urban JH, Peterson DA. Acute exposure to predator odor elicits a robust increase in corticosterone and a decrease in activity without altering proliferation in the adult rat hippocampus. Exp Neurol. 2006;201:308–315 [DOI] [PubMed] [Google Scholar]
- 38. Ferland CL, Schrader LA. Cage mate separation in pair-housed male rats evokes an acute stress corticosterone response. Neurosci Lett. 2011;489:154–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rygula R, Abumaria N, Flügge G, Fuchs E, Rüther E, Havemann-Reinecke U. Anhedonia and motivational deficits in rats: impact of chronic social stress. Behav Brain Res. 2005;162:127–134 [DOI] [PubMed] [Google Scholar]
- 40. Willner P, Towell A, Sampson D, Sophokleous S, Muscat R. Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology (Berl). 1987;93:358–364 [DOI] [PubMed] [Google Scholar]
- 41. Touma C, Gassen NC, Herrmann L, et al. FK506 binding protein 5 shapes stress responsiveness: modulation of neuroendocrine reactivity and coping behavior. Biol Psychiatry. 2011;70:928–936 [DOI] [PubMed] [Google Scholar]
- 42. Mahon PB, Zandi PP, Potash JB, Nestadt G, Wand GS. Genetic association of FKBP5 and CRHR1 with cortisol response to acute psychosocial stress in healthy adults. Psychopharmacology (Berl). 2013;227:231–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ising M, Depping AM, Siebertz A, et al. Polymorphisms in the FKBP5 gene region modulate recovery from psychosocial stress in healthy controls. Eur J Neurosci. 2008;28:389–398 [DOI] [PubMed] [Google Scholar]
- 44. Binder EB. The role of FKBP5, a co-chaperone of the glucocorticoid receptor in the pathogenesis and therapy of affective and anxiety disorders. Psychoneuroendocrinology. 2009;34(suppl 1):S186–S195 [DOI] [PubMed] [Google Scholar]
- 45. Gray JD, Rubin TG, Hunter RG, McEwen BS. Hippocampal gene expression changes underlying stress sensitization and recovery. Mol Psychiatry. 2013. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Datson NA, van den Oever JM, Korobko OB, Magarinos AM, de Kloet ER, McEwen BS. Previous history of chronic stress changes the transcriptional response to glucocorticoid challenge in the dentate gyrus region of the male rat hippocampus. Endocrinology. 2013;154:3261–3272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Buckingham JC, Loxley HD, Christian HC, Philip JG. Activation of the HPA axis by immune insults: roles and interactions of cytokines, eicosanoids, glucocorticoids. Pharmacol Biochem Behav. 1996;54:285–298 [DOI] [PubMed] [Google Scholar]
- 48. Mellman T, Lydiard RB. Posttraumatic stress disorder: characteristics and treatment. J Clin Psychiatry. 2008;69:e2. [DOI] [PubMed] [Google Scholar]